Abstract
Transforming growth factor-beta 1 (TGF-β1) stimulates a broad range of effects which are cell type dependent, and it has been suggested to induce cellular senescence. On the other hand, long-term culture of multipotent mesenchymal stromal cells (MSCs) has a major impact on their cellular physiology and therefore it is well conceivable that the molecular events triggered by TGF-β1 differ considerably in cells of early and late passages. In this study, we analyzed the effect of TGF-β1 on and during replicative senescence of MSCs. Stimulation with TGF-β1 enhanced proliferation, induced a network like growth pattern and impaired adipogenic and osteogenic differentiation. TGF-β1 did not induce premature senescence. However, due to increased proliferation rates the cells reached replicative senescence earlier than untreated controls. This was also evident, when we analyzed senescence-associated DNA-methylation changes. Gene expression profiles of MSCs differed considerably at relatively early (P 3 - 5) and later passages (P 10). Nonetheless, relative gene expression differences provoked by TGF-β1 at individual time points or in a time course dependent manner (stimulation for 0, 1, 4 and 12 h) were very similar in MSCs of early and late passage. These results support the notion that TGF-β1 has major impact on MSC function, but it does not induce senescence and has similar molecular effects during culture expansion.
Introduction
Transforming growth factor beta 1 (TGF-β1) triggers complex cellular responses, including activation of SMAD transcription factors, which regulate for example expression of inhibitors of DNA binding proteins 1-3 (ID1, ID2 and ID3) [1]. It has major impact on a multitude of other pathways such as mitogen-activated protein kinase (MAPK), Jun N-terminal kinase (JNK), and the phosphatidylinositol 3-kinase/Akt/mTOR pathways, as well as other down-stream targets of the small GTPases Rho, Rac, and Cdc42 [2–5]. TGF-β1 also up-regulates the cyclin-dependent kinase inhibitors CDKN1A (WAF1; CIP1, p21), CDKN2A (INK4A; p16) and CDKN2B (INK4B; p15) [4,6]. With regard to this variety of implications on the molecular network it may be not surprising that the effects of TGF-β1 are largely dependent on the cell type, the cellular environment and the differentiation state [7,8].
Multipotent mesenchymal stromal cells (MSCs) are concurrently tested in a multitude of clinical trials for a broad range of diseases [9]. They comprise a multipotent subset of cells which is capable of differentiation towards the mesodermal lineages such as adipocytes, osteocytes and chondrocytes [10]. It has been shown that TGF-β is essential for chondrogenic differentiation and supports myogenic differentiation [11,12–12], whereas it negatively effects adipogenic differentiation of MSCs [13,14]. Furthermore, the effect of TGF-β1 on differentiation of MSCs is influenced by substrate elasticity [15,16]. TGF-β alone or in a combination with platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) was suggested to be required to facilitate in vitro proliferation of MSCs [17–19], whereas other studies indicated that it induces cell-cycle arrest in mesodermal cells [20,21]. Some of these contradictory results may be due to the heterogeneous composition of different MSC preparations or culture conditions [22].
Even for defined cell preparations and under standardized culture conditions the cellular composition, morphology, and function changes continuously during culture: MSCs - such as all non-transformed primary cells - undergo a process of replicative senescence in the course of culture expansion. After a limited number of cell divisions they unequivocally stop proliferation, acquire a large and flattened cellular morphology, and they lose their in vitro differentiation potential [23,24]. These peculiar alterations in cellular physiology are reflected by global gene expression changes [23] and highly reproducible epigenetic modifications. Specific CpG sites in the genome become either hyper- or hypo-methylated upon long-term culture of MSCs [25], and can be used to track the process of cellular aging [26,27]. Thus, it is well conceivable, that effects of TGF-β1 differ considerably in cells of early and later passage. In fact, it has been suggested that the sensitivity towards TGF-β is influenced by the aging process [28–30] and it has been further suggested that this cytokine induces cellular senescence [20,21].
In this study, we have further analyzed the effect of TGF-β1 on human bone marrow MSCs, particularly during long-term expansion. Furthermore, we compared the global gene expression changes upon stimulation with TGF-β1 in MSCs of early and late passage to elucidate if the molecular response varies during culture expansion.
Methods
Ethics statement
All samples in this study were used after patient’s written consent using guidelines approved by the Ethic Committee of the University of Aachen (Permit number: EK128/09).
Isolation of MSC from human bone marrow
Multipotent mesenchymal stromal cells were isolated from mononuclear cells (MNCs) by plastic adherence. In brief, bone fragments from caput femoris or tibia plateau from patients undergoing clinical surgery were flushed with phosphate-buffered saline (PBS) and washed twice with PBS. MNC were then resuspended in culture medium consisting of DMEM (1 g/L glucose; PAA Laboratories, Pasching, Austria) supplemented with glutamine, penicillin/streptomycin (both Gibco / life Technologies, UK ) and 10% FSC (PAA) at 37°C in a humidified atmosphere with 5% CO2. Medium changes were performed twice per week and MSCs were passaged when reaching 80-90% of confluence. Re-seeding was performed at a density of 10,000 cells/cm2.
Long term cultivation of MSC
To analyze the effect of TGF-β1 on long-term expansion, MSCs of relatively early passage (P1 - P4) were cultured in parallel with or without 1ng/mL recombinant human TGF-β1 (R&D Systems, Inc., Minneapolis, MN 55413 USA) until they reached replicative senescence. After each passage, the cell number was determined using a Neubauer counting chamber (Brand, Wertheim, Germany) and cumulative population doublings (cPD) were calculated as previously described [31].
Proliferation assay
Cell proliferation was measured with the Thiazolyl Blue Tetrazolium Bromide (MTT) assay as described in our previous work [32]. Briefly, MSCs of passage 3 - 6 were seeded in 96-well plates (3,000 cells/cm2) with different concentrations of TGF-β1. After 7 days, cells were washed with PBS and incubated with 1 mM MTT (Sigma Aldrich, St. Louis, MO, USA) for 3.5 hours at 37°C. The excess solution was discarded and formazan crystals were resolved in 4 mM HCl in isopropanol (both from Roth, Karlsruhe, Germany). The absorbance was measured at 590 nm with a reference of 620 nm using a Tecan Infinite 200 plate reader (Tecan Trading, Switzerland). Each measurement included four technical replicas. Alternatively we estimated proliferation by counting of DAPI stained nuclei after 7 d in a 96-well format. Furthermore, we stimulated MSCs with different concentrations of TGF-β1 for 48 h and incubated with BrdU for additional 24h prior to analyzing BrdU incorporation by Cell Proliferation ELISA (Colorimetric; Roche Applied Science, Mannheim, Germany). Anti-BrdU Peroxidase incubation was performed for 90 min and substrate conversion was measured after 5 - 10 min.
Staining for senescence associated β-galactosidase activity
Activity of pH-dependent senescence-associated β-galactosidase (SA-β-gal) was analyzed simultaneously in different MSC passages using SA-β-gal staining kit (Cell Signaling Technology, Boston, MA, USA). In addition, pH dependent SA-β-gal activity was analyzed with a fluorescence-based method for quantitative and sensitive analysis by flowcytometry [33]. In brief, MSCs were incubated with Bafilomycin A1 (Sigma, St Louis, MO, USA) to prevent lysosomal acidification and subsequently 5-dodecanoylaminofluorescein di-β-D-galactopyranoside (C12FDG, Invitrogen, Eugene, OR, USA) was used as a fluorogenic substrate.
Immunophenotypic analysis
Expression of a panel of surface markers was analyzed in MSCs upon expansion for 4 - 5 passages with or without 1 ng/mL TGF-β1. Cells were stained in parallel with the following monoclonal mouse antihuman antibodies: CD14-APC (clone M5E2), CD29-PE (clone MAR4), CD31-PE (clone WM59), CD34-APC (clone 8G12), CD45-APC (clone HI30), CD73-PE (clone AD2), CD90-APC (clone 5E10) and CD325-PE (clone 8C11; all BD Bioscience, Heidelberg, Germany) and CD105-PE (clone MAR-226; ImmunoTools, Friesoythe, Germany). Analysis was performed using a FACS Canto II cytometer (BD Bioscience) and the collected data were analyzed with WinMDI 2.9 software (The Scripps Institute, Flow Cytometry Core Facility, CA, USA). Fluorescence intensities were normalized to the corresponding autofluorescence measurements for statistical comparison.
In vitro differentiation of MSC
The impact of TGF-β1 on in vitro differentiation was analyzed in MSCs which were pre-incubated either with or without 1 ng/mL TGF-β1 for 1 to 4 passages before induction of differentiation and during osteogenic and adipogenic differentiation [34]. After three weeks, calcium-phosphate deposition of osteogenic differentiated cells was analyzed by Alizarin Red stain and formation of fat droplets of adipogenic differentiated cells was analyzed using the green fluorescent dye BODIPY (4,4-difluoro-1,2,5,7,8-pentamethyl-4-bora-3a,4a-diaza-s-indacene; Molecular Probes, Eugene, USA) with DAPI counterstaining of nuclei. Chondrogenic differentiation was induced in pellet culture. The differentiation medium comprises TGF-β and therefore the same differentiation medium was used for TGF-β1-pretreated cells and un-treated controls [34]. After three weeks chondrogenic differentiation was analyzed by Alcian blue and Periodic acid shiff staining according to routine histology protocols and analyzed with a Leica DM IL HC fluorescence microscope (Leica, Wetzlar, Germany)[35].
Senescence-associated DNA-methylation changes
Culture expansion is associated with highly reproducible genomic hyper-methylation and hypo-methylation at specific CpG sites. We analyzed such senescence-associated DNA methylation (SA-DNAm) changes with our recently described Epigentic-Aging-Signature [26,36]. Briefly, DNA was isolated from 2x105 cells with the NucleoSpin Blood kit (Machery Nagel, Düren, Germany) and bisulfite converted. Pyrosequencing of six relevant CpG sites was performed at Varionostic GmbH (Ulm, Germany) as described before [37]. Beta-values (the percentage of DNAm at the respective sites) were then inserted in our online calculator to estimate passage number of cumulative population doublings (http://www.molcell.rwth-aachen.de/dms/).
Gene expression profiling
MSCs from three different donors at early passage (P3 - P5) and later passage (P10) were used to analyze the kinetic of TGF-β1 stimulation on gene expression. 1x106 cells were seeded into 6-well culture plates and after one day they were stimulated with TGF-β1 at concentrations and periods as indicated in the text. If indicated, we performed serum starvation with two washing steps and incubation for 12h in FCS free culture media before TGF-β1 treatment. RNA was isolated with the miRNeasy kit (Quiagen, Hilden, Germany) and DNAse digestion. RNA concentration and integrity was determined with an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA). Genome-wide gene expression analysis was then performed using GeneChip Human Gene 1.0 ST Arrays (Affymetrix, Santa Clara, CA, USA). The complete microarray data and additional information have been deposited in NCBIs Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE46019.
Real time PCR
cDNA synthesis was generated using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) and real time PCR was performed with the SYBR Green Method. Primers are listed in Table S1 in File S1. Fold change was compared to un-stimulated MSCs and assessed with the ΔΔCt method.
Bioinformatics
Analysis of gene expression profiles was performed via R and Bioconductor packages [38]. Raw probe intensities were summarized and normalized using Factor Analysis for Robust Microarray Summarization (FARMS) [39]. Differential gene expression analysis was performed for the probe sets, which could be mapped to Entrez gene IDs according to the Bioconductor package “hugenetransclusterst10.db”. Gene expression of late and early cell passages without TGF-β stimulation was compared via Linear Models for Microarray Data (Limma) [40] using a factorial design, which takes into account the correlation of the samples along the time course. An FDR of < 5 % was regarded as significant for differential expression [41]. Hierarchical Clustering of genes was performed using Euclidian distance as dissimilarity measure and complete linkage agglomeration. Whole time courses were compared at once using a random-effects model and the empirical Bayes method to estimate probabilities for differential time course expression [42]. A probability of > 0.95 was assumed to indicate differential time-course expression. For further gene set association studies using Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and Gene Ontology (GO) terms the p-values resulting from a Limma analysis and probabilities resulting from the time-course analysis were taken as ranking scores for genes. Gene sets (KEGG pathways and GO terms) were then tested for their association with these ranking scores by performing a univariate logistic regression [43]. Adjustment of resulting p-values was subsequently done according to Benjamini & Yekutieli’s false discovery rate control under dependency [44]. Significant functional associations to GO terms and KEGG pathways are regarded at FDR ≤ 0.05.
Statistics
Results are expressed as mean ± standard deviation of independent experiments. The number of biological replica (n) is depicted in the figure legends. Normal distribution of the data was checked using qq-plots and Shapiro-Wilk test. If the data were approximately normally distributed we have estimated the probability of differences using the paired two-sided Student’s T-test. If data were not normally distributed – e.g. due to natural numbers – we used non-parametric Wilcoxon test as indicated in the text. For all tests, p < 0.05 was used as level of significance.
Results
TGF-β1 influences growth pattern and in vitro differentiation of MSCs
Culture media for MSCs is usually supplemented with fetal calf serum (FCS) which is contains TGF-β1 - although predominantly in the biologically inactive latent form [45]. Since MSC growth is stalled under serum-free conditions we tested if we could observe similar effects of TGF-β1 in MSCs cultured either with 10% FCS or under serum starvation for 12 h. Under both culture conditions we observed a similar immediate early and transient up-regulation of ID1, ID2 and ID3 (1 h) followed by a repression of the corresponding genes (4 h) (Figure S1A in File S1) and this is in line with previous studies [1]. Furthermore, we compared different concentrations of TGF-β1 (0, 0.01, 0.1, 0.3, 1, 3, and 10 ng/mL) and the highest up-regulation of ID1 and ID3 was observed with 1 ng/mL TGF-β1 (Figure S1B in File S1). Therefore, we decided to perform all subsequent experiments in normal growth medium and with the addition of 1 ng/mL recombinant TGF-β1, if not indicated otherwise.
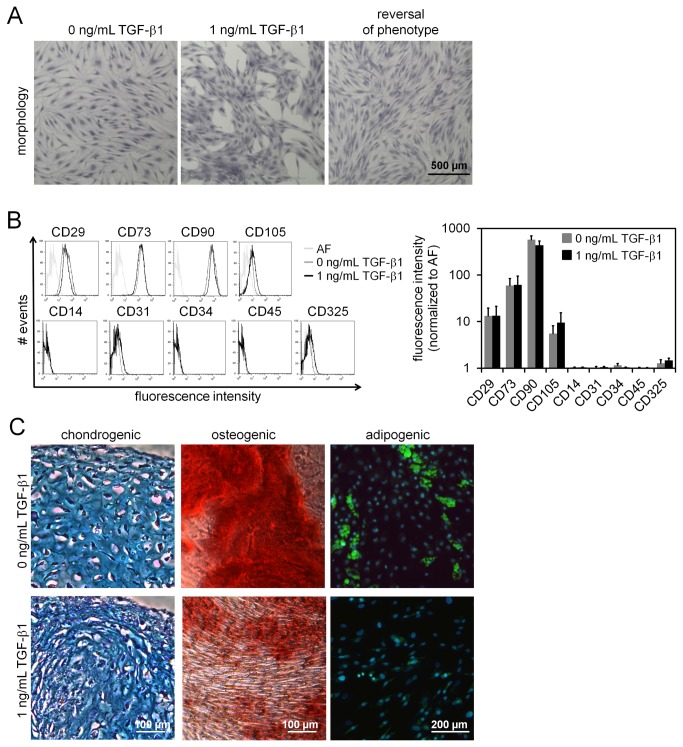
Notably, MSCs cultured with TGF-β1 always revealed a network-like growth pattern whereas untreated cells formed a more homogeneous cellular layer. This effect on growth pattern was reversible when TGF-β1 treated cells were re-seeded in normal culture medium (Figure 1A). All MSC preparations revealed the same typical immunophenotype (CD14-, CD29+, CD31-, CD34-, CD45-, CD73+, CD90+, CD105+, and CD325-), whether they were cultured with or without continuous TGF-β1 stimulation over four passages (Figure 1B). Furthermore, MSCs could be differentiated towards chondrogenic, osteogenic and adipogenic lineage and thus fulfilled standard criteria for definition of MSCs [10]. However, we observed that osteogenic and particularly adipogenic differentiation was greatly impaired if treated with TGF-β1 for several passages and during differentiation (Figure 1C). These results reflect that TGF-β1 has major impact on MSC growth and function.
Figure 1. Influence of TGF-β1 on MSC growth and in vitro differentiation.
Treatment with 1 ng/mL TGF-β1 induces a network-like growth pattern of MSCs within 7 days, which is reversed if the cells are re-seeded in media without TGF-β1 (A; crystal violet staining of fixed cells). Immunophenotypic analysis of MSCs upon continuous culture either with or without TGF-β1 for 4 to 5 passages was performed by flow cytometry. Exemplary histograms are depicted and analysis of mean fluorescence intensity (normalized to auto-fluorescence) did not reveal significant differences upon treatment with TGF-β1 (B; n = 5). MSCs that had been cultured with or without 1 ng/mL TGF-β1 for 1 to 4 passages were differentiated towards chondrogenic, osteogenic and adipogenic lineage. Particularly adipogenic differentiation was impaired by TGF−β1 (C; n = 3).
TGF-β1 increases proliferation and enhances replicative senescence of MSCs
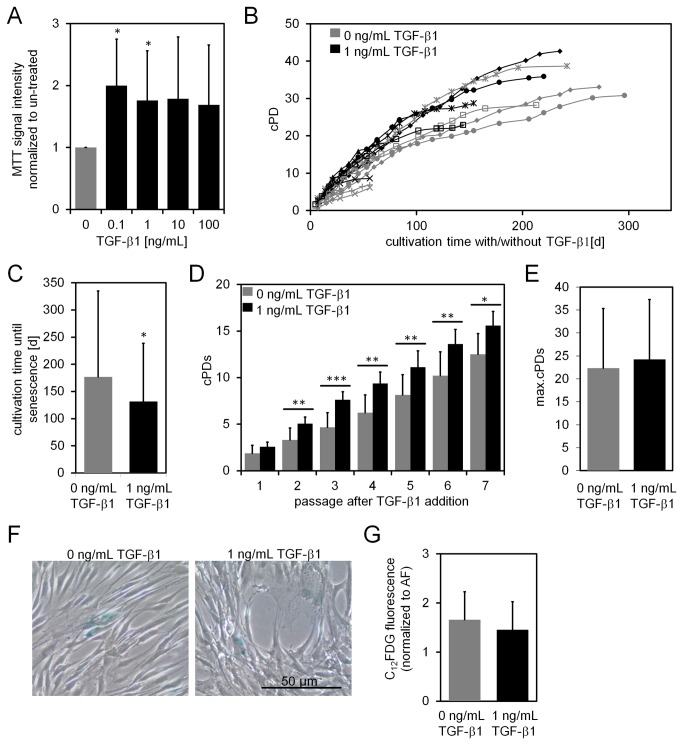
To analyze the impact of TGF-β1 on proliferation of MSCs we stimulated the cells with different concentrations of this ligand (0.1 to 100 ng/mL) for seven days and then performed the MTT assay. In comparison to untreated controls proliferation has significantly increased with 0.1 ng/mL (p = 0.018) and with 1 ng/mL (p = 0.05; both Student’s T-test) of TGF-β1 (n = 5; Figure 2A). Similar results were obtained by counting DAPI stained nuclei after seven days of culture (n = 3) or by quantification of BrdU incorporation after three days (n = 6; Figure S2 in File S1). Furthermore, we observed up-regulation of CDKN2B after 12 h whereas we did not detect cell cycle arrest in G0/G1 phase (Figure S3 in File S1). This is in contrast to observations of other groups which demonstrated that TGF-β1 rather impairs proliferation and induces cell cycle arrest in MSCs and fibroblasts [20].
Figure 2. Influence of TGF-β1 on short- and long-term expansion and senescence.
MSCs were stimulated for 7 days with increasing concentrations of TGF-β1 and proliferation was estimated using the MTT assay (A; n = 5). MSCs were cultured with or without 1 ng/mL TGF-β1 until they stopped proliferation. Cumulative population doublings (cPDs) were calculated throughout culture expansion and depicted by symbols for each passage (B; n = 6). The average culture period until proliferation arrest is shorter if cells are continuously cultured with TGF-β1 (C; n = 6). Comparison of cPDs for the first seven passages for TGF-β1 treated and un-treated MSC revealed a proliferative advantage particularly in the initial three passages (D; n = 6). The maximal cPDs at the time of ultimate proliferation arrest were similar with and without TGF-β1 treatment (E; n = 6). SA-β-gal activity was measured in MSCs cultured either with or without TGF-β1 for 7 passages by histochemical analysis with X-gal staining (E) or flowcytometric analysis of C12FDG (F; n = 3; *p < 0.05; **p < 0.01).
Subsequently, we assessed the effect of TGF-β1 on replicative senescence during long-term culture. To this end, we cultured MSCs of early passage in parallel with or without 1 ng/mL TGF-β1 until the cells reached proliferation arrest. Notably, TGF-β1-treated cells entered senescence on average 46 days earlier than untreated controls (p = 0.05; Wilcoxon test; Figure 2C). When we compared cumulative population doublings (cPDs) over each consecutive passage we observed the above mentioned growth promoting effect of TGF-β1, particularly in the initial three passages: supplementation of TGF-β1 increased the number of cPDs by 0.7, 1.73, and 2.94, respectively. In later passages this effect was no more evident, proliferation of non-treated cells slowly caught up until there was no significant difference in the maximal number of cPDs (Student’s T-test; Figure 2D,E). Staining with senescence-associated beta galactosidase (SA-β-Gal), a surrogate marker for senescent cells, did not reveal significant differences after stimulation with TGF-β1 for up to eight weeks (Student’s T-test; Figure 2F,G).
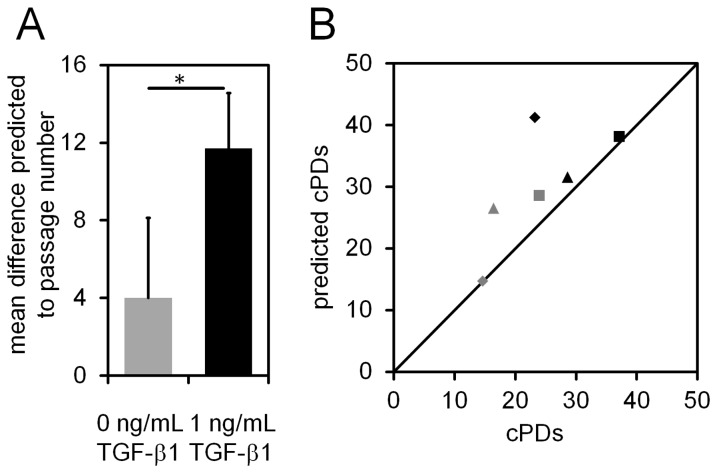
To further analyze if TGF-β1 induces epigenetic senescence-associated changes we used our recently described Epigenetic-Senescence-Signature [26,37]. This method is based on DNA methylation level at six CpG sites and facilitates predictions of passage numbers and cPDs. Cells that were treated with TGF-β1 for five passages were significantly over-estimated in their passage number (p = 0.031; Student’s T-test; Figure 3A), but this can be attributed to the higher number of cell divisions that the cells undergo during this period which is also reflected by the higher numbers of cPDs at the time (Figure 3B). Thus, in relation to the real number of cPDs the predictions of the Epigenetic-Senescence-Signature were not significantly over-estimated. Taken together, TGF-β1 increases proliferation of MSCs and thus they enter replicative senescence after less time. However, we did not observe evidence that TGF-β1 directly induces cellular senescence of MSCs.
Figure 3. Effect of TGF-β1 on senescence-associated DNA-methylation signature.
MSCs were cultured for three passages either with or without TGF-β1. Then the state of cellular senescence was tracked using our recently described Epigenetic-Senescence-Signature which is based on DNA-methylation (DNAm) changes at six specific CpG sites in the genome [35,36]. Predicted passage numbers and real passage numbers correlated well for controls, whereas the passage number was significantly overestimated for TGF-β1 treated cells (A; *p < 0.05). Comparison of predicted and real cumulative population doublings (cPDs) reflected the proliferative advantage with TGF-β1. Overall, the number of cPDs was slightly overestimated by the Epigenetic-Aging-Signature but this cannot be attributed to TGF-β1 stimulation (B). Symbols represent different MSC preparations that were cultured with (black color) or without TGF-β1 (grey color).
TGF-β1 induces similar gene expression changes in early and late passages
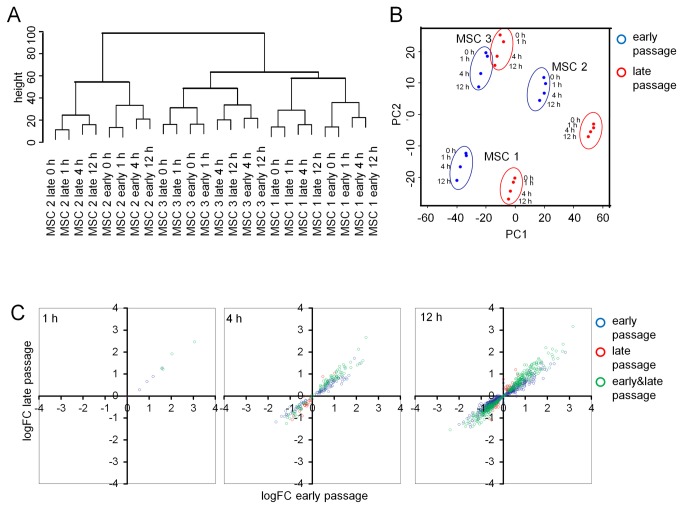
Global gene expression changes were analyzed upon stimulation of MSCs with TGF-β1 for 0, 1, 4 and 12 h. These experiments were performed with three MSC preparations at corresponding early (P3 - 5) and later passages (P10). These passage numbers corresponded to cPDs of 6.17 to 7.49 and 14.51 to 16.38, respectively. Microarray data reflected up-regulation of various TGF-β1 response genes, including transient induction of ID1, ID2 and ID3 which has also been demonstrated by qRT-PCR (Figure S4 in File S1) and is consistent with findings of Kang et al. [46]. Hierarchical clustering of gene expression profiles clearly demonstrated donor-associated variation. Furthermore, corresponding samples of early and late passages clustered together and in tendency gene expression profiles were also sorted according to the time of TGF-β1 stimulation (Figure 4A). These results are also reflected by principal component analysis of the gene expression profiles (Figure 4B). Thus, all three parameters – donor-specificity, passage number, and TGF-β1 – have reproducible impact on gene expression profiles.
Figure 4. Gene expression changes upon TGF-β1 treatment in early and later passages.
Hierarchical clustering of global gene expression profiles (Euclidean distance) revealed inter-donor variation, a close relationship of early and late passages, and continuous changes with TGF-β1 stimulation (A). This was also reflected by principal component analysis (B; components 1 [PC1] and 2 [PC2] are depicted). TGF-β1-induced gene expression changes were compared in MSCs of early passage (P3 - P5) and later passage (P10) upon stimulation for either 1, 4, or 12 hours. Some genes are predominantly induced in early passage (depicted in blue) or in late passage (depicted in red) but the induced gene expression changes were overall very similar in MSCs of early and later passage (C).
Comparison of gene expression profiles of MSCs at early and late passages (all without TGF-β1 stimulation) yielded 345 gene expression changes (File S2). This is in line with our previous work demonstrating that long-term culture of MSCs has major impact on gene expression profiles [23,37,47]. Subsequently, we analyzed differential gene expression upon stimulation with TGF-β1 for either 1, 4, or 12 h in comparison to non-treated controls. The number of differentially expressed genes increased continuously in MSCs of both early and late passages. Notably, there was a very high overlap of TGF-β1 induced genes in early and late passages (Table 1). This became also evident, when we plotted log fold changes in early versus late passages (Figure 4C). Noteworthy, the number of senescence-associated genes remained similar throughout the time course (File S2).
Table 1. Number of regulated genes after TGF-β1 treatment.
| 1 h | 4 h | 12 h | Time course | |
|---|---|---|---|---|
| early passage | 10 | 326 | 2108 | 2469 |
| late passage | 5 | 336 | 952 | 1966 |
| overlap (early and late passage) | 4 | 129 | 646 | 1285 |
Numbers of differentially expressed genes after TGF-β1 stimulation compared to untreated MSCs (Limma T-test; FDR < 5%). These changes were either analyzed in MSCs of early passage or MSCs of later passage (gene lists are provided in File S2). The overlap depicts the number of genes which are regulated in early and late passages.
We then analyzed TGF-β1 induced gene expression changes with regard to the whole time course. 2,469 transcripts and 1,966 transcripts revealed continuous gene expression changes in early and late passages, respectively (probability > 95%; Table 1; File S2). The overlap of these time course-associated genes was remarkably high (1,282 genes). When we specifically looked for genes with significantly differential time-courses between early and late passage, only four genes were identified (probability > 95%) – SULF1, PAQR5, RPLP1, and THBS4 – and none of them was more than two-fold differentially expressed upon TGF-β1 stimulation at any time point.
A follow-up functional analysis for association with KEGG pathways and GO terms revealed very similar TGF-β1 induced gene expression changes in early and late passage (Tables S3 and S4 in File S1). Taken together, our results support the notion that TGF-β1 stimulation has great influence on gene expression in a time course-dependent manner, but there are hardly any differences in TGF-β1 induced gene expression in MSCs of early and late passages. This underlines the similarity of the transcriptional response to TGF-β1 stimulation in early and late passages.
Discussion
The effects of TGF-β1 are interdependent on a variety of different parameters such as culture media, pretreatment, incubation time and even more critical on cell type and state of differentiation [2]. The goal of this study was to analyze the effect of TGF-β1 in long-term culture of MSCs as it has been suggested that TGF-β1 induces cellular senescence [20]. On the other hand, we assumed that TGF-β1 exerts different gene expression changes in cells of early and late passage. Our results support the notion that TGF-β1 has impact on MSC growth and differentiation but there was no evidence that it induces cellular senescence. Notably, despite the profound alterations in cellular physiology during culture expansion the molecular sequel of TGF-β1 appears to be very similar in cells of early and later passages.
In this study, we describe that TGF-β1 induces a network-like growth pattern in MSCs. It was previously reported that TGF-β induces alterations of the actin-cytoskeleton of MSCs [12,48]. In line with these observations, TGF-β1 induced gene expression was particularly associated with KEGG pathways “regulation of actin cytoskeleton” and “focal adhesion” and to GO-Terms “cell adhesion” and “axon guidance”. Several previous studies have indicated that TGF-β promotes proliferation of MSCs [13,17,19] whereas other authors claimed that it rather induces cell-cycle arrest and even cellular senescence [20,21]. Here, we describe that TGF-β1 significantly enhanced proliferation, particularly if applied in low concentrations. These contradictory results might be due to differences in culture conditions. It may also be attributed to differences between individual MSC preparations which vary based on the different starting material, isolation procedures and even between different donors [22].
Adipogenic differentiation was completely inhibited in TGF-β1 pre-cultured MSCs in the presence of TGF-β1 which has been demonstrated in similar studies before [14]. Notably, TGF-β1 treatment also resulted in a significant down-regulation of the peroxisome proliferator-activated receptor gamma (PPAR-γ) even without induction of adipogenic differentiation (0.5-fold down-regulation, p = 0.002). PPAR-γ is a nuclear hormone receptor which plays a central role in adipogenic differentiation [49]. Thus, down-regulation of this master regulator by TGF-β1 may be one of the reasons for impaired adipogenic differentiation. Osteogenic differentiation was also reduced in TGF-β1 pre-cultured MSCs although this effect was less pronounced and varied between experiments. The osteogenic differentiation marker RUNX2 was significantly up-regulated by TGF-β1 even without induction medium (2.5-fold up-regulation, p = 0.003). TGF-β1 may stimulate the early stages of osteogenic differentiation whereas it negatively affects subsequent differentiation steps which are characterized by calcium deposition [13,50,51]. Notably, in vitro differentiation of MSCs is also affected by long-term culture. Various groups have demonstrated that particularly the adipogenic differentiation decays in long-term culture [23,24,52]. In this regard, there may be a functional association of TGF-β1 stimulation and long-term culture associated changes.
TGF-β1 induced cellular senescence has been discussed as a mechanism to prevent malignant cell transformation into e.g. hepatocellular carcinoma [53] or lymphoma cells [54]. It has also been shown to induce apoptosis or senescence in un-transformed cells, like epithelial or T cells [55,56]. On the other hand, the growth stimulatory effect of TGF-β was discussed to activate malignant or non-malignant cell types, like glioma or smooth muscle cells [57,58]. So far, the senescence-stimulatory effect of TGF-β1 has been particularly detected by cell-cycle analysis and estimation of the SA-β-Gal activity [20]. In this study, we could not observe cell-cycle arrest or signs of premature cellular senescence upon treatment with TGF-β1.
For clinical applications MSCs are usually used before passage 5 - due to the functional implications of long-term culture and to the risk of malignant transformation [59]. On the other hand, in vitro expansion is necessary to obtain enough cells and this applies also to the experiments described in this study. In this regard, the term “early passage” might be misleading – we have used it to discern from MSCs which were expanded for much longer time. We even performed long-term culture experiments with or without addition of TGF-β1 for up to 300 days until the cells reached replicative senescence.
Some signs of cellular senescence, such as higher prediction of passage numbers with the Epigenetic-Senescence-Signature [26], may be attributed to higher proliferation rates and this may also be the reason for earlier growth arrest with TGF-β1. There was no significant effect on the maximal number of cPDs, or on the predictions of cPDs. These results support the notion, that cPDs are the more appropriate measure for cellular senescence in comparison to passage numbers [60]. TGF-β1 may enhance replicative senescence due to the growth stimulatory effect but it does not directly induce cellular senescence. Usually other growth factors, such as FGF2, bFGF or PDGF-BB are considered to stimulate MSC growth [61,62]. Addition of TGF-β1 to MSC culture media may enhance culture expansion, too [63] – but it interferes with in vitro differentiation potential.
Senescence has major impact on cellular physiology and epigenetics [23] and it has been shown, that epigenetic modifications may lead to alterations in the TGF-β mediated gene expression [64]. Therefore, we anticipated that the signaling cascades of TGF-β1 are also greatly influenced by the state of replicative senescence during culture expansion. So far, only few studies addressed the role of age-related effects on TGF-β signaling in tissues and cells [28–30]. By monitoring the effect on TGF-β1 during cellular senescence it may be possible to better understand age-related effects in vivo. As demonstrated by several previous studies, gene expression is greatly affected by TGF-β1 in a time-course dependent manner [65,66]. However, we hardly observed any differences between MSCs of early and later passages. These results indicate that long-term culture associated changes may not be a major parameter for the heterogeneous functions of TGF-β1.
Supporting Information
Combined PDF of Figure S1 - S4, and Tables S1, S3 and S4. Figure S1: Assessment of TGF-β1 stimulation conditions; Figure S2: TGF-β1 promotes MSC proliferation. Figure S3: TGF-β1 does not induce cell cycle arrest; Figure S4: Validation of microarray data by RT-PCR; Table S1: List of primers that were used for real time PCR; Table S3: KEGG pathways for differential gene expression in time course analysis; Table S4: Changes in gene expression after TGF-β1 stimulation are associated with very similar GO terms in early and late passages.
(PDF)
Excel table of significant gene expression changes. Gene lists are provided for TGF-β1 induction for 1, 4, 12 h; in early and late passages; and at individual time points or throughout the time course. Furthermore, differences between early and late passages are depicted with or without TGF-β1 stimulation.
(XLS)
Acknowledgments
The authors would like to thank Sabina van Heerlen, Maastricht and Sandra Jätzhold for excellent technical assistance.
Funding Statement
This work was supported by the Stem Cell Network North Rhine Westphalia (to WW), by the Else-Kröner-Fresenius Stiftung (to WW), and particularly by Interregional Program Alma in Silico (http://www.alma-in-silico.com; to HS, KH, MZ, RW, and WW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Liang YY, Brunicardi FC, Lin X (2009) Smad3 mediates immediate early induction of Id1 by TGF-beta. Cell Res 19: 140-148. doi: 10.1038/cr.2008.321. PubMed: 19079362. [DOI] [PubMed] [Google Scholar]
- 2. Massagué J (2000) How cells read TGF-beta signals. Nat Rev Mol Cell Biol 1: 169-178. doi: 10.1038/35042034. PubMed: 11252892. [DOI] [PubMed] [Google Scholar]
- 3. Mu Y, Gudey SK, Landström M (2012) Non-Smad signaling pathways. Cell Tissue Res 347: 11-20. doi: 10.1007/s00441-011-1201-y. PubMed: 21701805. [DOI] [PubMed] [Google Scholar]
- 4. Siegel PM, Massagué J (2003) Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 3: 807-821. doi: 10.1038/nrc1208. PubMed: 14557817. [DOI] [PubMed] [Google Scholar]
- 5. Moustakas A, Heldin CH (2005) Non-Smad TGF-beta signals. J Cell Sci 118: 3573-3584. doi: 10.1242/jcs.02554. PubMed: 16105881. [DOI] [PubMed] [Google Scholar]
- 6. Frippiat C, Chen QM, Zdanov S, Magalhaes JP, Remacle J et al. (2001) Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem 276: 2531-2537. doi: 10.1074/jbc.M006809200. PubMed: 11060295. [DOI] [PubMed] [Google Scholar]
- 7. Massagué J, Chen YG (2000) Controlling TGF-beta signaling. Genes Dev 14: 627-644. PubMed: 10733523. [PubMed] [Google Scholar]
- 8. Mizutani A, Koinuma D, Tsutsumi S, Kamimura N, Morikawa M et al. (2011) Cell type-specific target selection by combinatorial binding of Smad2/3 proteins and hepatocyte nuclear factor 4alpha in HepG2 cells. J Biol Chem 286: 29848-29860. doi: 10.1074/jbc.M110.217745. PubMed: 21646355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sensebé L, Krampera M, Schrezenmeier H, Bourin P, Giordano R (2009) Mesenchymal stem cells for clinical application. Vox Sang 98: 93-107. PubMed: 19663934. [DOI] [PubMed] [Google Scholar]
- 10. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315-317. doi: 10.1080/14653240600855905. PubMed: 16923606. [DOI] [PubMed] [Google Scholar]
- 11. Gao L, McBeath R, Chen CS (2010) Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cells 28: 564-572. PubMed: 20082286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Park JS, Chu JS, Krakowski A, Luo K et al. (2004) Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor beta1 stimulation. J Biol Chem 279: 43725-43734. doi: 10.1074/jbc.M407368200. PubMed: 15302865. [DOI] [PubMed] [Google Scholar]
- 13. Koli K, Ryynänen MJ, Keski-Oja J (2008) Latent TGF-beta binding proteins (LTBPs)-1 and -3 coordinate proliferation and osteogenic differentiation of human mesenchymal stem cells. Bone 43: 679-688. doi: 10.1016/j.bone.2008.06.016. PubMed: 18672106. [DOI] [PubMed] [Google Scholar]
- 14. Kumar A, Ruan M, Clifton K, Syed F, Khosla S et al. (2012) TGF-beta mediates suppression of adipogenesis by estradiol through connective tissue growth factor induction. Endocrinology 153: 254-263. doi: 10.1210/en.2011-1169. PubMed: 22067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS (2012) Matrix rigidity regulates a switch between TGF-beta1-induced apoptosis and epithelial-mesenchymal transition. Mol Cell Biol 23: 781-791. doi: 10.1091/mbc.E11-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park JS, Chu JS, Tsou AD, Diop R, Tang Z et al. (2011) The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials 32: 3921-3930. doi: 10.1016/j.biomaterials.2011.02.019. PubMed: 21397942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ng F, Boucher S, Koh S, Sastry KS, Chase L et al. (2008) PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 112: 295-307. doi: 10.1182/blood-2007-07-103697. PubMed: 18332228. [DOI] [PubMed] [Google Scholar]
- 18. Strutz F, Zeisberg M, Renziehausen A, Raschke B, Becker V et al. (2001) TGF-beta 1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2). Kidney Int 59: 579-592. doi: 10.1046/j.1523-1755.2001.059002579.x. PubMed: 11168939. [DOI] [PubMed] [Google Scholar]
- 19. Jian H, Shen X, Liu I, Semenov M, He X et al. (2006) Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev 20: 666-674. doi: 10.1101/gad.1388806. PubMed: 16543220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ito T, Sawada R, Fujiwara Y, Seyama Y, Tsuchiya T (2007) FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta2. Biochem Biophys Res Commun 359: 108-114. doi: 10.1016/j.bbrc.2007.05.067. PubMed: 17532297. [DOI] [PubMed] [Google Scholar]
- 21. Debacq-Chainiaux F, Borlon C, Pascal T, Royer V, Eliaers F et al. (2005) Repeated exposure of human skin fibroblasts to UVB at subcytotoxic level triggers premature senescence through the TGF-beta1 signaling pathway. J Cell Sci 118: 743-758. doi: 10.1242/jcs.01651. PubMed: 15671065. [DOI] [PubMed] [Google Scholar]
- 22. Wagner W, Ho AD (2007) Mesenchymal stem cell preparations-comparing apples and oranges. Stem. Cell Res 3: 239-248. [DOI] [PubMed] [Google Scholar]
- 23. Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S et al. (2008) Replicative Senescence of Mesenchymal Stem Cells - a Continuous and Organized Process. PLOS ONE 5: e2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schellenberg A, Stiehl T, Horn P, Joussen S, Pallua N et al. (2012) Population dynamics of mesenchymal stromal cells during culture expansion. Cytotherapy 14: 401-411. doi: 10.3109/14653249.2011.640669. PubMed: 22149184. [DOI] [PubMed] [Google Scholar]
- 25. Bork S, Pfister S, Witt H, Horn P, Korn B et al. (2010) DNA Methylation Pattern Changes upon Long-Term Culture and Aging of Human Mesenchymal Stromal Cells. Aging Cell 9: 54-63. doi: 10.1111/j.1474-9726.2009.00535.x. PubMed: 19895632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koch CM, Joussen S, Schellenberg A, Lin Q, Zenke M et al. (2012) Monitoring of cellular senescence by DNA-methylation at specific CpG sites. Aging Cell 11: 366-369. doi: 10.1111/j.1474-9726.2011.00784.x. PubMed: 22221451. [DOI] [PubMed] [Google Scholar]
- 27. Schellenberg A, Lin Q, Schüler H, Koch CM, Joussen S et al. (2011) Replicative senescence of mesenchymal stem cells causes DNA-methylation changes which correlate with repressive histone marks. Aging (Albany NY) 3: 873-888. PubMed: 22025769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iqbal J, Dudhia J, Bird JL, Bayliss MT (2000) Age-related effects of TGF-beta on proteoglycan synthesis in equine articular cartilage. Biochem Biophys Res Commun 274: 467-471. doi: 10.1006/bbrc.2000.3167. PubMed: 10913361. [DOI] [PubMed] [Google Scholar]
- 29. van Beuningen HM, van der Kraan PM, Arntz OJ, van den Berg WB (1994) In vivo protection against interleukin-1-induced articular cartilage damage by transforming growth factor-beta 1: age-related differences. Ann Rheum Dis 53: 593-600. doi: 10.1136/ard.53.9.593. PubMed: 7979598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCaffrey TA, Falcone DJ (1993) Evidence for an age-related dysfunction in the antiproliferative response to transforming growth factor-beta in vascular smooth muscle cells. Mol Cell Biol 4: 315-322. doi: 10.1091/mbc.4.3.315. PubMed: 8387357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cholewa D, Stiehl T, Schellenberg A, Bokermann G, Joussen S et al. (2011) Expansion of adipose mesenchymal stromal cells is affected by human platelet lysate and plating density. Cell Transplant 20: 1409-1422. doi: 10.3727/096368910X557218. PubMed: 21396157. [DOI] [PubMed] [Google Scholar]
- 32. Horn P, Bokermann G, Cholewa D, Bork S, Walenda T et al. (2010) Comparison of Individual Platelet Lysates for Isolation of Human Mesenchymal Stromal Cells. Cytotherapy 12: 888-898. doi: 10.3109/14653249.2010.501788. PubMed: 20662607. [DOI] [PubMed] [Google Scholar]
- 33. Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O (2009) Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4: 1798-1806. doi: 10.1038/nprot.2009.191. PubMed: 20010931. [DOI] [PubMed] [Google Scholar]
- 34. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143-147. doi: 10.1126/science.284.5411.143. PubMed: 10102814. [DOI] [PubMed] [Google Scholar]
- 35. Koch CM, Suschek CV, Lin Q, Bork S, Goergens M et al. (2011) Specific age-associated DNA methylation changes in human dermal fibroblasts. PLOS ONE 6: e16679. doi: 10.1371/journal.pone.0016679. PubMed: 21347436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koch CM, Wagner W (2013) Epigenetic Biomarker to Determine Replicative Senescence of in vitro-Cultured Cells. Methods Mol Biol (in press) . [DOI] [PubMed] [Google Scholar]
- 37. Koch CM, Reck K, Shao K, Lin Q, Joussen S et al. (2013) Pluripotent stem cells escape from senescence-associated DNA methylation changes. Genome Res 23: 248-259. doi: 10.1101/gr.141945.112. PubMed: 23080539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80. doi: 10.1186/gb-2004-5-10-r80. PubMed: 15461798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hochreiter S, Clevert DA, Obermayer K (2006) A new summarization method for Affymetrix probe level data. Bioinformatics 22: 943-949. doi: 10.1093/bioinformatics/btl033. PubMed: 16473874. [DOI] [PubMed] [Google Scholar]
- 40. Smyth G (2005) limma: Linear Models for Microarray Data. In: Gentleman R, Carey V, Dudoit S, Huber W, Irizarry RA. Bioinformatics and Computational Biology Solutions using R and Bioconductor. New York: Springer Verlag; pp. 397-420. [Google Scholar]
- 41. Benjamini Y, Hochberg Y (1995) Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc B Stat Methodol) 57: 289-300. [Google Scholar]
- 42. Aryee MJ, Gutiérrez-Pabello JA, Kramnik I, Maiti T, Quackenbush J (2009) An improved empirical bayes approach to estimating differential gene expression in microarray time-course data: BETR (Bayesian Estimation of Temporal Regulation. BMC Bioinformatics 10: 409. doi: 10.1186/1471-2105-10-409. PubMed: 20003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Montaner D, Dopazo J (2010) Multidimensional gene set analysis of genomic data. PLOS ONE 5: e10348. doi: 10.1371/journal.pone.0010348. PubMed: 20436964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Benjamini Y, Yekutieli D (2001) The Control of the False Discovery Rate in Multiple Testing under Dependency. Ann Statist 29: 1165-1188. doi: 10.1214/aos/1013699998. [DOI] [Google Scholar]
- 45. Oida T, Weiner HL (2010) Depletion of TGF-beta from fetal bovine serum. J Immunol Methods 362: 195-198. doi: 10.1016/j.jim.2010.09.008. PubMed: 20837018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kang Y, Chen CR, Massagué J (2003) A self-enabling TGFbeta response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol Cell 11: 915-926. doi: 10.1016/S1097-2765(03)00109-6. PubMed: 12718878. [DOI] [PubMed] [Google Scholar]
- 47. Schallmoser K, Bartmann C, Rohde E, Bork S, Guelly C et al. (2010) Replicative senescence-associated gene expression changes in mesenchymal stromal cells are similar under different culture conditions. Haematologica 95: 867-874. doi: 10.3324/haematol.2009.011692. PubMed: 20053868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kinner B, Zaleskas JM, Spector M (2002) Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp Cell Res 278: 72-83. doi: 10.1006/excr.2002.5561. PubMed: 12126959. [DOI] [PubMed] [Google Scholar]
- 49. Brun RP, Tontonoz P, Forman BM, Ellis R, Chen J et al. (1996) Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev 10: 974-984. doi: 10.1101/gad.10.8.974. PubMed: 8608944. [DOI] [PubMed] [Google Scholar]
- 50. Bonewald LF, Dallas SL (1994) Role of active and latent transforming growth factor beta in bone formation. J Cell Biochem 55: 350-357. doi: 10.1002/jcb.240550312. PubMed: 7962167. [DOI] [PubMed] [Google Scholar]
- 51. Zhao L, Jiang S, Hantash BM (2010) Transforming growth factor beta1 induces osteogenic differentiation of murine bone marrow stromal cells. Tissue Eng A 16: 725-733. doi: 10.1089/ten.tea.2009.0495. PubMed: 19769530. [DOI] [PubMed] [Google Scholar]
- 52. Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A et al. (2006) Aging of mesenchymal stem cell in vitro. BMC Cell Biol 7: 14. doi: 10.1186/1471-2121-7-14. PubMed: 16529651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Senturk S, Mumcuoglu M, Gursoy-Yuzugullu O, Cingoz B, Akcali KC et al. (2010) Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology 52: 966-974. doi: 10.1002/hep.23769. PubMed: 20583212. [DOI] [PubMed] [Google Scholar]
- 54. Müller J, Samans B, van RJ, Fagà G, Peh KR et al. (2010) TGFbeta-dependent gene expression shows that senescence correlates with abortive differentiation along several lineages in Myc-induced lymphomas. Cell Cycle 9: 4622-4626. doi: 10.4161/cc.9.23.14211. PubMed: 21127397. [DOI] [PubMed] [Google Scholar]
- 55. Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ (2007) CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol 8: 1353-1362. doi: 10.1038/ni1536. PubMed: 17982458. [DOI] [PubMed] [Google Scholar]
- 56. Schuster N, Krieglstein K (2002) Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res 307: 1-14. doi: 10.1007/s00441-001-0479-6. PubMed: 11810309. [DOI] [PubMed] [Google Scholar]
- 57. Stouffer GA, Owens GK (1994) TGF-beta promotes proliferation of cultured SMC via both PDGF-AA-dependent and PDGF-AA-independent mechanisms. J Clin Invest 93: 2048-2055. doi: 10.1172/JCI117199. PubMed: 8182136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Massagué J (2008) TGFbeta in Cancer. Cell 134: 215-230. doi: 10.1016/j.cell.2008.07.001. PubMed: 18662538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wagner W, Ho AD, Zenke M (2010) Different Facets of Aging in Human Mesenchymal Stem Cells. Tissue Eng B Rev 16: 445-453. doi: 10.1089/ten.teb.2009.0825. PubMed: 20196648. [DOI] [PubMed] [Google Scholar]
- 60. Wagner W, Bork S, Lepperdinger G, Joussen S, Ma N et al. (2010) How to track cellular aging of mesenchymal stromal cells? Aging (Albany NY) 2: 224-230. PubMed: 20453259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gharibi B, Hughes FJ (2012) Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells. J Transl Med 1: 771-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chieregato K, Castegnaro S, Madeo D, Astori G, Pegoraro M et al. (2011) Epidermal growth factor, basic fibroblast growth factor and platelet-derived growth factor-bb can substitute for fetal bovine serum and compete with human platelet-rich plasma in the ex vivo expansion of mesenchymal stromal cells derived from adipose tissue. Cytotherapy 13: 933-943. doi: 10.3109/14653249.2011.583232. PubMed: 21623669. [DOI] [PubMed] [Google Scholar]
- 63. Chase LG, Lakshmipathy U, Solchaga LA, Rao MS, Vemuri MC (2010) A novel serum-free medium for the expansion of human mesenchymal stem cells. Stem Cell Res Ther 1: 8. doi: 10.1186/scrt8. PubMed: 20504289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ikushima H, Miyazono K (2012) TGF-beta signal transduction spreading to a wider field: a broad variety of mechanisms for context-dependent effects of TGF-beta. Cell Tissue Res 347: 37-49. doi: 10.1007/s00441-011-1179-5. PubMed: 21618142. [DOI] [PubMed] [Google Scholar]
- 65. Xie L, Law BK, Aakre ME, Edgerton M, Shyr Y et al. (2003) Transforming growth factor beta-regulated gene expression in a mouse mammary gland epithelial cell line. Breast Cancer Res 5: R187-R198. doi: 10.1186/bcr640. PubMed: 14580254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kloth JN, Fleuren GJ, Oosting J, de Menezes RX, Eilers PH et al. (2005) Substantial changes in gene expression of Wnt, MAPK and TNFalpha pathways induced by TGF-beta1 in cervical cancer cell lines. Carcinogenesis 26: 1493-1502. doi: 10.1093/carcin/bgi110. PubMed: 15878915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Combined PDF of Figure S1 - S4, and Tables S1, S3 and S4. Figure S1: Assessment of TGF-β1 stimulation conditions; Figure S2: TGF-β1 promotes MSC proliferation. Figure S3: TGF-β1 does not induce cell cycle arrest; Figure S4: Validation of microarray data by RT-PCR; Table S1: List of primers that were used for real time PCR; Table S3: KEGG pathways for differential gene expression in time course analysis; Table S4: Changes in gene expression after TGF-β1 stimulation are associated with very similar GO terms in early and late passages.
(PDF)
Excel table of significant gene expression changes. Gene lists are provided for TGF-β1 induction for 1, 4, 12 h; in early and late passages; and at individual time points or throughout the time course. Furthermore, differences between early and late passages are depicted with or without TGF-β1 stimulation.
(XLS)