Abstract
Stem cells exert precise regulation to maintain a balance of self-renewal and differentiation programs to sustain tissue homeostasis throughout the life of an organism. Recent evidence suggests that this regulation is modulated, in part, via metabolic changes and modifications of nutrient-sensing pathways such as mTOR and AMPK. It is becoming increasingly clear that stem cells inhibit oxidative phosphorylation in favor of aerobic glycolysis for energy production. Recent progress has detailed the molecular mechanisms of this metabolic phenotype and has offered insight into new metabolic pathways that may be involved in stem cell homeostasis.
Introduction
During development and organogenesis there is a continuous need to generate specialized cell types, while maintaining tissue homeostasis throughout the lifespan of an organism. “Stem” cells meet this need via two key properties: (1) self-renewal, and (2) the ability to produce a subset of various differentiated cells. Mammals generate multiple stem cell types, including embryonic stem cells (ESCs) and adult stem cells. Both of these share the key properties listed above; however, they differ in their potency, or ability to differentiate. ESCs are pluripotent and produce all cells within the three embryonic germ layers (ectoderm, endoderm, and mesoderm). In contrast, adult stem cells are multipotent and exclusively generate differentiated cells of a particular organ or tissue, typically where they reside. For example, adult stem cells responsible for the formation of all blood cells, i.e., hematopoietic stem cells (HSCs), are located in the bone marrow, the site of hematopoiesis in adults.
The origin and differentiation of HSCs has been well characterized through detailed studies in mice. HSCs are formed within a very narrow time frame of embryogenesis, after which point the HSC pool is maintained strictly through self-renewal. The first appearance of HSCs occurs at embryonic day (E) 10.5 in the aorta-gonad-mesonephros (AGM) region of the conceptus. HSCs then migrate to the fetal liver at approximately E11.5; placental HSCs also appear at this time (Gekas et al., 2005). After E13.5 the placental pool of HSCs declines and the fetal liver remains the principal source of HSC production until migration to the bone marrow (the permanent site of hematopoiesis) at E16.5 (Gekas et al., 2010). HSCs constitute one adult stem cell type with a high rate of turnover, similar to intestinal and hair follicle stem cells, whereas neural stem cells exhibit low turnover rates (Hsu and Fuchs, 2012). Mechanisms determining the rate of adult stem cell turnover and differentiation are complex, but recent evidence suggests epigenetic modifications (especially DNA methylation) are key regulators of this process (Ji et al., 2010; Challen et al., 2012). Epigenetic changes affect HSC differentiation, and specific metabolic alterations influence this process (see subsequent discussion of 2-hydroxyglutarate).
Pluripotent ESCs, on the other hand, exhibit a specific developmental program that controls cell lineages produced at specific times during gestation. Mouse ESCs are derived from blastocysts, early embryonic structures that form after several rounds of cell division 4–5 d post-fertilization (Thomson et al., 1998). The epiblast, a tissue component of the early embryo and source of human ESCs, is obtained via immunosurgery or mechanical dissection (Vazin and Freed, 2010). After isolation, ESCs can be cultured in vitro indefinitely using either a feeder layer of fibroblast cells or an artificial substrate such as Matrigel with proper supplementation of necessary growth factors (Stojkovic et al., 2005; Wang et al., 2005). Because ESCs can be cultured indefinitely and have the ability to produce most somatic cells, ESCs hold therapeutic promise for a multitude of regenerative medicine and tissue engineering applications.
Characterizing the molecular determinants of multipotent and pluripotent stem cell differentiation is critical to develop the therapeutic potential of these cells. Recently, metabolic regulation of central pathways, such as glycolysis, has been demonstrated to be an important modulator of stem cell quiescence in adult stem cells and in maintaining ESC pluripotency. Using nutrient-sensing pathways, like those regulated by mTOR and AMPK, stem cells maintain energy production by inhibiting key processes (e.g., oxidative phosphorylation, OXPHOS) and enhancing others (e.g., glycolysis), and this interplay is key to the maintenance of “stem-ness.” This review will describe the nutrient-sensing pathways involved in stem cell homeostasis and how specific changes in metabolic flux affect stem cell differentiation.
Nutrient-sensing pathways in stem cell maintenance
PI3K/AKT and mTOR in HSCs.
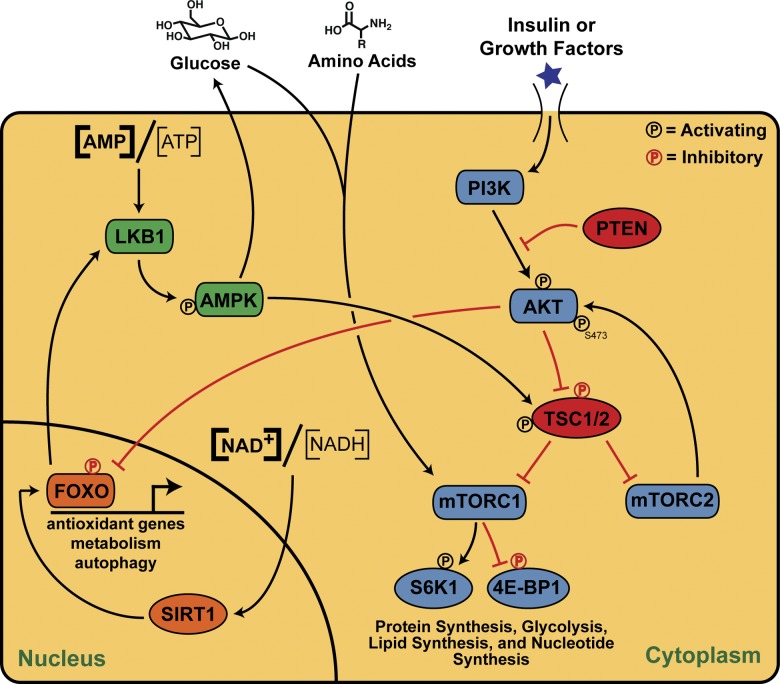
The mammalian target of rapamycin (mTOR) kinase plays a central role in cellular sensing of O2, nutrients, and growth factors through the phosphatidylinositol 3-kinase (PI3K)/AKT pathway (Fig. 1). It exists in two distinct complexes, mTORC1 and mTORC2, which have overlapping yet distinct functions. Growth factors such as insulin, insulin-like growth factor 1 (IGF-1), epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF) stimulate PI3K, activating AKT and mTORC1 through inhibition of mTOR inhibitory proteins, the tuberous sclerosis complex 1/2 (TSC1/2; Inoki et al., 2002; Altomare and Khaled, 2012; J. Lee et al., 2012). Nutrients (e.g., glucose) and amino acids (e.g., leucine) are potent mTORC1 stimulators (Kim and Guan, 2011). Upon activation, mTORC1 phosphorylates its downstream targets 4E-BP1 and S6K1 to promote mRNA translation, glycolysis, and lipid and nucleotide synthesis (Yecies and Manning, 2011). Less is known about mTORC2 regulation; however, upon growth factor treatment, mTORC2 phosphorylates AKT at Ser473, allosterically activating it (Oh and Jacinto, 2011).
Figure 1.
Pathways involved in stem cell hemostasis via regulation of nutrient sensing. The LKB1/AMPK pathway senses cellular energy levels and, when low, activates glucose uptake and inhibits mTOR. SIRT1 senses when NAD+ levels become high (oxidative environment) and activates the expression of FOXO proteins, which activate genes to combat oxidative stress. The PI3K/AKT pathway is activated by insulin or other growth factors to culminate in protein synthesis through mTOR, which can also be activated by glucose and amino acids. Items in red denote inhibitory paths or processes.
In the hematopoietic system, mTOR levels play dual roles in HSC maintenance. TSC1 deletion (a model of constitutive mTORC1 activation) in HSCs leads to loss of quiescence through increased mitochondrial biogenesis and production of reactive oxygen species (ROS; Chen et al., 2008). Furthermore, mTOR activation in HSCs by inflammatory cytokines leads to defective hematopoiesis (Chen et al., 2010), whereas mTOR stimulation through phosphatase and tensin homologue deleted on chromosome 10 (PTEN) deletion depletes HSCs (Yilmaz et al., 2006; Zhang et al., 2006). The latter finding was supported by Lee et al. (2010), who demonstrated that PTEN deletion induces both p16Ink4a and p53 expression in HSCs with a concomitant depletion of the stem cell pool, and that these effects were attenuated with rapamycin treatment. In another setting of PTEN ablation in HSCs, Magee et al. (2012) demonstrated that additional deletion of the mTORC2 binding partner Rictor prevented HSC loss, suggesting mTORC2 activation specifically depletes HSCs.
Although mTOR activation is clearly linked to decreased HSC numbers, Kalaitzidis et al. (2012) demonstrated that mTORC1 inhibition through ablation of its binding partner Raptor leads to defective hematopoiesis and reduced ability to reconstitute irradiated mice. In addition to fine-tuning mTOR activation in HSCs, the age of the organism also confers mTOR-dependent phenotypes in the hematopoietic system. For instance, the long-term reconstitutive capacity of HSCs in neonatal mice is unaffected by PTEN deletion, while adult HSCs suffering from PTEN deletion lose this ability (Magee et al., 2012). Ultimately, mTOR signaling is directly responsible for HSC aging; rapamycin treatment in aged mice increases lifespan and restores self-renewal and hematopoiesis to HSCs (Chen et al., 2009). The summation of these studies indicates that mTOR is essential in HSC maintenance, but only when enzymatically active at a specific level, and this holds true for ESCs as well (discussed next).
mTOR in other stem cells.
During early gestation, mTOR is crucial for embryonic growth, development, and cell proliferation, as its elimination causes defects in cell size and lethality between E6.5 and 7.5 (Murakami et al., 2004). Furthermore, mTOR inhibition prevents proliferation and impairs pluripotency in human ESCs (Zhou et al., 2009, 2012). Microarray analysis demonstrated that mTOR deletion results in enhanced expression of differentiation-promoting transcription factors such as Mix1 homeobox-like 1 (MIXL1), T gene, and the homeobox protein PITX2, resulting in loss of pluripotency (Zhou et al., 2009). Furthermore, mTOR promotes proliferation by suppressing growth inhibitory molecules such as cyclin G2 (Zhou et al., 2009). These data indicate that mTOR is centrally responsible for maintaining ESC pluripotency by preventing the expression of transcription factors that promote differentiation and repressing the expression of genes impacting developmental programs.
Paradoxically, increased expression of the direct mTOR target p70 S6 kinase (S6K1) induces human ESC differentiation (Easley et al., 2010). This discrepancy is likely due to the constitutive activation of S6K1, as wild-type S6K1 did not induce differentiation in this context (Easley et al., 2010). As with HSCs, these results point to an activation level–dependent effect of mTOR on ESC maintenance; either constitutive mTOR activation or complete mTOR deletion has detrimental consequences for the ESC pool. Because mTOR inhibitors, such as rapamycin, have already shown promise in restoring HSC homeostasis when mTOR is hyperactivated, perhaps mTOR stimulation could be useful when the basal levels of mTOR are abnormally low. It is important to note that in ESCs, mTOR inhibition with rapamycin has deleterious effects, indicating that ESCs are exquisitely sensitive to precise levels of mTOR activity. Additional studies should provide more details on mechanisms that finely tune mTOR activity levels, such that maintenance of HSC and ESC homeostasis can be maximized.
In addition to the “fine-tuning” effects of mTOR on HSCs and ESCs, it also plays a pivotal role in coupling calorie intake to stem cell function. In the intestine, Paneth cells are a key component of the stem cell niche, which modulate intestinal stem cell (ISC) homeostasis through mTOR activity (Yilmaz et al., 2012). Yilmaz et al. (2012) recently determined that calorie restriction reduces mTORC1 signaling in Paneth cells, but not ISCs, resulting in an increase in both stem cell and niche cell numbers. Furthermore, calorie restriction enhances intestinal regeneration via environmental actions of the intestinal niche on ISCs (Yilmaz et al., 2012). Stem cells often integrate microenvironmental signals with their maintenance and self-renewal, and it will be interesting to learn if other stem cell pools possess similar nutrient-sensing cues regulating their fate.
AMPK and LKB1.
AMP-activated protein kinase (AMPK) is an αβγ heterotrimeric enzyme, master regulator of metabolism, and key energy sensor of intracellular AMP to ATP ratios (Fig. 1). When this ratio becomes high (low energy conditions), two molecules of AMP bind to four cystathione β-synthase domains in the γ subunit, causing a conformation change that exposes Thr172 in the catalytic α subunit, allowing its phosphorylation and subsequent AMPK activation (Ellingson et al., 2007). AMPK inhibits anabolic pathways, such as lipogenesis and mTOR-regulated protein synthesis, while simultaneously activating catabolic pathways such as fatty acid oxidation and glucose uptake (Winder and Hardie, 1999). AMPK is also necessary for the prevention of type 2 diabetes (Li et al., 2011) and promotes longevity in several species (I. Lee et al., 2012; Slack et al., 2012; Stenesen et al., 2013).
Given that AMPK is crucial to cellular metabolism and energy status, one would expect it to have consequences for stem cell maintenance. The two catalytic subunits of AMPK, Prkaa1 and Prkaa2, are more highly expressed in HSC populations compared with unfractionated whole bone marrow, and deletion of these two subunits leads to increased mTORC1 activity in the HSC population (Nakada et al., 2010). Although inactivation of AMPK via genetic means reduces HSC frequency in mice, AMPK-deficient HSCs were still able to long-term reconstitute the bone marrow of irradiated recipients, indicating they are still functional (Nakada et al., 2010). This study suggests that AMPK-dependent and -independent effects are observed in HSCs, while the upstream activator of AMPK, LKB1, has substantial and direct effects in HSCs.
LKB1 is a constitutively active kinase that phosphorylates Thr172 of AMPK, only after it has sensed low energy levels and undergone a conformational change to expose this residue (Ellingson et al., 2007). LKB1 acts as a tumor suppressor that is mutated in Peutz-Jeghers syndrome patients (Hemminki et al., 1998; Jenne et al., 1998). Deletion of LKB1 leads to malignant transformation and invasiveness in endometrial adenocarcinomas (Contreras et al., 2008), and LKB1 has similar roles in other cancers (Pearson et al., 2008; Krock et al., 2011). Despite this seemingly inhibitory role for LKB1 in proliferation, it has a distinctly different impact on stem cell biology. Independently, three studies showed that LKB1 deletion in the HSC population leads to loss of HSC quiescence, coupled with an increase in multipotent progenitor cell numbers (Gan et al., 2010; Gurumurthy et al., 2010; Nakada et al., 2010). Because LKB1 is a direct activator of AMPK, deletion of LKB1 in HSCs, as expected, results in reduced AMPK phosphorylation with a concomitant increase in mTOR activity, based on phosphorylation of its downstream target S6 (Nakada et al., 2010). Furthermore, LKB1 deletion led to an inability of HSCs to long-term reconstitute the bone marrow of irradiated recipient mice. Intriguingly, however, these three studies concluded that the effects of LKB1 on HSCs were independent of AMPK, mTOR, and FOXO (see subsequent section). The effects of LKB1 on HSCs are instead likely mediated via mitochondrial biogenesis, as LKB1 deletion leads to down-regulation of PGC-1 coactivators (Gan et al., 2010). Cumulatively, these studies suggest that LKB1 is an essential regulator of HSCs that couples energy metabolism with stem cell homeostasis.
FOXO transcription factors.
The DNA-binding forkhead box O (FOXO) transcription factors are involved in a multitude of cellular processes from proliferation to longevity and they regulate the cell cycle, DNA repair, and apoptosis (Hedrick et al., 2012). FOXOs lie downstream of insulin/IGF-1 signaling and the PI3K/AKT pathway (Fig. 1), such that PI3K/AKT activation leads to inhibition of the FOXO family via AKT phosphorylation and subsequent nuclear exportation, ubiquitination, and degradation (Gross et al., 2008; Huang and Tindall, 2011). Decreases in insulin signaling (e.g., during starvation) or mutations in the PI3K/AKT pathway allow FOXOs to remain in the nucleus where they stimulate transcriptional programs to control metabolism and other processes (Gross et al., 2008).
In HSCs, FOXOs, especially FOXO3, are crucial for the maintenance of the stem cell pool (Miyamoto et al., 2007; Tothova et al., 2007). Conditional deletion of FOXO1, FOXO3, and FOXO4 in the hematopoietic compartment leads to a decrease in HSC numbers, an increase in differentiated effector cells, and defective reconstitution capability (Tothova et al., 2007). FOXO3 ablation reduces HSC quiescence and results in impaired reconstitution functionality (Miyamoto et al., 2007). Importantly, both of these studies hypothesized that FOXO’s role in HSC maintenance stems from its ability to blunt ROS production, as in vivo treatment with a potent antioxidant N-acetyl-l-cysteine ameliorates the FOXO3-deficient phenotype (Tothova et al., 2007). Accordingly, two follow-up studies further elucidated FOXO’s role in stem cells (Yalcin et al., 2008, 2010). FOXO3 was shown to suppress ROS production in HSCs by regulating the tumor suppressor ataxia telangiectasia mutated (ATM); furthermore, increased ROS in FOXO3-null HSCs activates p53 (Yalcin et al., 2008). Additionally, FOXO3 deletion in mice results in a myeloproliferative syndrome caused by an over-activation of AKT/mTOR via accumulated ROS. Moreover, N-acetyl-l-cysteine treatment relieved the myeloproliferative phenotype (Yalcin et al., 2010). In addition to regulating redox homeostasis, FOXOs protect HSCs from metabolic stress via the induction of autophagy (Warr et al., 2013). During both calorie restriction and cytokine withdrawal in mouse HSCs, FOXO3A induces autophagy, which allows HSCs to avoid an energy crisis (Warr et al., 2013).
There is considerable cross talk between the FOXO family of proteins and the nutrient-sensing LKB1/AMPK pathway. FOXO3 and FOXO4 bind to cis-acting elements in the LKB1 promoter to activate its transcription, and siRNA-mediated knockdown of these FOXO proteins results in reduced LKB1 expression and subsequent loss of AMPK phosphorylation (Lützner et al., 2012). Furthermore, under glucose deprivation AMPK phosphorylates FOXO3, leading to its mitochondrial accumulation and the expression of OXPHOS machinery (Peserico et al., 2013). Taken together, FOXOs play a crucial role in controlling stem cell homeostasis and coupling it with cellular metabolism. It will be interesting to further elucidate the role of FOXO proteins in controlling OXPHOS, as this pathway is generally suppressed in the highly glycolytic HSC population (see next section).
Metabolic regulation of stem cell homeostasis
Glucose, hypoxia, and the glycolytic pathway in HSCs.
The proliferation of terminally differentiated cells relies on a significant production of ATP, which is ordinarily sustained through OXPHOS. For each molecule of glucose metabolized by OXPHOS, 36–38 ATPs can theoretically be generated, providing the required energy to sustain protein synthesis, biomass accumulation, growth, and proliferation (Balaban, 1990). Stem cells do not rely solely on OXPHOS, however, as initial studies demonstrated that human pluripotent stem cells exhibit decreased oxygen consumption and increased media acidification (indicating excess lactate production), and that these phenotypes are independent of glucose uptake (Zhang et al., 2011). HSCs rely on glycolysis, rather than OXPHOS, for energy production (Simsek et al., 2010). Although glycolysis only yields a net of 2 ATP per mole of glucose consumed (Fig. 2), glycolysis can produce ATP at a rapid rate; in the presence of excess glucose, glycolysis can produce a larger percentage of ATP than OXPHOS (Guppy et al., 1993). Under hypoxia (see below), the rate of ATP production increases up to 100-fold compared with mitochondrial ATP generation under normoxic conditions, indicating ample energy can be generated via glycolysis (Takubo et al., 2013). It is important to note that this glycolytic phenotype precedes the expression of pluripotent markers, suggesting that metabolism changes occur before changes in stem-ness (Folmes et al., 2011).
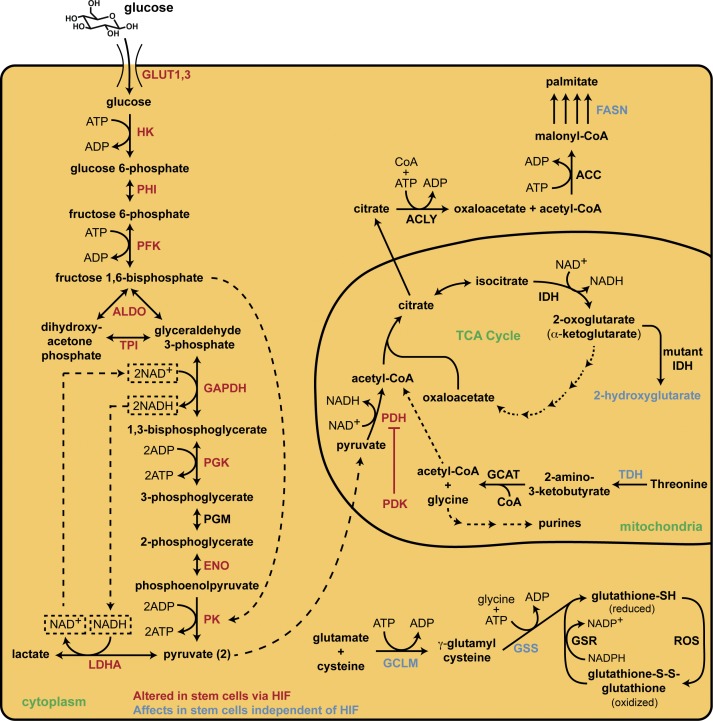
Figure 2.
Metabolic pathways that regulate stem cell homeostasis. HSCs use anaerobic glycolysis (left side of figure) to produce a net of 2 ATP for each glucose molecule consumed, and this metabolic adaptation is accomplished via HIF protein regulation of glycolytic genes. Specifically, HIF controls the expression of PDK, which inhibits the activity of PDH and impedes flux through the TCA cycle, promoting glycolysis. Furthermore, glycolysis is sustained through the increased activity of pyruvate kinase (PK) and lactate dehydrogenase A (LDHA) to generate ATP and NAD+, which is required for the GAPDH reaction to maintain glycolytic flux. Glutathione is the main antioxidant in all cells and the enzymes controlling its synthesis, GCLM and GSS, are elevated in stem cells to prevent oxidative stress, which promotes loss of quiescence. Threonine is metabolized by TDH in the mitochondria to produce acetyl-CoA, which enters the TCA cycle, and glycine, which is a building block for purine biosynthesis; this enzyme is elevated dramatically in stem cells. 2-hydroxyglutarate is an oncometabolite produced by mutant IDH enzymes that inhibits histone demethylases, causing a block in stem cell differentiation. Lastly, in neural stem cells, fatty acid synthase (FASN) is required for lipogenesis and its deletion leads to impaired neurogenesis. Collectively, this illustration demonstrates that maintaining stem cell homeostasis is a complex process and that several metabolic pathways, functioning in both a coordinated and independent manner, contribute to quiescence.
HSCs reside in a hypoxic bone marrow niche in vivo (Simsek et al., 2010), which necessitates their metabolic adaptation to producing energy under low O2 conditions. Indeed, O2 concentrations can regulate the differentiation of various types of cells, such as skeletal muscle progenitors and neural stem cells (Mazumdar et al., 2010; Majmundar et al., 2012). The main regulatory proteins responsible for cellular adaptation to a hypoxic environment are the hypoxia-inducible factors, or HIF transcription factors, and these proteins play a central role in stem cell homeostasis (Mazumdar et al., 2009; Lee and Simon, 2012). HIF-1α mRNA and protein levels are highly expressed in HSCs and HIF-1α knockout in mice leads to a loss in HSC quiescence (Takubo et al., 2010). The HSCs derived from these HIF-1α knockout mice are unable to reconstitute the bone marrow of irradiated recipients and show a reduced tolerance to stresses such as aging. These results demonstrate that hypoxia, via HIF-1α, maintains HSCs by decreasing proliferation and keeping cells quiescent (Eliasson et al., 2010). On the other hand, treatment of HSCs with a small molecule HIF stabilizer, FG-4497 (Bernhardt et al., 2009), or expression of a constitutively active form of HIF-1α led to a reduction in HSC reconstituting ability (Eliasson et al., 2010). These contradictory data point to regulation of HIF-1α levels as important for the fine-tuning of HSC homeostasis; complete ablation of HIF-1α leads to a loss of HSC quiescence and inability to reconstitute the bone marrow, and over-activation or stabilization of HIF-1α has the same consequences. Another HIF isoform, HIF-2α, controls the expression of stem cell markers such as OCT4 (Covello et al., 2006). It will be important to determine the appropriate range with which modulation of HIF-1α will be successful for stem cell therapies, and to further elucidate the role of HIF-2α in maintaining stem-ness.
It is clear that HSCs rely on both glycolysis and HIF-1α to maintain quiescence in the hypoxic bone marrow niche. The precise molecular details for the requirement of HIF-1α are currently being elucidated, with several studies demonstrating that HIF-1α mediates cellular reprogramming to a glycolytic phenotype. HIF-1α directly controls the expression of numerous glycolytic enzymes, including hexokinase (HK), phosphofructokinase (PFK), phosphoglycerate kinase (PGK), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), enolase (ENO), and pyruvate kinase (PK), as well as glucose transporters 1 and 3 (GLUT1 and 3, see Fig. 2; Gordan et al., 2007). Furthermore, HIF-1α regulates lactate dehydrogenase A (LDHA; Firth et al., 1995) and pyruvate dehydrogenase kinase (PDK; Kim et al., 2006), which, although neither is directly involved in the glycolytic pathway, have major influences on metabolism in stem cells. PDK phosphorylates and inactivates pyruvate dehydrogenase (PDH), preventing the conversion of pyruvate to acetyl-CoA and entry into the TCA cycle (Kim et al., 2006). Simultaneously, the increased expression of LDHA in hypoxic stem cells enhances the conversion of pyruvate to lactate by using cytosolic NADH generated in glycolysis as a cofactor (Vander Heiden et al., 2009). Because the regeneration of NAD+ is a rate-limiting step in glycolysis (Wheaton and Chandel, 2011), the high rate of conversion of pyruvate to lactate by LDHA maintains a constant supply of NAD+ for the GAPDH reaction and allows a high glycolytic flux to continue (Fig. 2; Vacanti and Metallo, 2013).
Recently, an elegant study by Takubo et al. (2013) provided more insight into the regulation of HSC metabolism via PDK. Using metabolome analysis of mouse HSCs, the authors found that fructose 1,6-bisphosphate (F1,6BP) is only present in HSCs, and that because F1,6BP allosterically activates PK, pyruvate levels were also drastically elevated exclusively in HSCs. This adaptation occurs to maximize glycolytic flux for this ATP-producing step of glycolysis (Fig. 2). It is important to note that TCA cycle-related metabolites such as 2-oxoglutarate, acetyl-CoA, and succinyl-CoA were not detected in any hematopoietic fraction, supporting the role of a diminished TCA cycle in HSCs (Takubo et al., 2013). The mechanism by which the TCA cycle is down-regulated lies in PDK, as alluded to in the previous paragraph. Takubo et al. (2013) found that HIF-1α is essential to maintain anaerobic glycolysis in HSCs by driving the expression of PDK isoforms 2 and 4, which prevent pyruvate from entering the TCA cycle through PDH phosphorylation. Deletion of PDK2 or 4 led to loss of transplantation capacity and cell cycle quiescence, similar to HIF-1α deletion (Takubo et al., 2010, 2013).
Cumulatively, these studies on HSC metabolism demonstrate that HSCs residing in the hypoxic bone marrow niche rely on HIF-1α to use a metabolic program that prevents pyruvate entry into the TCA cycle (and thus OXPHOS, through PDK) while simultaneously promoting ATP generation via glycolysis. Oxidative phosphorylation can still occur at low O2 tensions, albeit with less efficiency (Gnaiger, 2001), so one critical question arising from these studies is why HSCs seemingly expend so much effort to reduce OXPHOS. Interestingly, completely ablating OXPHOS by deletion of a mitochondrial phosphatase PTPMT1 results in hematopoietic failure due to an inability of HSCs to differentiate, indicating the OXPHOS is still required in this setting (Yu et al., 2013). Another hypothesis for the reduction in OXPHOS is that HSCs do this to minimize ROS levels in the cell (see section on ROS and redox status).
Energy production in ESCs via glycolysis.
Pluripotent stem cells, including ESCs, maintain rapid proliferation due to a shortened cell cycle, as compared with their somatic counterparts, with a shortened G1 and an extended S phase (Becker et al., 2006). Due to this increased rate of proliferation, ESCs exert substantial energy demands that one might predict to be fulfilled by OXPHOS; however, ESCs have limited oxidative capacity and rely on anaerobic respiration due to their localization in a hypoxic environment before implantation (Facucho-Oliveira and St John, 2009). The expression of stem cell markers OCT4, SOX2, and NANOG is increased under hypoxia, as well as is the consumption of glucose and the production of lactate (Forristal et al., 2013).
Because ESCs produce more lactate and consume more glucose than differentiated cells, this suggests that glycolysis is a more active component of their metabolism (Zhang et al., 2011). Indeed, ESCs exhibit a lower oxygen consumption rate, have a lower mitochondrial membrane potential, and have increased levels of glycolytic enzymes (Varum et al., 2011). Varum et al. (2011) demonstrated that this glycolytic phenotype of ESCs occurs to maximize energy production during rapid proliferation and is the result of increased hexokinase II levels and an inactivation of the PDH complex. In addition, Zhang et al. (2011) demonstrated that uncoupling protein 2 (UCP2) facilitates glycolysis by preventing mitochondrial glucose oxidation via pyruvate entry into the TCA. Using 13C6-labeled glucose, the authors confirmed that ESCs use UCP2 to increase the glycolytic flux and shunt pyruvate away from oxidation in the mitochondria for use in the pentose phosphate pathway.
Using both PDH inactivation and UCP2 expression mechanisms allows ESCs to ensure energy production via glycolysis. Although HSCs inactivate PDH through activation of PDK, as discussed previously, ESCs display no difference in PDK gene expression when compared with differentiated cells; however, ESCs still contain higher levels of phospho-PDH (Varum et al., 2011). This suggests that other complexes are regulating the phosphorylation of PDH in ESCs, and further study into this mechanism of PDH inactivation is warranted.
ROS and redox status.
The mitochondria of stem cells are fewer in number and exhibit decreased activity when compared with differentiated cells (Cho et al., 2006; Prigione et al., 2010). All of these features result in decreased ROS levels in stem cells. “Reactive oxygen species” is a general term for reduced forms of molecular oxygen that are highly reactive, such as superoxide (•O2−) and the hydroxyl radical (•OH−). ROS are naturally produced during mitochondrial respiration and have detrimental consequences when present in excess, such as DNA, protein, and lipid damage. High levels of ROS can lead to HSC exhaustion, as elevated ROS limit the lifespan, inhibit reconstitution capacity, and reduce the quiescence of HSCs (Ito et al., 2006). Paradoxically, the presence of low levels of ROS in HSCs maintains their quiescence and endows them with higher self-renewal potential (Jang and Sharkis, 2007). Low basal levels of ROS are needed to maintain genomic stability by activating DNA repair pathways (Li and Marbán, 2010) and to mediate differentiation of stem cells (Chaudhari et al., 2012; Ho et al., 2013). It appears that stem cells suffer detrimental effects from excess ROS, but are dependent on low basal ROS levels to maintain signaling activity and homeostasis.
Stem cells regulate ROS through multiple pathways. One mechanism is through the FOXO proteins (Fig. 1). FOXO3 expression is critical for stem cell maintenance (see section on FOXO transcription factors) and controls the expression of manganese superoxide dismutase (MnSOD), endowing quiescent cells with resistance to oxidative damage (Kops et al., 2002). Another ROS regulatory mechanism involves the polycomb proteins, which control the expression of genes through histone modifications. The polycomb complex component BMI1 promotes HSC self-renewal in part by inhibiting ROS production. Furthermore, mouse mammary tumor stem cells express higher levels of glutamate-cysteine ligase and glutathione synthetase, enzymes involved in the synthesis of the principal cellular antioxidant glutathione (Fig. 2; Diehn et al., 2009). These data suggest that stem cells maintain a transcriptional program to reduce OXPHOS levels, which minimizes ROS, while simultaneously expressing genes involved in the antioxidant response to maintain quiescence and self-renewal potential.
Another important intracellular molecule controlling cellular redox status is nicotinamide adenine dinucleotide (NAD). NAD serves as an electron transfer molecule for oxidative and reductive reactions in the cell, cycling between NAD+ (oxidized species—accepts electrons) and NADH (reduced species—donates electrons). The NAD+/NADH ratio represents an important measure of the redox environment in cells, which is typically between 3 and 10 in mammals, indicating an oxidative environment (Lin and Guarente, 2003). In stem cells, NAD+ must be constantly regenerated to supply the GAPDH reaction and maintain glycolytic flux; this occurs during the conversion of pyruvate to lactate, when NADH is used as a coenzyme and converted back to NAD+ (Fig. 2; Vacanti and Metallo, 2013). In addition to its role in glycolysis, high levels of NAD+ activate Sirtuin 1 (SIRT1), a histone deacetylase involved in transcriptional regulation of inflammatory pathways and mitochondrial biogenesis (Fig. 1; Haigis and Sinclair, 2010). SIRT1 maintains HSC pools via elimination of ROS, FOXO activation, and p53 inhibition (Matsui et al., 2012). As stated above, redox regulation in HSCs is crucial to their maintenance of quiescence and homeostasis. An interesting question that remains to be answered is what NAD+/NADH ratio exists in HSCs, and how this ratio changes in response to glycolytic reprogramming to maintain appropriate redox balance.
Lipid metabolism and fatty acid oxidation.
Lipids are important to all cells for aspects ranging from structural support to energy storage and signaling, and their specific role in stem cell biology is only beginning to be elucidated. De novo lipogenesis through the enzyme fatty acid synthase (FASN) has been recently shown to control adult neurogenesis (Fig. 2; Knobloch et al., 2013). The authors demonstrated that in neural stem cells FASN is highly active, while FASN deletion impairs mouse adult neurogenesis. They identified SPOT14 as a modulator of lipogenesis and showed that it decreases malonyl-CoA levels, which lower substrate availability for FASN to synthesize lipids (Knobloch et al., 2013). Neural stem cells require a high level of lipid synthesis for proper proliferation (Knobloch et al., 2013), but the breakdown of fatty acids is also important in stem cell maintenance.
For example, Ito et al. (2012) identified a fatty acid oxidation (FAO) pathway whose ablation resulted in a loss of HSC maintenance, whereas agonists of the FAO pathway improved HSC maintenance. FAO is operational in LSK stem cells but undetected in differentiated cells, and inhibition of mitochondrial FAO reduces both HSC numbers and reconstitution capacity of bone marrow cells. The authors find that this phenotype is the result of a loss of asymmetric HSC division in favor of symmetric cell divisions, leading to stem cell exhaustion (Ito et al., 2012). The amount of unsaturated fatty acids, the extent of de novo lipogenesis, and the efficiency of FAO are all implicated in HSC maintenance and homeostasis (Ito et al., 2012).
In ESCs, unsaturated lipids are important for homeostasis. Using a mass spectrometry–based metabolomics approach, Yanes et al. (2010) found that ESCs have a different lipid profile from differentiated cells and are characterized by an accumulation of unsaturated lipids and fatty acids whose levels decrease upon differentiation. The authors found that inhibiting the eicosanoid pathway, which is involved in fatty acid oxidation, promoted pluripotency in ESCs and maintained the levels of unsaturated fatty acids. Because lipids are involved in diverse signaling pathways, it will be important to delineate the effects of specific lipids on stem cell metabolism and identify a therapeutic window to target fatty acid homeostasis such that modulation provides beneficial effects on the stem cell pool without negative consequences.
Threonine metabolism.
Threonine is an essential amino acid subjected to multiple post-translational modifications when incorporated into proteins, such as phosphorylation and O-linked glycosylation. Using an LC-MS/MS–based metabolomics approach, Wang et al. (2009) profiled common metabolites in mouse ESCs and found that threonine levels increased with time as the ESCs underwent differentiation; this metabolic profile was associated with an increase in one-carbon metabolism for purine biosynthesis. Additionally, mRNA levels of the catabolic enzyme threonine dehydrogenase (TDH) were 1,000-fold higher in ESCs versus differentiated cells. TDH is localized in the mitochondria and catalyzes the two-step breakdown of threonine into glycine and acteyl-CoA, with glycine feeding the one-carbon metabolism of purine nucleotide biosynthesis and acetyl-CoA entering the TCA cycle (Fig. 2). Using systematic depletion of all 20 amino acids in tissue culture media, Wang et al. (2009) found that mouse ESCs are critically dependent on threonine, with no dependence on other amino acids, due to an impediment in thymidine biosynthesis when this amino acid is absent. Threonine-depleted media also causes a reduction in self-renewal gene expression in mouse ESCs, which rely on threonine to regulate the G1/S transition of the cell cycle (Ryu and Han, 2011). Moreover, purine nucleotides accumulate at lower levels in ESCs than somatic cells (Panopoulos et al., 2012). These studies demonstrate that in ESCs purine nucleotides are essential for homeostasis. Recently, Shyh-Chang et al. (2013) further elucidated the molecular details for the threonine dependence in ESCs. The authors found that threonine and S-adenosylmethionine (SAM) metabolism are coupled in pluripotent cells, and decreased threonine levels lead to a simultaneous decrease in SAM, the principle methyl-donating molecule. Decreased SAM levels manifest as a reduction in histone methylation, causing slowed growth and decreased differentiation (Shyh-Chang et al., 2013).
Exploiting the fact that TDH uses NAD+ as a cofactor and generates NADH, which is autofluorescent, Alexander et al. (2011) screened 200,000 molecules in an in vitro fluorescent assay for TDH inhibitors. The authors identified a class of compounds called “quinazolinecarboxamides” that inhibit TDH, impair mouse ESC cell growth, and have no effect on nonstem cells. Although these studies present interesting possibilities for the therapeutic modulation of ESCs, several questions remain. In addition to producing glycine used in purine biosynthesis, TDH also produces acetyl-CoA for the TCA cycle. With an abundance of mitochondrial acetyl-CoA through dramatically increased TDH levels (Wang et al., 2009), is the level of OXPHOS or TCA cycle flux increased? A partial answer to this question appears to be that levels of the TCA intermediate succinate are unchanged during ESC differentiation (Wang et al., 2009). Therefore, the TCA flux is not overwhelmingly increased in this context. The most important question, however, is the therapeutic relevance of TDH. It is expressed in all metozoans, but in humans TDH contains three mutations that give rise to variable splice mutants (Edgar, 2002). In 20 individuals, mutant TDH was confirmed by sequencing analysis, predicting that it is inactive in most humans, although this conclusion is based on a small sample size and no TDH functional assay (Edgar, 2002). These questions need to be investigated further to determine if TDH is a viable target for stem cell modulation in humans.
IDH mutants.
Isocitrate dehydrogenase (IDH) converts isocitrate to 2-oxoglutarate (α-ketoglutarate) in the TCA cycle. IDH mutants result in a gain-of-function enzymatic activity that converts 2-oxoglutarate to 2-hydroxyglutarate (2HG), a putative “oncometabolite” (Fig. 2; Ward et al., 2010, 2012). 2HG inhibits 2-oxoglutarate–dependent enzymes, including histone demethylases, resulting in a block to cell differentiation of nontransformed cells (Lu et al., 2012). Introduction of either mutant IDH or cell-permeable 2HG represses the expression of adipogenic differentiation genes CEBPα, PPARγ, and adiponectin, due to an inhibition of histone demethylation (Lu et al., 2012). The effects of 2HG are enantiomer specific, as (R)-2HG blocks differentiation in hematopoietic cells, whereas (S)-2HG is unable to do so despite being a more potent inhibitor of epigenetic enzymes like the 5′-methylcytosine hydroxylase TET2 (Losman et al., 2013), which controls DNA methylation via hydroxylation of methylcytosine.
Sasaki et al. (2012) generated a hematopoietic cell-specific IDH R132H mutant (the most common form) knock-in mouse strain and demonstrated increased early hematopoietic progenitor cell numbers in this setting. These mice were characterized by anemia and extramedullary hematopoiesis, suggestive of a dysfunctional bone marrow niche. Importantly, myeloid lineage cells expressing mutant IDH exhibit hypermethylated histones and changes in DNA methylation that resemble IDH mutant acute myeloid leukemia (AML; Sasaki et al., 2012).
IDH1 and IDH2 mutants occur in ∼17% of newly diagnosed AML, and IDH1 mutation is associated with decreased survival (Abbas et al., 2010). The available evidence suggests that IDH mutations producing 2HG may induce a block in differentiation to promote tumorigenesis, likely through epigenetic mechanisms (Wang et al., 2013). An inhibitor that is selective for mutant IDH2 over wild-type IDH2 was recently reported, named AGI-6780 (Wang et al., 2013). This inhibitor demonstrates remarkable selectivity exclusively toward IDH2 R140Q and has a low nanomolar IC50 when measuring 2HG formation in mutant IDH2-expressing cell lines. Importantly, using primary human AML cells ex vivo that contain the IDH2 mutation AGI-6780 showed a dose-dependent reduction in 2HG levels and increased differentiation (Wang et al., 2013). Because preleukemic HSCs (in which serial mutations have occurred in self-renewing cells) can lead to AML, targeting leukemic “drivers” should provide additional benefits for sustained remission by depleting the quiescent HSC pool harboring IDH mutations (Jan et al., 2012).
Conclusions
Stem cells have distinct metabolic requirements from their differentiated progeny. For instance, HSC and ESC localization in a hypoxic environment renders them dependent upon anaerobic glycolysis to produce ATP and minimize oxidative stress from OXPHOS. The metabolic adaptation of stem cells from an oxidative phenotype to one dependent upon glycolytic metabolism represents specific advantages that promote stem cell homeostasis. At the same time, stem cells remain metabolically plastic, in that they are able to rapidly change to an oxidative phenotype during differentiation to support the large amounts of ATP needed for this process. Metabolomic studies have begun to pave the way for an improved understanding of specific metabolites that modulate stem cell homeostasis. For example, ESCs contain a distinctly different lipid profile (increased unsaturated lipids) than differentiated cells, and the amino acid threonine is crucial to their survival to promote purine biosynthesis. Because changes in cellular metabolism precede changes in stem-ness, small perturbations in a particular pathway or metabolite, such as threonine, can have drastic consequences on the ability of the stem cell to self-renew and differentiate. Elucidating these molecular details will be crucial to develop advanced therapies for metabolic diseases and for using stem cells in applications such as regenerative medicine and tissue engineering.
Acknowledgments
The authors would like to thank Joanna Balcerek and Bryan Krock for their insightful and valuable discussions.
J.D. Ochocki is supported by a National Cancer Institute Cancer Pharmacology grant (R25 CA101871), and M.C. Simon is an investigator of the Howard Hughes Medical Institute.
Footnotes
Abbreviations used in this paper:
- 2HG
- 2-hydroxyglutarate
- AMPK
- AMP-activated protein kinase
- E
- embryonic day
- ESC
- embryonic stem cell
- FOXO
- forkhead box O
- HSC
- hematopoietic stem cell
- IDH
- isocitrate dehydrogenase
- mTOR
- mammalian target of rapamycin
- NAD
- nicotinamide adenine dinucleotide
- OXPHOS
- oxidative phosphorylation
- PDH
- pyruvate dehydrogenase
- PDK
- pyruvate dehydrogenase kinase
- ROS
- reactive oxygen species
- TDH
- threonine dehydrogenase
References
- Abbas S., Lugthart S., Kavelaars F.G., Schelen A., Koenders J.E., Zeilemaker A., van Putten W.J., Rijneveld A.W., Löwenberg B., Valk P.J. 2010. Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: prevalence and prognostic value. Blood. 116:2122–2126 10.1182/blood-2009-11-250878 [DOI] [PubMed] [Google Scholar]
- Alexander P.B., Wang J., McKnight S.L. 2011. Targeted killing of a mammalian cell based upon its specialized metabolic state. Proc. Natl. Acad. Sci. USA. 108:15828–15833 10.1073/pnas.1111312108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomare D.A., Khaled A.R. 2012. Homeostasis and the importance for a balance between AKT/mTOR activity and intracellular signaling. Curr. Med. Chem. 19:3748–3762 10.2174/092986712801661130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban R.S. 1990. Regulation of oxidative phosphorylation in the mammalian cell. Am. J. Physiol. 258:C377–C389 [DOI] [PubMed] [Google Scholar]
- Becker K.A., Ghule P.N., Therrien J.A., Lian J.B., Stein J.L., van Wijnen A.J., Stein G.S. 2006. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J. Cell. Physiol. 209:883–893 10.1002/jcp.20776 [DOI] [PubMed] [Google Scholar]
- Bernhardt W.M., Gottmann U., Doyon F., Buchholz B., Campean V., Schödel J., Reisenbuechler A., Klaus S., Arend M., Flippin L., et al. 2009. Donor treatment with a PHD-inhibitor activating HIFs prevents graft injury and prolongs survival in an allogenic kidney transplant model. Proc. Natl. Acad. Sci. USA. 106:21276–21281 10.1073/pnas.0903978106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen G.A., Sun D., Jeong M., Luo M., Jelinek J., Berg J.S., Bock C., Vasanthakumar A., Gu H., Xi Y., et al. 2012. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat. Genet. 44:23–31 10.1038/ng.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari P., Ye Z., Jang Y.Y. 2012. Roles of reactive oxygen species in the fate of stem cells. Antioxid. Redox Signal. Nov 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Liu Y., Liu R., Ikenoue T., Guan K.L., Liu Y., Zheng P. 2008. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J. Exp. Med. 205:2397–2408 10.1084/jem.20081297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Liu Y., Liu Y., Zheng P. 2009. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci. Signal. 2:ra75 10.1126/scisignal.2000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Liu Y., Liu Y., Zheng P. 2010. Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice. J. Clin. Invest. 120:4091–4101 10.1172/JCI43873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.M., Kwon S., Pak Y.K., Seol H.W., Choi Y.M., Park J., Park K.S., Lee H.K. 2006. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem. Biophys. Res. Commun. 348:1472–1478 10.1016/j.bbrc.2006.08.020 [DOI] [PubMed] [Google Scholar]
- Contreras C.M., Gurumurthy S., Haynie J.M., Shirley L.J., Akbay E.A., Wingo S.N., Schorge J.O., Broaddus R.R., Wong K.K., Bardeesy N., Castrillon D.H. 2008. Loss of Lkb1 provokes highly invasive endometrial adenocarcinomas. Cancer Res. 68:759–766 10.1158/0008-5472.CAN-07-5014 [DOI] [PubMed] [Google Scholar]
- Covello K.L., Kehler J., Yu H., Gordan J.D., Arsham A.M., Hu C.J., Labosky P.A., Simon M.C., Keith B. 2006. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 20:557–570 10.1101/gad.1399906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehn M., Cho R.W., Lobo N.A., Kalisky T., Dorie M.J., Kulp A.N., Qian D., Lam J.S., Ailles L.E., Wong M., et al. 2009. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 458:780–783 10.1038/nature07733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easley C.A.t., Ben-Yehudah A., Redinger C.J., Oliver S.L., Varum S.T., Eisinger V.M., Carlisle D.L., Donovan P.J., Schatten G.P. 2010. mTOR-mediated activation of p70 S6K induces differentiation of pluripotent human embryonic stem cells. Cell Reprogram. 12:263–273 10.1089/cell.2010.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar A.J. 2002. The human L-threonine 3-dehydrogenase gene is an expressed pseudogene. BMC Genet. 3:18 10.1186/1471-2156-3-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson P., Rehn M., Hammar P., Larsson P., Sirenko O., Flippin L.A., Cammenga J., Jönsson J.I. 2010. Hypoxia mediates low cell-cycle activity and increases the proportion of long-term-reconstituting hematopoietic stem cells during in vitro culture. Exp. Hematol. 38:301–310: e2 10.1016/j.exphem.2010.01.005 [DOI] [PubMed] [Google Scholar]
- Ellingson W.J., Chesser D.G., Winder W.W. 2007. Effects of 3-phosphoglycerate and other metabolites on the activation of AMP-activated protein kinase by LKB1-STRAD-MO25. Am. J. Physiol. Endocrinol. Metab. 292:E400–E407 10.1152/ajpendo.00322.2006 [DOI] [PubMed] [Google Scholar]
- Facucho-Oliveira J.M., St John J.C. 2009. The relationship between pluripotency and mitochondrial DNA proliferation during early embryo development and embryonic stem cell differentiation. Stem Cell Rev. 5:140–158 10.1007/s12015-009-9058-0 [DOI] [PubMed] [Google Scholar]
- Firth J.D., Ebert B.L., Ratcliffe P.J. 1995. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J. Biol. Chem. 270:21021–21027 10.1074/jbc.270.49.29083 [DOI] [PubMed] [Google Scholar]
- Folmes C.D., Nelson T.J., Martinez-Fernandez A., Arrell D.K., Lindor J.Z., Dzeja P.P., Ikeda Y., Perez-Terzic C., Terzic A. 2011. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 14:264–271 10.1016/j.cmet.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forristal C.E., Christensen D.R., Chinnery F.E., Petruzzelli R., Parry K.L., Sanchez-Elsner T., Houghton F.D. 2013. Environmental oxygen tension regulates the energy metabolism and self-renewal of human embryonic stem cells. PLoS ONE. 8:e62507 10.1371/journal.pone.0062507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan B., Hu J., Jiang S., Liu Y., Sahin E., Zhuang L., Fletcher-Sananikone E., Colla S., Wang Y.A., Chin L., Depinho R.A. 2010. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature. 468:701–704 10.1038/nature09595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekas C., Dieterlen-Lièvre F., Orkin S.H., Mikkola H.K. 2005. The placenta is a niche for hematopoietic stem cells. Dev. Cell. 8:365–375 10.1016/j.devcel.2004.12.016 [DOI] [PubMed] [Google Scholar]
- Gekas C., Rhodes K.E., Van Handel B., Chhabra A., Ueno M., Mikkola H.K. 2010. Hematopoietic stem cell development in the placenta. Int. J. Dev. Biol. 54:1089–1098 10.1387/ijdb.103070cg [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnaiger E. 2001. Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir. Physiol. 128:277–297 10.1016/S0034-5687(01)00307-3 [DOI] [PubMed] [Google Scholar]
- Gordan J.D., Thompson C.B., Simon M.C. 2007. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 12:108–113 10.1016/j.ccr.2007.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D.N., van den Heuvel A.P., Birnbaum M.J. 2008. The role of FoxO in the regulation of metabolism. Oncogene. 27:2320–2336 10.1038/onc.2008.25 [DOI] [PubMed] [Google Scholar]
- Guppy M., Greiner E., Brand K. 1993. The role of the Crabtree effect and an endogenous fuel in the energy metabolism of resting and proliferating thymocytes. Eur. J. Biochem. 212:95–99 10.1111/j.1432-1033.1993.tb17637.x [DOI] [PubMed] [Google Scholar]
- Gurumurthy S., Xie S.Z., Alagesan B., Kim J., Yusuf R.Z., Saez B., Tzatsos A., Ozsolak F., Milos P., Ferrari F., et al. 2010. The Lkb1 metabolic sensor maintains haematopoietic stem cell survival. Nature. 468:659–663 10.1038/nature09572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis M.C., Sinclair D.A. 2010. Mammalian sirtuins: biological insights and disease relevance. Annu. Rev. Pathol. 5:253–295 10.1146/annurev.pathol.4.110807.092250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S.M., Hess Michelini R., Doedens A.L., Goldrath A.W., Stone E.L. 2012. FOXO transcription factors throughout T cell biology. Nat. Rev. Immunol. 12:649–661 10.1038/nri3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki A., Markie D., Tomlinson I., Avizienyte E., Roth S., Loukola A., Bignell G., Warren W., Aminoff M., Höglund P., et al. 1998. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 391:184–187 10.1038/34432 [DOI] [PubMed] [Google Scholar]
- Ho P.J., Yen M.L., Tang B.C., Chen C.T., Yen B.L. 2013. H2O2 accumulation mediates differentiation capacity alteration, but not proliferative decline, in senescent human fetal mesenchymal stem cells. Antioxid. Redox Signal. 18:1895–1905 10.1089/ars.2012.4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y.C., Fuchs E. 2012. A family business: stem cell progeny join the niche to regulate homeostasis. Nat. Rev. Mol. Cell Biol. 13:103–114 10.1038/nrm3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Tindall D.J. 2011. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim. Biophys. Acta. 1813:1961–1964 10.1016/j.bbamcr.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K., Li Y., Zhu T., Wu J., Guan K.L. 2002. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4:648–657 10.1038/ncb839 [DOI] [PubMed] [Google Scholar]
- Ito K., Hirao A., Arai F., Takubo K., Matsuoka S., Miyamoto K., Ohmura M., Naka K., Hosokawa K., Ikeda Y., Suda T. 2006. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 12:446–451 10.1038/nm1388 [DOI] [PubMed] [Google Scholar]
- Ito K., Carracedo A., Weiss D., Arai F., Ala U., Avigan D.E., Schafer Z.T., Evans R.M., Suda T., Lee C.H., Pandolfi P.P. 2012. A PML–PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat. Med. 18:1350–1358 10.1038/nm.2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan M., Snyder T.M., Corces-Zimmerman M.R., Vyas P., Weissman I.L., Quake S.R., Majeti R. 2012. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci. Transl. Med. 4:ra118 10.1126/scitranslmed.3004315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y.Y., Sharkis S.J. 2007. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 110:3056–3063 10.1182/blood-2007-05-087759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne D.E., Reimann H., Nezu J., Friedel W., Loff S., Jeschke R., Müller O., Back W., Zimmer M. 1998. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 18:38–43 10.1038/ng0198-38 [DOI] [PubMed] [Google Scholar]
- Ji H., Ehrlich L.I., Seita J., Murakami P., Doi A., Lindau P., Lee H., Aryee M.J., Irizarry R.A., Kim K., et al. 2010. Comprehensive methylome map of lineage commitment from haematopoietic progenitors. Nature. 467:338–342 10.1038/nature09367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaitzidis D., Sykes S.M., Wang Z., Punt N., Tang Y., Ragu C., Sinha A.U., Lane S.W., Souza A.L., Clish C.B., et al. 2012. mTOR complex 1 plays critical roles in hematopoiesis and Pten-loss-evoked leukemogenesis. Cell Stem Cell. 11:429–439 10.1016/j.stem.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.W., Tchernyshyov I., Semenza G.L., Dang C.V. 2006. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3:177–185 10.1016/j.cmet.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Kim J., Guan K.-L. 2011. Amino acid signaling in TOR activation. Annu. Rev. Biochem. 80:1001–1032 10.1146/annurev-biochem-062209-094414 [DOI] [PubMed] [Google Scholar]
- Knobloch M., Braun S.M., Zurkirchen L., von Schoultz C., Zamboni N., Araúzo-Bravo M.J., Kovacs W.J., Karalay O., Suter U., Machado R.A., et al. 2013. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 493:226–230 10.1038/nature11689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops G.J., Dansen T.B., Polderman P.E., Saarloos I., Wirtz K.W., Coffer P.J., Huang T.T., Bos J.L., Medema R.H., Burgering B.M. 2002. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 419:316–321 10.1038/nature01036 [DOI] [PubMed] [Google Scholar]
- Krock B., Skuli N., Simon M.C. 2011. The tumor suppressor LKB1 emerges as a critical factor in hematopoietic stem cell biology. Cell Metab. 13:8–10 10.1016/j.cmet.2010.12.015 [DOI] [PubMed] [Google Scholar]
- Lee I., Hendrix A., Kim J., Yoshimoto J., You Y.J. 2012. Metabolic rate regulates L1 longevity in C. elegans. PLoS ONE. 7:e44720 10.1371/journal.pone.0044720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y., Nakada D., Yilmaz O.H., Tothova Z., Joseph N.M., Lim M.S., Gilliland D.G., Morrison S.J. 2010. mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell. 7:593–605 10.1016/j.stem.2010.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y., Kim Y.R., Park J., Kim S. 2012. Inositol polyphosphate multikinase signaling in the regulation of metabolism. Ann. N. Y. Acad. Sci. 1271:68–74 10.1111/j.1749-6632.2012.06725.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.E., Simon M.C. 2012. From stem cells to cancer stem cells: HIF takes the stage. Curr. Opin. Cell Biol. 24:232–235 10.1016/j.ceb.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Li T.S., Marbán E. 2010. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells. 28:1178–1185 10.1002/stem.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xu S., Mihaylova M.M., Zheng B., Hou X., Jiang B., Park O., Luo Z., Lefai E., Shyy J.Y., et al. 2011. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 13:376–388 10.1016/j.cmet.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.-J., Guarente L. 2003. Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr. Opin. Cell Biol. 15:241–246 10.1016/S0955-0674(03)00006-1 [DOI] [PubMed] [Google Scholar]
- Losman J.A., Looper R.E., Koivunen P., Lee S., Schneider R.K., McMahon C., Cowley G.S., Root D.E., Ebert B.L., Kaelin W.G., Jr 2013. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science. 339:1621–1625 10.1126/science.1231677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Ward P.S., Kapoor G.S., Rohle D., Turcan S., Abdel-Wahab O., Edwards C.R., Khanin R., Figueroa M.E., Melnick A., et al. 2012. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 483:474–478 10.1038/nature10860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lützner N., De-Castro Arce J., Rösl F. 2012. Gene expression of the tumour suppressor LKB1 is mediated by Sp1, NF-Y and FOXO transcription factors. PLoS ONE. 7:e32590 10.1371/journal.pone.0032590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee J.A., Ikenoue T., Nakada D., Lee J.Y., Guan K.L., Morrison S.J. 2012. Temporal changes in PTEN and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell. 11:415–428 10.1016/j.stem.2012.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar A.J., Skuli N., Mesquita R.C., Kim M.N., Yodh A.G., Nguyen-McCarty M., Simon M.C. 2012. O(2) regulates skeletal muscle progenitor differentiation through phosphatidylinositol 3-kinase/AKT signaling. Mol. Cell. Biol. 32:36–49 10.1128/MCB.05857-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K., Ezoe S., Oritani K., Shibata M., Tokunaga M., Fujita N., Tanimura A., Sudo T., Tanaka H., McBurney M.W., et al. 2012. NAD-dependent histone deacetylase, SIRT1, plays essential roles in the maintenance of hematopoietic stem cells. Biochem. Biophys. Res. Commun. 418:811–817 10.1016/j.bbrc.2012.01.109 [DOI] [PubMed] [Google Scholar]
- Mazumdar J., Dondeti V., Simon M.C. 2009. Hypoxia-inducible factors in stem cells and cancer. J. Cell. Mol. Med. 13:4319–4328 10.1111/j.1582-4934.2009.00963.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J., O’Brien W.T., Johnson R.S., LaManna J.C., Chavez J.C., Klein P.S., Simon M.C. 2010. O2 regulates stem cells through Wnt/β-catenin signalling. Nat. Cell Biol. 12:1007–1013 10.1038/ncb2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Araki K.Y., Naka K., Arai F., Takubo K., Yamazaki S., Matsuoka S., Miyamoto T., Ito K., Ohmura M., et al. 2007. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 1:101–112 10.1016/j.stem.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Murakami M., Ichisaka T., Maeda M., Oshiro N., Hara K., Edenhofer F., Kiyama H., Yonezawa K., Yamanaka S. 2004. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol. Cell. Biol. 24:6710–6718 10.1128/MCB.24.15.6710-6718.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada D., Saunders T.L., Morrison S.J. 2010. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 468:653–658 10.1038/nature09571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh W.J., Jacinto E. 2011. mTOR complex 2 signaling and functions. Cell Cycle. 10:2305–2316 10.4161/cc.10.14.16586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panopoulos A.D., Yanes O., Ruiz S., Kida Y.S., Diep D., Tautenhahn R., Herrerías A., Batchelder E.M., Plongthongkum N., Lutz M., et al. 2012. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 22:168–177 10.1038/cr.2011.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson H.B., McCarthy A., Collins C.M., Ashworth A., Clarke A.R. 2008. Lkb1 deficiency causes prostate neoplasia in the mouse. Cancer Res. 68:2223–2232 10.1158/0008-5472.CAN-07-5169 [DOI] [PubMed] [Google Scholar]
- Peserico A., Chiacchiera F., Grossi V., Matrone A., Latorre D., Simonatto M., Fusella A., Ryall J.G., Finley L.W., Haigis M.C., et al. 2013. A novel AMPK-dependent FoxO3A-SIRT3 intramitochondrial complex sensing glucose levels. Cell. Mol. Life Sci. 70:2015–2029 10.1007/s00018-012-1244-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigione A., Fauler B., Lurz R., Lehrach H., Adjaye J. 2010. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cells. 28:721–733 10.1002/stem.404 [DOI] [PubMed] [Google Scholar]
- Ryu J.M., Han H.J. 2011. L-threonine regulates G1/S phase transition of mouse embryonic stem cells via PI3K/Akt, MAPKs, and mTORC pathways. J. Biol. Chem. 286:23667–23678 10.1074/jbc.M110.216283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Knobbe C.B., Munger J.C., Lind E.F., Brenner D., Brüstle A., Harris I.S., Holmes R., Wakeham A., Haight J., et al. 2012. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 488:656–659 10.1038/nature11323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyh-Chang N., Locasale J.W., Lyssiotis C.A., Zheng Y., Teo R.Y., Ratanasirintrawoot S., Zhang J., Onder T., Unternaehrer J.J., Zhu H., et al. 2013. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science. 339:222–226 10.1126/science.1226603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek T., Kocabas F., Zheng J., Deberardinis R.J., Mahmoud A.I., Olson E.N., Schneider J.W., Zhang C.C., Sadek H.A. 2010. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 7:380–390 10.1016/j.stem.2010.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C., Foley A., Partridge L. 2012. Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PLoS ONE. 7:e47699 10.1371/journal.pone.0047699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenesen D., Suh J.M., Seo J., Yu K., Lee K.S., Kim J.S., Min K.J., Graff J.M. 2013. Adenosine nucleotide biosynthesis and AMPK regulate adult life span and mediate the longevity benefit of caloric restriction in flies. Cell Metab. 17:101–112 10.1016/j.cmet.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojkovic P., Lako M., Stewart R., Przyborski S., Armstrong L., Evans J., Murdoch A., Strachan T., Stojkovic M. 2005. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells. 23:306–314 10.1634/stemcells.2004-0137 [DOI] [PubMed] [Google Scholar]
- Takubo K., Goda N., Yamada W., Iriuchishima H., Ikeda E., Kubota Y., Shima H., Johnson R.S., Hirao A., Suematsu M., Suda T. 2010. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 7:391–402 10.1016/j.stem.2010.06.020 [DOI] [PubMed] [Google Scholar]
- Takubo K., Nagamatsu G., Kobayashi C.I., Nakamura-Ishizu A., Kobayashi H., Ikeda E., Goda N., Rahimi Y., Johnson R.S., Soga T., et al. 2013. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 12:49–61 10.1016/j.stem.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. 1998. Embryonic stem cell lines derived from human blastocysts. Science. 282:1145–1147 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- Tothova Z., Kollipara R., Huntly B.J., Lee B.H., Castrillon D.H., Cullen D.E., McDowell E.P., Lazo-Kallanian S., Williams I.R., Sears C., et al. 2007. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 128:325–339 10.1016/j.cell.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Vacanti N.M., Metallo C.M. 2013. Exploring metabolic pathways that contribute to the stem cell phenotype. Biochim. Biophys. Acta. 1830:2361–2369 10.1016/j.bbagen.2012.08.007 [DOI] [PubMed] [Google Scholar]
- Vander Heiden M.G., Cantley L.C., Thompson C.B. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 324:1029–1033 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varum S., Rodrigues A.S., Moura M.B., Momcilovic O., Easley C.A., IV, Ramalho-Santos J., Van Houten B., Schatten G. 2011. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE. 6:e20914 10.1371/journal.pone.0020914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazin T., Freed W.J. 2010. Human embryonic stem cells: derivation, culture, and differentiation: a review. Restor. Neurol. Neurosci. 28:589–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Travins J., DeLaBarre B., Penard-Lacronique V., Schalm S., Hansen E., Straley K., Kernytsky A., Liu W., Gliser C., et al. 2013. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 340:622–626 10.1126/science.1234769 [DOI] [PubMed] [Google Scholar]
- Wang G., Zhang H., Zhao Y., Li J., Cai J., Wang P., Meng S., Feng J., Miao C., Ding M., et al. 2005. Noggin and bFGF cooperate to maintain the pluripotency of human embryonic stem cells in the absence of feeder layers. Biochem. Biophys. Res. Commun. 330:934–942 10.1016/j.bbrc.2005.03.058 [DOI] [PubMed] [Google Scholar]
- Wang J., Alexander P., Wu L., Hammer R., Cleaver O., McKnight S.L. 2009. Dependence of mouse embryonic stem cells on threonine catabolism. Science. 325:435–439 10.1126/science.1173288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P.S., Patel J., Wise D.R., Abdel-Wahab O., Bennett B.D., Coller H.A., Cross J.R., Fantin V.R., Hedvat C.V., Perl A.E., et al. 2010. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 17:225–234 10.1016/j.ccr.2010.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P.S., Cross J.R., Lu C., Weigert O., Abel-Wahab O., Levine R.L., Weinstock D.M., Sharp K.A., Thompson C.B. 2012. Identification of additional IDH mutations associated with oncometabolite R(-)-2-hydroxyglutarate production. Oncogene. 31:2491–2498 10.1038/onc.2011.416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr M.R., Binnewies M., Flach J., Reynaud D., Garg T., Malhotra R., Debnath J., Passegué E. 2013. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature. 494:323–327 10.1038/nature11895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton W.W., Chandel N.S. 2011. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am. J. Physiol. Cell Physiol. 300:C385–C393 10.1152/ajpcell.00485.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder W.W., Hardie D.G. 1999. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am. J. Physiol. 277:E1–E10 [DOI] [PubMed] [Google Scholar]
- Yalcin S., Zhang X., Luciano J.P., Mungamuri S.K., Marinkovic D., Vercherat C., Sarkar A., Grisotto M., Taneja R., Ghaffari S. 2008. Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J. Biol. Chem. 283:25692–25705 10.1074/jbc.M800517200 [DOI] [PubMed] [Google Scholar]
- Yalcin S., Marinkovic D., Mungamuri S.K., Zhang X., Tong W., Sellers R., Ghaffari S. 2010. ROS-mediated amplification of AKT/mTOR signalling pathway leads to myeloproliferative syndrome in Foxo3(-/-) mice. EMBO J. 29:4118–4131 10.1038/emboj.2010.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanes O., Clark J., Wong D.M., Patti G.J., Sanchez-Ruiz A., Benton H.P., Trauger S.A., Desponts C., Ding S., Siuzdak G. 2010. Metabolic oxidation regulates embryonic stem cell differentiation. Nat. Chem. Biol. 6:411–417 10.1038/nchembio.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies J.L., Manning B.D. 2011. Transcriptional control of cellular metabolism by mTOR signaling. Cancer Res. 71:2815–2820 10.1158/0008-5472.CAN-10-4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O.H., Valdez R., Theisen B.K., Guo W., Ferguson D.O., Wu H., Morrison S.J. 2006. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 441:475–482 10.1038/nature04703 [DOI] [PubMed] [Google Scholar]
- Yilmaz O.H., Katajisto P., Lamming D.W., Gültekin Y., Bauer-Rowe K.E., Sengupta S., Birsoy K., Dursun A., Yilmaz V.O., Selig M., et al. 2012. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 486:490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W.M., Liu X., Shen J., Jovanovic O., Pohl E.E., Gerson S.L., Finkel T., Broxmeyer H.E., Qu C.K. 2013. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell. 12:62–74 10.1016/j.stem.2012.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Grindley J.C., Yin T., Jayasinghe S., He X.C., Ross J.T., Haug J.S., Rupp D., Porter-Westpfahl K.S., Wiedemann L.M., et al. 2006. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 441:518–522 10.1038/nature04747 [DOI] [PubMed] [Google Scholar]
- Zhang J., Khvorostov I., Hong J.S., Oktay Y., Vergnes L., Nuebel E., Wahjudi P.N., Setoguchi K., Wang G., Do A., et al. 2011. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 30:4860–4873 10.1038/emboj.2011.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Su P., Wang L., Chen J., Zimmermann M., Genbacev O., Afonja O., Horne M.C., Tanaka T., Duan E., et al. 2009. mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 106:7840–7845 10.1073/pnas.0901854106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Li D., Wang F. 2012. Assessing the function of mTOR in human embryonic stem cells. Methods Mol. Biol. 821:361–372 10.1007/978-1-61779-430-8_23 [DOI] [PubMed] [Google Scholar]