Background: ThiC is a radical S-adenosylmethionine (AdoMet) enzyme that synthesizes the thiamine pyrimidine 4-amino-5-hydroxymethyl-2-methylpyrimidine phosphate (HMP-P).
Results: An increase in the ThiC catalytic rate was detected when product 5′-deoxyadenosine was hydrolyzed. ThiC was inhibited by AdoMet metabolites.
Conclusion: ThiC is a multiple-turnover enzyme and is product-inhibited.
Significance: This is the first report of ThiC catalytic turnover and the identification of two AdoMet metabolites that inhibit ThiC activity.
Keywords: Enzyme Inhibitors, Iron-Sulfur Protein, Radicals, S-adenosylmethionine (AdoMet), Thiamine, Thiamine Biosynthesis
Abstract
ThiC (4-amino-5-hydroxymethyl-2-methylpyrimidine phosphate synthase; EC 4.1.99.17) is a radical S-adenosylmethionine (AdoMet) enzyme that uses a [4Fe-4S]+ cluster to reductively cleave AdoMet to methionine and a 5′-deoxyadenosyl radical that initiates catalysis. In plants and bacteria, ThiC converts the purine intermediate 5-aminoimidazole ribotide to 4-amino-5-hydroxymethyl-2-methylpyrimidine phosphate, an intermediate of thiamine pyrophosphate (coenzyme B1) biosynthesis. In this study, assay conditions were implemented that consistently generated 5-fold molar excess of HMP, demonstrating that ThiC undergoes multiple turnovers. ThiC activity was improved by in situ removal of product 5′-deoxyadenosine. The activity was inhibited by AdoMet metabolites S-adenosylhomocysteine, adenosine, 5′-deoxyadenosine, S-methyl-5′-thioadenosine, methionine, and homocysteine. Neither adenosine nor S-methyl-5′-thioadenosine had been shown to inhibit radical AdoMet enzymes, suggesting that ThiC is distinct from other family members. The parameters for improved ThiC activity and turnover described here will facilitate kinetic and mechanistic analyses of ThiC.
Introduction
ThiC (HMP-P2 synthase, EC 4.1.99.17) is a radical S-adenosylmethionine (AdoMet) enzyme that catalyzes the intramolecular rearrangement of 5-aminoimidazole ribotide (AIR) into HMP-P, carbon monoxide, and formate (Fig. 1) (1–4). HMP-P is condensed with 4-methyl-5-β-hydroxyethylthiazole phosphate to generate thiamine phosphate, which is further phosphorylated to the biologically active cofactor thiamine pyrophosphate (reviewed in Refs. 5, 6). Metabolically, AIR is at the branch point of purine and thiamine biosynthesis and has been known for decades to be the sole source of carbon for HMP-P (7–9). ThiC binds a [4Fe-4S]2+ cluster with a CX2CX4C motif (1), a unique variation on the canonical radical AdoMet superfamily motif CX3CX2C (10). Once reduced, the [4Fe-4S]+ cluster reductively cleaves AdoMet, producing methionine (Met) and a 5′-deoxyadenosyl radical that initiates catalysis. In vitro work by Chatterjee et al. (4) suggested that ThiC catalysis used two sequential hydrogen abstractions by the 5′-deoxyadenosyl radical, a mechanism that had not been reported.
FIGURE 1.
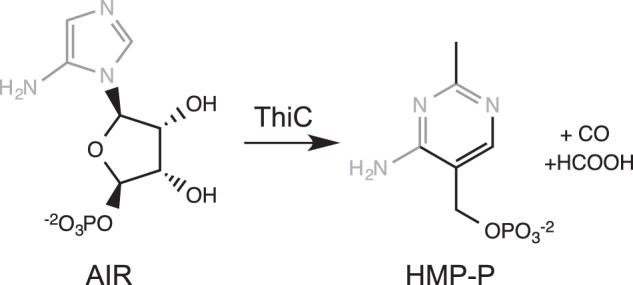
ThiC reaction.
Numerous radical AdoMet enzymes have been identified by bioinformatics analysis, and those that have been characterized carry out diverse reactions within metabolism, including nucleic acid modification and repair and synthesis of cofactors and antibiotics. Enzymes in the radical AdoMet superfamily can be divided into three classes (11–13). The first class uses AdoMet as a catalytic cofactor and includes spore photoproduct lyase and lysine 2,3-aminomutase (14, 15). The second class is made up of glycyl radical-activating enzymes that catalyze radical formation on glycines in other enzymes. This class includes pyruvate formate-lyase activating enzyme and ribonucleotide reductase-activating enzyme (16, 17). Enzymes in the third class use AdoMet as a substrate. The majority of radical AdoMet enzymes characterized to date fall into this class, including lipoyl synthase, tyrosine lyase, and biotin synthase (BioB) (18–20).
According to the literature, ThiC uses AdoMet as an oxidizing cosubstrate (1:1 stoichiometry) (4), making it a member of the third class described above. The activities of BioB, tyrosine lyase, and lipoyl synthase are inhibited by AdoMet cleavage products 5′-deoxyadenosine (5′-DOA) and Met (21, 22), whereas other enzymes in this class (including the maturase from Klebsiella pneumoniae AtsB and butirosin biosynthetic enzyme BtrN) are not product-inhibited (23, 24). S-methyl-5′-thioadenosine nucleosidase (MTAN, E.C. 3.2.2.9, 3.2.2.16) breaks down 5′-DOA to adenine and 5′-deoxyribose (25) and can improve activity when added to the assay mix of enzymes that are inhibited by 5′-DOA (21, 22).
This study was motivated by our interest in the complex metabolic context of the ThiC reaction in Salmonella enterica. In this organism, the conversion of AIR to HMP-P was decreased by perturbations in other metabolic processes, including the biosynthetic pathways for purines, Met, iron-sulfur clusters, and CoA (26–29). We sought to improve the in vitro assay for ThiC activity to allow us to obtain kinetic parameters that could help us rationalize the diverse metabolic connections identified in vivo. Here, we report assay conditions for the in vitro ThiC reaction that resulted in multiple turnovers and allowed the first kinetic measurements of this enzyme activity.
EXPERIMENTAL PROCEDURES
Media and Chemicals
Difco Luria Bertani (20 g/L) medium was used for routine Escherichia coli growth. For protein overexpression, Superbroth (tryptone (32 g/liter), yeast extract (20 g/liter), and NaCl (5 g/liter) with NaOH (0.05 N)) was used. Ampicillin and kanamycin were added to the medium as needed at 150 mg/liter and 50 mg/liter, respectively. Unless noted otherwise, all chemicals were purchased from Sigma-Aldrich, St. Louis, MO.
Protein Purification and ThiC Reconstitution
Proteins flavoprotein reductase (Fpr, E.C. 1.18.1.2), flavodoxin A (FldA), and TdPurE were expressed and purified as described previously (26, 30). TdPurE is Treponema denticola AIR carboxylase (E.C. 5.4.99.18) and was produced from pJK376 (a gift from J. Kappock). All ThiC purifications and manipulations were carried out in an anoxic glove box (Coy Laboratories, Grass Lake, MI) maintained at < 2 ppm O2. S. enterica His6-ThiC was produced from vector pET-28b(+) in a strain overexpressing Azotobacter vinelandii [Fe-S] cluster-loading genes from plasmid pDB1282 (31). ThiC was purified as described (26), except that the [4Fe-4S] cluster was reconstituted in vitro prior to freezing the protein at −80 °C. After purification, ThiC concentration was determined by Pierce 660 assay (Thermo Scientific, Rockford, IL) using BSA as the standard.
ThiC protein was reduced by adding a 50-fold excess of DTT in a vial that was then sealed and incubated on ice in the glove box overnight. A fresh stock solution of FeNH3SO4 (400 mm) was added in four aliquots to be 8-fold in excess of ThiC, and the vial was incubated at room temperature for 5 min. A fresh stock solution of Na2S (400 mm) was then added in four aliquots to reach an 8-fold excess over ThiC. Reduced ThiC was incubated for 1 h before desalting into freezing buffer (50 mm N-tris(hydroxymethyl)methyl-3-aminopropanesulfonic acid sodium-potassium salt, 3-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino}-1-propanesulfonic acid (TAPS) (pH 8.0), 0.2 m Na2SO4, 1.6 m glycerol) by a PD-10 Sephadex G-25 column (GE Healthcare Life Sciences, Piscataway, NJ). The desalted protein was concentrated in an Amicon 10,000 Da molecular weight cut off centrifugal filter unit (Millipore, Billerica, MA) at 2400 × g in sealed centrifuge tubes outside of the glove box. The protein concentration after reconstitution was 0.27 ± 0.03 mm, as determined by Bradford assay using purified ThiC with the concentration determined by amino acid analysis as a standard.
Iron Content Determination
The iron content of the ThiC protein was determined by a colorimetric assay using 3-(2-pyridyl)-5,6-bis(5-sulfo-2-furyl)-1,2,4-triazine disodium salt trihydrate adapted from Kennedy et al. (32). All reagents were prepared in double-distilled water and in new glassware or plasticware to prevent iron contamination. 25 μl of ThiC sample dilutions and iron standard solutions (Sigma) were mixed with 25 μl of HCl (0.12 N) in 1.5-ml microcentrifuge tubes and shaken gently. After incubation at 80 °C for 10 min, reagents were added to each tube sequentially with vortexing after each addition: 125 μl of ammonium acetate (0.96 m), 25 μl of ascorbic acid (0.2 m), 25 μl of sodium dodecyl sulfate (87 mm), and 25 μl of 3-(2-pyridyl)-5,6-bis(5-sulfo-2-furyl)-1,2,4-triazine disodium salt trihydrate (30 mm). The samples were then centrifuged for 5 min at 9000 × g, and the supernatant was analyzed for absorbance at 593 nm using a SpectraMax plate spectrophotometer (Molecular Devices, Sunnyvale, CA) because 3-(2-pyridyl)-5,6-bis(5-sulfo-2-furyl)-1,2,4-triazine disodium salt trihydrate absorbs at 593 nm when complexed to Fe2+. Iron content was 4.2 ± 0.7 mol iron/mol ThiC in the preparation used in the studies described herein.
Synthesis of CAIR, 4-Carboxyaminoimidazole Riboside, AIR, and Aminoimidazole Carboxamide Ribotide
CAIR and AIR were synthesized as described (26, 30, 33). The molar extinction coefficients ϵ250 = 10,980 M/cm and ϵ250 = 3,270 M/cm (34) were used to determine their respective concentrations. 4-carboxyaminoimidazole riboside and 5-aminoimidazole riboside were generated from stocks of CAIR and AIR (10–15 mm) treated with rAPid alkaline phosphatase (Roche) at 37° for 15 min. The alkaline phosphatase was then heat-inactivated by incubating at 80° for 3 min and cleared by centrifugation at 21,100 × g for 1 min. The supernatant was transferred to a new tube and degassed for 10 min before transfer to the glove box.
Purification of AdoMet from a Pharmaceutical Source
Commercial sources of AdoMet have been found to be as little as 43% biologically active S,S-AdoMet (22). We found previously that, of the compounds absorbing at 259 nm, SAMe (NatureMade, Mission Hills, CA) was ∼88% S,S-AdoMet by HPLC analysis (26). To further purify S,S-AdoMet, a SAMe pill was crushed, dissolved in double-distilled H2O, and filtered through a 0.22-μm Spin-X filter (Corning). The concentration of adenine compounds was determined using the extinction coefficient ϵ259 = 15,400 M/cm (35), and the concentration was adjusted to 100 mm in double-distilled H2O. 3-μl injections of the SAMe solution were separated by reverse phase-HPLC with a LC-20AT delivery system (Shimadzu, Kyoto, Japan) equipped with a 250 × 4.6 mm Luna C18 (2), 5-μm chromatography resolution column (Phenomenex, Torrance, CA). The column was equilibrated with 90% mobile phase A (13 mm TFA) and 10% mobile phase B (methanol). The separation used a flow rate of 1 ml/min with 90% A, 10% B for 10 min, followed by a linear gradient to 50% B over 20 min. Components eluted from the column were monitored with a SPD-M20A photodiode array detector (Shimadzu, wavelengths 190–350 nm) with data extracted at 259 nm. The 3.00- to 3.85-min fraction was collected using the FRC-10A fraction collector (Shimadzu) outfitted with a extruded polystyrene foam box filled with dry ice so the purified AdoMet was immediately frozen as it was collected in a 50-ml conical tube. The purified AdoMet was lyophilized and resuspended in double-distilled H2O sequentially three times to remove residual TFA. The purified AdoMet powder was resuspended in double-distilled H2O (∼22 mm), and samples were frozen at −20° until use. HPLC analysis determined that the purified AdoMet was 99% pure.
ThiC Activity Assays
Fpr, FldA, MTAN, AdoMet, and AIR were degassed with nitrogen for 10 min in 1.5-ml microcentrifuge tubes sealed with rubber stoppers prior to being placed in the glove box. Concentrations of AIR and AdoMet were determined with a Nanodrop spectrophotometer (Thermo Scientific) using the extinction coefficients listed above.
All components were resuspended in anoxic reaction buffer (50 mm TAPS (pH 8.0)). Each assay included ThiC (0.55 nmol monomer, 11 μm), MTAN (as indicated, 0.1 nmol), Fpr (0.5 nmol), and FldA (1 nmol). Under these conditions, HMP production was linear with respect to ThiC concentration, and MTAN, Fpr, and FldA were not rate-limiting. Reduced NADPH (0.8 mm) was added in excess, and the reaction mix was incubated for 10 min at room temperature before adding the substrate of interest. Substrates AdoMet (25–150 μm) and AIR (25–150 μm) were added to a final volume of 50 μl. The reactions were incubated at 37 °C in the anaerobic chamber for the specified time, stopped by heat treatment (65 °C for 3 min), and frozen at −20 °C if they were not analyzed immediately.
When included, inhibitors were preincubated with the ThiC reaction mixture for 10 min before the relevant substrates were added. Homocysteine, aminoimidazole carboxamide, Met, adenosine, and imidazole were brought into the glove box as powders and resuspended in anoxic reaction buffer. Adenosine was heated at 65 °C for 5 min to dissolve. All other potential inhibitors were made in reaction buffer, adjusted to pH 6–9, and degassed for 10 min prior to entering the glove box. In assays where we titrated specific inhibitors, the concentration of the inhibitor was determined after degassing using the relevant extinction coefficient. MTAN was not used in assays addressing inhibition.
HMP-P was dephosphorylated to HMP by alkaline phosphatase and quantified as described (26). In addition, samples were filtered through a 10,000- to 50,000-kDa cellulose membrane with an Amicon centrifugal filter (Millipore) to remove proteins prior to transferring the samples to autosampler vials (Macherey-Nagel, Düren, Germany).
Kinetic Data Analysis
Graphs were prepared, and data were analyzed using least squares analysis in Prism v. 6.0b (GraphPad Software Inc., La Jolla, CA). Kinetic constants are reported with the S.E. of the fit unless noted otherwise noted. For time course experiments, the data were fitted to a first-order kinetic equation, Equation 1, where [HMP] was the observed HMP produced (μm), [HMP]max was the predicted maximum HMP produced (μm), k was the observed first-order rate constant, and t was time in min.
The initial turnover number, kcat0, was determined by Equation 2 on the basis of the methods of Challand et al. (36).
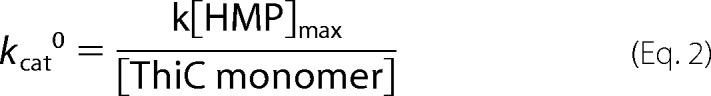 |
To determine the kinetics of ThiC inhibition, the initial velocity (v, nmol HMP/nmol ThiC/min) was estimated from reactions stopped after 20 min of incubation at 37 °C. The Km was determined from data titrating AdoMet (20–150 μm) and omitting MTAN. The data were fit to Equation 3.
 |
Data were first diagnosed as competitive, uncompetitive, or noncompetitive inhibition by their appearance when graphed as double reciprocal Lineweaver-Burk plots and fit by linear regression. The data were then analyzed according to the appropriate equation. For competitive inhibition, Equation 4 was used, where v is the velocity in nmol HMP/nmol ThiC/min, Vmax is the maximum velocity observed, KmObs is determined by the equation KmObs = Km(1 + [I]/Ki), and [S] is the concentration of substrate provided.
 |
For cooperative competitive inhibition by two different nonexclusive inhibitors, the data were fit to Equation 5 (37), where v is the velocity in nmol HMP/nmol ThiC/min, Vmax is the maximum velocity, [S] is the concentration of substrate, Ks is the Michaelis-Menten constant for the substrate, [I] is the concentration of one inhibitor and Ki is its inhibition constant, and [X] is the concentration of the second inhibitor and Kx is its inhibition constant, and α is the cooperativity factor.
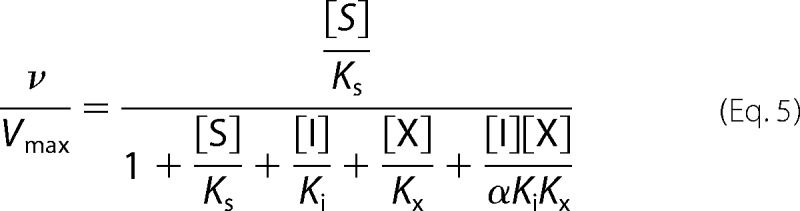 |
For uncompetitive inhibition, Equation 6 was used, where v is the velocity in nmol HMP/nmol ThiC/min, VmaxApp is the apparent maximum velocity, KmApp is the apparent Km, and the Ki′ inhibition constant is determined by the equations VmaxApp = Vmax/(1+[I]/(Ki′)) and KmApp = Km/(1 + [I]/(Ki′)). The Vmax for this dataset was determined by fitting the data for [Ado] = 0 μm to Equation 3.
 |
RESULTS AND DISCUSSION
ThiC Is a Multiple-turnover Enzyme
ThiC activity assays described elsewhere required high protein concentration and/or long incubations to quantify HMP-P production (1–4, 26). These conditions prevented mechanistic and kinetic analysis of ThiC. Changes were made to the assay protocol for ThiC to increase HMP production. The [4Fe-4S] cluster in ThiC was reconstituted in vitro, and pure sources of substrates AIR and AdoMet (99% pure) were used in the assay. With these modifications, ThiC produced 3.1 ± 0.1 nmol HMP/nmol ThiC monomer in 2 h, confirming that multiple turnovers were possible in vitro (Fig. 2). Under these conditions, steady-state turnover continued for 25 min. Data from technical duplicates were fit to the first-order kinetic equation (Equation 1) with a goodness of fit R2 of > 0.95. These results were then used in Equation 2 and yielded the following turnover number representing the mean ± S.E. of the constants determined by two independent experiments: kcat0 = 0.074 ± 0.014 min−1.
FIGURE 2.
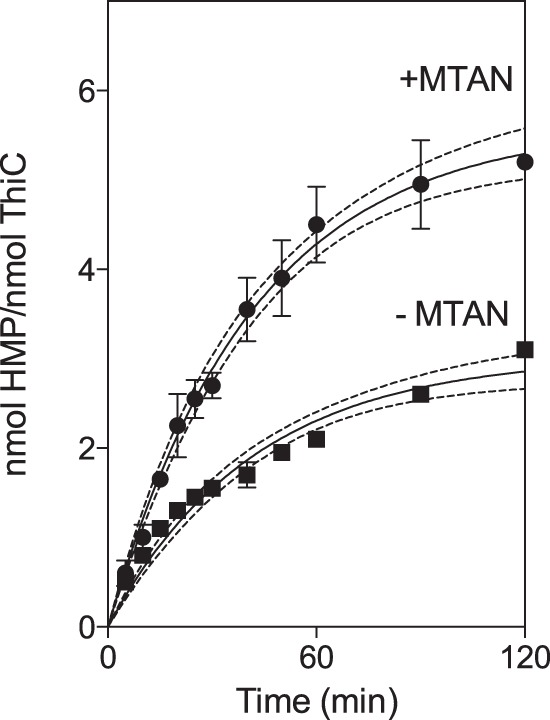
ThiC undergoes steady-state turnover. ThiC (0.55-nmol monomer) was incubated with flavoprotein reductase (0.5 nmol), flavodoxin A (1 nmol), NADPH (0.8 mm), AdoMet (100 μm), AIR (100 μm), and MTAN as indicated (0.1 nmol) at 37 °C. Each data point represents the mean ± S.D. of two replicates from a single experiment. The data were fit to a first-order rate equation, and the 95% confidence intervals of the regression analysis are represented by dotted lines.
The production of HMP was significantly enhanced by the addition of MTAN. When 0.1 nmol MTAN was included in the reaction mix, ThiC produced 5.2 ± 0.1 nmol HMP/nmol ThiC, and steady-state turnover continued for 1 h. The kinetic analysis of these data yielded the turnover number kcat0 = 0.14 ± 0.03 min−1. This value for kcat0 is within the range reported for other radical AdoMet enzymes in this class (22–24, 36, 38).
AdoMet-related Metabolites Inhibit the ThiC in Vitro Reaction
The finding that MTAN increased the reaction rate by ∼2-fold suggested that ThiC was inhibited by its product 5′-DOA. This conclusion was verified and extended by screening a number of potentially relevant metabolites for an effect on ThiC activity. Potential inhibitors tested included AdoMet-related metabolites, purines related to the substrate AIR, aminoimidazole carboxamide ribotide (AICAR)-related metabolites and CoA metabolites. The latter two represented metabolic pathways shown to impact the AIR to HMP-P conversion in vivo (28, 29).
Under the conditions tested, we saw no inhibition by purine biosynthetic intermediates related to AIR, including imidazole and the AIR riboside. These data support the conclusion that the in vivo findings reflect indirect metabolic effects of AICAR and CoA on the ThiC reaction. In contrast, several AdoMet-related metabolites inhibited ThiC, specifically 5′-DOA, Met, homocysteine, adenosine (Ado), S-adenosylhomocysteine (SAH), and S-methyl-5′-thioadenosine (MTA) (Fig. 3). Of these metabolites, 5′-DOA, Met, homocysteine, and SAH are known inhibitors of radical AdoMet enzymes (reviewed in Ref. 13). The data also showed that 5′-DOA acted additively with either Met or homocysteine to further inhibit ThiC activity.
FIGURE 3.
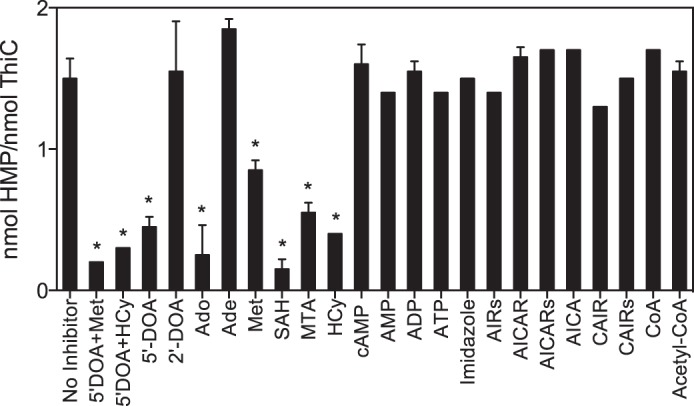
Metabolite inhibitors of ThiC activity. ThiC (0.55-nmol monomer) was preincubated with flavoprotein reductase (0.5 nmol), flavodoxin A (1 nmol), NADPH (0.8 mm), and potential inhibitor (0.5 mm) for 10 min at room temperature. Then AdoMet (100 μm) and AIR (100 μm) were added to initiate the reactions, which were incubated at 37 °C for 30 min. Data represent the mean ± S.D. of two replicates. The average is significantly different from the average with no inhibitor, as determined by an unpaired Student's t test (*, p < 0.05). 2′-DOA, 2′-deoxyadenosine; HCy, homocysteine; AIRs, 5-aminoimidazole riboside; AICARs, aminoimidazole carboxamide riboside; CAIRs, 5-amino-4-imidazolecarboxylic acid riboside.
S-Adenosylhomocysteine Inhibits ThiC Competitively with AdoMet
SAH has been reported to inhibit representatives of all three classes of radical AdoMet enzymes: lysine 2,3-aminomutase, ribonucleotide reductase-activating enzyme, BioB, and the nitrogenase cofactor biosynthetic enzyme NifB (22, 39–41). The mechanism of SAH inhibition of ThiC was investigated by adding SAH at different concentrations (0, 10, 25, and 50 μm) to reaction mixtures containing several concentrations of AdoMet (25–150 μm). The Lineweaver-Burk plot of these data showed that SAH inhibited ThiC competitively with AdoMet (Fig. 4). The Km of ThiC for AdoMet was determined by fitting data to Equation 3 from duplicate reactions of a titration of AdoMet (20–150 μm) carried out without MTAN. The data were fit with a global R2 value of 0.89, and the Km was 17 ± 3 μm. On the basis of the diagnosis of competitive inhibition, the data were fit to Equation 4 using the above Km with a global R2 value of 0.85 and generated the kinetic constant KiSAH = 5.6 ± 1.1 μm.
FIGURE 4.
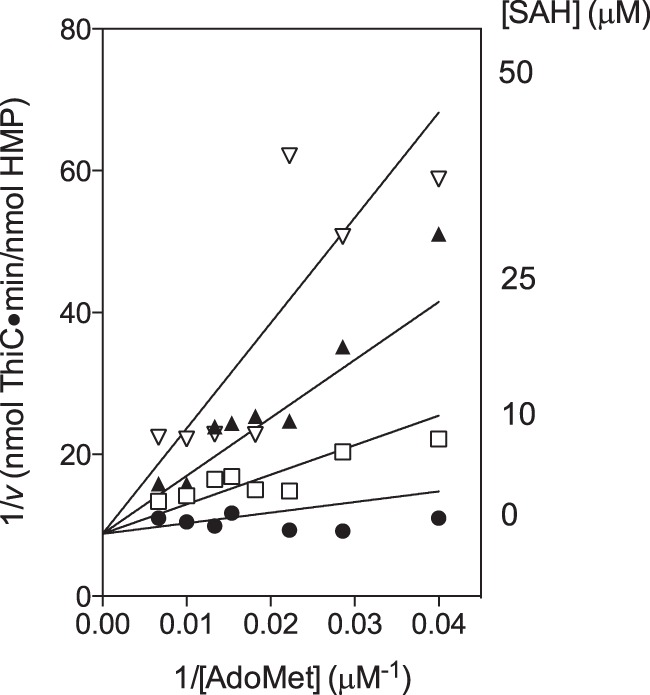
SAH inhibits ThiC competitively with respect to AdoMet. ThiC (0.55-nmol monomer) was preincubated with flavoprotein reductase (0.5 nmol), flavodoxin A (1 nmol), NADPH (0.8 mm), AIR (100 μm), and SAH (0, 10, 25, or 50 μm) for 10 min at room temperature. Then AdoMet (25–150 μm) was added to initiate the reactions, which were incubated at 37 °C for 20 min. The data were fit to Equation 4 by non-linear regression, constraining Km = 17 μm.
In the cell, SAH is produced by AdoMet methyltransferases and hydrolyzed by MTAN (42). SAH is present at ∼1 μm in wild-type E. coli and 50 μm in a mutant strain without MTAN (43). Together, these data suggest SAH could have a physiologically relevant role in regulating ThiC activity under conditions where MTAN activity is reduced.
5′-Deoxyadenosine and Methionine Cooperatively Inhibit ThiC
5′-DOA and Met were found to cooperatively inhibit BioB (22), and data from our inhibitor screen indicated that they also cooperatively inhibited ThiC. The reduction in activity by the addition of 5′-DOA and Met together (12% of activity with no inhibitor) was slightly greater than expected for linear combination of the inhibition caused by 5′-DOA (31%) or Met (55%) when either was the sole addition. To investigate the kinetics of this inhibition, several concentrations of 5′-DOA (0–500 μm) and Met (0–1000 μm) were added to ThiC reactions with AIR and AdoMet fixed at 100 μm (Fig. 5). Dixon replots of 1/v versus [5′-DOA] or [Met] intersected, confirming that 5′-DOA and Met were not mutually exclusive (37). 5′-DOA and Met were assumed to inhibit competitively with respect to AdoMet. The least squares analysis was constrained to [S] = 100 μm and Km = 17 μm and the data fit Equation 5 with a global R2 value of 0.94 and yielded Ki5′−DOA = 12 ± 2 μm, KiMet = 82 ± 13 μm, and α = 0.4 ± 0.1.
FIGURE 5.
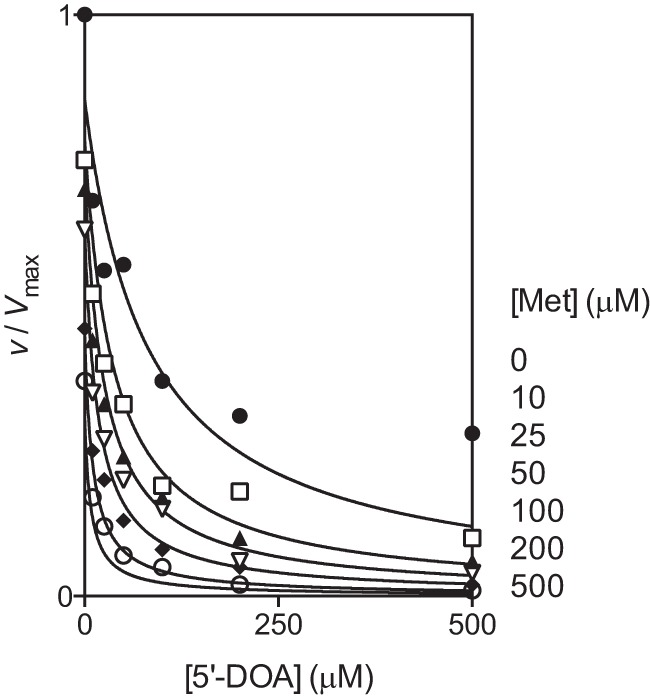
Cooperative inhibition by 5′-DOA and Met. ThiC (0.55-nmol monomer) was preincubated with flavoprotein reductase (0.4 nmol), flavodoxin A (1 nmol), NADPH (0.8 mm), 5′-DOA (0–500 μm), and Met (0–1000 μm) for 10 min at room temperature. Then AdoMet (100 μm) and AIR (100 μm) were added to initiate the reactions, which were incubated at 37 °C for 20 min. The data were fit to Equation 5 by non-linear regression, constraining Km = 17 μm and [AdoMet] = 100 μm.
Under normal metabolic conditions, product inhibition would be expected to be minimal. Met concentrations are estimated at 150–300 μm (43, 44), and MTAN is present to rapidly hydrolyze low levels of 5′-DOA produced. However, these constants suggest that product inhibition could be significant in in vitro assays, including those reported here. For example, after 2 h of incubation, product accumulation coupled with substrate depletion would cause ThiC to be 60% or 35% maximal activity with or without MTAN, respectively. These findings suggest that long incubation times will not allow accurate kinetic measurements of ThiC.
Adenosine Displays Uncompetitive Inhibition with AdoMet
If adenosine bound the site occupied by the adenosine moiety of AdoMet, adenosine should also inhibit ThiC competitively with respect to AdoMet. Adenosine was added at several concentrations (0, 100, 250, and 400 μm) to reactions containing several AdoMet concentrations (25–150 μm). Unexpectedly, the data with and without adenosine resulted in parallel lines in the Lineweaver-Burk plot (Fig. 6A), suggesting that adenosine was uncompetitive with AdoMet and bound the ThiC-AdoMet complex. To determine Vmax, the [Ado] = 0 μm data were fit to Equation 3 with an R2 value of 0.92 to yield Vmax = 0.1128 ± 0.0034 nmol HMP/nmol ThiC/min. The full dataset was fit to Equation 6, constraining the Km = 17 μm and Vmax = 0.1128 nmol HMP/nmol ThiC/min. The data fit Equation 6 with a global R2 value of 0.91 and produced the kinetic constant Ki'Ado = 99 ± 3 μm. However, the uncertainty in the inhibition constant is likely considerably higher. We found that Ki'Ado values of 55–140 μm were consistent with the data. Replots of the data from the reciprocal Lineweaver-Burk plot were also linear, confirming the diagnosis of uncompetitive inhibition (37). Experiments addressing adenosine inhibition with respect to AIR showed that adenosine is not competitive with AIR, which is consistent with the fact that AMP does not inhibit. The data did not distinguish between uncompetitive and noncompetitive inhibition (data not shown).
FIGURE 6.
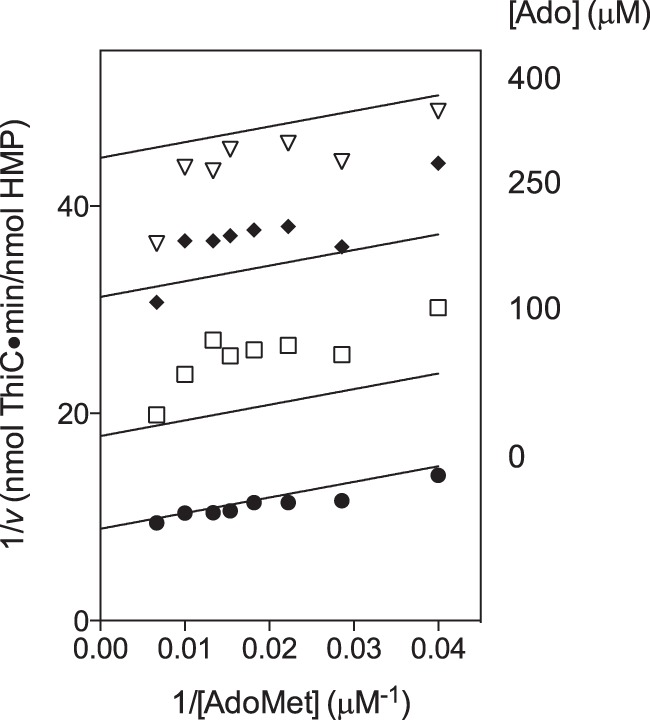
Adenosine is uncompetitive with AdoMet inhibiting ThiC. A, ThiC (0.55-nmol monomer) was preincubated with flavoprotein reductase (0.5 nmol), flavodoxin A (1 nmol), NADPH (0.8 mm), AIR (100 μm), and adenosine (0, 100, 250, or 400 μm) for 10 min at room temperature. Then AdoMet (AdoMet) (25–150 μm) was added to initiate the reactions, which were incubated at 37 °C for 20 min. The data were fit to Equation 6 by non-linear regression, constraining Km = 17 μm and Vmax = 0.1128 nmol HMP/nmol ThiC/min.
The adenosine concentration in E. coli was estimated at 0.13 μm (44), suggesting that adenosine inhibition is not physiologically relevant. However, direct inhibition of ThiC may be significant under conditions of increased adenosine levels, such as with AICAR accumulation (45) or when adenosine is present in the growth medium (46).
Conclusions
ThiC is the HMP-P synthase required for thiamine biosynthesis in bacteria and plants and is a member of the radical AdoMet superfamily of enzymes. Of numerous radical AdoMet enzymes predicted by bioinformatic analyses, relatively few have been characterized, and fewer still have been shown to turnover catalytically in vitro (10, 38, 47). The data presented here demonstrate that when product inhibition is relieved, ThiC undergoes steady-state turnover for up to 1 h.
To our knowledge, there are no other reports of radical AdoMet enzymes inhibited by adenosine or MTA, suggesting that this may be a unique property of ThiC. Although not many enzymes have been tested, BioB was not inhibited by adenosine or MTA (22), and MTA was reported to have no effect on lysine 2,3-aminomutase activity (39). Thus, ThiC has a distinct inhibitor profile in addition to its variant cysteine motif and proposed novel catalytic mechanism. The characterization of ThiC activity presented here, in particular achieving catalytic turnover in vitro, will contribute to future mechanistic studies of ThiC and further our understanding of the radical AdoMet enzyme superfamily.
Acknowledgments
We thank Jorge C. Escalante-Semerena for critical reading of the manuscript and George H. Reed and Michael G. Thomas for helpful discussions. We also thank T. Joseph Kappock for pJK376 expressing TdPurE, Jannell V. Bazurto for providing MTAN, and Mackenzie J. Parker and JoAnne Stubbe for authentic HMP used for quantification.
This work was supported, in whole or in part, by National Institutes of Health Grant GM47296 (to D. M. D.). This work was also supported by National Science Foundation Graduate Research Fellowship grant DGE-0718123 (to L. D. P.).
- HMP-P
- 4-amino-5-hydroxymethyl-2-methylpyrimidine phosphate
- AdoMet
- S-adenosylmethionine
- AIR
- 5-aminoimidazole ribotide
- BioB
- biotin synthase
- 5′-DOA
- 5′-deoxyadenosine
- MTAN
- S-methyl-5′-thioadenosine nucleosidase
- TAPS
- 3-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino}-1-propanesulfonic acid
- CAIR
- 5-Amino-4-imidazolecarboxylic acid ribotide
- HMP
- 4-amino-5-hydroxymethyl-2-methylpyrimidine
- AICAR
- aminoimidazole carboxamide ribotide
- Ado
- adenosine
- SAH
- S-adenosylhomocysteine
- MTA
- S-methyl-5′-thioadenosine.
REFERENCES
- 1. Martinez-Gomez N. C., Downs D. M. (2008) ThiC is an [Fe-S] cluster protein that requires AdoMet to generate the 4-amino-5-hydroxymethyl-2-methylpyrimidine moiety in thiamin synthesis. Biochemistry 47, 9054–9056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martinez-Gomez N. C., Poyner R. R., Mansoorabadi S. O., Reed G. H., Downs D. M. (2009) Reaction of AdoMet with ThiC generates a backbone free radical. Biochemistry 48, 217–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chatterjee A., Li Y., Zhang Y., Grove T. L., Lee M., Krebs C., Booker S. J., Begley T. P., Ealick S. E. (2008) Reconstitution of ThiC in thiamine pyrimidine biosynthesis expands the radical SAM superfamily. Nat. Chem. Biol. 4, 758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chatterjee A., Hazra A. B., Abdelwahed S., Hilmey D. G., Begley T. P. (2010) A “radical dance” in thiamin biosynthesis. Mechanistic analysis of the bacterial hydroxymethylpyrimidine phosphate synthase. Angew. Chem. Int. Ed. Engl. 49, 8653–8656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Begley T. P., Downs D. M., Ealick S. E., McLafferty F. W., Van Loon A. P., Taylor S., Campobasso N., Chiu H. J., Kinsland C., Reddick J. J., Xi J. (1999) Thiamin biosynthesis in prokaryotes. Arch. Microbiol. 171, 293–300 [DOI] [PubMed] [Google Scholar]
- 6. Begley T. P., Chatterjee A., Hanes J. W., Hazra A., Ealick S. E. (2008) Cofactor biosynthesis. Still yielding fascinating new biological chemistry. Curr. Opin. Chem. Biol. 12, 118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Estramareix B., David S. (1990) Conversion of 5-aminoimidazole ribotide to the pyrimidine of thiamin in enterobacteria. Study of the pathway with specifically labeled samples of riboside. Biochim. Biophys. Acta 1035, 154–160 [DOI] [PubMed] [Google Scholar]
- 8. Estramareix B., Lesieur M. (1969) Biosynthesis of the pyrimidine portion of thiamine. Source of carbons 2 and 4 in Salmonella typhimurium. Biochim. Biophys. Acta 192, 375–377 [PubMed] [Google Scholar]
- 9. Estramareix B., Therisod M. (1984) Biosynthesis of thiamine. 5-aminoimidazole ribotide as the precursor of all the carbon-atoms of the pyrimidine moiety. J. Am. Chem. Soc. 106, 3857–3860 [Google Scholar]
- 10. Sofia H. J., Chen G., Hetzler B. G., Reyes-Spindola J. F., Miller N. E. (2001) Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: Functional characterization using new analysis and information visualization methods. Nucleic Acids Res. 29, 1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frey P. A., Booker S. J. (1999) in Advances in Free Radical Chemistry (Zard S. Z. ed) pp. 1–43, JAI Press Inc., Stamford, CT [Google Scholar]
- 12. Booker S. J. (2009) Anaerobic functionalization of unactivated C-H bonds. Curr. Opin. Chem. Biol. 13, 58–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hiscox M. J., Driesener R. C., Roach P. L. (2012) Enzyme catalyzed formation of radicals from S-adenosylmethionine and inhibition of enzyme activity by the cleavage products. Biochim. Biophys. Acta 1824, 1165–1177 [DOI] [PubMed] [Google Scholar]
- 14. Cheek J., Broderick J. B. (2002) Direct H atom abstraction from spore photoproduct C-6 initiates DNA repair in the reaction catalyzed by spore photoproduct lyase. Evidence for a reversibly generated adenosyl radical intermediate. J. Am. Chem. Soc. 124, 2860–2861 [DOI] [PubMed] [Google Scholar]
- 15. Moss M. L., Frey P. A. (1990) Activation of lysine 2,3-aminomutase by S-adenosylmethionine. J. Biol. Chem. 265, 18112–18115 [PubMed] [Google Scholar]
- 16. Wagner A. F., Frey M., Neugebauer F. A., Schäfer W., Knappe J. (1992) The free radical in pyruvate formate-lyase is located on glycine-734. Proc. Natl. Acad. Sci. U.S.A. 89, 996–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamarit J., Mulliez E., Meier C., Trautwein A., Fontecave M. (1999) The anaerobic ribonucleotide reductase from Escherichia coli. The small protein is an activating enzyme containing a [4Fe-4S](2+) center. J. Biol. Chem. 274, 31291–31296 [DOI] [PubMed] [Google Scholar]
- 18. Miller J. R., Busby R. W., Jordan S. W., Cheek J., Henshaw T. F., Ashley G. W., Broderick J. B., Cronan J. E., Jr., Marletta M. A. (2000) Escherichia coli LipA is a lipoyl synthase. In vitro biosynthesis of lipoylated pyruvate dehydrogenase complex from octanoyl-acyl carrier protein. Biochemistry 39, 15166–15178 [DOI] [PubMed] [Google Scholar]
- 19. Kriek M., Martins F., Leonardi R., Fairhurst S. A., Lowe D. J., Roach P. L. (2007) Thiazole synthase from Escherichia coli. An investigation of the substrates and purified proteins required for activity in vitro. J. Biol. Chem. 282, 17413–17423 [DOI] [PubMed] [Google Scholar]
- 20. Ollagnier-de-Choudens S., Mulliez E., Fontecave M. (2002) The PLP-dependent biotin synthase from Escherichia coli. Mechanistic studies. FEBS Lett. 532, 465–468 [DOI] [PubMed] [Google Scholar]
- 21. Challand M. R., Ziegert T., Douglas P., Wood R. J., Kriek M., Shaw N. M., Roach P. L. (2009) Product inhibition in the radical S-adenosylmethionine family. FEBS Lett. 583, 1358–1362 [DOI] [PubMed] [Google Scholar]
- 22. Farrar C. E., Siu K. K., Howell P. L., Jarrett J. T. (2010) Biotin synthase exhibits burst kinetics and multiple turnovers in the absence of inhibition by products and product-related biomolecules. Biochemistry 49, 9985–9996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grove T. L., Lee K. H., St Clair J., Krebs C., Booker S. J. (2008) In vitro characterization of AtsB, a radical SAM formylglycine-generating enzyme that contains three [4Fe-4S] clusters. Biochemistry 47, 7523–7538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yokoyama K., Numakura M., Kudo F., Ohmori D., Eguchi T. (2007) Characterization and mechanistic study of a radical SAM dehydrogenase in the biosynthesis of butirosin. J. Am. Chem. Soc. 129, 15147–15155 [DOI] [PubMed] [Google Scholar]
- 25. Choi-Rhee E., Cronan J. E. (2005) A nucleosidase required for in vivo function of the S-adenosyl-l-methionine radical enzyme, biotin synthase. Chem. Biol. 12, 589–593 [DOI] [PubMed] [Google Scholar]
- 26. Palmer L. D., Dougherty M. J., Downs D. M. (2012) Analysis of ThiC variants in the context of the metabolic network of Salmonella enterica. J. Bacteriol. 194, 6088–6095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dougherty M. J., Downs D. M. (2006) A connection between iron-sulfur cluster metabolism and the biosynthesis of 4-amino-5-hydroxymethyl-2-methylpyrimidine pyrophosphate in Salmonella enterica. Microbiology 152, 2345–2353 [DOI] [PubMed] [Google Scholar]
- 28. Allen S., Zilles J. L., Downs D. M. (2002) Metabolic flux in both the purine mononucleotide and histidine biosynthetic pathways can influence synthesis of the hydroxymethyl pyrimidine moiety of thiamine in Salmonella enterica. J. Bacteriol. 184, 6130–6137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frodyma M., Rubio A., Downs D. M. (2000) Reduced flux through the purine biosynthetic pathway results in an increased requirement for coenzyme A in thiamine synthesis in Salmonella enterica serovar typhimurium. J. Bacteriol. 182, 236–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tranchimand S., Starks C. M., Mathews, Hockings S. C., Kappock T. J. (2011) Treponema denticola PurE is a bacterial AIR carboxylase. Biochemistry 50, 4623–4637 [DOI] [PubMed] [Google Scholar]
- 31. Cicchillo R. M., Lee K. H., Baleanu-Gogonea C., Nesbitt N. M., Krebs C., Booker S. J. (2004) Escherichia coli lipoyl synthase binds two distinct [4Fe-4S] clusters per polypeptide. Biochemistry 43, 11770–11781 [DOI] [PubMed] [Google Scholar]
- 32. Kennedy M. C., Kent T. A., Emptage M., Merkle H., Beinert H., Münck E. (1984) Evidence for the formation of a linear [3Fe-4S] cluster in partially unfolded aconitase. J. Biol. Chem. 259, 14463–14471 [PubMed] [Google Scholar]
- 33. Mehl R. A., Begley T. P. (2002) Synthesis of P-32-labeled intermediates on the purine biosynthetic pathway. J. Labelled Comp. Radiopharm. 45, 1097–1102 [Google Scholar]
- 34. Meyer E., Leonard N. J., Bhat B., Stubbe J., Smith J. M. (1992) Purification and characterization of the purE, purK, and purC gene-products. Identification of a previously unrecognized energy requirement in the purine biosynthetic-pathway. Biochemistry 31, 5022–5032 [DOI] [PubMed] [Google Scholar]
- 35. Bock R. M., Ling N. S., Morell S. A., Lipton S. H. (1956) Ultraviolet absorption spectra of adenosine-5′-triphosphate and related 5′-ribonucleotides. Arch. Biochem. Biophys. 62, 253–264 [DOI] [PubMed] [Google Scholar]
- 36. Challand M. R., Martins F. T., Roach P. L. (2010) Catalytic activity of the anaerobic tyrosine lyase required for thiamine biosynthesis in Escherichia coli. J. Biol. Chem. 285, 5240–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Segel I. H. (1975) Enzyme Kinetics, John Wiley & Sons, Inc., New York [Google Scholar]
- 38. Roach P. L. (2011) Radicals from S-adenosylmethionine and their application to biosynthesis. Curr. Opin. Chem. Biol. 15, 267–275 [DOI] [PubMed] [Google Scholar]
- 39. Chirpich T. P., Zappia V., Costilow R. N., Barker H. A. (1970) Lysine 2,3-aminomutase. Purification and properties of a pyridoxal phosphate and S-adenosylmethionine-activated enzyme. J. Biol. Chem. 245, 1778–1789 [PubMed] [Google Scholar]
- 40. Harder J., Eliasson R., Pontis E., Ballinger M. D., Reichard P. (1992) Activation of the anaerobic ribonucleotide reductase from Escherichia coli by S-adenosylmethionine. J. Biol. Chem. 267, 25548–25552 [PubMed] [Google Scholar]
- 41. Curatti L., Ludden P. W., Rubio L. M. (2006) NifB-dependent in vitro synthesis of the iron-molybdenum cofactor of nitrogenase. Proc. Natl. Acad. Sci. U.S.A. 103, 5297–5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walker R. D., Duerre J. A. (1975) S-adenosylhomocysteine metabolism in various species. Can. J. Biochem. 53, 312–319 [DOI] [PubMed] [Google Scholar]
- 43. Halliday N. M., Hardie K. R., Williams P., Winzer K., Barrett D. A. (2010) Quantitative liquid chromatography-tandem mass spectrometry profiling of activated methyl cycle metabolites involved in LuxS-dependent quorum sensing in Escherichia coli. Anal. Biochem. 403, 20–29 [DOI] [PubMed] [Google Scholar]
- 44. Bennett B. D., Kimball E. H., Gao M., Osterhout R., Van Dien S. J., Rabinowitz J. D. (2009) Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat. Chem. Biol. 5, 593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuramitsu H. K., Udaka S., Moyed H. S. (1964) Induction of inosine 5′-phosphate dehydrogenase and xanthosine 5′-phosphate aminase by ribosyl-4-amino-5-imidazolecarboxamide in purine-requiring mutants of Escherichia coli B. J. Biol. Chem. 239, 3425–3430 [PubMed] [Google Scholar]
- 46. Moyed H. S. (1964) Inhibition of the biosynthesis of the pyrimidine portion of thiamine by adenosine. J. Bacteriol. 88, 1024–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shisler K. A., Broderick J. B. (2012) Emerging themes in radical SAM chemistry. Curr. Opin. Struct. Biol. 22, 701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]


