Abstract
Extrinsic venous compression is caused by compression of the veins in tight anatomic spaces by adjacent structures, and is seen in a number of locations. Venous compression syndromes, including Paget–Schroetter syndrome, Nutcracker syndrome, May–Thurner syndrome and popliteal venous compression will be discussed. These syndromes are usually seen in young, otherwise healthy individuals, and can lead to significant overall morbidity. Aside from clinical findings and physical examination, diagnosis can be made with ultrasound, CT, or MR conventional venography. Symptoms and haemodynamic significance of the compression determine the ideal treatment method.
Extrinsic venous compression occurs by adjacent arterial, ligamentous, muscular or osseous structures in tight anatomic spaces [1]. Despite its relative infrequency, venous compression is most commonly seen in otherwise healthy and young individuals and may cause significant haemodynamic alterations that may lead to clinical symptoms and significant associated morbidity. Venous compression usually becomes clinically significant when there is a change in the flow direction or increased venous pressure, which lead to the formation of venous collaterals, varicosities and/or chronic venous insufficiency [2]. Repetitive endothelial injury at the site of extrinsic compression predisposes to acute or chronic venous thrombosis, which in turn may aggravate venous hypertension downstream [2].
Diagnosis of venous compression is based on a combination of clinical and imaging features. A wide range of signs and symptoms may be seen, including pain, swelling, venous thrombosis, varicosities and haematuria [1,2]. External venous compression incidentally demonstrated by imaging may not be of clinical significance on its own; therefore, imaging should be used for confirmation of the cause of the symptoms. Ultrasound with Doppler, contrast-enhanced CT, CT venography (CTV), contrast-enhanced MRI and MR venography (MRV) are helpful in the diagnosis of venous compression syndromes [3].
Ultrasound has been utilised to identify deep venous thrombosis (DVT) within the vein affected by the compression and allows for evaluation of the flow direction and dynamic examinations of the area of interest. Although MRV is more expensive than CTV, there has been an increasing use of MRV since the introduction of blood pool contrast agents and owing to concerns regarding radiation exposure with CTV. Variations in venous haemodynamics from patient to patient make the timing of CTV challenging in obtaining the appropriate venous phase for optimal visualisation of the venous system. MRI provides flow directionality with flow sensitive sequences that could be very valuable in evaluating the haemodynamic significance of the compression. MRV with blood pool contrast agents, such as gadofoveset trisodium, allows for evaluation of the vasculature (arterial and venous) during the steady-state phase. Isotropic high-resolution images can be obtained by allowing multiplanar reformats for better visualisation of compression, characterisation of acute vs chronic clot and venous mapping for procedure planning.
Ultimate diagnostic confirmation is obtained by conventional venography, demonstrating pressure gradients across the compressions, flow alteration and/or thrombus within the compressed vein. Several management options unique to the location of venous compression have been described, including conservative, endovascular and surgical treatment options.
In this review, we discuss the underlying anatomy, pathophysiology, diagnostic methods and management options of various venous compression syndromes namely May–Thurner syndrome (MTS), Paget–Schroetter syndrome (PSS), Nutcraker syndrome (NCS) and popliteal venous compression (PVC).
PAGET–SCHROETTER SYNDROME
Background
PSS (otherwise known as thoracic outlet syndrome and previously known as effort thrombosis) is an uncommon cause of DVT of the subclavian vein, most commonly seen as a consequence of chronic compression of the subclavian vein at the level of the thoracic outlet [4,5]. Primary axillary–subclavian vein thrombosis was first described by Paget in 1875 and Von Schroetter in 1884 and it was named the “Paget–Schroetter syndrome” by Hughes [6] in 1948. If not treated appropriately it may cause severe swelling and pain in the upper extremity, which may lead to post-thrombotic syndrome, causing significant disability.
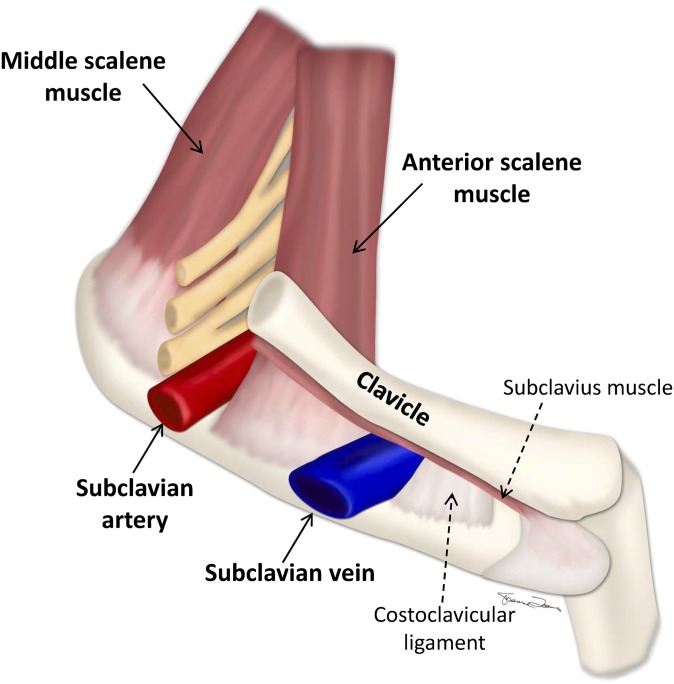
PSS is most commonly seen in the dominant arm of patients who are physically active and is provoked by excessive overhead activity. It is estimated to occur in approximately 2–11 out of 100 000 people [7]. Males are affected more than females. Its aetiology is complex and multifactorial. As opposed to the subclavian artery and the brachial plexus that get compressed in the interscalene triangle between the anterior and middle scaleneus muscles, the subclavian vein gets compressed in the costoclavicular triangle, which is bordered by the medial aspect of the clavicle superiorly, the first rib inferiorly and the insertion of the anterior scalene muscle posterolaterally (Figure 1) [8]. Compression in the costoclavicular space can occur secondary to a number of causes, including hypertrophy of the scalene muscles or tendons, callus formation from a prior fracture, hypertrophy of the costoclavicular ligament, a cervical rib and tumours that should be evaluated for with imaging.
Figure 1.
Interscalene triangle and the costoclavicular space. The subclavian vein can be seen coursing through the costoclavicular space, bordered by the clavicle superiorly, the first rib inferiorly and the anterior scalene muscle posteriorly. The relationship of the costoclavicular space to the interscalene triangle through which the subclavian artery and the brachial plexus travels can also be seen.
The pathophysiology is similar to that of the other compression syndromes. Excessive physical activity leads to extrinsic compression of the subclavian vein in the costoclavicular space that causes stagnation of blood flow in the vein and leads to venous hypertension. Repetitive microtrauma to the venous endothelium eventually initiates the coagulation cascade and causes acute thrombosis. With continuation of the same cascade, fibrotic changes in the vessel occur, resulting in perivenous adhesions and organised synechiae that eventually lead to chronic thrombosis of the subclavian vein and collateral vessel formation in the neck and upper chest.
The most common symptoms are unilateral arm pain and swelling. Hand cyanosis, enlarged shoulder and chest wall collateral veins are other occasional symptoms. Some patients can also be entirely asymptomatic.
Imaging findings
Venous thrombosis in the axillary and subclavian veins can be easily confirmed by ultrasound [9,10]. However, limitations such as the inability to see the proximal aspect of the subclavian vein by the overlying clavicle and to apply compression exist. Greyscale and Doppler ultrasound should be the initial test of choice in the work-up of these patients. Ultrasound has a sensitivity of 78–100% and a specificity of 88–100% in the identification of acute DVT of the subclavian vein that extends into the axillary vein and more distally [11].
CTV and MRV can be used when ultrasound findings are equivocal. The extent of the DVT, collateral vein formation, point of venous compression and any associated anatomical abnormality can be depicted [12–14].
A contrast venogram is the standard means of determining the anatomy and can be carried out in preparation for interventions such as catheter-directed thrombolysis and percutaneous transluminal angioplasty (PTA) [15,16]. The goal of venography is to evaluate the presence of stenosis or compression of the subclavian vein that may be better depicted with changing the arm position and to identify the chest wall and neck collaterals, the presence of which usually signifies the chronic nature of venous occlusion. Images should be obtained with positional manoeuvres of the arm that include arm neutral position, abduction and external rotation to demonstrate costoclavicular compression of the subclavian vein. Positional venography is essential for accurate depiction of the compression and more accurately reflects the severity, the location and the haemodynamic effects of the compression.
Management
In PSS, earlier diagnosis and treatment provides the best results. The treatment goals are to relieve the acute symptoms of venous occlusion, to prevent pulmonary embolism and to reduce the likelihood of recurrent thrombosis in an attempt to avoid development of post-thrombotic syndrome. Treatment methods include conservative, endovascular and surgical treatments.
Conservative therapy consists of bed rest, limb elevation, warm compresses and anticoagulation. Conservative treatment options are usually offered in addition to more definitive treatments or in cases where there is a contraindication to catheter-directed thrombolysis. In cases of acute DVT, catheter-directed pharmacological or mechanical thrombolysis is accepted as the initial treatment. The thrombus is lysed more rapidly than with systemic administration of a thrombolytic, thus decreasing the administered dose and ultimately reducing the risk of a serious bleeding complication. Repeat venograms should be performed at least daily to check for clot resolution and to reposition the catheter within the remaining clot. Complete lysis has been reported as at high as 88% on repeat venograms. With any treatment, patients should be placed on long-term anticoagulation to prevent further thrombus deposition and reduce the risk of recurrent thrombosis.
Catheter-directed thrombolysis is followed by surgical treatment to provide decompression of the vein. Depending on the cause, surgery could include release of the tendons and resection of the compressing rib, clavicle or muscle (Figure 2) [17,18]. In cases where surgical decompression is not performed, the underlying cause is left untreated, and patients are subjected to recurrence of the symptoms and DVT. If symptoms recur following surgery, a repeat venogram should be obtained. The cause in this situation is usually stenosis of the subclavian vein. PTA after first rib resection and venous stenting for short segment venous strictures that persist after PTA provide long-term patency of the subclavian vein and symptomatic relief. PTA could also be performed during surgery or within the immediate post-operative period that lessens the time away from work and provides faster resumption of normal activities [19].
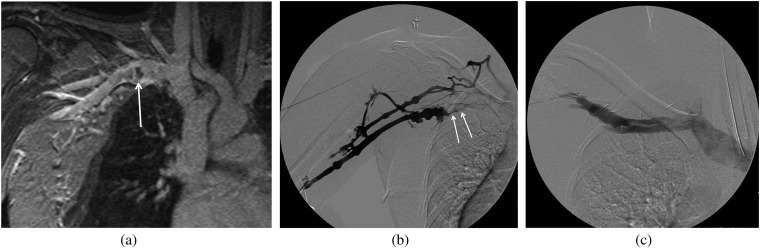
Figure 2.
A 27-year-old female athlete presenting with right arm swelling. Initial ultrasound showed deep venous thrombosis in the subclavian vein. (a) Coronal MR venography demonstrates focal thrombus (arrow) in the subclavian vein with focal dilatation and surrounding collaterals in the chest wall. (b) Right upper extremity venogram was performed showing acute thrombus (arrows), luminal irregularity and collaterals surrounding the subclavian vein that was treated with catheter-directed thrombolysis. (c) Repeat venogram following first rib resection shows resolution of the findings.
NUTCRACKER SYNDROME
Background
NCS, also known as renal vein entrapment syndrome or mesoaortic compression of the left renal vein (LRV), results from compression of the LRV between the superior mesenteric artery (SMA) and the aorta [20,21]. In cases where compression is seen on imaging but there is no clinical manifestation, it is named Nutcracker phenomenon. The compression leads to increased pressure in the LRV with subsequent development of venous varicosities surrounding the renal pelvis, ureter and the gonadal vein. Occasionally, rupture of the thin-walled vein into the renal calyceal fornix can be seen.
The syndrome was first described in 1950 by El Sadr and Mina [22] but first introduced into the literature by De Shepper [23]. It consists of two variants; the more commonly described anterior NCS, also known as mesoaortic compression of the LRV (compression of the LRV between the aorta and the SMA) and the more rare posterior NCS or retro-aortic or pseudonutcracker syndrome (compression of the retro-aortic LRV between the aorta and vertebral bodies). The retro-aortic LRV is a venous variation characterised by the LRV crossing behind the aorta, with an incidence of 0.5–3.7%. Retro-aortic entrapment of the LRV can also result in elevation of venous pressure, distention and left renal congestion, but its clinical significance is still unclear [24].
NCS is most commonly seen in relatively healthy and thin females in the third or fourth decade of life. Owing to the paucity of the fat in the aorto-mesenteric region, there is a reduction in the “take-off” angle of the SMA from the aorta, which is believed to be a factor in the pathophysiology. It is manifested by left flank and abdominal pain, with or without unilateral haematuria or orthostatic proteinuria and chronic fatigue. Because the left gonadal vein drains into the LRV, males may experience left testicular pain and females may experience symptoms similar to pelvic congestion, such as pelvic pain dysmenorrhoea, dysuria and dyspareunia. It can lead to varcicocele formation in males, and vulvar and pelvic varices in females [25]. Varicose veins of the lower extremities can also develop as a result of increased pelvic pressure.
Imaging findings
Doppler ultrasound is a non-invasive diagnostic modality commonly utilised in the diagnosis of NCS with a sensitivity and specificity of 78% and 100%, respectively [26]. Recent literature reported the usefulness of determination of peak velocities in the LRV [27]. The flow velocity of the LRV at the point of compression by the SMA is mostly faster than 100 cm s−1 [27]. Pre- and post-compression velocity ratios can also be utilised. The ratio of the inner diameter of the LRV at the hilum to the diameter at the compression point has also been utilised [28]. A ratio of more than three in the supine position, and a ratio of more than five in the standing position (for more than 15 min) are considered abnormal [29]. It is helpful to keep in mind that potential errors and interperformer differences could be seen in making these calculations in practice. Ultrasound can also be utilised to evaluate for enlarged gonadal veins, pelvic varices in females and varicocele formation in males [30].
Axial or sagittal images from a CT or MR venogram would clearly depict the compression of the LRV between the aorta and the SMA (Figure 3) [31,32]. Distention of the LRV can also be seen [33]. The course of the LRV, whether it is preaortic, retroaortic or circumaortic should be delineated. Determination of pelvic varices and their extrapelvic connections is also important, as it would indicate haemodynamic significance of the venous compression.
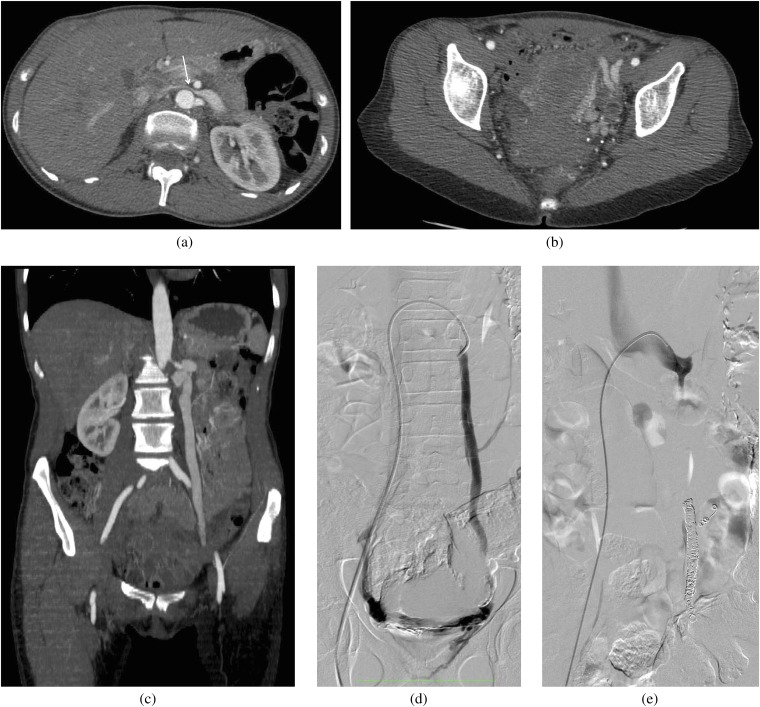
Figure 3.
A 25-year-old female who presented with pelvic pain and was also found to have haematuria. (a–c) A contrast-enhanced CT scan was performed to evaluate the cause of pain. An axial scan at the level of (a) the left renal vein (LRV) and (b) in the pelvis, and (c) a coronal scan through the gonadal vein demonstrate compression of the LRV (arrow) by the superior mesenteric artery anteriorly and the aorta posteriorly, multiple pelvic collateral vessels and an enlarged gonadal vein. (d–e) The patient later underwent a venogram to demonstrate the reflux and to coil embolise the gonadal vein. (d) Venogram via a right common femoral vein access and injection of the gonadal vein demonstrates an enlarged gonadal vein and cross pelvic collaterals with partial opacification of the right gonadal vein. (e) Venogram following coil embolisation of the left gonadal vein shows absence of reflux.
Retrograde venography supplemented with reno-caval pressure gradient measurements is accepted as the gold standard diagnostic test for NCS. Retrograde venography allows visualisation of the point of compression of the LRV at the meso-aortic crossing, stagnation of contrast in the renal vein and demonstration of perirenal and/or periureteral venous collaterals with reflux of contrast into the adrenal and gonadal veins. The normal reno-caval pullback mean pressure gradient across the point of compression ranges from 0 to 3 mmHg. The pressure gradient between the LRV and the inferior vena cava should be >3 mmHg to clinch the diagnosis of NCS [31,34]. Gradient measurements can also be used to assess the severity of the obstruction and to monitor the therapeutic results.
Management
Management options include conservative, endovascular and surgical treatments that depend on the duration and severity of the symptoms. Most patients are treated conservatively, particulary younger ones, as there is a high chance of spontaneous remission in this patient group. Patients who are very thin may benefit from modest weight gain. Patients complaining of mild pelvic or flank pain or asymptomatic haematuria are treated with pain medication, hydration, elastic compression stockings and occasionally hormonal therapy. However, more invasive treatment options may be necessary for patients who have significant haematuria, persistent orthostatic proteinuria, more severe flank or pelvic pain or renal functional impairment or who are unresponsive to conservative treatment options [35].
Several surgical procedures have been described in the literature, including medial nephropexy, gonadocaval bypass, transposition of the SMA, renal autotransplantation and LRV transposition [36,37] to relieve the compression. LRV transposition offers the best outcomes with significantly less morbidity, as it requires only limited exposure of the retroperitoneum, avoids the additional arterial anastomosis and has shorter renal ischemia times [38].
Endovascular stenting of the renal vein has been emerging as an alternative method of treatment [39,40]. There have been reports with good results in the use of expandable metallic stents. However, serious complications can occur, including stent occlusion with proximal migration of the thrombus and pulmonary embolism. In the event of documented reflux of contrast into gonadal, periureteral or pelvic varices, coil embolisation of the varices provides excellent symptomatic relief. The varices may be embolised with a combination of chemical sclerosants and coils. External stenting of the LRV laparoscopically with polytetra fluoroethylene reinforced stents have also been described [41].
MAY–THURNER SYNDROME
Background
MTS (otherwise known as the iliac vein compression syndrome) is the result of compression of the left common iliac vein between the right common iliac artery and the overlying spine, with subsequent development of DVT in the left lower extremity, which may lead to chronic venous insufficiency in the long term [42]. Virchow [43] first described this entity in 1851 and noted a left-sided predominance of iliofemoral DVT secondary to venous stasis. Compression of the left iliac vein with detailed anatomic description was later described by May and Thurner [44]. The overall prevalence of symptomatic MTS ranges from 18% to 49% among patients with left-sided lower extremity DVT [45]. It is predominantly seen in young to middle-aged females (20–40 years).
The right common iliac artery crosses over the left common iliac vein and then runs adjacent to the right iliac vein, where it continues as the right external iliac artery parallel to the right external iliac vein. The left iliac vein is compressed between the right common iliac artery anteriorly and the sacral promontory or the fifth lumbar vertebra posteriorly just before the iliocaval junction. Variations of MTS have been described, such as the right common iliac vein getting compressed by the right common iliac artery [46] or the compression of the left iliac vein by the left common iliac artery (Figure 4) [47]. The left common iliac vein can also be compressed by an aneurysmal right common iliac artery; however, these cases are extremely rare.
Figure 4.
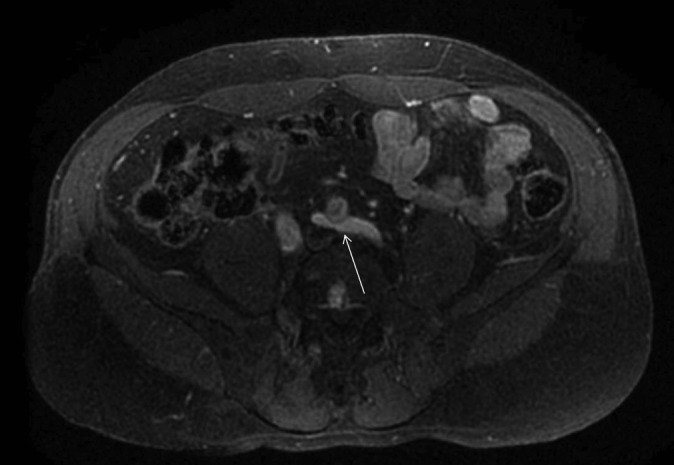
A 38-year-old female with pelvic pain and left lower extremity swelling. MR venography (MRV) was obtained to evaluate for May–Thurner syndrome (MTS). Axial image from the contrast-enhanced MRV demonstrates a variation of MTS, with compression of the left iliac vein (arrow) by the left common iliac artery.
Iliac vein compression is a frequent anatomic variant and is incidentally commonly observed on many cross-sectional imaging studies. More than 50% luminal compression of the left iliac vein can be seen in up to 25% of asymptomatic healthy individuals [48]. Compression becomes clinically significant only if it causes haemodynamic changes in venous flow or venous pressure, evident by flow reversal and/or the presence of varices, or if it leads to acute or chronic DVT.
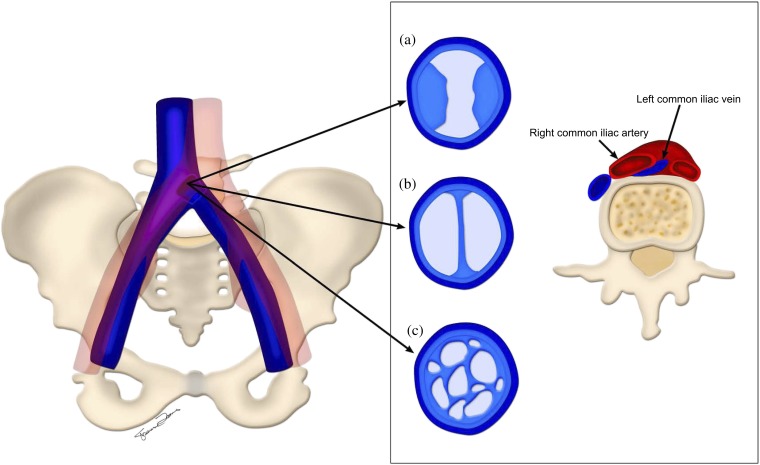
The combination of chronic mechanical compression and pulsatile vibratory pressure from the adjacent artery causes chronic repetitive microtrauma leading to endothelial injury of the vein. Endothelial damage leads to deposition of elastin and collagen, which over the long term leads to intraluminal webs, channels and spurs (Figure 5). These spurs may have several morphological features, which cause a predisposition to DVT.
Figure 5.
Left common iliac vein compression by the right common iliac artery. Chronic changes in the left common iliac vein secondary to endothelial damage with (a) intraluminal spurs, (b) webs and (c) channels are depicted.
The syndrome most commonly presents as acute iliofemoral DVT with sudden onset of left leg swelling and pain. In the acute phase, patients can also present with pulmonary embolism. Prolonged immobility, recent surgery and pregnancy are well-known predisposing factors. MTS could also present with chronic symptoms of left lower extremity venous hypertension, venous insufficiency without thrombosis. Patients usually complain of left lower extremity pain and swelling in the acute phase and varicose veins, skin discoloration and ulceration around the ankle owing to chronic venous stasis.
Imaging findings
Doppler ultrasound is accurate in identifying the presence of DVT in the lower extremity; however, iliac vein thrombosis may be technically challenging to depict and compressibility may not be possible to assess [42]. Lack of respiratory variations and the absence of response to Valsalva manoeuvre in the common femoral vein could be a sign of more proximal compression or obstruction (Figure 6).
Figure 6.
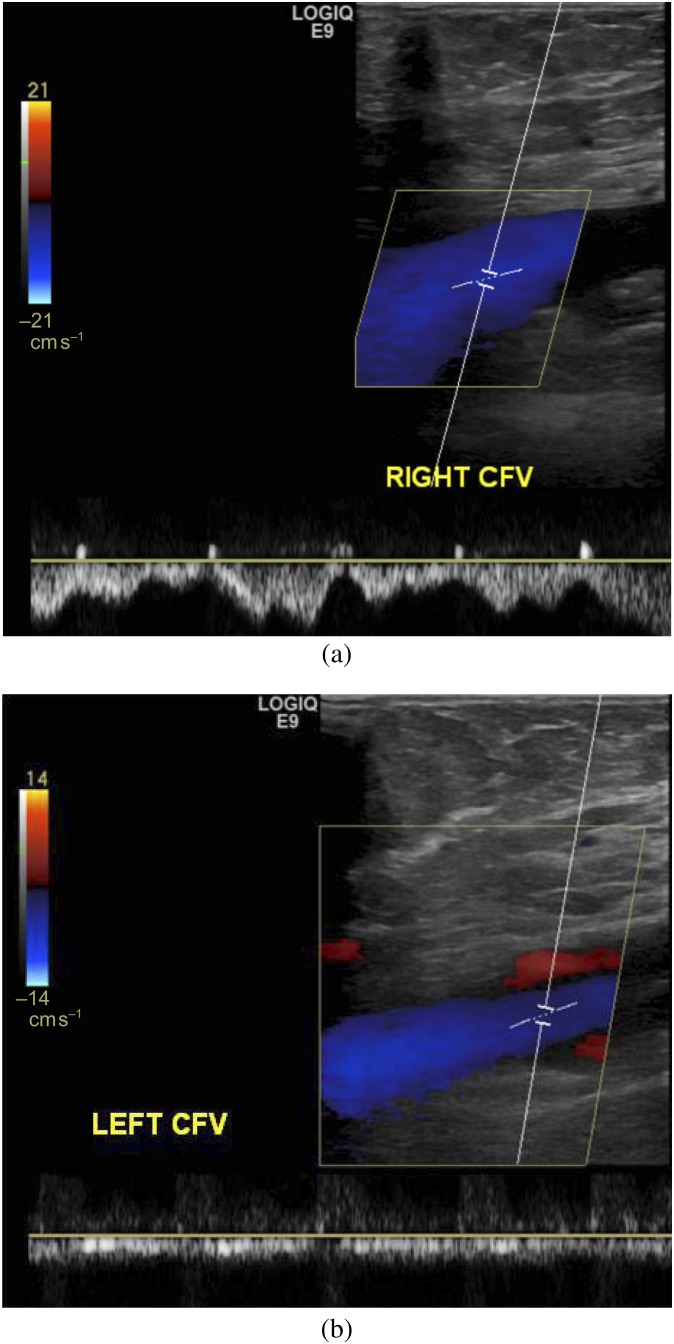
A 28-year-old female with left lower extremity swelling and varices. (a, b) Doppler ultrasound was performed to evaluate for deep venous thrombosis. (a) Right common femoral vein shows normal respiratory variation. (b) Left common femoral vein (CFV) study shows the absence of normal respiratory variation, suggesting more central (left iliac vein) obstruction. This patient was later found to have May–Thurner syndrome.
Both CTV and MRV have high sensitivity and specificity in evaluating DVT [3,46,47]; however, MRV has the advantage of better differentiation of intraluminal thrombus from contrast mixing. Both imaging modalities allow identification of the venous compression and pelvic venous collaterals. MRI, in particular, is highly useful in assessing the haemodynamic significance of venous compression as it has the ability to demonstrate retrograde flow in the ipsilateral iliac vein and in the collateral veins (Figure 7). The most common venous collateral pathways include the ascending lumbar, presacral, transpelvic and abdominal wall veins.
Figure 7.
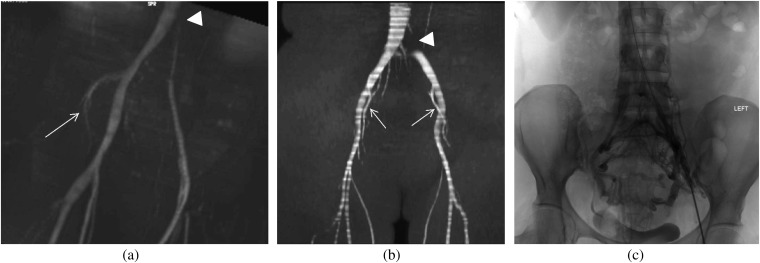
A 33-year-old female with unilateral, left-sided lower extremity pain, swelling and heaviness as well as chronic pelvic pain. (a) Coronal maximum intensity projection from the time of flight sequence of the MR venography obtained to further evaluate the cause of her symptoms demonstrates compression of the left common iliac vein which is diminished in calibre with a focal signal void (arrowhead). The left internal iliac vein is not visualised consistent with retrograde flow, which suggests haemodynamically significant left common iliac venous compression. However, the right internal iliac vein is seen (arrow). (b) The same sequence in another patient given for comparison purposes shows a focal signal void in the left common iliac artery at the point of compression (arrowhead), but a signal is seen in the internal iliac arteries (arrows), suggesting antigrade direction of flow. (c) The patient underwent a venogram for further evaluation and possible stenting, which shows some luminal irregularity of the left common iliac vein with multiple pelvic collaterals.
Left common femoral or iliac venography confirms the presence of venous obstruction and allows the assessment of haemodynamic significance. It is the ultimate diagnostic test and allows treatment to be offered in the same setting. The presence of flow reversal in the ipsilateral internal iliac vein, depiction of multiple cross pelvic collaterals, and an enlarged ascending lumbar vein can all be seen secondary to the underlying chronic venous hypertension. Pressure gradients could be measured across the compression, with a gradient of >2 mmHg indicating haemodynamic significance [45,48].
Intravascular ultrasound is another diagnostic tool that could be utilised for direct visualisation of iliac vein compression just before the iliocaval junction (Figure 8) [49]. It may identify mural abnormalities (spurs), intraluminal webs and channels in the vessel wall. During endovascular treatments, it facilitates accurate placement of a wire across the stenosed region.
Figure 8.
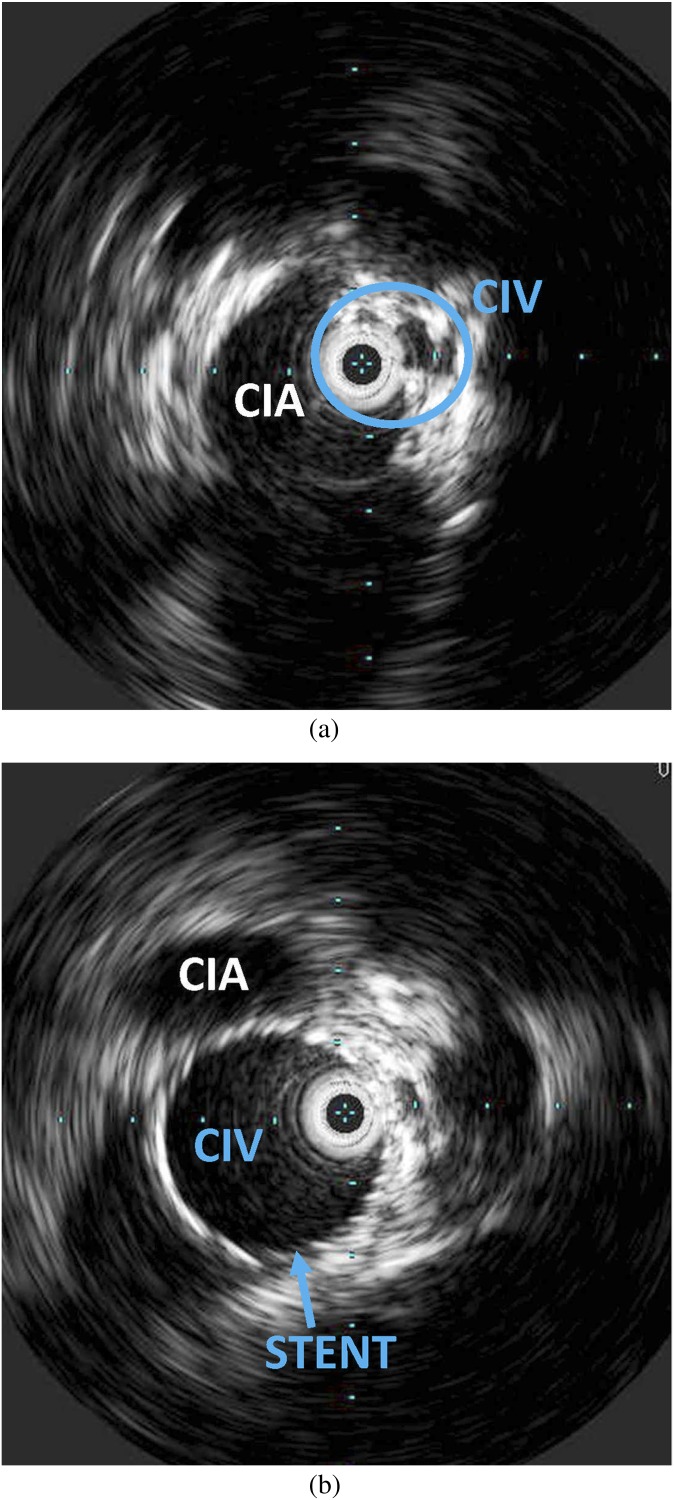
A 32-year-old female with an established diagnosis of May–Thurner syndrome. Patient has had recurrent episodes of unilateral deep venous thrombosis and swelling in the left lower extremity. (a, b) Intravascular ultrasound was performed before and after stenting of the left common iliac vein (CIV) to relieve the compression. (a) Wall thickening and intraluminal irregularity of the left CIV and extrinsic compression by the adjacent right common iliac artery (CIA) are seen obtained prior to stenting. The intravascular ultrasound probe can be seen in the encircled CIV. (b) Following stenting, the lumen is widely patent.
Management
Treatment is dependent on the presence of DVT [50]. In the absence of DVT, conservative treatments are preferred. Compression stockings alone may be effective. In the presence of acute DVT, the standard therapy is anticoagulation with compression stockings.
Currently, endovascular thrombolysis with treatment of the venous compression with stents is advocated [51,52]. Endovascular therapy involves catheter-directed thrombolysis with the use of pharmacological agents (tissue plasminogen activator) and/or various mechanical thrombectomy devices. These devices allow rapid removal of clot burden and rapid restoration of blood flow. They are helpful in preventing post-thrombotic syndrome, as early thrombolysis allows preservation of valve function. Subsequent to thrombolysis, the predisposing factor to the DVT, the underlying venous compression is usually treated with stenting. Self-expanding stents are most commonly used. Long-term anticoagulation and compression stockings are required to prevent recurrent DVT formation and stent occlusion. At 1 year, 79% and 93% primary and secondary patency rates are seen, respectively [42]. If symptoms recur following stent placement, stent thrombosis should be suspected and investigated.
Surgical treatment options can also be offered, which include thrombectomy of the iliofemoral veins with iliac venous reconstruction [52]. Another surgical treatment method that has been described is placement of a cross-femoral venous bypass graft (Palma procedure) [53,54]. An arteriovenous fistula is often created to assist long-term patency of the bypass grafts or reconstructed veins, and the fistula is ligated in 6 weeks. Patients usually receive long-term anticoagulation and compression stockings therapy.
POPLITEAL VENOUS COMPRESSION
Background
PVC can be seen in association with popliteal artery compression in popliteal entrapment syndrome. Venous compression can be seen in approximately 27% of healthy individuals and is considered to have no pathological significance unless it causes symptoms [55,56]. As the popliteal artery and vein course adjacent to each other in the popliteal fossa, popliteal entrapment can cause venous as well as arterial compression. The culprit is usually an aberrant course of the medial head of the gastrocnemius muscle [57]. The cause of venous compression can also be an aneurysmal popliteal artery, popliteal cysts or, rarely, a popliteus muscle sling or an adjacent fibrous band. The incidence of popliteal venous entrapment with or without co-existing arterial compression is unknown; however, in a series of 35 patients investigating arterial entrapment, concomitant venous entrapment was found in 9 of the limbs (12.9%) [58]. In cases of arterial compression, males are more commonly affected and the disease is bilateral in 22–67% of the cases [59].
Compression is position dependent and usually occurs with active plantar flexion with a fully extended knee. Patients can be entirely asymptomatic or, in the event of haemodynamically significant compression, they could present with varices below the knee and venous thrombosis. In the long term, chronic venous compression may lead to symptoms of chronic venous insufficiency with pain and oedema.
Imaging findings
Ultrasound with colour Doppler can be utilised to detect DVT and may demonstrate compression with dynamic manoeuvres, showing the change in the calibre of the vein with and without plantar flexion. Reflux into the lesser saphenous vein can be shown as well.
CTV and MRV can provide additional information in the evaluation of PVC (Figure 9) [60]. Anatomical abnormalities such as abnormal muscle insertions and fibrous bands can be detected. MRI with blood pool contrast agents allows for evaluation of the vasculature for about 45 min after the injection of contrast. The ability to have extended scanning time provides the opportunity to obtain images performed with stress manoeuvres (such as plantar flexion), thus allowing the diagnosis of any significant stenoses that may be present only during stress.
Figure 9.
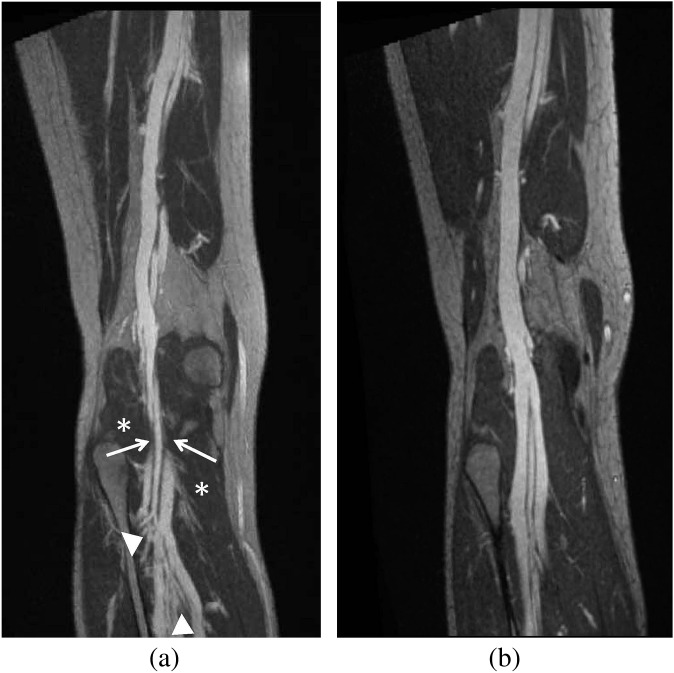
A 32-year-old female with right lower extremity, below the knee swelling, varicosities and venous ulceration at the medial malleolus. (a, b) Three-dimensional-curved maximum intensity projection coronal images from a high-resolution MR venography study using gadofoveset trisodium. (a) Pre-operative image shows popliteal venous compression (arrow) at the level of the popliteal fossa with multiple varices (arrowheads) below the point of compression. The gastrocnemius muscle is seen (*). (b) Post-operative image obtained at the same level following surgical release of the medial head of the gastrocnemius muscle shows resolution of the compression.
Positional ascending venography remains the definitive diagnostic test. Pressure measurements could also be obtained to detect venous hypertension and to show alteration in pressure in response to positional changes.
Management
Patients who do not have significant symptoms can be managed with compression stockings. In cases of acute DVT complicating venous compression, anticoagulation is the treatment of choice. Management is also dependent upon the existence of arterial compression. In cases of both arterial and venous compression, management should be directed to whichever is the presenting or dominating problem. Given that the ultimate curative procedure is surgical decompression, which involves resection of abnormal muscles, tendons or fibrous bands, both arterial and venous compression can be treated at the same setting.
CONCLUSION
Venous compression syndromes can be seen in a number of anatomical locations and can lead to DVT and significant morbidity. In patients presenting with DVT or symptoms of venous stasis, such as pelvic congestion, it is important to realise that the underlying cause could be related to an underlying anatomical compression especially in young, otherwise healthy individuals. Diagnosis should be made only after systemic vasculopathies and compression by other space occupying lesions are ruled out. A combination of conservative and endovascular therapy usually provides the best treatment in most settings.
REFERENCES
- 1.Eliahou R, Sosna J, Bloom AI. Between a rock and a hard place: clinical and imaging features of vascular compression syndromes. Radiographics 2012;32:e33–49 10.1148/rg.321115011 [DOI] [PubMed] [Google Scholar]
- 2.Musani MH, Matta F, Yaekoub AY, Liang J, Hull RD, Stein PD. Venous compression for prevention of postthrombotic syndrome: a meta-analysis. Am J Med 2010;123:735–40 10.1016/j.amjmed.2010.01.027 [DOI] [PubMed] [Google Scholar]
- 3.McDermott S, Oliveira G, Ergül E, Brazeau N, Wicky S, Oklu R. May-Thurner syndrome: can it be diagnosed by a single MR venography study? Diagn Interv Radiol 2013;19:44–8 10.4261/1305-3825.DIR.5939-12.1 [DOI] [PubMed] [Google Scholar]
- 4.Thompson JF, Winterborn RJ, Bays S, White H, Kinsella DC, Watkinson AF. Venous thoracic outlet compression and the Paget-Schroetter syndrome: a review and recommendations for management. Cardiovas Intervent Radiol 2011;34:903–10 10.1007/s00270-011-0148-4 [DOI] [PubMed] [Google Scholar]
- 5.Illig KA, Doyle AJ. A comprehensive review of Paget-Schroetter syndrome. J Vasc Surg 2010;51:1538–47 10.1016/j.jvs.2009.12.022 [DOI] [PubMed] [Google Scholar]
- 6.Hughes ES. Venous obstruction in the upper extremity; Paget–Schroetter's syndrome: a review of 320 cases. Surg Gynecol Obstet 1949;88:89–127 [PubMed] [Google Scholar]
- 7.Feugier P, Chevalier JM. The Paget-Schroetter syndrome. Acta Chir Belg 2005;105:256–64 [DOI] [PubMed] [Google Scholar]
- 8.Atasoy E. Thoracic outlet syndrome: anatomy. Hand Clin 2004;20:7–14 [DOI] [PubMed] [Google Scholar]
- 9.Ariyoshi M, Shiraishi J, Matsubara H. Novel ultrasonography technique for Paget-Schroetter syndrome. Intern Med 2011;50:2237–8 [DOI] [PubMed] [Google Scholar]
- 10.Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e351S–418S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin EE, Zimmerman PT, Grant EG. Sonographic evaluation of upper extremity deep venous thrombosis. J Ultrasound Med 2005;24:829–38 [DOI] [PubMed] [Google Scholar]
- 12.Demondion X, Herbinet P, Van Sint Jan S, Boutry N, Chantelot C, Cotten A. Imaging assessment of thoracic outlet syndrome. Radiographics 2006;26:1735–50 10.1148/rg.266055079 [DOI] [PubMed] [Google Scholar]
- 13.Demondion X, Bacqueville E, Paul C, Duquesnoy B, Hachulla E, Cotten A. Thoracic outlet: assessment with MR imaging in asymptomatic and symptomatic populations. Radiology 2003;227:461–8 10.1148/radiol.2272012111 [DOI] [PubMed] [Google Scholar]
- 14.Remy-Jardin M, Remy J, Masson P, et al. Helical CT angiography of thoracic outlet syndrome: functional anatomy. AJR Am J Roentgenol 2000;174:1667–74 10.2214/ajr.174.6.1741667 [DOI] [PubMed] [Google Scholar]
- 15.Landry GJ, Liem TK. Endovascular management of Paget-Schroetter syndrome. Vascular 2007;15:290–6 [DOI] [PubMed] [Google Scholar]
- 16.Kreienberg PB, Chang BB, Darling RC, Roddy SP, Paty PS, Lloyd WE, et al. Long-term results in patients treated with thrombolysis, thoracic inlet decompression, and subclavian vein stenting for Paget-Schroetter syndrome. J Vasc Surg 2001;33:S100–5 [DOI] [PubMed] [Google Scholar]
- 17.Melby SJ, Vedantham S, Narra VR, Paletta GA, Jr, Khoo-Summers L, Driskill M, et al. Comprehensive surgical management of the competitive athlete with effort thrombosis of the subclavian vein (Paget-Schroetter syndrome). J Vasc Surg 2008;47:809–20 10.1016/j.jvs.2007.10.057 [DOI] [PubMed] [Google Scholar]
- 18.Urschel HC, Jr, Patel AN. Surgery remains the most effective treatment for Paget-Schroetter syndrome: 50 years' experience. Ann Thorac Surg 2008;86:254–60 [DOI] [PubMed] [Google Scholar]
- 19.Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg 2007;45:328–34 10.1016/j.jvs.2006.09.052 [DOI] [PubMed] [Google Scholar]
- 20.Ahmed K, Sampath R, Khan MS. Current trends in the diagnosis and management of renal nutcracker syndrome: a review. Eur J Vasc Endovasc Surg 2006;31:410–16 10.1016/j.ejvs.2005.05.045 [DOI] [PubMed] [Google Scholar]
- 21.Rudloff U, Holmes RJ, Prem JT, Faust GR, Moldwin R, Siegel D. Mesoaortic compression of the left renal vein (nutcracker syndrome): case reports and review of the literature. Ann Vasc Surg 2006;20:120–9 10.1007/s10016-005-5016-8 [DOI] [PubMed] [Google Scholar]
- 22.El-Sadr AR, Mina E. Anatomical and surgical aspects in the operative management of varicocele. Urol Cutaneous Rev 1950;54:257–62 [PubMed] [Google Scholar]
- 23.De Schepper A. Nutcracker phenomenon of the renal vein causing left renal vein pathology. J Belg Rad 1972;55:507–11 [PubMed] [Google Scholar]
- 24.Preza Fernandes J, Amorim R, Gomes MJ, Oliveira V, Reis A, Ribeiro-Castro J. Posterior nutcracker syndrome with left renal vein duplication: a rare cause of haematuria in a 12-year-old boy. Case Rep Urol 2012;2012:849681 10.1155/2012/849681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scultetus AH, Villavicencio JL, Gillespie DL. The nutcracker syndrome: its role in the pelvic venous disorders. J Vasc Surg 2001;34:812–19 10.1067/mva.2001.118802 [DOI] [PubMed] [Google Scholar]
- 26.Takebayashi S, Ueki T, Ikeda N, Fujikawa A. Diagnosis of the nutcracker syndrome with color Doppler sonography: correlation with flow patterns on retrograde left renal venography. AJR Am J Roentgenol 1999;172:39–43 10.2214/ajr.172.1.9888735 [DOI] [PubMed] [Google Scholar]
- 27.Park SJ, Lim JW, Cho BS, Yoon TY, Oh JH. Nutcracker syndrome in children with orthostatic proteinuria: diagnosis on the basis of Doppler sonography. J Ultrasound Med 2002;21:39–45 [DOI] [PubMed] [Google Scholar]
- 28.Unlu M, Orguc S, Serter S, Pekindil G, Pabuscu Y. Anatomic and hemodynamic evaluation of renal venous flow in varicocele formation using color Doppler sonography with emphasis on renal vein entrapment syndrome. Scand J Urol Nephrol 2007;41:42–6 [DOI] [PubMed] [Google Scholar]
- 29.Shin JI, Park JM, Lee JS, Kim MJ. Doppler ultrasonographic indices in diagnosing nutcracker syndrome in children. Pediatr Nephrol 2007;22:409–13 10.1007/s00467-006-0319-8 [DOI] [PubMed] [Google Scholar]
- 30.Park SJ, Shin JI. Renal Doppler ultrasonography in the diagnosis of nutcracker syndrome. Eur J Pediatr 2013;172:135–6 10.1007/s00431-012-1859-5 [DOI] [PubMed] [Google Scholar]
- 31.Kim KW, Cho JY, Kim SH, Yoon JH, Kim DS, Chung JW, et al. Diagnostic value of computed tomographic findings of nutcracker syndrome: correlation with renal venography and renocaval pressure gradients. Eur J Radiol 2011;80:648–54 10.1016/j.ejrad.2010.08.044 [DOI] [PubMed] [Google Scholar]
- 32.Wong HI, Chen MC, Wu CS, Fu KA, Lin CH, Weng MJ, et al. The usefulness of fast-spin-echo T2-weighted MR imaging in Nutcracker syndrome: a case report. Korean J Radiol 2010;11:373–7 10.3348/kjr.2010.11.3.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buschi AJ, Harrison RB, Norman A, Brenbridge AG, Williamson BR, Gentry RR, et al. Distended left renal vein: CT/sonographic normal variant. AJR Am J Roentgenol 1980;135:339–42 10.2214/ajr.135.2.339 [DOI] [PubMed] [Google Scholar]
- 34.Kim WS, Cheon JE, Kim IO, Kim SH, Yeon KM, Kim KM, et al. Hemodynamic investigation of the left renal vein in pediatric varicocele: Doppler US, venography, and pressure measurements. Radiology 2006;241:228–34 10.1148/radiol.2411050271 [DOI] [PubMed] [Google Scholar]
- 35.Menard MT. Nutcracker syndrome: when should it be treated and how? Perspect Vasc Surg Endovasc Ther 2009;21:117–24 10.1177/1531003509338402 [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Yi L, Yang L, Liu Z, Rao J, Liu L, et al. Diagnosis and surgical treatment of nutcracker syndrome: a single-center experience. Urology 2009;73:871–6 [DOI] [PubMed] [Google Scholar]
- 37.Marone EM, Psacharopulo D, Kahlberg A, Coppi G, Chiesa R. Surgical treatment of posterior nutcracker syndrome. J Vasc Surg 2011;54:844–77 10.1016/j.jvs.2011.01.038 [DOI] [PubMed] [Google Scholar]
- 38.Hohenfellner M, D'Elia G, Hampel C, Dahms S, Thüroff JW. Transposition of the left renal vein for treatment of the nutcracker phenomenon: long-term follow-up. Urology 2002;59:354–7 [DOI] [PubMed] [Google Scholar]
- 39.Neste MG, Narasimham DL, Belcher KK. Endovascular stent placement as a treatment for renal venous hypertension. J Vasc Interv Radiol 1996;7:859–61 [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Zhang Y, Li C, Zhang H. Results of endovascular treatment for patients with nutcracker syndrome. J Vasc Surg 2012;56:142–8 10.1016/j.jvs.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 41.Barnes RW, Fleisher HL, 3rd, Redman JF, Smith JW, Harshfield DL, Ferris EJ. Mesoaortic compression of the left renal vein (the so-called nutcracker syndrome): repair by a new stenting procedure. J Vasc Surg 1988;8:415–21 [DOI] [PubMed] [Google Scholar]
- 42.Shebel ND, Whalen CC. Diagnosis and management of iliac vein compression syndrome. J Vasc Nurs 2005;23:10–17 10.1016/j.jvn.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 43.Virchow R. Uber die Erweiterung Kleiner Gefasse. Arch Path Anat 1851;3:427 10.10070BF0196/918 [DOI] [Google Scholar]
- 44.May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology 1957;8:419–27 [DOI] [PubMed] [Google Scholar]
- 45.Oğuzkurt L, Ozkan U, Tercan F, Koç Z. Ultrasonographic diagnosis of iliac vein compression (May–Thurner) syndrome. Diagn Interv Radiol 2007;13:152–5 [PubMed] [Google Scholar]
- 46.Molloy S, Jacob S, Buckenham T, Khaw KT, Taylor RS. Arterial compression of the right common iliac vein; an unusual anatomical variant. Cardiovasc Surg 2002;10:291–2 [DOI] [PubMed] [Google Scholar]
- 47.Morita S, Kimura T, Masukawa A, Saito N, Suzuki K, Mitsuhashi N. Flow direction of ascending lumbar veins on magnetic resonance angiography and venography: would “descending lumbar veins” be a more precise name physiologically? Abdom Imaging 2007;32:749–53 10.1007/s00261-006-9166-0 [DOI] [PubMed] [Google Scholar]
- 48.Oguzkurt L, Ozkan U, Ulusan S, Koc Z, Tercan F. Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol 2008;19:366–70 10.1016/j.jvir.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 49.Wu WL, Tzeng WS, Wu RH, Tsai WL, Chen MC, Lin PC, et al. Comprehensive MDCT evaluation of patients with suspected May–Thurner syndrome. Am J Roentgenol 2012;199:W638–45 10.2214/AJR.11.8040 [DOI] [PubMed] [Google Scholar]
- 50.Wolpert LM, Rahmani O, Stein B, Gallagher JJ, Drezner AD. Magnetic resonance venography in the diagnosis and management of May–Thurner syndrome. Vasc Endovascular Surg 2002;36:51–7 [DOI] [PubMed] [Google Scholar]
- 51.Cil BE, Akpinar E, Karcaaltincaba M, Akinci D. Case 76: May–Thurner syndrome. Radiology 2004;233:361–5 10.1148/radiol.2332030152 [DOI] [PubMed] [Google Scholar]
- 52.Ahmed HK, Hagspiel KD. Intravascular ultrasonographic findings in May–Thurner syndrome (iliac vein compression syndrome). J Ultrasound Med 2001;20:251–6 [DOI] [PubMed] [Google Scholar]
- 53.Lou WS, Gu JP, He X, Chen L, Su H-B, Chen G-P, et al. Endovascular treatment for iliac vein compression syndrome: a comparison between the presence and absence of secondary thrombosis. Korean J Radiol 2009;10:135–43 10.3348/kjr.2009.10.2.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Sullivan GJ, Semba CP, Bittner CA, Kee ST, Razavi MK, Sze DY, et al. Endovascular management of iliac vein compression (May–Thurner) syndrome. J Vasc Interv Radiol 2000;11:823–36 [DOI] [PubMed] [Google Scholar]
- 55.Hölper P, Kotelis D, Attigah N, Hyhlik-Dürr A, Böckler D. Longterm results after surgical thrombectomy and simultaneous stenting for symptomatic iliofemoral venous thrombosis. Eur J Vasc Endovasc Surg 2010;39:349–55 10.1016/j.ejvs.2009.09.028 [DOI] [PubMed] [Google Scholar]
- 56.Passari G, Lentini S, Benedetto F, La Spada M, Spinelli F. Cross-femoral venous by-pass (Palma's procedure) to relieve venous hypertension due to retroperitoneal leiomyosarcoma: a case report. Acta Chir Belg 2010;110:383–6 [DOI] [PubMed] [Google Scholar]
- 57.Comerota AJ. The current role of operative venous thrombectomy in deep vein thrombosis. Semin Vasc Surg 2012;25:2–12 10.1053/j.semvascsurg.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 58.Raju S, Neglen P. Popliteal vein entrapment: a benign venographic feature or a pathologic entity? J Vasc Surg 2000;31:631–41 10.1067/mva.2000.103786 [DOI] [PubMed] [Google Scholar]
- 59.Leon M, Volteas N, Labropoulos N, Hajj H, Kalodiki E, Fisher C, et al. Popliteal vein entrapment in the normal population. Eur J Vasc Surg 1992;6:623–7 [DOI] [PubMed] [Google Scholar]
- 60.Bouhoutsos J, Daskalakis E. Muscular abnormalities affecting the popliteal vessels. Br J Surg 1981;68:501–6 [DOI] [PubMed] [Google Scholar]
- 61.di Marzo L, Cavallaro A. Popliteal vascular entrapment. World J Surg 2005;29:S43–5 10.1007/s00268-004-2058-y [DOI] [PubMed] [Google Scholar]
- 62.Collins PS, McDonald PT, Lim RC. Popliteal artery entrapment: an evolving syndrome. J Vasc Surg 1989;10:484–90 [DOI] [PubMed] [Google Scholar]
- 63.Beitzke D, Wolf F, Juelg G, Lammer J, Loewe C. Diagnosis of popliteal venous entrapment syndrome by magnetic resonance imaging using blood-pool contrast agents. Cardiovasc Intervent Radiol 2011;34:S12–16 10.1007/s00270-009-9702-8 [DOI] [PubMed] [Google Scholar]