Abstract
Powers and Trobridge examine the impact of fetal bovine serum (FBS) on viral vector production. They demonstrate that there can be significant variability between different FBS production lots in the resultant viral titer of both foamy and lentivirus vectors.
Introduction
The use of fetal bovine serum (FBS) as a supplement in cell culture medium is a common practice (Price and Gregory, 1982; Brunner et al., 2010). Although it is generally accepted that the quality of serum lots varies and that testing serum lots for specific applications is beneficial, the comparison of different serum lots for the production of lentiviral and foamy viral vector preparations has not to our knowledge been previously reported. Previous studies have assessed the effect of different serum samples, including the effect of heat inactivation and polycations on transduction efficiency with lentiviral vectors (Denning et al., 2013), as well as the effect of the pH of serum samples for Moloney murine leukemia virus (MMLV) and murine stem cell virus (MSCV) vector transduction efficiency (Jensen et al., 2003).
The use of retroviral vectors in gene therapy is expanding. Several trials using retroviral vectors have shown promise (Cartier et al., 2009; DiGiusto et al., 2010; Gaspar et al., 2011). However, producing high-titer vector for clinical and preclinical applications remains a challenge (Bouard et al., 2009; Maetzig et al., 2012). This challenge is of significant interest because of the cost consideration of materials and reagents, as well as the time required for viral production. Care must be taken because of the presence of heat-labile complement proteins and immunoglobulin G (IgG), which may inhibit viral production (Chu et al., 1973; Rossi and Kiesel, 1974; Rahman et al., 2011). Here we compared titers for both foamy virus and lentiviral vector preparations, using five different serum samples from four different vendors.
Materials and Methods
Foamy viral and lentiviral vector preparation and titering
Foamy virus transfection was performed as previously described (Kiem et al., 2010). Briefly, three plates of HEK293 cells per serum sample were transfected with vector and helper plasmid constructs. Medium was harvested from each transfected HEK293 dish, filtered, frozen, thawed, and added to HT1080 cells that contained the respective serum sample. Lentiviral transduction was performed as previously described (Trobridge et al., 2010). Medium was harvested, filtered, stored at 4°C, and added to HT1080 cells that contained the respective serum sample. Both foamy and lentiviral vectors contained a phosphoglycerate kinase (PGK) promoter driving enhanced green fluorescent protein (EGFP) and transduced cells were analyzed by flow cytometry. Additional details are available in an online supplement (Supplementary data are available online at www.liebertpub.com/hgtb).
Statistical analysis
Analysis of variance (ANOVA) was used for multiple comparisons and the Student's t-test was used for individual comparisons to determine statistical significance.
Results and Discussion
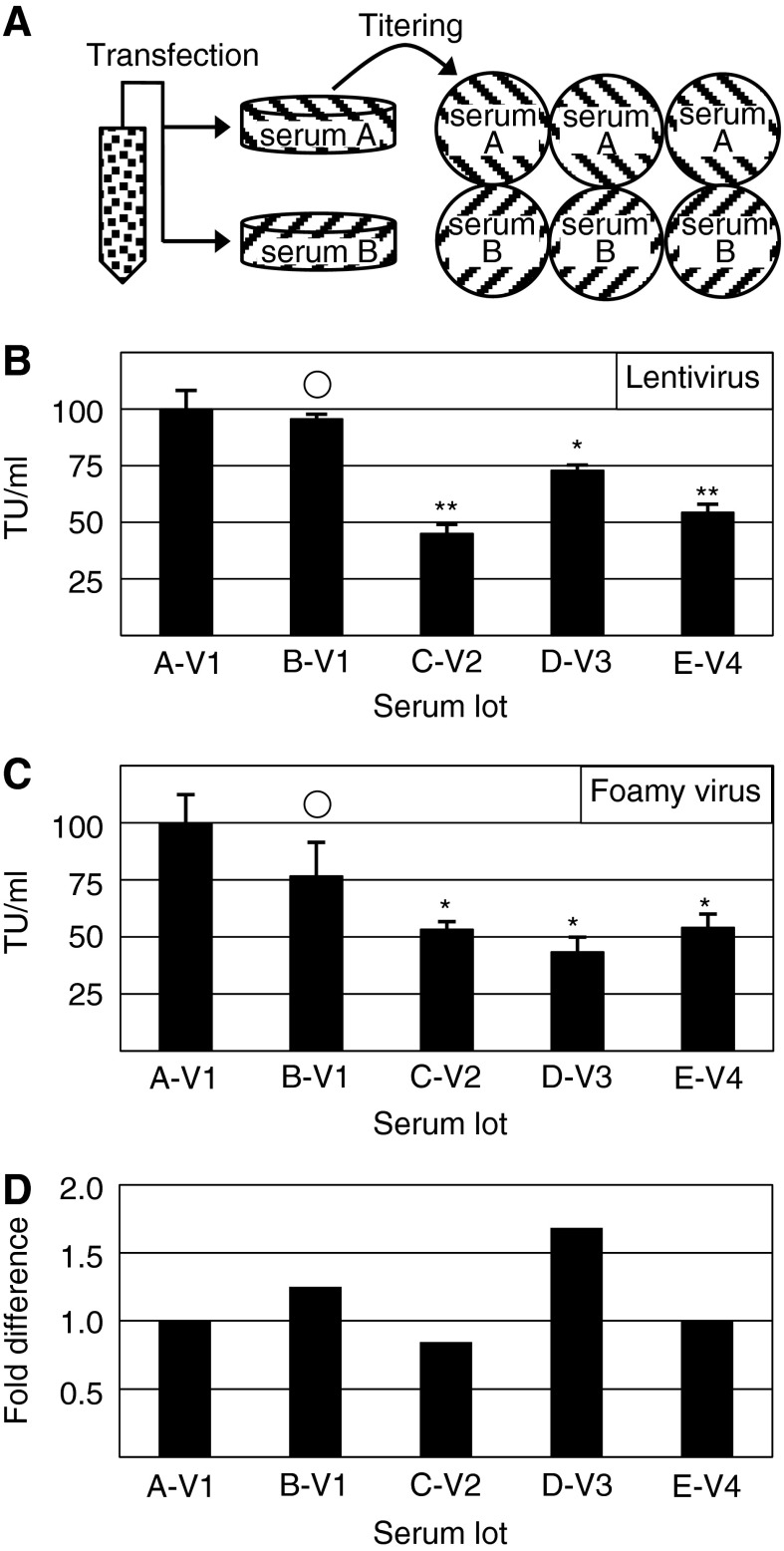
As the use of retroviral vectors expands, the ability to reliably produce high-titer vector stocks will be important to avoid increased production costs (McTaggart and Al-Rubeai, 2002). Our goal was to determine whether the choice of FBS production lots would have a significant effect on resulting viral transducing units (TU) per milliliter. Five lots were selected from four vendors and transfection and transduction occurred in triplicate as shown in Fig. 1A. When the resulting mean titers from each serum sample were compared for the lentiviral vector, the results were significantly different (p<0.0001) and the fold difference between highest and lowest titer, A-V1 and C-V2 respectively, was 2.23 (Fig. 1B). There was also a significant difference for foamy vectors (p=0.012) with a 2.35-fold difference between the highest and lowest titers, A-V1 and D-V3, respectively (Fig. 1C). Between both vector preparations, the two highest titer-producing sera were consistent (A-V1 and B-V1), whereas the remaining three sera showed variability. When production lots were compared between foamy and lentiviral vector productions, fold differences ranged from 0.84-fold lower (C-V2) to 1.68-fold higher (D-V3) when comparing normalized titers. One sample (E-V4) had identical normalized titers between both retroviral preparations (Fig. 1D).
FIG. 1.
Serum effect on foamy and lentiviral titer. (A) After culturing HEK293 cells in one of five serum sample lots, cells were transfected in triplicate. Medium was harvested up to 72 hr posttransfection. Collected medium from each plate was pooled and used to determine vector titer on HT1080 cells in the respective serum sample in triplicate. HT1080 cells were exposed to viral vector for 72 hr before being collected and analyzed by flow cytometry for enhanced green fluorescent protein (EGFP) expression. (B) Normalized lentiviral vector titer for plasmid encoding phosphoglycerate kinase (PGK) promoter-driven EGFP. (C) Normalized foamy vector titer for plasmid encoding phosphoglycerate kinase (PGK) promoter-driven EGFP. (D) Fold difference between lentivirus and foamy virus vector normalized titer for each serum. ○p>0.05, *p<0.05, **p<0.01 by Student t-test. Standard error bars are shown for (B) and (C). Serum sample lots are shown as (A–E) and vendors as V1–V4 for (B–D). TU/ml, transducing units per milliliter of vector.
In summary, we have shown that there can be significant variability between different FBS production lots in the production of viral vectors. Our data are consistent with prior studies with lentiviral vectors (Denning et al., 2013) as well as MMLV and MSCV vectors (Jensen et al., 2003). Our data also indicate that when serum will be used for the production of more than one type of retroviral vector, it is prudent to test the production of all vectors. Although there has been a movement toward serum-free medium for the production of viral vectors (Falkner et al., 2006), FBS is still commonly used for the production of preclinical and clinical viral vector preparations (DiGiusto et al., 2010). The importance of macromolecules, specifically lipids such as cholesterol, to obtain high-quality vector preparations has been described for the production of MLV vectors (Rodrigues et al., 2009) and pH may also play a role (Jensen et al., 2003). The presence of antiviral antibodies such as bovine foamy virus (BFV) antibodies may have also affected the resulting foamy viral titers. Because of the high occurrence of BFV infections (Romen et al., 2007) and the maternal transmittance of antibodies (Linial, 1999), it is possible that BFV antibodies may be present in FBS production lot samples. Our study indicates that FBS production lots should be compared for all vector types that will be produced.
Supplementary Material
Acknowledgments
These studies were supported by grants AI097100 and AI102672 (G.D.T.) from the National Institutes of Health as well as start-up funds provided by Washington State University (G.D.T.).
Author Disclosure Statement
No competing financial interests exist.
References
- Bouard D. Alazard-Dany D. Cosset F.L. Viral vectors: From virology to transgene expression. Br. J. Pharmacol. 2009;157:153–165. doi: 10.1038/bjp.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D. Frank J. Appl H., et al. Serum-free cell culture: The serum-free media interactive online database. Altex. 2010;27:53–62. doi: 10.14573/altex.2010.1.53. [DOI] [PubMed] [Google Scholar]
- Cartier N. Hacein-Bey-Abina S. Bartholomae C.C., et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- Chu F.C. Johnson J.B. Orr H.C., et al. Bacterial virus contamination of fetal bovine sera. In Vitro. 1973;9:31–34. doi: 10.1007/BF02615986. [DOI] [PubMed] [Google Scholar]
- Denning W. Das S. Guo S., et al. Optimization of the transductional efficiency of lentiviral vectors: Effect of sera and polycations. Mol. Biotechnol. 2013;53:308–314. doi: 10.1007/s12033-012-9528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiusto D.L. Krishnan A. Li L., et al. RNA-based gene therapy for HIV with lentiviral vector-modified CD34+ cells in patients undergoing transplantation for AIDS-related lymphoma. Sci. Transl. Med. 2010;2:36ra43. doi: 10.1126/scitranslmed.3000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner E. Appl H. Eder C., et al. Serum free cell culture: The free access online database. Toxicol In Vitro. 2006;20:395–400. doi: 10.1016/j.tiv.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Gaspar H.B. Cooray S. Gilmour K.C., et al. Long-term persistence of a polyclonal T cell repertoire after gene therapy for X-linked severe combined immunodeficiency. Sci. Transl. Med. 2011;3:97ra79. doi: 10.1126/scitranslmed.3002715. [DOI] [PubMed] [Google Scholar]
- Jensen T.W. Chen Y. Miller W.M. Small increases in pH enhance retroviral vector transduction efficiency of NIH-3T3 cells. Biotechnol. Prog. 2003;19:216–223. doi: 10.1021/bp025604g. [DOI] [PubMed] [Google Scholar]
- Kiem H.P. Wu R.A. Sun G., et al. Foamy combinatorial anti-HIV vectors with MGMTP140K potently inhibit HIV-1 and SHIV replication and mediate selection in vivo. Gene Ther. 2010;17:37–49. doi: 10.1038/gt.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M.L. Foamy viruses are unconventional retroviruses. J. Virol. 1999;73:1747–1755. doi: 10.1128/jvi.73.3.1747-1755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maetzig T. Baum C. Schambach A. Retroviral protein transfer: Falling apart to make an impact. Curr. Gene Ther. 2012;12:389–409. doi: 10.2174/156652312802762581. [DOI] [PubMed] [Google Scholar]
- McTaggart S. Al-Rubeai M. Retroviral vectors for human gene delivery. Biotechnol. Adv. 2002;20:1–31. doi: 10.1016/s0734-9750(01)00087-8. [DOI] [PubMed] [Google Scholar]
- Price P.J. Gregory E.A. Relationship between in vitro growth promotion and biophysical and biochemical properties of the serum supplement. In Vitro. 1982;18:576–574. doi: 10.1007/BF02810081. [DOI] [PubMed] [Google Scholar]
- Rahman H. Qasim M. Schultze F.C., et al. Fetal calf serum heat inactivation and lipopolysaccharide contamination influence the human T lymphoblast proteome and phosphoproteome. Proteome Sci. 2011;9:71. doi: 10.1186/1477-5956-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues A.F. Carmo M. Alves P.M. Coroadinha A.S. Retroviral vector production under serum deprivation: The role of lipids. Biotechnol. Bioeng. 2009;104:1171–1181. doi: 10.1002/bit.22499. [DOI] [PubMed] [Google Scholar]
- Romen F. Backes P. Materniak M., et al. Serological detection systems for identification of cows shedding bovine foamy virus via milk. Virology. 2007;364:123–131. doi: 10.1016/j.virol.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Rossi C.R. Kiesel G.K. Antibody to viruses affecting cattle in commercial tissue culture grade fetal calf serum. Appl. Microbiol. 1974;27:114–117. doi: 10.1128/am.27.1.114-117.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge G.D. Wu R.A. Hansen M., et al. Cocal-pseudotyped lentiviral vectors resist inactivation by human serum and efficiently transduce primate hematopoietic repopulating cells. Mol. Ther. 2010;18:725–733. doi: 10.1038/mt.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.