Abstract
The genetic modification of peripheral blood lymphocytes using retroviral vectors to redirect T cells against tumor cells has been recently used as a means to generate large numbers of antigen-specific T cells for adoptive cell therapy protocols. However, commonly used retroviral vector-based genetic modification requires T cells to be driven into cell division; this potent mitogenic stimulus is associated with the development of an effector phenotype that may adversely impact upon the long-term engraftment potential and subsequent antitumor effects of T cells. To investigate whether the cytokines used during culture impact upon the engraftment potential of gene-modified T cells, a humanized model employing T cells engrafted with a MART-1-specific T cell receptor adoptively transferred into NOD/Shi-scid IL-2rγ−/− (NSG) immune-deficient mice bearing established melanoma tumors was used to compare the effects of the common γ chain cytokines IL-2, IL-7, and IL-15 upon gene-modified T cell activity. MART-1-specific T cells cultured in IL-7 and IL-15 demonstrated greater relative in vitro proliferation and viability of T cells compared with the extensively used IL-2. Moreover, the IL-15 culture prolonged the survival of animals bearing melanoma tumors after adoptive transfer. However, the combination of IL-7 and IL-15 produced T cells with improved engraftment potential compared with IL-15 alone; however, a high rate of xenogeneic graft-versus-host disease prevented the identification of a clear improvement in antitumor effect of these T cells. These results clearly demonstrate modulation of gene-modified T cell engraftment in the NSG mouse, which supports the future testing of the combination of IL-7 and IL-15 in adoptive cell therapy protocols; however, this improved engraftment is also associated with the long-term maintenance of xenoreactive T cells, which limits the ultimate usefulness of the NSG mouse model in this situation.
In this study, Alcantar-Orozco and colleagues investigate whether the cytokines used during culture impact the engraftment potential of gene-modified T cells in a humanized animal model (the NSG immune-deficient mouse). Using this approach, they find that T cells cultured in IL-7 and IL-15 demonstrate greater relative in vitro proliferation and viability as compared to T cells cultured in IL-2. Moreover, they find that IL-7 and IL-15 cultured cells have higher engraftment potential compared to cells cultured in IL-15 alone.
Introduction
The adoptive transfer of antitumor lymphocytes to cancer patients represents a promising experimental treatment for solid malignancies such as melanoma and renal cell carcinoma (Rosenberg et al., 2008). Recent protocols of adoptive cell transfer (ACT) using tumor-infiltrating lymphocytes have achieved objective responses in a significant number of patients, and in some cases complete regression of large-sized tumors has been reported (Dudley et al., 2002, 2008; Besser et al., 2010). However, unavailability of tumor-infiltrating lymphocytes for all patients and the low frequency of naturally occurring tumor-specific T cells are both limiting factors for the wider application of ACT. The genetic modification of peripheral blood lymphocytes using retroviral vectors encoding T cell receptors (TCR) or chimeric antigen receptors (CAR) that effectively redirect T cells to recognize and eliminate tumor cells has been developed and can potentially overcome major technical obstacles, including the low frequency of natural tumor-specific T cells residing within the patient's T cell repertoire (Johnson et al., 2009; Gilham et al., 2012; Uttenthal et al., 2012). Indeed, protocols to generate gene-modified T cells for clinical application have been developed (Lamers et al., 2002; Yang et al., 2008, 2010, 2012b). To date, retroviral vectors have been the primary choice for gene delivery into primary human T cells. However, for effective transduction to occur, T cells must be driven into cell division in order to permit the retro-transcribed retroviral genome to access and integrate into the target T cell genome (Miller et al., 1990). To achieve this, a mitogenic stimuli involving antibodies (e.g., anti-CD3) or lectins (e.g., phytohaemagluttinin) are used, and this results in highly efficient gene transfer. Furthermore, to achieve sufficient numbers of cells for clinical application, the gene-modified T cells are cultured in cytokines to drive their expansion ex vivo with IL-2 being the predominant cytokine of choice.
Previous studies have shown that the long-term antitumor potential of ACT therapies depends on the ability of the adoptively transferred T cells to persist, self-renew, and differentiate into antitumor effectors and, thus, on the degree of differentiation of such T cells (Berger et al., 2008; Hinrichs et al., 2009, 2011; Gattinoni et al., 2011; Klebanoff et al., 2011). The downside of the mitogenic stimulation and ex vivo expansion of T cells is that it is associated with T cell differentiation in a similar fashion to that occurring upon antigen encounter, that is, from naive (TN) to central memory T cells (TCM) and effector memory T cells (TEM). Moreover, IL-2 has been associated with Fas-mediated T cell apoptosis, and it also has been observed to inhibit the proliferation of memory CD8+ T cells and to promote the growth of regulatory T cells (Refaeli et al., 1998; Ku et al., 2000). Although protocols based upon mitogenic T cell activation, retroviral transduction, and IL-2-driven expansion have been successful in generating T cells suitable for clinical application (Morgan et al., 2006; Kalos et al., 2011; Robbins et al., 2011), it is clear that improvements to the methods are required to generate T cells that possess improved engraftment potential and antitumor activity.
To this end, there is an emerging focus upon alternate gene delivery systems (Yang et al., 2008; Birkholz et al., 2009). However, there currently appears little scope to avoid mitogenic activation of T cells to facilitate retroviral gene transfer. Consequently, there has been a consideration of the roles of cytokines used during the ex vivo culture of T cells and their ability to maintain a less-differentiated phenotype. IL-2 shares structural similarity and some of the in vivo effects on T cells with other members of the common gamma chain (γc) cytokine family such as IL-7, IL-15, and IL-21. These and other cytokines have been progressively researched as alternative growth factors for the generation of efficient tumor reactive T cells. In particular, IL-7 and IL-15 are known to have a central role in homeostatic proliferation and survival of mature lymphocytes (Schluns and Lefrancois, 2003). Previous studies have shown that these two cytokines have the capacity to enhance CD8+ effector T cell responses and are key factors in maintaining the proliferation of memory CD8+ and CD4+ T cells (Berard et al., 2003; Melchionda et al., 2005; McKinlay et al., 2007; Colombetti et al., 2009), while exogenously administered IL-15 has been associated with enhanced in vivo antitumor activity of tumor-reactive T cells in mouse models (Klebanoff et al., 2004). Moreover, the combination of IL-7 and IL-15 drove the preferential expansion of mouse T cells with a TCM phenotype and supported enhanced in vivo antitumor function compared with IL-2-cultured T cells (Cha et al., 2010). Furthermore, IL-7 plus IL-15 also maintained a TCM phenotype on gene-modified human T cells (Kaneko et al., 2009).
Taken together, IL-7 and IL-15 are attractive candidate cytokines to test for the expansion of gene-modified human T cells. With a focus toward developing optimal clinical protocol to test gene-modified T cell ACT, we sought to test the effects of IL-7 and IL-15 upon T cells endowed with a HLA-A*0201-restricted MART-1 antigen-specific TCR (DMF5 TCR) (Johnson et al., 2006) and to determine whether these T cells demonstrated improved engraftment and antitumor activity compared with DMF5 TCR T cells cultured in standard IL-2 conditions when adoptively transferred in melanoma-bearing NOD/Shi-scid IL-2rγ−/− (NSG) animals.
Materials and Methods
Cell lines and culture media
All cell culture media and additives were purchased from Invitrogen unless where shown. The packaging cell line PG13 was used for production of the retroviral vector encoding the HLA-A*02-restricted DMF5 TCR (Johnson et al., 2006) and was kindly provided by Dr. Richard A. Morgan (National Cancer Institute, Bethesda, MD). The HLA-A*0201+ melanoma cell lines Mel624, Mel501, WM2664, and HLA-A*0201− Mel888 were kindly provided by Prof. Alan Melcher (Leeds Institute of Molecular Medicine, University of Leeds, Leeds, United Kingdom). PG13 cells and melanoma cell lines were routinely cultured in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal calf serum (FCS). TAP-deficient T2 cells were routinely cultured in complete RPMI 1640 (Lonza), 10% (v/v) FCS, 25 mM Hepes, 50 nM 2-mercaptoethanol, and 2 mM L-glutamine (hereafter called T cell media).
Generation of DMF5 TCR T cells
Peripheral blood mononuclear cells were isolated from blood obtained from healthy volunteers by Ficoll-hypaque density gradient centrifugation. Isolated peripheral blood mononuclear cells were plated at a concentration of 3–5×106 cells/ml of T cell media and T cells activated for 48 hr by the addition of 30 ng/ml antihuman CD3 (OKT3; OrthoBiotech) and CD28 (clone 37407.111; R&D Systems), and 100 IU/ml recombinant human IL-2 (Chiron). For retroviral transduction, non-tissue-culture six-well plates (BD Biosciences) were coated with retronectin (10 μg/ml; Takara, Invitrogen) and incubated at 4°C overnight. The following day, plates were washed and 2.5 ml of virus supernatant was added to each well. Plates were centrifuged for 30 min at 1200× g. Actively proliferating T cells were collected from culture and washed, and 1–2×106 cells in 1 ml of T cell media added to the 2.5 ml of viral supernatant, IL-2 was added to a final concentration of 100 IU/ml, and the plates were centrifuged again for 90 min at 1200× g before overnight incubation at 37°C/5% CO2. The following day, T cells were collected from each well, pelleted, and re-suspended in 1 ml of T cell media and added to a fresh retronectin-coated well containing 2.5 ml of fresh retroviral supernatant. After centrifugation at 1,200× g for 90 min, T cells were left to incubate for 4 hr at 37°C/5% CO2 and then collected, washed, and transferred to T cell media at a concentration of 5×105 cells/ml. IL-2 (100 IU/ml), IL-7, or IL-15 (10 ng/ml; Peprotech) were added, and cultures were adjusted to a concentration of 5×105 cells/ml every 2–3 days by the addition of fresh T cell media with cytokines.
Flow cytometric assessment of T cell transduction frequency and phenotype
Transduction efficiency was determined by staining cells collected from culture with a biotinylated MART-1 pentamer (ProImmune) and PE-Cy7 streptavidin (eBioscience). T cell phenotype was determined by antibody staining using FITC-anti-CD8, PE-anti-CD27, PE-anti-CD28 (all from BD Biosciences), and PE-Cy7-anti-CCR7 and APC-anti-CD62L (eBioscience). In brief, T cells (∼1×105) were incubated with the relevant antibodies for 30 min at 4°C in the dark and then washed with PBS/1% FCS and re-suspended in the same reagent. Samples were analyzed using a FACSCalibur flow cytometer (Becton Dickinson) with Flowjo software (Treestar).
Cytokine release assay
Target cell lines (1×105) were plated in flat-bottom 96-well plates and cultured overnight. The following day, DMF5 T cells were added (1:1 ratio) and cultures incubated for 24 hr. IFN-γ concentration in culture supernatants was determined by ELISA using the set of antibodies MAB285 and BAF285 from R&D Systems (Abingdon).
CD107a degranulation assay
The cytotoxic potential of DMF5 T cells was assessed measuring CD107a cell surface expression as a surrogate of T cell cytotoxic activity (Burkett et al., 2005). Target tumor cells or peptide pulsed T2 cells (1×105) and DMF5 TCR T cells or mock T cells (1:1 ratio) were plated in 200 μl of T cell media in a U-bottom 96-well plate including saturating concentrations of PE-conjugated antihuman CD107a or mouse IgG1 isotype control and protein transport inhibitor Golgistop (both from BD Biosciences). Cocultures were incubated for 4 hr at 37°C/5% CO2, and then washed and stained for 30 min at 4°C in the dark with antihuman CD8-FITC antibody (BD Pharmingen) and acquired in a FACScan flow cytometer (Becton Dickinson).
Intracellular cytokine staining
For intracellular staining of IFN-γ and IL-2, DMF5 TCR T cells (1×106) were stimulated with T2 cells (0.5:1 ratio) loaded with MART-1 peptide (ProImmune) in the presence of Golgiplug (BD Biosciences). After an 8 hr incubation, cells were collected, washed, and stained with PE-conjugated anti IFN-γ and IL-2 antibodies using fixation/permeabilization buffers (BD Biosciences), and FITC antihuman CD8 antibody (all antibodies from BD Biosciences). Samples were acquired in a FACSCalibur flow cytometer (Becton Dickinson) with Flowjo software (Treestar).
Adoptive cell therapy
NSG immunodeficient mice were obtained from Harlan Laboratories and housed in the animal facilities of the Biological Resources Unit of the Paterson Institute for Cancer Research. All experiments were conducted under the auspices of the Animals (Scientific Procedures) Act 1986 and under U.K. Coordinating Committee for Cancer Research guidelines. Cohorts of NSG mice were inoculated subcutaneously with 5×105 tumor cells with tumor growth assessed biweekly by calliper measurements; tumor volumes were determined by the following formula: volume=(length×width2)×0.5. Initial experiments were conducted using animals bearing 1-week established tumors. Subsequent experiments were performed on mice bearing 4–5-week established tumors.
After 14 days in culture, DMF5 TCR T cells were collected from culture flasks and dead cells removed by Ficoll hypaque gradient centrifugation. Cells were washed twice before being passed through a 100 μm cell strainer (BD Biosciences) to remove cell clumps. Each animal was injected intravenously with 2×107 T cells in 100 μl saline, and no systemic cytokines or vaccinations were employed. The persistence of adoptively transferred T cells was determined 1 week after injection using heparinized blood collected from the tail vein of mice. After red blood cell lysis (Pharm Lyse lysis buffer; BD Biosciences) and Fc-receptor blocking (Mouse BD Fc block; BD Biosciences), cells were stained with antihuman CD8-FITC, antihuman CD4-PE, and biotinylated MART-1 pentamer/streptavidin-PE-Cy7 (a representative flow cytometry profile of tailbleeds of mice receiving DMF5 TCR or control mock T cells is shown in Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/hgtb). The number of cells per milliliter of blood was enumerated by the addition of CountBright absolute counting beads according to the manufacturers protocol (Invitrogen) based upon the assumption that 1 g of blood is equivalent to 1 ml blood. Samples were acquired in a FACSCalibur flow cytometer (Becton Dickenson) equipped with CellQuest software and the results analyzed using the WinMDI v2.8 program (Scripps Research Institute). Mice were monitored regularly and culled when tumor achieved a volume greater than 1.24 cm3, if weight loss was greater that 20%, or should the animals develop symptoms such as hunched posture, inflamed skin and eyes, lethargy, and piloerection or other general signs of prolonged ill-health.
Results
Primary human T cells transduced with the DMF5 TCR fail to improve the survival of NSG mice bearing established HLA-A*0201 melanoma tumors
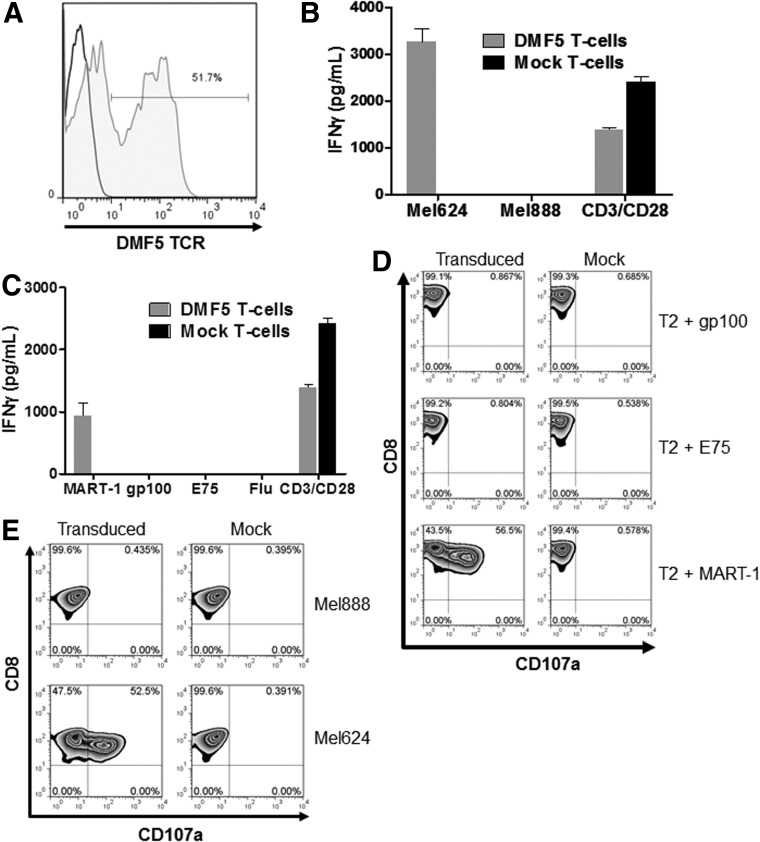
Previous studies have demonstrated that primary human T cells transduced with a retroviral vector encoding the DMF5 TCR can target tumor cells expressing the MART-1 antigen in the context of HLA-A*0201 restriction (Johnson et al., 2006). In agreement with this published work, primary human T cells were bulk-transduced to levels of greater than 50% (Fig. 1A), and these T cells demonstrated high levels of antigen-specific interferon gamma release (Fig. 1B and C) and CD107a degranulation (Fig. 1D and E) after coculture with HLA-A*0201+ melanoma cell lines but not HLA-A*0201− target cells.
FIG. 1.
Efficient transfer of the DMF5 TCR to peripheral blood lymphocyes and in vitro efficacy of DMF5 T cells. (A) MART-1 pentamer staining of T cells transduced with the retroviral vector encoding the DMF5 recombinant TCR. (B) IFN-γ release by DMF5 T cells and mock T cells after coculturing with HLA-A*02+ melanoma cell line Mel624 and HLA-A*02− Mel888, and plate-bound anti-CD3/anti-CD28 as a control. (C) IFN-γ release by DMF5 T cells and mock T cells after coculturing with peptide-pulsed T2 cells and plate-bound anti-CD3/anti-CD28 as a control. (D) Cytotoxicity by CD107a expression of DMF5 T cells when cocultured with peptide-pulsed T2 cells. (E) Cytotoxicity by CD107a expression of DMF5 T cells when cocultured with HLA-A*02+ melanoma cell line Mel624, and HLA-A*02− melanoma cell line Mel888. TCR, T cell receptors.
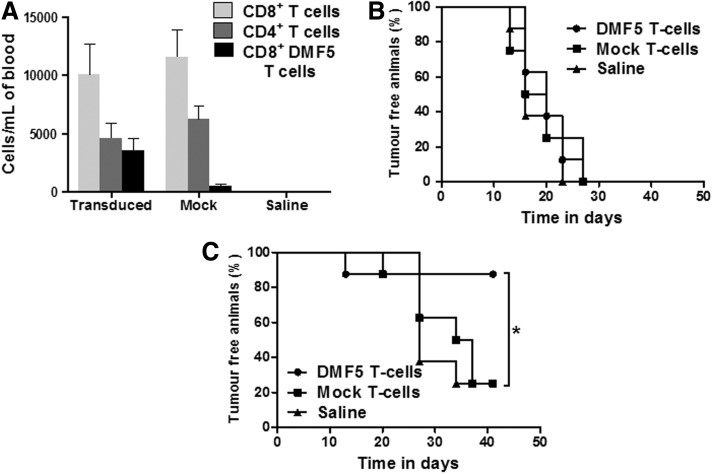
To test the in vivo potency of these DMF5 TCR T cells against melanoma tumor targets, cohorts of NSG mice were inoculated with HLA-A*0201+ Mel624 target cells in the left flank and control HLA-A*0201− Mel888 target cells in the right flank followed 1 week later by adoptive transfer of 2×107 DMF5 T cells (64% transduced and cultured in IL-2), mock T cells, or saline vehicle by the intravenous route. One week after adoptive transfer, human CD4+, CD8+, and DMF5 TCR T cells could be readily detected in the peripheral circulation of the treated mice (Fig. 2A). Importantly, all animals developed tumors on the right flank (i.e., HLA-A*0201− target cells) irrespective of adoptive transfer and this led to the demise of the mice (Fig. 2B). However, by the end of the experiment, only one of eight mice that received DMF5 TCR T cells developed tumor on the left flank compared with six of eight in the mice given mock T cells or saline (Fig. 2C), suggesting that the DMF5 TCR T cells were protecting against the development of HLA-A*0201 tumor growth in this experiment.
FIG. 2.
In vivo efficacy of DMF5 TCR T cells. (A) T cells recovered in the blood of tumor-bearing mice 1 week after systemic infusion of 2×107 DMF5 T cells, mock T cells, or saline vehicle (mean±SEM). (B) Percentage in time of tumor-free animals on the right flank (injected with Mel888) treated with DMF5 T cells, mock T cells, or saline vehicle. (C) Comparison of the percentage of tumor-free animals inoculated with Mel624 tumor cells after injection of DMF5 T cells, mock T cells, or saline.
We then examined the dose dependency of DMF5 TCR T cells engraftment and antitumor activity in NSG mice with established HLA-A*0201+ Mel624 melanoma target cells. Twenty-seven days after tumor inoculation, cohorts of NSG mice were adoptively transferred with four doses of DMF5 TCR T cells (0.1×107; 0.5×107; 1×107; and 2×107 total T cells, 34% transduced) or saline via the intravenous route. One week after T cell infusion, there was a clear dose-dependent increase in the level of engraftment of human T cells in the peripheral circulation of treated mice (Fig. 3A). At the lowest dose of T cells, CD8+ T cells were below the limit of detection in two animals and CD4+ T cells too few to detect in one. However, at cell doses of 0.5×107 and above, T cells could be clearly identified and enumerated. This dose-dependent engraftment appeared to impact upon the initial stages of tumor growth where none of the mice treated with the highest dose of DMF5 TCR T cells developed a tumor of greater than 400 mm3 within 50 days unlike that observed in each other cohort where within 50 days at least two of the five animals had a rapidly growing large tumor (Fig. 3B–F). Nevertheless, this delay in initial tumor growth of the animals treated with the highest dose of DMF5 TCR T cells did not translate to a statistically significant improvement in survival against any other treatment group (Fig. 3G). Furthermore, it was observed that at least one animal in each cohort was culled before the tumor had reached a size of greater than 600 mm3 as a result of symptoms associated with the development of xeno-graft-versus-host disease (x-GvHD), including weight loss, starry coats because of Piloerection, and general poor appearance as described in other studies (Schroeder and DiPersio, 2011; Ali et al., 2012). Consequently, animals that were culled because of x-GvHD symptoms before achieving a minimum tumor size of 600 mm3 were excluded from the survival analysis.
FIG. 3.
In vivo efficacy of adoptively transferred DMF5 T cells in a dose escalation model of ACT. (A) Number of CD4+ and CD8+ cells per ml of blood recovered in the blood of mice receiving escalating doses of T cells. Groups where no bars are shown had no detectable levels of human T cells. (B) Mice from Group A received saline only and were used as a control group. (C) Group B received 0.1×107 transduced T cells. (D) Group C received 0.5×107 transduced T cells. (E) Group D received 1×107 T cells. (F) Group E received 2×107 T cells. (G) Kaplan–Meier survival analysis of melanoma-bearing mice receiving escalating doses of DMF5 TCR-transduced T cells. Arrows indicate the day on which adoptive transfer of T cells was performed. Censored events are indicated by symbols identifying at which time point animals were culled because of x-GvHD symptoms. ACT, adoptive cell transfer; x-GvHD, xeno-graft-versus-host disease.
The effect of IL-7 or IL-15 upon the in vitro function of DMF5 TCR T cells
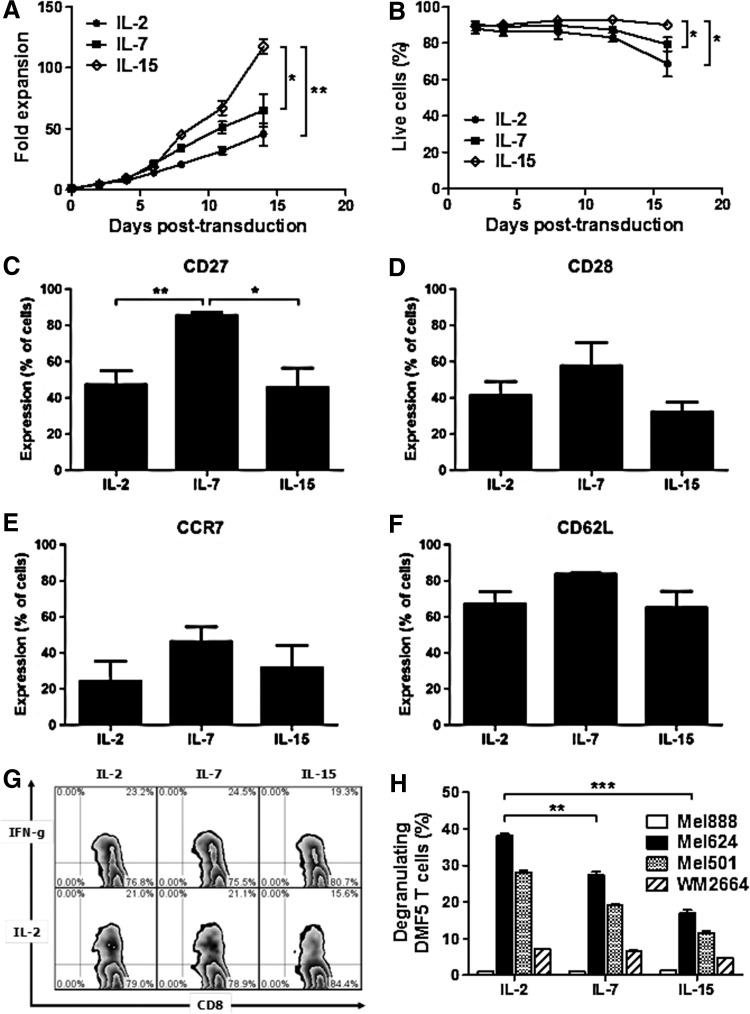
Given the structural similarity between IL-2 and other common gamma chain (γc) cytokines, we questioned whether including IL-7 and IL-15 during culture could improve the in vivo therapeutic activity of DMF5 TCR T cells. After a 3-week period of culture, DMF5 TCR T cells cultured in the presence of IL-15 (10 ng/ml) displayed a greater than 100-fold expansion, which was significantly greater than tat of DMF5 TCR T cells cultured in IL-7 (10 ng/ml) or IL-2 (100 IU/ml; Fig. 4A). Moreover, higher cell viability was also maintained in the IL-15-supplemented cultures compared with IL-7 and IL-2 cultures (Fig. 4B). Analysis of differentiation marker expression on DMF5 TCR T cells after 14 days in culture identified a significant increase in CD27 expression on CD8+ T cells cultured in IL-7 (Fig. 4C), but no other marker examined (CD28, CCR7, and CD62L) was found to significantly vary between CD8+ T cells (Fig. 4D–F) and CD4+ T cells (Supplementary Fig. S2A–D) in these cultures.
FIG. 4.
Effects of IL-7 and IL-15 on the proliferation, function, and phenotype of DMF5 T cells. (A) Proliferation of T cells cultured in IL-2, IL-7, and IL-15 after being activated with anti-CD3 and anti-CD28 antibodies, and transduced with the retroviral vector encoding the DMF5 TCR. (B) Viability of DMF5 T cells cultured in IL-2, IL-7, and IL-15 measured by flow cytometry according to the percentage of cells within the live lymphocyte gate. (C–F) Expression of costimulatory molecules CD27 and CD28 (C and D), and markers of homing to central lymphoid organs CCR7 and CD62L (E and F) on CD8+ DMF5 T cells after being cultured in IL-2, IL-7, and IL-15. (G) Representative flow cytometry plots showing production of IFN-γ and IL-2 by CD8+DMF5 T cells cultured in IL-2, IL-7, or IL-15 and stimulated with T2 cells loaded with MART-1 peptide. (H) Cytotoxicity of DMF5 T cells cultured in IL-2, IL-7, or IL-15 by CD107a expression after coculture with HLA-A*02+ cell lines Mel624, Mel501, and WM2664, and HLA-A*02− cell line Mel888. p-Values<0.05 by Student's t-test.
Additionally, no significant difference in the level of induced IFNγ and IL-2 was detected between DMF5 TCR T cells cultured in any of the three cytokines when challenged with antigen-pulsed T2 cells (Fig. 4G). However, DMF5 TCR T cells cultured in IL-2 showed a significant increase in the frequency of antigen-driven CD107a degranulation compared with both IL-7- and IL-15-cultured DMF5 TCR T cells (Fig. 4H). To summarize, DMF5 TCR T cells cultured in IL-2, IL-7, or IL-15 displayed similar phenotypic and in vitro functions except for cytotoxicity where T cells cultured in IL-2 appeared to have enhanced cytotoxic potential as determined by CD107a degranulation compared with T cells cultured in IL-7 or IL-15.
Adoptive transfer of IL-15-cultured DMF5 TCR T cells results in the improved survival of NSG mice bearing established melanoma
To investigate whether the culture of DMF5 TCR T cells in IL-7 or IL-15 impacted upon engraftment or antitumor activity, cohorts of NSG mice bearing 4-week established HLA-A*0201+ Mel624 tumors were adoptively transferred with 2×107 DMF5 TCR T cells cultured in IL-2, IL-7, or IL-15. At the time of adoptive transfer, there was some variation in tumor volume within each group but no significant difference was observed between groups (Fig. 5A). NSG mice given saline alone developed sizeable tumors and were culled because of excessive tumor growth within 45 days (Fig. 5B). In the NSG mice treated with DMF5 TCR T cells, at least 50% of animals were culled because of x-GvHD-related symptoms and before tumors achieved a volume of 600 mm3 (Fig. 5A). Allowing for censorship of these animals within all the cohorts, the survival analysis of these experiments indicated that only the mice receiving IL-15-cultured DMF5 TCR T cells achieved a statistically significant increase in survival compared with the saline control group (Fig. 5B). Analysis of tail bleeds drawn 1 week after adoptive transfer further showed that CD8+ and CD4+ T cells were readily detected (Fig. 5C and D); however, a later analysis of day 21 tail bleeds suggested that animals receiving IL-7-cultured DMF5 TCR T cells were significantly higher than background saline levels, while there was a widely spread degree of engraftment seen in animals receiving IL-15 DMF5 TCR T cells (Fig. 5E and F). The relative level of CD4+ and CD8+ T cells within the IL-2 and IL-15 groups was equivalent (∼20% CD4+; 80% CD8+; Supplementary Fig. S3A), while the IL-7-cultured T cell population had a close equivalence of both subsets (41.5 CD4+; 55.5% CD8+; Supplementary Fig. S3A) and overall transduction levels were approximately equal between groups (25–34%; Supplementary Fig. S3B). Taken together, these results suggest that the culture of DMF5 TCR T cells in IL-15 appeared to drive improved antitumor responses in vivo, while consistent longer-term engraftment appeared dependent upon IL-7 during culture potentially as a result of an altered CD4+/CD8+ ratio. Notwithstanding, x-GvHD is clearly a major issue when looking at long-term tumor responses to adoptive T cell therapy in the NSG model.
FIG. 5.
In vivo efficacy of DMF5 T cells cultured in IL-2, IL-7, and IL-15. (A) Comparison of the tumor volume on the day of adoptive transfer of T cells on NSG mice. Figures denote the number of animals that were culled because of x-GvHD as a proportion of the number of animals in the group. (B) Kaplan–Meier survival curve of tumor-bearing mice after systemic administration of DMF5 TCR T cells cultured in IL-2, IL-7, or IL-15, or saline vehicle as a control. Censored events are indicated by symbols identifying at which time point animals were culled due to x-GvHD symptoms. (C–F) Detection of systemically injected DMF5 T cells on tumor-bearing mice 7 days (C and D), and 3 weeks (E and F) after adoptive transfer. p-Values <0.05 by Student's t-test. NSG, NOD/Shi-scid IL-2rγ−/−.
The combination of IL-7 and IL-15 during the culture of DMF5 TCR T cells results in much improved levels of engraftment along with potent x-GvHD
Given the improved prolonged engraftment of IL-7-cultured DMF5 TCR T cells and the improved antitumor activity of IL-15-cultured DMF5 TCR T cells, it was obvious to test whether the combination of both cytokines could drive an advantage over the activity of the single cytokines. However, of the 10 NSG mice bearing established Mel624 tumors that were treated with IL-7/IL-15 DMF5 TCR T cells, 9 were culled because of the onset of x-GvHD symptoms well before developing tumors of a significant size. In comparison, only one of six animals developed these symptoms in the single cytokine IL-15 and IL-2 groups. This high degree of x-GvHD clearly impacted upon the ability to perform a survival analysis to determine whether the IL-7/IL-15 DMF5 TCR T cells demonstrated an improved antitumor activity compared with cells cultured in IL-15 or IL-2 alone (Fig. 6A). On the other hand, animals treated with control mock-transduced T cells cultured in IL-15 or IL-7/IL-15 had no improvement in survival compared with saline-treated control animals (Fig. 6B), confirming that the expression of the DMF5 TCR was driving antitumor responses. Interestingly, no animals receiving mock-transduced T cells developed symptoms of x-GvHD and they were culled because of tumors reaching the upper limit. However, the average survival of NSG mice receiving mock-transduced IL-15 or IL-7-/IL-15-cultured T cells was 57.7±15.6 days, while animals culled because of x-GvHD in the IL-7/IL-15 DMF5 TCR-treated cohort of NSG mice all were culled on 79.2±25.5 days. This suggested that the NSG mice receiving mock-transduced T cells were culled because of excessive tumor growth before the potential onset of x-GvHD symptoms.
FIG. 6.
In vivo efficacy of DMF5 T cells cultured in IL-7/IL-15 compared with IL-2 or IL-15 alone. (A) Kaplan–Meier survival curve of tumor-bearing mice after systemic administration of DMF5 T cells cultured in IL-2, IL-15, IL-7/IL-15, or saline as a control. (B) Kaplan–Meier survival curve of tumor-bearing mice after systemic administration of mock T cells cultured in IL-15, IL-7/IL-15, or saline as a control. Censored events are indicated by symbols identifying at which time point animals were culled because of x-GvHD symptoms. (C and D) Engraftment of systemically injected DMF5 T cells to tumor-bearing mice. T cells recovered in the blood of tumor-bearing mice 7 days after T cell injection. p-Values <0.05 by Student's t-test.
Analysis of tail bleeds taken 1 week after adoptive transfer showed that there was a trend toward improved engraftment in both the CD8+ and CD4+ compartments in NSG mice receiving IL-7/IL-15 DMF5 TCR T cells compared with IL-15-only-cultured T cells (Fig. 6C and D). While the difference in engraftment between IL-2 and IL-7/IL-15 DMF5 TCR T cells did not quite reach statistical significance at this time point, the level of significance for T cell engraftment in the IL-7/IL-15 cohort was far higher than that of the IL-2 cohort when compared with background control animals. Importantly, the relative ratio of CD4+ and CD8+ between all three groups was similar (Supplementary Fig. S3C), suggesting that a difference in the relative ratio of T cell subsets was not of major importance in this experiment. Taken together, the combination of IL-7 and IL-15 during the culture of DMF5 TCR T cells resulted in improved levels of engraftment compared with that achieved by DMF5 TCR T cells cultured in the presence of individual cytokines. Importantly, this improved level of engraftment is likely to have contributed to increased levels of x-GvHD in the melanoma-bearing NSG mouse, clearly impacting upon the ability to draw firm conclusions pertaining to the antitumor potency of these T cells.
Discussion
Clinical trials of T cells bearing tumor-specific TCR (Morgan et al., 2006; Robbins et al., 2011) or CAR (Kalos et al., 2011) demonstrate the principle that gene-modified T cell therapy is feasible and able to deliver objective clinical responses in patients with advanced cancer. However, improving on these initial responses is important to developing more potent therapies. Given recent discoveries regarding the likely importance of T cell phenotype and differentiation status in adoptive cell therapy, there is a focus upon optimizing the quality of T cells for therapy with respect to the development of systems that can produce gene-modified T cells bearing a less differentiated or naïve phenotype. Retroviral vectors require T cell activation for efficient gene transfer (Miller et al., 1990), which drives T cell differentiation. Current studies using lentiviral vectors also employ mitogenic activation in order to generate sufficient numbers of cells for therapy (Kalos et al., 2011). Consequently, focusing upon the ex vivo culture of T cells after transduction appears to be an immediate area that may be exploited to generate optimal therapeutic gene-modified T cells.
IL-7 and IL-15 are growth factors involved in the homeostasis of memory T cells (Schluns and Lefrancois, 2003). MART-1-specific DMF5 TCR T cells cultured in IL-15 possessed a greater proliferative potential than T cells cultured in IL-7 or IL-2, which reflects the observations of others (Klebanoff et al., 2004; Cha et al., 2010). Furthermore, IL-15-cultured DMF5 TCR also appeared to possess more potent antitumor activity in the NSG mouse compared with both IL-7- and IL-2-cultured DMF5 TCR T cells, while initial experiments suggested that IL-7 could maintain a less differentiated T cell phenotype. This improved in vivo activity of IL-15-cultured DMF5 TCR T cells is despite the better recognition of tumor target cells in vitro by DMF5 TCR T cells cultured in IL-2 (Fig. 4H). This may reflect an increased antioxidant capacity of IL-15-cultured T cells compared with those cultured in IL-2, resulting in improved in vivo functional activity (Kaur et al., 2011). Consequently, the combination of IL-7 and IL-15 during culture resulted in improved in vivo engraftment in the NSG mouse compared with IL-15 alone, which was highly encouraging. However, this high level of engraftment resulted in a strong x-GvHD response that negated the power of the model system to determine the efficacy of ACT upon mouse survival. Nonetheless, the combination of IL-7 and IL-15 clearly generated T cells that possess potent engraftment and in vivo effector potential.
The NSG mouse model is currently considered the gold-standard model for studies investigating human hemopoietic cell function (Ito et al., 2002). Previous studies using CD19-specific human CAR T cells to challenge NSG mice engrafted with Raji B cell lymphoma demonstrated effective tumor clearance but no reported evidence of an x-GvHD effect (Markley and Sadelain, 2010) although the dose of T cells used in this study was approximately one-hundredth of the T cell dose used in our study. Our study agrees with the findings of Markley and Sadelain (2010), where the combination of IL-7 with IL-15 or IL-21 resulted in enhanced antitumor activity. In our hands, IL-21 has issues with respect to optimally achieving in vitro dosing to avoid problems such as reduced cell growth (Yang et al., 2012a); hence, in our study, the combination of IL-7 and IL-15 offers major practical advantages during the ex vivo culture of gene-modified T cells. Moreover, this combination of cytokines to enhance gene-modified T cell therapy reflects the observations of others in vitro and in several mouse models (Kaneko et al., 2009; Markley and Sadelain, 2010; Pouw et al., 2010; Yang et al., 2012a). The sum of our observations is that a combination of IL-7 and IL-15 is able to generate a pool of T cells with a higher proportion of memory lymphocytes compared with IL-2 alone. As observed by the engraftment potential in our model, these cells will probably have the ability to persist for longer periods after adoptive transfer and maintain their antitumor competence clearing tumor cells.
Although less is known about the xenoreactive potential of memory T cell subsets, previous authors have suggested that TN and memory T cells have a higher alloreactive potential compared with effector T cells (Zhang et al., 2005; Zheng et al., 2009; Nadazdin et al., 2010; Ali et al., 2012). However, genetic modification of T cells by retroviral vectors is believed to diminish the alloreactive potential of T cells by generating a pool of mainly effector cells (Kaneko et al., 2009). Thus, these results suggest that culture in IL-7 and IL-15 restores the alloreactive potential of T cell lost during retroviral gene modification to a greater degree than T cells cultured in IL-2. Whether further mechanisms potentially underlie the driver of x-GvHD in this model system remains unclear. Recent studies have shown the rapid onset of GvHD resulting from TCR mispairing (Bendle et al., 2010), and further studies are warranted to determine whether such a mechanism enhanced by cytokines is at play in this model.
Clearly, T cell dose is important with respect to delivering an effective therapy and also the potential of x-GvHD induction in the NSG mouse model. In these studies, a dose of 2×107 total T cells was given per mouse. Assuming an average weight of 20 g per mouse, this equates to a T cell dose of 8×1010 total T cells for an 80 kg patient. In the field of gene-modified T cell therapy, trials to date (and particularly trials targeting solid tumors) have generally used T cell dose escalation with the final doses generally aimed to be in the 1010–1011 T cell dose; hence, the T cell doses used in these experiments reflect that of current trials. Indeed, the results of this study have contributed to a European Union Framework 7–supported phase II trial to investigate the activity of T cells engrafted with an NY-ESO-1-specific TCR in malignant melanoma and oesophageal carcinoma (ATTACK trial). This trial is based upon initial encouraging clinical responses of NY-ESO-1 TCR+ T cells in melanoma and synovial carcinoma (Robbins et al., 2011) with the melanoma part of the ATTACK trial investigating the potential of NY-ESO-1 TCR+ T cells produced using standard conditions (retroviral transduction, expansion in IL-2) against NY-ESO-1 TCR+ T cells produced under optimized conditions (CD62L-selected T cells transduced and expanded in the presence of IL-7 and IL-15) with the cell dose to be used in this trial between 2×109 and 2×1011 total T cells. In conclusion, studies seeking to optimize the engraftment and in vivo function of gene-modified T cells targeting slow-growing tumors in the NSG mouse may be limited because of the concomitant induction of x-GvHD. This is likely to be exacerbated in subcutaneous models where the effector T cells are required to deal with established tumor in the setting where allogeneic T cells can also drive x-GvHD. This is clearly related to the model being tested; targeting of hematological tumors in the NSG mouse is effective, most likely reflecting differences in the sensitivity of the target tumor cell to T cell killing and/or lower doses of effector T cells, highlighting that the therapeutic window will vary between models. The likely future importance of the NSG mouse in human gene-modified T cell studies targeting slowly growing tumors is to understand their engraftment potential and, in particular, developing strategies where studies investigating optimizing T cell activity would result in reduced overall T cell doses (Terakura et al., 2012; Yang et al., 2012a). Nonetheless, despite these limitations, the NSG model has proven of worth in this situation to develop protocols in support of the investigation of IL-7- and IL-15-cultured gene-modified T cells in clinical trial.
Supplementary Material
Acknowledgments
The authors thank Dr. Steven Rosenberg and Dr. Richard Morgan (Surgery Branch, NIH, Bethesda, MD) for kindly providing the DMF5 TCR. The authors also thank the biological resources and flow cytometry units of the Paterson Institute for Cancer Research for their help and efforts in supporting this study. E.M.A.-O. was supported by the National Council for Science and Technology of Mexico (CONACYT); H.G. was supported by the BBSRC; V.B. was supported by the European Union FP7 Marie-Curie ITN ATTRACT. This work has also been supported by the European Union FP6 Programme ATTACK, the Kay Kendall Leukaemia Fund, and Cancer Research UK.
Author Disclosure Statement
Robert E. Hawkins and David E. Gilham are both co-founders of Cellular Therapeutics Ltd. No other competing financial interests exist.
References
- Ali N. Flutter B. Sanchez Rodriguez R., et al. Xenogeneic graft-versus-host-disease in NOD-scid IL-2Rgammanull mice display a T-effector memory phenotype. PLoS One. 2012;7:e44219. doi: 10.1371/journal.pone.0044219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendle G.M. Linnemann C. Hooijkaas A.I., et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 2010;16:565–570. doi: 10.1038/nm.2128. , 561p following 570. [DOI] [PubMed] [Google Scholar]
- Berard M. Brandt K. Bulfone-Paus S. Tough D.F. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J. Immunol. 2003;170:5018–5026. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- Berger C. Jensen M.C. Lansdorp P.M., et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser M.J. Shapira-Frommer R. Treves A.J., et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin. Cancer Res. 2010;16:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- Birkholz K. Hombach A. Krug C., et al. Transfer of mRNA encoding recombinant immunoreceptors reprograms CD4+ and CD8+ T cells for use in the adoptive immunotherapy of cancer. Gene Ther. 2009;16:596–604. doi: 10.1038/gt.2008.189. [DOI] [PubMed] [Google Scholar]
- Burkett M.W. Shafer-Weaver K.A. Strobl S., et al. A novel flow cytometric assay for evaluating cell-mediated cytotoxicity. J. Immunother. 2005;28:396–402. doi: 10.1097/01.cji.0000165357.11548.6d. [DOI] [PubMed] [Google Scholar]
- Cha E. Graham L. Manjili M.H. Bear H.D. IL-7+ IL-15 are superior to IL-2 for the ex vivo expansion of 4T1 mammary carcinoma-specific T cells with greater efficacy against tumors in vivo. Breast Cancer Res. Treat. 2010;122:359–369. doi: 10.1007/s10549-009-0573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombetti S. Levy F. Chapatte L. IL-7 adjuvant treatment enhances long-term tumor-antigen-specific CD8+ T cell responses after immunization with recombinant lentivector. Blood. 2009;113:6629–6637. doi: 10.1182/blood-2008-05-155309. [DOI] [PubMed] [Google Scholar]
- Dudley M.E. Wunderlich J.R. Robbins P.F., et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M.E. Yang J.C. Sherry R., et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L. Lugli E. Ji Y., et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilham D.E. Debets R. Pule M., et al. CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol. Med. 2012;18:377–384. doi: 10.1016/j.molmed.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Hinrichs C.S. Borman Z.A. Cassard L., et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl. Acad. Sci. USA. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs C.S. Borman Z.A. Gattinoni L., et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117:808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Hiramatsu H. Kobayashi K., et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- Johnson L.A. Heemskerk B. Powell D.J., Jr., et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J. Immunol. 2006;177:6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L.A. Morgan R.A. Dudley M.E., et al. Gene therapy with human and mouse T cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos M. Levine B.L. Porter D.L., et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S. Mastaglio S. Bondanza A., et al. IL-7 and IL-15 allow the generation of suicide gene-modified alloreactive self-renewing central memory human T lymphocytes. Blood. 2009;113:1006–1015. doi: 10.1182/blood-2008-05-156059. [DOI] [PubMed] [Google Scholar]
- Kaur N. Naga O.S. Norell H., et al. T cells expanded in presence of IL-15 exhibit increased antioxidant capacity and innate effector molecules. Cytokine. 2011;55:307–317. doi: 10.1016/j.cyto.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff C.A. Finkelstein S.E. Surman D.R., et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc. Natl. Acad. Sci. USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff C.A. Gattinoni L. Palmer D.C., et al. Determinants of successful CD8+ T cell adoptive immunotherapy for large established tumors in mice. Clin. Cancer Res. 2011;17:5343–5352. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku C.C. Murakami M. Sakamoto A., et al. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- Lamers C.H. Willemsen R.A. Luider B.A., et al. Protocol for gene transduction and expansion of human T lymphocytes for clinical immunogene therapy of cancer. Cancer Gene Ther. 2002;9:613–623. doi: 10.1038/sj.cgt.7700477. [DOI] [PubMed] [Google Scholar]
- Markley J.C. Sadelain M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood. 2010;115:3508–3519. doi: 10.1182/blood-2009-09-241398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay A. Radford K. Kato M., et al. Blood monocytes, myeloid dendritic cells and the cytokines interleukin (IL)-7 and IL-15 maintain human CD4+ T memory cells with mixed helper/regulatory function. Immunology. 2007;120:392–403. doi: 10.1111/j.1365-2567.2006.02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchionda F. Fry T.J. Milliron M.J., et al. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J. Clin. Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.G. Adam M.A. Miller A.D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol. Cell Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.A. Dudley M.E. Wunderlich J.R., et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadazdin O. Boskovic S. Murakami T., et al. Phenotype, distribution and alloreactive properties of memory T cells from cynomolgus monkeys. Am. J. Transplant. 2010;10:1375–1384. doi: 10.1111/j.1600-6143.2010.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouw N. Treffers-Westerlaken E. Kraan J., et al. Combination of IL-21 and IL-15 enhances tumour-specific cytotoxicity and cytokine production of TCR-transduced primary T cells. Cancer Immunol. Immunother. 2010;59:921–931. doi: 10.1007/s00262-010-0818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refaeli Y. Van Parijs L. London C.A., et al. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–623. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- Robbins P.F. Morgan R.A. Feldman S.A., et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin. Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.A. Restifo N.P. Yang J.C., et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns K.S. Lefrancois L. Cytokine control of memory T cell development and survival. Nat. Rev. Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- Schroeder M.A. DiPersio J.F. Mouse models of graft-versus-host disease: advances and limitations. Dis. Model Mech. 2011;4:318–333. doi: 10.1242/dmm.006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terakura S. Yamamoto T.N. Gardner R.A., et al. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;119:72–82. doi: 10.1182/blood-2011-07-366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttenthal B.J. Chua I. Morris E.C. Stauss H.J. Challenges in T cell receptor gene therapy. J. Gene Med. 2012;14:386–399. doi: 10.1002/jgm.2637. [DOI] [PubMed] [Google Scholar]
- Yang S. Rosenberg S.A. Morgan R.A. Clinical-scale lentiviral vector transduction of PBL for TCR gene therapy and potential for expression in less-differentiated cells. J. Immunother. 2008;31:830–839. doi: 10.1097/CJI.0b013e31818817c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Dudley M.E. Rosenberg S.A. Morgan R.A. A simplified method for the clinical-scale generation of central memory-like CD8+ T cells after transduction with lentiviral vectors encoding antitumor antigen T cell receptors. J. Immunother. 2010;33:648–658. doi: 10.1097/CJI.0b013e3181e311cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Ji Y. Gattinoni L., et al. Modulating the differentiation status of ex vivo-cultured anti-tumor T cells using cytokine cocktails. Cancer Immunol. Immunother. 2012a;62:727–736. doi: 10.1007/s00262-012-1378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Karne N.K. Goff S.L., et al. A simple and effective method to generate lentiviral vectors for ex vivo gene delivery to mature human peripheral blood lymphocytes. Hum. Gene Ther. Methods. 2012b;23:73–83. doi: 10.1089/hgtb.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Joe G. Hexner E., et al. Alloreactive memory T cells are responsible for the persistence of graft-versus-host disease. J. Immunol. 2005;174:3051–3058. doi: 10.4049/jimmunol.174.5.3051. [DOI] [PubMed] [Google Scholar]
- Zheng H. Matte-Martone C. Jain D., et al. Central memory CD8+ T cells induce graft-versus-host disease and mediate graft-versus-leukemia. J. Immunol. 2009;182:5938–5948. doi: 10.4049/jimmunol.0802212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.