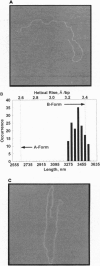
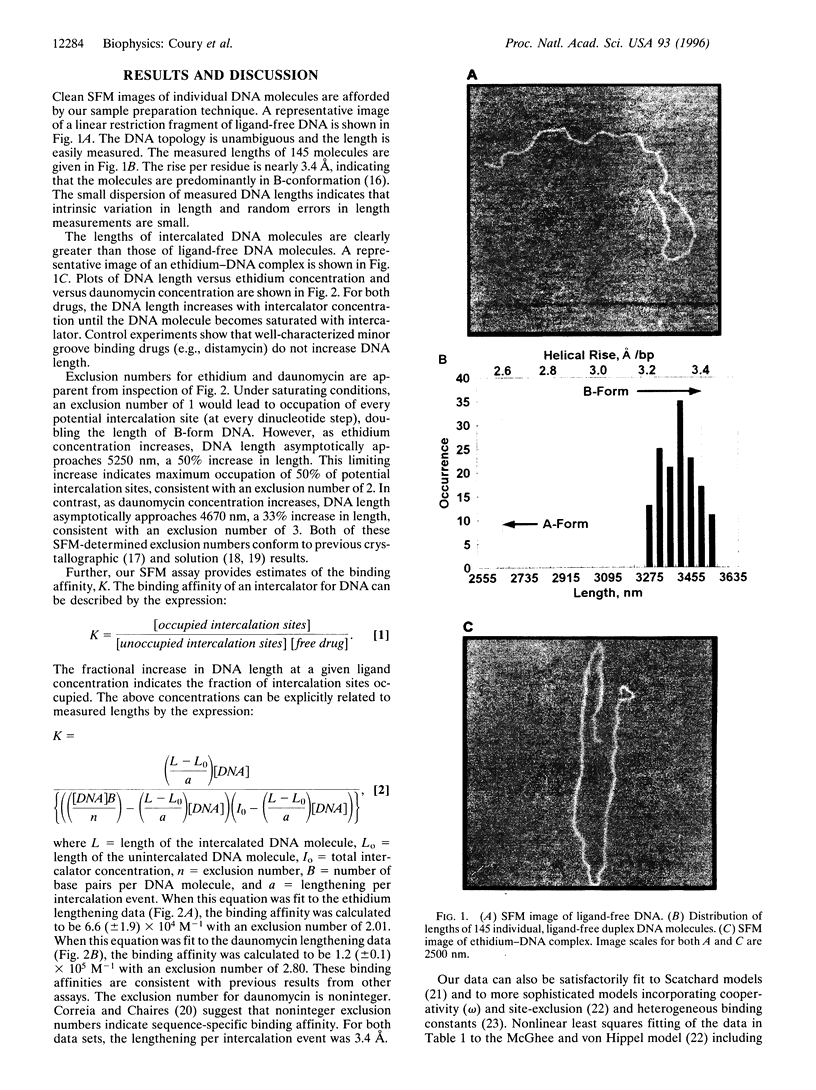
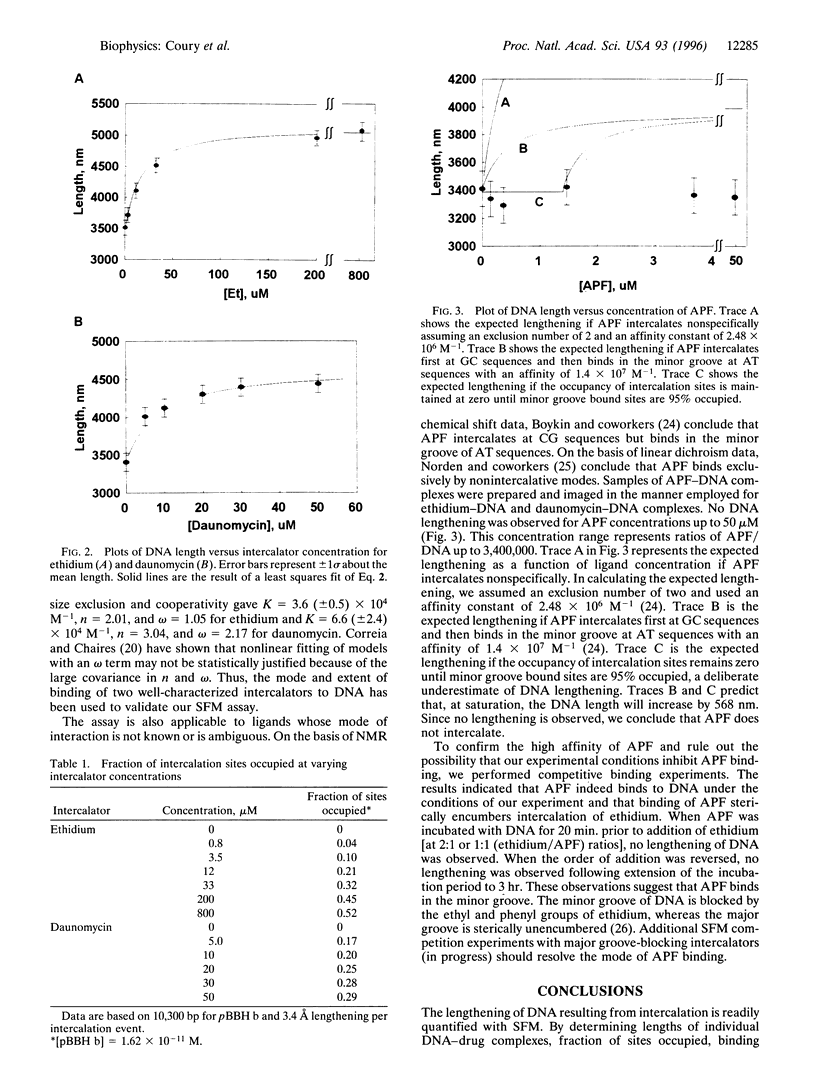
Abstract
Determining the mode-of-binding of a DNA ligand is not always straightforward. Here, we establish a scanning force microscopic assay for mode-of-binding that is (i) direct: lengths of individual DNA-ligand complexes are directly measured; (ii) rapid: there are no requirements for staining or elaborate sample preparation; and (iii) unambiguous: an observed increase in DNA length upon addition of a ligand is definitive evidence for an intercalative mode-of-binding. Mode-of-binding, binding affinity, and site-exclusion number are readily determined from scanning force microscopy measurements of the changes in length of individual drug-DNA complexes as a function of drug concentration. With this assay, we resolve the ambiguity surrounding the mode of binding of 2,5-bis(4-amidinophenyl) furan (APF) to DNA and show that it binds to DNA by nonintercalative modes. APF is a member of an important class of aromatic dicationic drugs that show significant activity in the treatment of Pneumocystis carinii pneumonia, an opportunistic infection that is the leading cause of death in AIDS patients.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Bresloff J. L., Crothers D. M. Equilibrium studies of ethidium--polynucleotide interactions. Biochemistry. 1981 Jun 9;20(12):3547–3553. doi: 10.1021/bi00515a038. [DOI] [PubMed] [Google Scholar]
- Bustamante C., Vesenka J., Tang C. L., Rees W., Guthold M., Keller R. Circular DNA molecules imaged in air by scanning force microscopy. Biochemistry. 1992 Jan 14;31(1):22–26. doi: 10.1021/bi00116a005. [DOI] [PubMed] [Google Scholar]
- Correia J. J., Chaires J. B. Analysis of drug-DNA binding isotherms: a Monte Carlo approach. Methods Enzymol. 1994;240:593–614. doi: 10.1016/s0076-6879(94)40065-2. [DOI] [PubMed] [Google Scholar]
- Frederick C. A., Williams L. D., Ughetto G., van der Marel G. A., van Boom J. H., Rich A., Wang A. H. Structural comparison of anticancer drug-DNA complexes: adriamycin and daunomycin. Biochemistry. 1990 Mar 13;29(10):2538–2549. [PubMed] [Google Scholar]
- Geierstanger B. H., Mrksich M., Dervan P. B., Wemmer D. E. Design of a G.C-specific DNA minor groove-binding peptide. Science. 1994 Oct 28;266(5185):646–650. doi: 10.1126/science.7939719. [DOI] [PubMed] [Google Scholar]
- Hansma H. G., Sinsheimer R. L., Li M. Q., Hansma P. K. Atomic force microscopy of single- and double-stranded DNA. Nucleic Acids Res. 1992 Jul 25;20(14):3585–3590. doi: 10.1093/nar/20.14.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. C., Sobell H. M. Visualization of drug-nucleic acid interactions at atomic resolution. VIII. Structures of two ethidium/dinucleoside monophosphate crystalline complexes containing ethidium: cytidylyl(3'-5') guanosine. J Biomol Struct Dyn. 1984 Mar;1(5):1179–1194. doi: 10.1080/07391102.1984.10507511. [DOI] [PubMed] [Google Scholar]
- Jansen K., Lincoln P., Nordén B. Binding of DAPI analogue 2,5-bis(4-amidinophenyl)furan to DNA. Biochemistry. 1993 Jul 6;32(26):6605–6612. doi: 10.1021/bi00077a013. [DOI] [PubMed] [Google Scholar]
- Lipscomb L. A., Zhou F. X., Presnell S. R., Woo R. J., Peek M. E., Plaskon R. R., Williams L. D. Structure of DNA-porphyrin complex. Biochemistry. 1996 Mar 5;35(9):2818–2823. doi: 10.1021/bi952443z. [DOI] [PubMed] [Google Scholar]
- Lyubchenko Y., Shlyakhtenko L., Harrington R., Oden P., Lindsay S. Atomic force microscopy of long DNA: imaging in air and under water. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2137–2140. doi: 10.1073/pnas.90.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Moskowitz L., Hensley G. T., Chan J. C., Adams K. Immediate causes of death in acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1985 Aug;109(8):735–738. [PubMed] [Google Scholar]
- Niedt G. W., Schinella R. A. Acquired immunodeficiency syndrome. Clinicopathologic study of 56 autopsies. Arch Pathol Lab Med. 1985 Aug;109(8):727–734. [PubMed] [Google Scholar]
- Oakley M. G., Turnbull K. D., Dervan P. B. Synthesis of a hybrid protein containing the iron-binding ligand of bleomycin and the DNA-binding domain of Hin. Bioconjug Chem. 1994 May-Jun;5(3):242–247. doi: 10.1021/bc00027a009. [DOI] [PubMed] [Google Scholar]
- Suh D., Chaires J. B. Criteria for the mode of binding of DNA binding agents. Bioorg Med Chem. 1995 Jun;3(6):723–728. doi: 10.1016/0968-0896(95)00053-j. [DOI] [PubMed] [Google Scholar]
- Tanious F. A., Spychala J., Kumar A., Greene K., Boykin D. W., Wilson W. D. Different binding mode in AT and GC sequences for unfused-aromatic dications. J Biomol Struct Dyn. 1994 Apr;11(5):1063–1083. doi: 10.1080/07391102.1994.10508053. [DOI] [PubMed] [Google Scholar]
- Williams L. D., Goldberg I. H. Selective strand scission by intercalating drugs at DNA bulges. Biochemistry. 1988 Apr 19;27(8):3004–3011. doi: 10.1021/bi00408a051. [DOI] [PubMed] [Google Scholar]