Abstract
Hydrogen sulfide, an important gaseous signaling agent generated in numerous biological tissues, influences many physiological processes. This biological profile appears reminiscent of nitric oxide, another important endogenously synthesized gaseous signaling molecule. Hydrogen sulfide reacts with nitric oxide or oxidized forms of nitric oxide and nitric oxide donors in vitro to form species that display distinct biology compared to both hydrogen sulfide and NO. The products of these interesting reactions may include small molecule S-nitrosothiols or nitroxyl, the one-electron reduced form of nitric oxide. In addition, thionitrous acid or thionitrite, compounds structurally analogous to nitrous acid and nitrite may constitute a portion of the reaction products. Both the chemistry and biology of thionitrous acid and thionitrite, compared to nitric oxide or hydrogen sulfide, remain poorly defined. General mechanisms for the formation of S-nitrosothiols, nitroxyl and thionitrous acid based upon the ability of hydrogen sulfide to act as a nucleophile and reducing agent with reactive nitric oxide-based intermediates are proposed. Hydrogen sulfide reactivity appears extensive and could impact numerous areas of redox controlled biology and chemistry warranting more work in this exciting and developing area.
Keywords: hydrogen sulfide, nitric oxide, nitroxyl, S-nitrosothiol, redox chemistry
Introduction
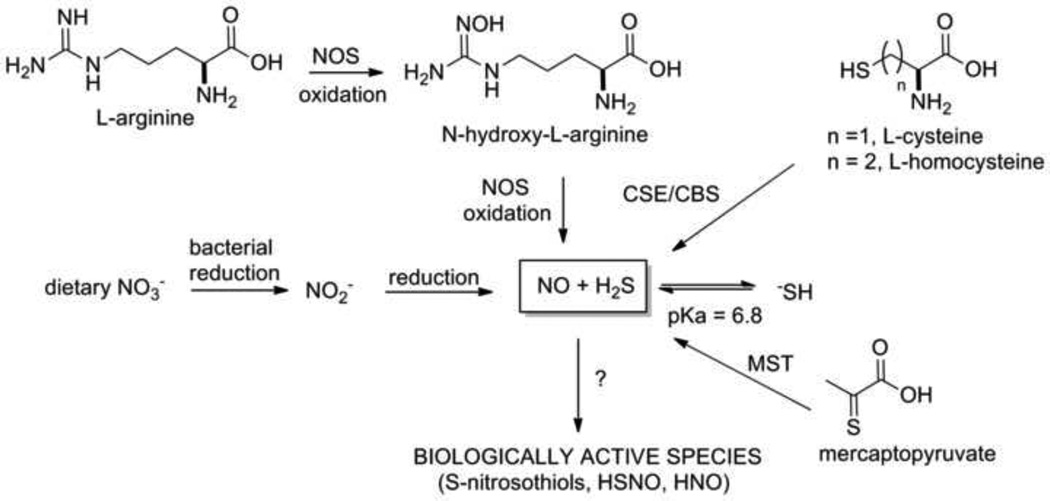
The identification of nitric oxide (NO) as a biological signaling agent established the validity of endogenously produced gases as mediators of numerous physiological processes. [1, 2] Once considered only an environmental toxin, NO plays important roles in blood pressure and flow control through soluble guanylate cyclase (sGC) activation, the immune response and neurotransmission. [1, 3] Early work on NO biosynthesis reveals the nitric oxide synthase (NOS) catalyzed oxidation of L-arginine to form NO and L-citrulline through the intermediacy of N-hydroxy-L-arginine (Figure 1). [3, 4] This pathway to NO (N formal oxidation state = +2) proceeds through a substrate at the ammonia oxidation state (N = −3) and an intermediate at the hydroxylamine oxidation state (N = −1). [4] The conversion of dietary nitrate (N formal oxidation state = +5) to nitrite (N formal oxidation state = +3) by oral bacteria in humans and the subsequent chemical or enzymatic reduction to NO provides an important concurrent reductive pathway to NO formation (Figure 1). [5] Together these pathways produce NO in humans from both oxidative and reductive pathways that utilize nearly all of the established oxidation states of nitrogen. The chemical and biological aspects of NO signaling have been thoroughly reviewed. [1, 3, 6]
Figure 1.
Biological production of nitric oxide and hydrogen sulfide and their possible reactions.
Similar to NO, hydrogen sulfide (H2S) displays increasing importance as an endogenously produced gaseous biological signaling agent. [2, 7–9] Like NO, H2S demonstrates toxicity at higher concentrations but mediates numerous physiological processes associated with the cardiovascular, nervous and immune (inflammatory response) systems. [7–11] Despite this activity, specific mechanisms for the action of H2S remain to be elucidated and precise H2S levels in biology remain debated. [2, 10, 11] Hydrogen sulfide forms during normal L-cysteine metabolism from the pyridoxal-5’-phosphate (PLP)-dependent cystathione β-synthetase (CBS) and cystathione γ-lyase (CSE) catalyzed reactions of cysteine, homocysteine, and cystathione or the action of 3-mercaptopyruvate sulfur transferase (MST) on 3-mercaptopyruvate (Figure 1). [10, 12, 13] Hydrogen sulfide formation occurs in a variety of tissues and excellent reviews regarding H2S chemistry and biology exist. [6, 10–15] This paper reviews the limited literature regarding the chemistry and biology resulting from the interaction of H2S and NO and proposes potential reactions between H2S and NO and its redox forms to generate other biologically active species that may form the chemical basis of NO/H2S “cross-talk”. [15, 16] Much chemical and biological work remains to completely define the chemistry and biology of this system.
Chemically, hydrogen sulfide represents the sulfur analog of water and the smallest, structurally simplest thiol. Both the organic and biological chemical reactivity of H2S have been reviewed. [6, 10, 11, 17] The size and electronegativity of the sulfur atom increase the acidity of H2S compared to water giving a first pKa of 6.8, which results in about two-thirds of H2S existing in the anionic form −SH at pH 7.4 (Figure 1). [6] The second pKa of 14.1 reveals that only small amounts of S−2 exist at pH 7.4. [6] The polarizability of the sulfur atom makes the sulfide anion (−SH) an excellent nucleophile that reacts with numerous electrophilic organic substrates. [6, 17] Hydrogen sulfide and the −SH anion reduce a variety of organic substrates. [17] Electrochemical evidence indicates −SH acts as a weaker two electron reducing agent (forming S0) than glutathione and cysteine but the redox potential of H2S appears similar to both cysteine and glutathione. [13, 18] Hydrogen sulfide preferentially (compared to cysteine and glutathione) reduces aromatic azides and nitro compounds to the amines forming the basis of new and selective fluorescent H2S probes indicating H2S acts as a better reducing agent than biological thiols under these conditions. [19, 20] Both the solution pH and the ability of H2S to act as a nucleophile (donating electrons) will influence the observed reaction chemistry of H2S, which merits further exploration based on recent biological discoveries. Generally, H2S/−SH acts as a nucleophile and reducing agent and should form addition complexes with electrophilic nitrogen oxides and reduce NO or its oxidized forms. [6, 16] Species susceptible to reaction include nitrate, nitrite, S-nitrosothiols, N2O3, peroxynitrite, NO, HNO and even NO-based metabolites, such as the electrophilic nitrated fatty acids (Figure 1). [16] These reactions allow the conversion of higher oxidation state nitrogen oxides to lower oxidation state nitrogen oxides that include NO, HNO and NH3, providing another mechanism for H2S-mediated biology.
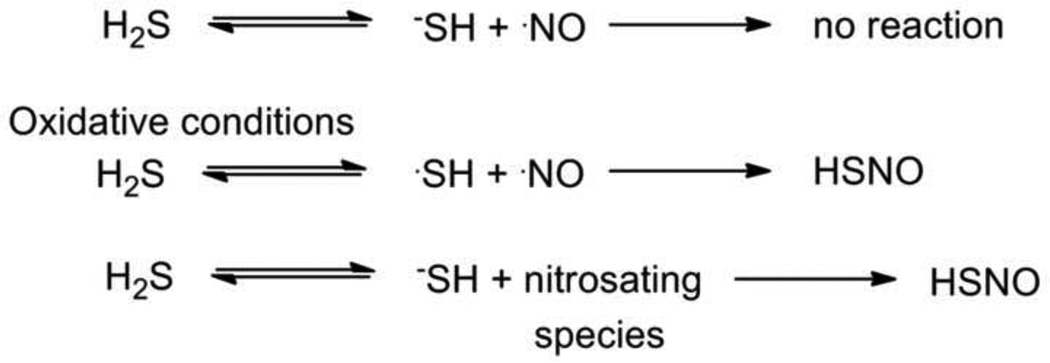
Given that H2S exists as the diamagnetic acid/base pair of H2S/−SH at physiological pH and the paramagnetic nature of NO, the direct reaction of H2S and NO remains unlikely, similar to the direct reaction of thiols with NO (Figure 2). [21–23] Calculations provide a H2S bond dissociation energy of 90 kcal/mol making H atom abstraction by NO to form HNO (H-N bond dissociation energy 47 kcal/mol) thermodynamically unfavorable. [6, 24] Direct reaction of NO with H2S requires oxidation of either H2S to the HS˙ radical followed by coupling with NO or oxidation of NO to a nitrosating species followed by reaction with −SH (formal transfer of the nitrosonium ion, a species too reactive to exist in aqueous environments) reactions that both would be predicted to yield the simplest S-nitrosothiol (HNSO, Figure 2), similar to thiol nitrosation to generate S-nitroso thiols. [21, 22, 25, 26]
Figure 2.
Potential reactions of nitric oxide and hydrogen sulfide.
Reactions of Hydrogen Sulfide with NO Donors
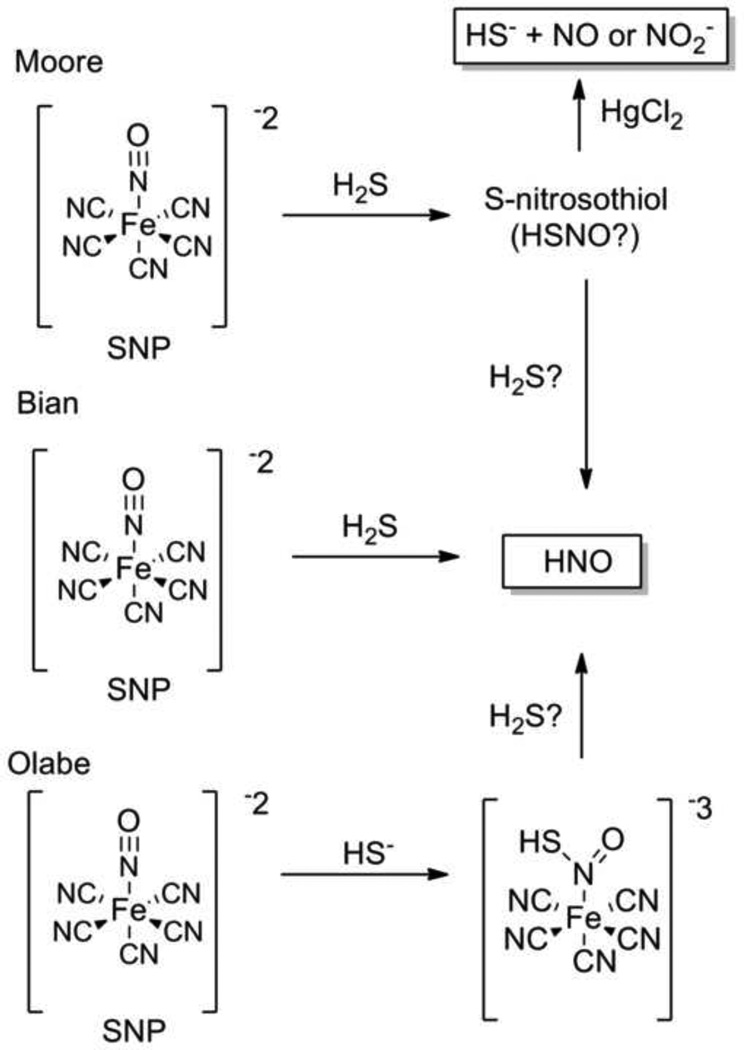
The discovery of peroxynitrite (−OONO) scavenging of H2S prompted the study of the reactions of NO donors and H2S by Moore and co-workers. [27] In this work, the mixture of various NO donors and H2S (in the form of sodium sulfide, NaSH) forms a new species that demonstrates the general chemical and biochemical characteristics of an S-nitrosothiol. [28] Specifically, the mixture of sodium nitroprusside (SNP, 2 Na+ [Fe(CN)5NO]−2) and other mechanistically distinct NO donors with NaSH generates nitrite in a time, concentration and mercuric chloride (HgCl2), which facilitates S-nitrosothiol decomposition to nitrite, dependent manner. [28] Similar amperometric and electron paramagnetic resonance (EPR) studies show that addition of NaSH to NO donors decreases the amount of released NO suggesting formation of an intermediate S-nitrosothiol that decomposes to NO upon treatment with HgCl2 (Figure 3). [28] Treatment of RAW264.7 cells with a mixture of the NO donor, SNP (100 µM) and NaSH (100 µM) does not result in an increase of cyclic guanosine monophosphate (cGMP) suggesting NaSH blocks NO release from SNP due to intermediate formation. [28] Addition of CuCl2 to these cells produces significant increases in cGMP again supporting an S-nitrosothiol intermediate. Experiments with the liver homogenates of lipo-polysaccharide (LPS) treated rats, a tissue model capable of both NO and H2S biosynthesis, show similar trends. [28] Addition of L-cysteine and PLP (for endogenous H2S production) to this tissue does not increase nitrite formation as a measure of NO, but the addition of HgCl2 increases baseline nitrite levels providing further evidence of an S-nitrosothiol intermediate. [28]
Figure 3.
Reactions of hydrogen sulfide with sodium nitroprusside. Top: S-nitrosothiol formation. (Moore), Middle: HNO formation (Bian), Bottom: Direct reaction with sodium nitrosoprusside (Olabe).
This work reveals two particularly important findings: 1) the addition of H2S (as NaSH) to different NO donors decreases the amount of released NO or blocks NO release and 2) the addition of H2S to NO donors changes the expected NO-based biological function. The treatment of these reaction mixtures with HgCl2 or CuCl2 produces nitrite or NO and restores the expected biological function leading the authors to suggest S-nitrosothiol formation. [28] While not explicitly defined structurally, a likely candidate for this species would be the simplest S-nitrosothiol, HSNO (thionitrous acid, Figure 3). Chemical mechanisms for S-nitrosothiol formation were not proposed, but the direct reaction between NO and H2S appears unlikely (Figure 2). The presence of oxygen during these experiments (aerobic conditions) may allow NO oxidation to give a nitrosating species capable of reacting with H2S and forming HSNO as an intermediate. Such a pathway remains consistent with the chemical ability of H2S to act as either a nucleophile or reducing agent by reacting with an oxidized NO species.
A recent report by Bian and co-workers similarly reveals that administration of NO donors and H2S (as NaSH) to cardiac myocytes elicits responses distinct to either the NO donor or NaSH alone. [29] Specifically, NaSH does not affect myocyte contractility and three mechanistically distinct NO donors (including SNP) decrease contractility (negative inotropic effect). [29] Addition of both NaSH and the NO donors however yields an increase in myocyte contractility (positive inotropic effect). [29] Other experiments show that SNP (50 µM) and NaSH (50 µM) increase the efficiency of electrically stimulated cell contraction and relaxation by significantly increasing the amplitude of the calcium transient while decreasing the delay time constant of the calcium amplitude, indicative of increased cytosolic calcium removal. [29] The SNP + NaSH system also increases the resting calcium level in the cell, which depends on intracellular calcium stores, suggesting increased calcium cycling within these cells. [29]
These results mimic the effects of nitroxyl (HNO), the one-electron reduced form of HNO (N oxidation state N = +1), on cardiac myocytes leading to the suggestion that the reaction of NO and H2S generates HNO (Figure 3). [29, 30] Experiments using Angeli’s salt (AS, Na2N2O3), the most common HNO donor, show a response identical to the mixture of SNP and H2S. [29] Nitroxyl demonstrates electrophilic reactivity with itself and thiols (necessitating the use of HNO donors) and addition of various thiols blocks both the AS and the SNP + NaSH mixture response suggesting HNO intermediacy. [29, 31] Further experiments show that the effects of SNP and NaSH do not involve cGMP or cyclic adenylate monophosphate (cAMP)-mediated pathways. [29]
Nitroxyl demonstrates a distinct chemistry and biology compared to NO and HNO donors have drawn considerable attention as potential therapies for congestive heart failure. [24, 31, 32] This interest stems from their ability (compared to NO donors) to increase cardiac tissue contractility through improved calcium cycling and enhanced calcium sensitivity of the myofilament contractile proteins, [30, 33–35] nearly identical responses to those observed in the Bian study. [29] Despite interest in HNO biochemistry and pharmacology, [24, 36] endogenous production of HNO remains poorly understood and only proposed pathways exist including: NOS-catalyzed reactions, [37] oxidation of hydroxylamine-derivatives, [38] reductions of NO, [39] and the direct reaction of S-nitrosothiols with thiols. [40] Given the previous evidence of S-nitrosothiol formation from the NO and H2S reaction, direct displacement of HSNO by H2S (a reduction) would in principle generate HNO and HSSH (Figure 3). [40] These biological results strongly support HNO generation from the reaction of SNP and NaSH and provide the basis for the chemical exploration of these speculative H2S-mediated pathways to HNO. Chemically, the two-electron reduction of nitrogen oxides at the nitrite level of oxidation (N = +3, nitrite, NO+, RSNO) with H2S would generate HNO (N = +1). These results also highlight the need for improved methods of HNO detection to better demonstrate HNO formation in these biological experiments and new fluorescent and phosphine-based methods have been reported. [41–44]
These studies examining the biological reactions between NO and H2S raise some concerns, including the physiological significance of the in vitro experiments with exogenously added reactants. [28, 29] The described experiments utilize relatively high concentrations of simultaneously added NO and H2S that likely exceed actual physiological concentrations of these species. Endogenous H2S and NO formation also remains subject to temporal and spatial control affecting the concentration and reactivity of NO and H2S under physiological conditions. These studies also utilize NO donors, particularly sodium nitroprusside (SNP), rather than endogenously generated NO as the NO source. [28, 29] Sodium nitrosprusside does not spontaneously release NO, but requires reduction or photolysis and its chemistry with thiols has been reviewed. [45] Hydrogen sulfide actively adds to SNP to form a red violet complex, the basis of the “Gmelin” test of sulfide and this reaction also facilitates cyanide ion dissociation. [46] A recent re-examination of the H2S/−SH reaction with the metal coordinated NO of SNP reveals initial addition of −SH and SNP to produce a nitrosyl addition product with a pKa of 10.5 (Figure 3). [47] This product/anion pair decomposes to HS2·−2 and [Fe(CN)5NO]·−3 radicals or further reacts with H2S to give addition products that ultimately yield NH3, N2O and HS2−, which may indicate HNO generation (Figure 3). [47] The known chemistry of SNP and H2S complicates interpretation of these biological results and should preclude the use of SNP as an NO source for examining the biological reactions of H2S and NO. [28, 29] Whether the mixture of SNP and H2S produces this complex chemistry under the ratios of reactants, pH and other conditions of the described studies remains to be elucidated, but the known complex chemistry of H2S and SNP requires consideration as these reactions may generate different products from the reaction of H2S and NO or its oxidized products. The reactivity of H2S with SNP also clearly identifies metal-NO complexes of proteins as potential targets of H2S reactivity. While a well-known NO-dependent vasodilator, the structure, mechanism of NO release and known reactivity with H2S of SNP should be borne in mind while interpreting these exciting biological discoveries as more complex reaction chemistry may be occurring in these systems.
Reactions of Hydrogen Sulfide with S-Nitrosothiols
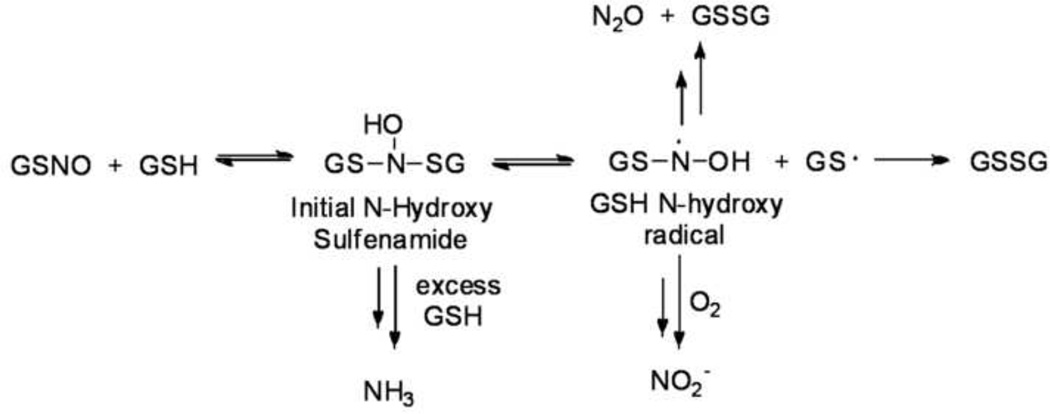
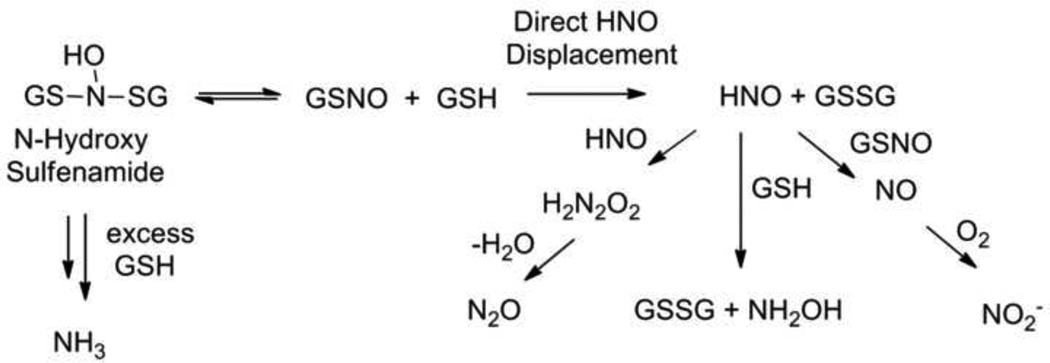
The above work strongly implicates a role for S-nitrosothiols during the interaction of NO and H2S and necessitates a brief review of the important early papers on reactions between S-nitrosothiols with thiols. Tannenbaum and co-workers provide the initial comprehensive report on the reaction of glutathione-S-nitrosothiol (GSNO) and glutathione (GSH). [48] Incubation of this biologically relevant thiol and S-nitroso thiol pair, which simplifies the analysis, yields varying amounts of nitrite, nitrous oxide and ammonia as the nitrogen-based products depending on the ratio of GSNO:GSH (Figure 4). [48] The yield of nitrite and nitrous oxide appear inversely proportional to the amount of GSH and the amount of ammonia proportional to GSH. [48] Oxidized glutathione (GSSG) constitutes the only observed peptide-based product in these reactions revealing overall sulfur oxidation and nitrogen reduction. [48] The proposed mechanism includes the initial generation of an N-hydroxysulfenamide from the addition of GSH to the nitrogen atom of GSNO (Figure 4). [48] Sulfur-nitrogen bond homolysis of the N-hydroxysulfenamide yields a GSH-based N-hydroxy radical and the gluthionyl radical (Figure 4). [48] Nitrite formation occurs through oxidative reactions of the GSH N-hydroxy radical under aerobic conditions and includes both NO oxidation and an NO-independent route (Figure 4). [48] Coupling of the GSH N-hydroxy radicals followed by GSSG elimination produces nitrous oxide and ammonia formation occurs through the GSH reduction of the initial N-hydroxysulfenamide to GSNH2 and GSNH2 to GSSG (Figure 4). Electrospray mass spectrometry provides evidence for unstable sulfenamides (GSNH-SG) and N-hydroxysulfenamides (GS-NOH-SG) as critical intermediates for the observed nitrogen-based products. [48] The N-hydroxysulfenamide represents the same intermediate required for thiol/S-nitrosothiol trans-nitrosation but this proposed mechanism does not evoke HNO in N2O formation or the reaction of S-nitrosothiols with thiols. [6] These well-characterized experiments reveal the extreme complexity of the reaction of thiols with S-nitrosothiols. [48]
Figure 4.
Summary of reactions of glutathione with glutathione S-nitrosothiol (Tannenbaum).
Work by Nagasawa and co-workers shows similar products from the reaction of GSNO and GSH including GSSG as the major GSNO-derived product and nitrous oxide, nitrite, NO, hydroxylamine and ammonia as the primary nitrogen-containing products depending on conditions (aerobic/anaerobic). [40] In contrast to the previously discussed study, [48] this paper proposes a major role for nitroxyl (HNO) in the formation of the observed products. [40] In addition to the direct formation of a reversible N-hydroxysulfenamide intermediate that further reacts with GSH to give GSSG and ammonia, this mechanism includes the direct displacement of HNO during the reaction of GSNO with GSH along with GSSG formation (Figure 5). [40] Dimerization of the nascent HNO followed by dehydration gives nitrous oxide and further reactions of HNO with both GSH and GSNO yield nitric oxide, hydroxylamine and ammonia with nitrite being generated from NO oxidation (Figure 5). [40] The identification of hydroxylamine and sulfinamides provides some support for HNO involvement in these reactions. [40] Despite these results, clear HNO production from the reaction of thiols with S-nitrosothiols remains debated due to the high reactivity of HNO with thiols and current limitations of HNO detection. [6, 24] The direct displacement of HNO from the reaction of GSH and GSNO competes with N-hydroxysulfenamide formation and further complicates these reactions and at present the consideration of both mechanisms for the reactions of S-nitrosothiols with thiols appears prudent. Displacement reaction of a thiol and S-nitrosothiol would provide a direct means of HNO generation from the reaction of HSNO and H2S that may warrant consideration (Figure 3).
Figure 5.
Summary of reactions of glutathione with glutathione S-nitrosothiol (Nagasawa).
Despite the proposed mechanistic differences, many similarities exist between these pathways providing guidance for considering the potential reactions of H2S with S-nitrosothiols. [40, 48] Following these mechanisms and substituting H2S for GSH in reactions with GSNO should yield glutathione persulfide (GSSH) as the major sulfur containing product regardless of the mechanism (Figure 6). These mechanisms also predict the formation of reduced nitrogen species including NO, N2O (possibly HNO) and ammonia from the reaction of S-nitrosothiols and H2S and nitrite under aerobic conditions and earlier work clearly shows a reaction between GSNO and H2S. [40, 48, 49] Indeed, an H2S-based chemiluminescence method of S-nitrosothiol detection shows NO formation upon excess H2S addition to an S-nitrosothiol. [50] The addition of H2S to GSNO (or any S-nitrosothiol) should yield an initial N-hydroxysulfenamide adduct that could undergo trans-nitrosation to yield GSH and HSNO (thionitrous acid) or decompose to products as described in Figure 4 (Figure 6). [48] Alternatively, direct displacement by the reaction of H2S with GSNO (or any S-nitrosothiol) would yield HNO and GSSH (Figure 6). [40] Such reactivity may yield profound biological activity as persulfides appear to play important roles in the modification of thiol-containing enzyme activity and have drawn considerable recent interest as a unique redox controlled protein thiol modification. [51, 52] While persulfides chemically demonstrate enhanced acidity and nucleophilicity, [6] they also represent the sulfur analog of sulfenic acids (RSOH), which act as important electrophilic/oxidized sulfur species involved in the regulation of various redox mediated cellular signaling events. [53–55] Alternatively, trans-nitrosation provides a mechanism for the formation of HSNO, the simplest S-nitrosothiol, whose basic chemistry and biological reactivity remains relatively unknown. The small size and enhanced basicity of H2S should enhance its reactivity compared to other larger and less acidic biological thiols and may facilitate reactions not available to these thiols. [6] The reactions of H2S and S-nitrosothiols require further examination in terms of kinetics, product identification and differences in reactivity between small molecule and protein S-nitrosothiols.
Figure 6.
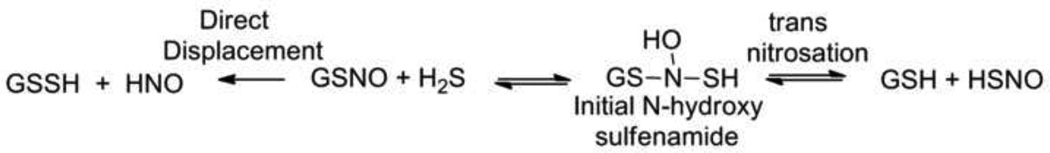
Proposed reactions of hydrogen sulfide with glutathione S-nitrosothiol.
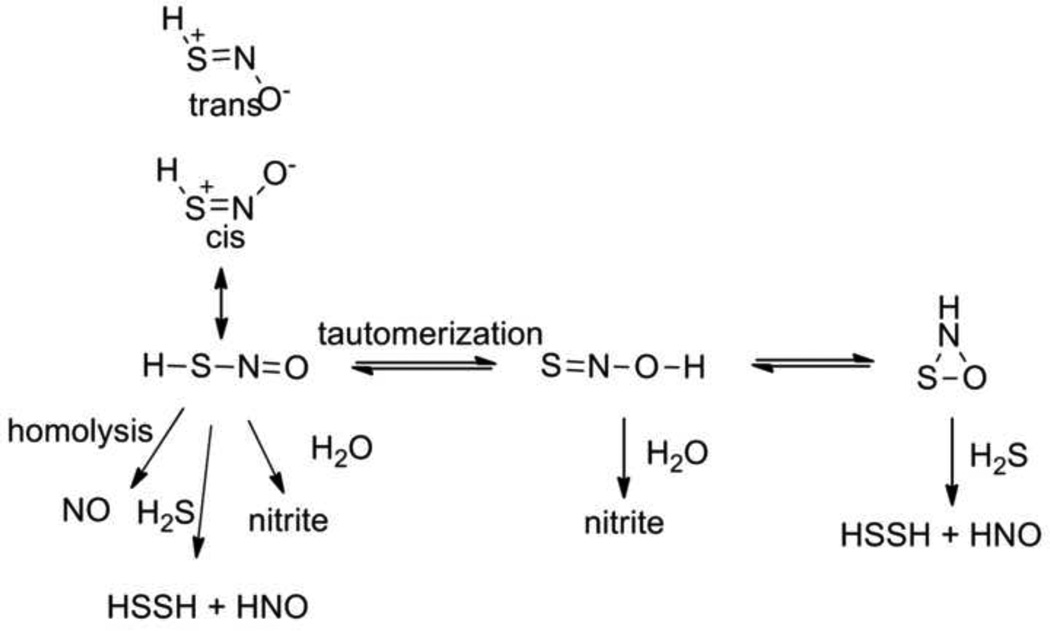
The trans-nitrosation of any small molecule or protein S-nitrosothiol with H2S should yield an equilibrium mixture of HSNO (thionitrous acid), an extremely interesting species given the known biological activities of NO, H2S, and nitrite. [16] HSNO, the simplest S-nitrosothiol and the sulfur analog of nitrous acid, would be expected to be more acidic than HNO2 (pKa = 3.5) indicating the likely formation of thionitrite (−SNO) at physiological pH. Unlike nitrite, HSNO could exist as a tautomeric pair with HONS, and cis and trans stereoisomers of both HSNO and HONS theoretically exist (Figure 7). [56–58] Both HSNO and HONS appear extremely unstable and have only been isolated by low temperature photolysis reactions of HNSO in an argon matrix and examined by infrared spectroscopy and theoretical calculations. [56–58] Free cis and trans HNSO and SNO− result from this method and ab initio calculations reveal the stability in terms of ground state energy to be HNSO > HOSN > HSNO > HNOS but no reported reaction chemistry of these nitrous acid and nitrite analogs exists. [56–58] Further theoretical calculations indicate an S-N bond dissociation energy of 29.2 kcal/mol in HSNO, indicating the weakness of this bond and suggesting homolytic S-N bond cleavage to NO. [59] These calculations also examine HSNO’s structure as a combination of covalent non-charged, zwitterionic and ion pair species to predict S-nitrosothiol structure. [60] While not proposed, HSNO could potentially exist as a reactive cyclic three-membered ring structure (Figure 7). The chemical reactivity of HSNO or SNO− remains poorly described but many reactions appear possible including sulfur-nitrogen bond homolysis to yield NO, reactions with nucleophiles to yield HNO, hydrolysis to nitrite and various dimerization and disproportionation pathways to other products. The biology and chemistry of these highly unstable species have not been clearly defined and whether HSNO/NSO− can be generated and detected under biological conditions remains to be discovered. Recent work shows the reaction of H2S with peroxynitrite generates sulfinyl nitrite (HS(O)NO), which was characterized by spectroscopic and computation methods and may act as a biological NO donor. [61] During the preparation of this paper, Filipovic described the formation of HSNO from the trans-nitrosation reaction of H2S and GSNO by a mechanism similar to Figure 6. [62] Using a variety of analytical methods to measure nitrogen oxide formation, HSNO further reacts to yield NO and HNO and that HSNO freely diffuses through membranes providing a mechanism for protein trans-nitrosation. [62] This exciting new work should stimulate more in-depth investigation of the complex chemistry and biology of HSNO that provides a point of convergence for H2S and NO-based signaling. [62]
Figure 7.
Consideration of the reactivity of HSNO and its tautomers.
Other H2S Reactions
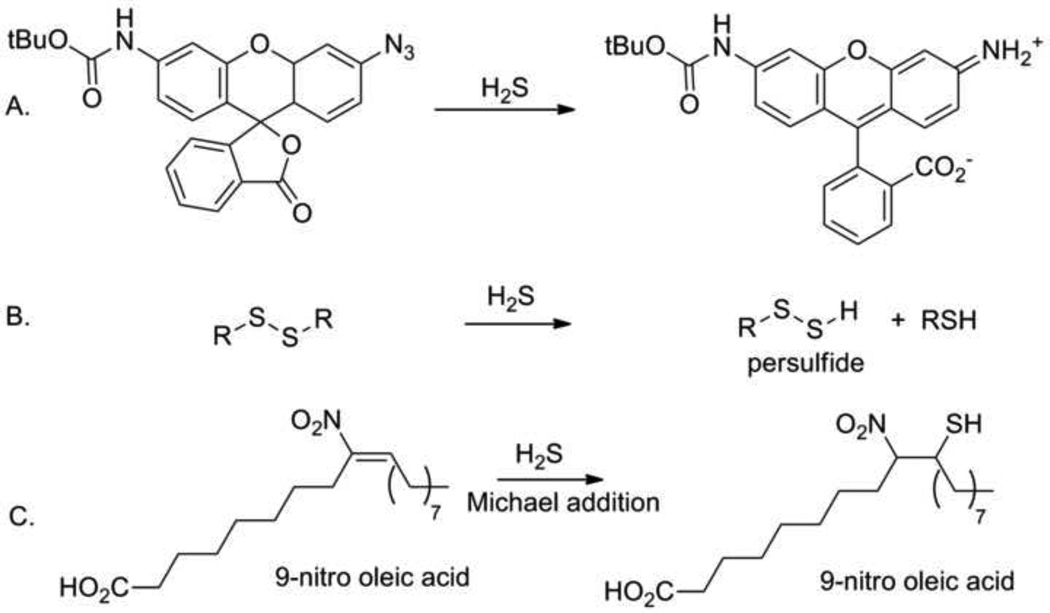
Treatment of aryl or sulfonyl azides with H2S results in azide group reduction to form an aromatic amine or sulfonamide, respectively (Figure 8). [17] Using a properly constructed azide, this reduction generates a fluorescent molecule permitting the development of a new group of H2S sensors based on this chemistry (Figure 8). [19, 63] Most importantly, H2S preferentially reduces these azides compared to cysteine and glutathione making mew H2S detectors selective in biological systems. [19, 63] These results also demonstrate the strength of H2S as a reducing agent comparable to these thiols under these conditions. Recently, this reactivity has been applied to H2S detection using genetically-modified fluorescent proteins in both aqueous solutions and mammalian cells. [64] Similarly H2S reduces aromatic nitro groups to amines and these compounds can selectively detect H2S. [20] Hydrogen sulfide or −SH reacts with disulfides in a disulfide exchange reaction to generate a persulfide, a pathway of potential importance in biological persulfide formation that also forms the basis of a new H2S detection method (Figure 8). [6, 65] The acidic and nucleophilic properties of H2S make it an excellent nucleophile in Michael reactions with electron deficient olefins and this chemistry forms the basis of another new H2S detection method. [66] This type of reactivity suggests reactions of H2S with the electrophilic nitrated fatty acids, another group of electrophilic nitrogen oxide-derived signaling molecules (Figure 8). [67] New work shows that H2S reacts with nitrated oleic acid to yield a thioether product by a double Michael reaction sequence and also forms an adduct with 8-nitro cGMP to alter redox signaling through electrophile sulfhydration. [68] In general, the diverse chemistry of H2S opens numerous avenues for selective chemical detection and such probes may provide insight into determining precise biological H2S levels.
Figure 8.
Summary of hydrogen sulfide reactivity with organic substrates. A) fluorescent H2S sensor; B) disulfide exchange reaction; and C) Michael reaction of nitrated fatty acids with H2S.
Conclusion
Hydrogen sulfide (H2S) has taken a place among other recognized gaseous transmitters (NO and CO) and elicits specific biological effects in the cardiovascular, immune and nervous systems making the control of H2S production and degradation an important therapeutic opportunity. [2, 6, 8–11, 15] Despite the emergence of H2S as an important biological mediator (numerous H2S donating drugs are being explored), [10, 69] the interaction of hydrogen sulfide with NO and its multiple precursors and metabolites has received little attention. Recent work shows a cooperative interaction of H2S and NO which increases and maintains cellular cGMP levels essential for vasorelaxation and angiogenesis and reveals the potential for each of these molecules to control the actions and effective concentration of the other. [70] Interestingly, a dual NO and H2S donor drug (NOSH-aspirin) is currently being evaluated, particularly for its anti-cancer actions. [71, 72] Chemically, H2S acts as a strong nucleophile and reducing agent and efficiently reduces a number of organic substrates, [6, 73] which now form the basis of H2S detection strategies. [74] Under biological conditions, H2S continues to act as a nucleophile and reducing agent that conceptually should react with a variety of NO-derived species. [16] Early work shows that NO donors and H2S react to likely yield HNO or S-nitrosothiol, which may be chemically interconverted, but these reactions elicit different biological responses compared to NO donors. [28, 29] The biological chemistry of H2S remains complex and reactions with S-nitrosothiols (both direct displacement of HNO and/or trans-nitrosation) may reveal a portion of the biological effects of these compounds. Much work remains including defining: the biological sources of NO and H2S, their generation and kinetics; the direct products of the reaction of H2S and NO and their biology; whether HSNO forms during these reactions; and the biological effects of this chemistry. Despite these questions, the reactions of these basic nitrogen and sulfur species likely play important roles in biology and warrants further examination.
Highlights.
Hydrogen sulfide behaves as a nucleophile and reducing agent
Hydrogen sulfide decreases the amount of nitric oxide released by NO donors
Hydrogen sulfide alters the normally observed biological response of nitric oxide
S-Nitrosothiols and nitroxyl likely form from hydrogen sulfide and nitric oxide
Hydrogen sulfide reacts with S-nitrosothiols by trans-nitrosation or displacement
Acknowledgements
The author wishes to thank Dr. Martin Feelisch for discussion that directed his attention to this interesting area. Preliminary work performed in the author’s laboratories was supported by the National Institutes of Health (HL62198) and Wake Forest University. The author wishes to thank Drs. Julie Reisz, Susan Mitroka and Jenna DuMond, as well as Mr. Ryan Daly and Ms. Nicole Irving for preliminary work on hydrogen sulfide chemistry and Dr. Angela King for proofreading the manuscript.
List of Abbreviations
- AS
Angeli’s Salt
- cAMP
cyclic adenylate monophosphate
- cGMP
cyclic guanylate monophosphate
- CBS
cystathione β-synthetase
- CSE
cystathione γ-lyase
- EPR
electron paramagnetic resonance
- GSH
glutathione
- GSNO
glutathione S-nitrosothiol
- GSSG
oxidized glutathione
- GSSH
glutathione persulfide
- LPS
lipo-polysaccharide
- MST
3-mercaptopyruvate sulfur transferase
- NO
nitric oxide
- NOS
nitric oxide synthase
- PLP
pyridoxal-5’-phosphate
- sGC
soluble guanylate cyclase
- SNP
sodium nitroprusside
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moncada S, Palmer RMJ, Higgs EA. NITRIC-OXIDE - PHYSIOLOGY, PATHOPHYSIOLOGY, AND PHARMACOLOGY. Pharmacological Reviews. 1991;43:109–142. [PubMed] [Google Scholar]
- 2.Li L, Hsu A, Moore PK. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation - a tale of three gases! Pharmacology & Therapeutics. 2009;123:386–400. doi: 10.1016/j.pharmthera.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Kerwin JF, Lancaster JR, Feldman PL. Nitric-Oxide - a New Paradigm for 2nd-Messengers. Journal of Medicinal Chemistry. 1995;38:4343–4362. doi: 10.1021/jm00022a001. [DOI] [PubMed] [Google Scholar]
- 4.Stuehr DJ. Mammalian nitric oxide synthases. Biochimica Et Biophysica Acta-Bioenergetics. 1999;1411:217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 5.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature Reviews Drug Discovery. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 6.Fukuto JM, Carrington SJ, Tantillo DJ, Harrison JG, Ignarro LJ, Freeman BA, Chen A, Wink DA. Small Molecule Signaling Agents: The Integrated Chemistry and Biochemistry of Nitrogen Oxides, Oxides of Carbon, Dioxygen, Hydrogen Sulfide, and Their Derived Species. Chemical Research in Toxicology. 2012;25:769–793. doi: 10.1021/tx2005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. Journal of Neurochemistry. 2010;113:14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mustafa AK, Gadalla MM, Snyder SH. Signaling by Gasotransmitters. Science Signaling. 2009;2 doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang GD, Wu LY, Jiang B, Yang W, Qi JS, Cao K, Meng QH, Mustafa AK, Mu WT, Zhang SM, Snyder SH, Wang R. H(2)S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson KR. The therapeutic potential of hydrogen sulfide: separating hype from hope. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2011;301:R297–R312. doi: 10.1152/ajpregu.00045.2011. [DOI] [PubMed] [Google Scholar]
- 11.Olson KR. A Practical Look at the Chemistry and Biology of Hydrogen Sulfide. Antioxidants & Redox Signaling. 2012;17:32–44. doi: 10.1089/ars.2011.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caliendo G, Cirino G, Santagada V, Wallace JL. Synthesis and Biological Effects of Hydrogen Sulfide (H2S): Development of H2S-Releasing Drugs as Pharmaceuticals. Journal of Medicinal Chemistry. 2010;53:6275–6286. doi: 10.1021/jm901638j. [DOI] [PubMed] [Google Scholar]
- 13.Kabil O, Banerjee R. Redox Biochemistry of Hydrogen Sulfide. Journal of Biological Chemistry. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li HY, Shimizu H, Flinspach M, Jamal J, Yang WP, Xian M, Cai TW, Wen EZ, Jia QA, Wang PG, Poulos TL. The novel binding mode of N-Alkyl-N '-hydroxyguanidine to neuronal nitric oxide synthase provides mechanistic insights into NO biosynthesis. Biochemistry. 2002;41:13868–13875. doi: 10.1021/bi020417c. [DOI] [PubMed] [Google Scholar]
- 15.Wang R. Shared signaling pathways among gasotransmitters. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8801–8802. doi: 10.1073/pnas.1206646109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fago A, Jensen FB, Tota B, Feelisch M, Olson KR, Helbo S, Lefevre S, Mancardi D, Palumbo A, Sandvik GK, Skovgaard N. Integrating nitric oxide, nitrite and hydrogen sulfide signaling in the physiological adaptations to hypoxia: A comparative approach. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology. 2012;162:1–6. doi: 10.1016/j.cbpa.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Dittmer DC. Encyclopedia of Reagents for Organic Synthesis. John Wiley and Sons; 2010. Hydrogen Sulfide. [Google Scholar]
- 18.Walsh C. Enzymatic Reaction Mechanisms. New York: W.H. Freeman; 1979. [Google Scholar]
- 19.Lippert AR, New EJ, Chang CJ. Reaction-Based Fluorescent Probes for Selective Imaging of Hydrogen Sulfide in Living Cells. Journal of the American Chemical Society. 2011;133:10078–10080. doi: 10.1021/ja203661j. [DOI] [PubMed] [Google Scholar]
- 20.Montoya LA, Pluth MD. Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chemical Communications. 2012;48:4767–4769. doi: 10.1039/c2cc30730h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. Journal of Biological Chemistry. 1996;271:18596–18603. doi: 10.1074/jbc.271.31.18596. [DOI] [PubMed] [Google Scholar]
- 22.Keszler A, Zhang YH, Hogg N. Reaction between nitric oxide, glutathione, and oxygen in the presence and absence of protein: How are S-nitrosothiols formed? Free Radical Biology and Medicine. 2010;48:55–64. doi: 10.1016/j.freeradbiomed.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demaster EG, Quast BJ, Redfern B, Nagasawa HT. REACTION OF NITRIC-OXIDE WITH THE FREE SULFHYDRYL-GROUP OF HUMAN SERUM-ALBUMIN YIELDS A SULFENIC ACID AND NITROUS-OXIDE. Biochemistry. 1995;34:11494–11499. doi: 10.1021/bi00036a023. [DOI] [PubMed] [Google Scholar]
- 24.Fukuto JM, Carrington SJ. HNO Signaling Mechanisms. Antioxidants & Redox Signaling. 2011;14:1649–1657. doi: 10.1089/ars.2010.3855. [DOI] [PubMed] [Google Scholar]
- 25.Zhang YH, Hogg N. S-nitrosohemoglobin: A biochemical perspective. Free Radical Biology and Medicine. 2004;36:947–958. doi: 10.1016/j.freeradbiomed.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Keshive M, Singh S, Wishnok JS, Tannenbaum SR, Deen WM. Kinetics of S-nitrosation of thiols in nitric oxide solutions. Chemical Research in Toxicology. 1996;9:988–993. doi: 10.1021/tx960036y. [DOI] [PubMed] [Google Scholar]
- 27.Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite 'scavenger'? Journal of Neurochemistry. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 28.Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochemical and Biophysical Research Communications. 2006;343:303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- 29.Yong QC, Hu LF, Wang SH, Huang DJ, Bian JS. Hydrogen sulfide interacts with nitric oxide in the heart: possible involvement of nitroxyl. Cardiovascular Research. 2010;88:482–491. doi: 10.1093/cvr/cvq248. [DOI] [PubMed] [Google Scholar]
- 30.Tocchetti CG, Stanley BA, Murray CI, Sivakumaran V, Donzelli S, Mancardi D, Pagliaro P, Gao WD, van Eyk J, Kass DA, Wink DA, Paolocci N. Playing with Cardiac "Redox Switches": The "HNO Way" to Modulate Cardiac Function. Antioxidants & Redox Signaling. 2011;14:1687–1698. doi: 10.1089/ars.2010.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flores-Santana W, Salmon DJ, Donzelli S, Switzer CH, Basudhar D, Ridnour L, Cheng R, Glynn SA, Paolocci N, Fukuto JM, Miranda KM, Wink DA. The Specificity of Nitroxyl Chemistry Is Unique Among Nitrogen Oxides in Biological Systems. Antioxidants & Redox Signaling. 2011;14:1659–1674. doi: 10.1089/ars.2010.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feelisch M. Nitroxyl gets to the heart of the matter. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4978–4980. doi: 10.1073/pnas.1031571100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai TY, Tian Y, Tocchetti CG, Katori T, Murphy AM, Kass DA, Paolocci N, Gao WD. Nitroxyl increases force development in rat cardiac muscle. Journal of Physiology-London. 2007;580:951–960. doi: 10.1113/jphysiol.2007.129254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paolocci N, Katori T, Champion HC, St John ME, Miranda KM, Fukuto JM, Wink DA, Kass DA. Positive inotropic and lusitropic effects of HNO/NO− in failing hearts: Independence from beta-adrenergic signaling. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5537–5542. doi: 10.1073/pnas.0937302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tocchetti CG, Wang W, Froehlich JP, Huke S, Aon MA, Wilson GM, Di Benedetto G, O'Rourke B, Gao WD, Wink DA, Toscano JP, Zaccolo M, Bers DM, Valdivia HH, Cheng HP, Kass DA, Paolocci N. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circulation research. 2007;100:96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irvine JC, Ritchie RH, Favaloro JL, Andrews KL, Widdop RE, Kemp-Harper BK. Nitroxyl (HNO): the Cinderella of the nitric oxide story. Trends in Pharmacological Sciences. 2008;29:601–608. doi: 10.1016/j.tips.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Wei CC, Wang ZQ, Hemann C, Hille R, Stuehr DJ. A tetrahydrobiopterin radical forms and then becomes reduced during N-omega-hydroxyarginine oxidation by nitric-oxide synthase. Journal of Biological Chemistry. 2003;278:46668–46673. doi: 10.1074/jbc.M307682200. [DOI] [PubMed] [Google Scholar]
- 38.Reisz JA, Bechtold E, King SB. Oxidative heme protein-mediated nitroxyl (HNO) generation. Dalton Transactions. 2010;39:5203–5212. doi: 10.1039/c000980f. [DOI] [PubMed] [Google Scholar]
- 39.Saleem M, Ohshima H. Xanthine oxidase converts nitric oxide to nitroxyl that inactivates the enzyme. Biochemical and Biophysical Research Communications. 2004;315:455–462. doi: 10.1016/j.bbrc.2004.01.081. [DOI] [PubMed] [Google Scholar]
- 40.Wong PSY, Hyun J, Fukuto JM, Shirota FN, DeMaster EG, Shoeman DW, Nagasawa HT. Reaction between S-nitrosothiols and thiols: Generation of nitroxyl (HNO) and subsequent chemistry. Biochemistry. 1998;37:5362–5371. doi: 10.1021/bi973153g. [DOI] [PubMed] [Google Scholar]
- 41.Rosenthal J, Lippard SJ. Direct Detection of Nitroxyl in Aqueous Solution Using a Tripodal Coppe(II) BODIPY Complex. Journal of the American Chemical Society. 2010;132:5536. doi: 10.1021/ja909148v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Y, Liu K, Li JY, Fang YA, Zhao TC, Yao C. Visualization of Nitroxyl in Living Cells by a Chelated Coppe(II) Coumarin Complex. Organic Letters. 2011;13:1290–1293. doi: 10.1021/ol103077q. [DOI] [PubMed] [Google Scholar]
- 43.Reisz JA, Klorig EB, Wright MW, King SB. Reductive Phosphine-Mediated Ligation of Nitroxyl (HNO) Organic Letters. 2009;11:2719–2721. doi: 10.1021/ol900914s. [DOI] [PubMed] [Google Scholar]
- 44.Reisz JA, Zink CN, King SB. Rapid and Selective Nitroxyl (HNO) Trapping by Phosphines: Kinetics and New Aqueous Ligations for HNO Detection and Quantitation. Journal of the American Chemical Society. 2011;133:11675–11685. doi: 10.1021/ja203652z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butler AR, Glidewell C. RECENT CHEMICAL STUDIES OF SODIUM-NITROPRUSSIDE RELEVANT TO ITS HYPOTENSIVE ACTION. Chemical Society Reviews. 1987;16:361–380. [Google Scholar]
- 46.Butler AR, Calsyharrison AM, Glidewell C, Sorensen PE. THE PENTACYANONITROSYLFERRATE ION .5. THE COURSE OF THE REACTIONS OF NITROPRUSSIDE WITH A RANGE OF THIOLS. Polyhedron. 1988;7:1197–1202. [Google Scholar]
- 47.Quiroga SL, Almaraz AE, Amorebieta VT, Perissinotti LL, Olabe JA. Addition and Redox Reactivity of Hydrogen Sulfides (H2S/HS-) with Nitroprusside: New Chemistry of Nitrososulfide Ligands. Chemistry-a European Journal. 2011;17:4145–4156. doi: 10.1002/chem.201002322. [DOI] [PubMed] [Google Scholar]
- 48.Singh SP, Wishnok JS, Keshive M, Deen WM, Tannenbaum SR. The chemistry of the S-nitrosoglutathione glutathione system. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14428–14433. doi: 10.1073/pnas.93.25.14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munro AP, Williams DLH. Reactivity of sulfur nucleophiles towards S-nitrosothiols. Journal of the Chemical Society-Perkin Transactions. 2000;2:1794–1797. [Google Scholar]
- 50.Teng XJ, Isbell TS, Crawford JH, Bosworth CA, Giles GI, Koenitzer JR, Lancaster JR, Doeller JE, Kraust DW, Patel RP. Novel method for measuring S-nitrosothiols using hydrogen sulfide. In: Cadenas E, Packer L, editors. Nitric Oxide, Pt G: Oxidative and Nitrosative Stress in Redox Regulation of Cell Signaling. 2008. pp. 161–172. [DOI] [PubMed] [Google Scholar]
- 51.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu WT, Gazi SK, Barrow RK, Yang GD, Wang R, Snyder SH. H2S Signals Through Protein S-Sulfhydration. Science Signaling. 2009;2 doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sen N, Snyder SH. Protein modifications involved in neurotransmitter and gasotransmitter signaling. Trends in Neurosciences. 2010;33:493–502. doi: 10.1016/j.tins.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roos G, Messens J. Protein sulfenic acid formation: From cellular damage to redox regulation. Free Radical Biology and Medicine. 2011;51:314–326. doi: 10.1016/j.freeradbiomed.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 54.Reddie KG, Carroll KS. Expanding the functional diversity of proteins through cysteine oxidation. Current Opinion in Chemical Biology. 2008;12:746–754. doi: 10.1016/j.cbpa.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 55.Klomsiri C, Karplus PA, Poole LB. Cysteine-Based Redox Switches in Enzymes. Antioxidants & Redox Signaling. 2011;14:1065–1077. doi: 10.1089/ars.2010.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tchir PO, Spratley RD. INFRARED-SPECTRUM AND FORCE-FIELD OF MATRIX-ISOLATED CIS-THIONYLIMIDE (HNSO) Canadian Journal of Chemistry-Revue Canadienne De Chimie. 1975;53:2311–2317. [Google Scholar]
- 57.Tchir PO, Spratley RD. PHOTOLYSIS OF MATRIX-ISOLATED CIS-THIONYLIMIDE .1. IDENTIFICATION AND INFRARED-SPECTRA OF CIS-HOSN, HSNO, AND SNO. Canadian Journal of Chemistry-Revue Canadienne De Chimie. 1975;53:2318–2330. [Google Scholar]
- 58.Tchir PO, Spratley RD. PHOTOLYSIS OF MATRIX-ISOLATED CIS-THIONYLIMIDE .2. IDENTIFICATION AND INFRARED-SPECTRA OF TRANS-HNSO AND NSO. Canadian Journal of Chemistry-Revue Canadienne De Chimie. 1975;53:2331–2336. [Google Scholar]
- 59.Timerghazin QK, Peslherbe GH, English AM. Structure and stability of HSNO, the simplest S-nitrosothiol. Physical Chemistry Chemical Physics. 2008;10:1532–1539. doi: 10.1039/b715025c. [DOI] [PubMed] [Google Scholar]
- 60.Timerghazin QK, English AM, Peslherbe GH. On the multireference character of S-nitrosothiols: A theoretical study of HSNO. Chemical Physics Letters. 2008;454:24–29. [Google Scholar]
- 61.Filipovic MR, Miljkovic J, Allgauer A, Chaurio R, Shubina T, Herrmann M, Ivanovic-Burmazovic I. Biochemical insight into physiological effects of H2S: reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. Biochemical Journal. 2012;441:609–621. doi: 10.1042/BJ20111389. [DOI] [PubMed] [Google Scholar]
- 62.Filipovic MR, Miljkovic JL, Nauser T, Royzen M, Klos K, Shubina T, Koppenol WH, Lippard SJ, Ivanovic-Burmazovic I. Chemical Characterization of the Smallest S-Nitrosothiol, HSNO; Cellular Cross-talk of H2S and S-Nitrosothiols. Journal of the American Chemical Society. 2012;134:12016–12027. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peng HJ, Cheng YF, Dai CF, King AL, Predmore BL, Lefer DJ, Wang BH. A Fluorescent Probe for Fast and Quantitative Detection of Hydrogen Sulfide in Blood. Angewandte Chemie-International Edition. 2011;50:9672–9675. doi: 10.1002/anie.201104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen SC, Ren Z, W Ai H. Reaction-Based Genetically Coded Fluorescent Hydrogen Sulfide Sensors. Journal of the American Chemical Society. 2012;134:9589–9592. doi: 10.1021/ja303261d. [DOI] [PubMed] [Google Scholar]
- 65.Liu CR, Pan J, Li S, Zhao Y, Wu LY, Berkman CE, Whorton AR, Xian M. Capture and Visualization of Hydrogen Sulfide by a Fluorescent Probe. Angewandte Chemie-International Edition. 2011;50:10327–10329. doi: 10.1002/anie.201104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu CR, Peng B, Li S, Park CM, Whorton AR, Xian M. Reaction Based Fluorescent Probes for Hydrogen Sulfide. Organic Letters. 2012;14:2184–2187. doi: 10.1021/ol3008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schopfer FJ, Cipollina C, Freeman BA. Formation and Signaling Actions of Electrophilic Lipids. Chemical Reviews. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishida M, Sawa T, Kitajima N, Ono K, Inoue H, Ihara H, Motohashi H, Yamamoto M, Suematsu M, Kurose H, van der Vliet A, Freeman BA, Shibata T, Uchida K, Kumagai Y, Akaike T. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nature Chemical Biology. 2012;8:714–724. doi: 10.1038/nchembio.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wallace JL, Del Soldato P. The therapeutic potential of NO-NSAIDs. Fundamental & Clinical Pharmacology. 2003;17:11–20. doi: 10.1046/j.1472-8206.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 70.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chattopadhyay M, Kodela R, Olson KR, Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model. Biochemical and Biophysical Research Communications. 2012;419:523–528. doi: 10.1016/j.bbrc.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 72.Kodela R, Chattopadhyay M, Kashfi K. NOSH-Aspirin: A Novel Nitric Oxide-Hydrogen Sulfide-Releasing Hybrid: A New Class of Anti-inflammatory Pharmaceuticals. Acs Medicinal Chemistry Letters. 2012;3:257–262. doi: 10.1021/ml300002m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sidgwick NV. The Chemical Elements and Their Compounds. London: Oxford University Press; 1950. [Google Scholar]
- 74.Xuan WM, Sheng CQ, Cao YT, He WH, Wang W. Fluorescent Probes for the Detection of Hydrogen Sulfide in Biological Systems. Angewandte Chemie-International Edition. 2012;51:2282–2284. doi: 10.1002/anie.201107025. [DOI] [PubMed] [Google Scholar]