Abstract
Objective:
To investigate CT findings in patients with pathologically proven mesenteric ischaemia post-cardiopulmonary bypass surgery and compare them with the control group of patients without ischaemia.
Methods:
68 patients were identified by a search of local surgical and pathological databases; these patients met the inclusion criteria of a laparotomy within 1 month of a procedure requiring cardiopulmonary bypass and a CT abdomen/pelvis within 1 week of the pathological diagnosis. Two radiologists independently reviewed the studies, evaluating 17 separate findings relating to the bowel, the vasculature or other structures; consensus was subsequently reached. The diagnostic value of CT findings was assessed using logistic regression.
Results:
52 of 68 patients had pathologically proven ischaemia. Portal venous gas, mesenteric venous gas and small bowel faeces sign all had specificities of >0.94 for ischaemia but low sensitivity (<0.27). Differential mural enhancement had high sensitivity (0.92) but poor specificity (0.50). The combination of pneumatosis, bowel loop dilatation and differential mural enhancement predicted bowel ischaemia with a probability of 98%. The hardest signs to interpret based on poor interreader kappa agreement were bowel wall thinning, mesenteric stranding and differential mural enhancement.
Conclusion:
A combination of CT signs was predictive of ischaemic bowel; however, the more specific findings lacked sensitivity. If clinical suspicion is high for bowel ischaemia, prompt surgical intervention is warranted, regardless of CT findings.
Advances in knowledge:
Arterial occlusion was uncommon and venous occlusion was not present, which is supportive of a predominantly non-occlusive aetiology for ischaemia in this patient group.
Mesenteric ischaemia with resulting bowel infarction is a potentially life-threatening complication following cardio-pulmonary bypass (CPB) surgery. The frequency following such operations is rare (0.49–2.00%) [1–3]; however, the mortality from acute mesenteric ischaemia of any aetiology is high at 70–100%, even for patients managed in specialist tertiary referral centres [4–6]. Although there has been a recent move away from coronary artery bypass graft (CABG) surgery to percutaneous transluminal coronary intervention, there has been an increase in the number of complex CABG surgical procedures performed, e.g. CABG with mixed valve replacement. This, combined with an ageing patient population with associated increased co-morbidities and risk factors, may lead to a rise in the incidence of ischaemic bowel in patients following CPB surgery [3]. Furthermore, definitive radiological diagnosis is known to be difficult in such patients [7]. The most common CT findings lack specificity, whereas the more specific findings are rarely present [8], thus knowledge of such CT findings and their diagnostic value would be beneficial.
Mesenteric angiography was previously considered the gold standard radiological test for the diagnosis of mesenteric ischaemia of any aetiology. Although this offers the additional benefit of treatment in certain cases [9], the technique is invasive, availability may be limited in the acute setting, and it may be challenging in unstable post-operative patients [10]. CT overcomes some of these issues and provides additional diagnostic information about the bowel wall, solid intra-abdominal organs and vessel walls. In our selected patient group, non-occlusive mesenteric ischaemia owing to hypoperfusion associated with a low cardiac output postoperatively would be expected to be more prevalent than occlusive ischaemia [11,12].
There have been several studies with a small number of patients looking at the multidetector CT features of patients presenting with mesenteric ischaemia [7,9,13–15], but to our knowledge, apart from a small case series [16], there are no studies specifically investigating the CT signs of bowel ischaemia in a post-cardiac surgery cohort. Thus, the aim of our study was to investigate the CT findings following pathologically proven mesenteric ischaemia/infarction in a retrospective group of patients postcardiac bypass surgery and compare this with the known features of acute mesenteric ischaemia.
MATERIALS AND METHODS
Patients
Institutional review board approval was obtained for retrospective data collection and analysis. Patients were identified by searches of local surgical and pathological databases. 119 patients were identified who had a laparotomy following a procedure requiring CPB, and 87 patients were identified from the pathological database who had bowel ischaemia/infarction listed as either part 1a (the immediate cause of death) or part 1b (condition that directly preceded part 1a). The following inclusion criteria were then applied: CT abdomen/pelvis within 1 month of an operative procedure requiring CPB, with laparotomy and/or postmortem within 1 week of CT (Figure 1). All clinical indications for CT were included regardless of suspicion for ischaemic bowel.
Figure 1.
Patients’ selection flow chart. CPB, cardio-pulmonary bypass.
Imaging acquisition
The CT examinations were performed on either a 64-slice (n=45) multidetector CT or a 4-slice (n=23) multidetector CT (Siemens Definition and Siemens Plus 4 Volume Zoom, respectively; Siemens Medical, Forchheim, Germany). All studies were performed following the administration of 90–120 ml iopamidol intravenous contrast medium (Niopam 300; Bracco UK Ltd, High Wycombe, UK), bolus injection via a power injector at a rate of 2.0–3.0 ml s−1. Images were acquired in either the arterial phase (n=3), the portal phase (n=59) or both (n=6). Acquisition was performed 25 s and 70 s from the start of injection for arterial phase and portal phase imaging, respectively. Tube voltage was 120 kV, the reference mAs values were 165 mAs (Siemens Plus 4) and 210 mAs [Siemens Definition, using CareDOSE™ (Siemens Medical)], collimation 4.0×2.5 (Siemens Plus 4) and 64.0×0.6 (Siemens Definition), slice thickness 1–2 mm.
Image interpretation
The data were retrospectively analysed by two readers (SU, ADT) independently; the readers were aware of the selection process but were blinded to the original CT reports. Images were viewed on a picture archiving and communication system workstation (Cadran Diagnostic Viewer; Cadran Imaging, Cambridge, UK) using axial slices, with post-processing software including maximum intensity projection and multiplanar reformatting available. At the time of interpretation, Reader 1 (SU) was a radiology consultant with a special interest in gastrointestinal radiology and 4 years’ experience and Reader 2 (ADT) was a radiology consultant at a tertiary cardiothoracic centre with 14 years’ experience.
CT images were assessed for parameters relating directly to the bowel (n=7), those relating to the vasculature (n=4), and other factors (n=6). These are listed in Table 1 and selected features are shown in Figures 2–7. Additionally, the positioning of an intra-aortic balloon pump (IABP) device was evaluated if present. The tip of an IABP should be positioned 2–3 cm distal to the left subclavian artery, with the balloon above the origins of the coeliac axis or superior mesenteric artery; a distal position of the balloon can compromise blood flow within these vessels. Patients with IABPs also receive inotrope support with or without vasoconstrictors, but the use of these agents was not separately recorded. Consensus agreement was subsequently agreed upon by three readers (T Barrett, SU, ADT).
Table 1.
Signs assessed
| Sign assessed | Notes |
| Bowel | |
| Mural thickening | If distended: >3 mm considered abnormal |
| If collapsed >10 mm considered abnormal | |
| Mural thinning | |
| Pneumatosis | |
| Bowel wall oedema | Wall thickening with fluid attenuation |
| Small bowel faeces sign | The presence of particulate (colon-like) faeculent matter mingled with gas bubbles in the lumen of dilated loops of the small intestine |
| Bowel obstruction | |
| Bowel-loop dilatation | Small bowel: >2.5 cm considered abnormal |
| Large bowel: >6 cm considered abnormal (Caecum: >8 cm) | |
| Vascular | |
| Differential mural enhancement | Considered pathological when absent, inhomogeneous, or marked and persistent |
| Venous thrombus | |
| Arterial occlusion | SMA/IMA/coeliac |
| Arterial calcification | SMA/IMA/coeliac |
| Other | |
| Mesenteric stranding | |
| Ascites | Not quantified |
| Solid organ infarction | |
| Free intraperitoneal air | Allowing for expected post-operative gas |
| Portal venous gas | |
| Mesenteric venous gas | Including presence of gas within the mesenteric venules |
| IABP position | See text |
IABP, intra-arterial balloon pump; IMA, inferior mesenteric artery; SMA, superior mesenteric artery.
Figure 2.
Extensive intramural pneumatosis. CT imaging in a 69-year-old male, 3 days post redo aortic valve replacement bioprosthesis surgery. Coronal reformats (a,b) and axial slices (c,d) show extensive pneumatosis in the bowel wall (arrows). The gas is more clearly demonstrated using bone window presets (b,d) than with the equivalent slices on standard abdominal window settings (a,c).
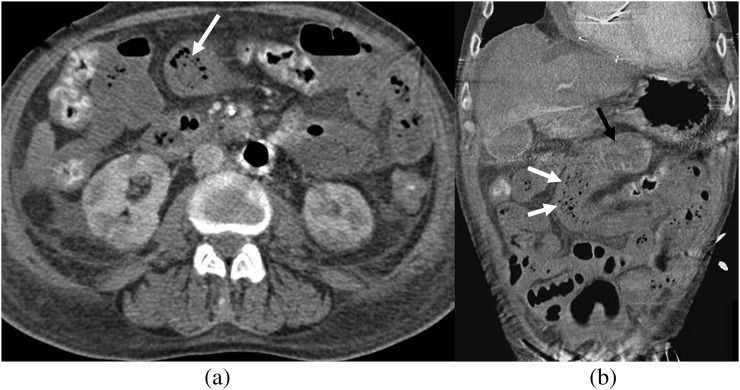
Figure 7.
Gas within mesenteric vessel. Portal phase imaging in a 61-year-old male patient, 2 days post coronary artery bypass graft surgery. Multiplanar coronal reformats (a,c) and axial CT (b) show multiple locules of gas within small peripheral mesenteric veins (arrows).
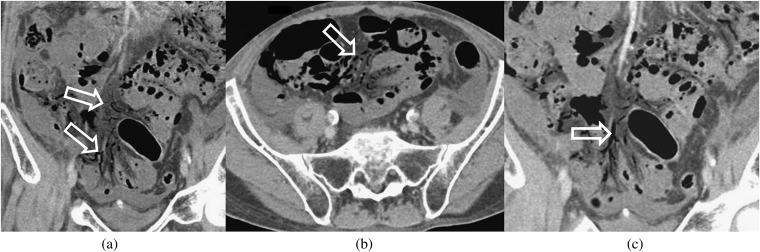
Figure 3.
Small bowel faeces sign. Axial (a) and coronal (b) reformatted CT images in an 80-year-old male patient, 3 days following coronary artery bypass graft surgery, show small bowel faeces sign (white arrows), a more proximal loop of jejunum is seen to be fluid-filled (black arrow).
Figure 4.
Differential bowel wall enhancement. (a) A 79-year-old female patient, 1 day post coronary artery bypass graft. Adjacent loops of ileum show differential enhancement with hypo- (white arrows) and hyperenhancement (black arrow). (b) Axial CT in a different patient showing differential enhancement of the descending colon with hyperenhancement of the medial wall (black arrow) and reduced enhancement and oedema of the lateral wall (white arrow).
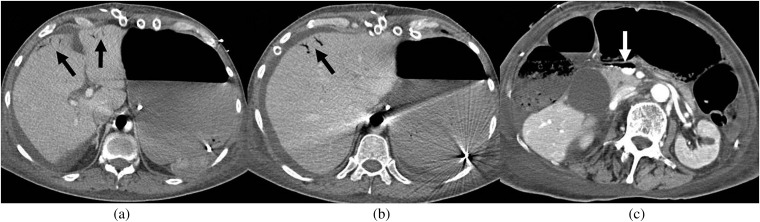
Figure 5.
Portal venous gas. (a,b) Axial CT images from a 79-year-old male, 4 days following coronary artery bypass graft (CABG) with aortic valve replacement (AVR), show locules of gas within peripheral portal vein branches (black arrows). (c) Axial CT slice from a 61-year-old male patient, 3 days post combined CABG/AVR surgery, shows gas in the extrahepatic portion of the main portal vein (white arrow); the gas lies anteriorly with the patient positioned supine.
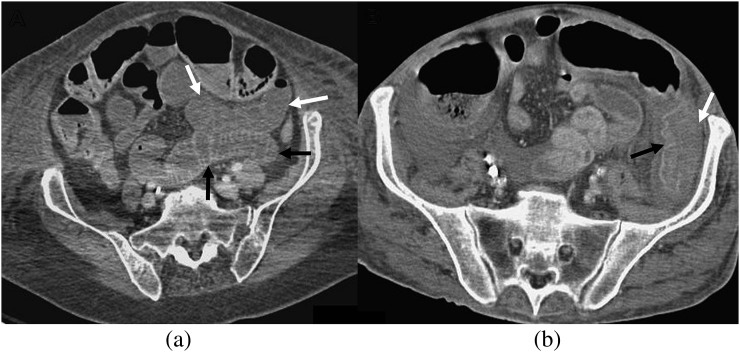
Figure 6.
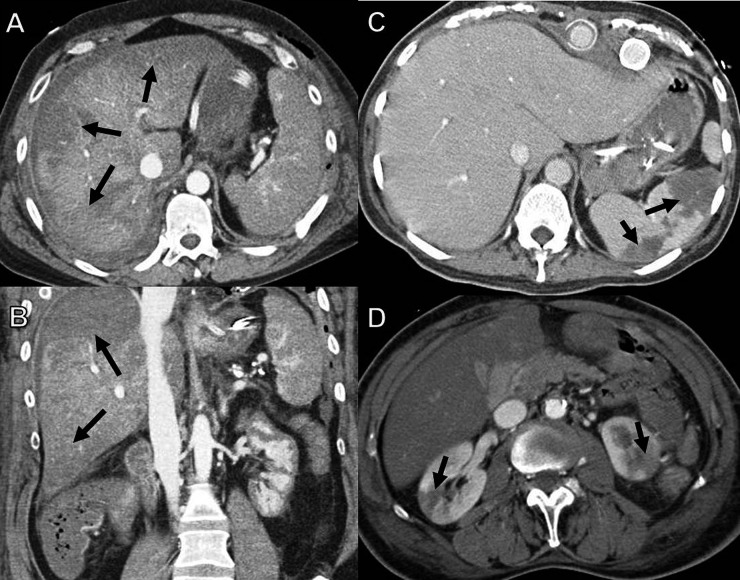
Solid organ hypoperfusion/infarction. (a,b) Axial CT imaging (a) with coronal reformats (b) in a 41-year-old female patient, 4 days post coronary artery bypass graft surgery, shows peripheral wedge-shaped areas of hypoperfusion in the liver (arrows). (c) Axial CT in an 82-year-old female patient, 10 days post aortic valve replacement (AVR) with multiple splenic infarcts (arrows). (d) A 65-year-old male patient, 14 days post mitral and AVR surgery with bilateral renal infarcts (arrows).
Statistical analysis
The interobserver agreement for the two readers was assessed using Cohen's kappa coefficient, with resulting agreement characterised according to Landis and Koch [17] as poor/slight for ≤0.2, fair for 0.21–0.40, moderate for 0.41–0.60, substantial for 0.61–0.80 and almost perfect for 0.81–1.00. Sensitivity and specificity and their 95% confidence intervals (CIs) of each individual CT finding were estimated based on the binomial distribution. The total number of signs present on CT was calculated, and the diagnostic value was assessed using the receiver operating characteristic (ROC) curve and the C-statistic (area under the ROC curve). In order to identify the most useful signs and to weight them, a logistic regression model was developed. The response variable used was the indicator of ischaemic bowel (52 cases and 16 controls), and the explanatory variables were the consensus score for each of the 16 signs (omitting venous thrombosis that was deemed not to be present). We used a forward selection strategy, including the most statistically significant sign at each stage until no additional variables increased the fit significantly according to the likelihood ratio test.
RESULTS
A total of 68 patients satisfied the selection criteria (46 males, 22 females). The average age of the patient group was 70.6 years (median 73 years; range 23–87 years). Pathologically proven ischaemic bowel was present in 52 patients (76%); 16 patients (24%) had no signs of bowel ischaemia at laparotomy or autopsy. The definitive diagnosis was provided in 33 patients by laparotomy, in 18 by autopsy alone and in 17 patients by both laparotomy and autopsy. Of the 16 patients without bowel ischaemia, the diagnosis was shown to be: no pathology (6 patients), pseudomembranous colitis (3 patients), a perforated duodenal ulcer, a chest drain perforating the stomach, a retroperitoneal haematoma, diverticulitis, congested large bowel (but no signs of ischaemia), splenic infarcts only and appendicitis. The initial CT reports were reviewed and suggested that ischaemic bowel was probable in 31 and possible in 6 of 52 cases where ischaemia was proven and probable in 5 and possible in 6 of the 16 cases where no ischaemia was demonstrated. Allowing for “probable” or “possible” results to be indicative of bowel ischaemia, this gives an overall sensitivity of 71.2% (37/52 cases) and specificity of 37.5% (6/16 cases) for the initial CT reports.
The most frequent operation requiring CPB was CABG (n=38), followed by CABG with aortic valve replacement (n=11). The average time from initial CPB operation to CT was 6.82 days (median 5 days; range 1–25 days). For patients undergoing laparotomy, the average time after CT was 1.16 days (range 0–7 days). The majority of patients had laparotomy within 48 h of the CT (42/50); 26 of these were within 24 h. There was a higher mortality within 1 month of CT in patients with proven mesenteric ischaemia than with controls (39/52; 75% compared with 4/16; 25%, Fisher’s exact test p=0.001).
Overall interreader agreement was substantial or excellent for 6 of the assessed signs (kappa coefficient >0.6) and moderate for 9 signs (kappa coefficient 0.41–0.60) (Table 2). There was poor kappa agreement for bowel wall thinning and venous thrombus (two patients were initially thought to have this sign, but on consensus reading, these cases were considered to be owing to artefact).
Table 2.
Cohen’s kappa statistics for interreader agreement on CT signs of ischaemic bowel
| CT finding | Cohen’s kappa (95% CI) |
| Mural thickening | 0.735 (0.57, 0.90) |
| Mural thinning | −0.011 (−0.31, 0.29) |
| Pneumatosis | 0.592 (0.40, 0.78) |
| Bowel wall oedema | 0.523 (0.33, 0.72) |
| Small bowel faeces sign | 0.529 (0.24, 0.82) |
| Mesenteric stranding | 0.450 (0.24, 0.66) |
| Bowel obstructiona | NA |
| Bowel loop dilatation | 0.516 (0.31, 0.72) |
| Differential mural enhancement | 0.493 (0.23, 0.75) |
| Venous thrombosis | −0.020 (−1.15, 1.00) |
| Aterial occlusion | 0.560 (0.31, 0.81) |
| Arterial calcification | 0.624 (0.44, 0.81) |
| Ascites | 0.577 (0.30, 0.85) |
| Solid organ infarction | 0.822 (0.69, 0.96) |
| Free intraperitoneal air | 0.915 (0.80, 1.00) |
| Portal venous gas | 0.859 (0.67, 1.00) |
| Mesenteric venous gas | 0.576 (0.25, 0.90) |
| IABP | 1.000 (1.00, 1.00) |
CI, confidence interval; IABP, intra-arterial balloon pump.
Not observed in this patient group.
Sensitivity and specificity for each individual sign are given in Table 3. The most common findings in those with proven ischaemic bowel were differential bowel wall enhancement, seen in 48/52 patients (92.3%), ascites in 45/52 (86.5%) and mesenteric stranding in 34/52 (65.4%). Neither bowel obstruction nor venous thrombus was demonstrated in any of the patients. The highest specificity results were seen with portal venous and mesenteric venous gas (specificities both 1.00), mural thinning (0.88), arterial occlusion (0.88) and pneumatosis (0.81), but CIs were wide owing to the small number of patients. In those with proven ischaemic bowel, the lowest number of CT signs present was 3, and the highest 13 (average 7.5, median 7.0); for those with no ischaemia pathologically, the range was 0–12 signs (average 5.2, median 6.0).
Table 3.
Sensitivity and specificity for each sign by consensus score
| Description | Occurrence among cases (n=52) | Occurrence among controls (n=16) | Sensitivity (95% CI) | Specificity (95% CI) |
| Mural thickening | 26 | 10 | 0.50 (0.36, 0.64) | 0.38 (0.15, 0.65) |
| Mural thinning | 21 | 2 | 0.40 (0.27, 0.55) | 0.88 (0.62, 0.98) |
| Pneumatosis | 31 | 3 | 0.60 (0.45, 0.73) | 0.81 (0.54, 0.96) |
| Bowel wall oedema | 27 | 11 | 0.52 (0.38, 0.66) | 0.31 (0.11, 0.59) |
| Small bowel faeces sign | 14 | 1 | 0.27 (0.16, 0.41) | 0.94 (0.70, 1.00) |
| Mesenteric stranding | 34 | 8 | 0.65 (0.51, 0.78) | 0.50 (0.25, 0.75) |
| Bowel loop dilatation | 28 | 3 | 0.54 (0.39, 0.68) | 0.81 (0.54, 0.96) |
| Differential mural enhancement | 48 | 8 | 0.92 (0.81, 0.98) | 0.50 (0.25, 0.75) |
| Occlusion SMA/IMA/coeliac | 11 | 2 | 0.21 (0.11, 0.35) | 0.88 (0.62, 0.98) |
| Calcification SMA/IMA/coeliac | 31 | 8 | 0.60 (0.45, 0.73) | 0.50 (0.25, 0.75) |
| Ascites | 45 | 11 | 0.87 (0.74, 0.94) | 0.31 (0.11, 0.59) |
| Solid organ infarction | 31 | 8 | 0.60 (0.45, 0.73) | 0.50 (0.25, 0.75) |
| Free air | 12 | 4 | 0.23 (0.13, 0.37) | 0.75 (0.48, 0.93) |
| Portal venous gas | 9 | 0 | 0.17 (0.08, 0.30) | 1.00 (0.79, 1.00) |
| Mesenteric venous gas | 11 | 0 | 0.21 (0.11, 0.35) | 1.00 (0.79, 1.00) |
| IABP | 11 | 2 | 0.21 (0.11, 0.35) | 0.88 (0.62, 0.98) |
CI, confidence interval; IABP, intra-arterial balloon pump; IMA, inferior mesenteric artery; SMA, superior mesenteric artery.
The sign “venous thrombosis” was not considered because it was not observed in any patient by the consensus score.
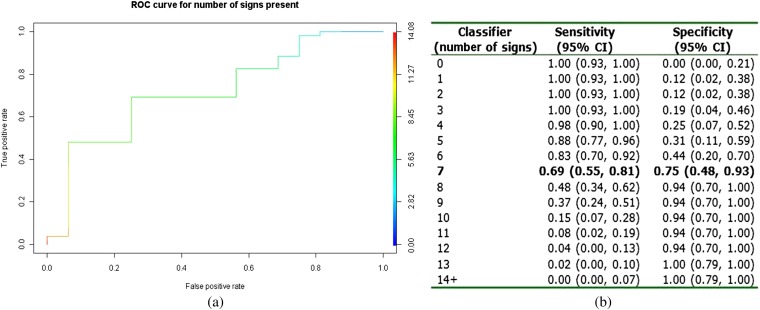
Plotting the diagnostic summaries for each number of signs present in an ROC curve gives a C-statistic of 0.77 (95% CI: 0.63, 0.90) (Figure 8a), implying that the total number of the 16 selected signs is a moderate diagnostic tool for ischaemic bowel. The optimum cut-off for the number of signs present was 7, which predicted ischaemic bowel with a sensitivity of 0.69 and a specificity of 0.75 in this patient group (Figure 8b).
Figure 8.
(a) Receiver operating characteristic (ROC) curve using the number of signs present (maximum observed=13). (b) Sensitivity and specificity by number of signs present. CI, contidence interval.
We performed a logistic regression analysis in order to determine the combination of signs and the best weighting for prediction of ischaemic bowel. Pneumatosis, bowel loop dilatation and differential mural enhancement were included in the final model (Table 4). If a patient presents with the signs pneumatosis, bowel loop dilatation and differential mural enhancement, the estimated predicted probability of ischaemic bowel is 98%. If a patient presents with at least 2 of these signs (in combination with any of the other signs), ischaemic bowel is predicted with a sensitivity of 0.73 and a specificity of 0.81. Replotting a ROC curve using these 3 variables gives an area under the curve of 0.84 (0.74, 0.93) (data not shown).
Table 4.
Logistic regression summary
| Coefficients | Coefficient | Standard error | Odds ratio | p-value |
| Intercept | −1.75 | 0.80 | 0.17 | 0.030 |
| Pneumatosis | 1.53 | 0.79 | 4.63 | 0.052 |
| Bowel loop dilatation | 1.63 | 0.83 | 5.11 | 0.050 |
| Differential mural enhancement | 2.40 | 0.82 | 11.05 | 0.003 |
DISCUSSION
We investigated the CT findings present in patients with pathologically confirmed ischaemic bowel following operations involving CPB and compared these with patients with a similar history but without ischaemia. CT may be particularly useful in the investigation of such post-operative patients, given that features such as a raised lactate or metabolic acidosis are often less reliable owing to the use of extracorporal circulation [8].
The CT signs that better predicted the presence of ischaemic bowel based on specificity were portal venous gas, mesenteric gas and small bowel faeces sign, which all had specificities of at least 0.94 for ischaemia; however, sensitivity of each of these was <0.27. Although small bowel faeces sign is typically associated with bowel obstruction, some studies have also shown a significant association of the sign with the presence of small bowel ischemia [18,19]. Differential mural enhancement had the highest sensitivity for diagnosis (0.92), but specificity was only 0.50. Using a logistic regression, the best subset of markers to predict the presence of ischaemic bowel are pneumatosis, bowel loop dilatation and differential mural enhancement. Patients presenting with these three signs has a predicted probability of ischaemic bowel of 98%.
Based on the kappa correlation statistics for interreader agreement, it would appear that the following signs are the hardest to interpret: bowel wall thinning, venous thrombosis, mesenteric stranding (possibly owing to the frequent concurrent presence of ascites) and differential mural enhancement. Previous studies have also highlighted poor interreader agreement for the assessment of differential mural enhancement [18]. The poor result for venous thrombosis is likely explained by its low prevalence in the patient group; the two readers each identified the sign once in separate patients, but on consensus, this was deemed to be owing to artefact.
Overall, the sensitivity of each of the signs was low, reflecting the findings of previous authors investigating acute mesenteric ischaemia. Signs, such as pneumatosis, superior mesenteric artery occlusion, portal venous gas and reduced mural enhancement, have been shown to have specificities of 1.00 but sensitivities not >0.57 and more typically <0.30 [12,14,18,20]. The choice of a control group in which the diagnosis of ischaemic bowel was a clinical possibility serves to better parallel daily practice; undoubtedly, the respective specificities would be higher if a healthy patient control group was selected. The prevalence of arterial occlusion (23.1%) and venous thrombosis (0%) was relatively low in our study compared with other studies, in which arterial occlusion was seen in 45–67% of cases [14,21,22] and venous thrombosis in 15–33% [14,20,22]. This is not unexpected in a population of patients following CPB surgery, where poor cardiac output and hypoperfusion and secondary splanchnic vasoconstriction is more likely to lead to a non-occlusive type of ischaemia [5,23]. The incidence of solid organ ischemia/infarction (61.5%) in our study was relatively high, which may relate to hypoperfusion or may be owing to post-operative prothrombotic status, leading to subsequent microemboli. Other findings, including the high prevalence of ascites were consistent with the findings identified in patients presenting with acute ischaemia in previous studies.
There was a degree of overlap between both types of signs present at CT and the overall number of signs present in patients with and without proven ischaemia. Unsurprisingly, the more signs that were present, the higher the specificity for predicting ischaemic bowel, with the ROC curve suggesting the optimal number of signs to be 7, to balance sensitivity (0.69) and specificity (0.75). It has been suggested that prompt diagnosis and early treatment is key for improved outcomes in bowel ischaemia [24] and as such there should be a low threshold for prompt surgical intervention [6,25]. It is possible that patients with obvious clinical features of ischaemic bowel were operated on immediately without prior CT, which may explain the degree of overlap as some of the theoretically more advanced cases did not undergo imaging. CT has overtaken angiography as the first-line diagnostic imaging modality in suspected bowel ischaemia [21]; however, it is accepted that a negative test should not prevent laparotomy if clinical suspicion remains high [26]. This may be particularly true in patients following CPB surgery where clinical signs may be difficult to elicit. Our results support the fact that although CT is useful in identifying signs of bowel ischaemia, findings may lack sensitivity, and in such cases, a low threshold for exploratory laparotomy should be adopted. This necessitates good communication between radiology and surgical/intensive treatment unit specialties.
There are several limitations to our study. One important limitation is the relatively small number of patients, particularly in the control group, which can lead to imprecise calculation of sensitivity and specificity. The lack of an external group for validation of the logistic regression prediction means that results will be optimistic. A further limitation is the fact that no unenhanced series were acquired and only 9 patients underwent arterial phase imaging. Portal venous phase imaging is thought to improve evaluation of bowel wall enhancement and in depicting the mesenteric veins [8]; however, if only a portal phase study is performed, this may lead to an underestimation of the presence of arterial occlusion [27]. The lack of arterial phase imaging reflects the non-specific clinical presentation of this cohort of post-operative patients owing to problems gaining a history and eliciting signs on clinical examination. Additionally, the lack of an unenhanced series makes assessment of intramural haemorrhage and differentiation from hyperenhancement on the post-contrast series difficult.
CONCLUSION
In this cohort of patients’ post-CPB surgery, arterial occlusion was uncommon and venous occlusion was not present, which is supportive of a predominantly non-occlusive aetiology for ischaemia. In addition, the incidence of solid organ infarction was relatively high, which may also relate to hypoperfusion or microemboli owing to a prothrombotic post-surgical status. The most predictive CT findings in this post-CPB surgery group were pneumatosis, bowel loop dilatation and differential mural enhancement; however, the findings lack sensitivity and, as such, a low threshold for exploratory laparotomy should be adopted.
FUNDING
T Benaglia was supported by funding from the Medical Research Council Biostatistics Unit (unit programme number U015232027).
REFERENCES
- 1.Allen KB, Salam AA, Lumsden AB. Acute mesenteric ischemia after cardiopulmonary bypass. J Vasc Surg 1992;16:391–6 [PubMed] [Google Scholar]
- 2.Venkateswaran RV, Charman SC, Goddard M, Large SR. Lethal mesenteric ischaemia after cardiopulmonary bypass: a common complication? Eur J Cardiothorac Surg 2002;22:534–8 [DOI] [PubMed] [Google Scholar]
- 3.Filsoufi F, Rahmanian PB, Castillo JG, Scurlock C, Legnani PE, Adams DH. Predictors and outcome of gastrointestinal complications in patients undergoing cardiac surgery. Ann Surg 2007;246:323–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nonthasoot B, Tullavardhana T, Sirichindakul B, Suphapol J, Nivatvongs S. Acute mesenteric ischemia: still high mortality rate in the era of 24-hour availability of angiography. J Med Assoc Thai 2005;88:S46–50 [PubMed] [Google Scholar]
- 5.Kassahun WT, Schulz T, Richter O, Hauss J. Unchanged high mortality rates from acute occlusive intestinal ischemia: six year review. Langenbecks Arch Surg 2008;393:163–71 10.1007/s00423-007-0263-5 [DOI] [PubMed] [Google Scholar]
- 6.Abboud B, Daher R, Sleilaty G, Madi-Jebara S, El Asmar B, Achouch R, et al. Is prompt exploratory laparotomy the best attitude for mesenteric ischemia after cardiac surgery? Interact Cardio Vasc Thorac Surg 2008;7:1079–83 10.1510/icvts.2008.176271 [DOI] [PubMed] [Google Scholar]
- 7.Blachar A, Barnes S, Adam SZ, Levy G, Weinstein I, Precel R, et al. Radiologists’ performance in the diagnosis of acute intestinal ischemia, using MDCT and specific CT findings, using a variety of CT protocols. Emerg Radiol 2011;18:385–94 10.1007/s10140-011-0965-4 [DOI] [PubMed] [Google Scholar]
- 8.Wiesner W, Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology 2003;226:635–50 [DOI] [PubMed] [Google Scholar]
- 9.Bächler P, Moschetta M, Stabile Ianora AA, Pedote P, Scardapane A, Angelelli G. Prognostic value of loss of wall enhancement for bowel infarction. Radiol Med 2011;116:821. [DOI] [PubMed] [Google Scholar]
- 10.Cohn SM, Moller BA, Zieg PM, Milner KA, Angood PB. Angiography for preoperative evaluation in patients with lower gastrointestinal bleeding: are the benefits worth the risks? Arch Surg 1998;133:50–5 [DOI] [PubMed] [Google Scholar]
- 11.Trompeter M, Brazda T, Remy CT, Vestring T, Reimer P. Non-occlusive mesenteric ischemia: etiology, diagnosis, and interventional therapy. EurRadiol 2002;12:1179–87 10.1007/s00330-001-1220-2 [DOI] [PubMed] [Google Scholar]
- 12.McLeod R, Lindsay T, O’Malley M, Members of the Evidence Based Reviews in Surgery Group Canadian Association of General Surgeons and American College of Surgeons evidence based reviews in surgery. Biphasic CT with mesenteric CT angiography in the evaluation of acute mesenteric ischemia: initial experience. Can J Surg 2005;48:491–3 [PMC free article] [PubMed] [Google Scholar]
- 13.Chou CK, Mak CW, Tzeng WS, Chang JM. CT of small bowel ischemia. Abdom Imaging 2004;29:18–22 10.1007/s00261-003-0073-3 [DOI] [PubMed] [Google Scholar]
- 14.Aschoff AJ, Stuber G, Becker BW, Hoffmann MH, Schmitz BL, Schelzig H, et al. Evaluation of acute mesenteric ischemia: accuracy of biphasic mesenteric multi-detector CT angiography. Abdom Imaging 2009;34:345–57 10.1007/s00261-008-9392-8 [DOI] [PubMed] [Google Scholar]
- 15.Barmase M, Kang M, Wig J, Kochhar R, Gupta R, Khandelwal N. Role of multidetector CT angiography in the evaluation of suspected mesenteric ischemia. Eur J Radiol 2011;80:e582–7 10.1016/j.ejrad.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 16.Katz MG, Schachner A, Ezri T, Kravtsov V, Freidman V, Hauptman E, et al. Nonocclusive mesenteric ischemia after off-pump coronary artery bypass surgery: a word of caution. Am Surg 2006;72:228–31 [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74 [PubMed] [Google Scholar]
- 18.Sheedy SP, Earnest F, 4th, Fletcher JG, Fidler JL, Hoskin TL. CT of small-bowel ischemia associated with obstruction in emergency department patients: diagnostic performance evaluation. Radiology 2006;241:729–36 [DOI] [PubMed] [Google Scholar]
- 19.Mayo-Smith WW, Wittenberg J, Bennett GL, Gervais DA, Gazelle GS, Mueller PR. The CT small bowel faeces sign: description and clinical significance. Clin Radiol 1995;50:765–7 [DOI] [PubMed] [Google Scholar]
- 20.Taourel PG, Deneuville M, Pradel JA, Régent D, Bruel JM. Acute mesenteric ischemia: diagnosis with contrast-enhanced CT. Radiology 1996;199:632–6 [DOI] [PubMed] [Google Scholar]
- 21.Wiesner W, Hauser A, Steinbrich W. Accuracy of multidetector row computed tomography for the diagnosis of acute bowel ischemia in a non-selected study population. Eur Radiol 2004;14:2347–56 10.1007/s00330-004-2462-6 [DOI] [PubMed] [Google Scholar]
- 22.Moschetta M, Stabile Ianora AA, Pedote P, Scardapane A, Angelelli G. Prognostic value of multidetector computed tomography in bowel infarction. Radiol Med 2009;114:780–91 10.1007/s11547-009-0422-6 [DOI] [PubMed] [Google Scholar]
- 23.Duran R, Denys AL, Letovanec I, Meuli RA, Schmidt S. Multidetector CT features of mesenteric vein thrombosis. Radiographics 2012;32:1503–22 10.1148/rg.325115100 [DOI] [PubMed] [Google Scholar]
- 24.Inderbitzi R, Wagner HE, Seiler C, Stirnemann P, Gertsch P. Acute mesenteric ischaemia. Eur J Surg 1992;158:123–6 [PubMed] [Google Scholar]
- 25.Abboud B, Daher R, Boujaoude J. Acute mesenteric ischemia after cardio-pulmonary bypass surgery. World J Gastroenterol 2008;14:5361–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology 2010;256:93–101 10.1148/radiol.10091938 [DOI] [PubMed] [Google Scholar]
- 27.Kirkpatrick ID, Kroeker MA, Greenberg HM. Biphasic CT with mesenteric CT angiography in the evaluation of acute mesenteric ischemia: initial experience. Radiology 2003;229:91–8 10.1148/radiol.2291020991 [DOI] [PubMed] [Google Scholar]