Abstract
Objective:
Consumption of green-leafy vegetables is being advocated beneficial for type 2 diabetes mellitus individuals possibly because they are cost effective source of potent biological antioxidants. This research analyzed various phytochemicals, free radicals scavenging antioxidant potentials and starch digesting enzymes inhibitory activities in fresh juice of nine green-leafy vegetables. Furthermore, this study also investigated influence of these vegetables juice on starch and glucose induced postprandial glycemic load.
Materials and Methods:
Phytochemical constituents, in vitro free radicals scavenging antioxidant and enzymes inhibitory activities were evaluated applying various reported methods. Post-prandial glycemic excursion was induced in rats pretreated with vegetables juice by oral administration of starch and glucose.
Results:
All the leafy vegetables juice displayed potent free radicals scavenging activities. Juice of amaranthus, rumex, palak and raphanus displayed potential anti-oxidative property by reducing H2O2 induced hemolysis in rats red blood cells RBCs. Ajwain and rumex juice showed pancreatic α-amylase inhibitory activity. Alternanthera, ajwain, methi, amaranthus and sowa leaves juice displayed intestinal α-glucosidase inhibitory activity. Juice of raphanus, ajwain and sowa significantly mitigated starch-induced postprandial glycemic load. Amaranthus leaves juice potently mitigated glucose-induced postprandial glycemic load and also reduced hemoglobin glycation induced by glucose in vitro.
Conclusions:
This investigation finds that juice of leafy vegetables is potent source of biological antioxidants. In addition, juice of raphanus, ajwain and sowa leaves possess capacity to mitigate starch induced postprandial glycemic burden and amaranthus leaves’ juice can reduce glucose induced postprandial glycemic excursion.
Keywords: Antioxidants, enzyme inhibition, green-leafy vegetables juice, oxidative stress, postprandial hyperglycemia
INTRODUCTION
Metabolic overload and resultant postprandial hyperglycemic excursion (PPHGE) induces redox imbalance that leads to the development of complex chronic diseases.[1] Abnormal surge in postprandial glycemia results from excessive and frequent ingestion of calorie-dense, refined starch-rich easily digestible foods and sugar-sweetened beverages.[2,3] Postprandial hyperglycemia (PPHG) has been recognized as one of the earliest detectable abnormalities expressed in ensuing type 2 diabetes mellitus (T2DM),[4] better predictor of progression of T2DM and cardiovascular disorders (CVD),[5] an important atherogenic[6] and oxidative stress[7] inducing factor. In fact, PPHG or hyperglycemia induced oxidative stress has been identified recently as an important pathophysiological link between diabetes mellitus and development of CVD[8] and diabetic complications.[9] Implications of oxidative stress in increasing pathogenesis of diabetic complications are suggested not only due to increased free radicals generation and imbalances in antioxidant defense systems but also due to free radicals mediated non-enzymatic protein glycation and auto oxidation of glucose.[10,11] Therefore, therapies that can mitigate development of PPHG, scavenge free radicals, protect free radicals mediated biomolecular and cellular damage may produce better results in controlling epidemic of T2DM and development of diabetic complications.
Even though, the efforts with currently available therapies to monitor raised blood glucose level is continue,[12] it is being comprehended that resorting to drug therapy for an epidemic caused by maladaptive modern diet is less rational than simply realigning eating habits.[13] Clinical features of diabetes in population of tropical and subtropical countries are observed distinct in various ways than that observed in Western European population. Therefore, it is also being realized that antidiabetic prescriptions and dietary regimens developed based on European physiological conditions require different approach for these populations.[14] It appears reasonable thus to identify antioxidant and anti-hyperglycemic activity in dietary materials to which these populations were traditionally adapted. Increased consumption of green, leafy vegetables have been observed to reduce risk of T2DM development.[15,16,17,18] However, there are inadequate experimental evidences to substantiate these assertions.
In this research, we present analysis of various chemical components in juice of nine commonly consumed leafy vegetables in India, explore their in vitro free radicals scavenging antioxidant activities and anti-oxidative stress potentials, effect on pancreatic α-amylase and intestinal α-glucosidase enzymes responsible for the digestion of calorie dense starch rich foods and release of glucose in to the blood circulation. Furthermore, this research also investigated influence of these leafy vegetables’ juice on starch and glucose induced postprandial hyperglycemic excursion (PPHGE) in rats.
MATERIALS AND METHODS
Chemicals
Chemicals used in this study were of high purity grade and purchased from Sigma-Aldrich chemicals (St Louis, MO USA), Merck (India) Limited (Mumbai, India) and S.D. Fine Chemicals Ltd (Mumbai, India).
Green leafy vegetables and juice preparation
Green leafy vegetables namely amaranthus (Amaranthus viridis L. Fam: Amarantheceae), alternanthera (Alternanthera sessilis L. Fam: Amarantheceae), sowa (Anethum sowa Roxb. Fam: Apiaceae), basella (Basella alba L. Fam: Basellaceae), methi (Trigonella foenum graceum L. Fam: Fabaceae), palak (Spinacea oleracea L. Fam: Amarantheceae), raphanus (Raphanus sativus L Fam: Brassicaceae.), rumex (Rumex vesicarius L. Fam: Polygonoaceae) and ajwain (Trachyspermum ammi L. Fam: Apiaceae) were procured from local markets of Hyderabad city (India). Leaves were washed thoroughly in running water and paste was prepared by grinding weighed amount of leaves in food grade grinder. The paste was transferred on to sterilized clean muslin cloth and squeezed to the maximum to obtain juice. Clear supernatant of the juice was obtained by centrifugation (5000 rpm for 30 min at room temperature) as described earlier[19] for further analysis.
Analysis of chemical components in fresh juice
Total polyphenols
In 2.5 mL of distilled deionised water, 25 μL of juice was added followed by 250 μL of Folin-Ciocalteu reagent (1 M) and sodium carbonate (20%, w/v). Reaction mixture was incubated in dark for 60 minutes and total polyphenol content was measured spectrophotometrically at 765 nm. Results were expressed in terms of gallic acid equivalent per mL.[20]
Total flavonoids
Equal volume of vegetables juice was mixed with 2% aluminum chloride in a 96-well micro-plate. Absorbance was recorded spectrophotometrically at 430 nm. Results were expressed as milligrams of rutin equivalent.[19]
Total anthocyanins
Anthocyanins content in juices was determined by mixing equal volumes of potassium chloride (25 mM, pH 1.0) and sodium acetate buffer (0.4M, pH 4.5). Absorbance at 510 nm and 700 nm were recorded and results were expressed as milligrams of anthocyanins per 100 mL of juice using a molar extinction coefficient of 26900, molecular weight of 449.2 and an absorbance of A = [(A510- A700) pH 1.0 - (A510- A700) pH 4.5].[19]
Total protein
In 240 μL of 1X Bradford reagent, 10 μL of juice was mixed in 96-well micro-plate and absorbance of the samples was read at 595 nm with micro-plate reader. Protein content was expressed in terms of bovine serum albumin equivalent.[19]
Determination of free radicals scavenging antioxidant potentials
2, 20′-Azinobis (3-Ethylbenzthiazoline-6-Sulfonate) cation (ABTS+) scavenging activity
ABTS·+ cation (0.5mM) was prepared by reaction of 2, 2′-Azinobis (3-ethyl benzthiazoline-6-sulphonic acid) with potassium persulphate (6.89 mM in phosphate buffer, pH 8.0) for 16h in dark. Decolourization of dark blue ABTS+ cation due to the action of various dilutions of vegetable's juice was recorded spectrophotometrically at 734 nm. Percentage of radical scavenging was calculated applying following formula: % radical scavenging = [(Absorbancecontrol-Absorbancejuice)/Absorbancecontrol] ×100. Suitable regression analysis was applied for calculation of concentration of juice required to scavenge 50% radical (SC50). Results were expressed in terms of μg/mL ascorbic acid equivalent.[19]
2, 2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity
Decolorization of DPPH radical was determined by the method reported earlier.[19] Briefly, in a 96-well microplate, 25 μL of various dilutions of fresh vegetable juice, 100 μL of tris-Hydrochloric acid (HCl) buffer (0.1 M, pH 7.4), and 125 μL DPPH solution (0.5 mM in methanol) were added and incubated in dark for 30 min. Absorbance was recorded (517 nm) and the percentage of DPPH scavenging by juices and SC50 values were obtained in terms of ascorbic acid equivalent concentration as above.
Hydrogen peroxide (H2O2) scavenging potentials
The determination of H2O2 scavenging activity was performed as follows: 40 μL of fresh juice was mixed with 1.2 mL of H2O2 solution (21.5 mM H2O2 in 0.1 M phosphate buffer (pH 7.4)). Absorbance values (230nm) of the reaction mixture were recorded at ‘0’ min and then at every 10th min up to 30 min spectrophotometrically. For each test sample a separate blank sample (devoid of H2O2) was used for background subtraction. The percentage of H2O2 scavenging was calculated by applying following formula [(Absorbancecontrol − Absorbancejuice)/Absorbancecontrol] ×100.
Prevention of glucose induced hemoglobin glycation
Blood was collected from adult male Wistar rats in tubes containing ethylenediaminetetraacetic acid (EDTA) and centrifuged at 1000 rpm for 20 min. Pellet was suspended with phosphate buffer saline (PBS, pH 7.4). Red Blood Corpuscles were lysed with 2 volumes of lysis buffer. Centrifugation was carried out to remove debris and supernatant containing hemoglobin was collected and diluted with PBS so as to get 5g/dL concentration. 0.5mL of the above solution was transferred to Eppendorf tube and incubated with 200μl juice for 10 min. 0.5 mL solution containing glucose (2g/100 mL) and gentamycin (20 mg/mL) was added and again incubated for 72 h. Amount of glycated hemoglobin in the reaction mixture was calculated by measuring absorbance at 443 nm.[21]
Ferric chloride reducing property
Ferric Chloride (FeCl3) reducing assay was adopted from Arumugam et al.[22] Briefly, 100 μL of various dilutions of fresh juice were mixed with 100 μL phosphate buffer and 100 μL potassium ferricyanide (1%). After 20 min incubation at 50°C, 10% trichloroacetic acid (TCA) was added to terminate reaction. Mixture was centrifuged at 3000 rpm for 10 min. 100 μL of supernatant was transferred into 96-well microplate and 100 μL of distilled water and 20 μL of 0.1% ferric chloride was added and mixed well. Absorbance was measured at 700 nm. Reducing power was expressed in terms of ascorbic acid equivalent concentration (mg/mL).
Prevention of H2O2 inducederythrocytes hemolysis
Blood sample was collected from Wistar rats in tubes containing EDTA and was stabilized by mixing with stabilizing solution (sodium citrate 13.2g, citric acid 4.8 g, and dextrose 14.7 g dissolved in 100 ml distilled water) in the ratio of 5:1 (v/v).[23] Stabilized blood was diluted to 0.4% with isotonic phosphate buffer (pH 7.4) and incubated with 100 μL ascorbic acid (100 mg/mL) or leafy vegetables juice before induction of oxidative stress by 600 μL H2O2 (0.5%). Total reaction mixture volume was made up to 2 mL with isotonic phosphate buffer in eppendorf tubes. Tubes were again incubated for 3h at 37°C and centrifuged (1000 g) for 5 min. Supernatant was read at 540/405 nm spectrophotometrically. The extent of hemolysis and inhibition were calculated accordingly.[24]
Enzyme inhibitory activity
Pancreatic α-amylase inhibition assay
Pancreatic α-amylase assay[25] was used with slight modification. In brief, 100 μl of juice was reconstituted in 100 μl phosphate buffer (20 mM, pH 6.8) containing 6.7 mM Sodium Chloride in 2ml Eppendorf tubes and incubated with 200μl porcine pancreatic α-amylase (4 U/ml prepared in ice cooled distilled water) for 10 min. The reaction was started by addition of 100 μl of soluble potato starch solution (0.5% w/v in 20 mM phosphate buffer, pH 6.9). Exactly after 10 min incubation, 400 μl of DNS color reagent (1.0 g of 3, 5-Di Nitro salicylic acid, 30 g of sodium potassium tartarate and 20 ml of 2N sodium hydroxide (NaOH) to a final volume of 100 ml in distilled water) was added. Closed tubes were placed in water bath (85-90°C) for 10 min to develop color and cooled. Reaction mixture (50 μL) was diluted with 175 μL of distilled water in a 96-well micro plate and absorbance (540 nm) was read spectrophotometrically. Percentage of inhibition was expressed in terms of acarbose equivalent concentration (mg/mL solution).
Rat intestinal α-glucosidase inhibition assay
Inhibition of rat intestinal α-glucosidase enzyme was done as reported earlier.[20] Briefly, in a 96-well microplate, 20 μL of juice was incubated with 50 μL of crude intestinal α-glucosidase for 5 min and then reacted with 50 μL of substrate (5 mM, p-nitrophenyl- α- glucopyranoside in 100 mM phosphate buffer pH, 6.8). Release of p-nitrophenol was measured spectrophotometrically at 405 nm. Percent enzyme inhibition was obtained applying formula mentioned above.
Animal experiments
Animal experiments were performed using male adult Wistar rats (180-220 g body weight). Institutional Animal Ethical Committee (CPCSEA Reg. No. 97/1999, Government of India) approval for experimental protocol was obtained. All experiments with live animals were performed in compliance with relevant laws and institutional guidelines. Experiments were performed as reported earlier.[19] All animals were kept for overnight fasting. The next day forenoon blood was collected from the retro orbital plexus in EDTA-containing tubes. Plasma glucose levels for basal (‘0’ hour) value were measured by glucose-oxidase test method using auto-blood analyzer. Rats were divided into various groups (six rats in each group of starch-induced postprandial glycemia, and five rats in each group of glucose-induced postprandial glycemia). The dose of vegetable's juice (7.5 mL/kg body weight) was selected according to earlier report[19] and was administered orally through gastric intubation in respective group of animals. Control group of animals were administered normal saline at the place of vegetables juice. Fifteen minutes after green leafy vegetables juice administration starch tolerance test[19] and 30 minutes after leafy vegetable juice administration to rats’ glucose tolerance test[26] was performed. Blood was collected at intervals of 30, 60, 90, and 120 minute post-starch or glucose feeding. Plasma was separated out for glucose measurement as described above. Two-hour postprandial glycemic load in terms of area under the curve (AUC0-120 minutes mg/dL) was calculated following trapezoidal rules.
Statistical analysis
One way ANOVA followed by Tukey's multiple comparison tests was applied to compare difference in animal study groups. The criterion for statistical significance was P < 0.05. Statistical analyses were performed by using GraphPad PRISM® Version 5.01 program.
RESULTS
Yield and chemical compositions
Yield and pH of juice from nine leafy vegetables is presented in Table 1. Leaves of rumex and ajwain were five times juicier than alternanthera. Juice of alternanthera and basella leaves was viscous. The pH of rumex and ajwain leaves was highly acidic (pH 3.5-3.75) than other leaves (pH 5.8-6.9). It is important to mention here that juice of rumex leaves tasted sour, methi leaves juice slightly bitter, and ajwain juice hot. Raphanus leaves juice smelled pungent. Smell of sowa leaves juice was pleasant.
Table 1.
Percentage yield of juice from different green-leafy vegetables and pH of the juice

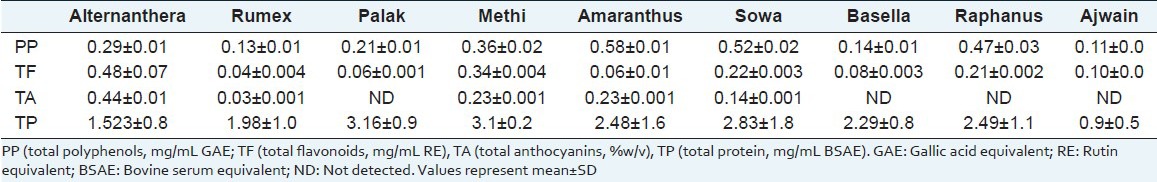
Table 2 presents concentration of different phytochemicals in juice of green-leafy vegetables. Wide quantitative differences in polyphenols, flavonoids, anthocyanins and proteins content was observed in juice [Table 2]. Juice of amaranthus, sowa, raphanus and methi leaves were rich in polyphenols. Anthocyanins could be detected only in juice of alternanthera, methi, amaranthus, sowa and rumex leaves. Juice from leaves of alternanthera, sowa and amaranthus were rich in protein content. High total flavonoids content was detected in alternanthera and methi leaves juice.
Table 2.
Concentration of phytochemicals in green leafy-vegetables
Free radicals scavenging and antioxidant activities
Table 3 summarizes various free radicals scavenging capacity and antioxidant potentials present in green-leafy vegetables juice. Methi and amaranthus juice were most potent in scavenging ABTS·+ cation. Ajwain juice was most potent in scavenging DPPH radical followed by amaranthus, sowa and raphanus. Similarly, amaranthus, basella, alternanthera, and sowa were potent in reducing FeCl3. Rumex, amaranthus, alternanthera and palak leaves juice were more potent in scavenging H2O2 than sowa, ajwain, methi and basella leave's juice.
Table 3.
Free radicals scavenging, antioxidant, pancreatic α-amylase, and intestinal α-glucosidase inhibitory activities of different leafy vegetables juice
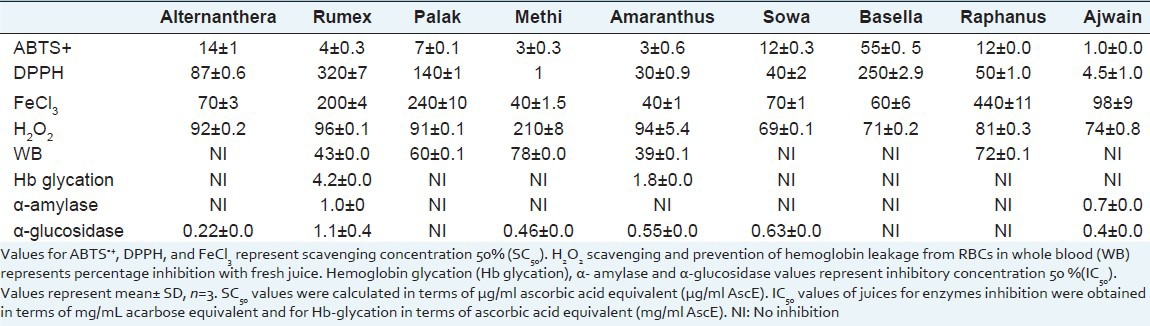
Fresh juice of raphanus and palak leaves prevented H2O2 induced hemolysis by more than 50% [Table 3]. However, prevention of hemolysis was less than 50% by juices of rumex and amaranthus leaves. Amaranthus leaves juice potently prevented glucose induced hemoglobin glycation followed by rumex juice [Table 3].
Enzyme inhibition
Inhibitory activity of leafy vegetables juice on these carbohydrate digestive enzymes is presented in Table 3. Juice of rumex and ajwain leaves inhibited porcine pancreatic α-amylase. Juice of alternanthera, ajwain, methi, amaranthus, sowa and rumex inhibited rat intestinal α-glucosidase [Table 3]. It was observed that in the order of potency, juice of alternanthera leaves was most potent followed by ajwain, methi and amaranthus leaves. Rumex and sowa leaves juice were observed to be mild inhibitors of rat intestinal α-glucosidase.
Oral starch and glucose tolerance test
Shape of plasma glucose concentration curve and 2 h glycemic load (AUC0-120 min mg/dL) following starch tolerance test in rats is presented in Figure 1. Juice of raphanus (12%, P < 0.001), ajwain (12%, P < 0.001) and sowa leaves (9%, P < 0.05) significantly mitigated starch induced glycemic load in rats [Figure 1f]. Juice of rumex [Figure 1a], methi and amaranthus [Figure 1d] moderately reduced starch induced postprandial glycemia. Juice of amaranthus leaves was potent in mitigating starch induced glycemic load (P < 0.05, Figure 1d) than palak juice.
Figure 1.
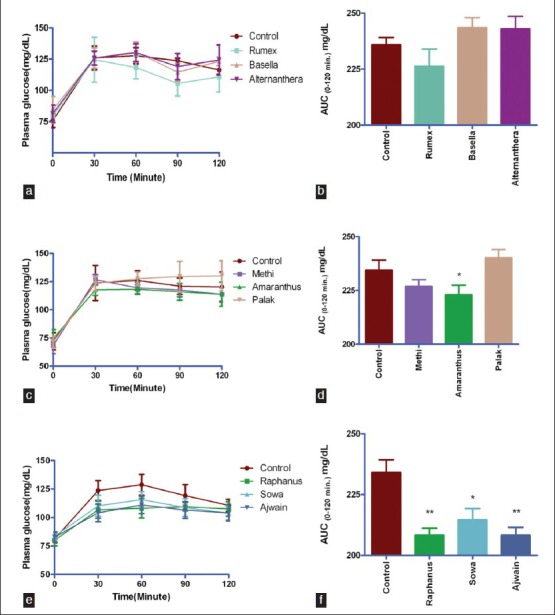
Shape of plasma glucose concentration curve overtime (min) and 2 h glycemic load (AUC0-120 min mg/dL)) following starch tolerance test in rats fifteen min after juice feeding. Values represent mean ± SE, n = 6. **P < 0.001, *P < 0.05 when compared with control (Figure f) and *P < 0.05 when compared with palak (Figure d)
Figure 2 presents shape of plasma glucose concentration curve and 2 h glycemic load (AUC0-120 min mg/dL) following glucose tolerance test in rats. It was observed that only amaranthus leaves juice could significantly (9%, P < 0.05) tone down glucose induced postprandial glycemic load [Figure 2d]. Rumex displayed moderate activity (4%, Figure 2b).
Figure 2.
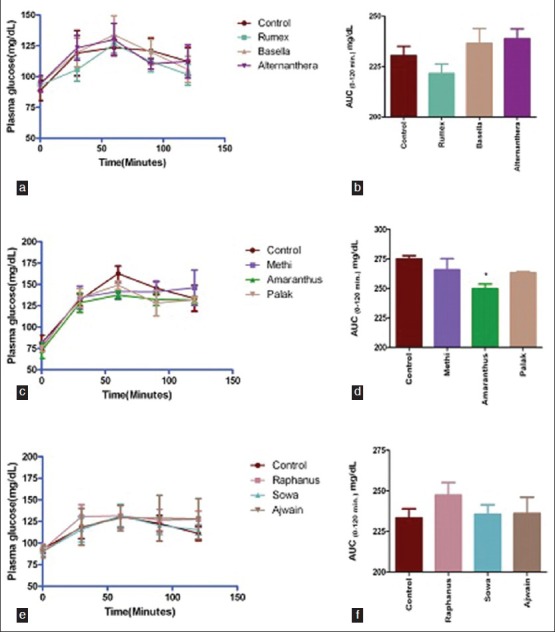
Shape of plasma glucose concentration curve overtime (minutes) and 2 h glycemic load (AUC0-120 min mg/dL)) following glucose tolerance test in rats 30 min after juice feeding. Values represent mean ± SE, n = 5. *P < 0.05 when compared with control (Figure d)
DISCUSSIONS
Polyphenols, flavonoids, phenolic acids, anthocyaninins and proteins constitute large and heterogeneous group of phytochemicals present in plant foods[19] and have been identified as major components responsible for antioxidant and other biological activities by various mechanisms.[27] It is important to mention here that although culinary plants materials are consumed daily as part of regular diet in large amounts, differences in terms of content and chemical composition can widely affect antioxidant properties of culinary plant materials as observed in our study.
Biological system has been equipped with multiple types of chemical and enzymatic antioxidants defense because it generates variety of free radicals through various redox systems operating in body.[19] Depending on the redox system and free radicals generated there in, antioxidants perform their activities by various mechanisms.[28,29] Due to these multiplicities involved in characteristics as well as mechanism of antioxidants, no single assay can reflect true nature and characteristics of an antioxidant in a mixture. Therefore, different free radicals based test methods were selected in this study. Analysis of this research finds that although all the juices studied displayed different activity potentials on various test models, only juice of amaranthus and rumex could display free radicals scavenging antioxidant activities on all the test systems studied.
Presence of different class of antioxidant phytochemicals like polyphenols, flavonoids, anthocyanins, and proteins in leafy vegetables juice might be held responsible for varying degree of antioxidant potentials. Similarly, variations in their antioxidant mechanisms may originate by virtue of their synergistic and cooperative interactions with structural diversities present in free radicals generation systems. However, despite their chemical and mechanistic diversities, health benefit of vegetable antioxidants have been unequivocally reported for their health protective benefits, including decreased risk of developing various forms of chronic diseases[30] and greater likelihood of healthy aging.[31]
Starch is primary component of digestible carbohydrate in human diet and major source of glucose that appears at relatively high concentration in blood circulation following intestinal digestion of a starch-rich meal. Therefore, attenuation of postprandial hyperglycemic excursion following starch rich diets have become important aspect for prevention and treatment of life-style associated diseases like T2DM, CVD and also in management of obesity.[32] Salivary and pancreatic α-amylase and intestinal α-glucosidase are key enzymes responsible for digestion of dietary carbohydrates into glucose. Inhibitions of these enzymes suppress postprandial hyperglycemic excursion. Juice of ajwain and rumex leaves were found potent inhibitor of pancreatic α-amylase and intestinal α-glucosidase. Alternanthera, ajwain, methi, amaranthus and sowa leaves inhibited only intestinal α-glucosidase.
Unusual surge in postprandial glycemia results from excessive and frequent ingestion of calorie-dense, refined starch-rich easily digestible foods and sugar-sweetened beverages,[2] and leads to development of impaired glucose tolerance (IGT). IGT is intermediate pre-diabetes state which often progresses to overt diabetes within a few years.[33] It has been demonstrated that early lifestyle modifications can regress prediabetic IGT to normal state and also decrease diabetes development rates in prediabetic individuals.[33] Dietary adjunct that can mitigate starch or glucose induced postprandial glycemic spikes therefore, may help prevent development of starch-rich diet or sugar-sweetened beverages induced IGT development.[20,26] Furthermore, consumption of such dietary adjuncts may also help prevent or delay progression of IGT into diabetes. Analysis of nine leafy vegetables juice in this study reveals that consumption of raphanus, ajwain and sowa leaves juice may help mitigate starch induced PPHGE and PPHG. Increased consumption of amaranthus over palak leaves may be advantageous for, amaranthus leaves juice significantly toned down starch induced glycemic load than palak juice.
Juice of rumex and ajwain leaves displayed potent pancreatic α-amylase and intestinal α-glucosidase inhibitory potentials in in-vitro assay. However, only ajwain could demonstrate antihyperglycemic activity in vivo. Moderation in starch induced postprandial glycemic spikes by rumex, methi and amaranthus may be partially ascribed to their intestinal α-glucosidase inhibitory activity. Antihyperglycemic activity of raphanus leaves juice in starch induced postprandial glycemia may be arising through some other mechanisms as it could inhibit neither pancreatic α-amylase nor intestinal α-glucosidase enzyme. On the other hand, only amaranthus leaves juice could significantly tone down the glucose induced postprandial glycemic burden. Therefore, consumption of amaranthus leaves juice could be beneficial in reducing glycemic burden either induced by starch and the glucose.
In conclusion analysis in this research finds that juice of green leafy vegetables are rich source of biological antioxidant and may help counterbalance through diverse mechanisms hyperglycemia induced imbalanced antioxidant defense. In addition to the potent source of biological antioxidants, juice of ajwain and sowa can help mitigate starch induced PPHG possibly by slowing down starch digestion and amaranthus leaves juice keep within bounds glucose induced PPHG. The mechanism by which raphanus and amaranthus leaves juice reduces starch and glucose induced PPHG respectively requires further investigation.
ACKNOWLEDGEMENTS
The authors thank Director, IICT, Hyderabad, for constant support, encouragements and personal support to carry out this research work. Authors also thank reviewers for their constructive comments and suggestions. This research was supported financially in part by project grant NaPAHA-CSC-0130 (CSIR- New Delhi).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kim JY, Kwon O. Culinary plants and their potential impact on metabolic overload. Ann N Y Acad Sci. 2011;1229:133–9. doi: 10.1111/j.1749-6632.2011.06090.x. [DOI] [PubMed] [Google Scholar]
- 2.Malik VS, Hu FB. Sweeteners and risk of obesity and type 2 Diabetes: The role of sugar-sweetened beverages. Curr Diab Rep. 2012;12:195–203. doi: 10.1007/s11892-012-0259-6. [DOI] [PubMed] [Google Scholar]
- 3.O’Keefe JH, Gheewala NM, O’Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008;51:249–55. doi: 10.1016/j.jacc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Gerich JE. Pathogenesis and treatment of type 2 (non insulin-dependent) diabetes mellitus (NIDDM) Horm Metab Res. 1996;28:404–12. doi: 10.1055/s-2007-979828. [DOI] [PubMed] [Google Scholar]
- 5.Davidson J. Should post prandial glucose Be measured and treated to a particular target? Yes. Diabetes Care. 2003;26:1919–21. doi: 10.2337/diacare.26.6.1919. [DOI] [PubMed] [Google Scholar]
- 6.Ceriello A. The post-prandial state and cardio vascular disease; relevance to diabetes mellitus. Diabetes Metab Res Rev. 2000;16:125–32. doi: 10.1002/(sici)1520-7560(200003/04)16:2<125::aid-dmrr90>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Ceriello A. Cardiovascular effects of acute hyperglycemia pathophysiological underpinnings. Diabetes Vasc Dis Res Rev. 2008;5:260–8. doi: 10.3132/dvdr.2008.038. [DOI] [PubMed] [Google Scholar]
- 8.Dokken BB. The pathophysiology of cardiovascular disease and diabetes: Beyond blood pressure and lipids. Diabetes Spectr. 2008;21:160–5. [Google Scholar]
- 9.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 10.Mullarkey CJ, Edelstein D, Brownlee L. Free radical generation by early glycation products: A mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Comm. 1990;173:932–9. doi: 10.1016/s0006-291x(05)80875-7. [DOI] [PubMed] [Google Scholar]
- 11.Wassmann S, Wassmann K, NIckenig G. Modulation of oxidant and antioxidant enzyme and function in vascular cells. Hypertension. 2004;44:381–6. doi: 10.1161/01.HYP.0000142232.29764.a7. [DOI] [PubMed] [Google Scholar]
- 12.Type 2 diabetes – time to change our approach. Lancet. 2010;375:2193. doi: 10.1016/S0140-6736(10)61011-2. [DOI] [PubMed] [Google Scholar]
- 13.Mitrou PN, Kipnis V, Thiebaut CM, Reedy J, Subar AF, Wirfalt E, et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: Results from the NIN-AARP diet and health study. Arch Intern Med. 2007;167:2461–8. doi: 10.1001/archinte.167.22.2461. [DOI] [PubMed] [Google Scholar]
- 14.Hoskote SS, Joshi SR. Are Indians destined to be diabetic? J Assoc Physicians India. 2008;56:225–6. [PubMed] [Google Scholar]
- 15.Schmidt BM, Ribnicky DM, Lipsky PE, Raskin I. Revisiting the ancient concept of botanical therapeutics. Nat Chem Biol. 2007;3:360–6. doi: 10.1038/nchembio0707-360. [DOI] [PubMed] [Google Scholar]
- 16.Bogaert YE, Schrier RW. Into the future: Prevention of diabetes. Contrib Nephrol. 2011;170:256–63. doi: 10.1159/000325781. [DOI] [PubMed] [Google Scholar]
- 17.Asif M. The role of fruits, vegetables, and spices in diabetes. Int J Nutr Pharmacol Neurol Dis. 2011;1:27–35. [Google Scholar]
- 18.Carter P, Gray LJ, Troughton J, Khunti K, Davies NJ. Fruit and vegetable intake and incidence of type II diabetes mellitus: Systematic review and meta-analysis. Br Med J. 2010;341:c4229. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiwari AK, Reddy KS, Radhakrishnan J, Kumar DA, Zehra A, Agawane SB, et al. Influence of antioxidant rich fresh vegetable juices on starch induced postprandial hyperglycemia in rats. Food Funct. 2011;2:521–8. doi: 10.1039/c1fo10093a. [DOI] [PubMed] [Google Scholar]
- 20.Tiwari AK, Swapna M, Ayesha SB, Zehra A, Agawane SB, Madhusudana K. Identification of proglycemic and antihyperglycemic activity in antioxidant rich fraction of some common food grains. Int Food Res J. 2011;18:915–23. [Google Scholar]
- 21.Asgary S, Naderi GH, Sarrafzadegan N, Ghassemi N, Boshtam M, Rafie M, et al. Anti-oxidant effect of flavonoids on hemoglobin glycosylation. Pharm Acta Helv. 1991;73:223–6. doi: 10.1016/s0031-6865(98)00025-9. [DOI] [PubMed] [Google Scholar]
- 22.Arumugam P, Murugan R, Subathra M, Ramseh A. Superoxide radical scavenging and antibacterial activites of different fractions of ethanol extract of Mentha spicata (L.) Med Chem Res. 2010;19:664–73. [Google Scholar]
- 23.Jha HC, Bergmann C, Zilliken F. Inhibitors of hydrogen peroxide-induced haemolysis of bovine erythrocytes. Biochem Pharmacol. 1984;33:1893–5. doi: 10.1016/0006-2952(84)90544-6. [DOI] [PubMed] [Google Scholar]
- 24.Malagoli D. A full-length protocol to test hemolytic activity of palytoxin on human erythrocytes. Invertebrate Surviv J. 2007;4:92–4. [Google Scholar]
- 25.Gowri PM, Tiwari AK, Ali ZA, Rao JM. Inhibition of α-glucosidase and amylase by Bartogenic Acid isolated from Barringtonia racemosa Roxb. Seeds. Phytother Res. 2007;21:796–9. doi: 10.1002/ptr.2176. [DOI] [PubMed] [Google Scholar]
- 26.Tiwari AK, Anusha I, Sumangali M, Anand DK, Madhusudana K, Agawane SB. Preventive and therapeutic efficacies of Benincasa hispida and Sechium edule fruit's juice on sweet-beverages induced impaired glucose tolerance and oxidative stress. Pharmacologia. 2013;4:197–207. [Google Scholar]
- 27.Ignat I, Volf I, Popa VI. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–35. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 28.Prior RI, Wu X, Schaich K. Standardised methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2000;48:4290–302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 29.Ishige K, Schubert D, Sagara Y. Flavanoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radical Biol Med. 2001;30:433–46. doi: 10.1016/s0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 30.Chu YF, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem. 2002;50:6910–6. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- 31.Podda M, Grundmann-Kollmann M. Low molecular weight antioxidants and their role in skin ageing. Clin Exp Dermatol. 2001;26:578–82. doi: 10.1046/j.1365-2230.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 32.Butterworth PJ, Warren JF, Ellis PR. Human α-amylase and starch digestion: An interesting marriage. Starch/Strake. 2011;63:395–405. [Google Scholar]
- 33.Moutzouri E, Tsimihodimos V, Rizos E, Elisaf M. Prediabetes: To treat or not to treat? Eur J Pharmacol. 2011;672:9–19. doi: 10.1016/j.ejphar.2011.10.007. [DOI] [PubMed] [Google Scholar]


