Background: Cytosolic carboxypeptidase 5 (CCP5) was proposed to selectively cleave γ-linked glutamate from tubulin side chains, but not α-linked glutamates.
Results: Purified CCP5 removes α- and γ-linked glutamates from microtubules, soluble tubulin, and synthetic peptides.
Conclusion: CCP5 is not specific for γ-linked glutamate or for a specific form of tubulin.
Significance: CCP5 is an important tubulin code-modifying enzyme.
Keywords: Carboxypeptidase, Enzymes, Metalloenzymes, Peptidases, Tubulin, Cytosolic Carboxypeptidase
Abstract
Cytosolic carboxypeptidase 5 (CCP5) is a member of a subfamily of enzymes that cleave C-terminal and/or side chain amino acids from tubulin. CCP5 was proposed to selectively cleave the branch point of glutamylated tubulin, based on studies involving overexpression of CCP5 in cell lines and detection of tubulin forms with antisera. In the present study, we examined the activity of purified CCP5 toward synthetic peptides as well as soluble α- and β-tubulin and paclitaxel-stabilized microtubules using a combination of antisera and mass spectrometry to detect the products. Mouse CCP5 removes multiple glutamate residues and the branch point glutamate from the side chains of porcine brain α- and β-tubulin. In addition, CCP5 excised C-terminal glutamates from detyrosinated α-tubulin. The enzyme also removed multiple glutamate residues from side chains and C termini of paclitaxel-stabilized microtubules. CCP5 both shortens and removes side chain glutamates from synthetic peptides corresponding to the C-terminal region of β3-tubulin, whereas cytosolic carboxypeptidase 1 shortens the side chain without cleaving the peptides' γ-linked residues. The rate of cleavage of α linkages by CCP5 is considerably slower than that of removal of a single γ-linked glutamate residue. Collectively, our data show that CCP5 functions as a dual-functional deglutamylase cleaving both α- and γ-linked glutamate from tubulin.
Introduction
Microtubules play essential roles in many cell processes, including cell division, growth, motility, polarization, ciliary function, axonal transport, and distribution of cellular organelles. Defects in microtubule function are associated with many neurodegenerative diseases, including Huntington disease (1–3), Alzheimer disease (4–6), Parkinson disease (7), amyotrophic lateral sclerosis, and hereditary spastic paraplegia (8). Tubulin modifications regulate the various properties of microtubules and are important for different cellular processes. The post-translational modifications of tubulin have been referred to as the “tubulin code,” by analogy to the histone code (9). In both codes, the system is composed of writers (protein-modifying enzymes), readers (interacting proteins), and erasers (enzymes that remove the modifications). Many tubulin-interacting proteins, including microtubule-associated proteins and molecular motors, recognize changes of tubulin modifications, resulting in modulations of binding affinities to microtubules (10, 11).
The tubulin code writers include tubulin tyrosine ligase (TTL)2 and a number of tubulin tyrosine ligase-like proteins (TTLLs) (12). TTL adds a Tyr to the C-terminal Glu of α-tubulin encoded by the α4 gene or formed from the other α isoforms by the action of a tubulin tyrosine carboxypeptidase (discussed below). Some of the TTLLs add Glu residues to the γ-carboxyl group of a glutamate located near the C terminus of α and/or β tubulin (13–15), whereas other TTLLs add a Gly residue to this glutamate residue (16–18). The addition of the first Glu or Gly is termed “initiation,” which some TTLLs perform. Other TTLLs build on the side chain to create forms of tubulin with multiple Glu or Gly residues, resulting in elongation.
The amino acids added by the TTLLs to the side chain, as well as residues on the C terminus of α-tubulin are removed by carboxypeptidases that have been identified recently. Abnormal processing of tubulin was found in the pcd (Purkinje cell degeneration) mouse (17, 19). The pcd mouse represents a spontaneous mutation that causes loss of Purkinje cells starting 3 weeks after birth (20). The pcd mutation was mapped to the Agtpbp1gene locus (21), which encodes the protein named Nna1 (22) and renamed cytosolic carboxypeptidase 1 (CCP1) due to its homology to members of the M14 metallocarboxypeptidase gene family (23, 24). Five additional genes encoding CCP1-like proteins were discovered, and the proteins were named CCP2–CCP6 (23, 24).
Recent studies have implicated several CCPs as tubulin-processing carboxypeptidases. CCP1, -4, -5, and -6 were suggested to remove Glu residues from tubulin, and CCP2 was proposed to remove C-terminal Tyr groups (17, 25, 26). Further, CCP5 was reported to selectively cleave the γ-linked glutamate at the branch point of the glutamylated side chain on tubulin (17, 25). These conclusions were largely based on overexpression and/or knockdown of the CCPs in cell lines and analysis of the tubulin forms using antisera. In studies involving overexpression or knockdown, it is possible that the observed changes are due to secondary effects and not a direct consequence of the elevated or reduced levels of a protein. Furthermore, antisera do not reveal the precise molecular changes, especially with complex mixtures, such as tubulin. Using mass spectrometry to characterize the specific cleavages, we recently reported that purified CCP1 cleaves Glu residues from the C terminus of α-tubulin and from the poly(E) side chains of α- and β-tubulin (19).
In the present study, we examined the activity of purified CCP5 with different types of substrates: soluble tubulin, paclitaxel-stabilized microtubules, and synthetic peptides corresponding to the C-terminal region of β-tubulin. A range of techniques were used to detect the products, including antisera-based methods and mass spectrometry. A direct comparison of CCP5 with CCP1 showed that CCP5 has a broad role as a “dual-functional” deglutamylase.
EXPERIMENTAL PROCEDURES
CCP1 and CCP5 Enzyme Purification
The full-length mouse CCP5-His6 was subcloned into EcoRI-NotI sites of pVL1393 (Pharmingen, BD Biosciences). CCP1-His6 subcloning was performed as described (19). CCP1 (human) and CCP5 constructs were expressed in Sf9 cells using Baculoplatinum DNA (Orbigen, San Diego, CA). Cells infected with CCP1, CCP5, or wild-type (WT) baculovirus were lysed in 50 mm phosphate buffer, pH 7.0, containing protease inhibitors (10 μm leupeptin, 100 μm 4-(2-aminoethyl) benzenesulfonyl fluoride, and 3 μm E64) and centrifuged at 20,000 rpm for 30 min, and then 0.5 m NaCl was added to soluble fractions. Protein mixtures were incubated with TALON metal affinity resin (Clontech) for 30 min at 4 °C and washed with 50 mm phosphate buffer, pH 7.0, containing 0.5 m NaCl, and protease inhibitors (10 μm leupeptin, 100 μm 4-(2-aminoethyl) benzenesulfonyl fluoride, and 3 μm E64). Resin with bound proteins was eluted from the column stepwise with 50 mm phosphate buffer containing 0.1 m NaCl, protease inhibitors, and 10, 20, and 80 mm imidazole. Only 80 mm imidazole fractions were used in all assays.
Western Blotting
After purification using a metal affinity column, Sf9 cell extracts and column eluates were supplemented with lysis buffer (50 mm Tris-HCl, pH 8.0, 120 mm NaCl, 0.5% Nonidet P-40) containing 1% SDS, fractionated on a denaturing polyacrylamide gel, transferred to nitrocellulose membranes, and then probed with tetra-His antibody (1:2000; Qiagen, Germantown, MD). Bands were visualized using IRDye-800-conjugated anti-mouse antibody (1:10,000; Rockland Immunochemicals Inc., Gilbertsville, PA). In order to investigate the purity of the eluates, proteins were separated in a denaturing polyacrylamide gel and then visualized with GelCode Blue reagent (Thermo Scientific, Rockford, IL).
Slot Blotting
For the characterization of CCP5 enzymatic activity, 2 μl of CCP5 or WT control TALON eluate were incubated with 1.25 μg of purified porcine brain tubulin (Cytoskeleton Inc., Denver, CO) in 80 mm PIPES, pH 7, at 37 °C for 1 h in a final volume of 25 μl. For some enzymatic reactions, samples were supplemented with 100, 200, or 500 mm NaCl or in PIPES buffer at pH 6, 7, or 8, as indicated. To investigate the effect of paclitaxel on CCP5 activity, purified porcine brain tubulin (50 μg/ml final) was preincubated with 5 μm paclitaxel (Sigma) for 15 min at 37 °C before the addition of WT or CCP5 eluate. After incubation with tubulin, aliquots were spotted onto a nitrocellulose membrane (Whatman, GE Healthcare) using a slot blot apparatus (HYBRI-SLOTTM Manifold 1052MM, Invitrogen). After drying, the membrane was probed with GT335 antibody (1:3000; Enzo Life Sciences, Farmingdale, NY), visualized using IRDye-800-conjugated anti-mouse antibody (1:10,000; Rockland Immunochemicals), and the band intensity was measured using the Odyssey Infrared Imaging System (LI-COR, Lincoln, NE). The membranes were reprobed with an antiserum to α-tubulin that does not discriminate between isoforms or C-terminal modifications (1:1000; clone DM1A, Sigma). Relative activity of CCP5 was calculated from the loss of GT335 signal and adjusted for the amount of total α-tubulin in the sample.
Tubulin and Peptide Mass Spectrometry
All procedures were performed as described (19). Briefly, 25 μl of the 80 mm imidazole WT, CCP1, and CCP5 eluates were incubated with 5 μg of purified porcine brain tubulin (final concentration 50 μg/ml) in 80 mm PIPES, pH 7, at 37 °C for 1 h. Proteins were resolved in a denaturing polyacrylamide gel, and the gels were stained with GelCode Blue stain reagent. Tubulin bands were excised from the gel, destained at 37 °C for 30 min in 50% acetonitrile in water, dried, and treated with CNBr (150 mg/ml in 70% formic acid) in the dark at room temperature for 3.5 h. The peptides remaining in the gel pieces were extracted twice by the addition of 50% acetonitrile and incubation at 37 °C for 30 min. The resulting solution was added to the original digest solution and dried in a vacuum centrifuge. The residue was resuspended in a small volume of deionized distilled water and dried again in the vacuum centrifuge. A total of three cycles of drying and resuspension were performed to remove CNBr and formic acid. The sample was fractioned by reverse phase capillary HPLC (Ultimate 3000, Dionex, CA) and mixed with α-cyano matrix solution (5 mg/ml α-cyano in 70% acetonitrile containing 0.1% TFA) automatically with a robot (Probot, Dionex, CA) onto a MALDI target. Mass spectrometry analysis was performed on an Applied Biosystems ABI 4800 Proteomics TOF/TOF analyzer (Foster City, CA) in both positive and negative ion modes.
For peptide mass spectrometry, a series of 24-residue-long peptides corresponding to the C-terminal region of β3-tubulin with one, two, three, and four Glu residues on the side chain were custom-synthesized (Peptide Institute Inc., Osaka, Japan). The peptide sequence is DATAEEEGEMYE(*)DDDEESEAQGPK (with the asterisk indicating the Glu modified with side chain glutamate(s)). Approximately 1 μm each peptide was incubated with 25 μl of the 80 mm imidazole WT, CCP1, or CCP5 eluates in 80 mm PIPES, pH 7, at 37 °C for either 10, 100, or 1000 min. Samples were desalted using a ZipTip pipette tip (Millipore, Milford, MA), mixed with α-cyano matrix (5 mg/ml α-cyano in 70% acetonitrile containing 0.1% TFA), and spotted onto a MALDI plate. Peptide masses were measured in negative mode MALDI-TOF/TOF MS on an Applied Biosystems 4800 instrument.
Microtubule Assay and Immunocytochemistry
MDA-MB-231 cells were a gift of Dr. Jonathan Backer (Albert Einstein College of Medicine). Microtubules from MDA-MB-231 cells were prepared as described (19). Briefly, cells were permeabilized on a plate with “optimum for microtubule preservation” (OPT) buffer (80 mm PIPES, 1 mm MgCl2, 0.5% Triton X-100, 10% glycerol, 5 μm paclitaxel, pH 6.8) at 37 °C for 10 min. In order to convert tyrosinated into detyrosinated tubulin, microtubules were incubated with 40 ng/ml carboxypeptidase A1 (Sigma) in OPT buffer at 37 °C for 15 min, washed with warm OPT buffer, and then treated at 37 °C for 2 h with purified CCP1, CCP5, or control eluate from WT virus-infected cells, each diluted in OPT buffer (1:10). Microtubules were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) at room temperature for 15 min, washed with PBS, blocked in 5% bovine serum albumin in PBS for 1 h, and then probed with anti-detyrosinated tubulin (1:300; Abcam, Cambridge, MA), anti-Δ2-tubulin (1:500; Abcam, Cambridge, MA), GT335 (1:500; Enzo Life Sciences, Farmingdale, NY), or poly(E) (1:1500; a gift from Dr. Martin Gorovsky, University of Rochester, Rochester, NY). After washing with PBS supplemented with 0.2% Tween 20, secondary Cy3-conjugated antibodies (Jackson Immunoresearch, West Grove, PA) were applied for 1 h, and microtubules were washed and mounted in ProLong Gold antifade reagent (Invitrogen).
RESULTS
Characterization of CCP5 Enzymatic Properties
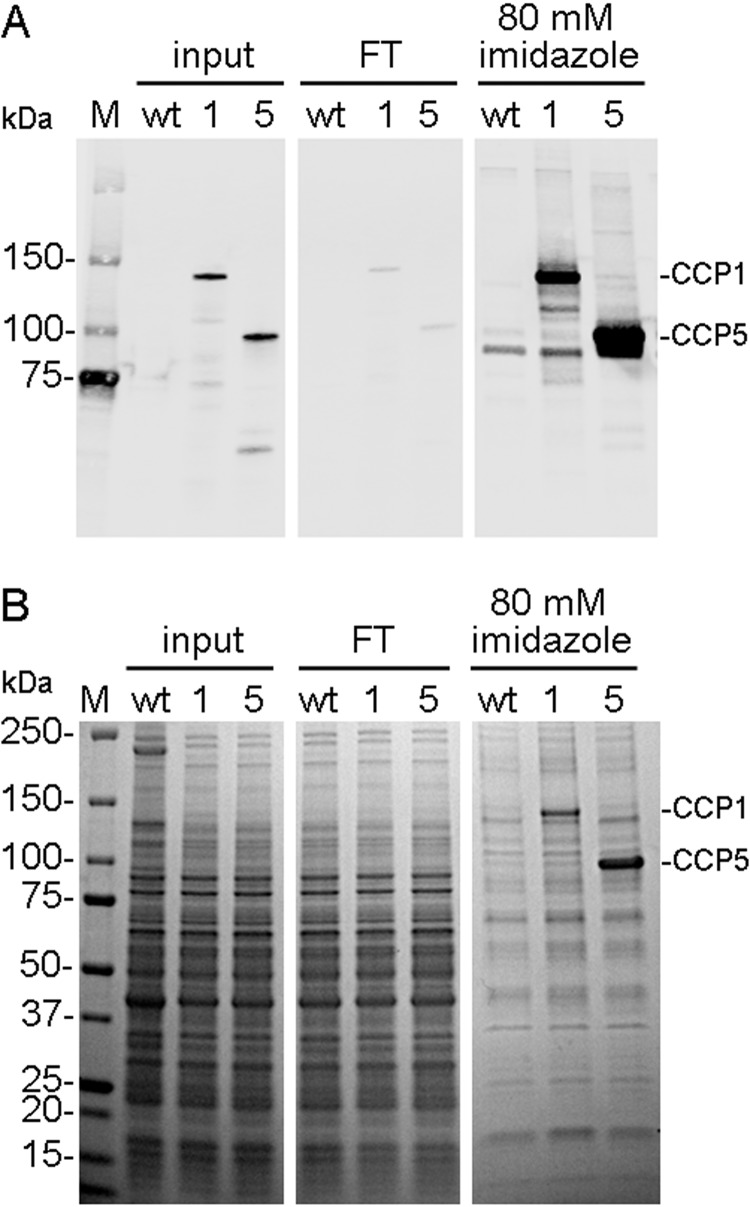
His6-tagged CCP5 was purified from baculovirus-infected Sf9 cells using a metal affinity column. Because CCP1 cleaves Glu residues from tubulin side chains and the C terminus of α-tubulin (19), we used CCP1 as a positive control in the present study in order to compare the specificity of the two enzymes. CCP1 and CCP5 proteins are enriched in the fractions containing 80 mm imidazole (Fig. 1A). Proteins corresponding to CCP1 and CCP5 are clearly visible on the Coomassie-stained protein gel, along with nonspecific proteins that are common to those detected when WT baculovirus-infected Sf9 cells were similarly fractionated on the metal affinity column (Fig. 1B). Because subsequent fractionation of the affinity-purified material on ion exchange columns provided further protein purification but eliminated enzymatic activity, we used the 80 mm imidazole eluates in our studies and compared the CCP-containing fraction with WT material to control for nonspecific proteins.
FIGURE 1.
Purification of recombinant CCP1 and CCP5. A, immunoblot analysis of metal affinity column fractions obtained following purification. The Sf9 insect cells were infected with WT baculovirus or with baculovirus expressing His-tagged CCP1 or His-tagged CCP5. Aliquots of cell extracts (input), non-bound fraction (flow-through; FT), and elute with 80 mm imidazole were separated by SDS-PAGE and probed with tetra-His antibody. B, protein staining of the samples used for the immunoblot. The expected molecular masses of CCP1 and CCP5 are 130 and 90 kDa, respectively. M, marker.
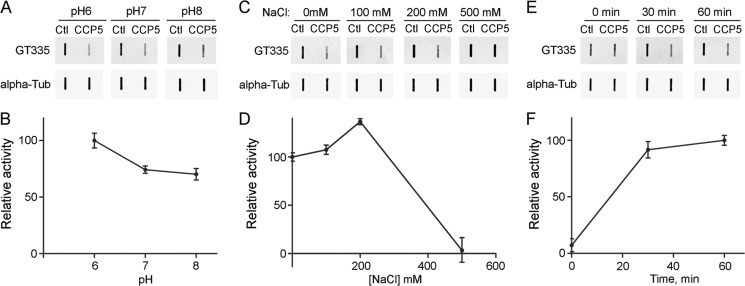
To determine the optimal conditions for CCP5 enzymatic activity, we incubated the enzyme with porcine brain tubulin at pH 6–8 and in the presence of various concentrations of NaCl. Deglutamylase activity was calculated from the loss of signal for the monoclonal antibody named GT335; this antibody recognizes the branch point Glu residue (13). The maximal activity of CCP5 toward branch point Glu was observed at pH 6 (Fig. 2, A and B), and in the presence of 200 mm NaCl (Fig. 2, C and D). To determine the time course of enzymatic reaction, CCP5 was incubated with brain tubulin at pH 7 for 0, 30, and 60 min, and the levels of GT335 were analyzed. CCP5 activity toward tubulin branch point Glu was generally similar at the 30 and 60 min time points (Fig. 2, E and F).
FIGURE 2.
Characterization of CCP5 enzymatic activity toward purified porcine brain tubulin. Purified brain tubulin was incubated with purified CCP5 or control eluates (Ctl) at 37 °C for 1 h at different conditions, slot-blotted into the nitrocellulose membrane, and then probed with GT335 and α-tubulin antibodies. A, C, and E, representative slot blots for GT335 and α-tubulin. B, D, and F, quantification of replicate slot blots. The GT335 band densities were normalized with the corresponding α-tubulin bands. CCP5 activity was measured as loss of GT335 signal. Error bars, S.E. (n = 3). A and B, effect of pH on CCP5 activity toward brain tubulin. C and D, effect of NaCl on CCP5 activity toward brain tubulin. E and F, reaction curve for CCP5 activity toward brain tubulin.
CCP5 Can Process Polymerized Tubulin and Remove Multiple Glutamates
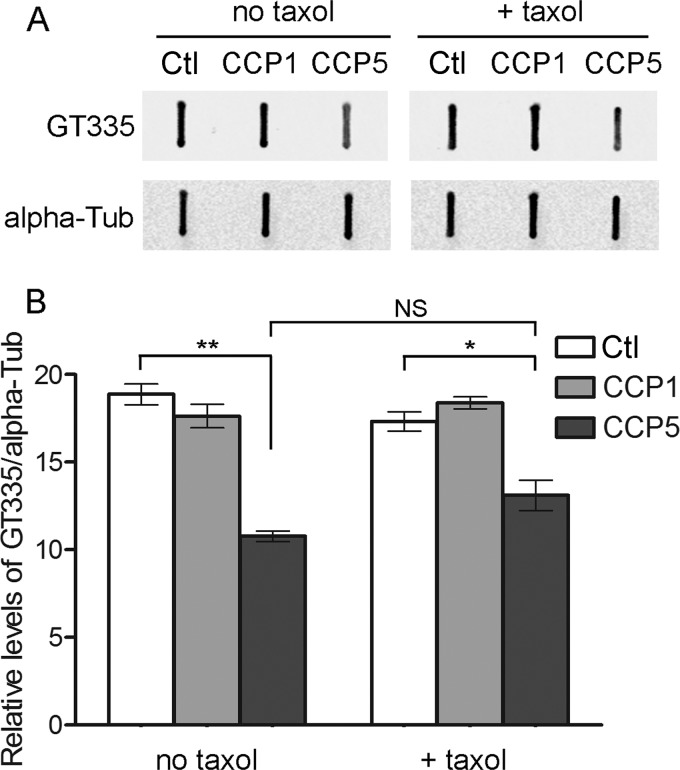
The studies described in Fig. 2 were performed with soluble tubulin at 50 μg/ml. Other tubulin-modifying enzymes show a preference for soluble or polymerized tubulin. For example, TTL specifically attaches tyrosine to tubulin and does not modify tubulin polymerized into microtubules (27). In contrast, glutamate ligases preferentially attach Glu to polymerized microtubules (14, 28). To test if CCP5 could remove the glutamate of the branch point from microtubules, we preincubated 50 μg/ml porcine brain tubulin in the presence or absence of paclitaxel, a microtubule-stabilizing drug (29, 30), and then added purified CCP5 and further incubated the samples. CCP1 and control eluate were also analyzed. Enzymatic activities were calculated from the loss of GT335 signal. CCP5 can process paclitaxel-treated tubulin, and the activity of CCP5 in the presence of paclitaxel is comparable with the activity in the absence of the drug (Fig. 3). CCP1 failed to cleave branched glutamate regardless of the addition of paclitaxel (Fig. 3).
FIGURE 3.
Effect of paclitaxel on CCP1 and CCP5 enzymatic activity toward purified porcine brain tubulin. Purified brain tubulin (50 μg/ml final concentration) was preincubated in the presence or absence of 5 μm paclitaxel (Taxol) for 15 min at 37 °C, and then CCP1, CCP5, or control eluate (Ctl) was added and incubated for a further 1 h at 37 °C. Samples were slot-blotted into nitrocellulose membrane and probed with GT335 antibody. A, representative slot blots. B, densitometric analysis of GT335. The GT335 band densities were normalized with the corresponding α-tubulin bands. Error bars, S.E. (n = 3). *, p ≤ 0.05; **, p ≤ 0.01 using Student's t test. NS, not significant.
We further tested CCP5 activity toward polymerized microtubules prepared from MDA-MB-231 cells grown on a coverslip. We also examined CCP5 activity against other modifications. Because MDA-MB-231 cells have very low levels of detyrosinated tubulin, we first treated microtubules with carboxypeptidase A1 to remove the C-terminal Tyr and form detyrosinated tubulin. After incubation with CCP5 or control eluate, the different forms of tubulin were analyzed by immunostaining. CCP5 eliminated GT335 signals (Fig. 4), consistent with the effect on soluble tubulin and on paclitaxel-treated tubulin. CCP5 diminished poly(E) signals on polymerized tubulin (Fig. 4). In addition, CCP5 produced a large decrease in the signal with the detyrosination-specific antiserum (Fig. 4). CCP5 also decreased levels of Δ2-tubulin (Fig. 4). The likely interpretation of these results is that CCP5 further cleaves polymerized Δ2-tubulin into Δ3-tubulin, indicating that the enzyme can remove branch point Glu as well as poly(E) from the tubulin side chain and C terminus.
FIGURE 4.
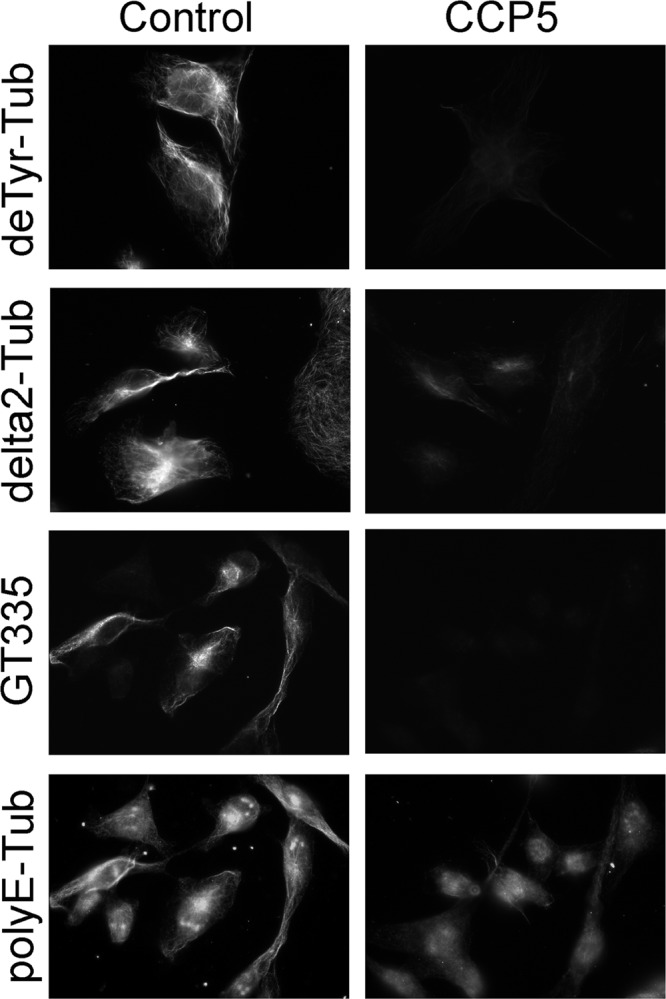
CCP5 enzymatic activity toward polymerized tubulin. Polymerized microtubules from MDA-MB-231 cells were prepared on plates using microtubule-stabilizing buffer containing 5 μm paclitaxel in the absence of GTP. For immunostaining with tyrosinated tubulin and Δ2-tubulin antibodies, microtubules were treated with carboxypeptidase A1 (40 ng/ml) to convert tyrosinated into detyrosinated tubulin. After washes, microtubules were incubated with CCP5 or control eluate for 2 h and then processed for immunocytochemistry.
CCP5 Processes Side Chains of α- and β-Tubulin and the C Terminus of α-Tubulin
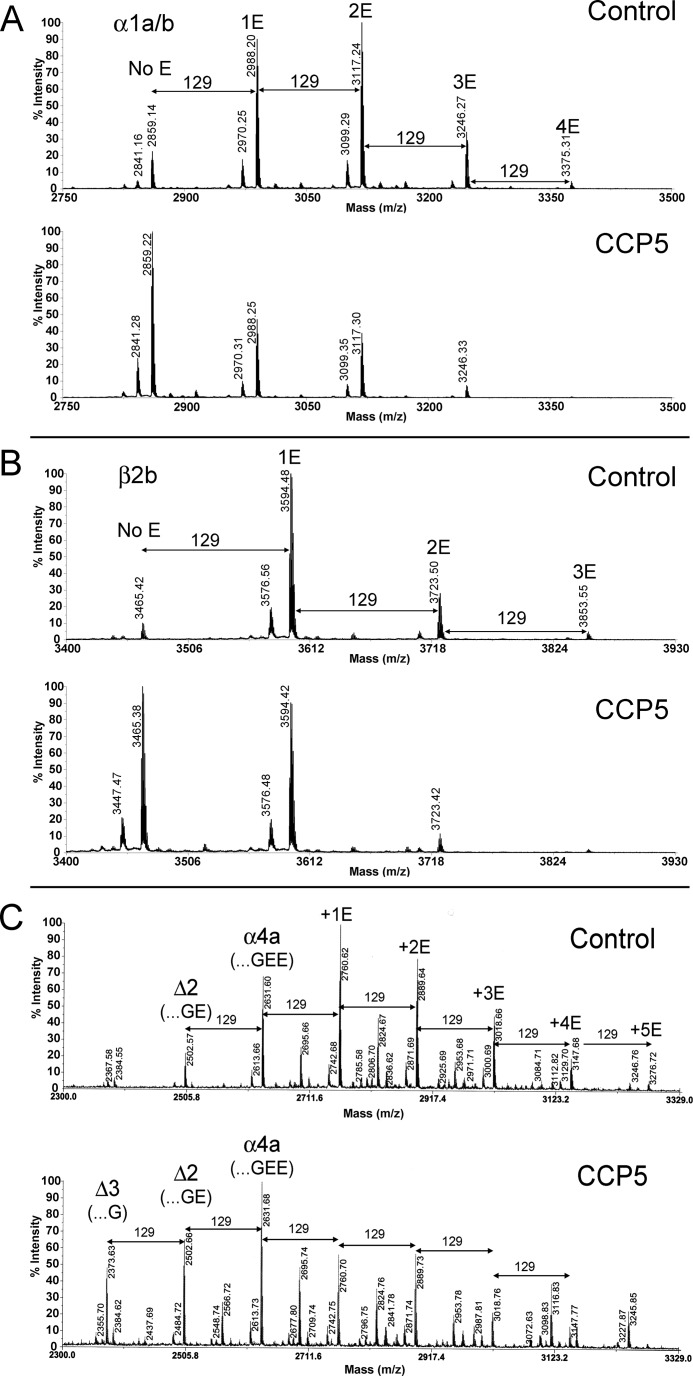
To further test if CCP5 cleaves both branch point Glu residues and α-linked Glu residues, we examined the reaction products of CCP5 and tubulin by mass spectrometry. For this, CCP5-treated tubulin was incubated with cyanogen bromide to cleave off the C-terminal region, and the products were analyzed by mass spectrometry. C-terminal peptides of both α- and β-tubulin were detected in our analysis.
The α1a/α1b/α3 tubulin isotype was detected with zero, one, two, three, and four Glu residues at the side chain (the peaks with m/z 2859.1, 2988.2, 3117.2, 3246.2, and 3375.3, respectively; Fig. 5A). These glutamylated forms of tubulin were observed in both control and CCP5-treated samples. Treatment of brain tubulin with CCP5 led to a decrease in the peak intensities of the multi- as well as monoglutamylated α-tubulin forms and an increase in the peak intensity of the form lacking all Glu residues on the side chain (m/z 2859.22; Fig. 5A). The same pattern of change was observed with the β2b form of tubulin; incubation of brain tubulin with CCP5 caused decreases of the peaks corresponding to multi- and monoglutamylated β2b-tubulin and a dramatic increase of the peak corresponding to β2b- tubulin without Glu on the side chain (m/z 3465.3; Fig. 5B). The form of the α4a tubulin isotype lacking C-terminal Tyr was detected and also showed a shift to lower numbers of Glu residues on the side chain after incubation with CCP5 (Fig. 5C). In addition, α4a tubulin lacking one C-terminal Glu residue and all side chain Glu residues (m/z 2502.6) was increased upon treatment with CCP5. A form of α4a-tubulin lacking two C-terminal Glu residues (m/z 2373.6) was dramatically elevated after CCP5 treatment (Fig. 5C). Taken together, these results indicate that CCP5 removes both α- and γ-linked glutamates from the side chains of α- and β-tubulin and cleaves C-terminal Glu residues from α-tubulin.
FIGURE 5.
Brain tubulin processing by purified CCP5. Purified porcine brain tubulin was incubated at 37 °C for 1 h with purified CCP5 or control eluates. After incubation, tubulin was separated by SDS-PAGE, dissected out from the gel, digested with CNBr, and analyzed by mass spectrometry. A, representative spectra of the C-terminal region of the α1a/α1b/α3 form of porcine α-tubulin after incubation with control eluate (top) or after incubation with CCP5 (bottom). The theoretical monoisotopic mass of the C-terminal fragment with no Glu side chain residues is 2859.19 (sequence AALEKDYEEVGVDSVEGEGEEEGEEY). The molecular mass of a Glu residue is 129 Da. B, representative spectra of the C-terminal region of β2b form of porcine β-tubulin after incubation with control eluate (top) or with CCP5 (bottom). The theoretical monoisotopic mass of the C-terminal fragment with no Glu side chain residues is 3465.36 (sequence NDLVSEYQQYQDATADEQGEFEEEEGEDEA). C, representative spectrum of the C-terminal region of α4a-tubulin after incubation with control eluate (top) or CCP5 (bottom). The theoretical monoisotopic mass of the C-terminal fragment with no Glu side chain residues is 2632.06 (sequence AALEKDYEEVGIDSYEDEDEGEE).
CCP5 Cleaves All Glu from Side Chains of Tubulin C-terminal Peptides
To confirm that CCP5 is a “dual-functional” enzyme, cleaving both α- and γ-linked glutamates, we used synthetic β3-tubulin-based 24-residue C-terminal peptides that had one, two, three, or four side chain Glu residues on a specified Glu residue. Synthetic peptides were incubated with purified CCP5, CCP1, or control eluate and analyzed by mass spectrometry. The same preparations of CCP1 and CCP5 were used for all of the assays so that relative differences between peptides could be compared. When tested with the peptide containing a single Glu side chain, CCP5 completely removed the Glu from this peptide after a 10-min incubation. In contrast, CCP1 was not able to produce deglutamylated peptide even after overnight incubation (Fig. 6, top panels). The peptide with two side chain Glu residues was reduced by ∼30% after overnight incubation with CCP5 as with CCP1. CCP5 produced only the peptide lacking all Glu side chain residues, whereas CCP1 generated exclusively the peptide containing a single Glu side chain (Fig. 6). Based on the efficient cleavage of the peptide with one Glu side chain by CCP5, it is likely that the conversion of the substrate with two Glu residues into the peptide without any residues occurs by sequential cleavage to produce the intermediate with one Glu, which is then rapidly converted into the product lacking Glu side chains.
FIGURE 6.
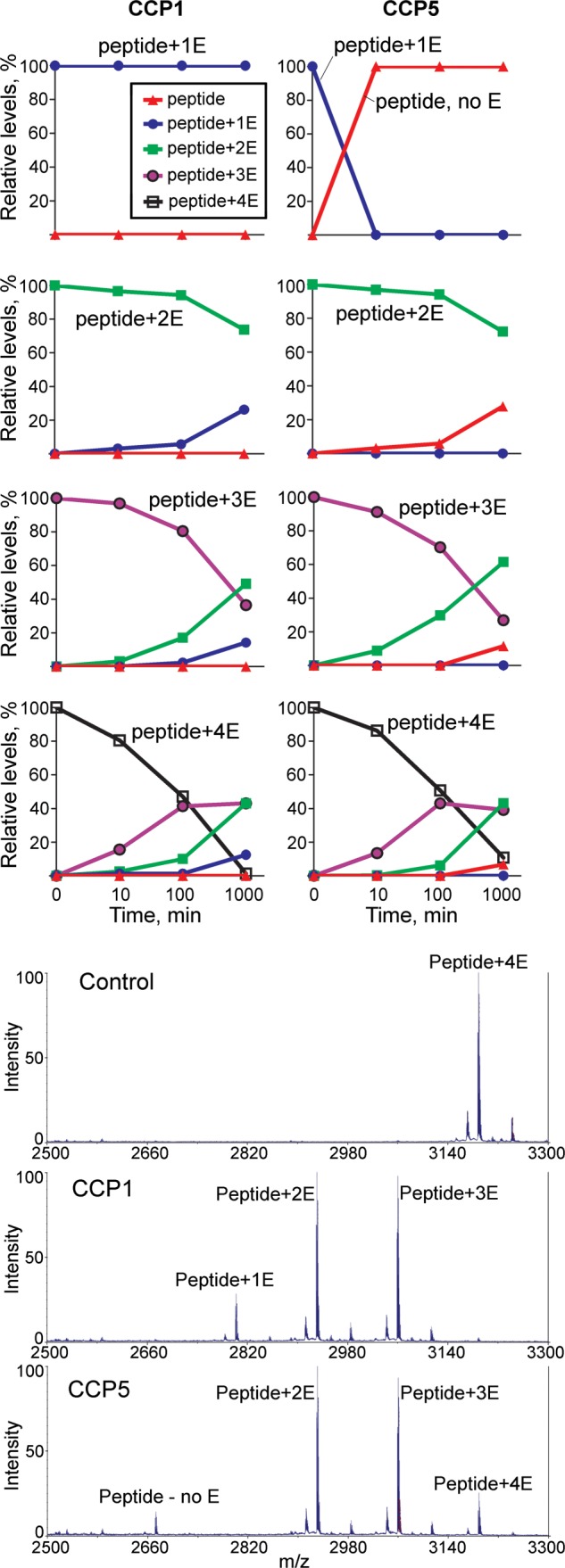
Time course of CCP1 and CCP5 activity toward β3-tubulin C-terminal peptides. Purified CCP1, CCP5, and control eluates were incubated with peptides corresponding to the C-terminal region of β3-tubulin and containing one, two, three, or four Glu residues at the side chain (sequence DATAEEEGEMYE*DDDEESEAQGPK, with the side chain glutamate(s) added to the E marked with an asterisk). After incubation for 10, 100, or 1000 min with 1 μm peptide, samples were desalted, mixed with matrix, and analyzed by MALDI MS. Top panels, peak heights representing different peptides within a spectrum were measured, and the relative abundance of each peptide was calculated as a percentage. Bottom panels, representative spectra for the peptide with four Glu residues incubated for 1000 min with control baculovirus, CCP1, or CCP5. Note that the CCP5 reaction shows more intact substrate (Peptide+4E) and also more of the fully processed product (Peptide, no E) as compared with the CCP1 reaction, indicating that CCP5 is less efficient than CCP1 at cleaving longer side chains but more active toward removing single Glu residues.
When tested with identical aliquots of the purified CCPs, the peptides with three and four Glu side chains were more efficiently processed than the peptide with only two Glu side chains (Fig. 6). Also, as found for the peptides with one and two Glu side chains, with CCP5, the product with no Glu residues is observed in place of the peptide with one Glu side chain, whereas the longer peptides are converted by CCP1 into shorter forms containing one or more Glu residues (Fig. 6). Incubation with control eluate produced no significant cleavages of any of the peptides.
DISCUSSION
The main finding of the present study is that CCP5 is a dual-functional enzyme that cleaves both α- and γ-linked Glu residues from the side chain of α- and β-tubulin and α-linked Glu from the C terminus of α-tubulin. Previous studies have reported that CCP5 is specific for cleaving branch point Glu from tubulin (17, 25). Although we also found that CCP5 could perform this step, the enzyme is not specific for this reaction and can also remove α-linked Glu, albeit at a reduced rate compared with the efficiency for removing branch point Glu. The previous studies relied on antisera to characterize the reaction products, in contrast to the mass spectrometric analysis performed in the present study. For this reason, the previous studies may have missed detecting the ability of CCP5 to cleave the longer poly(E) side chains of tubulin.
Results with antibodies can be difficult to interpret due to differences in reactivity of the various forms of tubulin with the antibody. For example, GT335, an antibody commonly used to detect γ-linked branch point Glu residues, shows a large variability in the signal strength depending on the number of side chain Glu residues (31). This antibody was raised against a peptide with a short γ-linked Glu side chain (13) and also recognizes longer Glu side chains. However, when tubulin containing long Glu side chains is digested with carboxypeptidase O, an enzyme that removes only α-linked Glu and not γ-linked Glu, the intensity of the tubulin signal on a Western blot probed with GT335 antibody shows an increase of ∼6-fold (31). Because of this variability, the time course of the incubation of purified CCP5 with brain tubulin (Fig. 2, E and F) is difficult to interpret. The decrease in GT335 signal after incubation for 30 min presumably reflects the removal of the short side chains of tubulin. The failure of the longer 60-min incubation to further decrease this signal may be due to the slow conversion of long side chains into shorter ones, which would cause an increase in GT335 signal that could offset the further activity toward the small side chains. An alternative explanation is that CCP5 is inactivated after 30 min of incubation with substrate, but this is not supported by the time course of CCP with the synthetic peptide substrates, which shows considerably more activity after 1000 min than after either 10 or 100 min (Fig. 6).
We also found that CCP5 functions on microtubules as well as soluble tubulin and can also remove Glu residues from the side chains of peptide substrates. This is in contrast to the various tubulin ligases. For example, TTL adds a Tyr to the C terminus of the detyrosinated form of soluble α-tubulin but does not function on microtubules (27). Initially, it was reported that TTL did not attach Tyr to synthetic peptides corresponding to the C-terminal region of α-tubulin (32), although a subsequent study found that TTL could perform this step, albeit at low efficiency (33). In contrast to TTL, the TTLLs that add Glu to tubulin more effectively function on tubulin polymerized into microtubules than on free tubulin (14, 28) or peptides (data not shown). The TTLLs also can be further differentiated by their preference for initiation versus elongation (15). The results from the present study as well as a recent study from our group also show that the CCPs have preferences for “undoing” initiation versus elongation, with CCP5 more efficient with single side chain Glu than 3–4 Glu on a side chain and CCP1 unable to remove a single side chain Glu from the test peptide. However, both CCP1 (19) and CCP5 (the present study) can perform both steps on soluble tubulin proteins, indicating greater redundancy within the CCP subfamily than within the TTLL subfamily.
Another finding of the present study is that purified CCP5 processes the C terminus of α-tubulin by removing all glutamates and generating the Δ3 form of α-tubulin. Recently, we found that CCP1 is also capable of producing the Δ3 form of tubulin (19). The biological function of the Δ3 form of tubulin is not known. A previous study investigating tubulin in mice deficient for PGs1, a subunit of α-tubulin-selective glutamyl ligase, found a form of tubulin that migrated on two-dimensional electrophoresis with an isoelectric point that was less acidic than the Δ2 form (10); this presumably corresponds to the Δ3 form. It is possible that the conversion of Δ2- to Δ3-tubulin has an effect on microtubule dynamics.
The finding that CCP1 and CCP5 are capable of removing side chain glutamates from a peptide corresponding to the C-terminal region of β3-tubulin is consistent with the activities of other members of the metallocarboxypeptidase family, which cleave both peptides and proteins. Recently, purified CCP1 was reported to release Glu from small peptides containing 2–3 Glu residues and a blocked N terminus (34). The structure of a bacterial CCP, as determined by x-ray crystallography, shows classical metallocarboxypeptidase-like motifs, including the presence of an active site and substrate-binding pockets generally similar to those of enzymatically active metallocarboxypeptidases, such as mammalian carboxypeptidases A and B (35). The purified CCP used for the crystallization studies was inactive toward all substrates that were tested, despite the presence of a functional active site pocket in the structure, possibly due to the presence of an N-terminal domain that occludes access of the substrate to the active site (35). The lack of enzymatic activity for the purified bacterial CCP is similar to the observation that mammalian CCPs lose activity upon purification to apparent homogeneity. For example, although the purified CCP5 used in the present study is the major protein band on polyacrylamide gels (Fig. 1), further fractionation on ion exchange columns that resulted in increased purity of the CCP5 protein led to the complete loss of activity toward either tubulin or peptide (data not shown). Similarly, we could not further purify CCP1 on ion exchange columns without complete loss of activity (19), and gel filtration of CCP1 does not result in homogenous protein (34). The CCPs are not inherently unstable and do not lose much activity after incubation for several h at 4 °C, the length of time required to run the ion exchange column. It is possible that a co-factor is present in the eluate from the metal affinity resin and subsequently lost upon further purification of the CCPs. Until this problem is solved, studies on partially purified CCPs, with appropriate controls (as done in the present study) represent the best approach to complement the previously used approaches of overexpression or knockdown of the CCPs in cell culture.
In comparing the activity of CCP5 toward peptides and proteins, there are some similarities and differences. With peptide substrates, CCP5 shows a marked preference for removing the γ-linked Glu of the side chain and more slowly cleaves longer side chains (Fig. 6). Incubation of purified CCP5 with peptides led to formation of products with zero, two, and three glutamates at the side chains. However, peptides with one glutamate were not observed. These results suggest that CCP5 prefers γ-linked Glu on peptides, and once the enzyme has access to the branch point residues, it cleaves them immediately. With tubulin, CCP5 also shows the ability to remove branch point Glu residues, but the difference is less striking. For example, both α-tubulin (1a/b isoform) and β-tubulin (2b isoform) show an increase in the forms lacking side chain Glu upon incubation with CCP5, but the forms containing a single Glu residue are still present (Fig. 5). It is possible that CCP5 prefers cleaving branch point Glu from peptide substrates rather than from tubulin. Alternatively, it is possible that differences in CCP5 function toward peptides and tubulin may be due to the tubulin isotypes; the peptide was based on the sequence of β3-tubulin, which was not detected in the analysis of the brain tubulin, and there are sequence differences between the various isotypes. In addition, the location of the poly(E) side chains can vary with each isotype, further complicating the comparison of peptide and tubulin. CCP1 also showed differences between cleavages of the peptide versus tubulin. With the tubulin-based peptide used in the present study, only long Glu side chains were processed by CCP1, and the γ-linked Glu was not removed even after overnight incubation (Fig. 6). In contrast, CCP1 was previously found to remove a single Glu from the side chain of α-1a/b and β-2b forms of tubulin (19). As with CCP5, the differences between peptide and tubulin forms may be due to sequence variation and/or the location of the Glu side chain. Further studies comparing a battery of different peptides will help address this issue.
Taken together with previous results, our studies show that CCP1 and CCP5 have overlapping but not identical substrate specificities. CCP4 and -6 have also been reported to cleave Glu from tubulin, and CCP2 has been reported to cleave C-terminal Tyr from α-tubulin, based on overexpression of the proteins in cell culture and analysis by Western blotting (17). Additional studies using purified enzymes and analysis using mass spectrometry are needed to confirm these previous results and learn precisely which steps each of the CCPs performs, as done for CCP5 in the present study. Collectively, these studies will help understand the processing of tubulin, which is important for normal cellular function. Altered tubulin polyglutamylation mislocalizes molecular motors, affects tubulin trafficking, impairs synaptic transmission, and causes neurodegeneration (9, 10, 19, 36). In their role as erasers of the tubulin code, the CCPs perform critical functions that are important for zebrafish development, as shown by Lyons et al. (37).
Acknowledgments
Antiserum to the polyglutamate form of tubulin was generously provided by Prof. Martin Gorovsky (University of Rochester, Rochester, NY). We thank Berta Burd for assistance with the cyanide bromide digestion of tubulin for mass spectrometry analysis.
This work was supported, in whole or in part, by National Institutes of Health Grants DA-004494 (to L. D. F.), 1P20 DA026149 (to R. H. A.), and 1R01 CA124898 (to S. B. Horwitz). This work was also supported by Grants-in-aid for Scientific Research on Innovative Areas 23117517 and 25113511 (K. I.).
- TTL
- tubulin tyrosine ligase
- TTLL
- tubulin tyrosine ligase-like protein
- CCP
- cytosolic carboxypeptidase
- OPT
- optimum for microtubule preservation.
REFERENCES
- 1. Gunawardena S., Her L. S., Brusch R. G., Laymon R. A., Niesman I. R., Gordesky-Gold B., Sintasath L., Bonini N. M., Goldstein L. S. (2003) Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron 40, 25–40 [DOI] [PubMed] [Google Scholar]
- 2. Her L. S., Goldstein L. S. (2008) Enhanced sensitivity of striatal neurons to axonal transport defects induced by mutant huntingtin. J. Neurosci. 28, 13662–13672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sinadinos C., Burbidge-King T., Soh D., Thompson L. M., Marsh J. L., Wyttenbach A., Mudher A. K. (2009) Live axonal transport disruption by mutant huntingtin fragments in Drosophila motor neuron axons. Neurobiol. Dis. 34, 389–395 [DOI] [PubMed] [Google Scholar]
- 4. Kanaan N. M., Morfini G. A., LaPointe N. E., Pigino G. F., Patterson K. R., Song Y., Andreadis A., Fu Y., Brady S. T., Binder L. I. (2011) Pathogenic forms of Tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J. Neurosci. 31, 9858–9868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peethumnongsin E., Yang L., Kallhoff-Muñoz V., Hu L., Takashima A., Pautler R. G., Zheng H. (2010) Convergence of presenilin- and Tau-mediated pathways on axonal trafficking and neuronal function. J. Neurosci. 30, 13409–13418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stokin G. B., Lillo C., Falzone T. L., Brusch R. G., Rockenstein E., Mount S. L., Raman R., Davies P., Masliah E., Williams D. S., Goldstein L. S. (2005) Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science 307, 1282–1288 [DOI] [PubMed] [Google Scholar]
- 7. Saha A. R., Hill J., Utton M. A., Asuni A. A., Ackerley S., Grierson A. J., Miller C. C., Davies A. M., Buchman V. L., Anderton B. H., Hanger D. P. (2004) Parkinson's disease α-synuclein mutations exhibit defective axonal transport in cultured neurons. J. Cell Sci. 117, 1017–1024 [DOI] [PubMed] [Google Scholar]
- 8. Perlson E., Maday S., Fu M. M., Moughamian A. J., Holzbaur E. L. (2010) Retrograde axonal transport. Pathways to cell death? Trends Neurosci. 33, 335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verdier-Pinard P., Pasquier E., Xiao H., Burd B., Villard C., Lafitte D., Miller L. M., Angeletti R. H., Horwitz S. B., Braguer D. (2009) Tubulin proteomics. Towards breaking the code. Anal. Biochem. 384, 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ikegami K., Heier R. L., Taruishi M., Takagi H., Mukai M., Shimma S., Taira S., Hatanaka K., Morone N., Yao I., Campbell P. K., Yuasa S., Janke C., Macgregor G. R., Setou M. (2007) Loss of α-tubulin polyglutamylation in ROSA22 mice is associated with abnormal targeting of KIF1A and modulated synaptic function. Proc. Natl. Acad. Sci. U.S.A. 104, 3213–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Konishi Y., Setou M. (2009) Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat. Neurosci. 12, 559–567 [DOI] [PubMed] [Google Scholar]
- 12. Janke C., Rogowski K., Wloga D., Regnard C., Kajava A. V., Strub J. M., Temurak N., van Dijk J., Boucher D., van Dorsselaer A., Suryavanshi S., Gaertig J., Eddé B. (2005) Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science 308, 1758–1762 [DOI] [PubMed] [Google Scholar]
- 13. Wolff A., de Néchaud B., Chillet D., Mazarguil H., Desbruyères E., Audebert S., Eddé B., Gros F., Denoulet P. (1992) Distribution of glutamylated α- and β-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur. J. Cell Biol. 59, 425–432 [PubMed] [Google Scholar]
- 14. Ikegami K., Mukai M., Tsuchida J., Heier R. L., Macgregor G. R., Setou M. (2006) TTLL7 is a mammalian β-tubulin polyglutamylase required for growth of MAP2-positive neurites. J. Biol. Chem. 281, 30707–30716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Dijk J., Rogowski K., Miro J., Lacroix B., Eddé B., Janke C. (2007) A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol. Cell 26, 437–448 [DOI] [PubMed] [Google Scholar]
- 16. Ikegami K., Setou M. (2009) TTLL10 can perform tubulin glycylation when co-expressed with TTLL8. FEBS Lett. 583, 1957–1963 [DOI] [PubMed] [Google Scholar]
- 17. Rogowski K., van Dijk J., Magiera M. M., Bosc C., Deloulme J. C., Bosson A., Peris L., Gold N. D., Lacroix B., Bosch Grau M., Bec N., Larroque C., Desagher S., Holzer M., Andrieux A., Moutin M. J., Janke C. (2010) A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell 143, 564–578 [DOI] [PubMed] [Google Scholar]
- 18. Wloga D., Webster D. M., Rogowski K., Bré M. H., Levilliers N., Jerka-Dziadosz M., Janke C., Dougan S. T., Gaertig J. (2009) TTLL3 is a tubulin glycine ligase that regulates the assembly of cilia. Dev. Cell 16, 867–876 [DOI] [PubMed] [Google Scholar]
- 19. Berezniuk I., Vu H. T., Lyons P. J., Sironi J. J., Xiao H., Burd B., Setou M., Angeletti R. H., Ikegami K., Fricker L. D. (2012) Cytosolic carboxypeptidase 1 is involved in processing α- and β-tubulin. J. Biol. Chem. 287, 6503–6517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mullen R. J., Eicher E. M., Sidman R. L. (1976) Purkinje cell degeneration, a new neurological mutation in the mouse. Proc. Natl. Acad. Sci. U.S.A. 73, 208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernandez-Gonzalez A., La Spada A. R., Treadaway J., Higdon J. C., Harris B. S., Sidman R. L., Morgan J. I., Zuo J. (2002) Purkinje cell degeneration (pcd) phenotypes caused by mutations in the axotomy-induced gene, Nna1. Science 295, 1904–1906 [DOI] [PubMed] [Google Scholar]
- 22. Harris A., Morgan J. I., Pecot M., Soumare A., Osborne A., Soares H. D. (2000) Regenerating motor neurons express Nna1, a novel ATP/GTP-binding protein related to zinc carboxypeptidases. Mol. Cell Neurosci. 16, 578–596 [DOI] [PubMed] [Google Scholar]
- 23. Kalinina E., Biswas R., Berezniuk I., Hermoso A., Aviles F. X., Fricker L. D. (2007) A novel subfamily of mouse cytosolic carboxypeptidases. FASEB J. 21, 836–850 [DOI] [PubMed] [Google Scholar]
- 24. Rodriguez de la Vega M., Sevilla R. G., Hermoso A., Lorenzo J., Tanco S., Diez A., Fricker L. D., Bautista J. M., Avilés F. X. (2007) Nna1-like proteins are active metallocarboxypeptidases of a new and diverse M14 subfamily. FASEB J. 21, 851–865 [DOI] [PubMed] [Google Scholar]
- 25. Kimura Y., Kurabe N., Ikegami K., Tsutsumi K., Konishi Y., Kaplan O. I., Kunitomo H., Iino Y., Blacque O. E., Setou M. (2010) Identification of tubulin deglutamylase among Caenorhabditis elegans and mammalian cytosolic carboxypeptidases (CCPs). J. Biol. Chem. 285, 22936–22941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sahab Z. J., Hall M. D., Me Sung Y., Dakshanamurthy S., Ji Y., Kumar D., Byers S. W. (2011) Tumor suppressor RARRES1 interacts with cytoplasmic carboxypeptidase AGBL2 to regulate the α-tubulin tyrosination cycle. Cancer Res. 71, 1219–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szyk A., Deaconescu A. M., Piszczek G., Roll-Mecak A. (2011) Tubulin tyrosine ligase structure reveals adaptation of an ancient fold to bind and modify tubulin. Nat. Struct. Mol. Biol. 18, 1250–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Regnard C., Audebert S., Desbruyères, Denoulet P., Eddé B. (1998) Tubulin polyglutamylase. Partial purification and enzymatic properties. Biochemistry 37, 8395–8404 [DOI] [PubMed] [Google Scholar]
- 29. Schiff P. B., Fant J., Horwitz S. B. (1979) Promotion of microtubule assembly in vitro by Taxol. Nature 277, 665–667 [DOI] [PubMed] [Google Scholar]
- 30. Schiff P. B., Horwitz S. B. (1980) Taxol stabilizes microtubules in mouse fibroblast cells. Proc. Natl. Acad. Sci. U.S.A. 77, 1561–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lyons P. J., Fricker L. D. (2011) Carboxypeptidase O is a glycosylphosphatidylinositol-anchored intestinal peptidase with acidic amino acid specificity. J. Biol. Chem. 286, 39023–39032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wehland J., Weber K. (1987) Tubulin-tyrosine ligase has a binding site on β-tubulin. A two-domain structure of the enzyme. J. Cell Biol. 104, 1059–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rudiger M., Wehland J., Weber K. (1994) Eur. J. Biochem. 220, 309–320 [DOI] [PubMed] [Google Scholar]
- 34. Wu H. Y., Wang T., Li L., Correia K., Morgan J. I. (2012) A structural and functional analysis of Nna1 in Purkinje cell degeneration (pcd) mice. FASEB J. 26, 4468–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Otero A., Rodríguez de la Vega M., Tanco S., Lorenzo J., Avilés F. X., Reverter D. (2012) The novel structure of a cytosolic M14 metallocarboxypeptidase (CCP) from Pseudomonas aeruginosa. A model for mammalian CCPs. FASEB J. 26, 3754–3764 [DOI] [PubMed] [Google Scholar]
- 36. Eschbach J., Dupuis L. (2011) Cytoplasmic dynein in neurodegeneration. Pharmacol. Ther. 130, 348–363 [DOI] [PubMed] [Google Scholar]
- 37. Lyons P. J., Sapio M. R., Fricker L. D. (2013) Cytosolic carboxypeptidase 5 removes α- and γ-linked glutamates from tubulin. J. Biol. Chem. 288, 30454–30462 [DOI] [PMC free article] [PubMed] [Google Scholar]