Background: eIF3m is a non-core subunit of eIF3.
Results: eIF3m deficiency in mice results in animal death and instability of other eIF3 subunits; haploinsufficiency reduces organ size.
Conclusion: eIF3m is critical for eIF3 structure and function.
Significance: Understanding the mechanism of eIF3 complex formation and its role in embryonic development and homeostasis is crucial for determining the physiological function of this eukaryotic translation factor.
Keywords: Embryo, Mouse Genetics, Protein Complexes, Protein Stability, Translation Initiation Factors, Eukaryotic Initiation Factor, Knock-out Mice
Abstract
Mammalian eIF3 is composed of 13 subunits and is the largest eukaryotic initiation factor. eIF3 plays a key role in protein biosynthesis. However, it is not fully understood how different subunits contribute to the structural integrity and function of the eIF3 complex. Whether eIF3 is essential for embryonic development and homeostasis is also not known. Here, we show that eIF3m null embryos are lethal at the peri-implantation stage. Compound heterozygotes (eIF3mflox/−) or FABP4-Cre-mediated conditional knock-out mice are lethal at mid-gestation stages. Although the heterozygotes are viable, they show markedly reduced organ size and diminished body weight. Acute ablation of eIF3m in adult mouse liver leads to rapidly decreased body weight and death within 2 weeks; these effects are correlated with a severe decline of protein biogenesis in the liver. Protein analyses reveal that eIF3m deficiency significantly impairs the integrity of the eIF3 complex due to down-regulation of multiple other subunits. Two of the subunits, eIF3f and eIF3h, are stabilized by eIF3m through subcomplex formation. Therefore, eIF3m is required for the structural integrity and translation initiation function of eIF3. Furthermore, not only is eIF3m an essential gene, but its expression level is also important for mouse embryonic development and the control of organ size.
Introduction
Protein synthesis is critical for all living organisms. Many eIFs are involved in the initial steps of protein translation. For instance, the eIF4F complex, which is composed of eIF4E, eIF4A, and eIF4G, binds directly to the 5′-cap structure of mRNA. eIF4G functions as a scaffold that links 5′-cap-binding eIF4E to poly(A)-binding protein. The interaction of eIF4G and eIF3 recruits the 40 S ribosomal subunit to the mRNA (1–3). The formation of the translation initiation complex can be activated by nutrients, growth factors, or hormones through mTOR (mammalian target of rapamycin) signaling (4). Modulation of the protein initiation machinery may promote longevity, death, or diseases, including cancer (4–8).
Among mammalian eIFs, eIF3 is the largest complex. It contains 13 subunits, which are named alphabetically as eIF3a–eIF3m (9). In addition to its role in recruiting the ribosome to mRNA, eIF3 stabilizes the binding of the eIF2-GTP-Met-tRNAiMet ternary complex to the 40 S ribosomal subunit (10). Furthermore, in response to nutrients, hormones, and mitogens, the protein kinases mTOR and S6K1 regulate translation initiation by associating with eIF3 (11). Recent interactome analyses suggest that eIF3 may also link the protein synthesis machinery to the protein degradation machinery for efficient protein quality control (12).
Cryo-electron microscopy has revealed that eIF3 exhibits a five-lobed structure (13). Three evolutionarily conserved subunits (eIF3a, eIF3b, and eIF3c) and three non-conserved subunits (eIF3e, eIF3f, and eIF3h) constitute its functional core (14). Other subunits also likely play important roles but have remained less characterized. For example, eIF3j is important for the tight association of eIF3 and the 40 S ribosomal subunit in vitro (15). eIF3m is a non-core subunit that interacts directly with eIF3f and eIF3h (9, 16). It is highly expressed in human colon cancer cells and has been implicated in cancer progression (17). eIF3m does not exist in budding yeast eIF3 (9, 18), but its counterpart in fission yeast is essential for viability (19). More importantly, in fission yeast, eIF3m and eIF3e define two distinct eIF3 complexes that share other subunits, and the eIF3m-containing complex appears to associate with the bulk of mRNAs (19). Despite these studies, the detailed role of eIF3m in the eIF3 complex remains unclear. Furthermore, the importance of mammalian eIF3m in embryonic development and homeostasis is completely unknown.
In this study, we used a knock-out approach to address the in vivo function of eIF3m in mice. We demonstrate that murine eIF3m is an essential gene for both embryonic development and homeostasis. Furthermore, it maintains the integrity of the eIF3 complex by stabilizing the core subunits eIF3c, and eIF3f, eIF3h.
EXPERIMENTAL PROCEDURES
Antibodies
Anti-eIF3m antibody was generated in rabbit using the full-length protein expressed in and purified from Escherichia coli. Primary antibodies against eIF4G (catalog no. 2498S), eIF4E (catalog no. 2067S), eIF3a (catalog no. 2538S), eIF3c (catalog no. 2068S), eIF3h (catalog no. 3413S), and eIF3j (catalog no. 3261S) were purchased from Cell Signaling Technology (Danvers, MA). Primary antibodies against eIF3b (sc-16377), eIF3i (sc-271539), eIF3d (sc-271516), and GFP (sc-8334) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-eIF3f antibody (catalog no. A303-005A) was obtained from Bethyl Laboratories (Montgomery, TX). Anti-Cre antibody (catalog no. 69050-3) was from Novagen (San Diego, CA). Anti-GAPDH antibody (catalog no. 10494-1-AP) was purchased from Proteintech (Chicago, IL). Anti-α-tubulin (catalog no. T5168), anti-FLAG (catalog no. F3165), and anti-HA (catalog no. H9658) antibodies were from Sigma.
Animals
Mice were maintained in a specific pathogen-free animal facility. eIF3m floxed mice were established at the Model Animal Research Center of Nanjing University and were maintained on the 129/Sv background. EIIa-cre mice were maintained on the FVB background. Fabp4-cre mice were purchased from the Model Animal Research Center of Nanjing University and maintained on the C57BL/6J background. Genotyping analyses were performed by PCR on genomic DNA extracted from tail tips. Pregnancies were obtained by natural mating and were timed from the day of the vaginal plug, which was defined as embryonic day (E)2 0.5. For acute liver-specific knock-out, 8–10-week-old mice were injected through the tail vein with ∼5 × 109 plaque-forming units of adenovirus in 0.1 ml of PBS as described (20, 21).
Cell Culture
Cells were cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum, 100 units/ml streptomycin, 100 units/ml penicillin, and 0.3 mg/ml l-glutamine at 37 °C in 5% CO2. Primary mouse embryonic fibroblasts (MEFs) were prepared from E13.5 embryos as described (22). Experiments were performed at early passages (passages 1–6). HEK293T cells were transfected using the conventional calcium phosphate method. Virus infections were performed as described (23).
Lentivirus Package and Adenovirus Preparation
Lentivirus was packaged in HEK293T cells as described (24). Briefly, HEK293T cells were transfected with vesicular stomatitis virus G packaging plasmid Delta8.9 and transfer vector. At 48 h post-transfection, the culture medium was harvested and prepared for ultracentrifugation. The pellet of lentivirus was resuspended in PBS. Adenovirus particles were prepared in AD-293 cells and concentrated using CsCl gradient centrifugation as described (25). Purified virus particles were stored at −80 °C.
Immunoprecipitation and Immunoblotting
For co-immunoprecipitation, cells were lysed in lysis buffer (20 mm Tris-HCl (pH 7.5), 100 mm KCl, 0.5% Nonidet P-40, 1 mm EDTA, 10% glycerol, 50 mm NaF, 10 mm sodium pyrophosphate, 1 mm sodium vanadate, 1 mm PMSF, 3 mm DTT, and protease inhibitors). Anti-FLAG M2 resin (Sigma) was added to the lysate and incubated for 2 h at 4 °C. The resin was washed three times with lysis buffer and three times with wash buffer (20 mm Tris-HCl (pH 7.5), 150 mm KCl, 0.5% Nonidet P-40, 1 mm EDTA, 10% glycerol, 50 mm NaF, 10 mm sodium pyrophosphate, 1 mm sodium vanadate, 1 mm PMSF, 3 mm DTT, and protease inhibitors). The immunoprecipitates were then eluted with FLAG peptide as described (26). For immunoblotting, proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Immunoblots were developed in chemiluminescence reagent (PerkinElmer Life Sciences) and exposed in a Fujifilm LAS 4000 imager.
Quantitative RT-PCR
Total RNA was extracted using TRIzol reagent (Invitrogen), and the SuperScript III first strand synthesis system with oligo(dT) primers (Invitrogen) was used for the reverse transcription reaction. Quantitative RT-PCR was performed using an Applied Biosystems 7500 HT sequence detection system with a Power SYBR Green PCR Master Mix kit (Applied Biosystems). GAPDH served as the internal control.
Histological Staining
Tissue samples were excised and immediately fixed in 4% paraformaldehyde in PBS, and 5-μm sections of paraffin-embedded tissue samples were stained with hematoxylin and eosin for morphological analysis. The stained samples were photographed with an Olympus BX51 microscope equipped with an Olympus DP71 cooled CCD camera.
Statistics
All data are presented as means ± S.D. Student's unpaired t test was used for statistical analyses. Differences were considered significant at p < 0.05.
RESULTS
eIF3m Deficiency in Mice Results in Embryonic Lethality
To gain insight into the physiological role of eIF3m, we examined its expression pattern in different organs of adult mice. Using GAPDH as the loading control, high levels of eIF3m were observed in the lung, spleen, testis, and fat tissue. Moderate levels of expression were observed in the liver, stomach, and kidney, and relatively low levels were observed in the brain, heart, and muscle (Fig. 1A).
FIGURE 1.
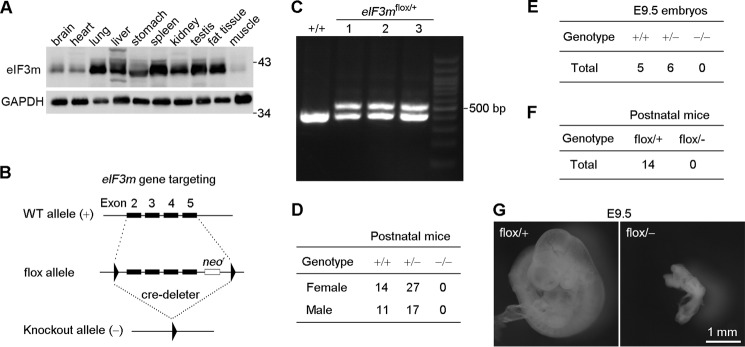
eIF3m is essential for mouse embryonic development. A, expression patterns of eIF3m in different mouse tissues. Cell lysates were prepared from an 8-week-old male mouse. GAPDH served as the loading control. B, strategy for eIF3m knock-out. Exons 2–5 of eIF3m, followed by a neomycin resistance cassette (neor), were flanked with two loxP sites (triangles). Exons 2–5 and neor were then deleted by Cre/loxP recombination, producing the eIF3m−/− (knock-out) allele. C, representative genotyping results obtained by PCR using tail DNA from one wild-type (eIF3m+/+) and three eIF3mflox/+ mice. D and E, genotyping of mice or embryos produced by crossing eIF3m+/− mice. F, genotyping of postnatal mice produced by crosses between eIF3mflox/flox and eIF3m+/− mice. G, representative images of eIF3mflox/+ and eIF3mflox/− embryos at E9.5.
To investigate the importance of eIF3m in mammalian development, we generated eIF3m knock-out mice using a Cre/loxP strategy. Exons 2–5 of eIF3m were flanked by two loxP sites and a neomycin resistance gene cassette (Fig. 1, B and C). Male mice of the eIF3m floxed strain (eIF3mflox/+) were crossed with EIIa-cre females (27) to obtain heterozygotes (eIF3m+/−). We then intercrossed the heterozygous mice to generate eIF3m null mice. The eIF3m+/+ and eIF3m+/− offspring were genotyped at term and displayed the expected Mendelian ratios (Fig. 1D). However, the intercrossing of eIF3m+/− mice did not produce any eIF3m−/− mice, suggesting that eIF3m is essential for normal embryonic development. We analyzed the embryos at E9.5 and again did not observe any eIF3m−/− embryos (Fig. 1E). Therefore, eIF3m is indispensable for early embryonic development.
As the very early embryonic death of eIF3m-deficient mice precluded disease-related studies, we explored whether compound mice (28) of eIF3m are viable. Insertion of the phosphoglycerate kinase promoter-driven neomycin cassette into intron 5 may have resulted in transcriptional interference; thus, the floxed allele may have been a hypomorphic eIF3m allele. Therefore, we crossed the eIF3mflox/flox mice with the eIF3m+/− mice to generate compound heterozygotes (eIF3mflox/−). The compound mice were expected to have lower eIF3m levels compared with the heterozygotes (eIF3m+/−) and may have shown disease phenotypes if the litters were viable. However, postnatal eIF3mflox/− mice were not obtained (Fig. 1F). We again examined embryos at E9.5 and found that eIF3mflox/− mice displayed severe developmental defects and growth retardation (Fig. 1G). These results indicate that eIF3m is an essential gene; sufficient expression levels of eIF3m are thus critical for normal mouse embryonic development.
eIF3m Heterozygous Mice Exhibit Decreased Body Weight and Organ Size
On the basis of the above results, we reasoned that even though eIF3m+/− mice were viable and fertile, they might display certain phenotypes due to reduced eIF3m levels compared with eIF3m+/+ mice. Immunoblotting confirmed that eIF3m+/− mice indeed expressed less eIF3m in a variety of organs (Fig. 2A). Accordingly, eIF3m+/− mice persistently exhibited decreased body weight compared with their wild-type littermates (Fig. 2B). We monitored food intake but did not observe significant differences between the heterozygous and wild-type littermates (Fig. 2C). The blood parameters of the heterozygous mice, including blood glucose, aspartate transaminase, and alanine transaminase, were also normal (data not shown).
FIGURE 2.
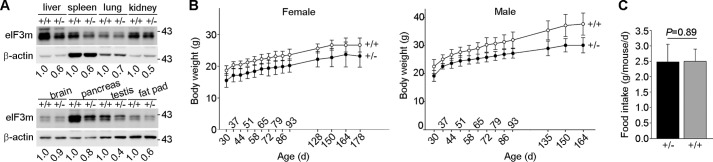
eIF3m haploinsufficiency results in decreased body weight in mice. A, immunoblot analysis of eIF3m levels in different organs of adult eIF3m+/+ and eIF3m+/− mice. The normalized relative intensity of eIF3m expression is shown at the bottom of each Western blot. B, body weight of female and male mice recorded 30 days (d) after birth. For each group, n = 8–12 mice. C, food intake recorded in heterozygous mice and control littermates. For each group, n = four to five mice.
We next examined organ size. The average weight of the liver, kidney, and fat pads of the heterozygotes was reduced by 22–63% compared with the wild-type mice (Fig. 3, A–C), with the fat pads exhibiting the most striking decline (Fig. 3, B and C). We performed histochemical staining of the subscapular fat pad and liver but did not observe significant differences in cell size between the wild-type and heterozygous mice (Fig. 3, D and E). These data suggest that the reduced organ weight was due to reduced cell numbers in the organs.
FIGURE 3.
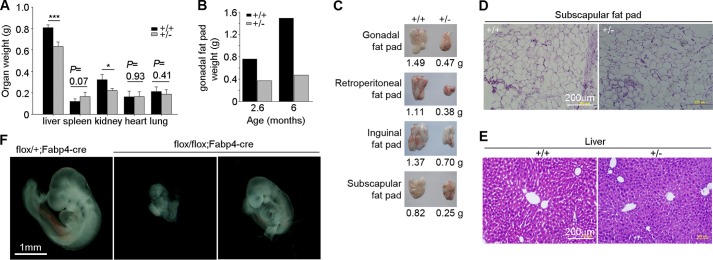
eIF3m haploinsufficiency results in decreased organ size. A, average weight of the indicated organs (n = 4–5) from mice at ∼5 months of age. *, p < 0.05 (Student's t test); ***, p < 0.001. B, weight of the gonadal fat pads from two pairs of littermates of the indicated ages. C, comparison of the fat pads dissected from a typical pair of littermates at 6 months of age. D and E, hematoxylin and eosin staining of fat and liver sections from 6-month-old littermates. Note that there is no obvious difference in cell size between the wild-type and heterozygous tissues. F, representative images of eIF3mflox/flox;Fabp4-cre and littermate control eIF3mflox/+;Fabp4-cre embryos at E9.5.
Because the fat tissues expressed high levels of eIF3m (Fig. 1A) and were sensitive to the amount of eIF3m (Fig. 3, B and C), we investigated the effect of a fat tissue-specific knock-out. eIF3mflox/flox mice were crossed with Fabp4-cre mice, which express Cre recombinase selectively in adipose tissues (29). Interestingly, eIF3mflox/flox;Fabp4-cre mice were also embryonic lethal. The eIF3mflox/flox;Fabp4-cre embryos at E9.5 displayed severe growth retardation compared with eIF3mflox/+;Fabp4-cre mice (Fig. 3F), although they were generally larger than the eIF3mflox/− embryos (Fig. 3F versus Fig. 1G).
Ablation of eIF3m in the Adult Mouse Liver Leads to Dramatically Decreased Protein Expression and Animal Death
We next investigated the importance of eIF3m in regulating homeostasis in adult mice. We used tail vein delivery of Cre recombinase adenovirus to achieve liver-specific knock-out of eIF3m in adult mice (20, 21). One week after adenoviral Cre administration, all four eIF3mflox/flox mice exhibited decreased body weight compared with the three wild-type control mice (Fig. 4A). Furthermore, all of the eIF3mflox/flox mice died within 2 weeks (Fig. 4B). To identify the cause of death, we extracted cell lysates from different tissues of an animal that died at 8 days post-injection. Ponceau S staining indicated a severe decline of total protein levels in the liver compared with uninjected eIF3mflox/flox littermates (Fig. 4C). Immunoblotting also confirmed specific ablation of eIF3m in the liver (Fig. 4C). Thus, eIF3m is required for homeostasis in adult mice due to its role in regulating liver protein synthesis.
FIGURE 4.
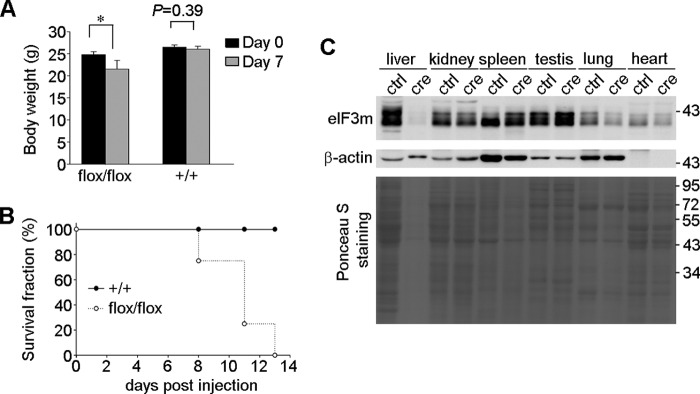
Acute ablation of eIF3m in mouse liver leads to death and a dramatic decrease in liver protein content. A, body weight at day 0 (before tail vein injection of Cre adenovirus) and day 7 (n = three to four mice). *, p < 0.05 (Student's t test). B, survival fraction of the mice after tail vein injection of Cre adenovirus. C, protein levels in different organs. Tissue lysates of a virus-injected eIF3mflox/flox mouse that died on day 8 were analyzed by Ponceau S staining, followed by immunoblotting. Lysates from an uninjected wild-type mouse of similar age served as a control (ctrl). We did not use any injected wild-type mice as a control because they were kept for the survival analysis in B.
Ablation of eIF3m Attenuates the Levels of the eIF3c, eIF3f, and eIF3h Subunits
To understand how eIF3m affects eIF3 complex function, we infected MEFs from eIF3mflox/flox mice with virus to express Cre or GFP (as control). We examined the protein levels of different eIF3 subunits by immunoblotting at 3 days post-infection, at which time the viability of Cre-expressing cells was still comparable to that of control cells. Coincident with the decline of eIF3m levels in the Cre virus-infected population, we observed that the levels of eIF3c, eIF3f, and eIF3h were similarly reduced (Fig. 5A). By contrast, other subunits, including eIF3a, eIF3b, eIF3d, eIF3i, and eIF3j, were not affected (Fig. 5A). In contrast to eIF3m, quantitative RT-PCR indicated that the mRNA levels of eIF3c, eIF3f, and eIF3h were not down-regulated in Cre virus-treated eIF3mflox/flox MEFs (Fig. 5B). Therefore, the reduced levels of eIF3c, eIF3f, and eIF3h were attributed to repressed protein translation and/or increased degradation.
FIGURE 5.
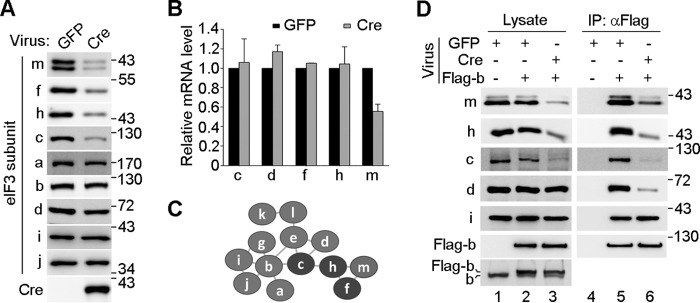
eIF3m deficiency selectively down-regulates eIF3f, eIF3h, and eIF3c and impairs the integrity of eIF3. A, immunoblotting for the indicated proteins in total cell lysates. eIF3mflox/flox MEFs were infected with Cre adenovirus or GFP for 72 h. B, eIF3m deficiency did not affect the mRNA levels of eIF3f, eIF3h, and eIF3c. eIF3mflox/flox MEFs were infected with lentiviral GFP or Cre for 120 h. Total RNA was extracted and used for quantitative RT-PCR. The results are from two independent experiments. C, diagram depicting subunit interactions within the mammalian eIF3 complex. The diagram was modified from models of Zhou et al. (16) and Pick et al. (9). Subunits are not drawn to scale. D, immunoprecipitation of the eIF3 complex. eIF3mflox/flox MEFs were infected with lentivirus for 72 h to express the indicated proteins. The eIF3 complex was immunoprecipitated (IP) with anti-FLAG beads. The indicated eIF3 subunits were detected by immunoblotting. Flag-b, FLAG-tagged eIF3b.
Mass spectrometry studies have shown that eIF3m, eIF3f, and eIF3h interact to form a tertiary subcomplex, which is further recruited into the eIF3 complex through the eIF3h-eIF3c interaction (Fig. 5C) (9, 16). Furthermore, eIF3c is known to be critical for linking the eIF3c-eIF3d-eIF3e-eIF3l-eIF3k subcomplex with the eIF3a-eIF3b-eIF3i-eIF3g subcomplex (Fig. 5C) (16). We thus speculated that the reduced eIF3c in the eIF3m−/− cells would further impair the integrity of eIF3. To validate this hypothesis, we ectopically expressed FLAG-tagged eIF3b in eIF3mflox/flox MEFs to levels comparable to the endogenous levels of eIF3b (Fig. 5D). After Cre-mediated knock-out, we performed co-immunoprecipitation to isolate the entire eIF3 complex (Fig. 5D) (19). The immunoblot indicated that the indirect association of eIF3d with eIF3b was markedly reduced in eIF3m−/− cells, although the direct eIF3b-eIF3i interaction was not affected (Fig. 5, C and D).
eIF3m Stabilizes eIF3f through Direct Interaction
The stability of protein complex subunits in vivo frequently requires proper subcomplex and/or complex formation (30–32). Therefore, eIF3m may stabilize the eIF3h and eIF3f subunits through subcomplex formation, which in turn bind and stabilize eIF3c (Fig. 5, A–C) (19). Alternatively, as eIF3 is critical for protein synthesis (9, 13), the reduced levels of the eIF3 complex in eIF3m knock-out cells (Fig. 5D) may result in inefficient protein translation, thereby reducing the levels of eIF3c and eIF3f. We reasoned that in the former case, eIF3m would be able to stabilize eIF3f or eIF3h but not eIF3c upon co-overexpression. In the latter case, no effect should be observed for any of the subunits because their overexpression is unlikely to alter the overall level of the eIF3 complex.
When HA-tagged eIF3c, eIF3d, eIF3f, or eIF3h was coexpressed with FLAG-eIF3m in HEK293T cells, only eIF3f and eIF3h exhibited clear up-regulation compared with their expression levels in the absence of FLAG-eIF3m (Fig. 6A). To further clarify that the effect was interaction-dependent, we constructed two C-terminal truncation mutants of eIF3m (N1 and N2). Co-immunoprecipitation analysis revealed that both mutants were incapable of binding eIF3f (Fig. 6B). Compared with wild-type eIF3m, neither mutant increased the levels of HA-eIF3f (Fig. 6C). These results indicate that the eIF3m-eIF3f interaction is required for the stabilization effect.
FIGURE 6.
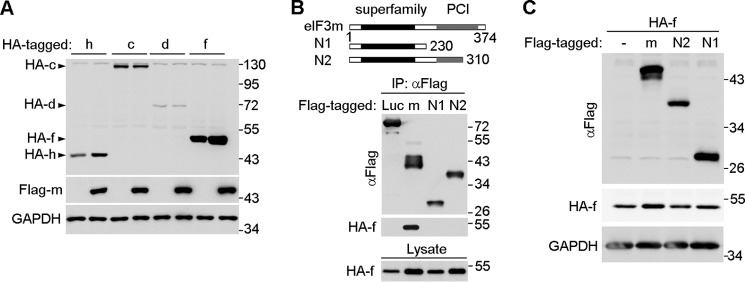
eIF3m stabilizes eIF3f and eIF3h through direct interaction. A, eIF3m up-regulated eIF3f and eIF3h but not eIF3c upon overexpression. HEK293T cells were transfected to express the indicated eIF3 subunits for 48 h and subjected to immunoblot analysis. GAPDH served as the loading control. B, mapping of regions of eIF3m that are critical for the interaction with eIF3f. HEK293T cells were transfected to coexpress HA-eIF3f with the indicated FLAG-tagged proteins for 48 h. Cell lysates were then subjected to immunoprecipitation (IP) using anti-FLAG beads. Firefly luciferase (Luc) served as a negative control. The eIF3m mutants (N1 and N2) are shown in the diagrams. C, the interaction-defective eIF3m mutants failed to up-regulate HA-eIF3f upon overexpression. HEK293T cells were cotransfected to express the indicated proteins for 48 h and then subjected to immunoblot analysis. GAPDH served as the loading control. PCI, proteasome-COP9-initiation factor 3 domain (268–357 amino acids).
DISCUSSION
Our results indicate that eIF3m is an essential gene for mouse embryonic development and homeostasis. First, whole animal knock-out of eIF3m resulted in early embryonic lethality. Because we were unable to identify any eIF3m−/− embryos at E9.5 (Fig. 1E), embryonic death might occur during the peri-implantation period, as occurs upon the knock-out of other essential genes (28, 33). Second, the eIF3mflox/flox;Fabp4-cre embryos started to die at ∼E9.5 and failed to develop to term (Fig. 3F). These results suggest that fetal fat tissue is essential for early embryonic development, in addition to its well recognized postnatal roles (34). FABP4-Cre was expressed on the dorsal side of E9.5 embryos and later on the peripheral ganglia, cartilage primordial, and vertebrae; these expression areas are in addition to FABP4-Cre expression in brown adipose tissue (35). Because adipocytes, neurons, chondrocytes, and osteocytes share a common lineage (36), an alternative possibility for the embryonic death was the loss of certain stem cell populations. Third, the ablation of eIF3m in the adult liver resulted in animal death within 2 weeks. This was likely due to the abolishment of liver protein synthesis, a critical process in the maintenance of homeostasis (Fig. 4).
A sufficient level of eIF3m expression is also important for organ size. Although eIF3mflox/flox mice were viable and normal, eIF3mflox/− embryos exhibited severe growth retardation at E9.5 and failed to further develop (Fig. 1, F and G). By contrast, the eIF3m+/− embryos, whose eIF3m levels were expected to be higher than those of eIF3mflox/− embryos but lower than wild-type levels, were fully viable. Nevertheless, after birth, the eIF3m+/− mice consistently displayed reduced body weight compared with their wild-type littermates (Fig. 2). Coincident with the decreased eIF3m expression, several organs were significantly smaller in eIF3m+/− mice, including the liver, kidney, and fat pad (Fig. 2A and Fig. 3, A–C). Notably, our results suggest that the reduced organ size was due to decreased cell number and not reduced cell size (Fig. 3, D and E). Thus, we propose that the level of eIF3m is an important determinant of the number of rounds of cell division required during organ construction. Consistent with this hypothesis, eIF3m has been reported to be important for the translation of proliferation-related proteins such as Cdc25A (17). It will be interesting to clarify the molecular mechanisms underlying the relationship between the expression level of eIF3m and organ size.
In this study, we demonstrated that eIF3m is critical for the stability of eIF3c, eIF3f, and eIF3h (Fig. 5). Consequently, the ablation of eIF3m severely impaired the integrity of the eIF3 complex (Fig. 5D). eIF3f and eIF3h are able to form a heterodimer and heterotrimer with eIF3m (9, 16). Our results suggest that subcomplex formation helps protect eIF3f and eIF3h from degradation (Fig. 6), similar to the actin regulatory WAVE complex (32). eIF3c binds directly to eIF3h but not eIF3m in the eIF3 complex (Fig. 5C) (9, 16). Therefore, down-regulation of eIF3c is likely an indirect effect resulting from the ablation of eIF3m (Fig. 5).
Acknowledgments
We thank Qiongping Huang and Lei Du for technical assistance.
This work was supported by Chinese Academy of Sciences Grant XDA01010107, Ministry of Science and Technology of China Grants 2012CB945003 and 2006BAI23B02, and Shanghai Municipal Science and Technology Commission Grant 12JC1409900.
- E
- embryonic day
- MEF
- mouse embryonic fibroblast.
REFERENCES
- 1. Jackson R. J., Hellen C. U., Pestova T. V. (2010) The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aitken C. E., Lorsch J. R. (2012) A mechanistic overview of translation initiation in eukaryotes. Nat. Struct. Mol. Biol. 19, 568–576 [DOI] [PubMed] [Google Scholar]
- 3. Hinnebusch A. G., Lorsch J. R. (2012) The mechanism of eukaryotic translation initiation: new insights and challenges. Cold Spring Harb. Perspect. Biol. 4, a011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoncu R., Efeyan A., Sabatini D. M. (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Santini E., Huynh T. N., MacAskill A. F., Carter A. G., Pierre P., Ruggero D., Kaphzan H., Klann E. (2013) Exaggerated translation causes synaptic and behavioural aberrations associated with autism. Nature 493, 411–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silvera D., Formenti S. C., Schneider R. J. (2010) Translational control in cancer. Nat. Rev. Cancer 10, 254–266 [DOI] [PubMed] [Google Scholar]
- 7. Rogers A. N., Chen D., McColl G., Czerwieniec G., Felkey K., Gibson B. W., Hubbard A., Melov S., Lithgow G. J., Kapahi P. (2011) Life span extension via eIF4G inhibition is mediated by posttranscriptional remodeling of stress response gene expression in C. elegans. Cell Metab 14, 55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Curran S. P., Ruvkun G. (2007) Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 3, e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pick E., Hofmann K., Glickman M. H. (2009) PCI complexes: beyond the proteasome, CSN, and eIF3 Troika. Mol. Cell 35, 260–264 [DOI] [PubMed] [Google Scholar]
- 10. Chaudhuri J., Chowdhury D., Maitra U. (1999) Distinct functions of eukaryotic translation initiation factors eIF1A and eIF3 in the formation of the 40 S ribosomal preinitiation complex. J. Biol. Chem. 274, 17975–17980 [DOI] [PubMed] [Google Scholar]
- 11. Holz M. K., Ballif B. A., Gygi S. P., Blenis J. (2005) mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123, 569–580 [DOI] [PubMed] [Google Scholar]
- 12. Sha Z., Brill L. M., Cabrera R., Kleifeld O., Scheliga J. S., Glickman M. H., Chang E. C., Wolf D. A. (2009) The eIF3 interactome reveals the translasome, a supercomplex linking protein synthesis and degradation machineries. Mol. Cell 36, 141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siridechadilok B., Fraser C. S., Hall R. J., Doudna J. A., Nogales E. (2005) Structural roles for human translation factor eIF3 in initiation of protein synthesis. Science 310, 1513–1515 [DOI] [PubMed] [Google Scholar]
- 14. Masutani M., Sonenberg N., Yokoyama S., Imataka H. (2007) Reconstitution reveals the functional core of mammalian eIF3. EMBO J. 26, 3373–3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fraser C. S., Lee J. Y., Mayeur G. L., Bushell M., Doudna J. A., Hershey J. W. (2004) The j-subunit of human translation initiation factor eIF3 is required for the stable binding of eIF3 and its subcomplexes to 40 S ribosomal subunits in vitro. J. Biol. Chem. 279, 8946–8956 [DOI] [PubMed] [Google Scholar]
- 16. Zhou M., Sandercock A. M., Fraser C. S., Ridlova G., Stephens E., Schenauer M. R., Yokoi-Fong T., Barsky D., Leary J. A., Hershey J. W., Doudna J. A., Robinson C. V. (2008) Mass spectrometry reveals modularity and a complete subunit interaction map of the eukaryotic translation factor eIF3. Proc. Natl. Acad. Sci. U.S.A. 105, 18139–18144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goh S. H., Hong S. H., Hong S. H., Lee B. C., Ju M. H., Jeong J. S., Cho Y. R., Kim I. H., Lee Y. S. (2011) eIF3m expression influences the regulation of tumorigenesis-related genes in human colon cancer. Oncogene 30, 398–409 [DOI] [PubMed] [Google Scholar]
- 18. Phan L., Zhang X., Asano K., Anderson J., Vornlocher H. P., Greenberg J. R., Qin J., Hinnebusch A. G. (1998) Identification of a translation initiation factor 3 (eIF3) core complex, conserved in yeast and mammals, that interacts with eIF5. Mol. Cell. Biol. 18, 4935–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou C., Arslan F., Wee S., Krishnan S., Ivanov A. R., Oliva A., Leatherwood J., Wolf D. A. (2005) PCI proteins eIF3e and eIF3m define distinct translation initiation factor 3 complexes. BMC Biol. 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akagi K., Sandig V., Vooijs M., Van der Valk M., Giovannini M., Strauss M., Berns A. (1997) Cre-mediated somatic site-specific recombination in mice. Nucleic Acids Res. 25, 1766–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Min L., Ji Y., Bakiri L., Qiu Z., Cen J., Chen X., Chen L., Scheuch H., Zheng H., Qin L., Zatloukal K., Hui L., Wagner E. F. (2012) Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nat. Cell Biol. 14, 1203–1211 [DOI] [PubMed] [Google Scholar]
- 22. Xu J. (2005) Preparation, culture, and immortalization of mouse embryonic fibroblasts. Curr. Protoc. Mol. Biol. Chapter 28, Unit 28.21, 10.1002/0471142727.mb2801s70 [DOI] [PubMed] [Google Scholar]
- 23. Wang F., Zhang Q., Cao J., Huang Q., Zhu X. (2008) The microtubule plus end-binding protein EB1 is involved in Sertoli cell plasticity in testicular seminiferous tubules. Exp. Cell Res. 314, 213–226 [DOI] [PubMed] [Google Scholar]
- 24. Lois C., Hong E. J., Pease S., Brown E. J., Baltimore D. (2002) Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295, 868–872 [DOI] [PubMed] [Google Scholar]
- 25. He T. C., Zhou S., da Costa L. T., Yu J., Kinzler K. W., Vogelstein B. (1998) A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. U.S.A. 95, 2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou Q., Chen D., Pierstorff E., Luo K. (1998) Transcription elongation factor P-TEFb mediates Tat activation of HIV-1 transcription at multiple stages. EMBO J. 17, 3681–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lakso M., Pichel J. G., Gorman J. R., Sauer B., Okamoto Y., Lee E., Alt F. W., Westphal H. (1996) Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. U.S.A. 93, 5860–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirotsune S., Fleck M. W., Gambello M. J., Bix G. J., Chen A., Clark G. D., Ledbetter D. H., McBain C. J., Wynshaw-Boris A. (1998) Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nat. Genet. 19, 333–339 [DOI] [PubMed] [Google Scholar]
- 29. He W., Barak Y., Hevener A., Olson P., Liao D., Le J., Nelson M., Ong E., Olefsky J. M., Evans R. M. (2003) Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. U.S.A. 100, 15712–15717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Derivery E., Gautreau A. (2010) Generation of branched actin networks: assembly and regulation of the N-WASP and WAVE molecular machines. BioEssays 32, 119–131 [DOI] [PubMed] [Google Scholar]
- 31. Wickner S., Maurizi M. R., Gottesman S. (1999) Posttranslational quality control: folding, refolding, and degrading proteins. Science 286, 1888–1893 [DOI] [PubMed] [Google Scholar]
- 32. Wu S., Ma L., Wu Y., Zeng R., Zhu X. (2012) Nudel is crucial for the WAVE complex assembly in vivo by selectively promoting subcomplex stability and formation through direct interactions. Cell Res. 22, 1270–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sasaki S., Mori D., Toyo-oka K., Chen A., Garrett-Beal L., Muramatsu M., Miyagawa S., Hiraiwa N., Yoshiki A., Wynshaw-Boris A., Hirotsune S. (2005) Complete loss of Ndel1 results in neuronal migration defects and early embryonic lethality. Mol. Cell. Biol. 25, 7812–7827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Satterfield M. C., Wu G. (2011) Brown adipose tissue growth and development: significance and nutritional regulation. Front. Biosci. 16, 1589–1608 [DOI] [PubMed] [Google Scholar]
- 35. Urs S., Harrington A., Liaw L., Small D. (2006) Selective expression of an aP2/fatty acid binding protein 4-Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res. 15, 647–653 [DOI] [PubMed] [Google Scholar]
- 36. Billon N., Monteiro M. C., Dani C. (2008) Developmental origin of adipocytes: new insights into a pending question. Biol. Cell 100, 563–575 [DOI] [PubMed] [Google Scholar]


