Background: The combined roles of intestinal MTP and ABCA1 in tissue and plasma lipid homeostasis are unknown.
Results: Intestine-specific ablation of MTP and ABCA1 severely impairs acute fat and cholesterol absorption.
Conclusion: MTP and ABCA1 are critical for lipid absorption.
Significance: Reducing both intestinal MTP and ABCA1 might reduce plasma lipid concentrations.
Keywords: Cholesterol, Intestine, Lipids, Lipid Absorption, Lipid Metabolism, Lipoprotein Secretion, Lipoprotein Structure, Triglyceride, ABCA1, MTP
Abstract
We have previously described apolipoprotein B (apoB)-dependent and -independent cholesterol absorption pathways and the role of microsomal triglyceride transfer protein (MTP) and ATP-binding cassette transporter A1 (ABCA1) in these pathways. To assess the contribution of these pathways to cholesterol absorption and to determine whether there are other pathways, we generated mice that lack MTP and ABCA1, individually and in combination, in the intestine. Intestinal deletions of Mttp and Abca1 decreased plasma cholesterol concentrations by 45 and 24%, respectively, whereas their combined deletion reduced it by 59%. Acute cholesterol absorption was reduced by 28% in the absence of ABCA1, and it was reduced by 92–95% when MTP was deleted in the intestine alone or together with ABCA1. MTP deficiency significantly reduced triglyceride absorption, although ABCA1 deficiency had no effect. ABCA1 deficiency did not affect cellular lipids, but Mttp deficiency significantly increased intestinal levels of triglycerides and free fatty acids. Accumulation of intestinal free fatty acids, but not triglycerides, in Mttp-deficient intestines was prevented when mice were also deficient in intestinal ABCA1. Combined deficiency of these genes increased intestinal fatty acid oxidation as a consequence of increased expression of peroxisome proliferator-activated receptor-γ (PPARγ) and carnitine palmitoyltransferase 1α (CPT1α). These studies show that intestinal MTP and ABCA1 are critical for lipid absorption and are the main determinants of plasma and intestinal lipid levels. Reducing their activities might lower plasma lipid concentrations.
Introduction
Lipids are carried in the blood by specialized transport particles called lipoproteins. There are two major types of lipoproteins in the plasma, low density apolipoprotein B (apoB)2-containing lipoproteins and non-apoB-containing high density lipoproteins (HDL). Increases and decreases in the plasma concentrations of apoB-containing lipoproteins and HDL, respectively, enhance risks for cardiovascular disorders (1). Hence, understanding their role in lipid transport is critical in devising ways to control plasma lipid concentrations. In fact, maintaining appropriate plasma lipid concentrations in the general population is a major goal of health care professionals (1). Changes in plasma concentrations of these lipoproteins occur either because of reduced catabolism or increased production. Production of apoB-containing lipoproteins and HDL is critically dependent on microsomal triglyceride transfer protein (MTP) and ATP-binding cassette transporter A1 (ABCA1), respectively (2). Intestine and liver are the major organs for lipoprotein production, and they play a critical role in the transport of dietary and endogenous lipids (2).
MTP deficiency in abetalipoproteinemia is associated with markedly lower plasma lipid concentrations, fat-soluble vitamin deficiency, stunted growth, and acanthocytosis (3, 4). Previous attempts to generate animal models of abetalipoproteinemia were hampered due to embryonic lethality of Mttp knock-out mice (5). However, liver- and intestine-specific ablations of MTP significantly reduced plasma triglycerides in apoB-containing lipoproteins and cholesterol in HDL (6–8). Thus, both intestinal and hepatic MTP contribute significantly to plasma lipids.
ABCA1 deficiency in Tangier disease is characterized by HDL deficiency, higher plasma triglycerides, increased tissue sterol levels, and premature coronary atherosclerosis in some kindreds (9). In rodent models, low plasma HDL concentrations in the absence of hepatic ABCA1 rise because of higher apoAI clearance via the kidney, because apoAI is not efficiently lipidated in these mice (10). Tissue-specific ablation studies show that hepatic and intestinal ABCA1 contribute ∼70 and 30% of plasma HDL, respectively (10, 11). Higher plasma triglycerides in the absence of hepatic ABCA1 have been attributed to the attenuation of PI3K-mediated inhibition of the assembly of triglyceride-rich larger VLDL particles (9). However, the role of ABCA1 deficiency in the assembly and secretion of intestinal triglyceride-rich lipoproteins has not been addressed.
Cholesterol absorption has been studied extensively because of its significant positive correlation with plasma cholesterol concentrations and atherosclerosis (12–14). Absorption of cholesterol is defined as the transport of dietary lipids from the intestinal lumen across the enterocytes into the plasma (13). Cholesterol uptake from the lumen by the enterocytes is the rate-limiting step in cholesterol absorption (15), and evidence indicates that Nieman Pick C1-like 1 (NPC1L1) is involved in this process (16–18). After uptake, enterocytes esterify cholesterol, a process mediated by acyl-coenzyme A:cholesterol acyltransferase enzymes (19, 20). Esterified and nonesterified cholesterol is then packaged into chylomicrons by MTP and secreted from the basolateral side of the enterocyte (21, 22). We showed that cholesterol secretion by differentiated Caco-2 cells and rat primary enterocytes occurs by apoB-dependent (chylomicrons) and apoB-independent (HDL) pathways (23). Cholesterol secretion with chylomicrons was induced by oleate and repressed by MTP inhibition, whereas secretion with HDL involves ABCA1 and apoA1 (23, 24). Evidence for multiple complementary mechanisms for cholesterol absorption also comes from studies in chow-fed Acat2−/− mice (25, 26). These mice absorb cholesterol as efficiently as wild-type mice despite the absence of cholesterol esterification, most likely because of transport of free cholesterol via the efflux pathway. In fact, 3-fold increased levels of ABCA1 mRNA have been detected in Acat2−/− mice (26). Temel et al. (27) have also shown that genetic ablation of either ACAT2 or ABCA1 in mice results in 44 or 13% reduction in cholesterol absorption, respectively. However, their combined deficiency results in 60% reduction, suggesting that the transport of free and esterified cholesterol occurs by two different additive mechanisms that contribute to efficient cholesterol absorption. A recent study showed that ∼30% of cholesterol is absorbed via the HDL pathway in the absence of MTP (7). Thus, there is significant evidence to suggest that these two pathways contribute to cholesterol absorption. It is unknown whether other mechanisms exist besides these two pathways. Here, we evaluated the contribution of intestinal MTP and ABCA1 in the absorption of dietary lipids using intestine-specific knock-out mouse models.
MATERIALS AND METHODS
Generation of Intestine-specific MTP, ABCA1, and MTP/ABCA1 Double Deficient Mice
Abca1flox/flox (Abca1f/f) mice (10) were crossed with Villin-Cre mice (The Jackson Laboratory) to generate intestinal ABCA1-deficient (Villin-Cre;Abca1−/−) mice. To generate intestine-specific MTP ablation, Mttpf/f(exon5,6) mice (28) were crossed with estrogen receptor T2 (ERT2)-Villin-Cre deletor line (29), and ERT2-Villin-Cre;Mttpf/f(exon5,6) mice were obtained. To create mice deficient in both MTP and ABCA1, ERT2-villin-Cre;Mttpf/f mice were crossed with Abca1f/f mice to generate ERT2-villin-Cre;Mttpf/fAbca1f/f. To induce gene ablation, tamoxifen (0.5 mg/mouse) was injected intraperitoneally three times on alternate days in 200 μl of corn oil. Control mice were injected with corn oil (200 μl) used to dissolve tamoxifen. Male 12-week-old mice on a C57Bl6J background were used in this study. All studies were approved by the Institutional Animal Care and Use Committee of State University of New York Downstate Medical Center.
In these studies, three different sets of knock-out mice and three different sets of controls were used. In initial studies, the appropriate controls were used for comparison. However, no significant differences were observed among the three different controls. Therefore, in later studies, these controls were combined to represent wild-type controls.
Plasma Lipid Measurements
Plasma and tissue total cholesterol and triglyceride (Thermo-Fisher Scientific), free cholesterol and free fatty acids (Wako Chemicals), and glycerol (Sigma) levels were measured using commercial kits. Esterified cholesterol was calculated by subtracting free cholesterol from total cholesterol. Plasma and tissue free glycerol levels were subtracted from triglyceride levels. HDL lipid levels were measured after precipitating apoB lipoproteins using phosphotungstate/MgCl2 reagent (23). Plasma lipoproteins were separated by gel filtration (flow rate of 0.2 ml/min) using a Superose 6-column, and 200-μl fractions were collected. Fractions were used to measure cholesterol and triglycerides.
Short Term Lipid Absorption Studies
Twelve-week-old male mice (n = 3 per group) on a chow diet were fasted overnight and injected intraperitoneally with poloxamer 407 (30 mg/mouse). After 1 h, mice were gavaged with 0.5 μCi of either [14C]triolein or [3H]cholesterol with 0.2 mg of unlabeled cholesterol in 15 μl of olive oil (24). After 2 h, plasma was collected to measure radioactivity.
Uptake and Secretion of [3H]Oleic Acid and [3H]Cholesterol by Primary Enterocytes
To study uptake, primary enterocytes were isolated from overnight fasted mice (n = 3) as described previously (23, 24). They were suspended in 4 ml of DMEM containing 0.5 μCi/ml [3H]oleic acid or [3H]cholesterol and incubated at 37 °C. After 1 h, enterocytes were washed, and cellular lipids were extracted to determine the uptake of radiolabeled lipids. For characterization of secreted lipoproteins, enterocytes were radiolabeled for 1 h with 0.5 μCi/ml [3H]oleic acid or [3H]cholesterol, washed, and incubated with fresh media containing 1.4 mm oleic acid and 0.5 mm taurocholate micelles (23, 30). After 2 h, enterocytes were centrifuged, and supernatants were collected. In [3H]oleic acid radiolabeling experiments, lipids were extracted from cells and media and separated by thin layer chromatography to quantify incorporation of [3H]oleic acid into triglycerides. In [3H]cholesterol radiolabeling experiments, media were subjected to density gradient ultracentrifugation to determine radiolabeled cholesterol distribution among lipoprotein classes (23).
Determination of MTP Activity
Small pieces (0.1 g) of liver and proximal small intestine (∼1-cm) were homogenized in low salt buffer (1 mm Tris-HCl, pH 7.6, 1 mm EGTA, and 1 mm MgCl2) and centrifuged, and supernatants were used for protein determination and the MTP assay (31, 32) using a commercial kit (Chylos, Inc.).
Histology
Aliquots of liver were fixed overnight in 10% formalin, dehydrated in 30% sucrose, embedded in M1 cryo-preservation media at −20 °C, and stored at −70 °C. Sections (7 μm) were placed on Tissue-Tack (Polysciences) slides, dehydrated in 60% isopropyl alcohol, immersed in 1% Oil Red O for 30 min at 22 °C, washed in 60% isopropyl alcohol, rinsed with tap water for 10 s, counterstained with Gill's hematoxylin for at least 20 min, rinsed with tap water until clear, acidified in alcohol (0.4% HCl in 95% ETOH), rinsed with tap water again, and dipped in basic solution (0.03 n NaOH) until sections visibly darkened. Images were taken with a SPOT RT3 Digital Camera. Image analysis was performed using SPOT software from Imaging Diagnostics.
Fatty Acid Oxidation
For fatty acid oxidation measurements, 50 mg of intestine and liver were incubated with [14C]oleic acid (0.3 μCi) for 2 h, and the radiolabeled CO2 was captured with filter paper soaked with phenylethylamine (33–35). Radiolabel was quantified using a scintillation counter (Beckman LS 6000TA). Oxidation was presented per mg of protein.
mRNA Quantification
Total RNA from tissues was isolated using TRIzolTM (Invitrogen). The purity of RNA was assessed by the A260/A280 ratio. RNA preparations with A260/A280 ratios more than 1.7 were used for cDNA synthesis. The first strand cDNA was synthesized using Omniscript RT (Qiagen) kit. Each reaction of quantitative PCR was carried out in a volume of 20 μl, consisting of 5 μl of cDNA sample (1:100 dilution of the first strand cDNA sample) and 15 μl of PCR master mix solution containing 1× PCR buffer (qPCRTM core kit for SYBR Green I, Eurogentec). The PCR was carried out by incubating the reaction mixture first for 10 min at 95 °C followed by 40 cycles of 15-s incubations at 95 °C and 1 min at 60 °C in an ABI 7000 SDS PCR machine. Data were analyzed using ΔΔCT method, according to the manufacturer's instructions, and presented as arbitrary units that were normalized to ARPp0 mRNA.
Statistics
Data are presented as mean ± S.D. Statistical significance (p < 0.05) was determined using Student's t test (GraphPad Prism 5).
RESULTS
Temporal Increases in Intestinal Triglycerides with Mttp Gene Ablation
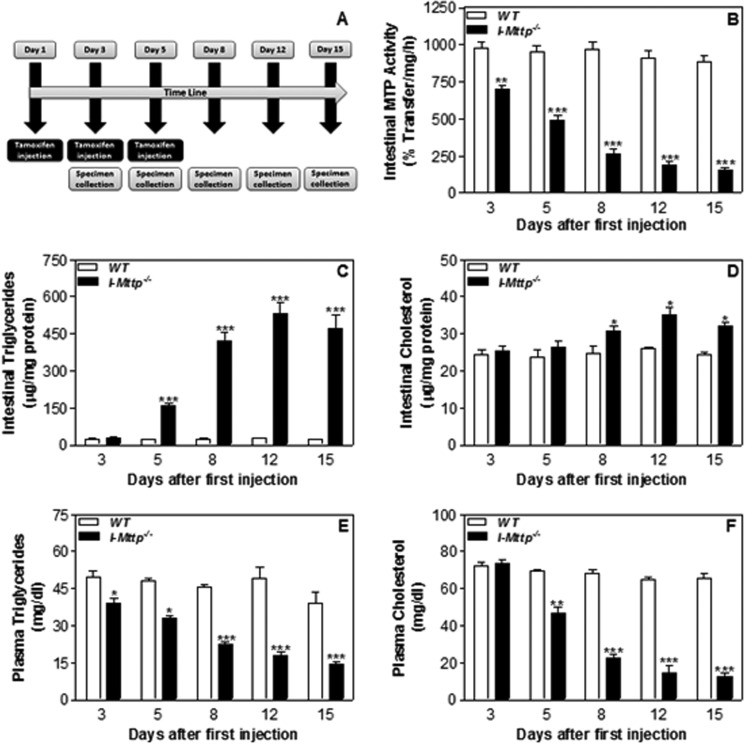
To determine optimal conditions for the intestine-specific Mttp gene ablation, we injected tamoxifen on alternate days in chow-fed, male, wild-type (WT, Mttpf/f), and ERT2-Villin-Cre;Mttpf/f mice, and plasma and intestines were collected (Fig. 1A). There was a time-dependent decrease in intestinal MTP activity by 28%, 48%, 73%, 80%, and 82% on days 3, 5, 8, 12, and 15, respectively, in tamoxifen-injected ERT2-Villin-Cre;Mttpf/f mice (I-Mttp−/−) mice but not in WT mice (Fig. 1B). These reductions were accompanied by significant increases in intestinal triglycerides (Fig. 1C). In contrast, there was a small increase in cholesterol in the intestine on days 8, 12, and 15 (Fig. 1D). Furthermore, decreases in intestinal MTP activity were associated with significant reductions in plasma triglycerides (Fig. 1E) and cholesterol (Fig. 1F). These studies showed that reductions in intestinal MTP are associated with increases in intestinal triglycerides and decreases in plasma triglyceride and cholesterol. Because there was no significant difference in residual MTP activity between day 12 and day 15, all the subsequent experiments were performed on day 12 after the first tamoxifen injection.
FIGURE 1.
Time-dependent changes in intestinal and plasma lipids with MTP gene deletion. A, schematic diagram showing the time points of tamoxifen injection (0.5 mg/mouse) and specimen collection from 12-week-old chow-fed, male, wild-type (WT, Mttpf/f) and ERT2-Villin-Cre;Mttpf/f (I-Mttp−/−) mice. At each time point, three mice from each group were sacrificed. Plasma and intestines were collected as described under “Materials and Methods.” Intestines were used to measure MTP activity (B), triglycerides (C), and cholesterol (D). Plasma was used to measure triglycerides (E) and cholesterol (F). Values are mean ± S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with WT. The data are representative of three independent experiments.
Intestinal MTP Ablation Increases Intestinal Lipids and Reduces Plasma Lipoproteins
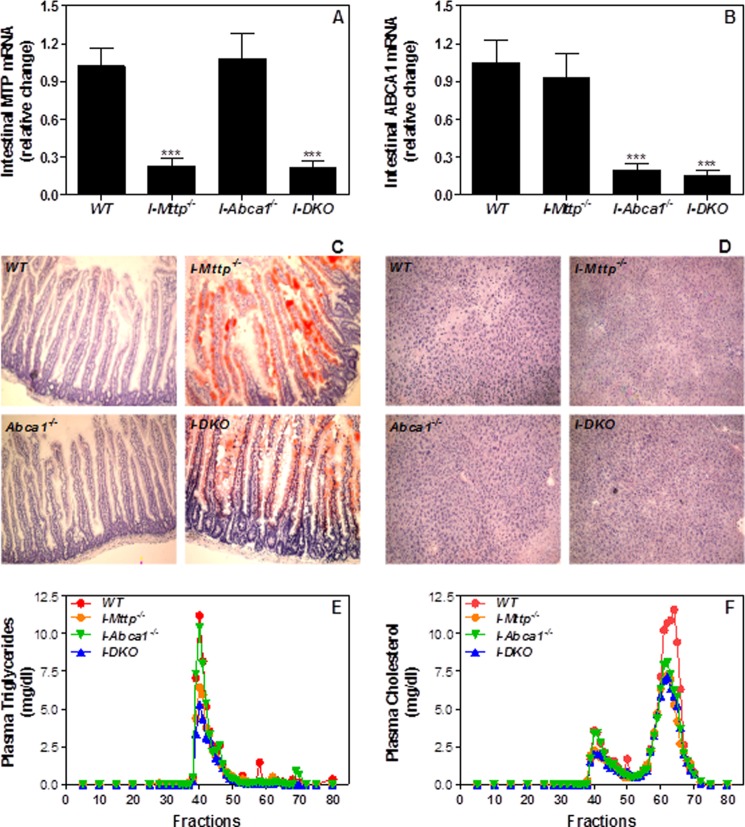
In a separate experiment, tamoxifen injection reduced MTP activity (Table 1) and mRNA (Fig. 2A) by ∼80% but had no effect on intestinal ABCA1 mRNA (Fig. 2B) and hepatic MTP activity (Table 1). To determine the consequences of MTP deletion, we quantified lipids in the intestine and liver of these mice and also performed Oil Red O staining of the frozen tissue sections. Conditional ablation of MTP was associated with a 22-fold, 153 and 80% increase in intestinal triglycerides, FFA, and cholesterol, respectively (Table 1). The increase in cholesterol was attributable to increases in both free as well as esterified cholesterol. Increases in triglycerides were corroborated by enhanced Oil Red O staining of the intestinal sections (Fig. 2C). Intestine-specific MTP ablation reduced hepatic triglyceride and FFA, increased cholesterol (Table 1), and had no effect on lipid staining (Fig. 2D). These studies showed that intestine-specific MTP ablation is associated with significant lipid accumulation in the intestine.
TABLE 1.
Effect of intestinal MTP and ABCA1 gene deletion on tissue lipids
Intestinal and hepatic tissues from 12-week-old wild-type (WT), I-Mttp−/−, I-Abca1−/−, and I-DKO mice (n = 5) were used to measure MTP activity, triglycerides, FFA, total, free, and esterified cholesterol mass. Values are mean ± S.D. Values in parentheses show percent change compared with WT.
| Injected with | Characteristics | MTP activity | Triglycerides | FFA | Cholesterol |
|||
|---|---|---|---|---|---|---|---|---|
| Total | Free | Esterified | ||||||
| % transfer/mg/h | μg/mg protein | μg/mg protein | μg/mg protein | |||||
| Intestine | ||||||||
| Mttpf/f | Tamoxifen | WT | 922.1 ± 93.6 | 18.7 ± 5.4 | 51.5 ± 10.5 | 13.2 ± 2.5 | 3.2 ± 1.1 | 10.0 ± 2.1 |
| ERT2-villin-Cre;Mttpf/f | Tamoxifen | I-Mttp−/− | 179.4 ± 68.2 | 436.7 ± 48.2 | 130.6 ± 5.5 | 23.8 ± 4.6 | 7.4 ± 1.7 | 16.4 ± 2.9 |
| (−80.5)a | (+2235.3)a | (+153.6)a | (+80.3)b | (+131.3)b | (+64.0)b | |||
| Abca1f/f | None | WT | 832.8 ± 79.3 | 21.9 ± 6.4 | 57.1 ± 12.4 | 16.1 ± 1.9 | 4.8 ± 1.5 | 11.3 ± 2.2 |
| Villin-Cre;Abca1f/f | None | I-Abca1−/− | 909.5 ± 103.4 | 16.4 ± 6.2 | 52.8 ± 2.2 | 12.3 ± 0.3 | 3.9 ± 0.4 | 8.4 ± 0.1 |
| (+9.2) | (−25.1) | (−7.5) | (−23.6)b | (−18.7) | (−25.7)b | |||
| ERT2-villin-Cre;Mttpf/f;Abca1f/f | Oil | WT | 983.3 ± 65.7 | 22.1 ± 6.5 | 58.2 ± 14.1 | 15.9 ± 2.0 | 4.2 ± 1.1 | 11.7 ± 1.8 |
| ERT2-villin-Cre, Mttpf/f;Abca1f/f | Tamoxifen | I-DKO | 180.6 ± 88.4 | 413.4 ± 25.9 | 45.6 ± 8.4 | 17.2 ± 1.4 | 3.0 ± 0.5 | 14.2 ± 0.9 |
| (−81.6)a | (+1770.6)a | (−21.6) | (+8.2) | (−28.6)b | (+21.4) | |||
| Liver | ||||||||
| Mttpf/f | Tamoxifen | WT | 493.1 ± 41.2 | 16.1 ± 3.2 | 10.8 ± 0.9 | 4.5 ± 1.2 | 0.9 ± 0.2 | 3.6 ± 1.5 |
| ERT2-villin, Cre-Mttpf/f | Tamoxifen | I-Mttp−/− | 546.2 ± 30.9 | 8.5 ± 1.4 | 8.1 ± 0.5 | 6.6 ± 0.5 | 1.4 ± 0.5 | 5.2 ± 0.0 |
| (+10.8) | (−47.2)a | (−25.0)c | (+46.7)b | (+55.6)b | (+44.4) | |||
| Abca1f/f | None | WT | 426.4 ± 56.9 | 19.8 ± 4.4 | 9.3 ± 1.2 | 5.3 ± 1.3 | 0.7 ± 0.2 | 4.6 ± 1.2 |
| Villin-Cre;Abca1f/f | None | I-Abca1−/− | 437.7 ± 67.8 | 25.3 ± 5.0 | 5.1 ± 0.3 | 7.3 ± 1.7 | 1.0 ± 0.1 | 6.4 ± 1.6 |
| (+2.7) | (+27.8) | (−45.2)a | (+37.7)b | (+42.9)b | (+39.1) | |||
| ERT2-villin-Cre;Mttpf/f;Abca1f/ff | Oil | WT | 526.2 ± 79.1 | 17.4 ± 3.2 | 10.1 ± 0.8 | 3.9 ± 1.7 | 0.5 ± 0.1 | 3.4 ± 1.4 |
| ERT2-villin-Cre;Mttpf/f;Abca1f/f | Tamoxifen | I-DKO | 706.8 ± 58.9 | 9.9 ± 1.4 | 3.4 ± 0.4 | 6.6 ± 0.9 | 1.7 ± 0.3 | 5.0 ± 0.8 |
| (+34.3)c | (−43.1)c | (−66.3)a | (+69.2)b | (+240.0)c | (+47.1) | |||
FIGURE 2.
Intestine-specific ablation of MTP and ABCA1 decreases plasma lipids. Total RNA isolated from the intestine of 12-week-old WT, I-Mttp−/−, I-Abca1−/−, and I-DKO (n = 5) male mice fed a chow diet was used to quantify mRNA levels of MTP (A) and ABCA1 (B). Oil Red O staining of proximal intestine (C) and liver (D) sections showing lipid staining in these tissues. Plasma was separated by gel filtration to determine mass of triglycerides (E) and cholesterol (F) in different lipoproteins. Values are mean ± S.D. ***, p < 0.001 compared with WT.
To assess the effect of intestinal MTP gene deletion on plasma lipids, we measured lipids in overnight fasted mice. I-Mttp−/− mice had 37–45% less plasma triglyceride, FFA, and cholesterol (Table 2). FPLC analysis of plasma showed lower triglycerides in VLDL/LDL fractions (Fig. 2E) and cholesterol in both VLDL/LDL and HDL fractions (Fig. 2F). These studies showed that intestinal MTP ablation significantly reduces plasma lipids and lipoproteins.
TABLE 2.
Effects of intestinal MTP and ABCA1 gene deletion on plasma lipids
Plasma from 12-week-old wild-type (WT), I-Mttp−/−, I-Abca1−/−, and I-DKO mice (n = 5) was used to measure triglycerides, FFA, total, free, and esterified cholesterol mass. Values are mean ± S.D. Values in parenthesis show percent change compared with WT.
| Injected with | Characteristics | Triglycerides | FFA | Cholesterol |
|||
|---|---|---|---|---|---|---|---|
| Total | Free | Esterified | |||||
| mg/dl | mg/dl | mg/dl | |||||
| Mttpf/f | Tamoxifen | WT | 53.2 ± 8.2 | 49.9 ± 5.2 | 76.8 ± 5.8 | 9.4 ± 1.7 | 67.4 ± 5.3 |
| ERT2-villin-Cre;Mttpf/f | Tamoxifen | I-Mttp−/− | 31.3 ± 4.8 | 31.4 ± 3.1 | 42.6 ± 4.5 | 5.4 ± 3.1 | 37.1 ± 3.4 |
| (−41.2)a | (−37.1)a | (−44.5)b | (−42.6) | (−45.0)b | |||
| Abca1f/f | None | WT | 45.3 ± 8.3 | 46.3 ± 6.2 | 78.3 ± 6.2 | 10.6 ± 2.1 | 67.7 ± 6.2 |
| Villin-Cre;Abca1f/f | None | I-Abca1−/− | 45.5 ± 4.1 | 49.4 ± 2.9 | 59.7 ± 3.8 | 6.9 ± 1.0 | 52.8 ± 2.8 |
| (−0.5) | (+6.7) | (−23.8)c | (−34.9) | (−22.0)b | |||
| ERT2-villin-Cre;Mttpf/f;Abca1f/f | Oil | WT | 50.3 ± 7.9 | 47.7 ± 5.4 | 77.6 ± 6.0 | 8.8 ± 1.4 | 68.8 ± 5.1 |
| ERT2-villin-Cre;Mttpf/f;Abca1f/f | Tamoxifen | I-DKO | 24.8 ± 7.0 | 26.3 ± 5.7 | 31.6 ± 6.7 | 3.5 ± 1.7 | 28.1 ± 5.5 |
| (−50.7)a | (−44.9)a | (−59.3)b | (−60.2)c | (−59.1)b | |||
a p < 0.01.
b p < 0.001 compared with WT mice.
c p < 0.05.
Effects of Intestine-specific ABCA1 Ablation on Plasma HDL and Tissue Lipids
About 80% reduction in intestinal ABCA1 mRNA (Fig. 2B) had no effect on intestinal and hepatic MTP (Table 1), suggesting that intestinal ABCA1 does not modulate MTP expression. Intestinal ABCA1 deficiency had no significant effect on intestinal triglyceride and FFA but reduced total and esterified cholesterol (Table 1). Hepatic triglyceride levels were not affected, but FFA levels were reduced, and cholesterol levels were increased in I-Abca1−/− mice (Table 1). No significant lipid accumulation was evident in the intestine and liver of ABCA1-deficient mice, as determined by Oil Red O staining (Fig. 2, C and D). Intestine-specific ABCA1 deficiency had no effect on plasma triglyceride and FFA, but reduced cholesterol by 24% (Table 2) consistent with an earlier study (11). FPLC analysis showed that intestinal ABCA1 deficiency has no effect on triglyceride and cholesterol in the VLDL/LDL fraction, but it reduced cholesterol in the HDL fraction (Fig. 2, E and F). Thus, the major effect of intestinal ABCA1 deficiency was on plasma HDL, consistent with an earlier study (11).
Effect of Combined Intestinal Deficiency of MTP and ABCA1 on Tissue and Plasma Lipids
As anticipated, intestine-specific ablation of MTP and ABCA1 (I-DKO) significantly reduced MTP and ABCA1 expression (Table 1 and Fig. 2, A and B). Individual ablation of intestinal MTP and ABCA1 had no effect on hepatic MTP activity, but double knock-out increased hepatic MTP activity (Table 1). I-DKO mice had significantly higher amounts of intestinal triglyceride similar to I-Mttp−/− mice, but unlike those mice, I-DKO mice had normal levels of FFA (Table 1). I-DKO mice had lower hepatic triglyceride, FFA, and higher total and free cholesterol, similar to I-Mttp−/− mice (Table 1). Intestinal, but not hepatic, sections from I-DKO mice had significant lipid staining (Fig. 2, C and D). I-DKO mice had significantly lower levels of plasma triglyceride, FFA, and cholesterol, similar to I-Mttp−/− mice (Table 2). Furthermore, they had reduced lipids in both VLDL and HDL fractions (Fig. 2, E and F). These studies indicate that intestinal lipid accumulation was similar in I-DKO and I-Mttp−/− mice, except for the absence of FFA accumulation in I-DKO.
Lowered Lipid Absorption Reduces Plasma Lipids in Intestine-specific MTP and ABCA1 Knock-out Mice
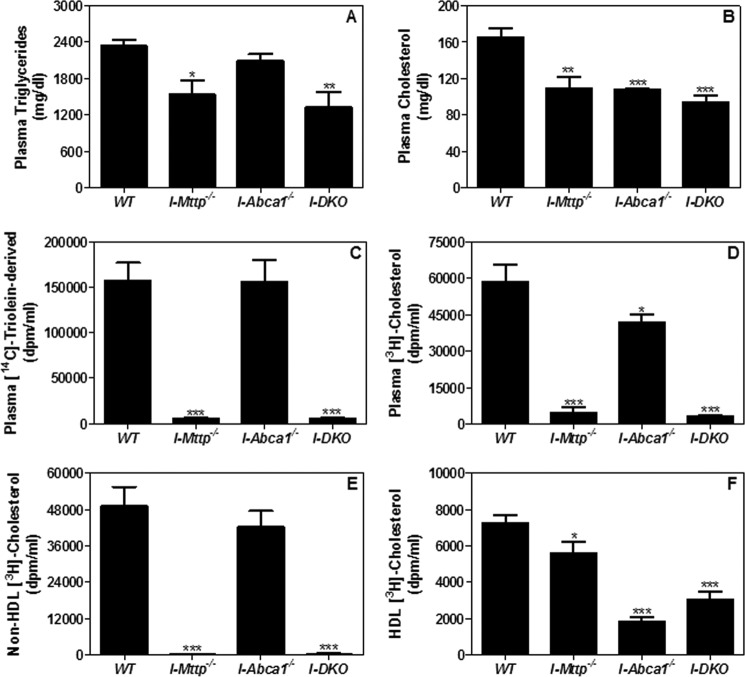
We hypothesized that significantly lower plasma triglyceride and cholesterol concentrations in mice deficient in intestinal MTP and ABCA1 might be secondary to lower lipid absorption during the postprandial state. To test this idea, I-Mttp−/−, I-Abca1−/−, and I-DKO mice were injected with P-407 to inhibit lipoprotein lipase and gavaged with radiolabeled triolein and cholesterol in olive oil. First, we measured total plasma triglyceride and cholesterol concentrations. Plasma triglyceride mass was significantly lower in I-Mttp−/− and I-DKO mice but was similar in I-Abca1−/− mice compared with WT mice (Fig. 3A), indicating that MTP, but not ABCA1, contributes significantly to plasma triglyceride. In contrast, plasma cholesterol concentrations were low in individual and I-DKO mice, suggesting that both MTP and ABCA1 contribute to plasma cholesterol concentrations during the postprandial state (Fig. 3B). As expected, reductions in plasma triglyceride and cholesterol were modest in these mice due to a smaller contribution of newly secreted lipoproteins during the 2-h time period.
FIGURE 3.
Chow diet-fed MTP and ABCA1 gene ablated mice absorb less lipids. 12-Week-old WT, I-Mttp−/−, I-Abca1−/−, and I-DKO male mice (n = 3) were fasted overnight and injected intraperitoneally with poloxamer 407 (30 mg/mouse). After 1 h, mice were gavaged with 0.5 μCi of [14C]triolein or [3H]cholesterol and 0.2 mg of cholesterol in 15 μl of olive oil. Plasma was collected 2 h after the gavage of radiolabeled lipids and used to measure triglycerides (A) and cholesterol (B) concentrations. Total plasma was also used to measure radioactivity to determine the absorption of [14C]triolein (C) and [3H]cholesterol (D). Plasma was precipitated as described under “Materials and Methods” to determine radioactivity cholesterol counts in non-HDL (E) and HDL (F) lipoproteins. Values are mean ± S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with WT.
To gain a better understanding of the contribution of these genes in the transport of newly absorbed lipids, we followed radiolabeled lipids. Appearance in the plasma of [14C]triolein- and [3H]cholesterol-derived lipids was reduced by >90% in mice deficient in MTP compared with WT (Fig. 3, C and D). In contrast, ABCA1 deficiency had no effect on [14C]triolein-derived lipids (Fig. 3C) but reduced cholesterol-derived lipids by 28% individually or by 95% in combination with MTP deficiency (Fig. 3D). MTP deficiency completely abolished cholesterol absorption with non-HDL lipoproteins and slightly reduced absorption with HDL (Fig. 3, E and F). In contrast, ABCA1 deficiency had no effect on the transport of cholesterol with non-HDL (Fig. 3E), but reduced cholesterol transport with HDL by 75% (Fig. 3F). Combined deficiency reduced cholesterol absorption via the HDL pathway by ∼70%. These studies indicate that MTP plays a significant role in triglyceride and cholesterol absorption via the non-HDL pathway, whereas ABCA1 contributes to cholesterol transport via the HDL pathway. Combined deficiency of MTP and ABCA1 reduces cholesterol absorption by ∼95% (Fig. 3D); thus, these two pathways are the major mechanisms involved in intestinal cholesterol absorption.
Effect of MTP and ABCA1 Gene Ablation on the Uptake of Fatty Acids and Secretion of Triglycerides by Enterocytes
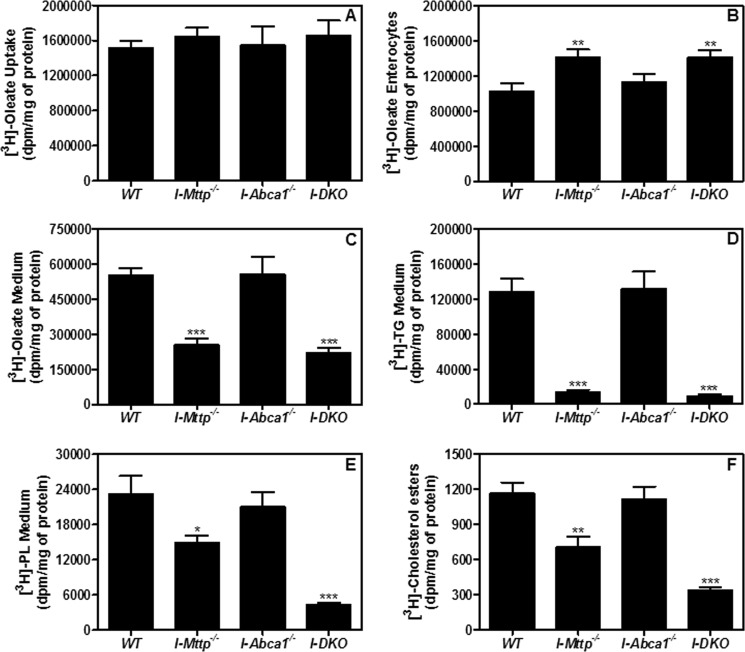
Reduced absorption of nascent triglycerides in I-Mttp−/− mice may either stem from decreased uptake of fatty acids and/or decreased secretion of triglycerides by the enterocytes. Enterocytes were incubated with [3H]oleic acid for 1 h. Fatty acid uptake was similar in control and all KO enterocytes (Fig. 4A). Thus, MTP and ABCA1 do not play a role in fatty acid uptake.
FIGURE 4.
Intestine-specific MTP and ABCA1 gene deletion decrease triglyceride secretion by enterocytes. To study lipid uptake, enterocytes were isolated from 12-week-old chow diet-fed, overnight-fasted mice and radiolabeled for 1 h with 0.5 μCi/ml of [3H]oleate. After 1 h, enterocytes were washed, and lipids were isolated to determine uptake of radiolabeled fatty acid (A). For characterization of secreted lipoproteins, after 1 h of uptake, enterocytes were washed and incubated with fresh media containing 1.4 mm oleic acid containing micelles for 2 h. Isolated lipids from the cells (B) and media (C) were counted to determine total fatty acid-derived radioactivity. Media from B were lipid-extracted, and lipids were separated by thin layer chromatography to determine radioactivity in triglycerides (D), phospholipids (E), and cholesteryl esters (F). Each measurement was done in triplicate with three mice per group. Mean ± S.D. *, p < 0.05; **, p < 0.01 and ***, p < 0.001 compared with WT.
Next, we studied retention and secretion of [3H]oleic acid-derived lipids by the enterocytes after pulsing for 1 h with radiolabeled oleic acid and chasing with 1.4 mm unlabeled oleic acid and 0.5 mm taurocholate micelles for 2 h. Enterocytes from I-Mttp−/− and I-DKO mice accumulated more [3H]oleic acid-derived lipids than WT mice (Fig. 4B) and secreted less into the media (Fig. 4C). Separation of lipids by thin layer chromatography revealed that these enterocytes secreted 89 and 93% less radiolabeled triglycerides (Fig. 4D), 36 and 81% less radiolabeled phospholipids (Fig. 4E), and 40 and 71% less cholesteryl esters (Fig. 4F) in the media. No change in [3H]oleic acid-derived lipid accumulation or secretion was seen in I-Abca1−/− enterocytes compared with WT. These data indicate that deletion of intestinal MTP does not affect fatty acid uptake but decreases fatty acid secretion, whereas deletion of intestinal ABCA1 affects neither uptake nor secretion of fatty acids.
MTP and ABCA1 Gene Ablations Reduce Cholesterol Secretion by Enterocytes via Chylomicron and HDL Pathways
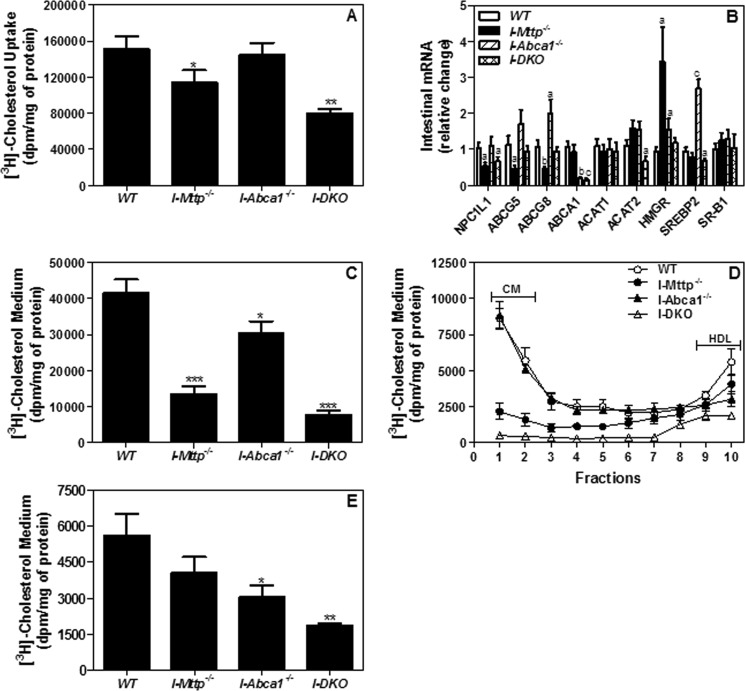
Next, we evaluated the role of both MTP and ABCA1 in the transport of cholesterol by enterocytes. Transport across the intestine involves uptake of cholesterol and subsequent secretion by enterocytes. Uptake of cholesterol by enterocytes was reduced by 24% in the absence of intestinal MTP but was not affected by ABCA1 deficiency; combined deficiency reduced uptake by 47% (Fig. 5A). The effect of MTP deficiency on cholesterol uptake was unanticipated. To determine the reasons for this, we measured mRNA levels of genes involved in cholesterol transport (Fig. 5B). Expression of NPC1L1, ATP -binding cassette subfamily G member 5 (ABCG5), and member 8 (ABCG8) was reduced in MTP-deficient intestinal tissue. By contrast, there was no change in scavenger receptor class B, type I (SR-BI) levels in I-Mttp−/−, I-Abca1−/−, or I-DKO mice. It is likely that reduced cholesterol uptake is secondary to reduced expression of NPC1L1. Significant cellular cholesterol accumulation might have down-regulated NPC1L1 to reduce further uptake. However, genes involved in cholesterol synthesis such as HMG-CoA reductase were increased in I-Mttp−/− mice. Why and how MTP deficiency affects NPC1L1 and HMG-CoA reductase expression were not the focus of this study. Sterol regulatory element-binding protein 2 (SREBP2), which regulates cholesterol homeostasis by controlling the genes involved in cholesterol synthesis, was increased in I-Abca1−/− mice.
FIGURE 5.
Intestine-specific MTP and ABCA1 gene deletion decrease secretion of cholesterol by enterocytes. A, to study cholesterol uptake, enterocytes were isolated from 12-week-old chow diet-fed, overnight-fasted mice and radiolabeled for 1 h with 0.5 μCi/ml [3H]cholesterol. After 1 h, enterocytes were washed, and lipids were isolated to determine uptake of radiolabeled cholesterol. B, in a separate experiment, total RNA isolated from the intestine of WT, I-Mttp−/−, I-Abca1−/−, and I-DKO (n = 5) male mice fed a chow diet was used to quantify mRNA levels of different genes. Mean ± S.D., n = 3. a, p < 0.05; b, p < 0.01, and c, p < 0.001 compared with WT. C–E, for characterization of secreted lipoproteins in an experiment described in A, after 1 h of uptake, enterocytes were washed and incubated with fresh media containing 1.4 mm oleic acid containing micelles for 2 h. Isolated lipids from the media (C) were counted to determine total cholesterol radioactivity. [3H]Cholesterol-radiolabeled media were used for separating lipoproteins by density gradient ultracentrifugation, and radioactivity was determined in each fraction (D). Fractions 1–3 and 8–10 represent chylomicrons (CM) and HDL, respectively. For better representation of HDL, fraction 10 was plotted separately (E). Each measurement was done in triplicate with three mice per group. Mean ± S.D., *, p < 0.05; **, p < 0.01 and ***, p < 0.001 compared with WT.
Cholesterol secretion was reduced by 68 and 27% in MTP- and ABCA1-deficient enterocytes, respectively, and their combined deficiency reduced cholesterol secretion by 82% (Fig. 5C). We considered the possibility that reductions in cholesterol secretion might be secondary to reduced esterification. However, there were no significant differences in ACAT1 and ACAT2 mRNA levels in these enterocytes (Fig. 5B), excluding this possibility. Thus, MTP and ABCA1 contribute significantly to cholesterol secretion by enterocytes.
It is known that enterocytes secrete cholesterol via chylomicron (apoB-dependent) or HDL (apoB-independent) pathways. Therefore, we subjected media to ultracentrifugation to determine secretion of cholesterol via these two different pathways. MTP deficiency significantly reduced secretion of cholesterol with chylomicrons but had no significant effect on HDL cholesterol (Fig. 5D). However, ABCA1 deficiency had no effect on cholesterol secretion with chylomicrons but significantly reduced secretion with HDL (Fig. 5D). Combined deficiency of MTP and ABCA1 further reduced cholesterol secretion with chylomicrons and HDL (Fig. 5D).
The effects of MTP and ABCA1 deficiencies on cholesterol secretion with HDL were plotted separately for better representation (Fig. 5E). Although MTP deficiency had no significant effect, ABCA1 deficiency significantly reduced cholesterol secretion with HDL. Combined deficiency of MTP and ABCA1 reduced secretion of cholesterol with HDL by ∼67% (Fig. 5, D and E). Thus, individual deficiencies of MTP and ABCA1 reduce secretion of cholesterol with chylomicron and HDL pathways, respectively, and their combined deficiency reduces cholesterol secretion with both chylomicrons and HDL.
Altered Free Fatty Acid Metabolism in MTP- and ABCA1-deficient Enterocytes
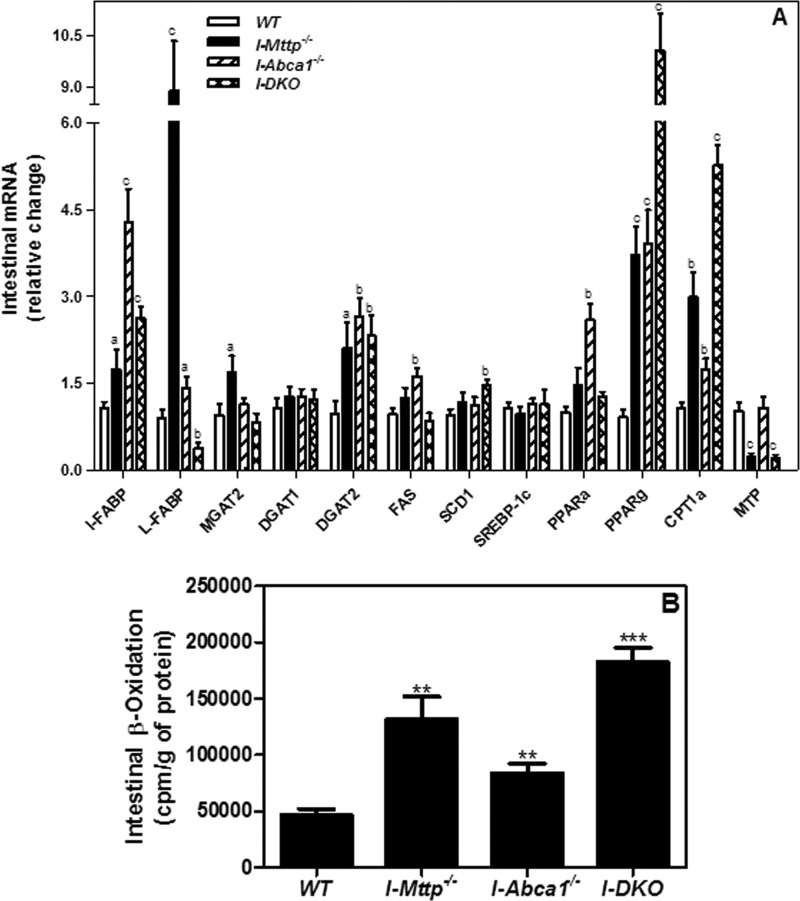
Triglyceride accumulation is universally observed in hepatic (6, 36) and in intestine-specific deficiencies of MTP (7). However, although FFA accumulation has not been observed in the liver-specific MTP knock-out mice (6, 36), intestine-specific MTP ablation has been reported to increase cellular free fatty acids (7). We also observed an ∼1.5-fold increase in intestinal free fatty acids (Table 1). Consistent with others (7), we also observed an ∼10-fold increase in L-FABP mRNA (Fig. 6A). Because there was no significant increase in fatty acid uptake (Fig. 4A), several causes for the increases in cellular FFA levels in the absence of MTP were considered. We reasoned that fatty acid levels might have increased secondary to reduced triglyceride synthesis. However, this was not the case, because mRNA levels of acyl-CoA:monoacylglycerol acyltransferase 2 (MGAT2) and diacylglycerol O-acyltransferase 2 (DGAT2) were increased in MTP-deficient intestines (Fig. 6A), and MTP-deficient enterocytes accumulated more oleic acid-derived lipids (Fig. 4B), suggesting increased rather than decreased triglyceride synthesis. Next, we considered the possibility that enterocytes might be synthesizing more fatty acids. However, expression of genes involved in fatty acid synthesis (FAS, SCD1, and SREBP1c) was not affected by MTP and ABCA1 deficiency (Fig. 6A). Hence, we studied β-oxidation by intestinal segments, anticipating significant reductions. Instead, I-Mttp−/− intestines showed higher β-oxidation (Fig. 6B) and expression of key proteins and transcription factors (CPT1α, PPARα, and PPARγ) involved in this process (Fig. 6A). Therefore, increases in triglyceride synthesis and β-oxidation were most likely a downstream response to increases in cellular FFA and were not the cause for their cellular accretion. More studies are needed to learn why FFA accumulate in the absence of MTP activity.
FIGURE 6.
Intestinal MTP deletion increases the expression of intestinal fatty acid oxidation genes. A, total RNA isolated from the intestine of 12-week-old WT, I-Mttp−/−, I-Abca1−/−, and I-DKO (n = 5) male mice fed a chow diet was used to quantify mRNA levels of different genes. Mean ± S.D., a, p < 0.05; b, p < 0.01, and c, p < 0.001 compared with WT. B, in a separate experiment, 12-week-old WT, I-Mttp−/−, I-Abca1−/−, and I-DKO male mice (n = 3) were fasted overnight and sacrificed. For fatty acid oxidation, 50-mg aliquots of intestine were incubated with [14C]oleic acid (0.3 μCi) for 2 h, and the radiolabeled CO2 was captured and quantified in a scintillation counter. Mean ± S.D. **, p < 0.01 and ***, p < 0.001 compared with WT.
ABCA1 deficiency had no significant effect on cellular FFA (Table 1) and slightly increased liver-fatty acid binding protein (L-FABP) (Fig. 6A); however, it increased intestinal (I)-FABP mRNA levels by ∼4.5-fold. ABCA1 deficiency increased β-oxidation (Fig. 6B) and mRNAs involved in this process (Fig. 6A). Surprisingly, increases in FFA and L-FABP mRNA seen in MTP-deficient intestines were not seen in mice deficient in both MTP and ABCA1. There was a significant increase in intestinal β-oxidation in the intestines of these I-DKO mice (Fig. 6B). Furthermore, there was an additive increase in the expression of PPARγ mRNA levels in I-DKO mice (Fig. 6A). These studies indicate that combined deficiency of MTP and ABCA1 additively enhances PPARγ expression and fatty acid β-oxidation, and this response might avoid accumulation of cellular FFA. Why and how the combined deficiency of these proteins enhances expression of PPARγ and augments free fatty acid need further investigation.
Compensatory Changes in Hepatic Lipid Metabolism in Intestine-specific MTP and ABCA1 Knock-out Mice
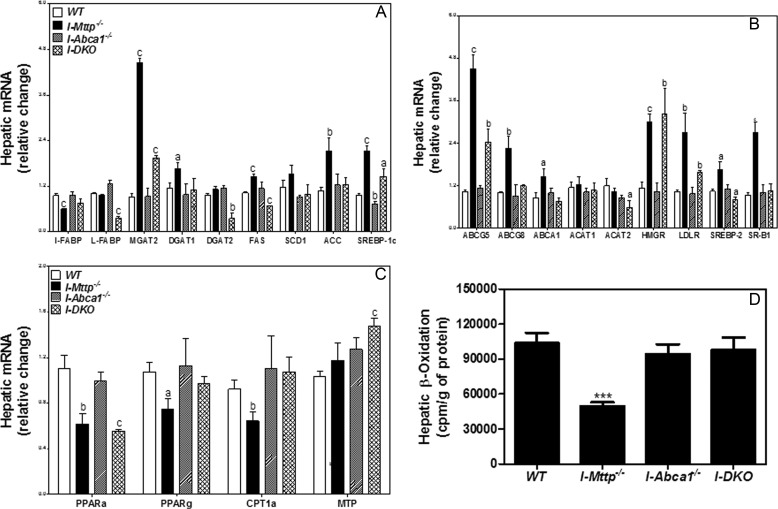
Xie et al. (7) reported increased hepatic lipogenesis in intestine-specific MTP-deficient mice. Consistent with these studies, we observed significant increases in mRNA levels of SREBP-1c, fatty-acid synthase (FAS), and acetyl-CoA carboxylase (ACC), but stearoyl-CoA desaturase-1 (SCD1) levels were unaltered (Fig. 7A). Increased lipogenesis might be a response to low levels of FFA found in the livers of I-Mttp−/− mice (Table 1). However, intestinal ABCA1 deficiency does not affect expression of these genes in the liver. In I-DKO mice, SREBP1c levels were high, but FAS and ACC levels were similar to controls.
FIGURE 7.
Relative mRNA levels of liver lipid metabolism genes. Total RNA isolated from liver of 12-week-old WT, I-Mttp−/−, I-Abca1−/−, and I-DKO (n = 5) male mice fed a chow diet was used to quantify mRNA levels of fatty acid and triglyceride biosynthesis genes (A), cholesterol metabolism genes (B), and fatty acid oxidation and triglyceride secretion genes (C). Mean ± S.D. a, p < 0.05; b, p < 0.01, and c, p < 0.001 compared with WT. D, in a separate experiment, 12-week-old WT, I-Mttp−/−, I-Abca1−/−, and I-DKO male mice (n = 3) were fasted overnight and sacrificed. For fatty acid oxidation, 50-mg aliquots of liver were incubated with [14C]oleic acid (0.3 μCi) for 2 h, and the radiolabeled CO2 was captured and quantified in a scintillation counter. Mean ± S.D. ***, p < 0.001 compared with WT.
Next, we studied the effects of intestinal MTP deficiency on the expression of selected genes involved in hepatic cholesterol metabolism. We observed significant increases in hepatic cholesterol levels in I-Mttp−/− mice and in the expression of ABCG5, ABCG8, SR-BI, HMG-CoA-reductase (HMGR), LDL receptor (LDLr), and SREBP-2 but not in ACAT1 and ACTA2. These studies suggest a significant effect on cholesterol transport but not on cholesterol esterification. Although there were increases in hepatic cholesterol in the setting of intestinal ABCA1 deficiency, significant changes in the expression of genes involved in cholesterol metabolism were absent. In I-DKO mice, hepatic cholesterol levels were significantly higher than controls, accompanied by significant increases in ABCG5, ABCG8, HMGR, LDLr, and SR-BI (Fig. 7B). These studies indicate that intestine-specific MTP deficiency augments hepatic cholesterol transport pathways, most likely in response to low delivery of cholesterol from the intestine.
Analysis of mRNA levels of genes involved in fatty acid oxidation showed that the livers of I-Mttp−/− mice had low levels of PPARα, PPARγ, and CPT1α, indicating reduced β-oxidation (Fig. 7C). Similarly, low hepatic β-oxidation was observed in the livers of these mice (Fig. 7D). In contrast, I-Abca1−/− mice had no significant effect on the expression of these genes and hepatic β-oxidation. In I-DKO mice, PPARα mRNA levels were low, although there were no significant differences in PPARγ and CPT1α compared with controls. Consistent with this, hepatic β-oxidation was similar to that seen in WT mice (Fig. 7D). These studies indicate that intestinal MTP deficiency most likely results in reduced hepatic fatty acid oxidation. Our findings suggest that intestine-specific deletion of MTP is accompanied by compensatory alterations in hepatic lipid metabolism.
DISCUSSION
Role of Intestinal MTP and ABCA1 in Lipid Absorption
These studies highlight the importance of intestinal MTP in triglyceride absorption and the contribution of intestinal MTP and ABCA1 to cholesterol absorption. Whereas intestinal Mttp ablation alone reduces >95% absorption of triolein-derived long chain fatty acids, demonstrating its essential role in fat absorption, both MTP and ABCA1 contribute to ∼95% of cholesterol absorption by two different pathways. Thus, MTP and ABCA1 are critical for and are the major determinants of dietary lipid absorption. There probably is no other mechanism of significance for their absorption besides these pathways.
Our studies indicate that intestinal MTP deficiency reduces cholesterol absorption by ∼92%, consistent with our previous studies (11). However, Xie et al. (7) reported that I-Mttp−/− decreased fractional cholesterol absorption by ∼60–70%. This discrepancy is the result of two different methods used to measure cholesterol absorption. We used a short term absorption method, in which the appearance of radiolabeled cholesterol was measured in the plasma after 2 h of oral gavage to simulate acute postprandial conditions. Xie et al. (7) used a dual isotope ratio method, in which unabsorbed cholesterol and plant sterols are measured in the feces collected over 48 h to determine cholesterol absorption. These two approaches show that cholesterol absorption during acute postprandial conditions is more dependent on MTP. In contrast, over a long period of time, other mechanisms also contribute to cholesterol absorption.
The other mechanism of cholesterol absorption might involve ABCA1. Despite no change in triglyceride absorption, acute cholesterol absorption in I-Abca1−/− mice was reduced by 28%. Further studies demonstrated that ABCA1 does not contribute to cholesterol secretion via an apoB-dependent chylomicron pathway; instead, ABCA1 contributes to cholesterol absorption via the apoB-independent HDL pathway. These in vivo studies are consistent with our previous studies demonstrating the presence of two cholesterol transport pathways in cultured cells (23).
In contrast to ∼92% inhibition of cholesterol absorption in acute absorption studies, data from I-Mttp−/− enterocytes show an ∼70% decrease in the secretion of cholesterol suggesting that ∼30% of cholesterol secretion occurs via an apoB-independent pathway. Combined deficiency of MTP and ABCA1 reduced cholesterol secretion by ∼80% in isolated enterocytes. Most likely, the remaining secretion represents efflux via the ABCG5/ABCG8 pathway. If this is true, then I-DKO enterocytes can be useful model systems to assess cholesterol efflux via these transporters, a process that previously has been difficult to quantify (37).
Roles of Intestinal MTP and ABCA1 in Tissue Lipid Homeostasis
Intestinal MTP deficiency significantly increases intestinal triglyceride and cholesterol levels, whereas ABCA1 deficiency decreases intestinal cholesterol. Combined deficiency of intestinal MTP and ABCA1 results in significant accumulation of triglyceride and cholesterol, similar to I-Mttp−/− mice. Therefore, MTP is the major determinant of tissue triglyceride and cholesterol levels.
Intestinal MTP deficiency results in significant accumulation of FFA and in increased expression of genes involved in fatty acid mobilization and oxidation, but ABCA1 deficiency has no effect on cellular FFA levels. Surprisingly, combined deficiency of MTP and ABCA1 ameliorates accumulation of intestinal FFA and changes in the expression of genes involved in β-oxidation associated with MTP deficiency. In other words, MTP deficiency enhances cellular FFA involving a novel mechanism, wherein ABCA1 is a co-participant. The reasons for intestinal FFA accumulation in I-Mttp−/− mice as well as remediation of this phenotype in the absence of ABCA1 are unknown. We considered the possibility that FFA accumulation in I-Mttp−/− mice might be caused by reduced triglyceride synthesis, reduced fatty acid β-oxidation, or increased synthesis of fatty acids. However, triglyceride synthesis was not reduced; β-oxidation was enhanced, and fatty acid synthesis was not augmented. Enhanced β-oxidation might be an adaptive response to the cellular accumulation of FFA. Thus, normal mechanisms do not explain this unusual phenotype. Triglyceride pools in cells are not inert; instead, they are constantly broken down and re-synthesized (38). Hence, triglyceride synthesis might not be affected by high FFA concentrations. However, in the absence of MTP, triglyceride hydrolysis and synthesis may be maximal, and FFA flux might be higher than the capacity of triglyceride-synthesizing enzymes, leading to accumulation of FFA in cells. FFA would then interact with fatty acid-binding protein and be transported to the nucleus to increase expression of genes (39) involved in fatty acid transport, e.g. L-FABP, and oxidation such as PPARα and PPARγ. Furthermore, L-FABP can target fatty acids to oxidative degradation (40). Therefore, higher β-oxidation of FFA might be secondary to high FFA concentrations.
ABCA1 is a membrane bound protein that modulates membrane raft domains (39, 41). Hence, it is possible that ABCA1 could sequester FFA in lipid rafts (42) making them inaccessible to β-oxidation. In the absence of ABCA1, FFA is not sequestered and remains available for transport to mitochondria for oxidation, leading to reduced cellular FFA levels. This is supported by the observation that intestinal segments from I-Abca1−/− mice show an 80% increase in fatty acid β-oxidation despite no significant change in intestinal FFA levels. Further studies are needed to explore these possibilities.
We previously reported that hepatic MTP deficiency increases free cholesterol in the livers of Western diet-fed mice or mice with hepatic LDL receptor deficiency, and this effect leads to endoplasmic reticulum stress and release of alanine and aspartate aminotransferases (36, 43). Surprisingly, free cholesterol did not accumulate significantly in the intestine of I-Mttp−/− mice. Xie et al. (7) have also reported that intestine-specific MTP deficiency does not significantly increase intestinal free cholesterol. Hyperlipidemic conditions may be needed for free cholesterol to accumulate in the absence of MTP. Alternatively, there could be different reasons for variable tissue-specific effects of MTP deficiencies on free cholesterol accumulation in the liver and intestine. We observed that I-Mttp−/− enterocytes took up less cholesterol, most likely secondary to reduced expression of NPC1L1. Furthermore, these enterocytes excreted free cholesterol with HDL. Thus, the intestine may have evolved different mechanisms to avoid free cholesterol accumulation in the absence of MTP.
The effects of intestine-specific MTP ablation on intestinal lipids might be pertinent to efforts underway to inhibit intestinal MTP to treat hyperlipidemia. MTP inhibition has been a favorite target to reduce plasma lipids. However, total MTP inhibition has been associated with hepatosteatosis. Hence, recent efforts have concentrated on inhibiting intestinal MTP (44, 45). Our studies in I-Mttp−/− mice suggest that intestine-specific MTP inhibition could result in significant reduction in plasma lipids. However, such a strategy might be associated with significant accretion of triglycerides, FFA, and cholesterol in the intestine. Therefore, there is a need to study the effects of these lipid accumulations on intestinal function and other physiologic parameters in animals exposed to intestine-specific MTP inhibitors.
Regulation of Plasma Lipids by Intestinal MTP and ABCA1
Intestinal MTP deficiency reduces plasma triglyceride, FFA, and cholesterol by 37–45%, and a combined deficiency of MTP and ABCA1 reduces these lipids by 45–60%. Thus, intestinal MTP is a major contributor to plasma lipids. Reductions in plasma triglyceride and cholesterol can be explained by the reduced production of intestinal lipoproteins. However, reductions in plasma FFA in 5-h fasted animals were unanticipated. Plasma FFA levels in fasting conditions result from mobilization of adipose tissue fat depots to the liver. There may be less adipose tissue in I-Mttp−/− mice available to contribute to plasma FFA mobilization; however, we did not see significant changes in body weight during the 12-day period of Mttp ablation. Therefore, reductions in plasma FFA in I-Mttp−/− mice might indicate that hydrolysis of intestine-derived lipoproteins also contributes significantly to plasma FFA levels.
Intestinal ABCA1 ablation had no effect on plasma triglyceride and FFA, but reduced plasma cholesterol levels by ∼24%, consistent with earlier studies (11). Combined deficiency with MTP reduced plasma cholesterol concentrations by ∼60%, suggesting that intestinal ABCA1 and MTP additively contribute significantly to plasma cholesterol levels. Hence, intestinal cholesterol absorption involving MTP and ABCA1 is a significant determinant of plasma cholesterol concentrations.
Effects of Intestinal MTP and ABCA1 Deficiencies on Hepatic Lipid Metabolism
Intestinal ABCA1 deficiency had no effect on hepatic triglyceride content, but intestinal MTP deficiency reduced hepatic triglyceride. Reductions in hepatic triglyceride in I-Mttp−/− mice might be due to reduced delivery of dietary fat from the intestine.
Intestinal MTP deficiency did not increase hepatic MTP expression. Xie et al. (7) also did not see any significant change in hepatic MTP expression after the conditional deletion of MTP in the intestine. Similarly, intestinal ABCA1 deficiency had no effect on hepatic MTP activity. However, ablation of both ABCA1 and MTP in the intestine elevated hepatic MTP expression and activity. This is likely a compensatory change to supply fat to other peripheral tissues.
Individual ablation of intestinal MTP and ABCA1 significantly reduced hepatic FFA levels, and combined deficiency appears to further reduce hepatic FFA levels. Reduced hepatic FFA in I-Mttp−/− can be explained by reduced delivery of dietary fat. However, the effect of intestinal ABCA1 deficiency on hepatic FFA was unanticipated and is hard to explain.
In short, the data presented here provide novel information about the combined effects of intestinal MTP and ABCA1 deficiencies on plasma and lipid tissue homeostasis and in lipid absorption. These studies establish that these two proteins are critical for the absorption of cholesterol via two independent pathways. Furthermore, these studies point out that these proteins participate, and might even co-operate, in controlling cellular FFA levels. It is likely that reducing their activities might be useful in reducing plasma lipids.
Acknowledgment
We gratefully acknowledge Karen Klein (Translational Science Institute, Wake Forest School of Medicine) for editing the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DK-46900 (to M. M. H.), HL049373, and HL119962 (to J. S. P.). This work was also supported by a grant-in-aid from the American Heart Association (to J. I.).
- apoB
- apolipoprotein B
- ABCA1
- ATP-binding cassette transporter subfamily A, member 1
- FFA
- free fatty acid
- MTP
- microsomal triglyceride transfer protein
- PPARγ
- peroxisome proliferator-activated receptor-γ
- SR-BI
- scavenger receptor class B, type I
- CPT1α
- carnitine palmitoyltransferase 1α
- NPC1L1
- Niemen Pick C1-like 1.
REFERENCES
- 1. Grundy S. M., Cleeman J. I., Merz C. N., Brewer H. B., Jr., Clark L. T., Hunninghake D. B., Pasternak R. C., Smith S. C., Jr., Stone N. J. (2004) Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation 110, 227–239 [DOI] [PubMed] [Google Scholar]
- 2. Iqbal J., Hussain M. M. (2009) Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 296, E1183–E1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hussain M. M., Rava P., Walsh M., Rana M., Iqbal J. (2012) Multiple functions of microsomal triglyceride transfer protein. J. Nutr. Metab. 9:14, 10.1186/1743-7075-9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berriot-Varoqueaux N., Aggerbeck L. P., Samson-Bouma M., Wetterau J. R. (2000) The role of the microsomal triglyceride transfer protein in abetalipoproteinemia. Annu. Rev. Nutr. 20, 663–697 [DOI] [PubMed] [Google Scholar]
- 5. Raabe M., Flynn L. M., Zlot C. H., Wong J. S., Véniant M. M., Hamilton R. L., Young S. G. (1998) Knockout of the abetalipoproteinemia gene in mice: Reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc. Natl. Acad. Sci. U.S.A. 95, 8686–8691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raabe M., Véniant M. M., Sullivan M. A., Zlot C. H., Björkegren J., Nielsen L. B., Wong J. S., Hamilton R. L., Young S. G. (1999) Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103, 1287–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xie Y., Newberry E. P., Young S. G., Robine S., Hamilton R. L., Wong J. S., Luo J., Kennedy S., Davidson N. O. (2006) Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J. Biol. Chem. 281, 4075–4086 [DOI] [PubMed] [Google Scholar]
- 8. Iqbal J., Li X., Chang B. H., Chan L., Schwartz G. J., Chua S. C., Jr., Hussain M. M. (2010) An intrinsic gut leptin-melanocortin pathway modulates intestinal microsomal triglyceride transfer protein and lipid absorption. J. Lipid Res. 51, 1929–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parks J. S., Chung S., Shelness G. S. (2012) Hepatic ABC transporters and triglyceride metabolism. Curr. Opin. Lipidol. 23, 196–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Timmins J. M., Lee J. Y., Boudyguina E., Kluckman K. D., Brunham L. R., Mulya A., Gebre A. K., Coutinho J. M., Colvin P. L., Smith T. L., Hayden M. R., Maeda N., Parks J. S. (2005) Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115, 1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brunham L. R., Kruit J. K., Iqbal J., Fievet C., Timmins J. M., Pape T. D., Coburn B. A., Bissada N., Staels B., Groen A. K., Hussain M. M., Parks J. S., Kuipers F., Hayden M. R. (2006) Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116, 1052–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kesäniemi Y. A., Miettinen T. A. (1987) Cholesterol absorption efficiency regulates plasma cholesterol level in the Finnish population. Eur. J. Clin. Invest. 17, 391–395 [DOI] [PubMed] [Google Scholar]
- 13. Wilson M. D., Rudel L. L. (1994) Review of cholesterol absorption with emphasis on dietary and biliary cholesterol. J. Lipid Res. 35, 943–955 [PubMed] [Google Scholar]
- 14. McGill H. C., Jr. (1979) The relationship of dietary cholesterol to serum cholesterol concentration and to atherosclerosis in man. Am. J. Clin. Nutr. 32, 2664–2702 [DOI] [PubMed] [Google Scholar]
- 15. Dawson P. A., Rudel L. L. (1999) Intestinal cholesterol absorption. Curr. Opin. Lipidol. 10, 315–320 [DOI] [PubMed] [Google Scholar]
- 16. Davis H. R., Jr., Zhu L. J., Hoos L. M., Tetzloff G., Maguire M., Liu J., Yao X., Iyer S. P., Lam M. H., Lund E. G., Detmers P. A., Graziano M. P., Altmann S. W. (2004) Niemann-pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole body cholesterol homeostasis. J. Biol. Chem. 279, 33586–33592 [DOI] [PubMed] [Google Scholar]
- 17. Altmann S. W., Davis H. R., Jr., Zhu L. J., Yao X., Hoos L. M., Tetzloff G., Iyer S. P., Maguire M., Golovko A., Zeng M., Wang L., Murgolo N., Graziano M. P. (2004) Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 303, 1201–1204 [DOI] [PubMed] [Google Scholar]
- 18. Cohen J. C., Pertsemlidis A., Fahmi S., Esmail S., Vega G. L., Grundy S. M., Hobbs H. H. (2006) Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc. Natl. Acad. Sci. U.S.A. 103, 1810–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang T. Y., Chang C. C., Cheng D. (1997) Acyl-coenzyme A:cholesterol acyltransferase. Annu. Rev. Biochem. 66, 613–638 [DOI] [PubMed] [Google Scholar]
- 20. Chang T. Y., Chang C. C., Lin S., Yu C., Li B. L., Miyazaki A. (2001) Roles of acyl-coenzyme A:cholesterol acyltransferase-1 and -2. Curr. Opin. Lipidol. 12, 289–296 [DOI] [PubMed] [Google Scholar]
- 21. Hussain M. M., Kancha R. K., Zhou Z., Luchoomun J., Zu H., Bakillah A. (1996) Chylomicron assembly and catabolism: role of apolipoproteins and receptors. Biochim. Biophys. Acta 1300, 151–170 [DOI] [PubMed] [Google Scholar]
- 22. Hussain M. M., Fatma S., Pan X., Iqbal J. (2005) Intestinal lipoprotein assembly. Curr. Opin. Lipidol. 16, 281–285 [DOI] [PubMed] [Google Scholar]
- 23. Iqbal J., Anwar K., Hussain M. M. (2003) Multiple, independently regulated pathways of cholesterol transport across the intestinal epithelial cells. J. Biol. Chem. 278, 31610–31620 [DOI] [PubMed] [Google Scholar]
- 24. Iqbal J., Hussain M. M. (2005) Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J. Lipid Res. 46, 1491–1501 [DOI] [PubMed] [Google Scholar]
- 25. Lee R. G., Kelley K. L., Sawyer J. K., Farese R. V., Jr., Parks J. S., Rudel L. L. (2004) Plasma cholesteryl esters provided by lecithin:cholesterol acyltransferase and acyl-coenzyme A:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circ. Res. 95, 998–1004 [DOI] [PubMed] [Google Scholar]
- 26. Repa J. J., Buhman K. K., Farese R. V., Jr., Dietschy J. M., Turley S. D. (2004) ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology 40, 1088–1097 [DOI] [PubMed] [Google Scholar]
- 27. Temel R. E., Lee R. G., Kelley K. L., Davis M. A., Shah R., Sawyer J. K., Wilson M. D., Rudel L. L. (2005) Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. J. Lipid Res. 46, 2423–2431 [DOI] [PubMed] [Google Scholar]
- 28. Chang B. H., Liao W., Li L., Nakamuta M., Mack D., Chan L. (1999) Liver-specific inactivation of the abetalipoproteinemia gene completely abrogates very low density lipoprotein low density lipoprotein production in a viable conditional knockout mouse. J. Biol. Chem. 274, 6051–6055 [DOI] [PubMed] [Google Scholar]
- 29. el Marjou F., Janssen K. P., Chang B. H., Li M., Hindie V., Chan L., Louvard D., Chambon P., Metzger D., Robine S. (2004) Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193 [DOI] [PubMed] [Google Scholar]
- 30. Anwar K., Iqbal J., Hussain M. M. (2007) Mechanisms involved in vitamin E transport by primary enterocytes and in vivo absorption. J. Lipid Res. 48, 2028–2038 [DOI] [PubMed] [Google Scholar]
- 31. Athar H., Iqbal J., Jiang X. C., Hussain M. M. (2004) A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J. Lipid Res. 45, 764–772 [DOI] [PubMed] [Google Scholar]
- 32. Rava P., Athar H., Johnson C., Hussain M. M. (2005) Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J. Lipid Res. 46, 1779–1785 [DOI] [PubMed] [Google Scholar]
- 33. Ibrahimi A., Bonen A., Blinn W. D., Hajri T., Li X., Zhong K., Cameron R., Abumrad N. A. (1999) Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J. Biol. Chem. 274, 26761–26766 [DOI] [PubMed] [Google Scholar]
- 34. Khatun I., Zeissig S., Iqbal J., Wang M., Curiel D., Shelness G. S., Blumberg R. S., Hussain M. M. (2012) Phospholipid transfer activity of MTP promotes assembly of phospholipid-rich apoB-containing lipoproteins and reduces plasma as well as hepatic lipids in mice. Hepatology 55, 1356–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Soh J., Iqbal J., Queiroz J., Fernandez-Hernando C., Hussain M. M. (2013) MicroRNA-30c reduces hyperlipidemia and atherosclerosis by decreasing lipid synthesis and lipoprotein secretion. Nat. Med. 19, 892–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Josekutty J., Iqbal J., Iwawaki T., Kohno K., Hussain M. M. (2013) Microsomal triglyceride transfer protein inhibition induces endoplasmic reticulum stress and increases gene transcription via Ire1α/cJun to enhance plasma ALT/AST. J. Biol. Chem. 288, 14372–14383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang J., Sun F., Zhang D. W., Ma Y., Xu F., Belani J. D., Cohen J. C., Hobbs H. H., Xie X. S. (2006) Sterol transfer by ABCG5 and ABCG8: in vitro assay and reconstitution. J. Biol. Chem. 281, 27894–27904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sturley S. L., Hussain M. M. (2012) Lipid droplet formation on opposing sides of the endoplasmic reticulum. J. Lipid Res. 53, 1800–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schroeder F., Petrescu A. D., Huang H., Atshaves B. P., McIntosh A. L., Martin G. G., Hostetler H. A., Vespa A., Landrock D., Landrock K. K., Payne H. R., Kier A. B. (2008) Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids 43, 1–17 [DOI] [PubMed] [Google Scholar]
- 40. Lagakos W. S., Gajda A. M., Agellon L., Binas B., Choi V., Mandap B., Russnak T., Zhou Y. X., Storch J. (2011) Different functions of intestinal and liver-type fatty acid-binding proteins in intestine and in whole body energy homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G803–G814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu Y., Tang C. (2012) Regulation of ABCA1 functions by signaling pathways. Biochim. Biophys. Acta 1821, 522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klappe K., Hummel I., Hoekstra D., Kok J. W. (2009) Lipid dependence of ABC transporter localization and function. Chem. Phys. Lipids 161, 57–64 [DOI] [PubMed] [Google Scholar]
- 43. Iqbal J., Rudel L. L., Hussain M. M. (2008) Microsomal triglyceride transfer protein enhances cellular cholesteryl esterification by relieving product inhibition. J. Biol. Chem. 283, 19967–19980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hussain M. M., Bakillah A. (2008) New approaches to target microsomal triglyceride transfer protein. Curr. Opin. Lipidol. 19, 572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wierzbicki A. S., Hardman T., Prince W. T. (2009) Future challenges for microsomal transport protein inhibitors. Curr. Vasc. Pharmacol. 7, 277–286 [DOI] [PubMed] [Google Scholar]