Abstract
Objective
To assess the contraceptive efficacy, user acceptability, cycle control, and tolerability of a combined contraceptive vaginal ring for up to 13 cycles.
Materials and Methods
Healthy women coming to the OPD for contraceptive advice were enrolled in this one-year study. Each ring was used for three weeks followed by a one-week ring-free period.
Results
A total of 184 women started treatment forming the intent to treat population. Subjects were followed for 13 cycles. Compliance was good with 99 % of cycles in full compliance with specified criteria. In the intent to treat population, no pregnancies occurred giving a Pearl Index of 0. The mean incidence of withdrawal bleeding was 99 % in all cycles. There was 0.16 % incidence of intermenstrual bleeding and 2 % incidence of early withdrawal bleeding. The ring was well tolerated with a low incidence of adverse events.
Conclusion
The ring is an effective contraceptive that is convenient, well tolerated with excellent cycle control, and highly acceptable to users.
Keywords: Combined vaginal contraceptive ring, Efficacy, Cycle control
Introduction
Combined oral contraceptive pills (COCPs) have been available for many years now. There has been a lot of development in the combined oral contraceptives, which focused on lowering the dosage of ethinylestradiol (EE) and progestogens and using newer, more selective progestogens [1]. Cycle control is a key factor affecting contraceptive compliance and it was found that reducing the dose of estrogen below 20 mcg/day of EE adversely affects cycle control [2]. There are some disadvantages with oral administration of contraceptives. The first is the hepatic first-pass metabolism of the oral contraceptives and the second is the reduced uptake because of vomiting or food interactions. Also, due to the daily pill intake of all combined oral contraceptives, there is fluctuation in hormone levels and poor compliance in some users [3–5].
Various hormonal contraceptive methods available are oral contraceptive pills, hormonal IUCD, injectables, implants, and the patch. Most of these widely available non-oral hormonal contraceptives contain only progestogen. These products need to be administered by medical personnel and there may be an unpredictable bleeding pattern. This makes them less acceptable to many women.
Because of these disadvantages, other approaches were investigated, which led to the development of vaginal rings to administer contraceptive steroids [6, 7]. The vaginal ring has an easy insertion and removal by the user herself, no need for daily intervention, regular cycles, and the possibility of administering both estrogens and progestogens. The vaginal ring also allows a lower dose of estrogen to be administered than with oral forms, which may reduce the occurrence of dose-related adverse events.
A combined contraceptive vaginal ring was developed which released 120 mcg of etonogestrel and 15 mcg of EE daily [8]. It is a flexible transparent ring made of ethinyl vinyl acetate (evatane). It is a monthly vaginal ring with an outer diameter of 54 mm and thickness of 4 mm and was used for three weeks followed by a one-week ring-free period. It was made available in India in December 2009 by the name of Nuvaring (henceforth all details are pertaining to Nuvaring). Published data show that the vaginal ring completely inhibits ovulation [9]. Dieben et al. [10] concluded that the combined contraceptive vaginal ring is an effective contraceptive with excellent cycle control and is well tolerated and highly acceptable to users.
A recent, open-label multicenter trial showed NuvaRing to be a valid contraceptive method to ensure optimal cycle control with low incidence of irregular bleeding and altered withdrawal bleeding [11]. It also confirmed previous data from the literature about the excellent efficacy, tolerability, and acceptability of NuvaRing [7, 10, 12].
The combined contraceptive vaginal ring has comparable efficacy and tolerability with a COC containing 150 mcg of levonorgestrel (LNG) and 30 mcg of EE and does not require daily dosing [12]. The combined contraceptive vaginal ring provides robust contraceptive protection with a low pearl index as demonstrated by large-scale trials [7, 10–12]. With a low daily dose of EE, the combined contraceptive vaginal ring has consistently been shown to have excellent cycle control in several large studies[7, 10, 11, 13]. Furthermore, cycle control with the vaginal ring has been shown to be superior to that with a combined oral contraceptive pill containing 30 mcg of EE and 150 mcg of levonorgestrel or 3 mg drosperinone [12, 13]. The vaginal ring proved to be a reliable and safe means of contraception for late reproductive age women with type-1 Diabetes Mellitus (DM)[14].
In our hospital, the cafeteria approach was offered to patients requesting contraception after proper counseling and many patients chose the vaginal contraceptive ring. This Open Observational Study was conducted at our tertiary care center with the objective to assess the contraceptive efficacy, user acceptability, cycle control, and tolerability of a combined contraceptive vaginal ring for up to 13 cycles.
Materials and Methods
Hundred and eighty-four healthy women of the age group 18–40 years who came to our OPD for contraceptive advice and opted for the vaginal ring were recruited for one year (13 cycles) for the study. The study was conducted over a period of about two years from Jan 2010 to Mar 2012.
Exclusion criteria for the study were contraindications to contraceptive steroids, the presence of certain conditions relevant to ring use such as cervicitis, vaginitis, bleeding cervical erosion, dyspareunia, or other coital problems.
All subjects were given instructions on ring use. The first insertion of the ring was done by the gynecologist in the OPD. The subjects were taught the method of insertion and removal of ring. Removal of the first ring and the subsequent reinsertion and removal were done by the subjects themselves.
For Starters, ring insertion was done on Day one/two of the menstrual cycle and for Switchers (from COCPs), ring insertion was done seven days after the start of the usual pill-free period. After temporary removal of the ring for coitus, it was allowed to be reinserted within two hours.
The intended period of this study was 13 cycles of ring use in a 28-day cycle with three weeks of use and a one-week ring-free period.
Study assessments were done at the time of Initial screening, in the first week after cycles one, three, six, and 13, or premature discontinuation. All subjects were asked to record the duration of ring use by recording the first insertion day and the dates of new ring insertions and removals in a diary. These data were documented on a Performa. A cycle was considered compliant if the ring use period did not deviate by more than 48 h from the usual three weeks and the ring-free period did not deviate by more than 24 h from the usual one week. Shortened and prolonged ring-free periods imply durations of less than six and more than eight days, respectively.
Contraceptive efficacy was assessed by the number of pregnancies and pearl index. Any pregnancy during the study period would be documented (Pearl Index is defined as the number of pregnancies per 100 woman years of exposure).
The cycle control was assessed daily by subjects by recording the following parameters in their diaries: spotting (requiring one pad per day) or bleeding (requiring two or more pads per day), withdrawal bleeding in the ring-free period, early or late withdrawal bleeding (i.e., any withdrawal bleeding starting before or continuing beyond the ring-free period), or any irregular bleeding.
Adverse events and device-related events like vaginal discomfort, coital problems, foreign body sensation, and expulsion were recorded at each study visit.
Discontinuation of the study could be due to adverse events, device-related events, irregular bleeding, pregnancy, no further requirement of contraception, or loss to follow-up.
The user’s acceptability of the vaginal ring was assessed by a questionnaire at cycles one, three, six, and 13, or on early discontinuation.
All analyses were done on both the intent to treat population (all women who started treatment) and the per-protocol population (all treated women without major protocol violation).
Our Intent to treat population was N = 184. The baseline characteristics like age distribution and parity of subjects are mentioned in Tables 1 and 2.
Table 1.
Age distribution of subjects
| 20–25 years | 51 |
| 26–30 years | 92 |
| 31–35 years | 41 |
Table 2.
Parity of the subjects
| Parity | No. of patients | Percentage |
|---|---|---|
| Nulligravida | 74 | 40.2 |
| Nullipara | 90 | 48.9 |
| Multipara | 20 | 10.8 |
Results
A total of 184 subjects were recruited for the study and started treatment and hence formed the Intent to treat population.
There was 100 % subject follow-up after one month. 10 subjects were lost to follow-up after three months. Another 15 subjects lost to follow-up by end of 13 cycles; two subjects discontinued ring use due to device-related events, two subjects switched over to the LNG intrauterine system for want of a long-term contraceptive. Out of these 25 subjects, 10 relocated to foreign locations and were satisfied with the usage and had taken enough quotas of rings with them and 12 subjects discontinued for want of pregnancy.
Hence, our per-protocol population consisted n = 143 subjects.
The contraceptive efficacy of the vaginal ring was assessed by calculating the pearl index and is mentioned in Table 3 along with the pearl index of other studies [7, 10–12].
Table 3.
Contraceptive Efficacy of combined vaginal contraceptive ring (Nuvaring)
| Study | Method | Pearl index |
|---|---|---|
| Our Study | Nuvaring (N = 184) | 0 |
| Roumen et al. 2001 | Nuvaring (N = 1145) | 0.65 |
| Dieben et al. 2002 | Nuvaring (N = 1177) | 1.75 |
| K. Oddsson et al. 2005 | Nuvaring (N = 512) | 1.23 |
| Bruni et al. 2008 | Nuvaring (N = 165) | 0 |
About 98 % of subjects never temporarily removed the ring during any of the ring periods. The cycle control was assessed by analyzing the diary recordings of the various parameters done by the subjects. The pattern of withdrawal bleeding in the per-protocol population has been shown in Table 4.
Table 4.
Incidence of withdrawal bleeding as a proportion of evaluable cycles (1–13) in the per-protocol population
| Mean incidence (%) | |
|---|---|
| Withdrawl bleeding | 99 |
| Early withdrawl bleeding | 2 |
| Intermenstrual bleeding | 0.16 |
Cycles were reported to be shorter with vaginal ring use with withdrawal bleeding starting as soon as the ring was removed. There was a very low incidence of irregular bleeding. Intermenstrual bleeding was recorded only in one subject. There was a high incidence of intended bleeding pattern. The discontinuation rate due to bleeding irregularities was extremely low (0.7 %). Excellent cycle control is probably due to the continuous release of hormones from the ring.
During the one-year study period, we analyzed the various adverse events recorded by the subjects. The most common treatment-related adverse events were headache and vaginitis. None of the subjects reported weight gain with vaginal ring use and only two subjects discontinued use of the ring due to foreign body sensation in the vagina. The incidence of typically estrogen-related events such as breast tenderness was very low. Table 5 shows the incidence of various adverse events with vaginal ring use.
Table 5.
Adverse events and device-related events with vaginal ring
| Adverse event | Percentage (N = 184) |
|---|---|
| Device-related events | 1 |
| Headache | 2.3 |
| Breast tenderness | 0.5 |
| Vaginitis | 3.8 |
| Bleeding irregularities | 0.5 |
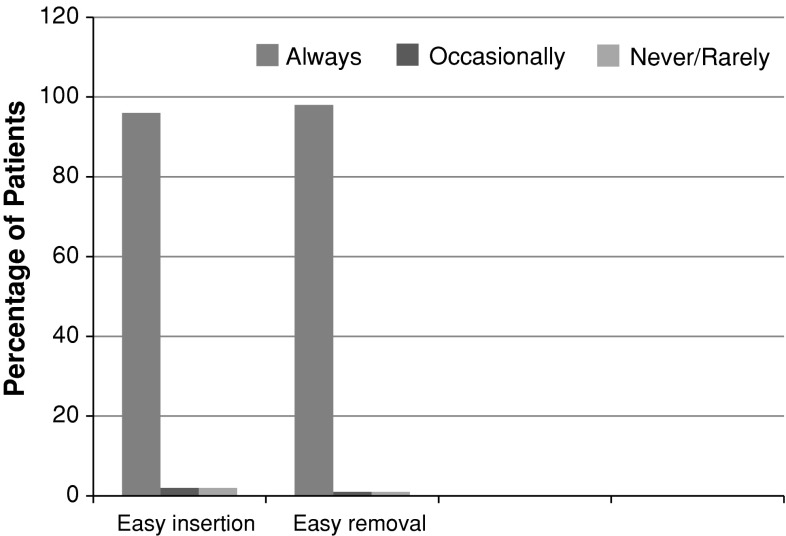
The questionnaires about tolerability and user acceptability were analyzed. Ninety-six percent subjects said that there was an easy insertion of ring and 98 % quoted easy removal as well. Ninety-four percent partners did not object to the ring during coitus. Ninety-seven percent of subjects said that they would recommend use of the vaginal ring to others. The user acceptability in terms of ease of insertion and removal of ring is shown in Fig. 1.
Fig. 1.
User acceptability: ease of insertion and removal of vaginal ring
Discussion
The analysis of this one-year study concludes that this combined vaginal contraceptive ring has a very good efficacy and excellent cycle control which is similar to previously published multicentric and large-scale studies [7, 10, 11, 13]. It is well tolerated and is acceptable to most users [7, 10–12].
It was observed that most women were compliant with the vaginal ring usage and used it correctly and there were negligible temporary ring removals. There were no ring fall outs.
During our study period, there were no pregnancies with a pearl index of zero. Hence, the Pearl index was similar to that previously demonstrated in large-scale trials (0.65, 1.75, 1.23, 0, respectively) [7, 10–12] and it demonstrates that the vaginal ring provides robust contraceptive protection.
Studies have shown that use of this ring causes effective inhibition of ovulation, which is comparable to that with a 30 mcg EE and 150 mcg desogestrel COC pill [9], and it has comparable efficacy and tolerability with a COC containing 150 mcg of LNG and 30 mcg of EE [12] .
Our study demonstrates that the combined contraceptive vaginal ring has excellent cycle control with regular menstrual bleeding which assures the absence of pregnancy. Cycle control with NuvaRing has been shown to be superior to that with a COC containing 30 mcg of EE and 150 mcg of levonorgestrel or drosperinone [12, 13]. There was a very low incidence of irregular bleeding (only 0.16 %), which was similar to or less than shown in previous multicenter trials [7, 10, 11] (5.01 % in study by Bruni et al.) [11]. The vaginal ring has good cycle control despite very low levels of hormones, possibly because of a continuous and sustained steroid release.
The ring was well tolerated by the users with a low incidence of side effects, the major side effects being vaginitis and headache, which were comparable with published studies [7, 10–12]. Only one percent subjects reported other device-related events and 0.5 % had breast tenderness. NuvaRing has previously been shown to have a neutral effect on body weight [10]. In our study as well, none of the patients had any weight gain.
Most women and their partners felt comfortable with the ring during coitus and most women were very satisfied with the use of the ring. Most of the users (97 %) would recommend it to others. These results were similar to previous large-scale studies [7, 10]. It was preferred by patients who frequently cross time zones and have busy lifestyles.
In summary, the combined vaginal contraceptive ring provides uniform release of hormones with the lowest EE dose in combined contraception. It is once a month convenient and requires discrete administration with lesser chances to forget than a daily dose. It has an excellent cycle control with high contraceptive efficacy. A high compliance and higher user satisfaction were observed.
Hence, the combined vaginal contraceptive ring is a safe and convenient combined hormonal contraceptive option.
References
- 1.Fotherby K, Caldwell AD. New progestogens in oral contraception. Contraception. 1994;49:1–32. doi: 10.1016/0010-7824(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg MJ, Waugh MS, Meehan TE. Use and misuse of oral contraceptives: risk indicators for poor pill taking and discontinuation. Contraception. 1995;51:283–288. doi: 10.1016/0010-7824(95)00074-K. [DOI] [PubMed] [Google Scholar]
- 3.Zibners A, Cromer BA, Hayes J. Comparison of continuation rates for hormonal contraception among adolescents. J Pediatr Adolesc Gynecol. 1999;12:90–94. doi: 10.1016/S1083-3188(00)86633-4. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg M, Waugh MS. Causes and consequences of oral contraceptive noncompliance. Am J Obstet Gynecol. 1999;180:276–279. doi: 10.1016/S0002-9378(99)70718-0. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg MJ, Waugh MS, Burnhill MS. Compliance, counseling and satisfaction with oral contraceptives: a prospective evaluation. Fam Plann Perspect. 1998;30:89–92. doi: 10.2307/2991665. [DOI] [PubMed] [Google Scholar]
- 6.Weisberg E, Fraser IS, Lacarra M, et al. Efficacy, bleeding patterns, and side effects of a 1-year contraceptive vaginal ring. Contraception. 1999;59:311–318. doi: 10.1016/S0010-7824(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 7.Roumen FJME, Apter D, Mulders TMT. Efficacy, tolerability and acceptability of a novel contraceptive vaginal ring releasing etonogestrel and ethinyl oestradiol. Hum Reprod. 2001;16:469–475. doi: 10.1093/humrep/16.3.469. [DOI] [PubMed] [Google Scholar]
- 8.Timmer CJ, Mulders TMT. Pharmacokinetics of etonogestrel and ethinylestradiol released from a combined contraceptive vaginal ring. Clin Pharmacokinet. 2000;39:233–242. doi: 10.2165/00003088-200039030-00005. [DOI] [PubMed] [Google Scholar]
- 9.Mudlers TMT, Dieben TOM. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril. 2001;75:865–870. doi: 10.1016/S0015-0282(01)01689-2. [DOI] [PubMed] [Google Scholar]
- 10.Theme OM Dieben, Frans JME Roumen, Dan Apter. Efficacy, cycle control, user acceptability of a novel contraceptive ring. Obstet Gynecol. 2002;100:585–93. [DOI] [PubMed]
- 11.Vincenzina B, Valentina P, Stefano L, et al. An open-label, multicentre trial to evaluate the vaginal bleeding pattern of the combined contraceptive vaginal ring NuvaRing. Eur J Obstet Gynecol Reprod Biol. 2008;139:65–71. [DOI] [PubMed]
- 12.Oddsson, Beate Leifels-Fischer, Nilson Roberto de Melo, et al. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: a 1-year randomized trial. Contraception. 2005;71:176–82. [DOI] [PubMed]
- 13.Milsom I, Lete I, Bjertnaes A, et al. Effects on cycle control and bodyweight of the combined contraceptive ring, NuvaRing, versus an oral contraceptive containing 30 mcg ethinyl estradiol and 3 mg drospirenone. Hum Reprod. 2006;21(9):2304–11. [DOI] [PubMed]
- 14.Grodnitskaya EE, Grigoryan OR, Klinyshkova EV, et al. Effect on carbohydrate metabolism and analysis of acceptability (menstrual cycle control) of extended regimens of the vaginally inserted hormone-releasing system ‘NuvaRing’ as compared with the standard 21/7 regimen in reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2010;26(9):663–8. [DOI] [PubMed]