Abstract
Synapse formation is a highly regulated process that requires the coordination of many cell biological events. Decades of research have identified a long list of molecular components involved in assembling a functioning synapse. Yet how the various steps, from transporting synaptic components to adhering synaptic partners and assembling the synaptic structure, are regulated and precisely executed during development and maintenance is still unclear. With the improvement of imaging and molecular tools, recent work in vertebrate and invertebrate systems has provided important insight into various aspects of presynaptic development, maintenance, and trans-synaptic signals, thereby increasing our understanding of how extrinsic organizers and intracellular mechanisms contribute to presynapse formation.
Chemical synapses are highly specialized, asymmetric intercellular junction structures that are the basic units of neuronal communication. Proper development of synapses determines appropriate connectivity for the assembly of functional neuronal circuits. Synaptic circuits arise during development through a series of intricate steps (Waites et al., 2005; McAllister, 2007; Jin and Garner, 2008). First, spatiotemporal cues guide axons through complex cellular environments to contact their appropriate postsynaptic targets. At their destination, synapse formation is specified and initiated through adhesive interactions between synaptic partner cells or by local diffusible signaling molecules. Stabilization of intercellular contacts and assembly into functional synapses involves cytoskeletal rearrangements, aggregation, and insertion of pre- and postsynaptic components at nascent synaptic sites. Maturation and modulation of these newly formed synapses can then occur by altering the organization or composition of synaptic proteins and post-translational modifications to achieve its required physiological responsiveness (Budnik, 1996; Lee and Sheng, 2000). Conversely, retraction of contacts and elimination of inappropriate synaptic proteins help to refine the neuronal circuitry (Goda and Davis, 2003; Sanes and Yamagata, 2009).
Over the last decade, new insights have furthered our understanding of synapse development through the identification of new molecular players and by advanced imaging technology that has allowed for high-resolution inspection of the dynamics and relative positions of synaptic proteins. This review will highlight recent results on the development of presynaptic specializations, and the roles of trans-synaptic organizers, intracellular synaptic proteins, and the cytoskeleton during the formation and maintenance of synapses.
Axonal transport of synaptic vesicle and active zone proteins
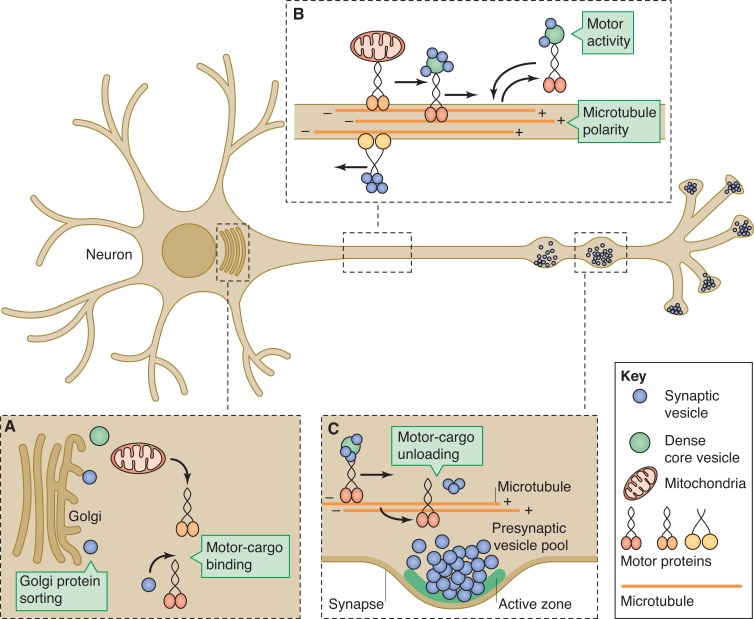
After cell fate determination and morphogenesis, neurons continue to differentiate by entering the phase of synapse formation. Most synaptic material required for this process is synthesized in the cell body of neurons and transported to synapses by microtubule (MT)-based molecular motors (Fig. 1). MTs are intrinsically polarized filaments with a plus and a minus end (Fig. 1 B). MT-based molecular motors use this polarity to transport cargoes to specific cellular locations. Examination of MTs by electron microscopy in dissociated cultured neurons showed that the organizations of MTs is different in axon and dendrite (Baas et al., 1988, 2006). In axons, all microtubules have their minus ends oriented toward the cell body and their plus ends extend distally. On the contrary, the MT polarity in dendrites is mixed. Recent studies tracking the movement of end-binding MT-capping proteins confirmed these results in vivo. Specifically, axonal MTs are uniformly organized with their plus ends pointing distally in all organisms. Dendrites of vertebrate neurons show more plus end–out MTs in vivo, whereas flies and worms have more minus end–out MTs in dendrites (Stepanova et al., 2003; Rolls et al., 2007; Stone et al., 2008).
Figure 1.
Regulatory steps during polarized motor-based transport of synaptic material. (A) At the Golgi apparatus, synaptic proteins have to be sorted into appropriate vesicles. These vesicles and other cargo such as mitochondria get loaded onto specific motor proteins. (B) Establishment of proper microtubule polarity along the axon determines anterograde and retrograde trafficking by plus end– and minus end–directed motor proteins such as kinesins and dynein. (C) At the appropriate destination, motor-cargo unloading occurs in a regulated fashion to achieve the appropriate distribution of synaptic boutons. At synapses, synaptic vesicle precursors give rise to mature synaptic vesicles. Proteins required for the SV cycle and trans-synaptic adhesion coalesce into the active zone (AZ) underneath the plasma membrane juxtaposed against the postsynaptic membrane.
Does the difference in microtubule organization and polarity help to segregate synaptic cargoes between axons and dendrites? Recent studies have started to identify some molecules that create these differences in MT polarity in different neuronal subcellular compartments and show how disruption of their function affects synapse formation. For example, a recent paper showed that kinesin-1 is required to establish the predominantly minus end–out organization in the dendrites of Caenorhabditis elegans motor neurons (Yan et al., 2013). In kinesin-1/unc-116 mutants, dendrites adopt the axon-like MT polarity causing presynaptic cargoes to mislocalize into dendrites (Seeger and Rice, 2010; Yan et al., 2013). Similarly, loss of the MT-binding CRMP protein UNC-33 or the actin–spectrin adaptor protein ankyrin/UNC-44 in worms also results in MT polarity defects, which also results in ectopic localization of synaptic vesicles and active zone proteins into dendrites (Maniar et al., 2012). These results support the idea that MT polarity ensures the faithful targeting of presynaptic components to the axon. However, another way motors can distinguish between axons and dendrites is through MT-associated proteins (MAPs). In a recent study, Banker and colleagues showed that plus end–orienting kinesins can differentiate axon and dendrite, likely due to specific MT-binding proteins in these compartments (Huang and Banker, 2012).
The direct regulation of motor activity by MTs or synaptic vesicle–associated proteins is likely to contribute to the trafficking of synaptic cargoes. Doublecortin, a MAP, binds to kinesin-3/KIF1A to affect the trafficking of the synaptic vesicle protein, synaptobrevin, in hippocampal neurons by altering the affinity of ADP-bound KIF1A to MTs (Liu et al., 2012). The Rab3 guanine nucleotide exchange factor, DENN/MADD, functions as an adaptor between kinesin-3 and GTP-Rab3–containing synaptic vesicles to promote the trafficking of synaptic vesicles in the axon (Niwa et al., 2008).
Precise regulation of motor-based transport ensures that synaptic cargoes are delivered to and maintained at synapses. Several recent studies have provided evidence that two postmitotic cyclin-dependent kinases are important regulators of anterograde and retrograde trafficking of presynaptic cargoes. The kinase CDK-5 is required in many aspects of nervous system function. In the context of presynaptic development and function, CDK-5 has been shown to regulate the transport of synaptic vesicles and dense core vesicles, which contain neuropeptides, by inhibiting a dynein-mediated pathway that mobilizes presynaptic components to the somatodendritic compartments in C. elegans neurons (Ou et al., 2010; Goodwin et al., 2012). A paralogue of CDK-5, the PCT-1 kinase acts in a partially redundant pathway to prevent the mislocalization of presynaptic material to dendrites. In animals lacking both kinases or their activators, synaptic cargoes completely mislocalize to the dendrites, leaving an “empty” axon (Ou et al., 2010). Vertebrate CDK-5 also plays profound roles in the regulation of synaptic vesicle pools by modifying Ca2+ channels. Genetic ablation or pharmacological inhibition of CDK-5 increases the pool of synaptic vesicles that are docked at the active zone, termed the readily releasable pool, and potentiates synaptic function (Kim and Ryan, 2010, 2013). These results suggest that CDK-5 and its paralogue control local and global vesicle pools. Regulation of the exchange between these pools can affect membrane trafficking at presynaptic terminals as well as the overall polarity of neurons.
To form synapses at defined locations, cargoes not only need to know how to “get on” the transport system but also need to know where to precisely “get off” at their destination (Fig. 1 C). Loss of a conserved small G-protein of the Arf-like family, ARL-8, in C. elegans, resulted in premature exit of synaptic cargoes during transport and showed ectopic aggregations of synaptic vesicles in the proximal axon. This causes a reduction in the number but an increase in the size of synapses (Klassen et al., 2010). ARL-8 localizes to both stable and trafficking synaptic vesicles and promotes trafficking by increasing kinesin-3 activity and suppressing aggregation-induced stoppage of synaptic cargoes along the axon (Wu et al., 2013). Hence, the balance between motor activity and aggregation propensity of trafficking cargoes may determine the number, size, and location of presynaptic terminals. Interestingly, the small GTPase Rab3, which normally associates with synaptic vesicles, has recently been shown to affect the distribution of active zone proteins at fly neuromuscular junction (NMJ) synapses, further suggesting that the trafficking of synaptic vesicles and formation of active zones are linked (Graf et al., 2009).
Besides synaptic material, another major organelle cargo that is often present at the presynaptic terminal is mitochondria. The Milton–Miro complex functions as an adaptor between kinesin-1 and mitochondria to support axonal transport of mitochondria. Interestingly, the coupling of the Milton–Miro complex to kinesin is regulated by Ca2+ (Macaskill et al., 2009; Wang and Schwarz, 2009), providing a mechanism for neuronal activity controlling transport of mitochondria along the axon.
Previous studies have suggested that components of the presynaptic active zone are transported in a preassembled form by Piccolo-Bassoon transport vesicles (PTVs) that may contain multiple components required to build a synapse (Zhai et al., 2001; Shapira et al., 2003). Recent studies found that Golgi-derived PTVs contain many active zone proteins including Piccolo, Bassoon, RIM1α, and ELKS2/CAST, but lack another active zone component, Munc-13, which may exit the Golgi on separate vesicles (Maas et al., 2012). Packing of various active zone components that have the propensity to self-assemble into separate vesicles may contribute a way to control synaptogenesis. This is interesting in light of the finding that Munc-13 can function as a protein scaffold for Bassoon and ELKS2 (Wang et al., 2009). The link between trafficking of synaptic vesicle and active zone components is not well understood. In vivo time-lapse imaging of synaptic vesicle and active zone trafficking showed that these components, possibly in the form of dense core vesicles, could be trafficked together in C. elegans neurons, suggestive of prepackaged presynaptic material during transport (Wu et al., 2013). Taken together, axonal transport of synaptic components is a necessary step for synapse formation and maintenance. The regulation of MTs, molecular motors, and synaptic cargoes ensure the targeting of appropriate proteins to synapses.
Role of the actin cytoskeleton in presynaptic assembly
Although MT-mediated transport is critical for long-range trafficking, actin-based mechanisms often organize local protein complexes in subcellular domains. A large body of work has described the role of the actin cytoskeleton in postsynaptic structure and function (Schubert and Dotti, 2007; Hotulainen and Hoogenraad, 2010). We will focus on more recent work that has highlighted the importance of the actin cytoskeleton in presynaptic formation.
F-actin is required for presynaptic assembly during the early stages of synaptogenesis. Depolymerization of F-actin in young hippocampal neuronal cultures results in a reduction in the size and number of synapses. This effect was not seen with older cultures when synapses are more mature (Zhang and Benson, 2001). This observation correlates with an increase in both pre- and postsynaptic F-actin levels in newly formed synapses compared with mature synapses (Zhang and Benson, 2002).
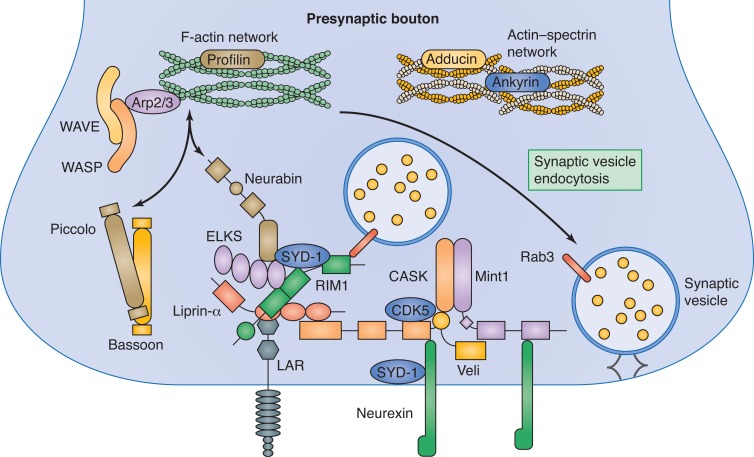
F-actin has been implicated in many steps of synapse assembly and function (Fig. 2; Cingolani and Goda, 2008). One of the roles that has been proposed for F-actin is to act as a scaffold for other presynaptic proteins (Sankaranarayanan et al., 2003). A recent study identified an F-actin–binding active zone molecule Neurabin/NAB-1 that is recruited by a presynaptic F-actin network (Chia et al., 2012). In addition, knockdown of Rac/Cdc42 GTPase exchange factor β-Pix resulted in a decrease in actin at synapses with a concomitant loss of synaptic vesicle clustering (Sun and Bamji, 2011). These studies demonstrate that F-actin at presynaptic sites can recruit and stabilize presynaptic components.
Figure 2.
Assembling the presynaptic active zone. Scaffolding proteins including Liprin, SYD-1, ELKS, Neurabin, Piccolo, and Bassoon form the dense protein network in the presynaptic cytomatrix that facilitates synaptic vesicle docking and fusion. The presynaptic F-actin networks are required for presynaptic assembly and maintenance.
Studies of Drosophila NMJs have found that the presynaptic spectrin–actin cytoskeleton is important for synapse stability. Loss of presynaptic spectrin led to retraction of synapses (Pielage et al., 2005). Intriguingly, loss of postsynaptic spectrin increased the total number of the active zone specializations, termed T-bars, and affected the size and distribution of presynaptic sites. Thus, the spectrin cytoskeleton can impose a trans-synaptic influence on synapse development (Pielage et al., 2006).
Given the importance of F-actin at synapses, it is crucial to understand the signaling pathways that instruct F-actin organization. Multiple studies have shown that signaling from synaptic cell adhesion molecules can lead to cytoskeletal rearrangements at synapses. Adhesion of hippocampal neurons to syndecan-2–coated beads is sufficient to induce F-actin clustering and downstream formation of presynaptic boutons (Lucido et al., 2009). In mice, the adhesion molecule L1CAM may bind to spectrin–actin adaptor ankyrin to mediate GABAergic synapse formation (Guan and Maness, 2010). Another adhesion molecule of the immunoglobulin superfamily SYG-1 in C. elegans has also been shown to be necessary and sufficient to recruit F-actin to synapses (Chia et al., 2012). In a recent study, secreted bone morphogenetic protein (BMP) can signal in a retrograde fashion to regulate Rac-GEF Trio expression in presynaptic neurons, which is important for controlling synaptic growth (Ball et al., 2010).
Interestingly, presynaptic active zone proteins can also affect F-actin assembly (Fig. 2). Knockdown of Piccolo reduced activity-dependent assembly of F-actin at synapses and enhanced dispersion of Synapsin1a and synaptic vesicles in hippocampal neurons. Loss of Piccolo also resulted in a loss of Profilin 2, a regulator of actin polymerization (Waites et al., 2011).
Various studies have begun to shed light on the actin regulators required for synaptic F-actin establishment and maintenance. Diaphanous, a formin-related gene that associates with barbed ends of F-actin, was found to function downstream of presynaptic receptor Dlar at fly NMJs. Spectrin–actin capping protein, Adducin, is enriched at presynaptic sites and is required to prevent synapse retraction and elimination (Bednarek and Caroni, 2011; Pielage et al., 2011). Activators of the Arp2/3 complex, WASP and WAVE, have also been implicated in the regulation of F-actin at synapses (Coyle et al., 2004; Stavoe et al., 2012; Zhao et al., 2013). This diversity of F-actin modulators suggests that there are probably different F-actin structures at different stages of development or even in subcellular domains within the synapse. This is supported by observations that F-actin can localize with synaptic vesicles, at the active zone and in the perisynaptic region (Bloom et al., 2003; Sankaranarayanan et al., 2003; Waites et al., 2011; Chia et al., 2012). Thus, much remains to be done in our understanding how distinct F-actin structures are formed and regulated to mediate various processes during synapse assembly and maintenance.
Assembly of the molecular network at presynaptic terminals
Although F-actin might help to initiate the presynaptic assembly process, many other ensuing molecular interactions are required to form the mature presynaptic apparatus (Fig. 2). The presynaptic active zone is comprised of a framework of scaffolding proteins that function as protein-binding hubs for other presynaptic components. Piccolo and Bassoon are important vertebrate multidomain proteins that traditionally have been widely used as active zone markers. Recent electrophysiology data on Piccolo mutant and Bassoon knockdown neurons showed that these molecules are dispensable for synaptic transmission but affect synaptic vesicle clustering (Mukherjee et al., 2010). Furthermore, Piccolo and Bassoon were found to be required for maintaining synapse integrity by regulating ubiquitination and degradation of presynaptic components (Waites et al., 2013).
Forward genetic approaches in worms and flies have made important contributions to our understanding of the presynaptic cytomatrix. Studies have found that two active zone scaffolding molecules, SYD-1 and Liprin-α/SYD-2, are required for proper synapse formation (Zhen and Jin, 1999; Patel et al., 2006; Astigarraga et al., 2010; Owald et al., 2010; Stigloher et al., 2011). Interestingly, at fly NMJs, SYD-1 is necessary for clustering presynaptic neurexin that in turn clusters postsynaptic neuroligin (Owald et al., 2012). The presynaptic assembly function of SYD-1 and SYD-2 appears to be conserved because mutation analysis of mammalian SYD-1 and knockdown of Liprin-α both caused defects in presynaptic development and function (Spangler et al., 2013; Wentzel et al., 2013). In flies, the active zone T-bar structure is comprised of ERC/CAST family protein bruchpilot (brp) as the major active zone organizing protein (Fouquet et al., 2009). Brp is not only present at the active zone but also plays important scaffolding roles in localizing Ca2+ channels. In C. elegans, the Brp homologue ELKS-1 is also localized to the active zone; however, the importance of ELKS-1 during development of synapses was only revealed in sensitized genetic backgrounds (Dai et al., 2006; Patel and Shen, 2009), suggesting that there are likely redundant molecular pathways for presynaptic assembly. In the vertebrate system, loss of one of the three ELKS genes, surprisingly, caused an increase in the inhibitory synaptic transmission (Kaeser et al., 2009). Besides Brp, Rab3-interacting molecule (RIM) binding protein (RBP) was found to be important for active zone structural integrity in flies. Using super-resolution microscopy, RBP was found to surround Ca2+ channels at T-bars and loss of RBP resulted in defective Ca2+ channel clustering and reduced evoked neurotransmitter release (Liu et al., 2011).
Assembly of the presynaptic active zone is subjected to several layers of regulation. The assembly process is balanced by inhibitory mechanisms that control the number and size of synapses. Loss of the E3 ubiquitin ligase Highwire/RPM-1 results in an increased number of synaptic boutons in flies and multiple active zones in worms (Wan et al., 2000; Zhen et al., 2000). Working together with F-box protein FSN-1, RPM-1 down-regulates the DLK MAP kinase signaling pathway (Liao et al., 2004; Nakata et al., 2005; Yan et al., 2009). Another E3 ubiquitin ligase, the SKP complex, has been shown to eliminate transient synapses during development in worms (Ding et al., 2007). Therefore, ubiquitin-mediated mechanisms play important roles in controlling the presynaptic assembly program.
Other inhibitory mechanisms include SRPK79D, a serine–arginine protein kinase discovered in flies that represses T-bar formation (Johnson et al., 2009). In the mutant, the T-bar component Brp is ectopically accumulated in the axonal shaft. Regulator of synaptogenesis, RSY-1, limits the extent of presynaptic assembly by directly binding to active zone scaffold molecule Liprin-α/SYD-2 and SYD-1 (Patel and Shen, 2009). In addition, Liprin-α/SYD-2 may inhibit its own activity via intramolecular interactions (Taru and Jin, 2011; Chia et al., 2013).
Taken together, the presynaptic assembly process driven by scaffolding molecules is controlled by complex inhibitory mechanisms to achieve the appropriate extent of aggregation in the process of synapse formation.
Trans-synaptic signals orchestrate pre- and postsynaptic formation
Coordinated pre- and postsynaptic development requires the precise apposition of presynaptic components to postsynaptic specializations. It is conceivable that signals from pre and postsynaptic sides functioning across the synaptic cleft coordinate synaptic differentiation reciprocally. Although a vast assortment of factors have been identified as synaptic organizers, the fact that genetic ablation of some synaptic organizers in vivo fails to elicit dramatic synaptic defects suggests the incomplete view of the trans-synaptic signaling. Moreover, the underlying mechanisms and the cross talk of these signaling pathways are still unclear. In recent years, an emerging body of literature has begun to shed light on trans-synaptic signaling and the importance of environmental cues in synapse formation.
Adhesion proteins instruct synaptic differentiation
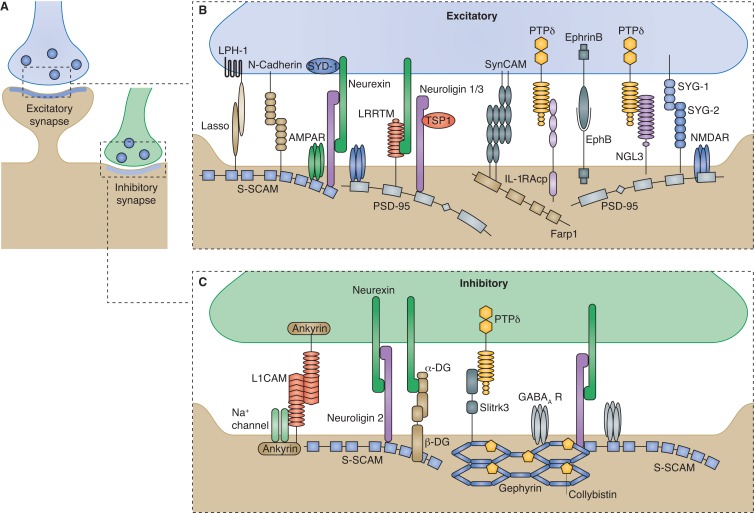
A large body of literature suggests that trans-synaptic interactions between synaptic adhesion molecules function bi-directionally for synapse formation and maturation (Fig. 3). Neurexin–neuroligin is the first pair to be shown to induce pre- and postsynapse formation (Scheiffele et al., 2000; Graf et al., 2004; Chih et al., 2005; Nam and Chen, 2005; Chubykin et al., 2007). Recent in vitro studies have unveiled more components interacting with neurexin or neuroligin in specific synaptic differentiation events (Fig. 3, B and C). In early developmental stages, a secreted synaptic organizer, thrombospondin 1 (TSP1, see next section) increases the speed of synaptogenesis through neuroligin 1 (Xu et al., 2010). At excitatory synapses, a retrograde signaling controls synaptic vesicle clustering, neurotransmitter release, and presynaptic maturation by cooperation of neuroligin and N-cadherin (Wittenmayer et al., 2009; Stan et al., 2010; Aiga et al., 2011). A leucine-rich repeat transmembrane (LRRTM) protein family was also identified as an organizer of the function of excitatory synapses through interactions with neurexin (Linhoff et al., 2009). Further studies showed that binding of LRRTMs and neuroligins to neurexin acts redundantly to maintain excitatory synapses by preventing activity and Ca2+-dependent synapse elimination during early development, while performing divergent functions upon synapse maturation (de Wit et al., 2009; Ko et al., 2009, 2011; Soler-Llavina et al., 2011).
Figure 3.
Adhesive trans-synaptic signalings orchestrate excitatory and inhibitory synaptic assembly. Multiple pairs of trans-synaptic adhesion molecules organize synaptic differentiation and function on both pre- and postsynaptic sites. Note that different adhesion molecules are used at excitatory and inhibitory synapses. LPH1, latrophilin 1; α-DG, α-dystroglycan; β-DG, β-dystroglycan; S-SCAM, synaptic scaffolding molecule; Lasso, LPH1-associated synaptic surface organizer; IL-1RAcp, interleukin-1 receptor accessory protein.
The function of neurexin and neuroligin in mediating synaptic differentiation has also been shown at Drosophila NMJs and mammalian CNS. In mammalian, although neither compound knockout of three neurexins nor two individual neuroligin knockout mice display severe defects in the number or morphology of synapses (Missler et al., 2003), the deletion of either neurexin or neuroligin affects the neurotransmitter release and in turn impairs the relevant behavior (Zhang et al., 2005; Blundell et al., 2009, 2010; Etherton et al., 2009; Jedlicka et al., 2011). Neurexin loss of function in fly leads to reduced number and defective morphology of synaptic boutons and active zones from early developmental stages (Li et al., 2007; Chen et al., 2010). In contrast, deletion of either neuroligin 1 or 2 causes NMJ defects and alternations of active zones only in the larval stage, indicating that they function mainly in the expansion of NMJs during development (Banovic et al., 2010; Sun et al., 2011). These abnormalities further impair synaptic transmission at the NMJs (Li et al., 2007; Banovic et al., 2010; Chen et al., 2010; Sun et al., 2011). Moreover, these phenotypes are enhanced when the Teneurin family of adhesion molecules is deleted, suggestive of functional redundancy between adhesion molecules (Mosca et al., 2012). Recently, it has been reported that an active zone protein, SYD-1, is required for the formation and function of the neurexin–neuroligin complex in flies (Fig. 2; Owald et al., 2012), providing an example of how trans-synaptic neurexin–neuroligin signaling orchestrates synaptic assembly bi-directionally. Interestingly, at postsynaptic sites, the NMDA receptor activity-triggered Ca2+-dependent cleavage of neuroligin 1 was found to destabilize presynaptic neurexin, reduce presynaptic release probability, and depress synaptic transmission (Peixoto et al., 2012). This observation raises a possibility that neurexin and neuroligin could fine-tune synaptogenesis both positively and negatively.
Although Drosophila neuroligin and neurexin mutants share many phenotypes in synaptic differentiation, there are some unique features for each mutant, suggesting that they play distinct roles. For example, some aspects of synaptic specificity are achieved by different pairs of neurexin–neuroligin interactions. Neuroligin 1 promotes the growth and differentiation of excitatory synapses by binding to PSD-95, whose amount balances the ratio of excitatory-to-inhibitory synaptic specializations (Prange et al., 2004; Banovic et al., 2010). Neuroligin 2, on the contrary, binds to a scaffold protein gephyrin at inhibitory synapses, instructing inhibitory postsynaptic assembly (Fig. 3, B and C; Poulopoulos et al., 2009). Different isoforms of neurexin also contribute to the differentiation of excitatory and inhibitory synapses (Fig. 3, B and C; Chih et al., 2006; Graf et al., 2006; Kang et al., 2008).
Other novel trans-synaptic interactions have also been identified to organize synaptic differentiation (Fig. 3, B and C). For example, Netrin-G ligand 3 (NGL-3), localized at postsynaptic region, induces excitatory synaptic differentiation by interacting with the receptor tyrosine phosphatase LAR family proteins, including PTPδ and PTPσ (Woo et al., 2009; Kwon et al., 2010). PTPδ can also trans-interact with Slitrk3 and IL-1 receptor accessory protein (IL-1RAcP) to promote presynaptic formation (Takahashi et al., 2012; Yoshida et al., 2012). Molecules that function in other neuronal developmental processes have also been shown to regulate synaptic differentiation. Farp1, essential for the dynamics of dendritic filopodia, regulates postsynaptic development and triggers a retrograde signal promoting active zone assembly by binding to SynCAM 1 (Cheadle and Biederer, 2012). Teneurins, instructing synaptic partner selection in fly olfactory system (Hong et al., 2012), act in synaptogenesis through trans-synaptic interaction at NMJs (Mosca et al., 2012). Another splice variant of a postsynaptic Teneurin-2 in rat, Lasso, binding with presynaptic Latrophilin 1 (LPH1), induces presynaptic Ca2+ signals and regulates synaptic function (Silva et al., 2011). Neural activity is also involved in controlling the growth of the presynapse. Conditioning or BDNF application induces presynaptic bouton development via an ephrin-B–dependent manner (Li et al., 2011), suggesting the role of EphB/ephrin-B signaling in activity-dependent synaptic modification.
Secreted molecules organize synapse differentiation
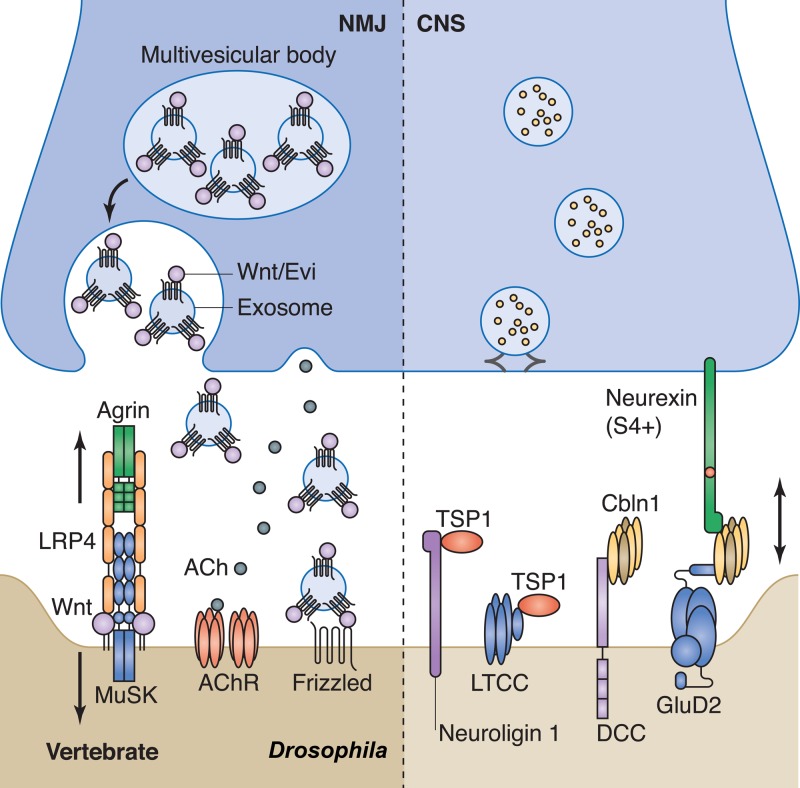
In addition to adhesion molecules, some secreted molecules also serve as synaptic organizers (Fig. 4). For example, the motor neuron–derived ligand agrin, which was the first identified secreted organizing molecule for postsynaptic differentiation, activates MuSK, a postsynaptic receptor tyrosine kinase, to regulate NMJ specialization (Glass et al., 1996; Zhou et al., 1999). Recently, a low-density lipoprotein receptor–related protein, LRP4, was identified as the co-receptor of agrin, forming a complex with MuSK and mediating MuSK signaling (Kim et al., 2008; Zhang et al., 2008). Several Wnts appears to act together with agrin to activate the LRP4–MuSK receptor complex to promote postsynaptic differentiation (Jing et al., 2009; Zhang et al., 2012). LRP4 also acts as a direct retrograde signal, functioning independently of MuSK for presynaptic differentiation (Yumoto et al., 2012), demonstrating that LRP4 acts as a bi-directional synaptic organizer (Fig. 4, left).
Figure 4.
Secreted trans-synaptic signaling at NMJs and CNS synapses. (Left) At Drosophila neuromuscular junctions (NMJs), Wnts are secreted from presynaptic terminals in association with Evi in the form of exosomes. In vertebrate NMJs, Wnt binds to the Agrin–LRP4–MuSK complex to regulate synapse formation. (Right) At CNS synapses, glia-derived thrombospondins (TSPs) and presynaptic neuron–derived cerebellin (Cbln) organize synapse differentiation and formation bi-directionally through binding to GluD2 and an isoform of neurexin (S4+) on the postsynaptic and presynaptic membranes, respectively. LTCC, L-type Ca2+ channel complex; AChR, acetyl choline receptor.
Wnt is another well-characterized signaling molecule regulating many developmental processes including synaptic differentiation bi-directionally. Wnt regulates synaptic assembly both positively and negatively. For example, Wnt3 collaborates with agrin to promote the clustering of acetyl choline receptor (AChR) at the vertebrate NMJs (Henriquez et al., 2008), while Wnt3a inhibits AChR aggregation through β-catenin signaling (Wang et al., 2008). In the C. elegans NMJ, a Wnt molecule, CWN-2, stimulates the delivery and insertion of AchR to the postsynaptic membrane through the activation of a Frizzled–CAM-1 receptor complex (Jensen et al., 2012). Local Wnt gradient can suppress synapse formation in both C. elegans and Drosophila (Inaki et al., 2007; Klassen and Shen, 2007). Interestingly, in these contexts, Wnts are secreted from nonneuronal or nonsynaptic partner cells, suggesting that environmental factors can shape synaptic connections. Wnt can also be secreted from presynaptic neurons. A recent study demonstrated the trans-synaptic transmission of Wnt by exosome-like vesicles containing the Wnt-binding protein Evi at Drosophila NMJs (Fig. 4, left; Korkut et al., 2009; Koles et al., 2012). Presynaptic vesicular release of Evi is required for the secretion of Wnt. Intriguingly, different Wnt ligands regulate synapse formation in distinct cellular contexts. Wnt3a promotes excitatory synaptic assembly through CaMKII, whereas Wnt5a mediates inhibitory synapse formation by stabilizing GABAA receptors (Cuitino et al., 2010; Ciani et al., 2011). This functional diversity indicates that different Wnts, receptors, and downstream pathways, as well as cell-specific contexts dictate the action of extracellular cues. Another conserved secreted molecule, netrin/UNC-6, can also pattern synapses by either promoting or inhibiting synapse formation (Colón-Ramos et al., 2007; Poon et al., 2008). Because Wnt and netrin often exist in gradients, these observations suggest that the localization of synapses can be specified by the gradient of extrinsic cues.
In mammalian, several glia-derived cues have been shown to play important roles in regulating synapse formation or elimination. Thrombospondins (TSPs) are trans-synaptic organizers secreted from immature astrocytes (Christopherson et al., 2005). Both in vitro and in vivo data demonstrate the capacity of TSPs to increase synapse number, promote the localization of synaptic molecules, and refine the pre- and postsynaptic alignment (Christopherson et al., 2005; Eroglu et al., 2009). Recently, two transmembrane molecules were uncovered in mediating TSP-induced synaptogenesis (Fig. 4, right). Neuroligin 1 interacts with TSP1 with its extracellular domain mediating the acceleration of synaptogenesis in hippocampal neurons (Xu et al., 2010). α2δ-1, a subunit of the L-type Ca2+ channel complex (LTCC), was also identified as the postsynaptic receptor of TSP in excitatory CNS neurons (Eroglu et al., 2009). Interaction between TSP and α2δ–1 triggers the conformational changes and sequentially recruits synaptic scaffolding molecules and initiates synapse formation (Eroglu et al., 2009). Interestingly, TSP-induced synapses, although structurally normal and presynaptically active, are postsynaptically silent due to the lack of AMPA receptors (Christopherson et al., 2005), indicating the existence of other glia-derived signals involved in synapse formation. In fact, in cultured hippocampal neurons, a glia-derived neurotrophic factor GDNF enhances the pre- and postsynaptic adhesion by triggering the trans-homophilic interaction of its receptors GFRα1 localized at both pre- and postsynaptic sites (Ledda et al., 2007). Several other glia-derived factors have been shown to play critical roles in synaptogenesis. Astrocytes secrete extracellular molecules hevin and SPARC to regulate synapse formation in vitro and in vivo (Kucukdereli et al., 2011). Astrocytes also express a transmembrane adhesion protein, protocadherin-γ, serving as a local cue to promote synapse formation (Garrett and Weiner, 2009). TGF-β secreted from the NMJ glia acts together with the muscle-derived TGF-β to control synaptic growth (Fuentes-Medel et al., 2012). In a similar fashion, secretion of BDNF by vestibular supporting cells is required for synapse formation between hair cells and sensory organs (Gómez-Casati et al., 2010).
Another important synaptic organizer is cerebellin (Cbln), a presynapse-derived complement protein, C1q-like family protein. In cbln1-null mice the number of parallel fibers (PF)–Purkinje synapses is dramatically reduced; the postsynaptic densities in the remaining synapses are larger than the apposite active zones (Hirai et al., 2005). Cbln was also found to regulate synaptic plasticity, as cbln1-null mice show impaired long-term depression in cerebellum (Hirai et al., 2005). These defects precisely resemble those in mice lacking a putative glutamate receptor, GluD2 (Kashiwabuchi et al., 1995; Kurihara et al., 1997), suggesting that Cbln1 and GluD2 function in synaptic differentiation through a common pathway. Interestingly, the C-terminal domain and N-terminal domains of GluD2 are indispensable for cerebella LTD and PF–Purkinje synaptic morphology, respectively (Kohda et al., 2007; Uemura et al., 2007; Kakegawa et al., 2008, 2009). Further studies suggested that Cbln1 directly binds to the N-terminal domain of GluD2 and recruits postsynaptic proteins by clustering GluD2 (Matsuda et al., 2010). Neurexin was recently reported as the presynaptic receptor of Cbln in promoting synaptogenesis (Uemura et al., 2010), which reinforces the understanding of Cbln-mediated trans-synaptic signaling: Cbln serves as a bi-directional synapse organizer by linking presynaptic neurexin and postsynaptic GluD2 (Fig. 4, right).
Besides being required for synapse formation at early stages, genetic ablation of GluD2 in adult cerebellum leads to loss of PF–Purkinje synapses (Takeuchi et al., 2005), indicating that Cbln1–GluD2 signaling is also important for the maintenance of PF–Purkinje synapses. Chronic stimulation of neural activity decreases Cbln1 expression and diminishes the number of PF–Purkinje synapses (Iijima et al., 2009), suggesting the importance of Cbln1–GluD2 signaling for synaptic plasticity and homeostasis.
Cbln subfamily proteins are widely expressed throughout the brain (Miura et al., 2006), suggesting that their synaptogenic roles may be wide spread in other regions of the brain. Cbln2 and 4 are also secreted proteins, whereas Cbln3 is retained in the cellular endomembrane system (Iijima et al., 2007). Cbln1 and 2, interacting with an isoform of presynaptic neurexin, induce synaptogenesis (Joo et al., 2011; Matsuda and Yuzaki, 2011). Notably, the cortical synapses induced by neurexin–Cbln signaling are preferentially inhibitory (Joo et al., 2011), distinguishing the effects of Cbln from neuroligin. GluD1 was recently found to be the postsynaptic receptor of Cbln1 and 2 in cortical neurons, mediating the differentiation of inhibitory presynapses (Yasumura et al., 2012). On the other side, Cbln4 selectively binds to the netrin receptor DCC in a netrin-displaceable manner (Fig. 4, right), suggesting a potential function of Cbln4 through DCC signaling pathway (Iijima et al., 2007). Intriguingly, C1q, although sharing similar structure with Cbln, serves an opposite role by regulating the synapse elimination: C1q released from retinal ganglion cells refines the retinogeniculate connections by eliminating unneeded synapses (Stevens et al., 2007).
Concluding remarks
Synapse development is regulated in multiple steps. Research over the last few years have uncovered many regulatory mechanisms on how trafficking of synaptic material is regulated and how scaffold proteins act with cytoskeleton networks and trans-synaptic signaling to instruct the synapse formation. Nevertheless, our understanding of the cellular and molecular mechanisms regulating synapse development is still incomplete. For example, how is the direction, speed, and amount of synaptic material being transported specified? How is a synapse’s size determined? How is synapse type and strength specified through adhesive and secreted trans-synaptic signaling? How do the redundant synapse-inducing pathways interact with each other? Given the rapidly emerging improvements of technologies, especially super-resolution microscopy and high-throughput genomics and proteomics, the synapse development field will likely rapidly evolve in the near future.
Acknowledgments
This work is supported by the Howard Hughes Medical Institute and the National Institutes of Health (1R01NS082208 and 5R01 NS048392). Illustrations were provided by Neil Smith, www.neilsmithillustration.co.uk.
Footnotes
Abbreviations used in this paper:
- Cbln
- cerebellin
- MAP
- MT-associated protein
- MT
- microtubule
- NMJ
- neuromuscular junction
- PF
- parallel fiber
- TSP
- thrombospondin
References
- Aiga M., Levinson J.N., Bamji S.X. 2011. N-cadherin and neuroligins cooperate to regulate synapse formation in hippocampal cultures. J. Biol. Chem. 286:851–858 10.1074/jbc.M110.176305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astigarraga S., Hofmeyer K., Farajian R., Treisman J.E. 2010. Three Drosophila liprins interact to control synapse formation. J. Neurosci. 30:15358–15368 10.1523/JNEUROSCI.1862-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P.W., Deitch J.S., Black M.M., Banker G.A. 1988. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc. Natl. Acad. Sci. USA. 85:8335–8339 10.1073/pnas.85.21.8335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P.W., Vidya Nadar C., Myers K.A. 2006. Axonal transport of microtubules: the long and short of it. Traffic. 7:490–498 10.1111/j.1600-0854.2006.00392.x [DOI] [PubMed] [Google Scholar]
- Ball R.W., Warren-Paquin M., Tsurudome K., Liao E.H., Elazzouzi F., Cavanagh C., An B.-S., Wang T.-T., White J.H., Haghighi A.P. 2010. Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron. 66:536–549 10.1016/j.neuron.2010.04.011 [DOI] [PubMed] [Google Scholar]
- Banovic D., Khorramshahi O., Owald D., Wichmann C., Riedt T., Fouquet W., Tian R., Sigrist S.J., Aberle H. 2010. Drosophila neuroligin 1 promotes growth and postsynaptic differentiation at glutamatergic neuromuscular junctions. Neuron. 66:724–738 10.1016/j.neuron.2010.05.020 [DOI] [PubMed] [Google Scholar]
- Bednarek E., Caroni P. 2011. β-Adducin is required for stable assembly of new synapses and improved memory upon environmental enrichment. Neuron. 69:1132–1146 10.1016/j.neuron.2011.02.034 [DOI] [PubMed] [Google Scholar]
- Bloom O., Evergren E., Tomilin N., Kjaerulff O., Löw P., Brodin L., Pieribone V.A., Greengard P., Shupliakov O. 2003. Colocalization of synapsin and actin during synaptic vesicle recycling. J. Cell Biol. 161:737–747 10.1083/jcb.200212140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J., Tabuchi K., Bolliger M.F., Blaiss C.A., Brose N., Liu X., Südhof T.C., Powell C.M. 2009. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav. 8:114–126 10.1111/j.1601-183X.2008.00455.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J., Blaiss C.A., Etherton M.R., Espinosa F., Tabuchi K., Walz C., Bolliger M.F., Südhof T.C., Powell C.M. 2010. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J. Neurosci. 30:2115–2129 10.1523/JNEUROSCI.4517-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V. 1996. Synapse maturation and structural plasticity at Drosophila neuromuscular junctions. Curr. Opin. Neurobiol. 6:858–867 10.1016/S0959-4388(96)80038-9 [DOI] [PubMed] [Google Scholar]
- Cheadle L., Biederer T. 2012. The novel synaptogenic protein Farp1 links postsynaptic cytoskeletal dynamics and transsynaptic organization. J. Cell Biol. 199:985–1001 10.1083/jcb.201205041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Gracheva E.O., Yu S.C., Sheng Q., Richmond J., Featherstone D.E. 2010. Neurexin in embryonic Drosophila neuromuscular junctions. PLoS ONE. 5:e11115 10.1371/journal.pone.0011115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P.H., Patel M.R., Shen K. 2012. NAB-1 instructs synapse assembly by linking adhesion molecules and F-actin to active zone proteins. Nat. Neurosci. 15:234–242 10.1038/nn.2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P.H., Patel M.R., Wagner O.I., Klopfenstein D.R., Shen K. 2013. Intramolecular regulation of presynaptic scaffold protein SYD-2/liprin-α. Mol. Cell. Neurosci. 56C:76–84 10.1016/j.mcn.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B., Engelman H., Scheiffele P. 2005. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 307:1324–1328 10.1126/science.1107470 [DOI] [PubMed] [Google Scholar]
- Chih B., Gollan L., Scheiffele P. 2006. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 51:171–178 10.1016/j.neuron.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Christopherson K.S., Ullian E.M., Stokes C.C., Mullowney C.E., Hell J.W., Agah A., Lawler J., Mosher D.F., Bornstein P., Barres B.A. 2005. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 120:421–433 10.1016/j.cell.2004.12.020 [DOI] [PubMed] [Google Scholar]
- Chubykin A.A., Atasoy D., Etherton M.R., Brose N., Kavalali E.T., Gibson J.R., Südhof T.C. 2007. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 54:919–931 10.1016/j.neuron.2007.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani L., Boyle K.A., Dickins E., Sahores M., Anane D., Lopes D.M., Gibb A.J., Salinas P.C. 2011. Wnt7a signaling promotes dendritic spine growth and synaptic strength through Ca²+/Calmodulin-dependent protein kinase II. Proc. Natl. Acad. Sci. USA. 108:10732–10737 10.1073/pnas.1018132108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani L.A., Goda Y. 2008. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 9:344–356 10.1038/nrn2373 [DOI] [PubMed] [Google Scholar]
- Colón-Ramos D.A., Margeta M.A., Shen K. 2007. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 318:103–106 10.1126/science.1143762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle I.P., Koh Y.-H., Lee W.-C.M., Slind J., Fergestad T., Littleton J.T., Ganetzky B. 2004. Nervous wreck, an SH3 adaptor protein that interacts with Wsp, regulates synaptic growth in Drosophila. Neuron. 41:521–534 10.1016/S0896-6273(04)00016-9 [DOI] [PubMed] [Google Scholar]
- Cuitino L., Godoy J.A., Farías G.G., Couve A., Bonansco C., Fuenzalida M., Inestrosa N.C. 2010. Wnt-5a modulates recycling of functional GABAA receptors on hippocampal neurons. J. Neurosci. 30:8411–8420 10.1523/JNEUROSCI.5736-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Taru H., Deken S.L., Grill B., Ackley B., Nonet M.L., Jin Y. 2006. SYD-2 Liprin-alpha organizes presynaptic active zone formation through ELKS. Nat. Neurosci. 9:1479–1487 10.1038/nn1808 [DOI] [PubMed] [Google Scholar]
- de Wit J., Sylwestrak E., O’Sullivan M.L., Otto S., Tiglio K., Savas J.N., Yates J.R., III, Comoletti D., Taylor P., Ghosh A. 2009. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 64:799–806 10.1016/j.neuron.2009.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M., Chao D., Wang G., Shen K. 2007. Spatial regulation of an E3 ubiquitin ligase directs selective synapse elimination. Science. 317:947–951 10.1126/science.1145727 [DOI] [PubMed] [Google Scholar]
- Eroglu C., Allen N.J., Susman M.W., O’Rourke N.A., Park C.Y., Ozkan E., Chakraborty C., Mulinyawe S.B., Annis D.S., Huberman A.D., et al. 2009. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 139:380–392 10.1016/j.cell.2009.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton M.R., Blaiss C.A., Powell C.M., Südhof T.C. 2009. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc. Natl. Acad. Sci. USA. 106:17998–18003 10.1073/pnas.0910297106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquet W., Owald D., Wichmann C., Mertel S., Depner H., Dyba M., Hallermann S., Kittel R.J., Eimer S., Sigrist S.J. 2009. Maturation of active zone assembly by Drosophila Bruchpilot. J. Cell Biol. 186:129–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Medel Y., Ashley J., Barria R., Maloney R., Freeman M., Budnik V. 2012. Integration of a retrograde signal during synapse formation by glia-secreted TGF-β ligand. Curr. Biol. 22:1831–1838 10.1016/j.cub.2012.07.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett A.M., Weiner J.A. 2009. Control of CNS synapse development by gamma-protocadherin-mediated astrocyte-neuron contact. J. Neurosci. 29:11723–11731 10.1523/JNEUROSCI.2818-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass D.J., Bowen D.C., Stitt T.N., Radziejewski C., Bruno J., Ryan T.E., Gies D.R., Shah S., Mattsson K., Burden S.J., et al. 1996. Agrin acts via a MuSK receptor complex. Cell. 85:513–523 10.1016/S0092-8674(00)81252-0 [DOI] [PubMed] [Google Scholar]
- Goda Y., Davis G.W. 2003. Mechanisms of synapse assembly and disassembly. Neuron. 40:243–264 10.1016/S0896-6273(03)00608-1 [DOI] [PubMed] [Google Scholar]
- Gómez-Casati M.E., Murtie J.C., Rio C., Stankovic K., Liberman M.C., Corfas G. 2010. Nonneuronal cells regulate synapse formation in the vestibular sensory epithelium via erbB-dependent BDNF expression. Proc. Natl. Acad. Sci. USA. 107:17005–17010 10.1073/pnas.1008938107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin P.R., Sasaki J.M., Juo P. 2012. Cyclin-dependent kinase 5 regulates the polarized trafficking of neuropeptide-containing dense-core vesicles in Caenorhabditis elegans motor neurons. J. Neurosci. 32:8158–8172 10.1523/JNEUROSCI.0251-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf E.R., Zhang X., Jin S.X., Linhoff M.W., Craig A.M. 2004. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 119:1013–1026 10.1016/j.cell.2004.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf E.R., Kang Y., Hauner A.M., Craig A.M. 2006. Structure function and splice site analysis of the synaptogenic activity of the neurexin-1 beta LNS domain. J. Neurosci. 26:4256–4265 10.1523/JNEUROSCI.1253-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf E.R., Daniels R.W., Burgess R.W., Schwarz T.L., DiAntonio A. 2009. Rab3 dynamically controls protein composition at active zones. Neuron. 64:663–677 10.1016/j.neuron.2009.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H., Maness P.F. 2010. Perisomatic GABAergic innervation in prefrontal cortex is regulated by ankyrin interaction with the L1 cell adhesion molecule. Cereb. Cortex. 20:2684–2693 10.1093/cercor/bhq016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez J.P., Webb A., Bence M., Bildsoe H., Sahores M., Hughes S.M., Salinas P.C. 2008. Wnt signaling promotes AChR aggregation at the neuromuscular synapse in collaboration with agrin. Proc. Natl. Acad. Sci. USA. 105:18812–18817 10.1073/pnas.0806300105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H., Pang Z., Bao D., Miyazaki T., Li L., Miura E., Parris J., Rong Y., Watanabe M., Yuzaki M., Morgan J.I. 2005. Cbln1 is essential for synaptic integrity and plasticity in the cerebellum. Nat. Neurosci. 8:1534–1541 10.1038/nn1576 [DOI] [PubMed] [Google Scholar]
- Hong W., Mosca T.J., Luo L. 2012. Teneurins instruct synaptic partner matching in an olfactory map. Nature. 484:201–207 10.1038/nature10926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P., Hoogenraad C.C. 2010. Actin in dendritic spines: connecting dynamics to function. J. Cell Biol. 189:619–629 10.1083/jcb.201003008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.F., Banker G. 2012. The translocation selectivity of the kinesins that mediate neuronal organelle transport. Traffic. 13:549–564 10.1111/j.1600-0854.2011.01325.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T., Miura E., Matsuda K., Kamekawa Y., Watanabe M., Yuzaki M. 2007. Characterization of a transneuronal cytokine family Cbln—regulation of secretion by heteromeric assembly. Eur. J. Neurosci. 25:1049–1057 10.1111/j.1460-9568.2007.05361.x [DOI] [PubMed] [Google Scholar]
- Iijima T., Emi K., Yuzaki M. 2009. Activity-dependent repression of Cbln1 expression: mechanism for developmental and homeostatic regulation of synapses in the cerebellum. J. Neurosci. 29:5425–5434 10.1523/JNEUROSCI.4473-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaki M., Yoshikawa S., Thomas J.B., Aburatani H., Nose A. 2007. Wnt4 is a local repulsive cue that determines synaptic target specificity. Curr. Biol. 17:1574–1579 10.1016/j.cub.2007.08.013 [DOI] [PubMed] [Google Scholar]
- Jedlicka P., Hoon M., Papadopoulos T., Vlachos A., Winkels R., Poulopoulos A., Betz H., Deller T., Brose N., Varoqueaux F., Schwarzacher S.W. 2011. Increased dentate gyrus excitability in neuroligin-2-deficient mice in vivo. Cereb. Cortex. 21:357–367 10.1093/cercor/bhq100 [DOI] [PubMed] [Google Scholar]
- Jensen M., Hoerndli F.J., Brockie P.J., Wang R., Johnson E., Maxfield D., Francis M.M., Madsen D.M., Maricq A.V. 2012. Wnt signaling regulates acetylcholine receptor translocation and synaptic plasticity in the adult nervous system. Cell. 149:173–187 10.1016/j.cell.2011.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Garner C.C. 2008. Molecular mechanisms of presynaptic differentiation. Annu. Rev. Cell Dev. Biol. 24:237–262 10.1146/annurev.cellbio.23.090506.123417 [DOI] [PubMed] [Google Scholar]
- Jing L., Lefebvre J.L., Gordon L.R., Granato M. 2009. Wnt signals organize synaptic prepattern and axon guidance through the zebrafish unplugged/MuSK receptor. Neuron. 61:721–733 10.1016/j.neuron.2008.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.L., III, Fetter R.D., Davis G.W. 2009. Negative regulation of active zone assembly by a newly identified SR protein kinase. PLoS Biol. 7:e1000193 10.1371/journal.pbio.1000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo J.Y., Lee S.J., Uemura T., Yoshida T., Yasumura M., Watanabe M., Mishina M. 2011. Differential interactions of cerebellin precursor protein (Cbln) subtypes and neurexin variants for synapse formation of cortical neurons. Biochem. Biophys. Res. Commun. 406:627–632 10.1016/j.bbrc.2011.02.108 [DOI] [PubMed] [Google Scholar]
- Kaeser P.S., Deng L., Chávez A.E., Liu X., Castillo P.E., Südhof T.C. 2009. ELKS2alpha/CAST deletion selectively increases neurotransmitter release at inhibitory synapses. Neuron. 64:227–239 10.1016/j.neuron.2009.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa W., Miyazaki T., Emi K., Matsuda K., Kohda K., Motohashi J., Mishina M., Kawahara S., Watanabe M., Yuzaki M. 2008. Differential regulation of synaptic plasticity and cerebellar motor learning by the C-terminal PDZ-binding motif of GluRdelta2. J. Neurosci. 28:1460–1468 10.1523/JNEUROSCI.2553-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa W., Miyazaki T., Kohda K., Matsuda K., Emi K., Motohashi J., Watanabe M., Yuzaki M. 2009. The N-terminal domain of GluD2 (GluRdelta2) recruits presynaptic terminals and regulates synaptogenesis in the cerebellum in vivo. J. Neurosci. 29:5738–5748 10.1523/JNEUROSCI.6013-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y., Zhang X., Dobie F., Wu H., Craig A.M. 2008. Induction of GABAergic postsynaptic differentiation by alpha-neurexins. J. Biol. Chem. 283:2323–2334 10.1074/jbc.M703957200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwabuchi N., Ikeda K., Araki K., Hirano T., Shibuki K., Takayama C., Inoue Y., Kutsuwada T., Yagi T., Kang Y., et al. 1995. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell. 81:245–252 10.1016/0092-8674(95)90334-8 [DOI] [PubMed] [Google Scholar]
- Kim S.H., Ryan T.A. 2010. CDK5 serves as a major control point in neurotransmitter release. Neuron. 67:797–809 10.1016/j.neuron.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Ryan T.A. 2013. Balance of calcineurin Aα and CDK5 activities sets release probability at nerve terminals. J. Neurosci. 33:8937–8950 10.1523/JNEUROSCI.4288-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N., Stiegler A.L., Cameron T.O., Hallock P.T., Gomez A.M., Huang J.H., Hubbard S.R., Dustin M.L., Burden S.J. 2008. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 135:334–342 10.1016/j.cell.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen M.P., Shen K. 2007. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 130:704–716 10.1016/j.cell.2007.06.046 [DOI] [PubMed] [Google Scholar]
- Klassen M.P., Wu Y.E., Maeder C.I., Nakae I., Cueva J.G., Lehrman E.K., Tada M., Gengyo-Ando K., Wang G.J., Goodman M., et al. 2010. An Arf-like small G protein, ARL-8, promotes the axonal transport of presynaptic cargoes by suppressing vesicle aggregation. Neuron. 66:710–723 10.1016/j.neuron.2010.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J., Fuccillo M.V., Malenka R.C., Südhof T.C. 2009. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron. 64:791–798 10.1016/j.neuron.2009.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J., Soler-Llavina G.J., Fuccillo M.V., Malenka R.C., Südhof T.C. 2011. Neuroligins/LRRTMs prevent activity- and Ca2+/calmodulin-dependent synapse elimination in cultured neurons. J. Cell Biol. 194:323–334 10.1083/jcb.201101072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda K., Kakegawa W., Matsuda S., Nakagami R., Kakiya N., Yuzaki M. 2007. The extreme C-terminus of GluRdelta2 is essential for induction of long-term depression in cerebellar slices. Eur. J. Neurosci. 25:1357–1362 10.1111/j.1460-9568.2007.05412.x [DOI] [PubMed] [Google Scholar]
- Koles K., Nunnari J., Korkut C., Barria R., Brewer C., Li Y., Leszyk J., Zhang B., Budnik V. 2012. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J. Biol. Chem. 287:16820–16834 10.1074/jbc.M112.342667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkut C., Ataman B., Ramachandran P., Ashley J., Barria R., Gherbesi N., Budnik V. 2009. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 139:393–404 10.1016/j.cell.2009.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukdereli H., Allen N.J., Lee A.T., Feng A., Ozlu M.I., Conatser L.M., Chakraborty C., Workman G., Weaver M., Sage E.H., et al. 2011. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc. Natl. Acad. Sci. USA. 108:E440–E449 10.1073/pnas.1104977108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara H., Hashimoto K., Kano M., Takayama C., Sakimura K., Mishina M., Inoue Y., Watanabe M. 1997. Impaired parallel fiber—>Purkinje cell synapse stabilization during cerebellar development of mutant mice lacking the glutamate receptor delta2 subunit. J. Neurosci. 17:9613–9623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S.K., Woo J., Kim S.Y., Kim H., Kim E. 2010. Trans-synaptic adhesions between netrin-G ligand-3 (NGL-3) and receptor tyrosine phosphatases LAR, protein-tyrosine phosphatase delta (PTPdelta), and PTPsigma via specific domains regulate excitatory synapse formation. J. Biol. Chem. 285:13966–13978 10.1074/jbc.M109.061127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledda F., Paratcha G., Sandoval-Guzmán T., Ibáñez C.F. 2007. GDNF and GFRalpha1 promote formation of neuronal synapses by ligand-induced cell adhesion. Nat. Neurosci. 10:293–300 10.1038/nn1855 [DOI] [PubMed] [Google Scholar]
- Lee S.H., Sheng M. 2000. Development of neuron-neuron synapses. Curr. Opin. Neurobiol. 10:125–131 10.1016/S0959-4388(99)00046-X [DOI] [PubMed] [Google Scholar]
- Li J., Ashley J., Budnik V., Bhat M.A. 2007. Crucial role of Drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron. 55:741–755 10.1016/j.neuron.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zheng Z., Keifer J. 2011. Transsynaptic EphB/Ephrin-B signaling regulates growth of presynaptic boutons required for classical conditioning. J. Neurosci. 31:8441–8449 10.1523/JNEUROSCI.6343-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao E.H., Hung W., Abrams B., Zhen M. 2004. An SCF-like ubiquitin ligase complex that controls presynaptic differentiation. Nature. 430:345–350 10.1038/nature02647 [DOI] [PubMed] [Google Scholar]
- Linhoff M.W., Laurén J., Cassidy R.M., Dobie F.A., Takahashi H., Nygaard H.B., Airaksinen M.S., Strittmatter S.M., Craig A.M. 2009. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 61:734–749 10.1016/j.neuron.2009.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.S., Schubert C.R., Fu X., Fourniol F.J., Jaiswal J.K., Houdusse A., Stultz C.M., Moores C.A., Walsh C.A. 2012. Molecular basis for specific regulation of neuronal kinesin-3 motors by doublecortin family proteins. Mol. Cell. 47:707–721 10.1016/j.molcel.2012.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.S.Y., Siebert M., Mertel S., Knoche E., Wegener S., Wichmann C., Matkovic T., Muhammad K., Depner H., Mettke C., et al. 2011. RIM-binding protein, a central part of the active zone, is essential for neurotransmitter release. Science. 334:1565–1569 10.1126/science.1212991 [DOI] [PubMed] [Google Scholar]
- Lucido A.L., Suarez Sanchez F., Thostrup P., Kwiatkowski A.V., Leal-Ortiz S., Gopalakrishnan G., Liazoghli D., Belkaid W., Lennox R.B., Grutter P., et al. 2009. Rapid assembly of functional presynaptic boutons triggered by adhesive contacts. J. Neurosci. 29:12449–12466 10.1523/JNEUROSCI.1381-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas C., Torres V.I., Altrock W.D., Leal-Ortiz S., Wagh D., Terry-Lorenzo R.T., Fejtova A., Gundelfinger E.D., Ziv N.E., Garner C.C. 2012. Formation of Golgi-derived active zone precursor vesicles. J. Neurosci. 32:11095–11108 10.1523/JNEUROSCI.0195-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaskill A.F., Rinholm J.E., Twelvetrees A.E., Arancibia-Carcamo I.L., Muir J., Fransson A., Aspenstrom P., Attwell D., Kittler J.T. 2009. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 61:541–555 10.1016/j.neuron.2009.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniar T.A., Kaplan M., Wang G.J., Shen K., Wei L., Shaw J.E., Koushika S.P., Bargmann C.I. 2012. UNC-33 (CRMP) and ankyrin organize microtubules and localize kinesin to polarize axon-dendrite sorting. Nat. Neurosci. 15:48–56 10.1038/nn.2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Yuzaki M. 2011. Cbln family proteins promote synapse formation by regulating distinct neurexin signaling pathways in various brain regions. Eur. J. Neurosci. 33:1447–1461 10.1111/j.1460-9568.2011.07638.x [DOI] [PubMed] [Google Scholar]
- Matsuda K., Miura E., Miyazaki T., Kakegawa W., Emi K., Narumi S., Fukazawa Y., Ito-Ishida A., Kondo T., Shigemoto R., et al. 2010. Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science. 328:363–368 10.1126/science.1185152 [DOI] [PubMed] [Google Scholar]
- McAllister A.K. 2007. Dynamic aspects of CNS synapse formation. Annu. Rev. Neurosci. 30:425–450 10.1146/annurev.neuro.29.051605.112830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M., Zhang W., Rohlmann A., Kattenstroth G., Hammer R.E., Gottmann K., Südhof T.C. 2003. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 423:939–948 10.1038/nature01755 [DOI] [PubMed] [Google Scholar]
- Miura E., Iijima T., Yuzaki M., Watanabe M. 2006. Distinct expression of Cbln family mRNAs in developing and adult mouse brains. Eur. J. Neurosci. 24:750–760 10.1111/j.1460-9568.2006.04950.x [DOI] [PubMed] [Google Scholar]
- Mosca T.J., Hong W., Dani V.S., Favaloro V., Luo L. 2012. Trans-synaptic Teneurin signalling in neuromuscular synapse organization and target choice. Nature. 484:237–241 10.1038/nature10923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K., Yang X., Gerber S.H., Kwon H.B., Ho A., Castillo P.E., Liu X., Südhof T.C. 2010. Piccolo and bassoon maintain synaptic vesicle clustering without directly participating in vesicle exocytosis. Proc. Natl. Acad. Sci. USA. 107:6504–6509 10.1073/pnas.1002307107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K., Abrams B., Grill B., Goncharov A., Huang X., Chisholm A.D., Jin Y. 2005. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 120:407–420 10.1016/j.cell.2004.12.017 [DOI] [PubMed] [Google Scholar]
- Nam C.I., Chen L. 2005. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc. Natl. Acad. Sci. USA. 102:6137–6142 10.1073/pnas.0502038102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa S., Tanaka Y., Hirokawa N. 2008. KIF1Bβ- and KIF1A-mediated axonal transport of presynaptic regulator Rab3 occurs in a GTP-dependent manner through DENN/MADD. Nature. 10:1269–1279 [DOI] [PubMed] [Google Scholar]
- Ou C.-Y., Poon V.Y., Maeder C.I., Watanabe S., Lehrman E.K., Fu A.K.Y., Park M., Fu W.-Y., Jorgensen E.M., Ip N.Y., Shen K. 2010. Two cyclin-dependent kinase pathways are essential for polarized trafficking of presynaptic components. Cell. 141:846–858 10.1016/j.cell.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D., Fouquet W., Schmidt M., Wichmann C., Mertel S., Depner H., Christiansen F., Zube C., Quentin C., Körner J., et al. 2010. A Syd-1 homologue regulates pre- and postsynaptic maturation in Drosophila. J. Cell Biol. 188:565–579 10.1083/jcb.200908055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D., Khorramshahi O., Gupta V.K., Banovic D., Depner H., Fouquet W., Wichmann C., Mertel S., Eimer S., Reynolds E., et al. 2012. Cooperation of Syd-1 with Neurexin synchronizes pre- with postsynaptic assembly. Nat. Neurosci. 15:1219–1226 10.1038/nn.3183 [DOI] [PubMed] [Google Scholar]
- Patel M.R., Shen K. 2009. RSY-1 is a local inhibitor of presynaptic assembly in C. elegans. Science. 323:1500–1503 10.1126/science.1169025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M.R., Lehrman E.K., Poon V.Y., Crump J.G., Zhen M., Bargmann C.I., Shen K. 2006. Hierarchical assembly of presynaptic components in defined C. elegans synapses. Nat. Neurosci. 9:1488–1498 10.1038/nn1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto R.T., Kunz P.A., Kwon H., Mabb A.M., Sabatini B.L., Philpot B.D., Ehlers M.D. 2012. Transsynaptic signaling by activity-dependent cleavage of neuroligin-1. Neuron. 76:396–409 10.1016/j.neuron.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielage J., Fetter R.D., Davis G.W. 2005. Presynaptic spectrin is essential for synapse stabilization. Curr. Biol. 15:918–928 10.1016/j.cub.2005.04.030 [DOI] [PubMed] [Google Scholar]
- Pielage J., Fetter R.D., Davis G.W. 2006. A postsynaptic spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J. Cell Biol. 175:491–503 10.1083/jcb.200607036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielage J., Bulat V., Zuchero J.B., Fetter R.D., Davis G.W. 2011. Hts/Adducin controls synaptic elaboration and elimination. Neuron. 69:1114–1131 10.1016/j.neuron.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon V.Y., Klassen M.P., Shen K. 2008. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature. 455:669–673 10.1038/nature07291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulopoulos A., Aramuni G., Meyer G., Soykan T., Hoon M., Papadopoulos T., Zhang M., Paarmann I., Fuchs C., Harvey K., et al. 2009. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 63:628–642 10.1016/j.neuron.2009.08.023 [DOI] [PubMed] [Google Scholar]
- Prange O., Wong T.P., Gerrow K., Wang Y.T., El-Husseini A. 2004. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc. Natl. Acad. Sci. USA. 101:13915–13920 10.1073/pnas.0405939101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls M.M., Satoh D., Clyne P.J., Henner A.L., Uemura T., Doe C.Q. 2007. Polarity and intracellular compartmentalization of Drosophila neurons. Neural Dev. 2:7 10.1186/1749-8104-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J.R., Yamagata M. 2009. Many paths to synaptic specificity. Annu. Rev. Cell Dev. Biol. 25:161–195 10.1146/annurev.cellbio.24.110707.175402 [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S., Atluri P.P., Ryan T.A. 2003. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat. Neurosci. 6:127–135 10.1038/nn1002 [DOI] [PubMed] [Google Scholar]
- Scheiffele P., Fan J., Choih J., Fetter R., Serafini T. 2000. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 101:657–669 10.1016/S0092-8674(00)80877-6 [DOI] [PubMed] [Google Scholar]
- Schubert V., Dotti C.G. 2007. Transmitting on actin: synaptic control of dendritic architecture. J. Cell Sci. 120:205–212 10.1242/jcs.03337 [DOI] [PubMed] [Google Scholar]
- Seeger M.A., Rice S.E. 2010. Microtubule-associated protein-like binding of the kinesin-1 tail to microtubules. J. Biol. Chem. 285:8155–8162 10.1074/jbc.M109.068247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira M., Zhai R.G., Dresbach T., Bresler T., Torres V.I., Gundelfinger E.D., Ziv N.E., Garner C.C. 2003. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron. 38:237–252 10.1016/S0896-6273(03)00207-1 [DOI] [PubMed] [Google Scholar]
- Silva J.P., Lelianova V.G., Ermolyuk Y.S., Vysokov N., Hitchen P.G., Berninghausen O., Rahman M.A., Zangrandi A., Fidalgo S., Tonevitsky A.G., et al. 2011. Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities. Proc. Natl. Acad. Sci. USA. 108:12113–12118 10.1073/pnas.1019434108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Llavina G.J., Fuccillo M.V., Ko J., Südhof T.C., Malenka R.C. 2011. The neurexin ligands, neuroligins and leucine-rich repeat transmembrane proteins, perform convergent and divergent synaptic functions in vivo. Proc. Natl. Acad. Sci. USA. 108:16502–16509 10.1073/pnas.1114028108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler S.A., Schmitz S.K., Kevenaar J.T., de Graaff E., de Wit H., Demmers J., Toonen R.F., Hoogenraad C.C. 2013. Liprin-α2 promotes the presynaptic recruitment and turnover of RIM1/CASK to facilitate synaptic transmission. J. Cell Biol. 201:915–928 10.1083/jcb.201301011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan A., Pielarski K.N., Brigadski T., Wittenmayer N., Fedorchenko O., Gohla A., Lessmann V., Dresbach T., Gottmann K. 2010. Essential cooperation of N-cadherin and neuroligin-1 in the transsynaptic control of vesicle accumulation. Proc. Natl. Acad. Sci. USA. 107:11116–11121 10.1073/pnas.0914233107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavoe A.K.H., Nelson J.C., Martínez-Velázquez L.A., Klein M., Samuel A.D.T., Colón-Ramos D.A. 2012. Synaptic vesicle clustering requires a distinct MIG-10/Lamellipodin isoform and ABI-1 downstream from Netrin. Genes Dev. 26:2206–2221 10.1101/gad.193409.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova T., Slemmer J., Hoogenraad C.C., Lansbergen G., Dortland B., De Zeeuw C.I., Grosveld F., van Cappellen G., Akhmanova A., Galjart N. 2003. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein). J. Neurosci. 23:2655–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., Micheva K.D., Mehalow A.K., Huberman A.D., Stafford B., et al. 2007. The classical complement cascade mediates CNS synapse elimination. Cell. 131:1164–1178 10.1016/j.cell.2007.10.036 [DOI] [PubMed] [Google Scholar]
- Stigloher C., Zhan H., Zhen M., Richmond J., Bessereau J.L. 2011. The presynaptic dense projection of the Caenorhabditis elegans cholinergic neuromuscular junction localizes synaptic vesicles at the active zone through SYD-2/liprin and UNC-10/RIM-dependent interactions. J. Neurosci. 31:4388–4396 10.1523/JNEUROSCI.6164-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M.C., Roegiers F., Rolls M.M. 2008. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol. Biol. Cell. 19:4122–4129 10.1091/mbc.E07-10-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Xing G., Yuan L., Gan G., Knight D., With S.I., He C., Han J., Zeng X., Fang M., et al. 2011. Neuroligin 2 is required for synapse development and function at the Drosophila neuromuscular junction. J. Neurosci. 31:687–699 10.1523/JNEUROSCI.3854-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Bamji S.X. 2011. β-Pix modulates actin-mediated recruitment of synaptic vesicles to synapses. J. Neurosci. 31:17123–17133 10.1523/JNEUROSCI.2359-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Katayama K., Sohya K., Miyamoto H., Prasad T., Matsumoto Y., Ota M., Yasuda H., Tsumoto T., Aruga J., Craig A.M. 2012. Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction. Nat. Neurosci. 15:389–398: S1–S2 10.1038/nn.3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T., Miyazaki T., Watanabe M., Mori H., Sakimura K., Mishina M. 2005. Control of synaptic connection by glutamate receptor delta2 in the adult cerebellum. J. Neurosci. 25:2146–2156 10.1523/JNEUROSCI.4740-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taru H., Jin Y. 2011. The Liprin homology domain is essential for the homomeric interaction of SYD-2/Liprin-α protein in presynaptic assembly. J. Neurosci. 31:16261–16268 10.1523/JNEUROSCI.0002-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Kakizawa S., Yamasaki M., Sakimura K., Watanabe M., Iino M., Mishina M. 2007. Regulation of long-term depression and climbing fiber territory by glutamate receptor delta2 at parallel fiber synapses through its C-terminal domain in cerebellar Purkinje cells. J. Neurosci. 27:12096–12108 10.1523/JNEUROSCI.2680-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Lee S.J., Yasumura M., Takeuchi T., Yoshida T., Ra M., Taguchi R., Sakimura K., Mishina M. 2010. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 141:1068–1079 10.1016/j.cell.2010.04.035 [DOI] [PubMed] [Google Scholar]
- Waites C.L., Craig A.M., Garner C.C. 2005. Mechanisms of vertebrate synaptogenesis. Annu. Rev. Neurosci. 28:251–274 10.1146/annurev.neuro.27.070203.144336 [DOI] [PubMed] [Google Scholar]
- Waites C.L., Leal-Ortiz S.A., Andlauer T.F.M., Sigrist S.J., Garner C.C. 2011. Piccolo regulates the dynamic assembly of presynaptic F-actin. J. Neurosci. 31:14250–14263 10.1523/JNEUROSCI.1835-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites C.L., Leal-Ortiz S.A., Okerlund N., Dalke H., Fejtová A., Altrock W.D., Gundelfinger E.D., Garner C.C. 2013. Bassoon and Piccolo maintain synapse integrity by regulating protein ubiquitination and degradation. EMBO J. 32:954–969 10.1038/emboj.2013.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H.I., DiAntonio A., Fetter R.D., Bergstrom K., Strauss R., Goodman C.S. 2000. Highwire regulates synaptic growth in Drosophila. Neuron. 26:313–329 10.1016/S0896-6273(00)81166-6 [DOI] [PubMed] [Google Scholar]
- Wang J., Ruan N.J., Qian L., Lei W.L., Chen F., Luo Z.G. 2008. Wnt/beta-catenin signaling suppresses Rapsyn expression and inhibits acetylcholine receptor clustering at the neuromuscular junction. J. Biol. Chem. 283:21668–21675 10.1074/jbc.M709939200 [DOI] [PubMed] [Google Scholar]
- Wang X., Schwarz T.L. 2009. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell. 136:163–174 10.1016/j.cell.2008.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hu B., Zieba A., Neumann N.G., Kasper-Sonnenberg M., Honsbein A., Hultqvist G., Conze T., Witt W., Limbach C., et al. 2009. A protein interaction node at the neurotransmitter release site: domains of Aczonin/Piccolo, Bassoon, CAST, and rim converge on the N-terminal domain of Munc13-1. J. Neurosci. 29:12584–12596 10.1523/JNEUROSCI.1255-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzel C., Sommer J.E., Nair R., Stiefvater A., Sibarita J.-B., Scheiffele P. 2013. mSYD1A, a mammalian synapse-defective-1 protein, regulates synaptogenic signaling and vesicle docking. Neuron. 78:1012–1023 10.1016/j.neuron.2013.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenmayer N., Körber C., Liu H., Kremer T., Varoqueaux F., Chapman E.R., Brose N., Kuner T., Dresbach T. 2009. Postsynaptic Neuroligin1 regulates presynaptic maturation. Proc. Natl. Acad. Sci. USA. 106:13564–13569 10.1073/pnas.0905819106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J., Kwon S.K., Choi S., Kim S., Lee J.R., Dunah A.W., Sheng M., Kim E. 2009. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat. Neurosci. 12:428–437 10.1038/nn.2279 [DOI] [PubMed] [Google Scholar]
- Wu Y.E., Huo L., Maeder C.I., Feng W., Shen K. 2013. The balance between capture and dissociation of presynaptic proteins controls the spatial distribution of synapses. Neuron. 78:994–1011 10.1016/j.neuron.2013.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Xiao N., Xia J. 2010. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat. Neurosci. 13:22–24 10.1038/nn.2459 [DOI] [PubMed] [Google Scholar]
- Yan D., Wu Z., Chisholm A.D., Jin Y. 2009. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell. 138:1005–1018 10.1016/j.cell.2009.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Chao D.L., Toba S., Koyasako K., Yasunaga T., Hirotsune S., Shen K. 2013. Kinesin-1 regulates dendrite microtubule polarity in Caenorhabditis elegans. Elife. 2:e00133 10.7554/eLife.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumura M., Yoshida T., Lee S.J., Uemura T., Joo J.Y., Mishina M. 2012. Glutamate receptor δ1 induces preferentially inhibitory presynaptic differentiation of cortical neurons by interacting with neurexins through cerebellin precursor protein subtypes. J. Neurochem. 121:705–716 10.1111/j.1471-4159.2011.07631.x [DOI] [PubMed] [Google Scholar]
- Yoshida T., Shiroshima T., Lee S.J., Yasumura M., Uemura T., Chen X., Iwakura Y., Mishina M. 2012. Interleukin-1 receptor accessory protein organizes neuronal synaptogenesis as a cell adhesion molecule. J. Neurosci. 32:2588–2600 10.1523/JNEUROSCI.4637-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumoto N., Kim N., Burden S.J. 2012. Lrp4 is a retrograde signal for presynaptic differentiation at neuromuscular synapses. Nature. 489:438–442 10.1038/nature11348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai R.G., Vardinon-Friedman H., Cases-Langhoff C., Becker B., Gundelfinger E.D., Ziv N.E., Garner C.C. 2001. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron. 29:131–143 10.1016/S0896-6273(01)00185-4 [DOI] [PubMed] [Google Scholar]
- Zhang W., Benson D.L. 2001. Stages of synapse development defined by dependence on F-actin. J. Neurosci. 21:5169–5181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Benson D.L. 2002. Developmentally regulated changes in cellular compartmentation and synaptic distribution of actin in hippocampal neurons. J. Neurosci. Res. 69:427–436 10.1002/jnr.10313 [DOI] [PubMed] [Google Scholar]
- Zhang W., Rohlmann A., Sargsyan V., Aramuni G., Hammer R.E., Südhof T.C., Missler M. 2005. Extracellular domains of alpha-neurexins participate in regulating synaptic transmission by selectively affecting N- and P/Q-type Ca2+ channels. J. Neurosci. 25:4330–4342 10.1523/JNEUROSCI.0497-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Luo S., Wang Q., Suzuki T., Xiong W.C., Mei L. 2008. LRP4 serves as a coreceptor of agrin. Neuron. 60:285–297 10.1016/j.neuron.2008.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Liang C., Bates R., Yin Y., Xiong W.C., Mei L. 2012. Wnt proteins regulate acetylcholine receptor clustering in muscle cells. Mol. Brain. 5:7 10.1186/1756-6606-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Wang D., Wang Q., Rodal A.A., Zhang Y.Q. 2013. Drosophila cyfip regulates synaptic development and endocytosis by suppressing filamentous actin assembly. PLoS Genet. 9:e1003450 10.1371/journal.pgen.1003450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen M., Jin Y. 1999. The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans. Nature. 401:371–375 [DOI] [PubMed] [Google Scholar]
- Zhen M., Huang X., Bamber B., Jin Y. 2000. Regulation of presynaptic terminal organization by C. elegans RPM-1, a putative guanine nucleotide exchanger with a RING-H2 finger domain. Neuron. 26:331–343 10.1016/S0896-6273(00)81167-8 [DOI] [PubMed] [Google Scholar]
- Zhou H., Glass D.J., Yancopoulos G.D., Sanes J.R. 1999. Distinct domains of MuSK mediate its abilities to induce and to associate with postsynaptic specializations. J. Cell Biol. 146:1133–1146 10.1083/jcb.146.5.1133 [DOI] [PMC free article] [PubMed] [Google Scholar]