Background: A fine balance between the anti- and pro-apoptotic multidomain Bcl-2 family proteins controls hepatocyte apoptosis in the healthy liver.
Results: Disruption of the BH3-only proteins Bim and Bid prevents spontaneous hepatocyte apoptosis in the absence of anti-apoptotic Bcl-2 family proteins.
Conclusion: Hepatocyte integrity is maintained by the well orchestrated Bcl-2 network.
Significance: We demonstrated the novel involvement of BH3-only proteins in the healthy Bcl-2 network of the liver.
Keywords: Bcl-2 Family Proteins, Cell Biology, Hepatocyte, Liver Injury, Mitochondrial Apoptosis, Bcl-xl, Mcl-1
Abstract
An intrinsic pathway of apoptosis is regulated by the B-cell lymphoma-2 (Bcl-2) family proteins. We previously reported that a fine rheostatic balance between the anti- and pro-apoptotic multidomain Bcl-2 family proteins controls hepatocyte apoptosis in the healthy liver. The Bcl-2 homology domain 3 (BH3)-only proteins set this rheostatic balance toward apoptosis upon activation in the diseased liver. However, their involvement in healthy Bcl-2 rheostasis remains unknown. In the present study, we focused on two BH3-only proteins, Bim and Bid, and we clarified the Bcl-2 network that governs hepatocyte life and death in the healthy liver. We generated hepatocyte-specific Bcl-xL- or Mcl-1-knock-out mice, with or without disrupting Bim and/or Bid, and we examined hepatocyte apoptosis under physiological conditions. We also examined the effect of both Bid and Bim disruption on the hepatocyte apoptosis caused by the inhibition of Bcl-xL and Mcl-1. Spontaneous hepatocyte apoptosis in Bcl-xL- or Mcl-1-knock-out mice was significantly ameliorated by Bim deletion. The disruption of both Bim and Bid completely prevented hepatocyte apoptosis in Bcl-xL-knock-out mice and weakened massive hepatocyte apoptosis via the additional in vivo knockdown of mcl-1 in these mice. Finally, the hepatocyte apoptosis caused by ABT-737, which is a Bcl-xL/Bcl-2/Bcl-w inhibitor, was completely prevented in Bim/Bid double knock-out mice. The BH3-only proteins Bim and Bid are functionally active but are restrained by the anti-apoptotic Bcl-2 family proteins under physiological conditions. Hepatocyte integrity is maintained by the dynamic and well orchestrated Bcl-2 network in the healthy liver.
Introduction
Apoptosis via the intrinsic pathway, which is known as the mitochondrial pathway, is regulated by Bcl-2 family members. These members are divided into two groups as follows: core Bcl-2 family proteins, which possess three or four Bcl-2 homology domains (BH1–BH4)2 and the Bcl-2 homology domain 3 (BH3)-only proteins (1). The former, which are multidomain proteins, are subdivided into pro- and anti-apoptotic proteins. Pro-apoptotic core Bcl-2 family members, such as Bax and Bak, serve as effector molecules of this apoptotic machinery. Upon activation, these members can form pores to permeabilize the mitochondrial outer membrane. Apoptogenic factors, such as cytochrome c, can then be released through this membrane into the cytosol, leading to the activation of the caspase cascade and to cellular demise (2). Anti-apoptotic core Bcl-2 family members, including Bcl-2, Bcl-xL, Mcl-1, Bcl-w, and Bfl-1/A1, inhibit the intrinsic pathway of apoptosis by either directly or indirectly antagonizing Bak/Bax activity (3–5). In the original rheostasis model, cellular life and death are regulated by a balance between these anti- and pro-apoptotic core Bcl-2 family proteins (6). We previously reported that the hepatocyte-specific deletion of the bcl-x gene resulted in spontaneous hepatocyte apoptosis, and this effect could be completely prevented by the additional deletion of the bak and bax genes (7). These findings elucidated the importance of the rheostatic balance of the core Bcl-2 family proteins in controlling hepatocyte apoptosis in the healthy liver.
The BH3-only proteins, which include at least eight members, are considered to function as pro-apoptotic sensors, and these proteins set this rheostatic balance toward apoptosis upon activation by a variety of apoptotic stimuli (8, 9). It has been reported that hepatocyte apoptosis through the activation of these BH3-only proteins is involved in the pathophysiology of various liver diseases (10–12). Alternatively, we previously reported that the slight activation of Bid, which can trigger hepatocyte apoptosis, occurs even in the healthy liver and that the inactivation of Bid partially ameliorated spontaneous hepatocyte apoptosis in Bcl-xL- or Mcl-1-knock-out mice (7, 13). In the present study, we focused on another BH3-only protein, Bim, which promotes hepatocyte apoptosis upon activation by free fatty acids or by reactive oxygen species in pathological settings, and we further clarified the orchestration of the Bcl-2 network, which governs hepatocyte life and death in the physiological state (10, 11, 14, 15). We found that the disruption of Bim ameliorated hepatocyte apoptosis in Bcl-xL- or Mcl-1-knock-out mice, indicating the involvement of Bim in this hepatocyte apoptosis machinery in the healthy liver as well as that of Bid. Additionally, the deletion of both Bim and Bid prevented the massive hepatocyte apoptosis caused by the inhibition of both Bcl-xL and Mcl-1, suggesting that Bim and Bid are functionally active in the healthy liver and are essential regulators for promoting the intrinsic pathway of apoptosis in hepatocytes in the absence of anti-apoptotic Bcl-2 family proteins. Our present study unveiled the fine and dynamic Bcl-2 networks, the orchestration of which determines hepatocyte life and death in the healthy liver.
EXPERIMENTAL PROCEDURES
Mice
Mice carrying a bcl-x gene with two loxP sequences at the promoter region and a second intron (bcl-xflox/flox), mice carrying an mcl-1 gene encoding amino acids 1–179 flanked by two loxP sequences, and heterozygous alb-cre transgenic mice expressing the Cre recombinase gene under regulation of the albumin gene promoter have been described previously (16–18). Hepatocyte-specific Bcl-xL-knock-out mice (bcl-xflox/floxalb-cre) (17), hepatocyte-specific Mcl-1-knock-out mice (bcl-xflox/floxalb-cre) (13), systemic Bid-knock-out mice (bid−/−) (12), and Bcl-xL/Bid double knock-out mice (bid−/−bcl-xflox/floxalb-cre) (7) have also been described previously. We purchased C57BL/6J mice from Charles River (Osaka, Japan), systemic Bim-knock-out mice (bim−/−) from the Jackson Laboratory (Bar Harbor, ME), and NOD/ShiJic-scid Jcl mice from Clea Japan Inc. (Osaka, Japan). We generated Bcl-xL/Bim double knock-out mice (bim−/−bcl-xflox/floxalb-cre), Mcl-1/Bim double knock-out mice (bim−/−mcl-1flox/floxalb-cre), Bcl-xL/Bim/Bid triple knock-out mice (bim−/−bid−/−bcl-xflox/floxalb-cre), and Bim/Bid double knock-out mice (bim−/−bid−/−) by mating the strains. We generated mice with a hepatocyte-specific deletion of Mcl-1 and homozygote severe combined immune deficiency (SCID) mutations (mcl-1flox/floxprkdcscid/scidalb-cre) by mating hepatocyte-specific Mcl-1-knock-out mice (bcl-xflox/floxalb-cre) and NOD/ShiJic-scid Jcl mice. Genotyping of prkdcscid gene mutation was performed by the PCR-confronting two-pair primer (PCR-CTPP) method reported previously (19). The mice were maintained in a specific pathogen-free facility and were afforded humane care under approval from the Animal Care and Use Committee of Osaka University Medical School.
Histological Analyses
Liver sections were stained with hematoxylin and eosin (H&E). To detect apoptotic cells, the liver sections were also subjected to staining by terminal deoxynucleotidyltransferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) according to a procedure reported previously (20). For immunohistochemical detection of cleaved caspase-3, the liver sections were incubated with the polyclonal rabbit anti-cleaved caspase-3 antibody (Cell Signaling Technology, Beverly, MA) according to a procedure reported previously (20).
Caspase-3/7 Activity
Serum caspase-3/7 activity was measured by a luminescent substrate assay for caspase-3 and caspase-7 (Caspase-Glo assay, Promega) according to the manufacturer's protocol.
Western Blot Analysis
Liver tissue was lysed in lysis buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1× protein inhibitor mixture (Nacalai tesque, Kyoto, Japan), 1× phosphatase inhibitor mixture (Nacalai tesque), and phosphate-buffered saline, pH 7.4). The liver lysates were cleared by centrifugation at 10,000 × g for 15 min at 4 °C. The protein concentrations were determined using a bicinchoninic acid protein assay kit (Pierce). The protein lysates were electrophoretically separated with SDS-polyacrylamide gels and were transferred onto a polyvinylidene fluoride membrane. For immunodetection, the following antibodies were used: a rabbit polyclonal antibody to Bcl-xL (Santa Cruz Biotechnology, Inc.), a rabbit polyclonal antibody to Bid, a rabbit polyclonal antibody to Bax, a rabbit polyclonal antibody to cleaved caspase-3, a rabbit polyclonal antibody to cleaved caspase-7, a rabbit polyclonal antibody to Puma (Cell Signaling Technology, Beverly, MA), a rabbit monoclonal antibody to Bad, a rabbit polyclonal antibody to Noxa (Abcam, Cambridge, MA), a rabbit polyclonal antibody to Bak (Millipore, Billerica, MA), a rabbit polyclonal antibody to Bim (Enzo Life Sciences Inc., Farmingdale, NY), a rabbit polyclonal antibody to Mcl-1 (Rockland, Gilbertsville, PA), and a mouse monoclonal antibody to β-actin (Sigma-Aldrich).
Real-time Reverse Transcription Polymerase Chain Reaction (Real-time RT-PCR) for mRNA
Total RNA was extracted from liver tissues using an RNeasy minikit (Qiagen, Valencia, CA), was reverse-transcribed, and was subjected to real-time RT-PCR as described previously (21). The mRNA expression of specific genes was quantified using TaqMan gene expression assays (Applied Biosystems, Foster City, CA) as follows: murine bcl2l11 (assay ID: Mm00437796_m1), murine fas (assay ID: Mm01204974_m1), murine bik (assay ID: Mm00476123_m1), murine hrk (assay ID: Mm01208086_m1), murine bmf (assay ID: Mm00506773_m1), and murine actb (assay ID: Mm02619580_g1 or Mm00607939_s). The transcript levels are presented as -fold inductions.
siRNA-mediated in Vivo Knockdown
The hepatocyte-specific Bcl-xL-knock-out mice (bcl-xflox/floxalb-cre) and the Bcl-xL/Bim/Bid triple knock-out mice (bim−/−bid−/−bcl-xflox/floxalb-cre) were injected with 5 mg/kg in vivo grade siRNA against mcl-1 (MSS275671_e0N), which was mixed with Invivofectamine (Invitrogen), via the tail vein according to the manufacturer's protocol. The mice were sacrificed and examined as indicated by the time courses. The Stealth RNAi negative control with low GC content (Invitrogen) was used as the control.
In Vivo ABT-737 Experiment
ABT-737 was dissolved in a mixture of 30% propylene glycol, 5% Tween 80, and 65% D5W (5% dextrose in water) with pH 4–5. ABT-737 (100 mg/kg) was intraperitoneally administered to the Bim/Bid double knock-out mice (bim−/−bid−/−) or to the Bid-knock-out mice (bid−/−). The mice were sacrificed and examined 6 h later.
Statistical Analysis
All of the data are expressed as means ± S.D. unless otherwise indicated. Statistical analyses were performed using an unpaired Student's t test or a one-way analysis of variance unless otherwise indicated. When the analyses of variance were applied, the differences in the mean values among the groups were examined by Scheffe's post hoc correction unless otherwise indicated. p < 0.05 was considered statistically significant.
RESULTS
The Disruption of Bim Alleviated Spontaneous Hepatocyte Apoptosis in Hepatocyte-specific Bcl-xL-knock-out Mice
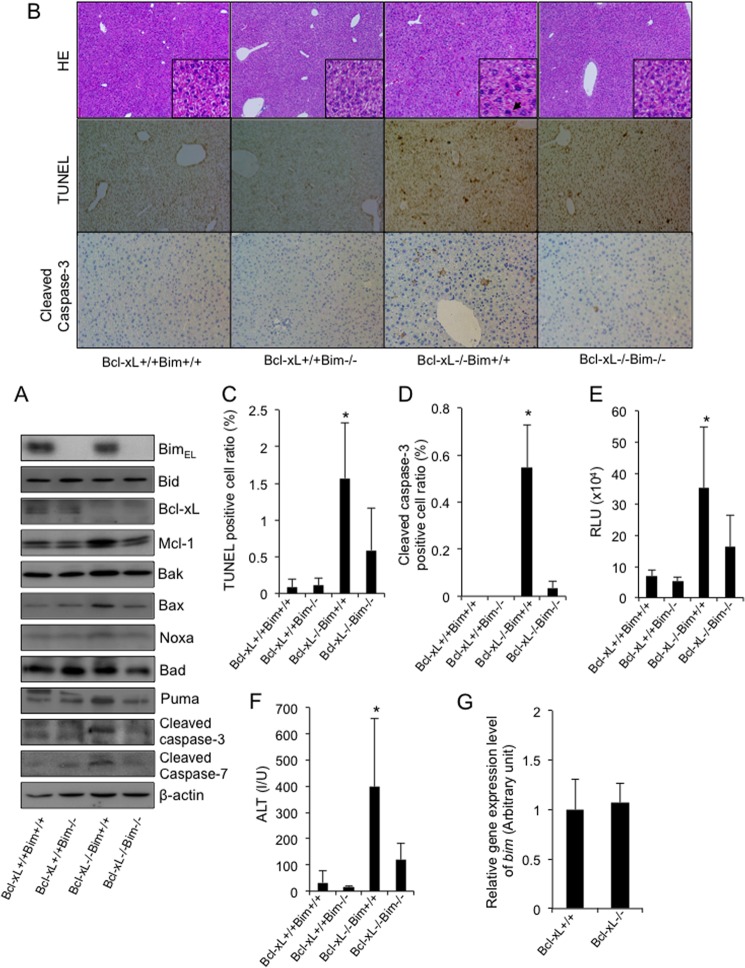
To investigate the involvement of the BH3-only protein Bim in the hepatocyte apoptosis caused by Bcl-xL deficiency, hepatocyte-specific Bcl-xL-knock-out mice (bcl-xfl/flalb-cre) were mated with systemic Bim-knock-out mice (bim−/−). Offspring from the mating of bim+/−bcl-xfl/flalb-cre mice and bim+/−bcl-xfl/fl mice were examined at 6 weeks of age. A Western blot study confirmed the disappearance of both Bcl-xL and Bim protein expression in the liver tissue of the double knock-out mice (bim−/−bcl-xfl/flalb-cre) (Fig. 1A). In agreement with our previous report (7, 17), H&E staining of the liver sections showed an increase in the number of hepatocytes, with chromatin condensation and cytosolic shrinkage in the liver lobules of the Bcl-xL-knock-out mice (Fig. 1B). The staining also showed a significant increase in TUNEL-positive cells and cleaved caspase-3-positive cells in the liver (Fig. 1, B–D). Consistent with these histological observations, the levels of serum caspase-3/7 activity and serum alanine aminotransferase (ALT), which can be used as indicators of hepatocyte apoptosis (22, 23), were significantly higher in the Bcl-xL-knock-out mice than in their wild-type littermates (Fig. 1, E and F). Additionally, cleaved caspse-3 and -7 were detected in the livers of the Bcl-xL-knock-out mice by Western blotting (Fig. 1A). All of these findings indicated spontaneous hepatocyte apoptosis in these mice. Bim-knock-out mice did not show any phenotypes in the liver under physiological conditions (Fig. 1, B–F). Alternatively, the disruption of Bim significantly improved all of the parameters that are indicative of hepatocyte apoptosis in Bcl-xL-knock-out mice, including the TUNEL-positive cell counts, cleaved caspase-3-positive cell counts, serum ALT levels, and serum caspase-3/7 activity (Fig. 1, B–F). These findings clearly demonstrated that Bim was involved in the hepatocyte apoptosis caused by Bcl-xL disruption. It should be noted that the gene and protein expression levels of Bim were not different between the Bcl-xL-knock-out mice and their wild-type littermates (Fig. 1, A and G), indicating that the Bim expression levels observed in the healthy liver could induce hepatocyte apoptosis in the absence of the Bcl-2 family proteins.
FIGURE 1.
The disruption of Bim alleviated spontaneous hepatocyte apoptosis in the absence of Bcl-xL. A–F, the offspring from the mating of bim±bcl-xflox/floxalb-cre mice with bim±bcl-xflox/flox mice were examined at 6 weeks of age. Bcl-xL+/+ and Bcl-xL−/−, bcl-xflox/flox and bcl-xflox/floxalb-cre, respectively. A, Western blot analysis of whole liver lysates for the expression of BimEL, Bid, Bcl-xL, Mcl-1, Bak, Bax, Noxa, Bad, Puma, cleaved caspase-3, cleaved caspase-7, and β-actin. B, representative images for liver histology stained with hematoxylin-eosin (HE), TUNEL, and cleaved caspase-3 (original magnifications, ×100 (large panels) and ×400 (insets)); black arrows indicate apoptotic bodies. C, TUNEL-positive cell ratio; n = 8 mice/group; *, p < 0.05 versus all. D, cleaved caspase-3-positive cell ratio; n = 3 mice/group; *, p < 0.05 versus all. E, serum caspase-3/7 activity; n = 11 mice/group; *, p < 0.05 versus all. F, serum ALT levels; n = 13 mice/group; *, p < 0.05 versus all. G, offspring from the mating of bcl-xflox/floxalb-cre mice with bcl-xflox/flox mice were examined at 6 weeks of age. Bcl-xL+/+ and Bcl-xL−/−, bcl-xflox/flox and bcl-xflox/floxalb-cre, respectively. bim mRNA levels in the whole liver tissue were determined by real-time RT-PCR; n = 6 mice/group. Error bars, S.D. RLU, relative light units; I/U, international units.
The Disruption of Bim Alleviated Spontaneous Hepatocyte Apoptosis in Hepatocyte-specific Mcl-1-knock-out Mice
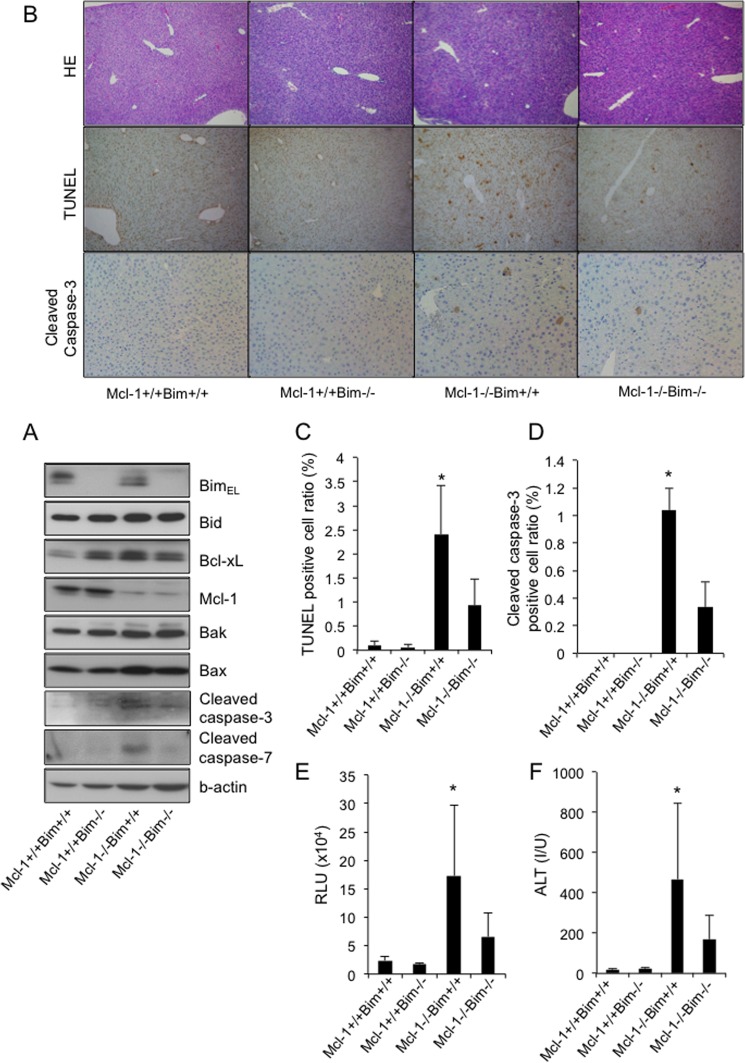
Of the five members of the anti-apoptotic Bcl-2 family proteins, we previously reported that Mcl-1 and Bcl-xL played a pivotal anti-apoptotic role in maintaining hepatocyte integrity in the healthy liver (13). We thus examined the role of Bim in the hepatocyte apoptosis caused by Mcl-1 deficiency. We generated Mcl-1/Bim double knock-out mice (bim−/−mcl-1fl/flalb-cre) by mating the hepatocyte-specific Mcl-1-knock-out mice (mcl-1fl/flalb-cre) with the systemic Bim-knock-out mice (bim−/−). A Western blot study confirmed the disappearance of both Mcl-1 and Bim protein expression in the liver tissue of the double knock-out mice (bim−/−mcl-1fl/flalb-cre) (Fig. 2A). Consistent with our previous report (13), hepatocyte-specific Mcl-1-knock-out mice showed apoptosis phenotypes very similar to those of the Bcl-xL-knock-out mice, as assessed by TUNEL staining (Fig. 2, B and C), cleaved caspase-3 staining (Fig. 2, B and D), serum caspase-3/7 activity (Fig. 2E), and serum ALT levels (Fig. 2F). In contrast, Mcl-1/Bim double knock-out mice showed significant improvement in these parameters (Fig. 2, B–F), indicating that Bim is also involved in the hepatocyte apoptosis induced by the disruption of Mcl-1.
FIGURE 2.
The disruption of Bim alleviated spontaneous hepatocyte apoptosis in the absence of Mcl-1. The offspring from the mating of bim+−mcl-1flox/floxalb-cre mice with bim+/−mcl-1flox/flox mice were examined at 6 weeks of age. Mcl-1+/+ and Mcl-1−/−, mcl-1flox/flox and mcl-1flox/floxalb-cre, respectively. A, Western blot analysis of whole liver lysates for the expression of BimEL, Bid, Bcl-xL, Mcl-1, Bak, Bax, cleaved caspase-3, cleaved caspse-7, and β-actin. B, representative images for liver histology stained with hematoxylin-eosin (HE), TUNEL, and cleaved caspase-3 (original magnification, ×100). C, TUNEL-positive cell ratio; n = 3–6 mice/group; *, p < 0.05 versus all. D, cleaved caspase-3-positive cell ratio; n = 3 mice/group; *, p < 0.05 versus all. E, serum caspase-3/7 activity; n = 9–15 mice/group; *, p < 0.05 versus all. F, serum ALT levels; n = 9–15 mice/group; *, p < 0.05 versus all. Error bars, S.D. RLU, relative light units; I/U, international units.
The Disruption of Bim and Bid Prevented Spontaneous Hepatocyte Apoptosis in Hepatocyte-specific Bcl-xL-knock-out Mice
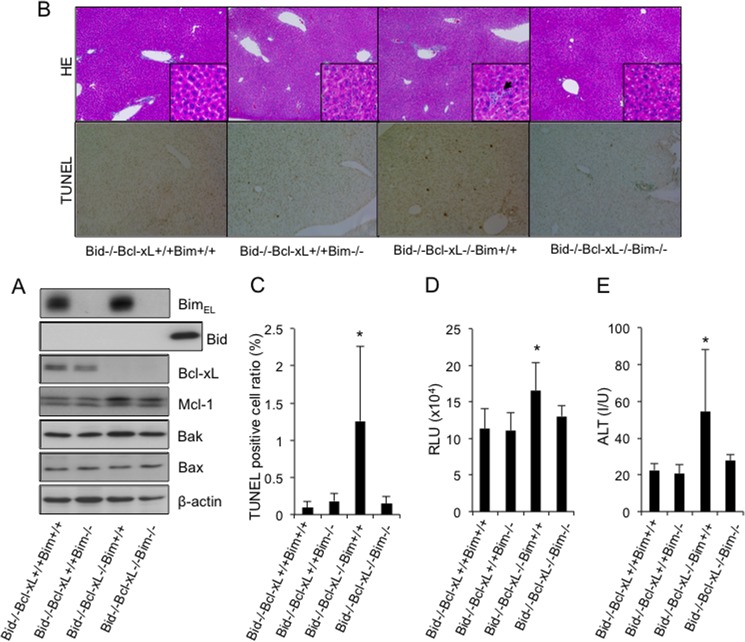
We previously reported that a small amount of Bid, which is another BH3-only protein, was constitutively active and was involved in the spontaneous hepatocyte apoptosis in Bcl-xL- or Mcl-1-knock-out mice (7, 13). We thus examined whether these BH3-only proteins redundantly or cooperatively promoted hepatocyte apoptosis in the absence of Bcl-xL. To this end, Bim/Bid/Bcl-xL triple knock-out mice (bim−/−bid−/−bcl-xfl/flalb-cre) were generated by mating the Bim/Bcl-xL double knock-out mice (bim−/−bcl-xfl/flalb-cre) with the Bid/Bcl-xL double knock-out mice (bid−/−bcl-xfl/flalb-cre). The offspring from the mating of bim+/−bid−/−bcl-xfl/flalb-cre mice with bim+/−bid−/−bcl-xfl/fl mice were examined at 6 weeks of age. A Western blot study confirmed that Bcl-xL, Bid, and Bim protein expression disappeared from the liver tissue of the triple knock-out mice (bim−/−bid−/−bcl-xfl/flalb-cre) (Fig. 3A). Liver sections of the Bim/Bid/Bcl-xL triple knock-out mice were histologically normal compared with those of the Bid/Bcl-xL double knock-out mice (bim+/+bid−/−bcl-xfl/flalb-cre), which still contained some hepatocytes with apoptotic morphologies (Fig. 3B). Both the number of TUNEL-positive cells and the serum caspase-3/7 activity in the triple knock-out mice were significantly lower than those in the Bid/Bcl-xL double knock-out mice and did not differ from their control Bid-knock-out or Bim/Bid double knock-out littermates (Fig. 3, B–D). Moreover, in contrast to the mild elevation of serum ALT levels in the Bid/Bcl-xL double knock-out mice, the levels in the triple knock-out mice were completely normal (Fig. 3E). These findings demonstrated that hepatocyte apoptosis in the absence of Bcl-xL was completely dependent on these two BH3-only proteins.
FIGURE 3.
The disruption of Bim and Bid prevented spontaneous hepatocyte apoptosis in the absence of Bcl-xL. The offspring from the mating of bim+/−bid−/−bcl-xflox/floxalb-cre mice with bim+/−bid−/−bcl-xflox/flox mice were examined at 6 weeks of age. Bcl-xL+/+ and Bcl-xL−/−, bcl-xflox/flox and bcl-xflox/floxalb-cre, respectively. A, Western blot analysis of whole liver lysates for the expression of BimEL, Bid, Bcl-xL, Mcl-1, Bak, Bax, and β-actin. B, representative images of liver histology stained with hematoxylin-eosin (HE) and TUNEL (original magnifications, ×100 (large panels) and ×400 (insets)). Black arrows indicate apoptotic bodies. C, TUNEL-positive cell ratio; more than 5 mice/group; *, p < 0.05 versus all. D, serum caspase-3/7 activity; more than 6 mice/group; *, p < 0.05 versus all. E, serum ALT levels; more than 6 mice/group; *, p < 0.05 versus all. Error bars, S.D. RLU, relative light units; I/U, international units.
Bim and Bid Are Essential Regulators for the Promotion of the Intrinsic Pathway of Apoptosis in Hepatocytes in the Absence of Anti-apoptotic Bcl-2 Family Proteins
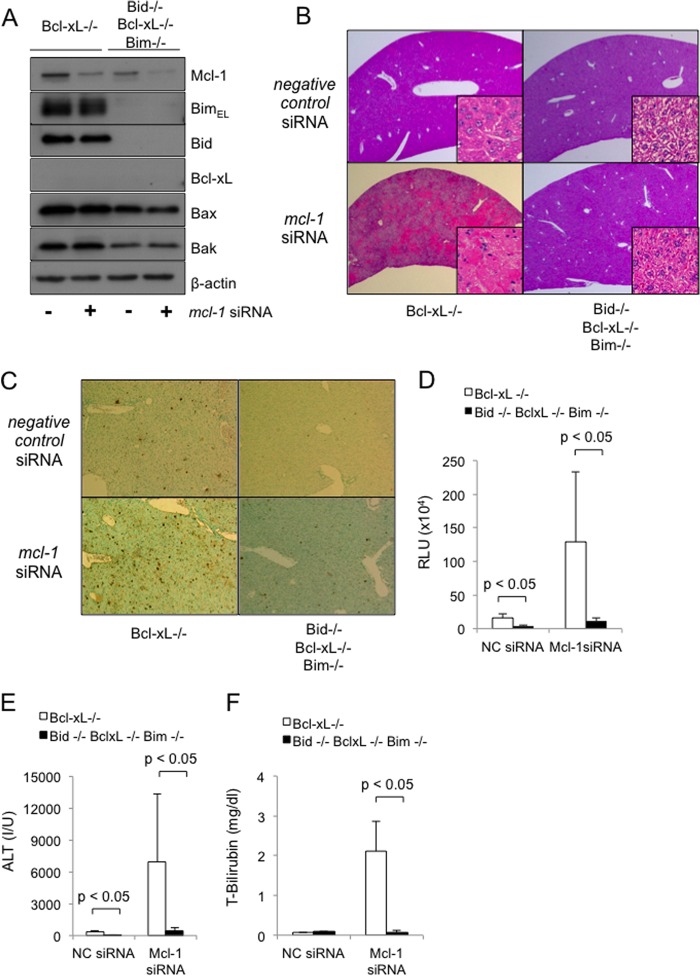
We then attempted to further examine the involvement of Bim and Bid in hepatocyte apoptosis in the absence of both Bcl-xL and Mcl-1, which are two major anti-apoptotic proteins in the liver. Because, as we reported (13), the hepatocyte-specific Bcl-xL and Mcl-1 double knock-out mice died within 1 day after birth due to impaired liver development, we performed an siRNA-mediated in vivo knockdown of mcl-1 in the Bcl-xL-knock-out mice and in the Bim/Bid/Bcl-xL triple knock-out mice. mcl-1 siRNA administration efficiently reduced Mcl-1 protein expression in the liver tissue of both mice (Fig. 4A), but it caused severe liver injury only in the Bcl-xL-knock-out mice (Fig. 4B) when assessed by the H&E staining of liver sections. Notably, mcl-1 siRNA administration caused massive hepatocyte apoptosis in the Bcl-xL-knock-out mice, but this apoptosis was weakened in the Bim/Bid/Bcl-xL triple knock-out mice, as evidenced by the TUNEL staining of the liver sections, serum caspase-3/7 activity, and serum ALT levels (Fig. 4, C–E). In agreement with these findings, mcl-1 siRNA treatment impaired the liver function of the Bcl-xL-knock-out mice, as evidenced by an increase in the serum bilirubin levels, but not the liver function of the triple knock-out mice (Fig. 4F). These findings demonstrated that the massive hepatocyte apoptosis and liver failure caused by decreases in these anti-apoptotic Bcl-2 family proteins were dependent on Bid and Bim.
FIGURE 4.
Bim and Bid are essential regulators involved in the intrinsic pathway of apoptosis in hepatocytes in the absence of anti-apoptotic Bcl-2 family proteins. bcl-xflox/floxalb-cre mice and bim−/−bid−/−bcl-xflox/floxalb-cre mice were injected with mcl-1 or with negative control siRNA via the tail vein and were sacrificed 24 h (A and C–F) or 48 h (B) later. Bcl-xL+/+ and Bcl-xL−/−, bcl-xflox/flox and bcl-xflox/floxalb-cre, respectively. NC, negative control. A, Western blot analysis of whole liver lysates for the expression of BimEL, Bid, Bcl-xL, Mcl-1, Bak, Bax, and β-actin. B, representative images of liver histology stained with hematoxylin-eosin (original magnifications, ×100 (large panels) and ×400 (insets)). C, representative images of liver histology stained with TUNEL (original magnification, ×100). D, serum caspase-3/7 activity; n = 3–4 mice/group. E, serum ALT levels; n = 4 mice/group; data are presented as means ± S.E. (error bars). F, serum T-bilirubin levels; n = 4 mice/group. RLU, relative light units; I/U, international units.
The Presence of Bim- and Bid-induced Constant BH3 Stress in the Healthy Liver Causes Hepatotoxicity with the Use of Anti-cancer Agents That Target the Anti-apoptotic Bcl-2 Family Proteins
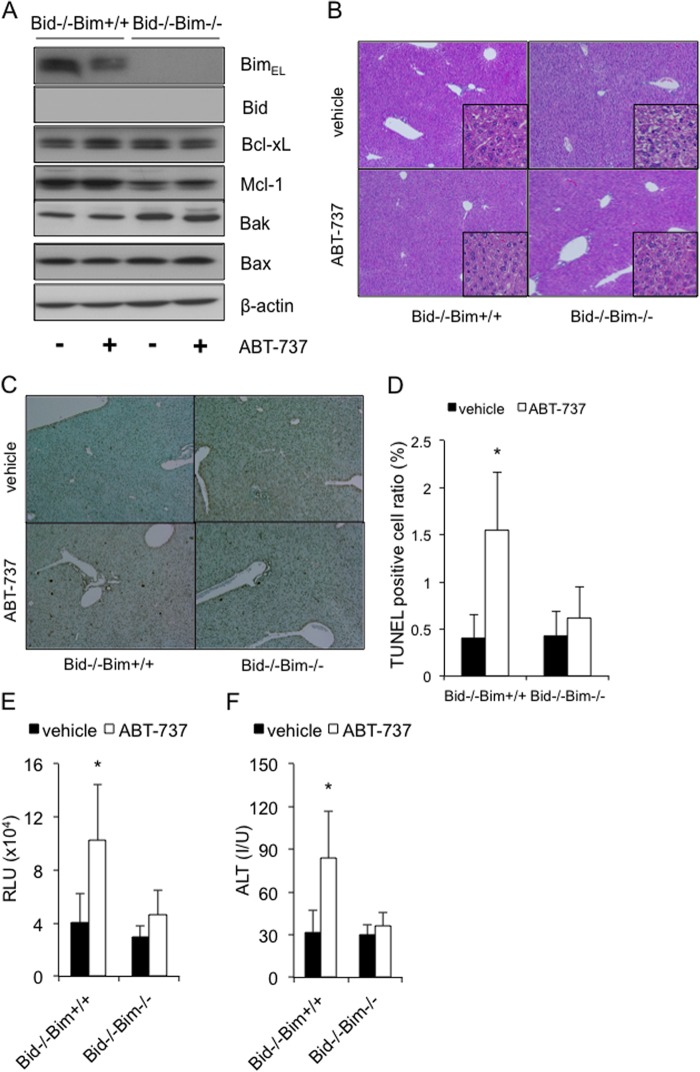
Recent advances in cancer therapy have enabled the selective targeting of some anti-apoptotic Bcl-2 family proteins, which are often dysregulated in malignant cells. ABT-737, which is a BH3 mimetic, could inhibit Bcl-xL, Bcl-2, and Bcl-w, and it has induced the regression of solid tumors (23). We previously reported that high dose ABT-737 administration caused hepatocyte apoptosis even in a normal liver, which was partly due to constitutive Bid-mediated BH3 stress (7). This finding led us to investigate the involvement of Bim and Bid in this ABT-737-mediated hepatotoxicity. Bim/Bid double knock-out mice (bim−/−bid−/−) were generated by mating Bim knock-out mice (bim−/−) with Bid knock-out mice (bid−/−), and the offspring were then treated with this drug. Western blot analysis confirmed the efficient deletion of Bim and Bid from the liver tissue of the double knock-out mice (Fig. 5A). Upon ABT-737 treatment, the Bim/Bid double knock-out mice showed complete prevention of ABT-737-induced hepatocyte apoptosis and hepatotoxicity (Fig. 5, B–F), in sharp contrast to their Bid-knock-out littermates, which still showed moderate hepatocyte apoptosis (Fig. 5, C–E) and increased serum ALT levels (Fig. 5F). These findings suggested that Bim- and Bid-mediated constant BH3 stress evoked hepatotoxicity by promoting the intrinsic pathway of apoptosis with the use of the inhibitors of the Bcl-2 family.
FIGURE 5.
The presence of Bim- and Bid-induced constant BH3 stress in the healthy liver causes hepatotoxicity with the use of anti-cancer agents that target anti-apoptotic Bcl-2 family proteins. The offspring from bim+/−bid−/− mating pairs were given an intraperitoneal injection of ABT-737 (100 mg/kg) or vehicle and were examined after 6 h. A, Western blot analysis of whole liver lysates for the expression of BimEL, Bid, Bcl-xL, Mcl-1, Bak, Bax, and β-actin. B and C, representative images of liver histology stained with hematoxylin-eosin and TUNEL (original magnifications, ×100 (large panels) and ×400 (insets)). D, TUNEL-positive cell ratio; n = 5–6 mice/group; *, p < 0.05 versus all. E, serum caspase-3/7 activity; more than 5 mice/group; *, p < 0.05 versus all. F, serum ALT levels; more than 5 mice/group; *, p < 0.05 versus all. Error bars, S.D. RLU, relative light units; I/U, international units.
DISCUSSION
At least eight BH3-only proteins are known, and five have been reported to exist in hepatocytes: Bid, Bim, Noxa, Puma, and Bad (22). We also confirmed these five proteins in the liver tissue of our mice (Fig. 1A), and we detected at least the mRNA expression of three other genes (supplemental Fig. 1). These proteins are considered to function as pro-apoptotic sensors upon activation by a variety of apoptotic stimuli, thereby promoting an intrinsic pathway of apoptosis in a manner that is dependent on the presence of Bak and Bax. In previous studies, bile acids or death receptor stimuli activated Bid and induced liver injury, which was alleviated by Bid disruption (12, 22). Bim activation was involved in hepatocyte lipoapoptosis, which is a critical feature of non-alcoholic steatohepatitis, and in reactive oxygen species-induced hepatocyte apoptosis (10, 11, 14). Additionally, a recent in vivo study revealed that the activation of Bid and Bim played a central pro-apoptotic role in fatal TNF-α-induced hepatitis (24). Taken together, these findings indicated the importance of these two BH3-only proteins in the pathogenesis of various liver diseases (12, 24, 25). Conversely, the systemic knock-out of Bid or Bim in mice did not result in any liver abnormalities under normal conditions; therefore, there has not been much interest in studying their physiological involvement in the healthy liver (12, 26). However, our present study showed that spontaneous hepatocyte apoptosis in the absence of Bcl-xL was alleviated by the deletion of either Bim or Bid, and it was diminished by the deletion of both. These results indicated that these BH3-only proteins are functionally active even in the healthy liver, but they are fully restrained by the anti-apoptotic Bcl-2 family proteins in the physiological state.
What type of stimuli constitutively activate these BH3-only proteins remains unknown. The liver is a specific organ that can be continuously exposed to a variety of stimuli, such as bile acids and enteric endotoxin, as well as interactions with immune cells. These stimuli might cause constitutive BH3-only stress through the activation of death receptors, such as Fas, tumor necrosis factor (TNF), and TNF-related apoptosis-inducing ligand (TRAIL) receptors. To explore the involvement of Fas signaling in generating this BH3-only stress, we studied the effect of fas inhibition in the hepatocyte apoptosis induced by the genetic disruption of Bcl-xL or ABT-737 administration. siRNA-mediated in vivo knockdown of fas did not alleviate their hepatocyte apoptosis (supplemental Fig. 2, B and D), suggesting that Fas signaling may not be the origin of this BH3-only stress. However, it should be noted here that siRNA administration only decreased fas mRNA levels to around half (supplemental Fig. 2, A and C). Therefore, genetic study is still necessary to clarify its involvement. In order to examine the involvement of T and B cells, which comprise about 50% of intrahepatic resident immune cells (27), in producing the BH3-only stress in the healthy liver, we crossed hepatocyte-specific Mcl-1 knock-out mice with homozygous SCID mutant mice, which are characterized by an absence of functional T cells and B cells (28). The spontaneous hepatocyte apoptosis of the Mcl-1 knock-out mice was unchanged even in the homozygous SCID mutant background, monitored by serum ALT levels and serum caspase-3/7 activity (supplemental Fig. 3, A–D). These data indicate that these immune cells are not the major source of the BH3-only stress in the liver under physiological conditions. Therefore, further study is required to identify the main source of constitutive BH3-only stress in the healthy liver. We previously reported that Mcl-1 and Bcl-xL individually worked as apoptotic antagonists in differentiated hepatocytes (13). However, the hepatocyte-specific deletion of both led to early postnatal death due to the failure of hepatocyte development in the fetal liver (13), thus hampering the clarification of their cooperative involvement in the adult liver. In the present study, the combination of genetically engineered mice and in vivo siRNA technology enabled the investigation of their cooperative roles for the first time, and we found that the inhibition of Mcl-1 caused sublethal liver injury with massive hepatocyte apoptosis in Bcl-xL-knock-out mice. Meanwhile, we also found that sublethal apoptosis was prevented in a Bim/Bid double knock-out background, suggesting that, of the BH3-only proteins, Bim and Bid are important for activating the intrinsic pathway of hepatocyte apoptosis in the absence of anti-apoptotic Bcl-2 family proteins. It would also be interesting to determine whether other anti-apoptotic Bcl-2 family proteins or BH3-only proteins are involved in this healthy Bcl-2 rheostasis.
The anti-apoptotic Bcl-2 family proteins are often dysregulated in a variety of malignancies, and they have been recognized as important oncogenes (29). ABT-737, which was recently developed to inhibit the Bcl-xL, Bcl-w, and Bcl-2 proteins, displays anti-tumor activity against lymphoid malignancies and small-cell lung carcinoma (23). These drugs were considered to selectively target tumor cells because malignant cells receive many genotoxic and environmental stress-induced BH3-only signals, so these cells are thus dependent on the anti-apoptotic Bcl-2 family members for their survival. However, we previously reported that the high-dose administration of ABT-737 (100 mg/kg) elicited hepatotoxicity via Bak/Bax-dependent apoptosis in normal hepatocytes (7), suggesting that dependence on the anti-apoptotic Bcl-2 family proteins is not a specific feature of tumor cells but is the case in healthy liver cells. In the present study, we demonstrated that the disruption of Bim and Bid completely prevented hepatocyte apoptosis and hepatotoxicity induced by high dose ABT-737 (100 mg/kg), suggesting that these proteins are responsible for this hepatotoxicity. Meanwhile, although 25 mg/kg ABT-737, which is relatively close to the clinical dose, caused moderate hepatocyte apoptosis, this apoptosis was completely blocked by Bid inhibition (supplemental Fig. 4). Therefore, it is unclear whether both Bid and Bim are truly involved in hepatotoxicity when using ABT-737 at clinically relevant doses.
This study demonstrated that Bim was also involved in the hepatocyte apoptosis caused by Mcl-1 deficiency in addition to Bid, which was noted in our previous report (13). Several previous human studies have reported that Mcl-1 proteins were down-regulated in the liver tissues of non-alcoholic steatohepatitis and primary biliary cirrhosis patients (30, 31), and experimental studies have demonstrated that Mcl-1 down-regulation by saturated fatty acids caused hepatocyte lipoapoptosis, which plays an important role in the development of fatty liver disease (32, 33). Taken together with our findings, these reports suggest the possibility that Bim- and Bid-mediated constant BH3 stresses might constitute therapeutic targets of the hepatotoxicity observed in these human liver diseases.
In conclusion, we have demonstrated that the novel rheostatic balance between the pro-apoptotic BH3-only proteins Bim and Bid and the anti-apoptotic Bcl-2 family proteins Bcl-xL and Mcl-1 regulates hepatocyte life and death in the physiological state. Our present study sheds new light on the dynamic and well orchestrated Bcl-2 networks in the healthy liver.
Acknowledgments
We thank Lothar Hennighausen (National Institutes of Health) and Dr. You-Wen He (Duke University) for providing the floxed bcl-x mice and floxed mcl-1 mice, respectively. We also thank Abbott Laboratories for providing ABT-737.
This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to T. Takehara).

This article contains supplemental Figs. 1–4.
- BH1–BH4
- Bcl-2 homology domains 1–4
- SCID
- severe combined immune deficiency
- ALT
- alanine aminotransferase.
REFERENCES
- 1. Youle R. J., Strasser A. (2008) The BCL-2 protein family. Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9, 47–59 [DOI] [PubMed] [Google Scholar]
- 2. Chipuk J. E., Green D. R. (2008) How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 18, 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adams J. M., Cory S. (2007) Bcl-2-regulated apoptosis. Mechanism and therapeutic potential. Curr. Opin. Immunol. 19, 488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim H., Rafiuddin-Shah M., Tu H. C., Jeffers J. R., Zambetti G. P., Hsieh J. J., Cheng E. H. (2006) Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 8, 1348–1358 [DOI] [PubMed] [Google Scholar]
- 5. Willis S. N., Fletcher J. I., Kaufmann T., van Delft M. F., Chen L., Czabotar P. E., Ierino H., Lee E. F., Fairlie W. D., Bouillet P., Strasser A., Kluck R. M., Adams J. M., Huang D. C. (2007) Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315, 856–859 [DOI] [PubMed] [Google Scholar]
- 6. Korsmeyer S. J., Shutter J. R., Veis D. J., Merry D. E., Oltvai Z. N. (1993) Bcl-2/Bax. A rheostat that regulates an anti-oxidant pathway and cell death. Semin. Cancer Biol. 4, 327–332 [PubMed] [Google Scholar]
- 7. Hikita H., Takehara T., Kodama T., Shimizu S., Hosui A., Miyagi T., Tatsumi T., Ishida H., Ohkawa K., Li W., Kanto T., Hiramatsu N., Hennighausen L., Yin X. M., Hayashi N. (2009) BH3-only protein bid participates in the Bcl-2 network in healthy liver cells. Hepatology 50, 1972–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giam M., Huang D. C., Bouillet P. (2008) BH3-only proteins and their roles in programmed cell death. Oncogene 27, Suppl. 1, S128–S136 [DOI] [PubMed] [Google Scholar]
- 9. Lomonosova E., Chinnadurai G. (2008) Oncogene 27, Suppl. 1, S2–S19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barreyro F. J., Kobayashi S., Bronk S. F., Werneburg N. W., Malhi H., Gores G. J. (2007) Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J. Biol. Chem. 282, 27141–27154 [DOI] [PubMed] [Google Scholar]
- 11. Ishihara Y., Takeuchi K., Ito F., Shimamoto N. (2011) Dual regulation of hepatocyte apoptosis by reactive oxygen species. Increases in transcriptional expression and decreases in proteasomal degradation of BimEL. J. Cell Physiol. 226, 1007–1016 [DOI] [PubMed] [Google Scholar]
- 12. Yin X. M., Wang K., Gross A., Zhao Y., Zinkel S., Klocke B., Roth K. A., Korsmeyer S. J. (1999) Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400, 886–891 [DOI] [PubMed] [Google Scholar]
- 13. Hikita H., Takehara T., Shimizu S., Kodama T., Li W., Miyagi T., Hosui A., Ishida H., Ohkawa K., Kanto T., Hiramatsu N., Yin X. M., Hennighausen L., Tatsumi T., Hayashi N. (2009) Mcl-1 and Bcl-xL cooperatively maintain integrity of hepatocytes in developing and adult murine liver. Hepatology 50, 1217–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishihara Y., Ito F., Shimamoto N. (2011) Increased expression of c-Fos by extracellular signal-regulated kinase activation under sustained oxidative stress elicits BimEL upregulation and hepatocyte apoptosis. FEBS J. 278, 1873–1881 [DOI] [PubMed] [Google Scholar]
- 15. Malhi H., Bronk S. F., Werneburg N. W., Gores G. J. (2006) Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 281, 12093–12101 [DOI] [PubMed] [Google Scholar]
- 16. Dzhagalov I., St John A., He Y. W. (2007) The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood 109, 1620–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takehara T., Tatsumi T., Suzuki T., Rucker E. B., 3rd, Hennighausen L., Jinushi M., Miyagi T., Kanazawa Y., Hayashi N. (2004) Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology 127, 1189–1197 [DOI] [PubMed] [Google Scholar]
- 18. Wagner K. U., Claudio E., Rucker E. B., 3rd, Riedlinger G., Broussard C., Schwartzberg P. L., Siebenlist U., Hennighausen L. (2000) Conditional deletion of the Bcl-x gene from erythroid cells results in hemolytic anemia and profound splenomegaly. Development 127, 4949–4958 [DOI] [PubMed] [Google Scholar]
- 19. Maruyama C., Suemizu H., Tamamushi S., Kimoto S., Tamaoki N., Ohnishi Y. (2002) Genotyping the mouse severe combined immunodeficiency mutation using the polymerase chain reaction with confronting two-pair primers (PCR-CTPP). Exp. Anim. 51, 391–393 [DOI] [PubMed] [Google Scholar]
- 20. Kodama T., Takehara T., Hikita H., Shimizu S., Shigekawa M., Tsunematsu H., Li W., Miyagi T., Hosui A., Tatsumi T., Ishida H., Kanto T., Hiramatsu N., Kubota S., Takigawa M., Tomimaru Y., Tomokuni A., Nagano H., Doki Y., Mori M., Hayashi N. (2011) Increases in p53 expression induce CTGF synthesis by mouse and human hepatocytes and result in liver fibrosis in mice. J. Clin. Invest. 121, 3343–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kodama T., Takehara T., Hikita H., Shimizu S., Li W., Miyagi T., Hosui A., Tatsumi T., Ishida H., Tadokoro S., Ido A., Tsubouchi H., Hayashi N. (2010) Thrombocytopenia exacerbates cholestasis-induced liver fibrosis in mice. Gastroenterology 138, 2487–2498, 2498.e2481–2487 [DOI] [PubMed] [Google Scholar]
- 22. Baskin-Bey E. S., Gores G. J. (2005) Death by association. BH3 domain-only proteins and liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G987–G990 [DOI] [PubMed] [Google Scholar]
- 23. Oltersdorf T., Elmore S. W., Shoemaker A. R., Armstrong R. C., Augeri D. J., Belli B. A., Bruncko M., Deckwerth T. L., Dinges J., Hajduk P. J., Joseph M. K., Kitada S., Korsmeyer S. J., Kunzer A. R., Letai A., Li C., Mitten M. J., Nettesheim D. G., Ng S., Nimmer P. M., O'Connor J. M., Oleksijew A., Petros A. M., Reed J. C., Shen W., Tahir S. K., Thompson C. B., Tomaselli K. J., Wang B., Wendt M. D., Zhang H., Fesik S. W., Rosenberg S. H. (2005) An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677–681 [DOI] [PubMed] [Google Scholar]
- 24. Kaufmann T., Jost P. J., Pellegrini M., Puthalakath H., Gugasyan R., Gerondakis S., Cretney E., Smyth M. J., Silke J., Hakem R., Bouillet P., Mak T. W., Dixit V. M., Strasser A. (2009) Fatal hepatitis mediated by tumor necrosis factor TNFα requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity 30, 56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higuchi H., Miyoshi H., Bronk S. F., Zhang H., Dean N., Gores G. J. (2001) Bid antisense attenuates bile acid-induced apoptosis and cholestatic liver injury. J. Pharmacol. Exp. Ther. 299, 866–873 [PubMed] [Google Scholar]
- 26. Bouillet P., Metcalf D., Huang D. C., Tarlinton D. M., Kay T. W., Köntgen F., Adams J. M., Strasser A. (1999) Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286, 1735–1738 [DOI] [PubMed] [Google Scholar]
- 27. Blom K. G., Qazi M. R., Matos J. B., Nelson B. D., DePierre J. W., Abedi-Valugerdi M. (2009) Isolation of murine intrahepatic immune cells employing a modified procedure for mechanical disruption and functional characterization of the B, T, and natural killer T cells obtained. Clin. Exp. Immunol. 155, 320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shultz L. D., Schweitzer P. A., Christianson S. W., Gott B., Schweitzer I. B., Tennent B., McKenna S., Mobraaten L., Rajan T. V., Greiner D. L. (1995) Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 154, 180–191 [PubMed] [Google Scholar]
- 29. Kirkin V., Joos S., Zörnig M. (2004) The role of Bcl-2 family members in tumorigenesis. Biochim. Biophys. Acta 1644, 229–249 [DOI] [PubMed] [Google Scholar]
- 30. García-Monzon C., Lo Iacono O., Mayoral R., González-Rodriguez A., Miquilena-Colina M. E., Lozano-Rodríguez T., García-Pozo L., Vargas-Castrillón J., Casado M., Boscá L., Valverde A. M., Martín-Sanz P. (2011) Hepatic insulin resistance is associated with increased apoptosis and fibrogenesis in nonalcoholic steatohepatitis and chronic hepatitis C. J. Hepatol. 54, 142–152 [DOI] [PubMed] [Google Scholar]
- 31. Iwata M., Harada K., Kono N., Kaneko S., Kobayashi K., Nakanuma Y. (2000) Expression of Bcl-2 familial proteins is reduced in small bile duct lesions of primary biliary cirrhosis. Hum. Pathol. 31, 179–184 [DOI] [PubMed] [Google Scholar]
- 32. Ibrahim S. H., Kohli R., Gores G. J. (2011) Mechanisms of lipotoxicity in NAFLD and clinical implications. J. Pediatr. Gastroenterol. Nutr. 53, 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Masuoka H. C., Mott J., Bronk S. F., Werneburg N. W., Akazawa Y., Kaufmann S. H., Gores G. J. (2009) Mcl-1 degradation during hepatocyte lipoapoptosis. J. Biol. Chem. 284, 30039–30048 [DOI] [PMC free article] [PubMed] [Google Scholar]