Abstract
Objective:
To determine the prognostic value of pre-treatment apparent diffusion coefficient (ADC) of colorectal liver metastases in predicting disease response, progression-free survival (PFS) and overall survival (OS).
Methods:
We retrospectively reviewed 102 patients who underwent pre-treatment diffusion-weighted MRI using a breath-hold (b=0, 150, 500) or a free-breathing (b=0, 50, 100, 250, 500, 750) technique. The mean ADC (b=0–500) and mean flow-insensitive ADC (ADChigh) values (breath-hold: b=150 and 500; free-breathing: b=100 and 500) of up to three hepatic lesions were evaluated in each patient. Clinical and laboratory parameters were recorded. Tumour response was assessed by Response Evaluation Criteria in Solid Tumors (RECIST) criteria at 12 weeks after treatment. Associations between tumour response, ADC values and clinical/laboratory parameters were examined by one-way analysis of variance. The relationship of ADC with PFS and OS was determined by Kaplan–Meier analysis.
Results:
62 patients responded to chemotherapy at 12 weeks. The pre-treatment mean ADC and mean ADChigh were higher in the non-responding group than in the responding group (1.55 vs 1.36, p=0.033; 1.40 vs 1.16, p=0.024). However, the PFS and OS of the two groups of patients stratified by the median of mean ADC values or threshold derived by receiver operating characteristic analysis were not statistically significant. By multivariate Cox regression analysis, patients with ≤2 metastases and response to chemotherapy showed better PFS; white cell count ≤10 and surgical treatment were associated with better OS.
Conclusion:
Colorectal liver metastasis with higher pre-treatment mean ADC and mean ADChigh was associated with poorer response to chemotherapy. However, ADC and ADChigh values did not predict the patient outcome in this study cohort.
Advances in knowledge:
High mean ADC values of colorectal liver metastases on pre-treatment diffusion-weighted MRI is associated with poorer response to chemotherapy.
Liver metastasis from colorectal cancer is common and is associated with poor survival. It has been shown that liver metastectomy [1], radiofrequency ablation [2] and good response to chemotherapy confer a favourable long-term outcome [3]. Given the impact of adverse effects of current treatments on quality of life, knowledge on the likelihood to respond to chemotherapy, progression-free survival (PFS) and overall survival (OS) will also facilitate clinical decision making on how aggressively to pursue the various therapeutic options.
There are several pre-treatment clinical factors that have been shown to affect the outcome in metastatic colorectal cancer [4]. Negative clinical predictors of outcome include platelets (plt) >400×109 l−1, alkaline phosphatase (ALP) >300 units per litre, white blood cell count (WCC) >10×109 l−1, and haemoglobin (Hb) <11×109 l−1. The presence of lung or lymph node metastases and the primary site being at the rectum are associated with better outlook. However, there is currently no imaging-derived prognostic index that is linked to treatment outcomes. An MRI prognostic feature is attractive because it can be derived non-invasively and may also be applied for response monitoring.
The apparent diffusion coefficient (ADC), derived from diffusion-weighted MRI (DW-MRI), provides information on the microscopic movement of water molecules [5–7]. In neoplasms, ADC informs on cell membrane integrity, cellular density, extracellular space tortuosity and microstructural organisation [8,9]. Solid tumours usually return lower ADC values than their tissue of origin. In brain tumours, a pre-treatment ADC value has been shown to predict tumour response [10] and disease survival [11]. Although studies have shown that a high pre-treatment ADC value of colorectal liver metastases predicts poor response to chemotherapy [12,13], the relationship of pre-treatment ADC value and the patient clinical outcome has not been examined in abdominal malignancies.
The aim of this study was to determine whether pre-treatment ADC of colorectal liver metastases is of prognostic value in predicting the patient outcome in terms of disease response, PFS and OS.
MATERIALS AND METHODS
Regional ethics and local scientific approval were obtained for this study. Informed consent was waived as this was a retrospective study.
Subjects
Patients who underwent DW-MRI as part of their standard diagnostic work-up for colorectal hepatic metastasis were identified retrospectively and sequentially from a list containing all the MR studies performed between 1 January 2004 and 31 December 2009. The inclusion criteria were (a) pathologically confirmed colorectal cancer, (b) hepatic metastases visible on MR study and measuring >1 cm, (c) that patients did not receive chemotherapy in the month prior to diagnosis of liver metastasis. The exclusion criterion was history of previous or concurrent non-colorectal cancer. Patients who had extrahepatic metastases were also excluded. Patients were not excluded on the basis of the type of treatment received. Hence, the final study population comprised 102 patients.
Clinical data
The following demographic data for each patient were collected: age at diagnosis of primary tumour, sex, date of diagnosis of primary tumour, date of diagnosis of liver metastasis, number of metastases, date of disease progression in the liver, date of disease progression outside the liver, date of death or last known contact, treatment modality [none, chemotherapy alone, chemotherapy with surgery or radiofrequency ablation (RFA)].
Clinical and laboratory parameters at the time of diagnosis of liver metastasis which may influence outcome were also collected: site of primary tumour (rectum vs other locations in the colon), Hb, WCC, plt, serum ALP, serum lactate dehydrogenase (LDH, above or below upper limit of normal) and serum carcionembryonic antigen (CEA).
MRI
MRI performed prior to the commencement of treatment was performed on either a Philips Intera (Koninklijke Philips N.V., Best, Netherlands) or a Siemens Avanto 1.5 T (Siemens Healthcare, Erlangen, Germany) MR systems using a phased array body coil. Qualitative assurance using sucrose phantom showed no significant difference in the ADC values obtained between the two scanners. The imaging sequences used on these scanners are summarised in Table 1. T1 weighted sequences with and without fat suppression were obtained after intravenous gadolinium contrast injection and were reviewed in conjunction with the DW-MR images for lesion localisation.
Table 1.
Parameters used in obtaining axial DW-MRI of the liver using breath-hold single-shot echo-planar imaging (EPI) or free-breathing EPI DW-MRI
| Scanning parameters | Breath-hold | Free-breathing |
| TR | 1850 | 2500 |
| TE | 56 | 76 |
| α | 90 | |
| Slice thickness (mm) | 7 | 6 |
| NEX | 1 | 4 |
| FOV (mm) | 340 | 340 |
| Matrix | 112×256 | 112×256 |
| GRAPPA/SENSE factor | 2 | 2 |
| b-values (s mm−2) | 0, 150, 500 | 0, 50, 100, 250, 500, 750 |
DW-MRI, diffusion-weighted MRI; FOV, field of view; GRAPPA, generalised autocalibrating partially parallel acquisition; NEX, number of excitations; SENSE, sensitivity encoding; TE, time of echo; TR, time of repetition.
Image analysis
Images were reviewed offline using an in-house software (DiffusionView; Royal Marsden Hospital, UK) by DMK and HT (with 7 and 3 years of experience in body DW-MRI, respectively) in consensus, blinded to the clinical details. In each patient, a marker metastasis >1 cm in diameter was chosen, avoiding vascular structures and areas with image artefacts. In patients where multiple metastases of different sizes were present, up to three metastases of different sizes (1–2 cm, >2–5 cm and >5 cm) were selected at random and analysed. If the multiple lesions were of similar sizes, then only an index lesion was identified and evaluated. Lesions situated at the left lobe of the liver or the dome of the liver adjacent to the diaphragm were avoided in order to minimise the impact on ADC values caused by cardiac or respiratory motion. Regions of interest (ROIs) encompassing selected metastases were drawn on b=500 s mm−2 images on the image section showing the widest diameter of the lesion and copied onto the ADC maps to record their values. For each metastasis, maps of flow-sensitive ADC (including b=0 s mm−2) and flow-insensitive ADC (ADChigh) (using only b-values of 150 and 500 or 100 and 500 s mm−2) were generated.
Statistical analysis
Analysis was performed using SPSS® v. 17 (SPSS Inc., Chicago, IL) by HT with contribution and supervision from DMK. A p-value of <0.05 was considered statistically significant. The following relationships were investigated.
Differences in apparent diffusion coefficient between responders and non-responders
One-way analysis of variance (ANOVA) was performed to compare the mean pre-treatment ADC and ADChigh with demographical, clinical and laboratory factors (sex, age at diagnosis, primary site, Hb, WCC, plt, ALP, LDH, CEA) between responders and non-responders. Receiver operating characteristic (ROC) analysis was performed to identify an optimum threshold ADC and ADChigh values for distinguishing responding from non-responding metastases.
Survival analysis
The PFS and OS (number of days from diagnosis of liver metastasis to date of progression and to date of death, respectively) were calculated. The median ADC values and the ADC values from the aforementioned ROC analysis were used as thresholds for outcome stratification: one group with ADC value less than or equal to the thresholds and the other group with ADC value higher than the thresholds. Kaplan–Meier survival analysis with log-rank test was performed to compare PFS and OS between these two groups of patients. For PFS, any imaging evidence of disease progression (both in the liver and elsewhere in the body) was recorded as an event. Stable patients not having any imaging evidence of disease progression were censored at last follow-up. For OS, patient death was recorded as an event while patients were otherwise censored at last follow-up.
RESULTS
A total of 109 patients with colorectal hepatic-only metastases were identified, of which 7 had previous or concurrent second malignancy and were excluded from the study. In the remaining 102 patients [60 males, 42 females, mean age at diagnosis of primary tumour 64.5 (27–88) years], 4 patients received no treatment, 52 received chemotherapy only and 46 received a combination of surgery/RFA and chemotherapy. A total of 147 metastases were analysed.
The median follow-up period was 630 (75–2063) days, during which there were 70 events of disease progression and 32 events of death. The median time to progression was 286 (43–791) days, and the median time to death was 609 (75–1807) days.
Differences in apparent diffusion coefficient between responders and non-responders
Of those who received chemotherapy, 62 patients showed partial response at 12 weeks. The mean ADC was 1.44×10−3 mm2 s−1, and mean ADChigh was 1.25×10−3 mm2−1. Mean lesion diameter was 20.4 mm.
One-way ANOVA (Table 2) showed that pre-treatment ADC was significantly higher in the non-responding group than in the responding group [1.55 vs 1.36×10−3 mm2 s−1, F(1, 95)=4.701, p=0.033, Figure 1], as was ADChigh [1.40 vs 1.16×10−3 mm2 s−1, F(1, 95)=5.302, p=0.024]. Lesion diameter (p=0.632) and the other demographical/clinical factors were not significantly different between the 2 groups.
Table 2.
One-way analysis of variance, comparing the different clinical and diffusion parameters between the responding and non-responding groups of patients
| Parameters | F (between group df, within group df) | Significance |
| Sex | 2.356 (1, 95) | 0.128 |
| Age | 0.007 (1, 95) | 0.931 |
| Primary site (rectum vs colon) | 0.306 (1, 95) | 0.581 |
| Haemoglobin | 0.561 (1, 92) | 0.456 |
| WCC | 0.003 (1, 92) | 0.954 |
| Platelets | 0.488 (1, 92) | 0.486 |
| LDH | 0.050 (1, 89) | 0.823 |
| ALP | 0.826 (1, 92) | 0.366 |
| CEA | 2.107 (1, 93) | 0.150 |
| Number of liver metastases | 0.104 (1, 95) | 0.748 |
| ADC | 4.701 (1, 95) | 0.033 |
| ADChigh | 5.302 (1, 95) | 0.024 |
| Diameter | 0.231 (1, 95) | 0.632 |
ADC, apparent diffusion coefficient; ALP, alkaline phosphatase; CEA, carcionembryonic antigen; df, degrees of freedom LDH, lactate dehydrogenase; WCC, white cell count.
Figure 1.
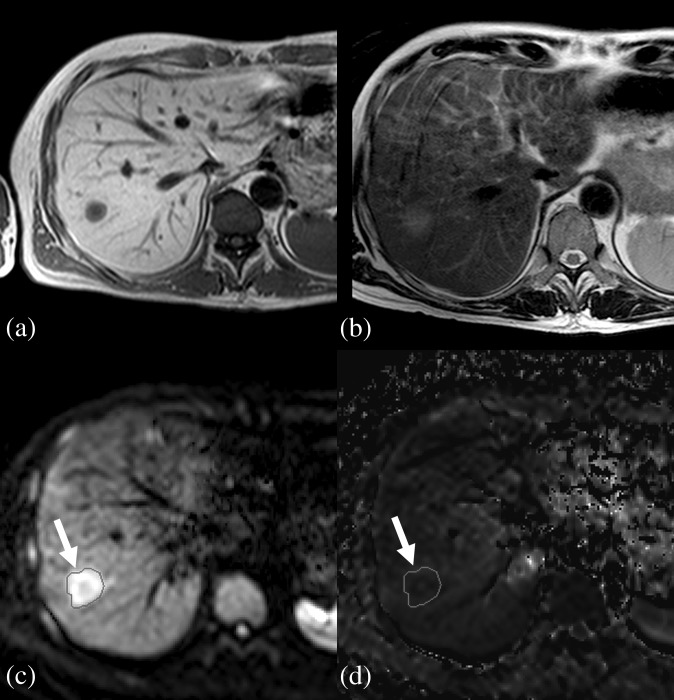
A liver metastasis in a 57-year-old male patient. (a) T1 weighted imaging. (b) T2 weighted imaging. (c) Regions of interest (ROI) (circle, indicated by arrows) drawn around the lesion on b=500 image. (d) The same ROI in (c) is copied onto the apparent diffusion coefficient map.
When stratified by the median value of ADChigh=1.13×10−3 mm2 s−1, the proportion of non-responders in the group with ADChigh lower than this threshold value was 0.3, whereas the proportion of non-responders in the group with ADChigh above this threshold value was 0.43; odds ratio 1.728.
Survival analysis for all patients regardless of treatment received
Threshold values
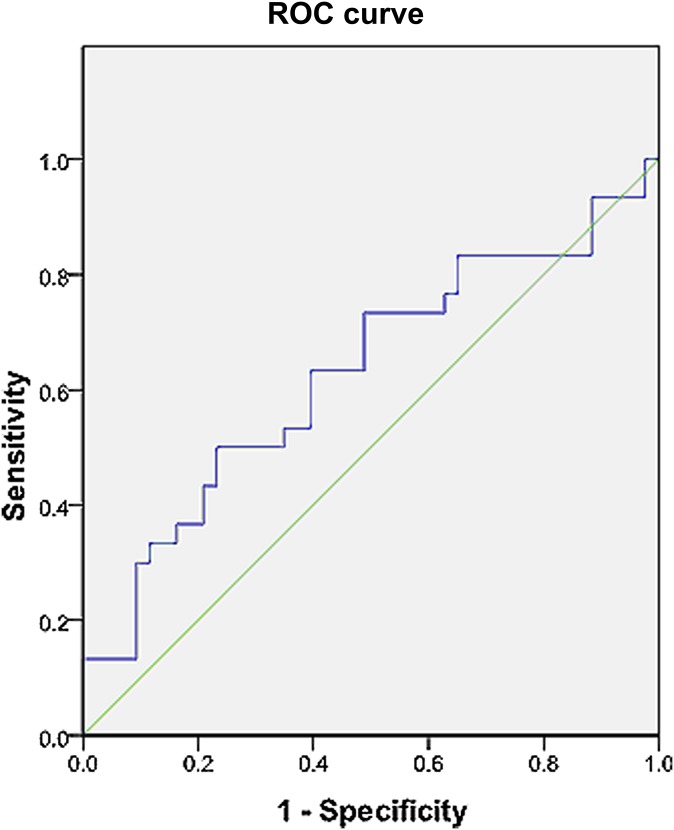
For ADChigh, the median value that divided the cohort into 2 groups was 1.13×10−3 mm2 s−1. From the ROC curve analysis, a threshold value of 1.41×10−3 mm2 s−1 was also found to have a relatively high specificity (sensitivity 0.343; specificity 0.790; area under the curve 0.626; asymptotic significance 0.04; Figure 2). Threshold values for dichotomising the study cohort using the other parameters are listed in Table 3.
Figure 2.
Pre-treatment receiver operating characteristic (ROC) curve of flow-insensitive apparent diffusion coefficient in predicting response to therapy. Area under the curve=0.626, asymptotic significance=0.04. A threshold value of 1.41×10−3 mm2 s−1 results in a sensitivity of 0.343 and a specificity of 0.790.
Table 3.
Threshold values for dichotomising patients in survival analysis
| Parameters | Group 0 | Group 1 |
| Sex | Female | Male |
| Age at diagnosis | ≤64 years | >64 years |
| Site of primary cancer | Rest of colon | Rectum |
| Haemoglobin | ≤11 | >11 |
| White cell count | ≤10 | >10 |
| Platelets | ≤400 | >400 |
| ALP | ≤300 | >300 |
| LDH | <Laboratory upper limit | >Laboratory upper limit |
| CEA | ≤13 | >13 |
| Number of liver metastases | 1 or 2 | >2 |
| Metastases diameter (mm) | ≤15.17 | >15.17 |
| Received metastatectomy or RFA | No | Yes |
| Response to chemotherapy | PD or SD | PR or CR |
ALP, alkaline phosphatase; CEA, carcionembryonic antigen; CR, complete response; LDH, lactate dehydrogenase; PD, progressive disease; PR, partial response; RFA, radiofrequency, ablation; SD, stable disease.
Threshold values for site of primary cancer, haemoglobin, white cell count, platelets and ALP were chosen according to previously published values. Median values were used for age at diagnosis, CEA levels, number of liver metastases and metastases diameter. Laboratory upper limit is used for LDH.
Progression-free survival
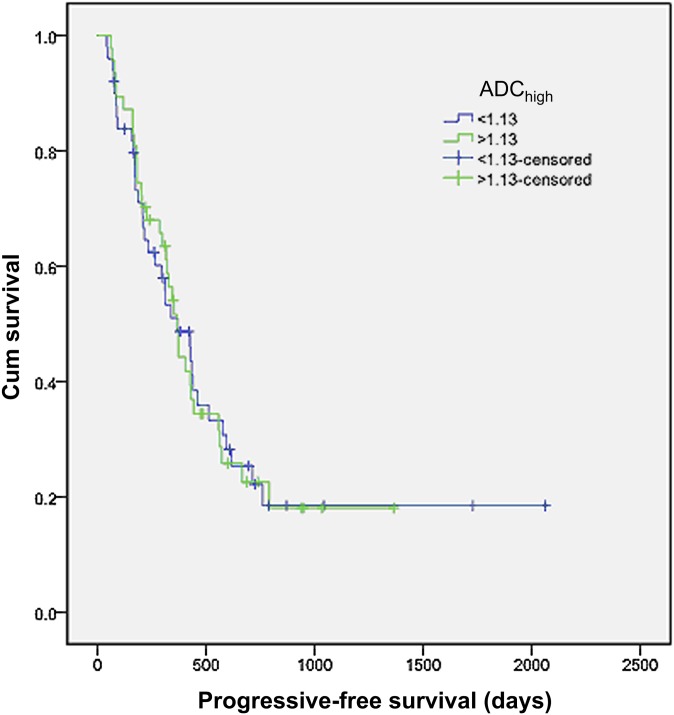
When patients were stratified using the ADChigh median value, there was no significant difference in PFS between the 2 groups (estimated median survival 369 vs 366 days; p=0.954; Figure 3). A similar result was obtained when patients were stratified by the threshold value from the ROC curve (estimated median survival 369 vs 366 days; p=0.431).
Figure 3.
Kaplan–Meier survival analysis of progression-free survival (PFS) with patients stratified into 2 groups using the threshold flow-insensitive apparent diffusion coefficient (ADChigh) value of 1.13. No significant difference in PFS was observed between these two groups.
Other parameters associated with better PFS at Kaplan–Meier analysis were LDH below laboratory upper limit, CEA ≤13, number of liver metastases ≤2, having received liver metastatectomy or RFA and response to chemotherapy at 12 weeks (Table 4). However, analysis using multivariate Cox regression showed that only the number of metastases (1 or 2 metastatic deposits demonstrated a hazard ratio of 0.55, p=0.022) and response to chemotherapy (non-responders demonstrate a hazard ratio of 2.78, p<0.001) remained significant (Table 4).
Table 4.
Kaplan–Meier (KM) and Cox regression (Cox) analyses on PFS and OS in patient groups dichotomised using threshold values of Table 3
| Parameters | PFS-KM | PFS-Cox | OS-KM | OS-Cox | ||||||
| Median survival (days) | p | Hazard ratio | p | Median survival (days) | p | Hazard ratio | p | |||
| Group 0 | Group 1 | Group 0 | Group 1 | |||||||
| Sex | 405 | 346 | 0.331 | 956 | 1610 | 0.685 | ||||
| Age | 346 | 369 | 0.659 | 1610 | 1010 | 0.987 | ||||
| Site of primary | 369 | 353 | 0.803 | 907 | 1709 | 0.069 | ||||
| Haemoglobin | 564 | 366 | 0.474 | 776 | 1254 | 0.795 | ||||
| White cell count | 373 | 286 | 0.241 | 1050 | 393 | 0.005 | 0.287 | 0.023 | ||
| Platelets | 373 | 227 | 0.508 | 1610 | 105 | 0.000 | 0.531 | 0.187 | ||
| ALP | 369 | 87 | 0.876 | 1050 | 941 | 0.556 | ||||
| LDH | 426 | 327 | 0.020 | 0.685 | 0.158 | 1709 | 850 | 0.048 | 1.056 | 0.904 |
| CEA | 428 | 339 | 0.049 | 0.858 | 0.571 | 1709 | 907 | 0.006 | 0.516 | 0.307 |
| Number of mets | 428 | 316 | 0.006 | 0.540 | 0.022 | 1610 | 956 | 0.048 | 0.748 | 0.496 |
| Surgery/RFA | 339 | 435 | 0.027 | 1.618 | 0.075 | 764 | 1807 | 0.000 | 6.119 | 0.001 |
| Response to chemotherapy | 183 | 435 | 0.000 | 2.780 | <0.001 | 907 | 1050 | 0.135 | ||
| Liver lesion diameter | 369 | 373 | 0.901 | 1807 | 941 | 0.031 | 1.404 | 0.606 | ||
ALP, alkaline phosphatase; CEA, carcionembryonic antigen; LDH, lactate dehydrogenase; OS, overall survival; PFS, progression-free survival; RFA, radiofrequency ablation.
After multivariate analysis, it was shown that number of metastases ≤2 and response to chemotherapy were associated with better PFS, whereas white cell count ≤10 and having received surgery or RFA to the liver metastases were associated with better OS.
Factors reaching statistical significance are highlighted in bold.
Overall survival
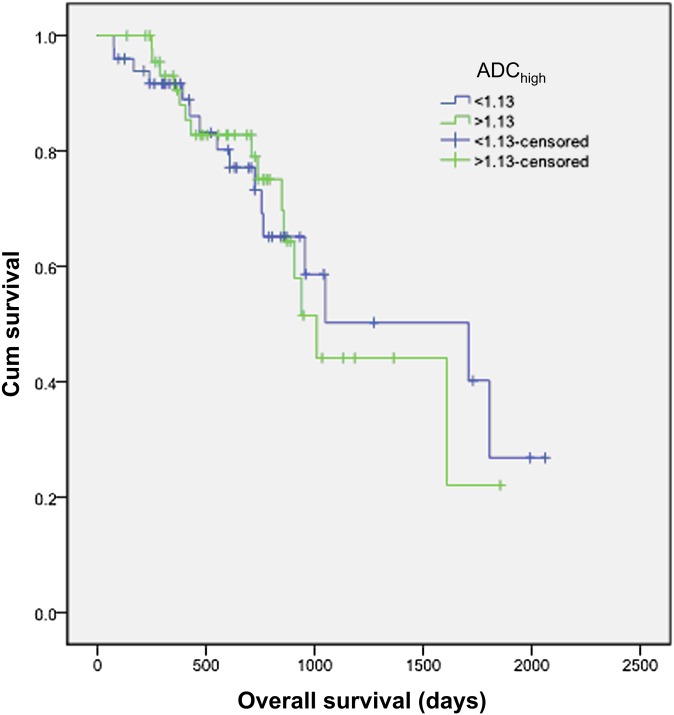
When patients were stratified by the ADChigh median value, the group with the lower ADChigh value shows a longer median survival than the higher value group, but the difference was not statistically significant (estimated median survival 1709 vs 1010 days; p=0.897; Figure 4). Results derived by applying a threshold value from the ROC curve were likewise not significant (p=0.324).
Figure 4.
Kaplan–Meier survival analysis of overall survival (OS) with patients stratified into 2 groups using the threshold flow-insensitive apparent diffusion coefficient (ADChigh) value of 1.13. No difference of OS was observed between these two groups.
Other parameters associated with better OS at Kaplan–Meier analysis were WCC ≤10, plt ≤400, LDH below laboratory upper limit, CEA ≤13, number of liver metastases ≤2, having received liver surgery or RFA and liver metastasis diameter ≤15.17 (Table 4). However, analysis using multivariate Cox regression showed that only WCC (≤13 demonstrates a hazard ratio of 0.287, p=0.023) and having received liver surgery or RFA (not having received surgery or RFA was associated with a hazard ratio of 6.119, p=0.001) remained significant (Table 4).
Survival analysis for patients who had received only chemotherapy but no surgery or radiofrequency ablation
Threshold values
52 patients received chemotherapy but did not undergo surgery or RFA. Threshold values for dichotomising the patients into 2 groups are the same as the whole cohort (listed in Table 3), except for age at diagnosis (median=65 years old), ADChigh (median=1.12 mm2 s−1), lesion diameter (median=16 mm) and number of liver metastases (median=3.5).
Progression-free survival
The group of patients who showed response to chemotherapy at 12 weeks after commencement of treatment had longer PFS (estimated median survival 428 days vs 87 days; p<0.001). Dichotomisation by the other clinical, laboratory or DW-MRI parameters did not show any difference in PFS between the two patient groups (Table 5).
Table 5.
Kaplan-Meier (KM) and Cox regression (Cox) analyses on PFS and OS in patients who received chemotherapy only
| Parameters | PFS-KM | OS-KM | OS-Cox | |||||
| Median survival (days) | p | Median survival (days) | p | Hazard ratio | p | |||
| Group 0 | Group 1 | Group 0 | Group 1 | |||||
| Sex | 405 | 227 | 0.590 | 764 | 710 | 0.767 | ||
| Age | 208 | 366 | 0.135 | 710 | 859 | 0.090 | ||
| Site of primary | 314 | 208 | 0.539 | 710 | 859 | 0.137 | ||
| Haemoglobin | 428 | 314 | 0.528 | 640 | 724 | 0.786 | ||
| White cell count | 339 | 208 | 0.496 | 859 | 167 | 0.002 | 0.262 | 0.013 |
| Platelets | 339 | 208 | 0.975 | 850 | 405 | 0.119 | ||
| ALP | 314 | 87 | 0.510 | 764 | 941 | 0.483 | ||
| LDH | 314 | 227 | 0.243 | 859 | 764 | 0.527 | ||
| CEA | 314 | 208 | 0.530 | 859 | 710 | 0.203 | ||
| Number of mets | 353 | 314 | 0.402 | 850 | 725 | 0.323 | ||
| Response to chemotherapy | 87 | 428 | 0.000 | 431 | 850 | 0.039 | 2.164 | 0.092 |
| Liver lesion diameter | 205 | 366 | 0.578 | 859 | 725 | 0.582 | ||
| ADChigh | 369 | 208 | 0.782 | 725 | 850 | 0.674 | ||
ADC, apparent diffusion coefficient; ALP, alkaline phosphatase; CEA, carcionembryonic antigen; LDH, lactate dehydrogenase; OS, overall survival; PFS, progression-free survival.
Patients who responded to chemotherapy had longer PFS. Patients whose white cells were <10 had prolonged OS.
Factors reaching statistical significance are highlighted in bold.
Overall survival
Patients who responded to chemotherapy at 12 weeks after commencement of treatment (estimated median survival 850 days vs 431 days; p=0.039) and patients whose WCC was lower than 10 (estimated median survival 859 days vs 167 days; p=0.002) had longer OS. The association of chemotherapy response with prolonged OS was lost in multivariate Cox regression analysis. The other clinical, laboratory or DW-MRI parameters did not predict OS (Table 5).
DISCUSSION
We found that in patients with colorectal liver metastases, lesions with higher pre-treatment ADC and ADChigh values are less likely to respond to treatment. This observation is consistent with previous reports [12,13]. However, in our study, DW-MRI was performed using two different MR scanners employing different imaging protocols. Despite these differences, the pre-treatment ADC and ADChigh values of the metastases were still useful in predicting disease response. Furthermore, the difference in the mean ADC and ADChigh between responders and non-responders was greater than the generally observed 15–30% ADC difference that could be attributed to measurement variability. This suggests that ADC values obtained from different scanners using different protocols can still be sufficiently robust as potential response biomarkers.
In the literature, the pre-treatment ADC value of primary colorectal cancer has been shown to correlate negatively with the percentage tumour size reduction post chemotherapy [14]. Although the biological basis for low ADC tumours showing better treatment response remains uncertain, two theories may account for this observation: tumour necrosis and variation in the local immune response.
As the normal cellular barriers are broken down in necrosis, water molecules are able to diffuse more freely in the necrotic area resulting in a higher ADC value. Tumour necrosis is an adverse prognostic feature. Necrotic tumours often have a dense neo-vasculature [15] that has abnormal structure and function. Together with the hypoxia and acidity found in necrotic regions, these changes in the tumour microenvironment can limit the effectiveness of chemotherapy and lead to drug resistance [16,17]. In breast cancer, tumour necrosis is associated with a poorer response to chemotherapy [18]. In colorectal hepatic metastasis, necrosis is also a common feature [19]. This may explain the higher ADC value observed in the group of non-responding patients in the present study.
Another hypothesis to explain poor chemotherapy response associated with high ADC value is variation in local immune response. As local immune response tends to both increase tissue cellularity and cause cellular swelling, water diffusion may become impeded, resulting in a lower ADC. Inflammation-induced reduction in ADC has been observed in inflammatory bowel disease [20]. In cancers, tumours with lymphocytic infiltration have a higher response rate to treatment than those that do not demonstrate this feature [21]. The result of the present study, where metastases with lower ADC showed better response to chemotherapy, may therefore thus reflect local immune response. Clearly, our hypothesis would need to be validated by histological correlation in future studies.
In theory, tumour necrosis and dampened immune response can lead to higher ADC, which may also have a bearing on disease survival. In fact, these histological features have been shown to adversely influence survival [22–26]. In malignant astrocytoma [27], glioblastoma [28] and primary central nervous system (CNS) lymphoma [29], a lower pre-treatment ADC has been shown to be associated with improved survival. However, the ability of ADC values in predicting survival in extracranial tumours and metastatic lesions has not yet been demonstrated. Such a result was also not observed in the present study. Several reasons may be offered as explanations. First, the discrepancy between the present study and those results observed in primary CNS tumours [27–29] may also be owing to histopathological differences between different tissue types. Second, there can be biological differences between primary lesions and metastatic deposits [30]. The response of disease in one organ (e.g. the liver) may not be the main determinant of disease survival in a patient with widespread metastatic disease. Third, single ADC values may not adequately reflect the complex interplay of different therapies administered over the lifetime of the patient, which would have a bearing on disease survival. In a previously unpublished interim analysis of pre-treatment ADChigh values in colorectal hepatic metastasis, a high pre-treatment value was associated with earlier disease progression, independent from other factors such as lesion size, number of metastasis and initial response to treatment [31]. Thus, a future prospective study conducted in a more selected study population could help to further ascertain the value of pre-treatment ADC values in predicting long-term outcome in colorectal liver metastasis.
There are several limitations to this study. First, this was a single centre retrospective study in a heterogeneous treatment population, which may confound any relation between ADC values and treatment outcomes. Hence, even though we have not demonstrated a positive relationship between ADC values and long-term outcome in our current study, it would be important to reappraise the prognostic value of ADC in a well-designed prospective multicentre study. Second, because of cardiac motion artefacts inherent to the non-cardiac gated protocol employed in this study (also respiratory motion artefact in the free-breathing protocol), ADC measurement is inaccurate for lesions in the left lobe of the liver and the dome of the liver adjacent to the diaphragm. These lesions were therefore not selected for analysis in order to avoid spurious results. Third, ADC cannot be accurately measured in small lesions <1 cm in diameter. Thus, only lesions >1 cm were selected for analysis. Although this may have lead to a selection bias, this is technically unavoidable. Last but not least, although ideally all lesions should be included for analysis, it is difficult to do so in a large cohort. It is also impractical to include all lesions when employing DW-MRI in a clinical situation. In addition, a large number of patients have solitary liver metastasis. Therefore, in the present study, it was felt that a maximum of three lesions per patient is a suitable compromise between practicality and representativeness.
In conclusion, our study confirms the value of pre-treatment DW-MRI in colorectal liver metastasis in predicting treatment response. However, we did not observe a significant relationship between pre-treatment ADC value and patient outcome in our study cohort. A larger prospective study in more defined study population should be undertaken to further ascertain the association between pre-treatment ADC value and disease survival.
FUNDING
CRUK and ESPRC Cancer Imaging Centre and National Health Service funding to the National Institute for Health Research Biomedical Research Centre (C1060/A10334).
REFERENCES
- 1.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 1990;77:1241–6 [DOI] [PubMed] [Google Scholar]
- 2.Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 2009;19:1206–13 10.1007/s00330-008-1258-5 [DOI] [PubMed] [Google Scholar]
- 3.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg 2004;240:1052–61; discussion 61–4 10.1016/j.recot.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Köhne CH, Cunningham D, Di CF, Glimelius B, Blijham G, Aranda E, et al. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol 2002;13:308–17 [DOI] [PubMed] [Google Scholar]
- 5.Le Bihan D. Molecular diffusion nuclear magnetic resonance imaging. Magn Reson Q 1991;7:1–30 [PubMed] [Google Scholar]
- 6.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168:497–505 [DOI] [PubMed] [Google Scholar]
- 7.Stejskal EO, Tanner JE. Spin diffusion measurements: spin-echo in the presence of a time dependent field gradient. J Chem Phys 1965;42:288–92 [Google Scholar]
- 8.Gauvain KM, McKinstry RC, Mukherjee P, Perry A, Neil JJ, Kaufman BA, et al. Evaluating pediatric brain tumor cellularity with diffusion-tensor imaging. AJR Am J Roentgenol 2001;177:449–54 10.2214/ajr.177.2.1770449 [DOI] [PubMed] [Google Scholar]
- 9.Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 1999;9:53–60 [DOI] [PubMed] [Google Scholar]
- 10.Moffat BA, Chenevert TL, Meyer CR, McKeever PE, Hall DE, Hoff BA, et al. The functional diffusion map: an imaging biomarker for the early prediction of cancer treatment outcome. Neoplasia 2006;8:259–67 10.1593/neo.05844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh J, Henry RG, Pirzkall A, Lu Y, Li X, Catalaa I, et al. Survival analysis in patients with glioblastoma multiforme: predictive value of choline-to-N-acetylaspartate index, apparent diffusion coefficient, and relative cerebral blood volume. J Magn Reson Imaging 2004;19:546–54 [DOI] [PubMed] [Google Scholar]
- 12.Koh DM, Scurr E, Collins D, Kanber B, Norman A, Leach MO, et al. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol 2007;188:1001–8 10.2214/AJR.06.0601 [DOI] [PubMed] [Google Scholar]
- 13.Cui Y, Zhang XP, Sun YS, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology 2008;248:894–900 10.1148/radiol.2483071407 [DOI] [PubMed] [Google Scholar]
- 14.Dzik-Jurasz A, Domenig C, George M, Wolber J, Padhani A, Brown G, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 2002;360:307–8 10.1016/S0140-6736(02)09520-X [DOI] [PubMed] [Google Scholar]
- 15.Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer 1999;79:991–5 10.1038/sj.bjc.6690158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 2007;99:1441–54 10.1093/jnci/djm135 [DOI] [PubMed] [Google Scholar]
- 17.Brown JM. The hypoxic cell: a target for selective cancer therapy—eighteenth Bruce F. Cain Memorial Award lecture. Cancer Res 1999;59:5863–70 [PubMed] [Google Scholar]
- 18.Uematsu T, Kasami M, Yuen S. Neoadjuvant chemotherapy for breast cancer: correlation between the baseline MR imaging findings and responses to therapy. Eur Radiol 2010;20:2315–22 10.1007/s00330-010-1813-8 [DOI] [PubMed] [Google Scholar]
- 19.Outwater E, Tomaszewski JE, Daly JM, Kressel HY. Hepatic colorectal metastases: correlation of MR imaging and pathologic appearance. Radiology 1991;180:327–32 [DOI] [PubMed] [Google Scholar]
- 20.Oto A, Zhu F, Kulkarni K, Karczmar GS, Turner JR, Rubin D. Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad Radiol 2009;16:597–603 10.1016/j.acra.2008.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010;28:105–13 10.1200/JCO.2009.23.7370 [DOI] [PubMed] [Google Scholar]
- 22.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–4 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 23.Klintrup K, Mäkinen JM, Kauppila S, Väre PO, Melkko J, Tuominen H, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer 2005;41:2645–54 10.1016/j.ejca.2005.07.017 [DOI] [PubMed] [Google Scholar]
- 24.Nagtegaal ID, Marijnen CA, Kranenbarg EK, Mulder-Stapel A, Hermans J, van de Velde CJ, et al. Local and distant recurrences in rectal cancer patients are predicted by the nonspecific immune response; specific immune response has only a systemic effect—a histopathological and immunohistochemical study. BMC Cancer 2001;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pflanz S, Brookman-Amissah S, Roigas J, Kendel F, Hoschke B, May M. Impact of macroscopic tumour necrosis to predict survival of patients with surgically resected renal cell carcinoma. Scand J Urol Nephrol 2008;42:507–13 10.1080/00365590802460633 [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003;348:203–13 10.1056/NEJMoa020177 [DOI] [PubMed] [Google Scholar]
- 27.Murakami R, Sugahara T, Nakamura H, Hirai T, Kitajima M, Hayashida Y, et al. Malignant supratentorial astrocytoma treated with postoperative radiation therapy: prognostic value of pretreatment quantitative diffusion-weighted MR imaging. Radiology 2007;243:493–9 [DOI] [PubMed] [Google Scholar]
- 28.Yamasaki F, Sugiyama K, Ohtaki M, Takeshima Y, Abe N, Akiyama Y, et al. Glioblastoma treated with postoperative radio-chemotherapy: prognostic value of apparent diffusion coefficient at MR imaging. Eur J Radiol 2010;73:532–7 10.1016/j.ejrad.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 29.Barajas RF, Jr, Rubenstein JL, Chang JS, Hwang J, Cha S. Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol 2010;31:60–6 10.3174/ajnr.A1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padera TP, Kuo AH, Hoshida T, Liao S, Lobo J, Kozak KR, et al. Differential response of primary tumor versus lymphatic metastasis to VEGFR-2 and VEGFR-3 kinase inhibitors cediranib and vandetanib. Mol Cancer Ther 2008;7:2272–9 10.1158/1535-7163.MCT-08-0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tam HH, Colins DJ, Brown G, Chau I, Cunningham D, Leach MO, et al. High pre-treatment apparent diffusion coefficient predicts poor disease survival in patients with colorectal hepatic metastasis. In: Joint Annual Meeting ISMRM-ESMRMB; 1–7 May 2010; Stockholm, Sweden [Google Scholar]