Background: PulD forms the dodecameric outer membrane portal in the type II secretion system and can self-assemble.
Results: Assembly is a multistep process initiated and modulated by protein contact with the membrane.
Conclusion: A prepore precedes formation of the native structure.
Significance: Understanding membrane protein biogenesis aids in the design of novel proteins/functions and drugs.
Keywords: Kinetics, Membrane Proteins, Phospholipid Vesicle, Protein Self-assembly, Protein Synthesis, Secretin, Type II Secretion
Abstract
Investigations into protein folding are largely dominated by studies on monomeric proteins. However, the transmembrane domain of an important group of membrane proteins is only formed upon multimerization. Here, we use in vitro translation-coupled folding and insertion into artificial liposomes to investigate kinetic steps in the assembly of one such protein, the outer membrane secretin PulD of the bacterial type II secretion system. Analysis of the folding kinetics, measured by the acquisition of distinct determinants of the native state, provides unprecedented evidence for a sequential multistep process initiated by membrane-driven oligomerization. The effects of varying the lipid composition of the liposomes indicate that PulD first forms a “prepore” structure that attains the native state via a conformational switch.
Introduction
Membrane protein folding is a rapidly expanding field of research in which experiment and computation on model systems deliver important insights into how membrane proteins are stabilized and interact with lipids (1–8) and how they assemble in vivo (9–11). In many such analyses, the protein under study is monomeric or is maintained in a monomeric form, often to reduce the complexity of the system (8, 12–14). However, membrane proteins are often oligomeric (15), and in some cases, oligomerization establishes the transmembrane topology. Typical examples of the latter are voltage-gated channels (16) and bacterial secretins (17) as well as soluble proteins such as bacterial toxins, viroporins, and membrane attack complex/perforin-like proteins, which interact with membranes to form pores (18–20). Investigations into the determinants and mechanisms of membrane protein oligomerization (21–23), therefore, probe an important feature of the assembly landscape.
Studies using monomeric model systems often require extensive optimization to prevent protein aggregation (24–26), a process likely to have an even greater negative impact on the folding of complex multimeric and/or multidomain membrane proteins. One way to overcome this problem is to couple protein production and folding in an in vitro translation system. Because of the vectorial nature of translation, independent domains can begin folding before it is complete, providing a means to reduce aggregation. Several membrane proteins reach their native state in such in vitro systems (27), including secretins from type II secretion systems (28, 29). Such tractability could allow a detailed analysis of the assembly of multimeric membrane proteins, particularly if ways can be found to trap assembly intermediates.
Here, we establish a detailed folding mechanism of the Klebsiella oxytoca outer membrane (OM)2 secretin PulD, a dodecameric, transmembrane, channel-forming protein. PulD is a prototype of the secretin protein family of large multidomain proteins that multimerize to form a portal for enzyme and virulence factor secretion and for the assembly of surface appendages called pili (30). PulD is targeted to the OM by its dedicated chaperone PulS, and its membrane insertion is independent of the β-barrel assembly machinery that facilitates the assembly of OM β-barrel proteins (31, 32). In the absence of PulS, PulD folds to its native pore but inserts into the inner membrane, inducing a phage shock response (32). Therefore, two competing pathways exist in the cell: one driven by PulS-mediated PulD trafficking to the OM and one leading to autoassembly into the inner membrane. A fragment of the PulD polypeptide primarily comprising the transmembrane domain and the last of three N-terminal periplasmic repeats (N3) (PulD28–42/259–660 (28)) can assemble spontaneously into artificial liposomes, indicating that the majority of the periplasmic part of the polypeptide (N0-N1–2) is not required for assembly (28). Two further characteristics render PulD28–42/259–660 well suited for folding and assembly studies. The full-length protein is prone to degradation, and the yield of full-length PulD (PulDfl) multimers is lower than that of PulD28–42/259–660 (33, 34). Here we investigate the second, cytotoxic autoassembly pathway that probably reflects faithfully the events that occur at the inner membrane. We demonstrate that folding and assembly of PulD28–42/259–660 is a sequential process that depends on the association of monomers on a membrane surface and that folding to the PulD native state requires a fluid membrane. We further characterize a membrane-associated intermediate state and speculate that a conformational switch occurs at the same time as native pore formation.
EXPERIMENTAL PROCEDURES
Liposome Preparation
Appropriate amounts of lecithin (Sigma), 1,2-dilauroyl-sn-glycero-3-phosphocholine, 1,2-dimiristoyl-sn-glycero-3-phosphocholine, diC16:0PC, or 1,2-distearoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids) in solvent (as supplied) were dried under a gentle stream of nitrogen, followed by evaporation of residual chloroform under a vacuum. Dried lipids were hydrated to 20–200 mg/ml (as appropriate), vortexed, and sonicated for 15 min in a water bath.
PulD Synthesis
PulD was synthesized by in vitro translation using an RTS100 Escherichia coli kit (5 Prime) as described (28) in the presence of 10 ng DNA (pCHAP3731 (PulDfl) or pCHAP3716 (PulD28–42/259–660)) and 2 μg of liposomes (soy bean l-α-lecithin (Sigma), 1,2-dilauroyl-sn-glycero-3-phosphocholine, 1,2-dimiristoyl-sn-glycero-3-phosphocholine, diC16:0PC, or 1,2-distearoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids)) or 1 ng detergent (Zwittergent 3-14)/μl RTS100 at 30 °C unless stated otherwise. Although the RTS100 kits were centrifuged at 100,000 × g for 30 min before use to remove most of the E. coli membranes, the trace amounts that remain are sufficient to allow limited PulD assembly (28, 35). Synthesis was arrested with 3 ng streptomycin/μl of reaction after 6 min for initial multimerization determinations, after 10 min in all other kinetic experiments, or after 6 h in all other experiments. Synthesis reactions that were arrested after 6 or 10 min were further incubated for 6 h at 30 °C for complete assembly to occur. Mixed multimers were produced by priming with appropriate ratios of pCHAP3731 and pCHAP3716. Monomeric and multimeric PulD were separated in SDS on a 10% polyacrylamide (37.5:1 acrylamide/bisacrylamide) gel without heating to 100 °C, transferred to nitrocellulose, and analyzed by immunoblotting with an antibody raised against native PulD multimers. Low solubility of the multimers at longer time scales is probably due to clustering of PulD in the membrane. This phenomenon results in occasional smearing on SDS-PAGE. Bands corresponding to multimeric and monomeric PulD were analyzed by densitometry. Resulting transients were fitted to a single or double exponential equation using Kaleidagraph 4.0.
Folding Kinetics followed by SDS Treatment
Folding transients of PulD28–42/259–660 were obtained by mixing aliquots of the synthesis reaction at the time points indicated with SDS-PAGE loading buffer (4% SDS, 62.5 mm Tris (pH 6.8), 20% glycerol) in a 1:1 ratio to arrest PulD folding and incubated on ice for 1 h before analysis by SDS-PAGE.
Folding Kinetics followed by Urea Treatment
PulD28–42/259–660 aliquots were mixed with 8 m urea (in 50 mm sodium phosphate buffer (pH 8.0), and 250 mm NaCl) to a final urea concentration of 7.2 m at the times indicated and incubated for 1 h on ice before mixing with SDS loading buffer for analysis.
Folding Kinetics followed by Limited Proteolysis by Trypsin Digestion
Trypsin was added to PulD28–42/259–660 aliquots at the indicated times to a final concentration of 4 μg/μl and incubated on ice for 5 min. Reactions were blocked using 150 ng/μl Pefabloc (Interchim) before mixing with SDS loading buffer.
Temperature Jump Experiments
Rescue of PulD28–42/259–660 assembly in diC16:0PC liposomes was achieved by initiating synthesis at 30 °C for 10 min and then increasing the temperature to 42 °C after the addition of streptomycin. Folding transients were obtained as above by removing aliquots at increasing time points before trypsin treatment and/or mixing with SDS loading buffer and incubation on ice for analysis.
Flotation of Liposome-associated PulD on a Discontinuous Sucrose Gradient
Liposomes containing PulD28–42/259–660 were collected by centrifugation (100,000 × g, 30 min), resuspended in 60% sucrose, and overlaid with 40, 20, and 0% sucrose. Liposomes were floated by centrifugation for 90 min at 100,000 × g. Aliquots were taken from the top of the tube and analyzed by gel electrophoresis followed by immunoblotting.
1-Azidopyrene Labeling
PulD28–42/259–660 synthesized in the presence of lecithin or diC16:0PC-liposomes and pelleted (13,000 × g, 1 h) was washed in 50 mm sodium phosphate buffer (pH 8.0) containing 2 m urea, collected by centrifugation (13,000 × g, 1 h), and washed in the same buffer without urea. Sedimented liposomes were resuspended in 100 μl of the same buffer containing 250 mm NaCl and 1 mm 1-azidopyrene (from a 200 mm stock solution in dimethyl sulfoxide, Hangzhou Sage Chemical Co., Hangzhou, China). Samples were shaken for 1 h followed by UV irradiation for 15 min (312 nm), centrifugation (13,000 × g, 1 h), resuspension in SDS loading buffer, and electrophoresis in SDS on a 4–15% polyacrylamide gel (Bio-Rad). Protein bands were visualized under UV light (312 nm) and by immunoblotting. Labeling controls were performed following the same procedure, but without additional washes, using the soluble proteins MalE (synthesized for 6 h by in vitro translation in the presence of liposomes) and BSA (1 μg pure BSA (Interchim) added to liposomes without synthesis reaction but with 6 h incubation) (data not shown). Gels (discontinuous 4/10% polyacrylamide) were visualized under UV light and stained with Oriole fluorescent gel stain (Bio-Rad) to reveal all proteins.
Glutaraldehyde Cross-linking and Blue Native PAGE
PulD28–42/259–660 synthesized in the presence of diC16:0PC liposomes was collected by ultracentrifugation (100,000 × g, 30 min). The pellet was resuspended in an equal volume of phosphate buffer (above) containing 250 mm NaCl and 100 mm glutaraldehyde and incubated at room temperature for 25 min before analysis by SDS-PAGE and immunoblotting.
For blue native PAGE (Invitrogen), lecithin or diC16:0PC liposome-associated PulD28–42/259–660 was solubilized in 1% digitonin (in phosphate buffer containing 250 mm NaCl) for 2 h at 4 °C. Insoluble material was removed by ultracentrifugation (100,000 × g, 1 h), and supernatants were analyzed by electrophoresis according to the instructions of the manufacturer, followed by immunoblotting on PVDF membranes (Millipore).
Circular Dichroism
Reaction mixtures containing PulD28–42/259–660 were centrifuged at 100,000 × g for 30 min to collect the liposomes. Pellets were washed in phosphate buffer containing NaCl (above) and 2 m urea to remove nonspecifically bound proteins from the synthesis kit (28), collected by centrifugation (100,000 × g, 30 min), and washed in the same buffer without urea. After centrifugation, the liposomes were resuspended at 1 mg of protein/ml in buffer. CD spectra were taken at room temperature on an AVIV spectrometer between 190–260 nm at a rate of 20 nm/min and a bandwidth of 1 nm in a 0.2-mm cuvette. Spectra were averaged over three measurements.
RESULTS
Multimerization Dependence on the Lipid Concentration
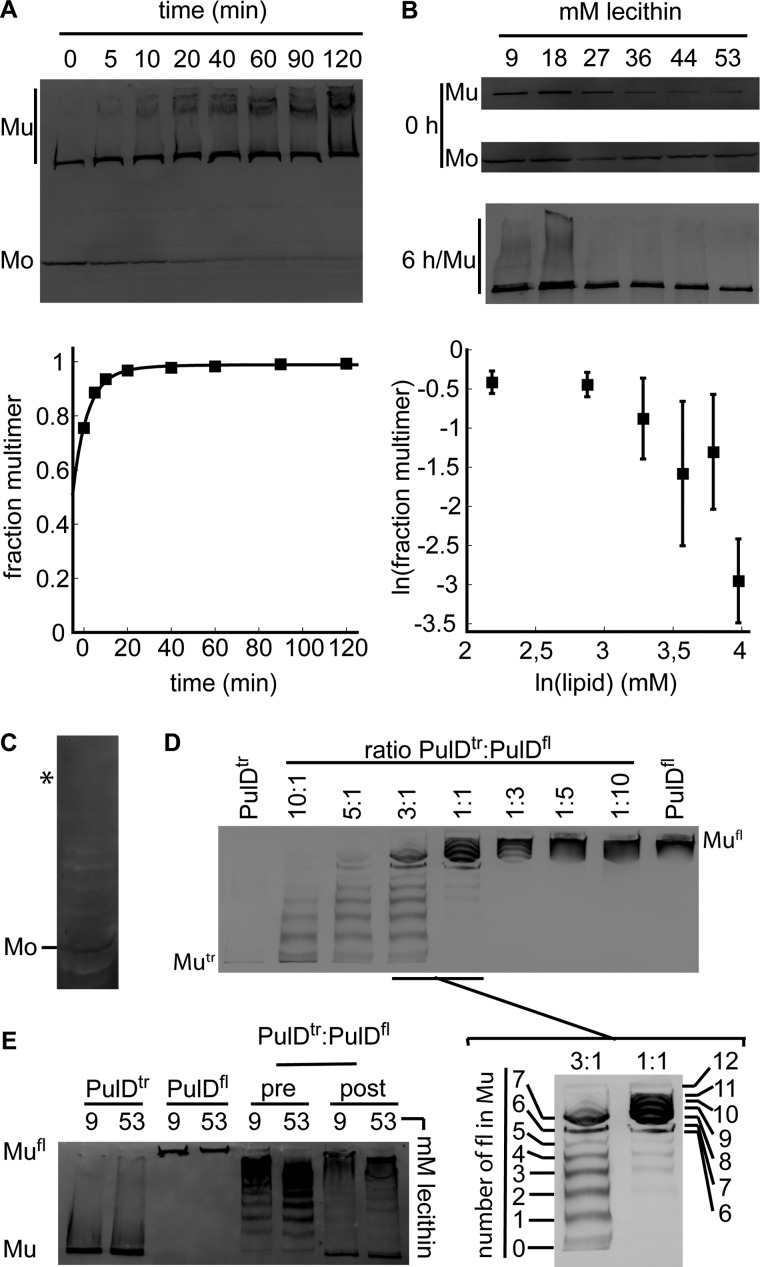
We previously reported efficient folding, assembly, and insertion of PulDfl and PulD28–42/259–660 into 4 mm lecithin liposomes at a lipid-to-protein ratio estimated to be 400:1 following 10 min of synthesis in vitro at 30 °C in the presence of 10 ng/μl DNA (28). Multimers obtained in this way are indistinguishable by all available criteria from those isolated from the outer membrane (28, 29, 36). Multimerization of PulD into its native state is assayed by resolving the multimeric and monomeric forms of the protein by SDS-PAGE (33). Under these conditions, > 75% of the synthesized protein becomes multimeric within 10 min (Fig. 1A). High molecular weight smears that appear likely correspond to PulD28–42/259–660 clusters that were poorly solubilized from the liposomes and/or separated by detergents (28). A more detailed kinetic analysis was facilitated if protein synthesis was limited to 6 min. The initial multimerization dependence on the protein concentration (determined by the amount of DNA in the reaction mixture) could not be measured, in part because longer synthesis times are required to visualize PulD with < 5 ng/μl DNA. However, the initial multimerization could be measured in the presence of increasing lecithin concentrations (9–53 mm) to separate PulD28–42/259–660 monomers over an increasing number of lecithin vesicles. On average, more PulD28–42/259–660 was synthesized at low lecithin concentrations (Fig. 1B), but PulD28–42/259–660 synthesis did not vary as markedly above 27 mm. Initial multimerization decreased above 27 mm lecithin and declined more markedly above 44 mm (Fig. 1B). All of the monomers were incorporated into multimers under all conditions after 6 h of incubation (Fig. 1B). PulD28–42/259–660 synthesis in the presence of the detergent Zwittergent 3-14 only yielded monomeric PulD28–42/259–660 (Fig. 1C), adding to the evidence that a multimerization requires a large surface such as provided by liposomes. The slope of a linear fit through the lipid dependence of the initial multimers formed determines the order of the multimerization reaction. Thus, determining the effect of the lipid concentration on PulD28–42/259–660 multimerization can detect the formation of oligomeric intermediates irrespective of whether such intermediates are SDS-resistant. At lecithin concentrations higher than 44 mm, the slope was 9.04 ± 2.50, reflecting the assembly of up to 11 monomers per multimer under these conditions. This is in reasonable agreement with the presence of 12 monomers in the native multimer (36, 37).
FIGURE 1.
The PulD multimerization mechanism. A, immunoblot analysis after SDS-PAGE of assembly kinetics of PulD28–42/259–660 after 10 min of synthesis. Mu, multimeric PulD28–42/259–660 species; Mo, monomeric PulD28–42/259–660 species. B, dependence of PulD multimerization on lecithin concentration. Synthesis was stopped after 6 min, and reactions were incubated further for 0 or 6 h. Error bars represent S.D. over five independent measurements. Only multimers are shown after 6 h of incubation because only trace amounts of monomers were left in all cases. C, PulD28–42/259–660 synthesis in the presence of 0.1% Zwittergent 3-14. Synthesis was allowed to continue for 6 h. (Zwittergent 3-14 inhibits synthesis, decreasing the quality of the blot.) The asterisk indicates the migration position of the PulD28–42/259–660 multimer that is not assembled in this reaction. D, mixed multimer formation between PulD28–42/259–660 and PulDfl in different ratios (indicated). Synthesis was stopped after 10 min, and reactions were incubated for a further 6 h. E, multimerization of PulD28–42/259–660 and PulDfl and of 1:1 mixtures before (pre) and after synthesis (post) in the presence of the indicated concentrations of lecithin. Synthesis was stopped after 10 min, and reactions were incubated for an additional 6 h. Only multimeric bands are shown in D and E. Mu, multimeric PulD28–42/259–660 (PulDtr) species; Mo, monomeric PulD28–42/259–660 (PulDtr) species; Mufl, PulDfl. All experiments were performed in the presence of 4 mm lecithin liposomes unless indicated otherwise. All synthesis reactions and incubations were performed at 30 °C. Bands were quantified by densitometry.
Because multimerization kinetics failed to measure the assembly of 12 monomers per multimer, the formation of smaller oligomers that might act as seeds could not be excluded. To facilitate detection of such seeds, PulDfl and PulD28–42/259–660 were cosynthesized at different ratios. Cosynthesis of PulDfl with PulD28–42/259–660 allowed their incorporation into mixed multimers containing from zero up to 12 copies of one form and, reciprocally, from 12 down to zero copies of the other, creating a regular 13-step ladder of mixed multimers and confirming the presence of 12 monomers per multimer (Fig. 1D). At limiting concentrations of either PulD-construct, the formation of seeds could result in the disappearance of steps from the ladder of mixed multimers, should such seeds be obligatory. Alternatively, mixing PulDfl and PulD28–42/259–660 following synthesis for 10 min in two separate reactions should also enable one to visualize seeds during the formation of mixed multimers. For example, should dimers form before multimerization, every other step in the ladder would disappear. However, both experiments yielded regular PulD ladders (Fig. 1, D and E), suggesting that neither of the two PulD constructs formed seeds prior to multimerization. Thus, the presence of N0-N1–2 domains in the full-length protein did not appear to change the assembly pathway at this stage. Together, the data indicate that rapid PulD monomer adsorption onto the lipid surface is followed by dodecamerization without prior formation of oligomeric seeds.
Folding Kinetics of PulD
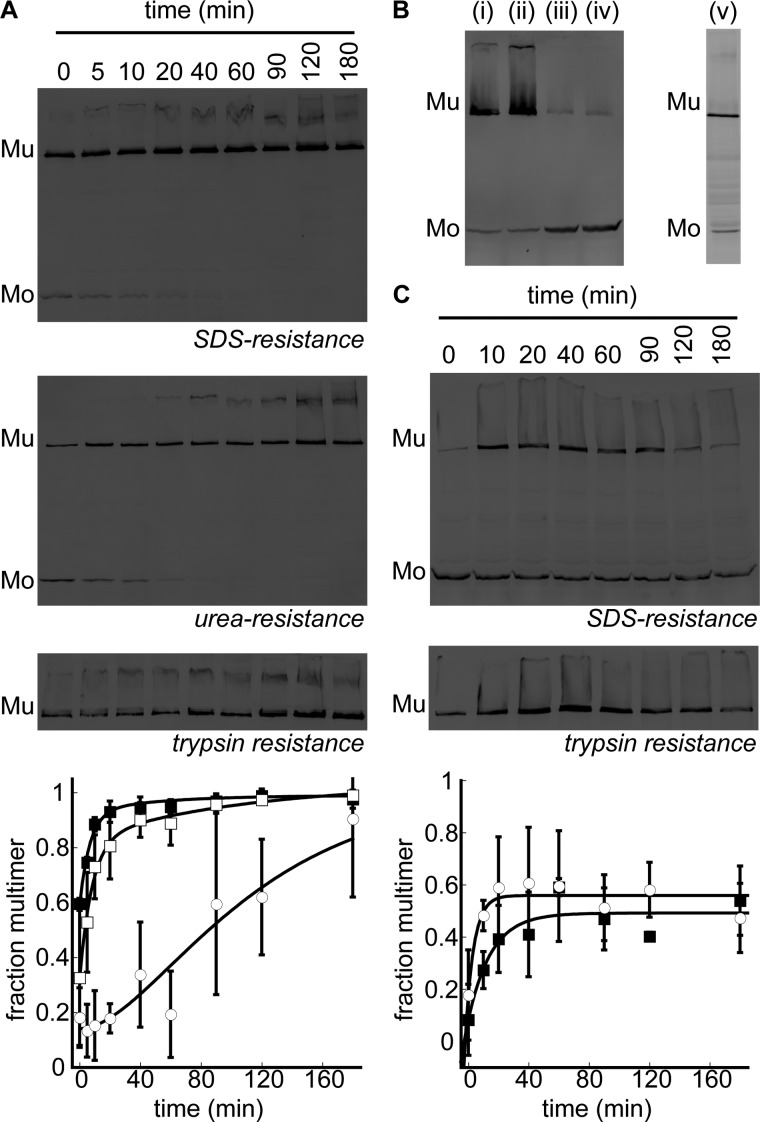
Native PulD dodecamers are not dissociated by SDS or by high concentrations of urea and have a trypsin-resistant core (monomers are digested completely) (28, 37). These criteria were used to identify PulD assembly steps in the presence of 53 mm lecithin liposomes after 10 min of PulD28–42/259–660 synthesis. Multimerization kinetics were fitted using a single or double exponential function, the latter resulting in a visually improved fit with smaller residuals (data not shown). Folding was initiated by a burst that accounted for 0.59 ± 0.02 of the total amplitude (Fig. 2A). The subsequent exponential phases were characterized by a fast rate constant of 0.14 ± 0.04 min with a partial amplitude of 0.35 ± 0.05 and a minor, poorly defined slow phase with a rate constant of 0.01 ± 0.04 min and a partial amplitude of 0.06 ± 0.13. Treating PulD28–42/259–660 with 8 m urea prior to the addition of SDS decreased the burst phase amplitude to 0.32 ± 0.03 of the total reaction amplitude (Fig. 2A). The transient also fitted to a double exponential function with rate constants of 0.12 ± 0.04 min and 0.01 ± 0.02 min, which agreed well with the rates determined by measuring SDS resistance without urea treatment. However, the partial amplitude of the slow phase increased to 0.21 ± 0.09, with the fast phase amplitude measuring 0.51 ± 0.10. Because the rate constants of the respective phases are equal regardless of the sample treatment and only the relative contributions of the amplitudes differ, the phases occur sequentially rather than in parallel (38). The delayed acquisition of trypsin resistance of PulD28–42/259–660 is also in agreement with a sequential folding mechanism (Fig. 2A). Thus, it appears that PulD28–42/259–660 multimerization and its consolidation into a urea-resistant form precede formation of the tightly packed trypsin resistant core, indicating that at least one intermediate is formed en route to the native state. These results indicate that trypsin resistance is the only true measure for attainment of the PulD native state.
FIGURE 2.
Kinetics of PulD28–42/259–660 assembly in lecithin or diC16:0PC liposomes. A, immunoblot analysis after SDS-PAGE of multimerization kinetics (■) and acquisition of urea (□) and trypsin resistance (○) of PulD28–42/259–660 in the presence of 53 mm lecithin at 30 °C. Synthesis (10 min) was performed before kinetics were monitored. Mu, multimeric PulD28–42/259–660 species; Mo, monomeric PulD28–42/259–660 species. B, PulD28–42/259–660 assembly in diCx:0PC liposomes (in i, ii, iii, and iv, x = 12, 14, 16, and 18, respectively) at 30 °C and in diC16:0PC liposomes upon warming to 42 °C (v). Synthesis was performed for 6 h, except in the latter sample, where synthesis was stopped after 10 min before increasing the temperature to 42 °C for 6 h. C, multimerization kinetics (■) and acquisition of the trypsin resistance (○) of PulD28–42/259–660 in the presence of diC16:0PC liposomes after a temperature jump from 30 to 42 °C. Synthesis was stopped after 10 min at 30 °C. Only multimers are shown for the trypsin-resistant state because monomers were digested completely. Bands were quantified by densitometry. Error bars represent S.D. over three independent measurements.
Effect of Membrane Fluidity on PulD Folding
Previous studies on the folding of OM proteins and pore-forming toxins indicate that the fluidity of the model membrane determines, in part, whether folding to a native state can occur (14, 39–41). To investigate whether PulD only assembles into fluid membranes, synthesis and folding were initiated at 30 °C in the presence of phosphatidylcholine liposomes with acyl chain lengths of 12–18 carbons. PulD multimerized efficiently into fluid liposomes of 1,2-dilauroyl-sn-glycero-3-phosphocholine (12 carbons) or 1,2-dimiristoyl-sn-glycero-3-phosphocholine lipids (14 carbons) (Fig. 2B). In contrast, when produced in the presence of diC16:0PC or 1,2-distearoyl-sn-glycero-3-phosphocholine (18 carbons) liposomes, for which the phase transition temperatures to the fluid phase are ∼41 and 59 °C, respectively, PulD migrated predominantly as a monomer upon SDS-PAGE, even after incubation for 6 h (Fig. 2B). However, the putative monomers accumulated at 30 °C in the presence of diC16:0PC-liposomes assembled into multimers with a yield of 54 ± 13% when the temperature was subsequently increased to 42 °C (Fig. 2B). Data scattering precluded a rigorous analysis of folding kinetics in the presence of diC16:0PC liposomes. Nonetheless, the data clearly show that trypsin resistance of the multimer was acquired simultaneously with SDS resistance (Fig. 2C), indicating that PulD folds and assembles in a single step under these conditions and suggesting that at least one folding step occurred prior to warming the liposomes above the phase transition temperature. Together, these observations indicate that early PulD folding/assembly steps can occur on non-fluid membranes.
PulD Is Multimeric in the Presence of diC16:0PC Liposomes at 30 °C
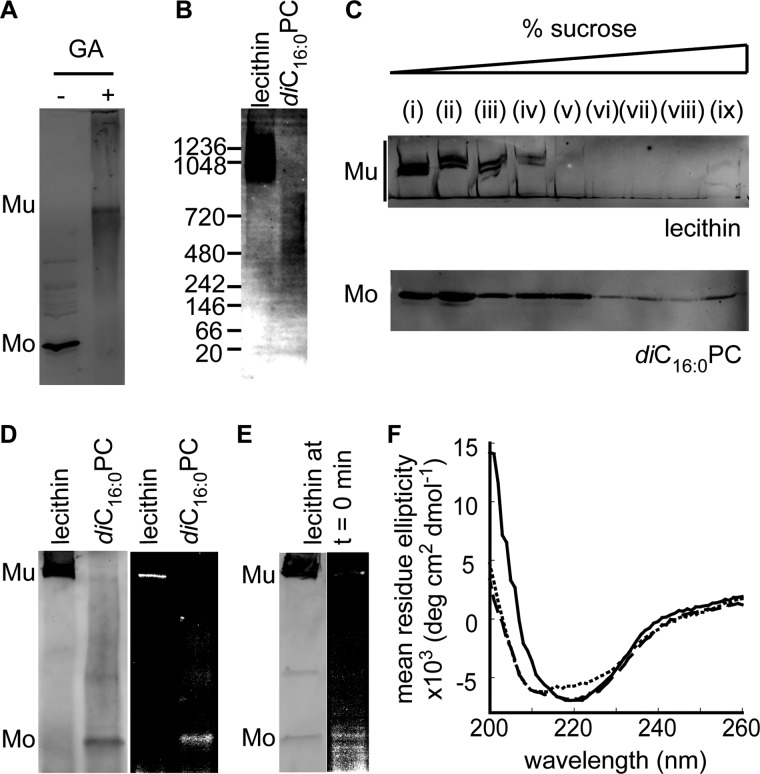
According to electrophoretic analysis, the normally hexameric Staphylococcus aureus α-toxin remains monomeric when it interacts with non-fluid membranes (41). Electrophoresis of PulD28–42/259–660 solubilized from diC16:0PC-liposomes suggested that PulD28–42/259–660 behaves similarly (Fig. 2B). However, the putative PulD28–42/259–660 monomers made in the presence of diC16:0PC-liposomes at 30 °C could be cross-linked by 100 mm glutaraldehyde, suggesting that they could, in fact, be SDS-sensitive multimers (Fig. 3A). Because nonspecific cross-linking can produce artifacts, blue native PAGE was performed on PulD28–42/259–660 solubilized from liposomes in the detergent digitonin. When solubilized from lecithin liposomes, the majority of PulD28–42/259–660 migrated in a broad band centered around 1200 kDa and a faint smear above 480 kDa. Monomeric PulD28–42/259–660 (45 kDa) was not observed (Fig. 3B). Solubilization of PulD28–42/259–660 from diC16:0PC liposomes was poor (data not shown), and only the band migrating above 480 kDa was observed (Fig. 3B). The size of this band is close to that expected for a PulD28–42/259–660 dodecamer (540 kDa), whereas the smear at 1200 kDa could represent dimers of PulD dodecamers, as reported previously in Zwittergent 3-14 (37). These results indicate that PulD28–42/259–660 can multimerize in the presence of non-fluid membranes.
FIGURE 3.
Characterization of PulD28–42/259–66 assembled in lecithin and diC16:0PC liposomes. Shown are glutaraldehyde (GA, 100 mm) cross-linking of PulD28–42/259–660 in diC16:0PC liposomes (A) and blue native PAGE in digitonin upon membrane solubilization (B). Mu, multimeric PulD28–42/259–660 species; Mo, monomeric PulD28–42/259–660 species. C, flotation on a discontinuous sucrose gradient after assembly in liposomes. D, cross-linking of 1-azidopyrene to PulD segments inserted into the membrane. E, immunoblot analysis and 1-azidopyrene fluorescence after SDS-PAGE following a 10 min synthesis reaction in the presence of lecithin liposomes. F, circular dichroism spectra in lecithin liposomes (solid line) and in diC16:0PC liposomes before (short dashed line) and after (long dashed line) a temperature jump from 30 to 42 °C. All electrophoresed samples were analyzed by immunoblotting. All synthesis reactions were performed for 6 h at 30 °C in the presence of 4 mm liposomes, except for PulD28–42/259–660 in diC16:0PC liposomes at 42 °C, for which synthesis at 30 °C was stopped after 10 min before incubating for 6 h at 42 °C.
PulD Arrested on diC16:0PC Liposomes Is Membrane-associated
The lipid association of multimeric PulD28–42/259–660 species assembled in the presence of diC16:0PC liposomes at 30 °C was demonstrated by separating membrane-bound and soluble or aggregated protein by centrifugation through a discontinuous sucrose gradient. Both PulD28–42/259–660 inserted into lecithin bilayers, and PulD28–42/259–660 produced in the presence of diC16:0PC liposomes appeared primarily in two parts of the sucrose gradient (Fig. 3C). The relatively small amounts of protein at high sucrose density (Fig. 3C, lanes viii and ix) likely represented PulD28–42/259–660 inserted into membrane fragments present in the E. coli lysate used for in vitro translation (42) or from aggregated protein that is not membrane-associated. The rest of PulD28–42/259–660 was found in fractions of lower sucrose density (Fig. 3C, lanes i–vii) with the majority floating at low densities (lanes i–iv/v), indicating association with the liposomes.
To determine whether diC16:0PC-associated PulD28–42/259–660 was inserted into the lipid bilayer of these liposomes at 30 °C, proteins were labeled post-assembly by UV irradiation with the membrane-soluble 1-azidopyrene, which labels only membrane-inserted proteins (43–45). Both PulD28–42/259–660 multimers and monomers fluoresced under UV light in 1-azidopyrene-treated lecithin and diC16:0PC liposomes, respectively (Fig. 3D). We could not unambiguously determine whether monomers could be labeled efficiently in lecithin as well. However, PulD28–42/259–660 multimers did not label efficiently immediately after synthesis, suggesting that PulD28–42/259–660 is not membrane-inserted at the initial stages during assembly (Fig. 3E). The low labeling efficiency of the multimeric form in lecithin relative to that of apparent PulD28–42/259–660 monomers in diC16:0PC liposomes at 30 °C suggested that both species were inserted into the membrane in different conformations. PulD28–42/259–660 monomers associated with diC16:0PC liposomes at 30 °C also remained trypsin-sensitive (Fig. 2C), indicating that they are distinct from the native state.
Secondary Structure of PulD in Liposomes
To characterize the structural differences between the membrane-associated and membrane-inserted PulD28–42/259–660 further, lecithin and diC16:0PC liposomes containing PulD28–42/259–660 were subjected to CD spectroscopy. In agreement with spectra obtained previously in detergent (36), PulD28–42/259–660 inserted into lecithin liposomes exhibited a minimum around 220 nm, characteristic of a structure rich in β-sheets (Fig. 3F). However, PulD associated with diC16:0PC liposomes contained an additional band with a minimum close to 210 nm, indicative of an increase in α-helical conformation (Fig. 3F). Consistent with the results obtained by SDS-PAGE, the CD spectrum obtained after warming PulD28–42/259–660 in diC16:0PC liposomes to 42 °C (followed by cooling to room temperature for spectral analysis) was closer to that of the native state, exhibiting features of both characterized states (Fig. 3F). Together with the 1-azidopyrene-labeling experiments above, these data strongly support the occurrence of a structural rearrangement during the transition of the membrane-embedded PulD28–42/259–660 oligomerization intermediate to its native state.
DISCUSSION
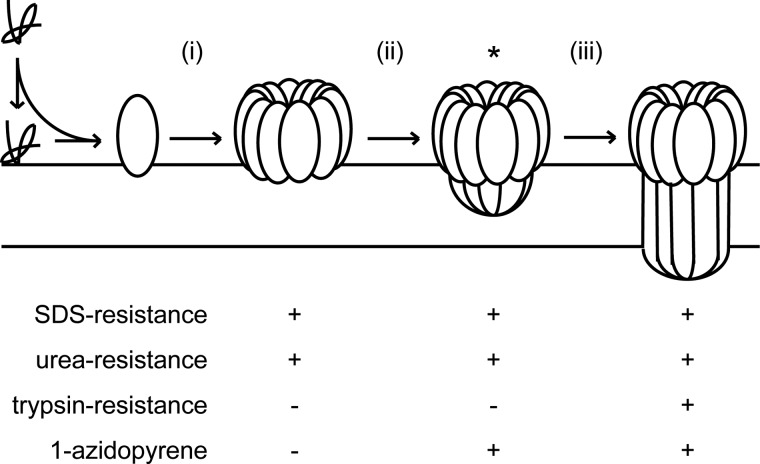
The data presented here provide important direct evidence for lipid-mediated assembly of a multimeric pore-forming protein (Fig. 4, stage i). By analyzing features of the PulD native state in model membranes, we show that PulD can form an alternative, probably loosely packed multimeric intermediate that is membrane-embedded (Fig. 4, stage ii) before a conformational switch triggers formation of the native state (stage iii). Distinct assembly steps occur in lecithin liposomes. An SDS- and urea-resistant structure that is formed first gradually attains the native, trypsin-resistant state. SDS and trypsin resistance appear simultaneously upon temperature-induced assembly of immature multimer trapped previously in non-fluid diC16:0PC liposomes. Clearly, SDS resistance of the immature multimer cannot be obtained in non-fluid membranes, suggesting that complete interaction between membrane-embedded parts of PulD is facilitated in fluid membranes. Differences in secondary structure between the intermediate and the native multimer are consistent with previous evidence that the C-domain of PulD is rich in β-sheets (36, 46). The conformational changes that occur upon conversion of the PulD intermediate to the native state are reminiscent of similar changes proposed to occur upon membrane insertion of a prepore in a group of non-constitutive pore forming proteins, including bacterial toxins and perforins (47–49). In accordance with this analogy, we suggest that the PulD intermediate represents a membrane-embedded prepore and conclude that the conformational switch associated with membrane penetration likely represents a widespread mechanism shared by a range of self-assembly pore-forming proteins. Alternatively, PulD could transverse the membrane in two conformations, one rich in α-helices and a second rich in β-sheets. Although we cannot exclude this hypothesis, we consider it unlikely that such a conformational exchange can occur within the membrane.
FIGURE 4.
Proposed model for PulD assembly in lecithin liposomes. Rapid monomer association with the liposomes leads to membrane-bound oligomerization (i) into a prepore structure (ii) that inserts (iii) upon the activation of a conformational switch to achieve the trypsin-resistant native state. The acquisition of native state determinants at each stage is also shown. The asterisk indicates the likely immature PulD multimer that can be isolated on non-fluid diC16:0PC liposomes. Note that SDS and urea resistance are not obtained in diC16:0PC liposomes, which likely prevent essential membrane-embedded interactions to achieve such a resistance.
Electron microscopy of the periplasmic vestibule of a secretin involved in type IV pilus assembly (50) and cross-linking of non-equivalent engineered cysteine residues in the N-domain of type II secretion system secretins (51, 52) led the authors to propose that secretin multimers consist of a hexameric arrangement of dimers. The majority of the N-domain is almost completely superfluous for PulD assembly (28), suggesting that it cannot play a major role in secretin assembly. The presence of the N0-N1–2 domains apparently provides only an apparent kinetic advantage during in vitro assembly. Dimers were not detected during the initial stages of the assembly of full-length and truncated PulD in our experiments. Attaining dodecameric symmetry could, therefore, be the first step, followed by differentiation into a hexamer of dimers upon membrane insertion after prepore formation or during the assembly of the entire secretion system. It is likely that PulD and secretins in general cycle through a range of conformations while performing their functions. Secretins with dodecameric symmetry and secretins with lower symmetry could, therefore, represent a particular stage in this cycle.
Genetic studies failed to identify key multimerization sites (53) other than the N3 domain (28). Together with the model proposed here, these data suggest that the N3 domain drives multimerization (Fig. 4, stage i) followed by organizing the C-domain to construct a prepore (stage ii) that is competent to form a transmembrane pore (stage iii). The CD spectrum of the prepore is similar to that of the N domains of PulD (36), suggesting that the N3 domain is folded in the prepore, allowing multimerization to occur. Previous studies also showed that PulD assembles into the inner membrane in the absence of its chaperone PulS (31, 32). Because PulD alone is able to autoassemble in vitro and in vivo, PulS might prevent premature multimerization. However, the PulS binding site is the C-terminal domain of PulD, distal to the N3 domain (54). PulS might prevent PulD multimerization if the highly flexible nature of the PulS binding domain facilitates the interaction with the N3 domain (55). A second unresolved question is raised by the discrepancy between the requirement for highly fluid membranes for the folding of outer membrane proteins in vitro (56, 57) and the presumed low fluidity of the outer membrane (58). Recent observations indicate that at least certain chaperones can help overcome this barrier (59, 60). PulS might play such a role. Indeed, PulD insertion into purified bacterial outer membranes in vitro was efficient only when PulS was present in those membranes (35). Future challenges will, therefore, include delineating the role of PulS in PulD assembly into the outer membrane and how PulS interferes with the PulD autoassembly pathway that leads to cytotoxicity. In addition, the unique tractability of PulD assembly that enables an assembly intermediate to be trapped validates it as a model protein for investigating the determinants of membrane assembly in this class of proteins.
Acknowledgments
We thank Patrick England and Bruno Baron for help with CD.
This work was supported in part by the French National Research Agency Agence Nationale de la Recherche Grant 09-BLAN-0291. This work was also supported by Marie Curie Intra-European Fellowship PIEF-GA-2010-272611 (to G. H. M. H.).
- OM
- outer membrane
- diC16:0PC
- 1,2-dipalmitoyl-sn-glycero-3-phosphocholine.
REFERENCES
- 1. Bondar A. N., White S. H. (2012) Hydrogen bond dynamics in membrane protein function. Biochim. Biophys. Acta 1818, 942–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bowie J. U. (2011) Membrane protein folding. How important are hydrogen bonds? Curr. Opin. Struct. Biol. 21, 42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cao Z., Bowie J. U. (2012) Shifting hydrogen bonds may produce flexible transmembrane helices. Proc. Natl. Acad. Sci. U.S.A. 109, 8121–8126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curnow P., Di Bartolo N. D., Moreton K. M., Ajoje O. O., Saggese N. P., Booth P. J. (2011) Stable folding core in the folding transition state of an α-helical integral membrane protein. Proc. Natl. Acad. Sci. U.S.A. 108, 14133–14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fiedler S., Broecker J., Keller S. (2010) Protein folding in membranes. Cell Mol. Life Sci. 67, 1779–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong H., Bowie J. U. (2011) Dramatic destabilization of transmembrane helix interactions by features of natural membrane environments. J. Am. Chem. Soc. 133, 11389–11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalli A. C., Campbell I. D., Sansom M. S. (2011) Multiscale simulations suggest a mechanism for integrin inside-out activation. Proc. Natl. Acad. Sci. U.S.A. 108, 11890–11895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moon C. P., Fleming K. G. (2011) Side-chain hydrophobicity scale derived from transmembrane protein folding into lipid bilayers. Proc. Natl. Acad. Sci. U.S.A. 108, 10174–10177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amaral M. D. (2004) CFTR and chaperones. Processing and degradation. J. Mol. Neurosci. 23, 41–48 [DOI] [PubMed] [Google Scholar]
- 10. Hagan C. L., Silhavy T. J., Kahne D. (2011) β-Barrel membrane protein assembly by the Bam complex. Annu. Rev. Biochem. 80, 189–210 [DOI] [PubMed] [Google Scholar]
- 11. Mogensen J. E., Otzen D. E. (2005) Interactions between folding factors and bacterial outer membrane proteins. Mol. Microbiol. 57, 326–346 [DOI] [PubMed] [Google Scholar]
- 12. Curnow P., Booth P. J. (2007) Combined kinetic and thermodynamic analysis of α-helical membrane protein unfolding. Proc. Natl. Acad. Sci. U.S.A. 104, 18970–18975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hong H., Tamm L. K. (2004) Elastic coupling of integral membrane protein stability to lipid bilayer forces. Proc. Natl. Acad. Sci. U.S.A. 101, 4065–4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huysmans G. H., Baldwin S. A., Brockwell D. J., Radford S. E. (2010) The transition state for folding of an outer membrane protein. Proc. Natl. Acad. Sci. U.S.A. 107, 4099–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cymer F., Schneider D. (2012) Oligomerization of polytopic α-helical membrane proteins. Causes and consequences. Biol. Chem. 393, 1215–1230 [DOI] [PubMed] [Google Scholar]
- 16. Clarke O. B., Gulbis J. M. (2012) Oligomerization at the membrane. Potassium channel structure and function. Adv. Exp. Med. Biol. 747, 122–136 [DOI] [PubMed] [Google Scholar]
- 17. Meng G., Fronzes R., Chandran V., Remaut H., Waksman G. (2009) Protein oligomerization in the bacterial outer membrane (Review). Mol. Membr. Biol. 26, 136–145 [DOI] [PubMed] [Google Scholar]
- 18. Dunstone M. A., Tweten R. K. (2012) Packing a punch. The mechanism of pore formation by cholesterol dependent cytolysins and membrane attack complex/perforin-like proteins. Curr. Opin. Struct. Biol. 22, 342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nieva J. L., Madan V., Carrasco L. (2012) Viroporins. Structure and biological functions. Nat. Rev. Microbiol. 10, 563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tilley S. J., Saibil H. R. (2006) The mechanism of pore formation by bacterial toxins. Curr. Opin. Struct. Biol. 16, 230–236 [DOI] [PubMed] [Google Scholar]
- 21. Cymer F., Schneider D. (2010) A single glutamate residue controls the oligomerization, function, and stability of the aquaglyceroporin GlpF. Biochemistry 49, 279–286 [DOI] [PubMed] [Google Scholar]
- 22. Hong H., Blois T. M., Cao Z., Bowie J. U. (2010) Method to measure strong protein-protein interactions in lipid bilayers using a steric trap. Proc. Natl. Acad. Sci. U.S.A. 107, 19802–19807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stanley A. M., Chuawong P., Hendrickson T. L., Fleming K. G. (2006) Energetics of outer membrane phospholipase A (OMPLA) dimerization. J. Mol. Biol. 358, 120–131 [DOI] [PubMed] [Google Scholar]
- 24. Dewald A. H., Hodges J. C., Columbus L. (2011) Physical determinants of β-barrel membrane protein folding in lipid vesicles. Biophys. J. 100, 2131–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ebie Tan A., Burgess N. K., DeAndrade D. S., Marold J. D., Fleming K. G. (2010) Self-association of unfolded outer membrane proteins. Macromol. Biosci. 10, 763–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masi M., Duret G., Delcour A. H., Misra R. (2009) Folding and trimerization of signal sequence-less mature TolC in the cytoplasm of Escherichia coli. Microbiology 155, 1847–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Junge F., Haberstock S., Roos C., Stefer S., Proverbio D., Dötsch V., Bernhard F. (2011) Advances in cell-free protein synthesis for the functional and structural analysis of membrane proteins. N. Biotechnol. 28, 262–271 [DOI] [PubMed] [Google Scholar]
- 28. Guilvout I., Chami M., Berrier C., Ghazi A., Engel A., Pugsley A. P., Bayan N. (2008) In vitro multimerization and membrane insertion of bacterial outer membrane secretin PulD. J. Mol. Biol. 382, 13–23 [DOI] [PubMed] [Google Scholar]
- 29. Nickerson N. N., Abby S. S., Rocha E. P., Chami M., Pugsley A. P. (2012) A single amino acid substitution changes the self-assembly status of a type IV piliation secretin. J. Bacteriol. 194, 4951–4958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McLaughlin L. S., Haft R. J., Forest K. T. (2012) Structural insights into the type II secretion nanomachine. Curr. Opin. Struct. Biol. 22, 208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Collin S., Guilvout I., Chami M., Pugsley A. P. (2007) YaeT-independent multimerization and outer membrane association of secretin PulD. Mol. Microbiol. 64, 1350–1357 [DOI] [PubMed] [Google Scholar]
- 32. Guilvout I., Chami M., Engel A., Pugsley A. P., Bayan N. (2006) Bacterial outer membrane secretin PulD assembles and inserts into the inner membrane in the absence of its pilotin. EMBO J. 25, 5241–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guilvout I., Nickerson N. N., Chami M., Pugsley A. P. (2011) Multimerization-defective variants of dodecameric secretin PulD. Res. Microbiol. 162, 180–190 [DOI] [PubMed] [Google Scholar]
- 34. Hardie K. R., Lory S., Pugsley A. P. (1996) Insertion of an outer membrane protein in Escherichia coli requires a chaperone-like protein. EMBO J. 15, 978–988 [PMC free article] [PubMed] [Google Scholar]
- 35. Collin S., Krehenbrink M., Guilvout I., Pugsley A. P. (2013) The targeting, docking and anti-proteolysis functions of the secretin chaperone PulS. Res. Microbiol. 164, 390–396 [DOI] [PubMed] [Google Scholar]
- 36. Chami M., Guilvout I., Gregorini M., Rémigy H. W., Müller S. A., Valerio M., Engel A., Pugsley A. P., Bayan N. (2005) Structural insights into the secretin PulD and its trypsin-resistant core. J. Biol. Chem. 280, 37732–37741 [DOI] [PubMed] [Google Scholar]
- 37. Nouwen N., Stahlberg H., Pugsley A. P., Engel A. (2000) Domain structure of secretin PulD revealed by limited proteolysis and electron microscopy. EMBO J. 19, 2229–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wallace L. A., Matthews C. R. (2002) Sequential vs. parallel protein-folding mechanisms. Experimental tests for complex folding reactions. Biophys. Chem. 101, 113–131 [DOI] [PubMed] [Google Scholar]
- 39. Kleinschmidt J. H., Tamm L. K. (1996) Folding intermediates of a β-barrel membrane protein. Kinetic evidence for a multi-step membrane insertion mechanism. Biochemistry 35, 12993–13000 [DOI] [PubMed] [Google Scholar]
- 40. Rodionova N. A., Tatulian S. A., Surrey T., Jähnig F., Tamm L. K. (1995) Characterization of two membrane-bound forms of OmpA. Biochemistry 34, 1921–1929 [DOI] [PubMed] [Google Scholar]
- 41. Tomita T., Watanabe M., Yasuda T. (1992) Influence of membrane fluidity on the assembly of Staphylococcus aureus α-toxin, a channel-forming protein, in liposome membrane. J. Biol. Chem. 267, 13391–13397 [PubMed] [Google Scholar]
- 42. Collin S., Guilvout I., Nickerson N. N., Pugsley A. P. (2011) Sorting of an integral outer membrane protein via the lipoprotein-specific Lol pathway and a dedicated lipoprotein pilotin. Mol. Microbiol. 80, 655–665 [DOI] [PubMed] [Google Scholar]
- 43. Blanton M. P., Cohen J. B. (1992) Mapping the lipid-exposed regions in the Torpedo californica nicotinic acetylcholine receptor. Biochemistry 31, 3738–3750 [DOI] [PubMed] [Google Scholar]
- 44. Perides G., Harter C., Traub P. (1987) Electrostatic and hydrophobic interactions of the intermediate filament protein vimentin and its amino terminus with lipid bilayers. J. Biol. Chem. 262, 13742–13749 [PubMed] [Google Scholar]
- 45. Smith D. P., Kilbourn M. R., McDowell J. H., Hargrave P. A. (1981) Topography of rhodopsin in retinal rod outer segment disk membranes. Photochemical labeling with L-azidopyrene. Biochemistry 20, 2417–2424 [DOI] [PubMed] [Google Scholar]
- 46. Bitter W., Koster M., Latijnhouwers M., de Cock H., Tommassen J. (1998) Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol. Microbiol. 27, 209–219 [DOI] [PubMed] [Google Scholar]
- 47. Dowd K. J., Tweten R. K. (2012) The cholesterol-dependent cytolysin signature motif. A critical element in the allosteric pathway that couples membrane binding to pore assembly. PLoS Pathog. 8, e1002787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Law R. H., Lukoyanova N., Voskoboinik I., Caradoc-Davies T. T., Baran K., Dunstone M. A., D'Angelo M. E., Orlova E. V., Coulibaly F., Verschoor S., Browne K. A., Ciccone A., Kuiper M. J., Bird P. I., Trapani J. A., Saibil H. R., Whisstock J. C. (2010) The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature 468, 447–451 [DOI] [PubMed] [Google Scholar]
- 49. Rosado C. J., Buckle A. M., Law R. H., Butcher R. E., Kan W. T., Bird C. H., Ung K., Browne K. A., Baran K., Bashtannyk-Puhalovich T. A., Faux N. G., Wong W., Porter C. J., Pike R. N., Ellisdon A. M., Pearce M. C., Bottomley S. P., Emsley J., Smith A. I., Rossjohn J., Hartland E. L., Voskoboinik I., Trapani J. A., Bird P. I., Dunstone M. A., Whisstock J. C. (2007) A common fold mediates vertebrate defense and bacterial attack. Science 317, 1548–1551 [DOI] [PubMed] [Google Scholar]
- 50. Lieberman J. A., Frost N. A., Hoppert M., Fernandes P. J., Vogt S. L., Raivio T. L., Blanpied T. A., Donnenberg M. S. (2012) Outer membrane targeting, ultrastructure, and single molecule localization of the enteropathogenic Escherichia coli type IV pilus secretin BfpB. J. Bacteriol. 194, 1646–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van der Meeren R., Wen Y., Van Gelder P., Tommassen J., Devreese B., Savvides S. N. (2013) New insights into the assembly of bacterial secretins. Structural studies of the periplasmic domain of XcpQ from Pseudomonas aeruginosa. J. Biol. Chem. 288, 1214-1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang X., Pineau C., Gu S., Guschinskaya N., Pickersgill R. W., Shevchik V. E. (2012) Cysteine scanning mutagenesis and disulfide mapping analysis of arrangement of GspC and GspD protomers within the type 2 secretion system. J. Biol. Chem. 287, 19082–19093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guilvout I., Hardie K. R., Sauvonnet N., Pugsley A. P. (1999) Genetic dissection of the outer membrane secretin PulD. Are there distinct domains for multimerization and secretion specificity? J. Bacteriol. 181, 7212–7220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Daefler S., Guilvout I., Hardie K. R., Pugsley A. P., Russel M. (1997) The C-terminal domain of the secretin PulD contains the binding site for its cognate chaperone, PulS, and confers PulS dependence on pIVf1 function. Mol. Microbiol. 24, 465–475 [DOI] [PubMed] [Google Scholar]
- 55. Nickerson N. N., Tosi T., Dessen A., Baron B., Raynal B., England P., Pugsley A. P. (2011) Outer membrane targeting of secretin PulD protein relies on disordered domain recognition by a dedicated chaperone. J. Biol. Chem. 286, 38833–38843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Burgess N. K., Dao T. P., Stanley A. M., Fleming K. G. (2008) β-barrel proteins that reside in the Escherichia coli outer membrane in vivo demonstrate varied folding behavior in vitro. J. Biol. Chem. 283, 26748–26758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huysmans G. H., Radford S. E., Baldwin S. A., Brockwell D. J. (2012) Malleability of the folding mechanism of the outer membrane protein PagP. Parallel pathways and the effect of membrane elasticity. J. Mol. Biol. 416, 453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nikaido H. (2003) Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67, 593–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McMorran L. M., Bartlett A. I., Huysmans G. H., Radford S. E., Brockwell D. J. (2013) Dissecting the effects of periplasmic chaperones on the in vitro folding of the outer membrane protein PagP. J. Mol. Biol 425, 3178–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patel G. J., Behrens-Kneip S., Holst O., Kleinschmidt J. H. (2009) The periplasmic chaperone Skp facilitates targeting, insertion, and folding of OmpA into lipid membranes with a negative membrane surface potential. Biochemistry 48, 10235–10245 [DOI] [PubMed] [Google Scholar]