Abstract
Background: Hyperhomocysteinemia is a risk factor for vascular diseases. This study aimed to investigate the serum total homocysteine (tHcy) level and nutritional status in elderly inpatients and determine the relationship between tHcy level and nutritional status. Methods: This cross sectional study was carried out in the Tongji hospital, and 142 subjects were consecutively recruited. Fasting blood was collected, and the liver and kidney function, blood glucose, glycosylated hemoglobin (HbA1c), plasma protein, lipid profile, folic acid, vitamin B12 and serum total tHcy were measured. Anthropometric measurements, grip strength and the shortened MNA form (MNA-SF) were used to assess the nutritional status. Results: Undernutrition was common in this population. Based on MNA-SF scores, 34.2% of subjects were at risk of malnutrition, and malnourished subjects accounted for 4.9%. The mean tHcy was 14.10±5.46 μmol/l, and the prevalence of hyperhomocysteinemia was 32.4% (46/142). Hyperhomocysteinemia was a risk factor of cerebral infarction (RR=1.636, 95% CI: 1.169-2.288); Serum tHcy was negatively correlated with serum folic acid, vitamin B12 and MNA-SF score (r=-0.348,P=0.000; r=-0.236, P=0.005; r=-0.208, P=0.014), and positively with BMI within normal range (18.5-23.9; r=0.232, P=0.044). Serum tHcy was negatively correlated with HbA1c, (r=-0.196, P=0.021) and positively with serum creatinine (r=0.327, P=0.000), but unrelated to fasting blood glucose (r=-0.098, P=0.250). Multivariate stepwise regression analysis showed serum folic acid, serum creatinine, MNA-SF score and HbA1c were independent determinants of serum tHcy. Conclusion: Elderly subjects have higher serum tHcy level. Compromised renal function, poor nutritional status and lower blood glucose are likely to influence the serum tHcy level.
Keywords: Homocysteine, nutritional status, MNA-SF, HbA1c
Introduction
Homocysteine (Hcy) is a sulphur-containing amino acid, an intermediary product in methionine metabolism. Serum Hcy occurs in three major forms: protein-integrated Hcy, di-sulfurated Hcy and Cys- di-sulfurated Hcy and free type Hcy, which are collectively known as total Hcy. In mammalian cells, catabolism of Hcy is done in two ways: under the condition of negative balance, Hcy is methylated into methionine in the presence of methionine synthesis enzyme (MS) or catalysis methyl transferase enzyme (BMHT) in the liver and kidney; under the condition of positive balance, cysteine is obtained in the presence of cystathionine-β-synthesis enzyme (CβS), Hcy and serine, and further transformed into sulphate which is then secreted into urine [1-4].
Elevated serum total homocysteine (tHcy) is a concern in a rare hereditary disease due to the deficiency of CβS and B vitamins in nutrition, diet with high methionine, aging, smoking, climacteric and sedentary lifestyle [5-7]. In 1969, Mcully reported that Hcy was related to atherosclerosis, and high tHcy level predicts a high risk for vascular diseases [7]. A series of study have shown that excessive tHcy may cause injury to the vascular endothelium, promote proliferation of smooth muscle cells, facilitate atherosclerosis, enrich in blood cells and enhance the risk for venous thrombosis [8]. According to epidemiological evidence, high tHcy level is an independent risk factor for cardiovascular diseases [9].
The elder population is at a high risk for malnutrition, which is worse in elderly inpatients (nearly 50% are malnourished in elderly inpatients). In addition, these patients have higher morbidity, poorer quality of life, prolonged hospital stay and higher hospital mortality [10,11]. Elder patients usually coexisting chronic diseases including cardiovascular diseases. Nevertheless, little is known about the influence of nutritional status on the Hcy level in this population. This study was to investigate the effect of metabolism of proteins, lipids and glucose on the serum total Hcy level.
Subjects and methods
Subjects
Consecutive patients aged 65 years or older were recruited from the Department of Internal Medicine and Geriatrics of Tongji Hospital (Shanghai, China). Exclusion criteria were: 1) Liver and kidney dysfunction; 2) malignant tumor; 3) Gastrointestinal absorption disorder; 4) Severe infection; 5) Unconsciousness or cognitive impairment; 6) medication with vitamin B or vegetarian. This study was approved by the Ethics Committee of Tongji Hospital. All subjects signed informed consent.
Nutritional assessment
Medical history was reviewed. Anthropometric parameters, body mass index (BMI, kg/m2), mid-arm circumference (AC, cm) and calf circumference (CC, cm) were measured using standard methods [12].
Nutritional status was determined using the Mini-Nutritional Assessment short form (MNA-SF) which includes six domains: food intake, weight change, activity, psychological stress, cognitive situation and BMI. The MNA-SF was completed using CC instead of BMI in cases of absence of weight and height information. Subjects were classified as malnourished (score ≤7), at risk of malnutrition (score 8-11) or well-nourished (score ≥12) ones [13].
Grip strength test was performed with a hand-held dynamometer (Camry, Electronic hand dynamometer, Model: EH101). Handgrip strength was measured in kilograms (kg) holding at least 3 seconds. Two tests were performed and the greater handgrip strength was scored. Scores were divided into approximate quartiles, separately, for men and women as described elsewhere [14].
Biochemical detection
Fasting blood was collected. Serum total Hcy was measured with cycling enzymatic method. Serum total Hcy concentration of <15 mol/L was considered normal. Serum folic acid and vitamin B12 were detected with electrochemiluminescence immunoassay. Glucose was determined enzymatically. Glycosylated hemoglobin (HbA1c) was measured using high performance liquid chromatography. Serum total cholesterol (TC), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) were measured by enzymatic clearance assay. Triglyceride (TG) was measured by TG LiquiColor Test. Serum creatinine was measured using standard technique. Estimate glomerular filtration rate (eGFR) was calculated with MDRD method.
Statistical analysis
Continuous data were expressed as mean and standard deviation (SD), and categorical data as percentages. tHcy distribution was tested for normality. Categorical data were compared using chi-square test. Among different age groups, comparisons were done with one way analysis of variance (ANOVA).
Hcy was used as dependent variable and other indicators as independent variables. The correlation analysis was performed after adjusting for age, gender and smoking. Age, folic acid, creatinine and other parameters related to Hcy were analyzed with stepwise regression equation and the independent factors affecting Hcy concentration were determined.
A value of 2-tailed P<0.05 was considered for statistical significance. Statistical analysis was performed using SPSS version 17 for windows (SPSS Inc., Chicago, Illinois).
Results
A total of 142 consecutive patients were recruited including 65 males and 77 females. The frequency distribution of Hcy in this population is shown in Figure 1. The Hcy distribution was skewed. The mean Hcy was 14.10±5.46 μmol/l. The prevalence of hyperhomocysteinemia was 32.4% (46/142). Hyperhomocysteinemia was a relative risk factor of cerebral infarction (relative risk =1.636, 95% confidence interval [CI]: 1.169-2.288, X2=7.505, P=0.006).
Figure 1.
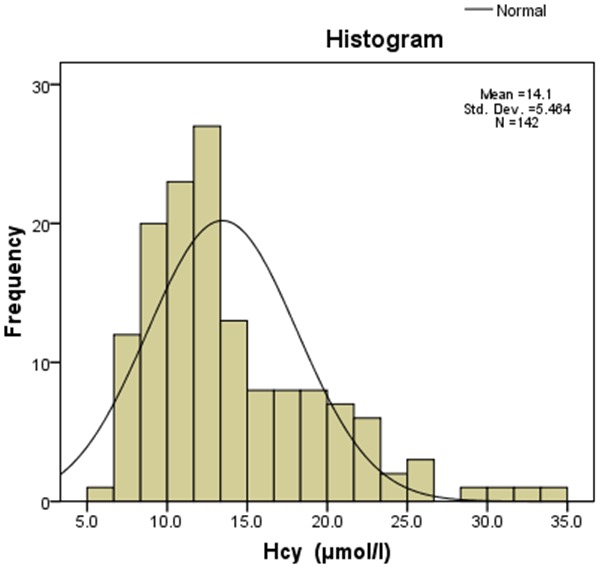
Homocysteine (Hcy) was skewed distribution, median was 12.80 μmol/l, the minimum was 5.9 μmol/l and maximum was 33.4 μmol/l.
The characteristics of these patients are shown in Table 1 according to the gender. The mean age was 77.1±7.6 years (range: 65-95 years). The Mean systolic and diastolic blood pressure was 136.2±19.5 mmHg and 79.7±8.4 mmHg, respectively. Men had higher education level, were more likely to smoke and drink and had higher handgrip strength.
Table 1.
Clinical characteristics of patients
| Parameters | Total (n=142) | Male (n=65) | Female (n=77) |
|---|---|---|---|
| Age (y) | 77.1±7.6 | 76.0±7.6 | 77.8±7.3 |
| Education level (primary and blow, n, %) | 53.5 | 41.5 | 63.6* |
| Current and former smoker (%) | 19.7 | 40.0 | 2.6** |
| Current and ever drink alcohol (%) | 12.0 | 21.5 | 3.9** |
| Body mass index (kg/m2) | 23.9±5.7 | 23.9±7.8 | 23.9±4.0 |
| Mid-arm circumference (cm) | 26.8±3.1 | 26.4±2.5 | 27.2±3.4 |
| Calf circumference (cm) | 32.4±3.7 | 33.2±3.5 | 31.8±3.7 |
| Systolic pressure (mmHg) | 136.2±19.5 | 134.2±15.5 | 138.0±22.3 |
| Diastolic pressure (mmHg) | 79.7±8.4 | 80.7±6.7 | 78.8±9.5 |
| Handgrip strength (kg) | 18.2±9.3 | 23.6±9.7 | 13.7±5.3** |
| MNA-SF score | 11.7±1.9 | 11.7±1.9 | 11.7±2.0 |
| Homocysteine (μmol/l) | 14.10±5.46 | 15.56±5.70 | 12.86±4.97* |
| Serum folic acid (ng/ml) | 9.91±4.93 | 9.69±4.89 | 10.09±4.99 |
| Serum vitamin B12 (pmol/l) | 322.4±266.2 | 299.5±287.9 | 341.7±246.6 |
| Hypertension (n, %) | 69.0 | 61.5 | 75.3 |
| CAD (n, %) | 45.8 | 40.0 | 50.6 |
| Cerebral infarction (n, %) | 46.5 | 52.3 | 41.6 |
MNA-SF score, Mini-Nutritional Assessment short form; CAD, coronary atherosclerotic heart disease; Continuous data are expressed as mean ± SD and categorical data as percentages. Comparisons were conducted using Student’s t-test or chi-square test.
P<0.05;
P<0.01.
The biochemical parameters are shown in different age groups (65-74 years, 75-84 years and ≥85 years) in Table 2. The mean Hb, ALB, PA, TC and TG decreased, but the BUN, Cr, UA and Hcy increased with age, showing significant differences among different age groups.
Table 2.
Serum biochemical parameters in different age groups
| Variables | Total (n=142) | Age (65-74 y) (n=51) | Age (75-84 y) (n=69) | Age (≥85 y) (n=22) | P value |
|---|---|---|---|---|---|
| HB (g/l) | 128.0±16.2 | 132.5±14.3 | 126.3±17.0 | 123.1±16.1 | 0.034 |
| ALB (g/l) | 34.7±3.8 | 35.7±4.7 | 34.4±3.0 | 33.3±3.2 | 0.032 |
| PA (g/l) | 0.285±0.062 | 0.302±0.060 | 0.281±0.062 | 0.257±0.053 | 0.011 |
| BUN (mmol/l) | 4.79±1.89 | 4.23±1.40 | 5.05±2.02 | 5.24±2.24 | 0.031 |
| Cr (μmol/l) | 73.8±17.1 | 67.5±15.5 | 75.9±16.6 | 80.7±16.7 | 0.002 |
| UA (μmol/l) | 324.3±86.1 | 318.2±82.5 | 322.1±86.6 | 345.1±93.2 | 0.455 |
| BG (mmol/l) | 6.53±2.49 | 6.88±2.78 | 6.55±2.54 | 5.65±1.09 | 0.153 |
| HbA1c (%) | 6.70±1.60 | 6.82±1.73 | 6.71±1.62 | 6.37±1.22 | 0.552 |
| TC (mmol/l) | 4.60±1.01 | 4.86±1.04 | 4.51±0.97 | 4.29±0.91 | 0.047 |
| TG (mmol/l) | 1.47±0.86 | 1.68±0.71 | 1.47±1.00 | 1.00±0.49 | 0.008 |
| HDL (mmol/l) | 1.06±0.32 | 1.02±0.28 | 1.05±0.35 | 1.14±0.31 | 0.323 |
| LDL (mmol/l) | 2.82±0.84 | 3.02±0.76 | 2.73±0.89 | 2.62±0.80 | 0.091 |
| Hcy (μmol/l) | 14.10±5.46 | 12.21±4.05 | 14.82±6.11 | 16.18±5.06 | 0.005 |
| Folic acid (ng/ml) | 9.91±4.93 | 10.33±5.02 | 10.13±4.66 | 8.36±5.47 | 0.253 |
| Vitamin B12 (pmol/l) | 322.4±266.2 | 313.5±161.9 | 313.8±276.7 | 367.6±386.7 | 0.676 |
HB, hemoglobin; ALB, albumin; PA, prealbumin; BUN, urea nitrogen; Cr, creatinine; UA, uric acid; FBG, fasting blood glucose; HbA1c, Glycosylated hemoglobin; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; Hcy, Homocysteine. Variables were expressed as mean ± SD, and comparisons were conducted using ANOVA.
Univariate analysis showed the Hcy level was negatively correlated with MNA-SF score (P<0.014), HbA1c (P<0.021) and eGFR (P=0.011), but positively correlated with Cr (P=0.000) in Table 3. The correlation was statistically significant after adjusting for age, gender and smoking. Multivariate stepwise regression analysis showed the serum Cr (β=0.436, P=0.000), folic acid (β=-0.385, P=0.000), MNA-SF score (β=-0.283, P=0.000) and HbA1c (β=-0.151, P=0.040) were independent determinants of tHcy.
Table 3.
Relationship between Hcy level and nutrition status
| Hcy | MNA-score | Hb | Alb | PA | Bun | Cr | UA | BG | HbAlc | TC | TG | HDL | LDL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.000 | -.208 | -.037 | .052 | -.091 | .077 | .327 | .127 | -.098 | -.196 | -.013 | .054 | -.047 | .047 |
| . | .014 | .660 | .542 | .285 | .363 | .000 | .136 | .250 | .021 | .883 | .528 | .583 | .584 |
| 1.000 | .333 | .309 | .114 | .086 | .151 | .057 | -.033 | -.094 | .064 | .142 | .006 | .022 | |
| . | .000 | .000 | .180 | .315 | .076 | .501 | .703 | .271 | .454 | .093 | .946 | .793 | |
| 1.000 | .440 | .227 | .130 | .074 | .147 | .054 | -.024 | .194 | .093 | .084 | .152 | ||
| . | .000 | .007 | .127 | .383 | .083 | .530 | .783 | .022 | .272 | .326 | .073 | ||
| 1.000 | .511 | .081 | .157 | .143 | .012 | -.050 | .199 | .076 | .151 | .150 | |||
| . | .000 | .342 | .065 | .092 | .884 | .554 | .018 | .371 | .075 | .077 | |||
| 1.000 | .132 | .071 | .126 | -.028 | -.012 | .242 | .292 | .075 | .168 | ||||
| . | .119 | .405 | .137 | .744 | .892 | .004 | .000 | .380 | .048 | ||||
| 1.000 | .421 | .336 | .089 | .084 | -.040 | .019 | .115 | -.088 | |||||
| . | .000 | .000 | .295 | .322 | .641 | .828 | .176 | .303 | |||||
| 1.000 | .354 | -.013 | -.146 | -.078 | .136 | -.084 | -.063 | ||||||
| . | .000 | .879 | .086 | .360 | .109 | .321 | .457 | ||||||
| 1.000 | .020 | -.145 | .114 | .242 | -.052 | .076 | |||||||
| . | .815 | .088 | .181 | .004 | .545 | .373 | |||||||
| 1.000 | .700 | .059 | .257 | -.197 | -.049 | ||||||||
| . | .000 | .486 | .002 | .020 | .565 | ||||||||
| 1.000 | .037 | .109 | -.128 | -.010 | |||||||||
| . | .661 | .201 | .131 | .904 | |||||||||
| 1.000 | .226 | .431 | .920 | ||||||||||
| . | .007 | .000 | .000 | ||||||||||
| 1.000 | -.344 | .042 | |||||||||||
| . | .000 | .626 | |||||||||||
| 1.000 | .290 | ||||||||||||
| . | .001 | ||||||||||||
| 1.000 |
Discussion
Our results showed elderly patients mainly developed hypertension, cerebral infarction, coronary heart disease and type 2 diabetes. The prevalence of hyperhomocysteinemia was 32.4%. Our findings revealed that the risk for cerebral infarction in elderly patients with elevated serum Hcy was 1.636 times higher than that in patients with normal Hcy. High tHcy level is an independent risk factor for cardiovascular diseases including ischemic heart disease, stroke and vascular lesions. A prospective meta-analysis showed that the risk for venous embolism was up to 27% while the plasma tHcy increased by 5 μmol/L. The risk for cardiovascular diseases was reduced by 11% and that for stroke by 19% when the plasma tHcy was decreased by 3 μmol/L [15]. The increased tHcy may cause stress, oxidation-reduction imbalance and inflammation, resulting in atherosclerosis [16]. The present study showed that tHcy was not related to blood lipids, which means tHcy has no relation with lipid metabolism on the affection for cardiovascular disease. In the elderly, body fat mass shows a increasing trend, while the body fat tends to be distributed in the abdomen. Our study showed the serum tHcy was positively related to BMI within normal range.
The prevalence of inadequate nutritional status was high as assessed by MNA-SF score (34.2% at risk for malnutrition and 4.9% malnourished status). Our results showed the tHcy level increased with age and had a negative relation with nutrition status, which means that good nutrition status may exert protective effects on cardiovascular system. According to the Ducloux’s study, the tHcy level had a negative relation with all-cause mortality in inflammation and malnutrition patients [17]. Our results indicated that tHcy was related to serum creatinine and GFR deducted by MDRD formula. This was also present after adjusting for age and gender. Decreased renal function may leads to the retention of metabolites, affecting metabolism of sulfur amino acid and causing high tHcy level. Multiple prospective cohort study showed that the tHcy level is associated with mortality due to end-stage renal disease and concurrent atherosclerosis but not with heart failure and severe inflammation [18]. Cardiovascular diseases are a major cause of morbidity and mortality in patients with chronic kidney disease (CKD). A moderate increase in plasma total Hcy occurs in the early stage of CKD and Hcy may increase as renal function decreases, indicating the important role of the kidney in Hcy metabolism [19,20].
ATP produced in the aerobic metabolism of glucose is major source of energy in the brain. The interrupted oxygen and nutrition supply may cause damage to the metabolism in the brain. The depletion of cell energy, release of excitatory amino acid, mitochondrial dysfunction and production of free radicals have been found to attributed to the pathogenesis of cerebral ischemia [21]. Study on hippocampal slices exposed to oxygen and glucose deprivation and subsequent re-oxygenation showed lactate dehydrogenase increased accompanied by cell death [22]. In the study of Sanchez et al on 20 patients, fasting plasma tHcy was positively related to fasting insulin. Another survey showed that higher plasma tHcy level was associated with higher blood glucose level in type 2 diabetes mellitus patients [23]. Our findings revealed that there was no significant relationship between serum tHcy and fasting blood glucose, but serum tHcy was negatively related to HbA1c. HbA1c reflects the average blood glucose level within past 3 months, which may affect cell metabolism, resulting in increased serum Hcy level in elderly patients with lower baseline blood glucose level. Our findings were different from those in the study of Passaro et al on diabetic patients [24], and further studies are required.
In conclusion, our study shows a high prevalence of hyperhomocysteinemia in elderly patients. Higher serum tHcy level is associated with age, serum folic acid, vitamin B12, renal function, nutritional status and baseline glucose level. There is no association between lipid profile and total Hcy.
Disclosure of conflict of interest
None.
References
- 1.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–46. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Fowler B. Homocysteine: overview of biochemistry, molecular biology, and role in disease processes. Semin Vasc Med. 2005;5:77–86. doi: 10.1055/s-2005-872394. [DOI] [PubMed] [Google Scholar]
- 3.Dudman NP, Guo XW, Gordon RB, Dawson PA, Wilcken DE. Human homocysteine catabolism: three major pathways and their relevance to development of arterial occlusive disease. J Nutr. 1996;126:1295S–300S. doi: 10.1093/jn/126.suppl_4.1295S. [DOI] [PubMed] [Google Scholar]
- 4.Matthews RG, Elmore CL. Defects in homocysteine metabolism: diversity among hyperhomocyst(e)inemias. Clin Chem Lab Med. 2007;45:1700–3. doi: 10.1515/CCLM.2007.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhoef P, Hennekens CH, Malinow MR, Kok FJ, Willett WC, Stampfer MJ. A prospective study of plasma homocyst(e)ine and risk of ischemic stroke. Stroke. 1994;25:1924–30. doi: 10.1161/01.str.25.10.1924. [DOI] [PubMed] [Google Scholar]
- 6.Arnesen E, Refsum H, Bonaa KH, Ueland PM, Forde OH, Nordrehaug JE. Serum total homocysteine and coronary heart disease. Int J Epidemiol. 1995;24:704–9. doi: 10.1093/ije/24.4.704. [DOI] [PubMed] [Google Scholar]
- 7.Garofolo L, Barros N Jr, Miranda F Jr, D’Almeida V, Cardien LC, Ferreira SR. Association of increased levels of homocysteine and peripheral arterial disease in a Japanese–Brazilian population. Eur J Vasc Endovasc Surg. 2007;34:23–8. doi: 10.1016/j.ejvs.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Faraci FM, Lentz SR. Hyperhomocysteinemia, oxidative stress and cerebral vascular disfunction. Stroke. 2004;35:354–47. doi: 10.1161/01.STR.0000115161.10646.67. [DOI] [PubMed] [Google Scholar]
- 9.Wierzbicki AS. Homocysteine and cardiovascular disease: a review of the evidence. Diab Vasc Dis Res. 2007;4:143–50. doi: 10.3132/dvdr.2007.033. [DOI] [PubMed] [Google Scholar]
- 10.Guigoz Y. The Mini Nutritional Assessment (MNA) review of the literature--What does it tell us? J Nutr Health Aging. 2006;10:466–85. discussion 85-7. [PubMed] [Google Scholar]
- 11.Kagansky N, Berner Y, Koren-Morag N, Perelman L, Knobler H, Levy S. Poor nutritional habits are predictors of poor outcome in very old hospitalized patients. Am J Clin Nutr. 2005;82:784–91. doi: 10.1093/ajcn/82.4.784. quiz 913-4. [DOI] [PubMed] [Google Scholar]
- 12.Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. 1981;34:2540–5. doi: 10.1093/ajcn/34.11.2540. [DOI] [PubMed] [Google Scholar]
- 13.Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF) J Gerontol A Biol Sci Med Sci. 2001;56:M366–72. doi: 10.1093/gerona/56.6.m366. [DOI] [PubMed] [Google Scholar]
- 14.Al Snih S, Markides KS, Ray L, Ostir GV, Goodwin JS. Handgrip Strength and Mortality in Older Mexican Americans. J Am Geriatr Soc. 2002;50:1250–56. doi: 10.1046/j.1532-5415.2002.50312.x. [DOI] [PubMed] [Google Scholar]
- 15.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–22. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 16.McCully KS. Chemical pathology of homocysteine. IV. Excitotoxicity, oxidative stress, endothelial dysfunction, and inflammation. Ann Clin Lab Sci. 2009;39:219–32. [PubMed] [Google Scholar]
- 17.Ducloux D, Klein A, Kazory A, Devillard N, Chalopin JM. Impact of malnutrition- inflammation on the association between homocysteine and mortality. Kidney Int. 2006;69:331–5. doi: 10.1038/sj.ki.5000096. [DOI] [PubMed] [Google Scholar]
- 18.van Guldener C. Homocysteine and the kidney. Curr Drug Metab. 2005;6:23–26. doi: 10.2174/1389200052997410. [DOI] [PubMed] [Google Scholar]
- 19.Lindner A, Bankson DD, Stehman-Breen S, Mahuren JD, Coburn SP. Vitamin B6 metabolism and homocysteine in end-stage renal disease and chronic renal insufficiency. Am J Kidney Dis. 2002;39:134–45. doi: 10.1053/ajkd.2002.29904. [DOI] [PubMed] [Google Scholar]
- 20.Arnadottir M, Hultberg B, Nilsson-Ehle P, Thyssel H. The effect of reduced glomerular filtration rate on plasma total homocysteine concentration. Scand J Clin Lab Invest. 1996;56:41–6. doi: 10.3109/00365519609088586. [DOI] [PubMed] [Google Scholar]
- 21.Tagliari LZ. Homocysteine increases neuronal damage in hippocampal slices receiving oxygen and glucose deprivation. Metab Brain Dis. 2006;21:273–8. doi: 10.1007/s11011-006-9029-y. [DOI] [PubMed] [Google Scholar]
- 22.Fontella FU, Cimarosti H, Crema LM, Thomazi AP, Leite MC, Salbego C, Gonçalves CA, Wofchuk S, Dalmaz C, Netto CA. Acute and repeated restraint stress influences cellular damage in rat hippocampal slices exposed to oxygen and glucose deprivation. Brain Res Bull. 2005;65:443–50. doi: 10.1016/j.brainresbull.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Sudchada P, Saokaew S, Sridetch S, Incampa S, Jaiyen S, Khaithong W. Effect of folic acid supplementation on plasma total homocysteine levels and glycemic control in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2012;98:151–8. doi: 10.1016/j.diabres.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Passaro A, Calzoni F, Volpato S, Nora ED, Pareschi PL, Zamboni PF, Fellin R, Solini A. Effect of metabolic control on homocysteine levels in type 2 diabetic patients: a 3-year follow-up. J Intern Med. 2003;254:264–71. doi: 10.1046/j.1365-2796.2003.01184.x. [DOI] [PubMed] [Google Scholar]


