Abstract
Failures of highly touted trials have caused experts to call for re-evaluation of the current approach toward sepsis. New research has revealed key pathogenic mechanisms; autopsy results have shown that most patients admitted to intensive care units for treatment of sepsis had unresolved septic foci at post mortem, suggesting that patients were unable to eradicate invading pathogens and were more susceptible to nosocomial organisms, or both. These results suggest that therapies that improve host immunity might increase survival. Additional work showed that cytokine production by splenocytes taken post mortem from patients who died of sepsis is profoundly suppressed, possibly because of so-called T-cell exhaustion—a newly recognised immunosuppressive mechanism that occurs with chronic antigenic stimulation. Results from two clinical trials of biomarker-guided therapeutic drugs that boosted immunity showed promising findings in sepsis. Collectively, these studies emphasise the degree of immunosuppression that occurs in sepsis, and explain why many previous sepsis trials which were directed at blocking inflammatory mediators or pathogen recognition signalling pathways failed. Finally, highly encouraging results from use of the new immunomodulatory molecules interleukin 7 and anti-programmed cell death 1 in infectious disease point the way for possible use in sepsis. We hypothesise that immunoadjuvant therapy represents the next major advance in sepsis.
Introduction
The failure of several high-profile clinical trials in sepsis has led researchers to state that sepsis studies need new direction.1–6 Experts have discussed important reasons for the failures of new investigative drugs and highlighted problems in design and conduct of sepsis trials.1–6 However, there might also be inadequate understanding of key pathophysiological mechanisms that operate in sepsis. Post-mortem studies of patients who died of sepsis have provided important insights into why septic patients die, and highlighted key immunological defects that impair host immunity.7,8 Several small phase 2 clinical trials of immune-enhancing drugs have shown benefit, thereby substantiating the concept that immunosuppression has a central role.9,10 Findings from studies of clinically relevant animal models of sepsis that mimic the protracted nature of the disease also support the premise that boosting immunity improves survival.11 Sepsis and cancer share many immunological defects, and therefore the recent successes of several immunomodulatory drugs in cancer provide hope for and insight into potential immunostimulatory therapies in sepsis.12–14
Sepsis as a cytokine storm
Patients with sepsis often present with high spiking fevers, shock, and respiratory failure. Partly because of this striking presentation, the prevailing theory of sepsis for many years was that it represented an uncontrolled inflammatory response.15 The discovery that various potent cytokines, including tumour necrosis factor (TNF) and interleukin 1, are at increased concentrations in patients with sepsis, and when injected into animals reproduced many clinical and laboratory features of sepsis, led to the concept of sepsis as a cytokine storm. On the basis of this theory and encouraging results in animal models, pharmaceutical companies initiated many clinical trials—eg, TNF and interleukin 1 antagonists, toll receptor blockers, and endotoxin antagonists in sepsis. The results of more than 30 trials of diverse anticytokine and anti-inflammatory drugs showed no benefit or, in some cases, reduced survival rates.1,5
Rigorous examination of previous studies provides evidence that both proinflammatory and an opposing anti-inflammatory response occur concomitantly in sepsis. Results of studies of circulating cytokines in patients showed that, in addition to pro-inflammatory cytokines, concentrations of the potent anti-inflammatory cytokine interleukin 10 were increased.16 Van Dissel and colleagues16 investigated cytokine profiles and mortality in 464 patients and reported that a high ratio of interleukin 10 to TNFα correlated with mortality in patients with community-acquired infection. Other investigators documented reduced production of both proinflammatory and anti-inflammatory cytokines—ie, global cytokine depression in sepsis.17–20 Ertel and coworkers17 stimulated whole blood from patients with and without sepsis with endotoxin and reported that production of TNFα, interleukin 1β, and interleukin 6 from patients with sepsis was frequently less than 10–20% of that found in patients without. Munoz and colleagues18 determined that lipopolysaccharide-stimulated monocytes from septic patients had profound decreases in production of interleukin 1β, TNFα, and interleukin 6 versus controls.17 Likewise, Sinistro and colleagues20 stimulated blood monocytes from septic or control patients and quantitated the proportion of cells producing proinflammatory cytokines. Fewer than 5% of monocytes from patients with sepsis produced cytokines compared with roughly 15–20% of monocytes from controls. Weighardt and colleagues21 investigated lipopolysaccharide-stimulated cytokine production by monocytes in patients with sepsis after abdominal surgery. Postoperative sepsis was associated with defects in production of both proinflammatory and anti-inflammatory cytokines. Survival correlated with recovery of inflammatory but not anti-inflammatory responses. Collectively, these results indicate that some patients with sepsis rapidly produce both proinflammatory and anti-inflammatory cytokines, whereas other patients have either predominance of anti-inflammatory cytokines or globally depressed cytokine production.
Why do patients with sepsis die?
Whereas some patients rapidly succumb to massive proinflammatory cytokine-driven inflammation as occurs, for example, in toxic shock syndrome and meningococcaemia, improved treatment algorithms have resulted in most patients surviving the early hyperinflammatory phase of sepsis and entering a more protracted phase.22,23 More than 70% of deaths in sepsis occur after the first 3 days of the disorder, with many deaths occurring weeks later. In a post-mortem study, Torgersen and colleagues7 reviewed findings in 235 patients in surgical intensive care who were admitted with sepsis. At death, about 80% of patients had unresolved septic foci. Only 52 of 97 autopsy-confirmed pneumonias were appropriately diagnosed during their intensive-care admission. Peritonitis also accounted for many unresolved septic foci. Such ongoing infections are not necessarily the main cause of death. In fact, the real cause of death and organ failure in most patients dying of sepsis is unknown. Postmortem study results have shown a relative paucity of cell death in most major organs in patients who died of sepsis.24 One theory is that much of the organ dysfunction in sepsis might be a result of a so-called cellular hibernation response.25,26 In many situations, death is due to the family’s decision to change from aggressive support measures to comfort measures because of the patient’s many, severe pre-existing comorbidities and small probability of meaningful recovery. However, the crucial message remains that many patients in intensive care units do not recover because there is ongoing infection. Despite broad-spectrum antibiotics and aggressive source control measures, many patients do not eradicate their infections and develop secondary hospital-acquired infections.27,28 Therefore, therapy that boosts immune competence could affect outcomes by leading to more rapid resolution of the primary infection and prevention of lethal secondary infections.
Sepsis as an immunosuppressive disorder
Although both proinflammatory and anti-inflammatory processes begin promptly after sepsis initiation, in general there is predominance of an initial hyperinflammatory phase, the scale of which is determined by many factors including pathogen virulence, bacterial load, host genetic factors, age, and host comorbidities. For example, a previously healthy young adult who develops meningococcaemia will likely have a profound hyper-inflammatory cytokine-storm-mediated response, that causes shock, high fevers, and multiple organ failure (figure 1). If the patient dies in the first few days, death will probably have been caused by cytokine-driven hyperinflammation and multiple organ failure, especially cardiovascular collapse. Conversely, an elderly patient with diabetes undergoing haemodialysis who develops pneumonia might not show any obvious signs of sepsis. The only clues to diagnosing sepsis in such a patient might be reduced mental status, inability to tolerate dialysis because of hypotension, hypothermia, and glucose intolerance—there could be no obvious response to infection or predominant anti-inflammatory reaction. Although patients can and do die in either the hyperinflammatory or the hypoinflammatory phase of sepsis, new therapies and treatment protocols have resulted in more prolonged disease with a shift toward the immunosuppressive phase. Also, sepsis is increasingly a disease of elderly people: 60% of patients who develop sepsis and 75% of the deaths in sepsis, in countries with advanced health-care delivery and modern intensive care units, are in patients older than 65 years.29 The immune systems of elderly people are less effective than earlier in life, so-called immunosenescence.30 Increased comorbidities and immunosenescence contribute to the greater incidence of and mortality from sepsis in elderly people.
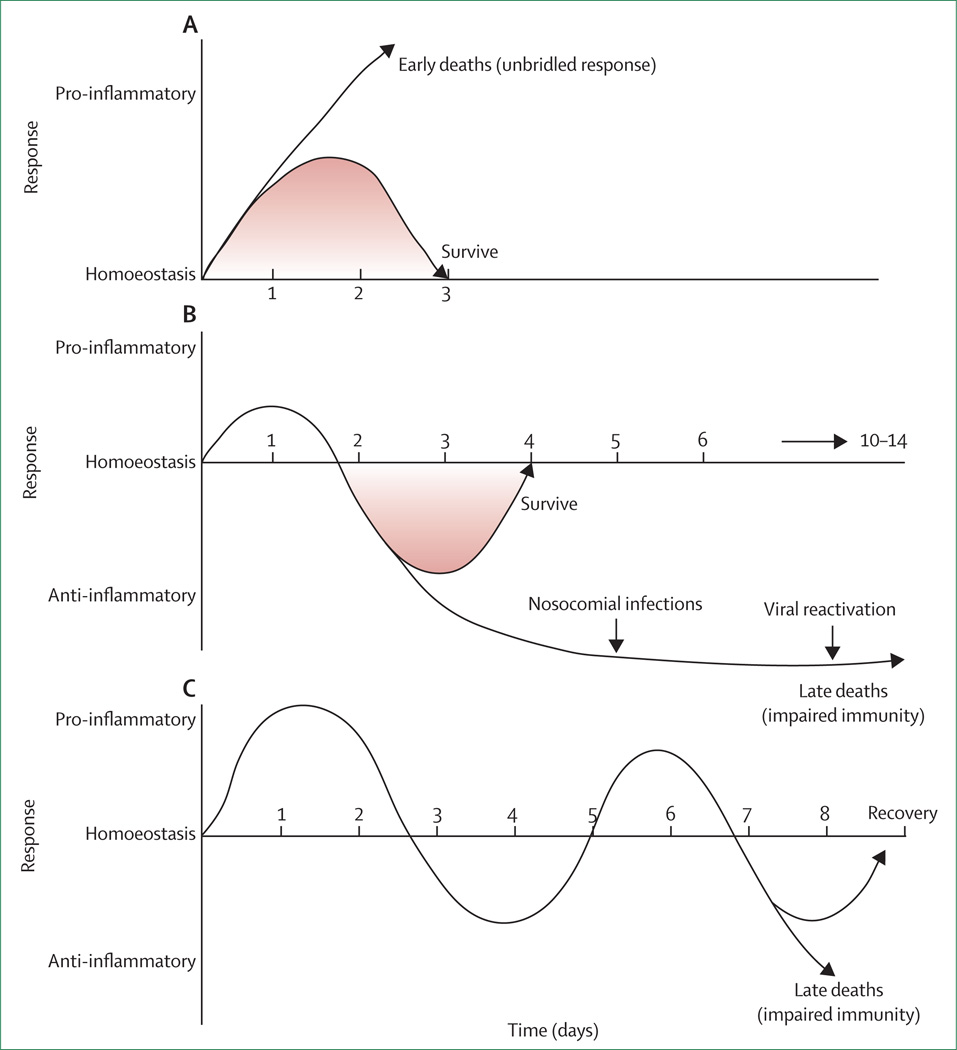
Figure 1. Potential inflammatory responses in sepsis.
Immune responses in sepsis are determined by many factors including pathogen virulence, size of bacterial inoculum, comorbidities, etc. (A) Although both proinflammatory and anti-inflammatory responses begin rapidly after sepsis, the initial response in previously healthy patients with severe sepsis is typified by an overwhelming hyperinflammatory phase with fever, hyperdynamic circulation, and shock. Deaths in this early phase of sepsis are generally due to cardiovascular collapse, metabolic derangements, and multiple organ dysfunction. Although no particular anti-inflammatory therapies have improved survival in large phase 3 trials, short acting anti-inflammatory or anticytokine therapies offer a theoretical benefit. (B) Many patients who develop sepsis are elderly with numerous comorbidities that impair immune response. When these individuals develop sepsis, a blunted or absent hyperinflammatory phase is common, and patients rapidly develop impaired immunity and an anti-inflammatory state. Immunoadjuvant therapy that boosts immunity offers promise in this setting. (C) A third theoretical immunological response to sepsis is characterised by cycling between hyperinflammatory and hypoinflammatory states. According to this theory, patients who develop sepsis have an initial hyperinflammatory response followed by a hypoinflammatory state. With the development of a new secondary infection, patients have a repeat hyperinflammatory response and may either recover or re-enter the hypoinflammatory phase. Patients can die in either state. There is less evidence for this theory, and the longer the sepsis continues the more likely a patient is to develop profound immunosuppression.
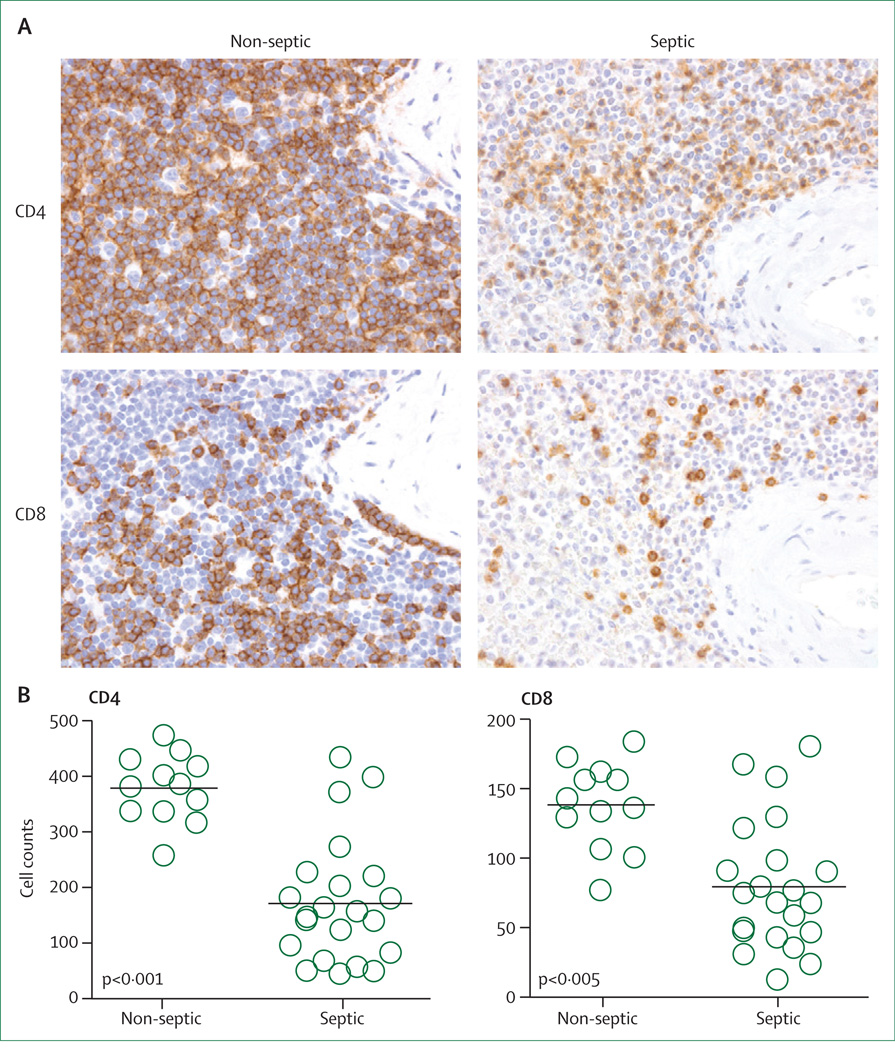
Increasing evidence supports a central role for immunosuppression in sepsis. Meakins and colleagues31 first noted that patients with sepsis and trauma had loss of delayed type hypersensitivity response to common recall antigens such as measles and mumps—a finding that correlated with mortality. Our group did rapid tissue harvesting at the bedsides of patients dying of sepsis and showed that patients had striking apoptosis-induced loss of cells of the innate and adaptive immune system including CD4 and CD8 T, B, and dendritic cells (figure 2).24,32,33 The loss of these immune cells is particularly noteworthy because it occurs during life-threatening infection when clonal expansion of lymphocytes should be occurring. Results of subsequent post-mortem studies of paediatric and neonatal patients dying of sepsis also showed substantial loss of immune cells.34,35 Therefore, severe depletion of immune effector cells is a universal finding in all age groups during sepsis. T regulatory cells are less vulnerable to sepsis-induced apoptosis, therefore the percentage of T regulatory cells increases in patients with sepsis.36–38 Myeloid derived suppressor cells are also immunosuppressive cells that are increased in sepsis.39 The net effect of these immunological changes is that the host’s ability to combat invading pathogens is severely compromised. A putative causative link between the loss of immune effector cells and mortality in sepsis was established when multiple independent groups showed that antiapoptotic therapies were effective at preventing death of immune effector cells and resulted in improved survival in clinically relevant animal models.40–42
Figure 2. Depletion of splenic lymphocytes in septic patients.
(A) Spleens from patients with or without sepsis were obtained by rapid post-mortem sampling and immunostained for CD4, or CD8 T cells. An investigator blinded to sample identity examined the slides. CD4 and CD8 T cells are brown in colour (400× magnification). (B) CD4 and CD8 T cells are decreased in patients with sepsis relative to control patients without sepsis. Cell counts for CD4 and CD8 T cells obtained by counting the number of cells or field in periarteriolar lymphoid sheaths. N=12 non-septic and N=22 septic. Figure modified with permission from the American Medical Association.8
Examination of pathogens that are common causes of nosocomial sepsis in patients in intensive care units can provide further evidence consistent with impaired host immunity in sepsis. Many of these pathogens—eg, Stenotrophomonas spp, Acinetobacter spp, Enterococus spp, Pseudomonas spp, and Candida spp—are weakly virulent or opportunistic organisms, or both, and thus are emblematic of severely depressed host immunity in patients with sepsis.28,43 Additional compelling evidence for immunosuppression in patients with sepsis is the high incidence of reactivation of cytomegalovirus and herpes simplex virus (HSV), latent viruses that host immunity normally holds in abeyance.44,45 Reactivation of cytomegalovirus and HSV has been reported to occur in roughly 33% and 21%, respectively, of immunocompetent critically ill patients with sepsis.44,45 Probably only a few patients with sepsis and viral reactivation had active invasive viral infections; however, these studies show that critically ill patients who had normal immunity before admission to an intensive care unit become profoundly immunocompromised during protracted sepsis, thereby enabling reactivation of latent viruses. The panel shows a summary of clinical and laboratory evidence for immunosuppression in sepsis.
Post-mortem and gene-expression clinical studies
Results from an important post-mortem study showed that sepsis-induced immunosuppression occurred in major organs, not just within circulating leucocytes.8 Rapid post-mortem spleen and lung harvest was done 30–180 min after death in 40 patients with sepsis. Cytokine secretion studies and immunophenotyping of cell-surface receptor or ligand expression profiles were done to discover potential mechanisms of immunosuppression. A striking finding was that lipopolysaccharide-stimulated splenocytes from patients with sepsis had reduced production of both proinflammatory and anti-inflammatory cytokines, less than 10% of that in patients without sepsis. Both spleen and lung showed upregulated expression of selected inhibitory receptors including programmed cell death 1 (PD-1), expansion of suppressor cells (T regulatory cells and myeloid derived suppressor cells), and concomitant downregulation of activation pathways.8
The results of this unique post-mortem study have significant implications. First sepsis clearly induces multiple overlapping mechanisms of immunosuppression in two vital organs, resulting in suppressed host immunity. Second, sepsis decreases the response of cells of both the innate and adaptive immune system. This finding contrasts with a large, multicentre study in patients with trauma that examined gene expression in circulating unfractionated white blood cells at 1, 4, 7, 14, 21, and 28 days after injury.46 Some of the patients developed hospital-acquired infections, although the proportion who developed sepsis is difficult to determine precisely, thus part of the genomic findings could be reflective of both trauma and sepsis. These researchers also compared genomic findings in patients with trauma with those in patients with burns and healthy volunteers who received endotoxin challenge. These three groups of patients had similar gene responses and results showed that patients had down regulation of genes controlling adaptive immunity but upregulation of genes controlling innate immunity. On the basis of these white blood cell transcriptome results, some investigators have concluded that sepsis causes sustained activation of innate immune cells (eg, macrophages and monocytes) and that this activation is causing tissue inflammation and injury.46 By contrast, the results of the post-mortem study of actual cytokine production rather than mRNA showed that both innate and adaptive immune cells are severely suppressed and produce only small amounts of proinflammatory and anti-inflammatory cytokines. One obvious explanation for this difference between the two studies is the much greater complexity of the host response in sepsis in comparison with trauma. In sepsis, there is a major systemic inflammatory response to ongoing infection or, at times, multiple infectious challenges. A second substantial difference between the two studies is that the trauma study measured mRNA whereas the postmortem study quantitated actual proteins (cytokines). Therefore, a potential limitation of the trauma study is the extensive regulation of transcription such that not all mRNA is ultimately translated into protein. Another potential reason for differences between the post-mortem tissue study and the blood genomic study is that the tissue study included some patients who had been septic for prolonged periods whereas the trauma genomic blood study was done on patients who were acutely injured or had shorter periods of trauma and sepsis.
We do not believe that genomic results implying a sustained, prolonged hyperactivation of the innate immune response are indicative of the actual immune status of most patients with sepsis. We believe that there is an initial hyperactivation of the innate immune response that persists for a variable period depending on patient’s age, comorbidities, organism virulence, and other factors, followed by defective innate and adaptive immunity. CD4 T cells are crucial regulators of monocyte and macrophage function. Therefore, given the profound loss and dysfunction of CD4 T cells in sepsis, envisioning how many innate immune cells (ie, monocytes or macrophages) could have sustained hyperactivation is difficult. Most importantly, the findings of sepsis-induced depression of cytokine production reported in the postmortem study are highly consistent with many studies that have examined peripheral blood mononuclear cells and whole-blood-stimulated cytokine production in patients with sepsis and documented substantially decreased cytokine production.16–20,47–52 Future clinical studies could resolve this important issue.
New approaches: immunomodulatory therapy
Sepsis can be thought of as a race to the death between the invading microbes and the host immune response, and the pathogens seek an advantage by incapacitating various aspects of host immunity. Most previous sepsis drug trials used compounds that blocked the host response to pathogens or limited inflammation. There is likely a role for drugs that block inflammatory cytokines in sepsis; however, such agents should be shortacting, applied early in sepsis, and used only in patients who have substantially elevated proinflammatory cytokines. Most patients will rapidly progress to an immunosuppressive state. Thus, in addition to development of protocols to improve timely antibiotic administration and development of clinical practices that avoid infections, focus should shift to the development of methods to augment host immunity (figure 3). A second important implication of this novel immunosuppression paradigm is that newer antibiotics alone are unlikely to substantially improve sepsis mortality because the major underlying defect is impaired patient immunity.
Figure 3. Immunostimulation therapy in sepsis: a new approach.
New biomarker-based methods to semi-quantitate the degree of immunosuppression in septic patients are now being used. For example, flow cytometric quantitation of circulating blood monocyte expression of HLA-DR has been used to identify patients who would respond to granulocyte macrophage colony stimulating factor (GM-CSF). In the future, other biomarkers that are currently used in cancer immunotherapy will probably be used. Monocyte expression of programmed cell-death ligand-1 (PD-L1) could be used to guide therapy with anti-PD-1 antibody. Patients who have persistently low absolute lymphocyte counts could be candidates for interleukin-7 therapy. Patients with infections caused by weakly virulent pathogens including Candida spp are also candidates for immunotherapy. Therapy refers to immunostimulation for most severely immunodepressed patients, identified via immunomonitoring.
Findings from two studies of granulocyte macrophage colony stimulating factor (GM-CSF), a cytokine that activates and induces production of neutrophils and monocytes or macrophages, show the potential for immunotherapy in sepsis.9,10 To ensure that only patients who had entered the immunosuppressive phase of sepsis were treated with GM-CSF, investigators restricted therapy to patients who had persistent decreases in monocyte HLA-DR expression, a common abnormality in sepsis. Results showed that patients with sepsis who were treated with GM-CSF had restoration of HLA-DR expression, fewer ventilatory days, and shorter hospital and intensive care unit days.9 GM-CSF also showed benefit in a paediatric sepsis study in which Hall and colleagues10 used lipopolysaccharide-stimulated TNFα production in whole blood to identify immunosuppressed patients with sepsis. Patients with TNFα production of less than 200 pg/mL were immunosuppressed and treated with GM-CSF, which restored TNFα production and decreased acquisition of new nosocomial infections.
Another immunotherapeutic agent with great potential is interleukin 7, a pleuripotent cytokine that has been termed the maestro of the immune system because of its diverse effects on immunity.53–60 Interleukin 7 induces proliferation of naive and memory T cells, thereby supporting replenishment of lymphocytes, which are relentlessly depleted during sepsis (figure 2).8,32,40 In clinical trials at the National Cancer Institute, it caused a doubling of circulating CD4 and CD8 T cells and an increase in size of spleen and peripheral lymph nodes by roughly 50%.57 Similarly, results of a trial of interleukin 7 in patients infected with HIV-1 who had persistently low CD4 T cells despite effective viral suppression showed that the cytokine induced an increase of two to three times in circulating CD4 and CD8 T cells.58 Thus, interleukin 7 reverses a major pathological abnormality in sepsis—ie, profound lymphopenia. Interleukin 7 has many additional actions that are highly beneficial in sepsis (figure 4):11,60–63 it increases the ability of T cells to become activated, potentially restoring functional capacity of hyporesponsive or exhausted T cells which typify sepsis;11,60–63 increases expression of cell-adhesion molecules, which enhance trafficking of T cells to sites of infection;11,59 and increases T-cell receptor diversity, leading to more potent immunity against pathogens.56,58
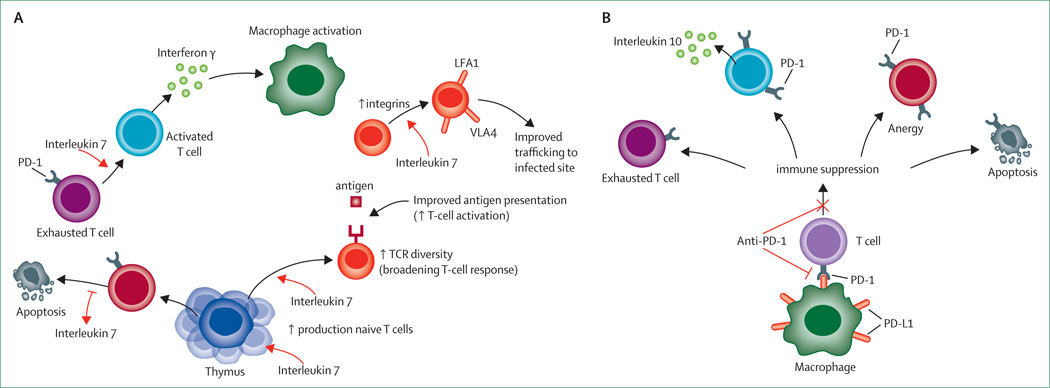
Figure 4. Interleukin 7 and anti-PD-1 immunotherapy in sepsis.
Interleukin 7 (A) acts to reverse immunosuppression by multiple mechanisms including increased production of CD4 and CD8 T cells, blockade of sepsis-induced apoptosis, reversal of T-cell exhaustion, increased interferon γ production leading to macrophage activation, increased integrin expression leading to improved T-cell recruitment to infected areas, and increased T-cell receptor (TCR) diversity. Anti-PD-1 antibody (B) will prevent interaction of programmed cell-death ligand-1 (PD-L1), which is expressed on macrophages with PD-1 receptor, which is expressed on T cells. Thus, anti-PD-1 antibody will prevent formation of exhausted T cells, decrease interleukin 10 production, prevent T-cell anergy, and decrease sepsis-induced apoptosis. LFA=leucocyte function-associated antigen. VLA=very late antigen. PD-1=programmed cell death 1.
Interleukin 7 has shown efficacy both clinically and in animal models of infection. A case report of a patient with idiopathic low CD4 T cells with progressive multifocal leukoencephalopathy (PML) showed that interleukin 7 caused rapid increases in lymphocytes, decreased circulating JC virus, and led to disease resolution.61 Pellegrini and colleagues59 gave interleukin 7 to mice that were chronically infected with lymphocytic chorio-meningitis. The treatment enhanced T-cell recruitment to the infected site and increased T-cell numbers, thereby easing viral clearance. Our group showed that interleukin 7 restored the delayed type hypersensitivity response, decreased sepsis-induced lymphocyte apoptosis, reversed sepsis-induced depression of interferon γ (a cytokine that is essential for macrophage activation), and improved survival in murine polymicrobial sepsis.11 Our group also reported that interleukin 7 is beneficial in a fungal sepsis model that reproduces the delayed secondary infections typical of patients in intensive care units.62 We also showed interleukin 7’s ability to reverse sepsis-induced T-cell alterations in septic shock patients.63 Ex-vivo treatment of patients’ cells with interleukin 7 corrected multiple sepsis-induced defects including CD4 and CD8 T cell proliferation, interferon γ production, STAT5 phosphorylation, and Bcl-2 induction to that of healthy controls. This functional restoration indicates that the interleukin 7 pathway remains fully operative during sepsis.
Interleukin 7 is in clinical trials in patients with cancer, HIV-1, and PML. It has been well tolerated in more than 200 patients and, unlike interleukin 2, a closely-related cytokine, it rarely induces fever, capillary leak syndrome, or other clinical abnormalities associated with excessive proinflammatory cytokines.56,57 Because of its diverse beneficial effects on immunity and excellent safety record, investigators at the National Cancer Institute have consistently ranked interleukin 7 as one of the top potential immunotherapeutic molecules.14 Because of its many beneficial effects on immunity, reported efficacy in bacterial, fungal, and animal sepsis models, and clinical track record, we believe that interleukin 7 should be clinically tested in sepsis, and that it has enormous promise.
Another exciting immunomodulatory therapy that holds much potential in sepsis involves blockade of negative costimulatory molecules present on T cells. The negative costimulatory molecule PD-1 is inducibly expressed on CD4 and CD8 T cells.64–67 Signalling through PD-1 inhibits the ability of T cells to proliferate, produce cytokines, or perform cytotoxic functions. Persistent antigenic exposure as occurs in chronic viral infections such as HIV-1 and viral hepatitis leads to excessive PD-1 expression and exhausted T cells.66,67 Antibody blockade of PD-1 or its ligand (PD-L1) can reverse T-cell dysfunction and induce pathogen clearance (figure 4).67 Similarly, three independent groups showed that blockade of the PD-1 pathway improves survival in clinically relevant animal models of bacterial and fungal sepsis.68–70 Our group showed that PD-1 over-expression on circulating T cells from patients with sepsis correlated with decreased T-cell proliferative capacity, increased secondary nosocomial infections, and mortality.50 Thus, expression of PD-1 or PD-L1 on circulating immune cells could function as a valuable biomarker for the selection of candidates for blockade therapy. Importantly, post-mortem study of patients with sepsis showed that PD-L1 was highly expressed on tissue parenchymal cells, including endothelial cells, thereby providing opportunity for pathway activation.8
Sepsis has many of the same immunosuppressive mechanisms that operate in cancer, including increased production of the immunosuppressive cytokine interleukin 10, T regulatory cells, myeloid derived suppressor cells, and PD-1 and PD-L1 with T-cell exhaustion.12–14 Therefore, immunotherapy that is effective in cancer might also be successful in sepsis. Thus, the extraordinary recent success of anti-PD-1 antibody in oncology is particularly noteworthy.13 Anti-PD-1 antibody produced excellent clinical responses in 20–25% of patients with diverse tumours including non-small-cell lung cancer (a malignant disease that has been extremely difficult to treat), melanoma, and renal-cell cancer.13 Although there are concerns about autoimmune reactions in patients on long-term anti-PD-1 or anti-PD-L1 therapy, serious reactions are very uncommon. Patients with sepsis would not need prolonged therapy with anti-PD-1 or anti-PD-L1 antibodies, therefore concerns about auto immune reactions would be diminished. If additional studies confirm its safety and efficacy, anti-PD-1 based therapy should be tested in clinical sepsis.
Another potential immunostimulatory cytokine receiving renewed interest as a potential therapeutic agent in sepsis is interferon γ, a potent monocyte and macrophage activator, which produced encouraging results in a small trial of patients with sepsis. Docke and colleagues71 treated patients with sepsis whose monocytes had reduced HLA-DR expression and produced decreased amounts of TNFα after lipopolysaccharide stimulation. Interferon γ treatment reversed the sepsis-induced monocyte dysfunction and resulted in eight of nine patients successfully resolving the septic insult. Nalos and associates reported on use of interferon γ in a patient with persistent staphylococcal sepsis.72 Interferon γ therapy resulted in increased monocyte expression of HLA-DR, increased numbers of interleukin-17-producing CD4 T cells, and clinical resolution of the sepsis. Interferon γ is approved for treatment of fungal sepsis in patients with chronic granulomatous disease. Jarvis and colleagues73 treated HIV patients who had cryptococcal meningitis with interferon γ in a randomised controlled trial. Patients treated with interferon γ had more rapid clearing of cerebrospinal fluid than did control patients.
Other immunoadjuvant molecules in early stages of testing have also shown efficacy in clinically relevant animal models of sepsis. Interleukin 15 is a pleuripotent cytokine closely related to interleukin774 that also acts on CD4 and CD8 T cells to induce proliferation and prevent apoptosis. A potential advantage of interleukin 15 compared with interleukin 7 is its potent immunostimulatory and proliferative effects on natural killer cells and dendritic cells. These cells have important roles in fighting infection and are also severely depleted in sepsis. Inoue and colleagues74 reported that interleukin 15 blocked sepsis-induced apoptosis of CD8 T cells, natural killer cells, and dendritic cells, and improved survival in sepsis due to caecal ligation and puncture and in primary pseudomonas pneumonia. The B and T lymphocyte attenuator (BTLA) is an immunoregulatory receptor expressed by various innate and adaptive immune cells. Activation of BTLA induces a potent immunosuppressive effect on T cells and other immune cells. Adler and coworkers75 reported that BTLA null mice showed reduced parasitaemia and faster clearing of malaria in a murine model of infection. Results in the caecal ligation and puncture model of murine sepsis show similar protective effects: BTLA-null mice have increased survival and reduced organ injury compared with wild-type mice.76 Thus, there are several immunoadjuvants that offer hope in the battle against sepsis.
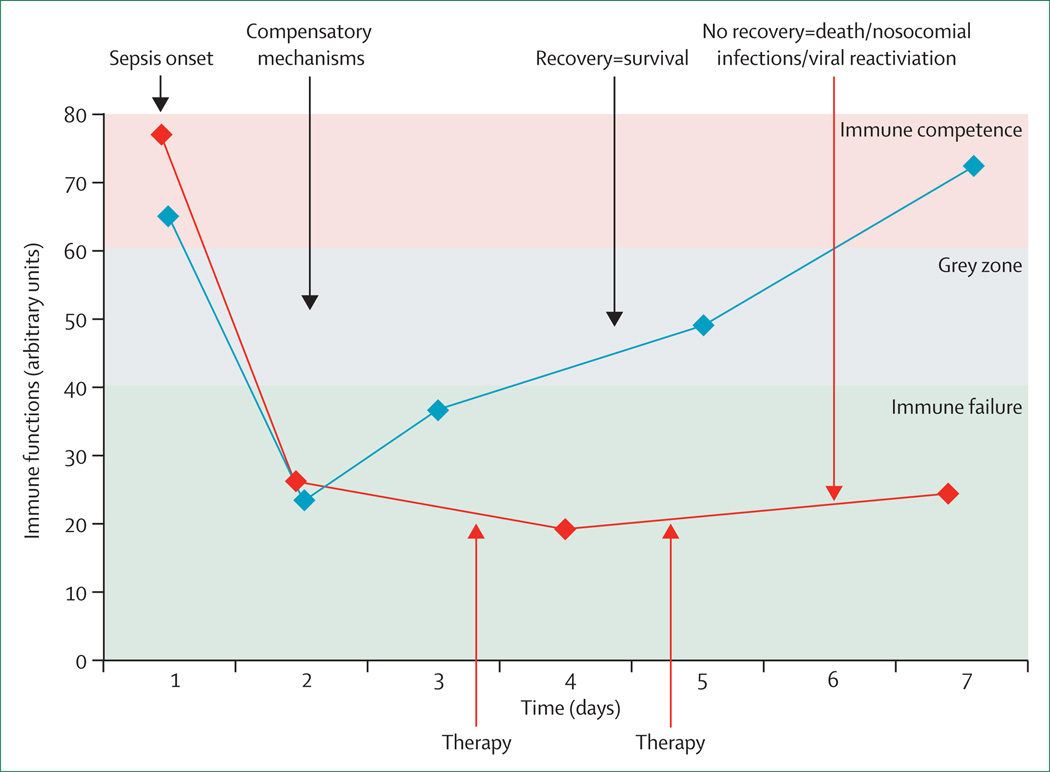
An immunostimulatory therapeutic approach relies on individual, targeted, and timed treatment:1,5,77–81 only those septic patients who are immunosuppressed will benefit. For each patient with sepsis, the scale, persistence over time, various mechanisms sustaining this immunosuppression (identified through laboratory monitoring, panel), or occurrence of some particular clinical event (eg, viral reactivation) will help to define the appropriate drug and time of administration.1–6,77–81 After onset of sepsis, every patient has activation of transient immunosuppressive mechanisms that normally reflect compensatory measures, which counterbalance the initial inflammatory response (figure 1B). Generally, after 2–3 days, most patients recover substantial immune function; however, some will have persistent immunosuppression associated with increased nosocomial infections and mortality— only these will benefit from immune-stimulatory therapy. This selective approach contrasts with previous non-specific trials aimed at modification of the pro-inflammatory and anti-inflammatory balance after sepsis. Indeed, these clinical trials were, for the most part, designed without stratification of patients.
Another approach to the selection of patients for individualised, targeted immunoadjuvant therapy in sepsis will likely be genetic screening. Evidence that the intense inflammatory response that occurs in sepsis and other disorders can alter gene expression is accumulating.81,82 Epigenetic gene regulation refers to all the mechanisms that modulate gene expression without changing the DNA sequence. Potent inflammatory responses that occur as a result of sepsis induce increases or decreases in gene expression by processes referred to as epigenetic changes that result in DNA methylation, histone modification, and chromatin remodelling. Results of studies indicate that epigenetic changes happen with intense immunoinflammatory responses such as sepsis and result in impaired expression of genes that regulate key immune activation responses, thereby rendering the host more susceptible to infection. Rapid detection of these sepsis-induced epigenetic changes in particular patients with sepsis could lead to early identification of an immunosuppressive state and allow more timely immune-boosting therapy.
Conclusion
In the future, immunomodulatory therapies in sepsis will be personalised on the basis of particular laboratory and clinical findings, or both—eg, the use of GM-CSF dependent on monocyte HLA-DR expression (table).1,9,10 Similarly, flow cytometry quantitation of circulating immune cell expression of PD-1/PD-L1 or rapid whole-blood stimulation assays of cytokine secretion could be used to guide immunotherapy. Finally, patients with infections caused by opportunistic pathogens (eg, Enterococcus spp, Candida spp, Stenotrophomonas spp), or patients with cytomegalovirus or HSV reactivation, are likely candidates for immune-enhancing therapy. Although immune-stimulatory drugs could possibly worsen the hyperinflammatory phase of sepsis or induce autoimmunity, this was not reported in clinical trials of interferon γ, a potent immunostimulatory agent, and G-CSF and GM-CSF in patients with various systemic inflammatory states including sepsis and trauma.71,83,84 Additionally, most patients with protracted sepsis are so immunosuppressed that they are unlikely to develop hyperinflammation.
Table.
Potential biomarker and clinical-laboratory findings for applied immunotherapy
| Immunotherapy | |
|---|---|
| Decreased monocyte HLA-DR expression | GM-CSF, interferon γ |
| Persistent severe lymphopenia | Interleukin 7 |
| Increased PD-1 or PD-L1 expression | Anti-PD1/Anti-PD-L1 antibody |
| Decreased TNFα production in stimulated blood | Many |
| Increased T-regulatory cells | Anti-T-regulatory cell agents |
| Infections with relatively avirulent or opportunistic pathogens (Enterococci spp, Acinetobacter spp, Candida spp, etc) |
Many |
| Reactivation of cytomegalovirus or HSV | Many |
| Elderly patients with malnutrition and multiple comorbidities | Many |
GM-CSF=granulocyte macrophage colony stimulating factor. PD-1=programmed cell death 1. PD-L1=programmed cell death 1 ligand 1. TNF=tumour necrosis factor. HSV=herpes simplex virus.
Advances in immunology and our understanding of the pathophysiological basis of sepsis provide exciting new therapeutic opportunities. Primum non nocere— first, do no harm—is a wise medical dictum. However, mortality due to sepsis has remained stubbornly high, and, as another aphorism states: desperate diseases require desperate means. Immunoadjuvants have been successfully applied clinically in both cancer and sepsis with acceptable safety profiles and some success. We postulate that immunotherapy will have wide-ranging beneficial effects in sepsis, and could be a major advance in infectious disease.
Panel: Clinical or laboratory evidence for sepsis being an immunosuppressive disorder.
Loss of delayed type hypersensitivity response to common recall antigens31
Apoptosis-induced depletion of immune effector cells, loss of CD4, CD8, B, and dendritic cells24,32,33
Reactivation of latent viruses including cytomegalovirus and herpes simplex virus occurs in roughly 25–35% of patients with44,45
Infection with relatively avirulent pathogens (eg, Enterococci spp, Acinetobacter spp, Stenotropomonas spp, Candida spp) 28,43
Autopsy study showing unresolved foci of infection in roughly 80% of patients with sepsis7
Small positive phase 2 studies of biomarker guided immune enhancing agents granulocyte-macrophage colony stimulating factor and interferon γ in patients with sepsis9,10
Blood studies from patients with and without sepsis show decreased production of proinflammatory cytokines, decreased monocyte HLA-DR expression, increased numbers of regulatory T cells, increased production of PD-1 or PD-L116–20
Autopsy study of spleens and lungs from patients with and without sepsis showed decreased cytokine production, decreased immune cell activation pathways, and upregulation of immune suppression pathways, decreased HLA-DR and CD28 expression, increased production of PD-1 and PD-L1, increased numbers of regulatory T cells)8
Clinically relevant animal models of sepsis showing increased survival with immune enhancing treatment (interleukin 7, anti-PD-1 antibody, interleukin 15)11,40,41
PD-1=programmed cell death 1. PD-L1=programmed cell death 1 ligand 1.
Search strategy and selection criteria.
References for this Review were identified through searches of PubMed for articles published from Jul, 1976, to Oct, 2012 by use of the terms “sepsis”, “immunosuppression”, “immunoparalysis”, and “immunotherapy”. Only papers published in English were used.
Acknowledgments
Conflicts of interest
RSH has received research funding from Bristol-Myers Squib, Medimmune, Pfizer, Aurigene, Agennix, and from the National Institutes of Health grants GM055194 and GM044118. GM has received research funding from Biomerieux. DP has received support from a grant from University Paris 7 Denis Diderot, Plan Quadriennal.
Contributor Information
Richard S Hotchkiss, Department of Anesthesiology, Medicine, and Surgery; Washington University School of Medicine, St Louis, MO, USA.
Guillaume Monneret, Hospices Civils de Lyon, Immunology Laboratory, Hôpital E, Herriot, Lyon, France.
Didier Payen, Department of Anaesthesiology and Critical Care and SAMU, Hôpital Lariboisière, Assistance Publique Hôpitaux de Paris, Paris, France.
References
- 1.Cohen J, Opal S, Calandra T. Sepsis studies need new direction. Lancet Infect Dis. 2012;12:503–505. doi: 10.1016/S1473-3099(12)70136-6. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel RP, Edmond MB. Septic shock: evaluating another failed treatment. N Engl J Med. 2012;366:2122–2124. doi: 10.1056/NEJMe1203412. [DOI] [PubMed] [Google Scholar]
- 3.Williams SC. After Xigris, researchers look to new targets to combat sepsis. Nat Med. 2012;18:1001. doi: 10.1038/nm0712-1001. [DOI] [PubMed] [Google Scholar]
- 4.Dolgin E. Trial failure prompts soul-searching for critical-care specialists. Nat Med. 2012;18:1000. doi: 10.1038/nm0712-1000. [DOI] [PubMed] [Google Scholar]
- 5.Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA. 2011;306:2614–2615. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 6.Vincent JL. The rise and fall of drotrecogin alfa (activated) Lancet Infect Dis. 2012;12:649–651. doi: 10.1016/S1473-3099(12)70175-5. [DOI] [PubMed] [Google Scholar]
- 7.Torgersen C, Moser P, Luckner G, et al. Macroscopic postmortem findings in 235 surgical intensive care patients with sepsis. Anesth Analg. 2009;108:1841–1847. doi: 10.1213/ane.0b013e318195e11d. [DOI] [PubMed] [Google Scholar]
- 8.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meisel C, Schefold JC, Pschowski R, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640–648. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]
- 10.Hall MW, Knatz NL, Vetterly C, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–532. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unsinger J, McGlynn M, Kasten KR, et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 2010;184:3768–3779. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 15.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 16.van Dissel JT, van Langevelde P, Westendorp RG, Kwappenberg K, Frolich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351:950–953. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 17.Ertel W, Kremer JP, Kenney J, et al. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood. 1995;85:1341–1347. [PubMed] [Google Scholar]
- 18.Munoz C, Carlet J, Fitting C, Misset B, Bleriot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rigato O, Salomao R. Impaired production of interferon-gamma and tumor necrosis factor-alpha but not of interleukin 10 in whole blood of patients with sepsis. Shock. 2003;19:113–116. doi: 10.1097/00024382-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Sinistro A, Almerighi C, Ciaprini C, et al. Downregulation of CD40 ligand response in monocytes from sepsis patients. Clin Vaccine Immunol. 2008;15:1851–1858. doi: 10.1128/CVI.00184-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weighardt H, Heidecke CD, Emmanuilidis K, et al. Sepsis after major visceral surgery is associated with sustained and interferon-gamma-resistant defects of monocyte cytokine production. Surgery. 2000;127:309–315. doi: 10.1067/msy.2000.104118. [DOI] [PubMed] [Google Scholar]
- 22.Barochia AV, Cui X, Vitberg D, et al. Bundled care for septic shock: an analysis of clinical trials. Crit Care Med. 2010;38:668–678. doi: 10.1097/CCM.0b013e3181cb0ddf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med. 2008;14:64–78. doi: 10.2119/2007-00102.Monneret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, Swanson PE, Freeman BD, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 2009;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Fink MP, Evans TW. Mechanisms of organ dysfunction in critical illness: report from a round table conference held in Brussels. Intensive Care Med. 2002;28:369–375. doi: 10.1007/s00134-001-1205-2. [DOI] [PubMed] [Google Scholar]
- 26.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. 2007;35:2408–2416. doi: 10.1097/01.ccm.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 27.Kethireddy S, Kumar A. Mortality due to septic shock following early, appropriate antibiotic therapy: can we do better? Crit Care Med. 2012;40:2228–2229. doi: 10.1097/CCM.0b013e318256bb99. [DOI] [PubMed] [Google Scholar]
- 28.Otto GP, Sossdorf M, Claus RA, et al. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care. 2011;15:R183. doi: 10.1186/cc10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 30.Reber AJ, Chirkova T, Kim JH, et al. Immunosenescence and challenges of vaccination against influenza in the aging population. Aging Dis. 2012;3:68–90. [PMC free article] [PubMed] [Google Scholar]
- 31.Meakins JL, Pietsch JB, Bubenick O, et al. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann Surg. 1977;186:241–250. doi: 10.1097/00000658-197709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 33.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 34.Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174:3765–3772. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- 35.Toti P, De Felice C, Occhini R, et al. Spleen depletion in neonatal sepsis and chorioamnionitis. Am J Clin Pathol. 2004;122:765–771. doi: 10.1309/RV6E-9BMC-9954-A2WU. [DOI] [PubMed] [Google Scholar]
- 36.Venet F, Chung CS, Monneret G, et al. Regulatory T cell populations in sepsis and trauma. J Leukoc Biol. 2008;83:523–535. doi: 10.1189/jlb.0607371. [DOI] [PubMed] [Google Scholar]
- 37.Venet F, Chung CS, Kherouf H, et al. Increased circulating regulatory T cells (CD4(+)CD25 (+)CD127 (-)) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med. 2009;35:678–686. doi: 10.1007/s00134-008-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leng FY, Liu JL, Liu ZJ, Yin JY, Qu HP. Increased proportion of CD4(+)CD25(+)Foxp3(+) regulatory T cells during the early-stage sepsis in ICU patients. J Microbiol Immunol Infect. 2012 doi: 10.1016/j.jmii.2012.06.012. published online Aug 23. http://dx.doi.org/10.1016/j.jmii.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Delano MJ, Scumpia PO, Weinstein JS, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hotchkiss RS, Swanson PE, Knudson CM, et al. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
- 41.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci USA. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wesche-Soldato DE, Swan RZ, Chung CS, Ayala A. The apoptotic pathway as a therapeutic target in sepsis. Curr Drug Targets. 2007;8:493–500. doi: 10.2174/138945007780362764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kollef KE, Schramm GE, Wills AR, Reichley RM, Micek ST, Kollef MH. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest. 2008;134:281–287. doi: 10.1378/chest.08-1116. [DOI] [PubMed] [Google Scholar]
- 44.Luyt CE, Combes A, Deback C, et al. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med. 2007;175:935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 45.Limaye AP, Kirby KA, Rubenfeld GD, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boomer JS, Shuherk-Shaffer J, Hotchkiss RS, Green JM. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit Care. 2012;16:R112. doi: 10.1186/cc11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukaszewicz AC, Grienay M, Resche-Rigon M, et al. Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med. 2009;37:2746–2752. doi: 10.1097/CCM.0b013e3181ab858a. [DOI] [PubMed] [Google Scholar]
- 49.Venet F, Lepape A, Monneret G. Clinical review: flow cytometry perspectives in the ICU - from diagnosis of infection to monitoring of injury-induced immune dysfunctions. Crit Care. 2011;15:231. doi: 10.1186/cc10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guignant C, Lepape A, Huang X, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15:R99. doi: 10.1186/cc10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monneret G, Venet F. Additional bad news from regulatory T cells in sepsis. Crit Care. 2010;14:453. doi: 10.1186/cc9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belikova I, Lukaszewicz AC, Faivre V, Damoisel C, Singer M, Payen D. Oxygen consumption of human peripheral blood mononuclear cells in severe human sepsis. Crit Care Med. 2007;35:2702–2708. doi: 10.1097/01.ccm.0000295593.25106.c4. [DOI] [PubMed] [Google Scholar]
- 53.Sprent J, Surh CD. Interleukin 7, maestro of the immune system. Semin Immunol. 2012;24:149–150. doi: 10.1016/j.smim.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 54.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11:330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim HR, Hwang KA, Park SH, Kang I. IL-7 and IL-15: biology and roles in T-cell immunity in health and disease. Crit Rev Immunol. 2008;28:325–339. doi: 10.1615/critrevimmunol.v28.i4.40. [DOI] [PubMed] [Google Scholar]
- 56.Morre M, Beq S. Interleukin-7 and immune reconstitution in cancer patients: a new paradigm for dramatically increasing overall survival. Target Oncol. 2012;7:55–68. doi: 10.1007/s11523-012-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenberg SA, Sportes C, Ahmadzadeh M, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levy Y, Sereti I, Tambussi G, et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis. 2012;55:291–300. doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pellegrini M, Calzascia T, Toe JG, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 60.Kasten KR, Prakash PS, Unsinger J, et al. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis. Infect Immun. 2010;78:4714–4722. doi: 10.1128/IAI.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel A, Patel J, Ikwuagwu J. A case of progressive multifocal leukoencephalopathy and idiopathic CD4+ lymphocytopenia. J Antimicrob Chemother. 2010;65:2697–2698. doi: 10.1093/jac/dkq359. [DOI] [PubMed] [Google Scholar]
- 62.Unsinger J, Burnham CA, McDonough J, et al. Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J Infect Dis. 2012;206:606–616. doi: 10.1093/infdis/jis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Venet F, Foray AP, Villars-Mechin A, et al. IL-7 restores lymphocyte functions in septic patients. J Immunol. 2012;189:5073–5081. doi: 10.4049/jimmunol.1202062. [DOI] [PubMed] [Google Scholar]
- 64.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 65.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 67.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 68.Huang X, Venet F, Wang YL, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci USA. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010;88:233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Zhou Y, Lou J, et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care. 2010;14:R220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Docke WD, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 72.Nalos M, Santner-Nanan B, Parnell G, Tang B, McLean AS, Nanan R. Immune effects of interferon gamma in persistent staphylococcal sepsis. Am J Respir Crit Care Med. 2012;185:110–112. doi: 10.1164/ajrccm.185.1.110. [DOI] [PubMed] [Google Scholar]
- 73.Jarvis JN, Meintjes G, Rebe K, et al. Adjunctive interferon-γ immunotherapy for treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS. 2012;26:1105–1113. doi: 10.1097/QAD.0b013e3283536a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Inoue S, Unsinger J, Davis CG, et al. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol. 2010;184:1401–1409. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adler G, Steeg C, Pfeffer K, et al. B and T lymphocyte attenuator restricts the protective immune response against experimental malaria. J Immunol. 2011;187:5310–5319. doi: 10.4049/jimmunol.1101456. [DOI] [PubMed] [Google Scholar]
- 76.Shubin NJ, Chung CS, Heffernan DS, Irwin LR, Monaghan SF, Ayala A. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. J Leukoc Biol. 2012;92:593–603. doi: 10.1189/jlb.1211641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hotchkiss RS, Opal S. Immunotherapy for sepsis—a new approach against an ancient foe. N Engl J Med. 2010;363:87–89. doi: 10.1056/NEJMcibr1004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ward PA. Immunosuppression in sepsis. JAMA. 2011;306:2618–2619. doi: 10.1001/jama.2011.1831. [DOI] [PubMed] [Google Scholar]
- 79.Christaki E, Anyfanti P, Opal SM. Immunomodulatory therapy for sepsis: an update. Expert Rev Anti Infect Ther. 2011;9:1013–1033. doi: 10.1586/eri.11.122. [DOI] [PubMed] [Google Scholar]
- 80.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Waterer GW. Community-acquired pneumonia: genomics, epigenomics, transcriptomics, proteomics, and metabolomics. Semin Respir Crit Care Med. 2012;33:257–265. doi: 10.1055/s-0032-1315637. [DOI] [PubMed] [Google Scholar]
- 82.Roger T, Lugrin J, Le Roy D, et al. Histone deacetylase inhibitors impair innate immune responses to Toll-like receptor agonists and to infection. Blood. 2011;117:1205–1217. doi: 10.1182/blood-2010-05-284711. [DOI] [PubMed] [Google Scholar]
- 83.Nelson S, Belknap SM, Carlson RW, et al. on behalf of the CAP Study Group. A randomized controlled trial of filgrastim as an adjunct to antibiotics for treatment of hospitalized patients with community-acquired pneumonia. J Infect Dis. 1998;178:1075–1080. doi: 10.1086/515694. [DOI] [PubMed] [Google Scholar]
- 84.Root RK, Lodato RF, Patrick W, et al. Multicenter, double-blind, placebo-controlled study of the use of filgrastim in patients hospitalized with pneumonia and severe sepsis. Crit Care Med. 2003;31:367–373. doi: 10.1097/01.CCM.0000048629.32625.5D. [DOI] [PubMed] [Google Scholar]