Abstract
OBJECTIVES:
To compare ocular surface changes induced via glaucoma treatment in patients using fixed combinations of prostaglandin analogues (travoprost, latanoprost and bimatoprost) with 0.5% timolol maleate
METHODS:
A prospective, multicenter, randomized, parallel group, single-blind clinical trial was performed in 33 patients with ocular hypertension or open angle glaucoma who had not been previously treated. The ocular surface was evaluated prior to and three months after treatment, with a daily drop instillation of one of the three medications. The main outcome measurements included the tear film break-up time, Schirmer's test, Lissamine green staining, the Ocular Surface Disease Index questionnaire, impression cytology using HE and PAS and immunocytochemistry for interleukin-6 and HLA-DR. Ensaiosclinicos.gov.br: UTN - U1111-1129-2872
RESULTS:
All of the drugs induced a significant reduction in intraocular pressure. Decreases in the Schirmer's test results were observed with all of the drugs. Decreases in tear-film break-up time were noted with travoprost/timolol and latanoprost/timolol. An increase in the Lissamine green score was noted with travoprost/timolol and bimatoprost/timolol. The Ocular Surface Disease Index score increased after treatment in the travoprost/timolol group. Impression cytology revealed a significant difference in cell-to-cell contact in the same group, an increase in cellularity in all of the groups and an increase in the number of goblet cells in all of the groups. The fixed combinations induced an increase in IL-6 expression in the travoprost/timolol group, in which there was also an increase in HLA-DR expression.
CONCLUSIONS:
All of the fixed combinations induced a significant reduction in intraocular pressure, and the travoprost/timolol group showed increased expression of the inflammatory markers HLA-DR and interleukin-6. All three tested medications resulted in some degree of deterioration in the ocular surface after three months of glaucoma treatment.
Keywords: Glaucoma, Conjunctiva, Immunohistochemistry, Inflammation, Treatment
INTRODUCTION
Glaucoma is a chronic, multifactorial, progressive optic neuropathy that requires long-term treatment with topical hypotensive medications (1). Several classes of drugs are currently available to treat this condition, including prostaglandin (PG) analogues as well as fixed combinations (FCs) of prostaglandin/prostamide analogues combined with 0.5% timolol maleate (2).
Beta-blockers are often used to treat glaucoma and were considered the “gold standard” for starting glaucoma treatment until recently, when they were replaced by prostaglandin analogues (2). Beta-blockers have many systemic side effects, especially bradycardia and bronchospasms, as well as effects on the central nervous system.
While the systemic side effects induced by topical PG analogues are rare, iris hyperpigmentation, excessive eyelash growth and conjunctival hyperemia have been reported among the local side effects caused by these drugs (3-5). Conditions suggestive of the stimulation or reactivation of ocular inflammatory responses, such as anterior uveitis or cystoid macular edema, have also been associated with the use of PG analogues (6).
Ocular surface dysfunction has also been related to glaucoma treatment. Beta-blockers have been known to induce conjunctival hyperemia, punctate keratitis and corneal anesthesia, as well as dry eye and allergic blepharoconjunctivitis (7-9).
Previous studies with patients who received long-term treatment with topical medications showed that both hypotensive drugs and their preservatives (especially benzalkonium chloride – BAK) could increase the number of inflammatory cells and fibroblasts in the substantia propria of the conjunctiva and reduce the number of goblet cells, thereby inducing ocular surface changes manifested clinically as dry eye (8-11). The length of administration, concentration and amount of these drugs have been related to the severity of the side effects. In addition, there has been strong evidence suggesting that these changes might increase the risk of trabeculectomy failure (12,13). However, most of the information on this subject was published prior to the introduction of PG analogues.
The purpose of this study was to evaluate (clinically, histologically and via immunocytochemistry) the ocular surface changes induced by glaucoma treatment with topical FCs of PG analogues and timolol.
MATERIALS AND METHODS
Inclusion criteria
Eligible patients were adults (≥18 years of age) with a clinical diagnosis of primary open-angle glaucoma (POAG) or ocular hypertension (OH) in at least one eye and with no previous topical hypotensive treatment. The selected patients had a open iridocorneal angle upon gonioscopy examination. POAG was diagnosed on the basis of characteristic optic disc changes and/or glaucomatous visual field loss demonstrated on the Humphrey visual field analyzer (HFA) (Humphrey Instruments, Inc., Zeiss Humphrey, San Leandro, California, USA). IOP, measured at 8 a.m., had to be between 26 mm Hg and 35 mm Hg in the study eye(s). In addition, patients were required to have a corrected distance visual acuity (CDVA) of 20/70 or better in each eye, and those with glaucoma had to have a recent (within three months) visual field examination showing a mean deviation greater than -15 dB and no fixation threat. Finally, the eligible patients were required to be able to follow instructions, to be willing and able to attend all of the study visits, and to provide informed consent prior to screening.
Exclusion criteria
Patients were excluded if they met any of the following criteria: previous ocular surgery; active ocular inflammation; or clinically diagnosed dry eye. Patients with hypersensitivity or poor tolerance to any components of the study medication; with bronchial asthma or history of bronchial asthma; with bronchial hyperreactivity or severe chronic obstructive pulmonary disease that would preclude the safe administration of a topical beta-blocker; sinus bradycardia, second-degree or third-degree atrioventricular block, sinoatrial block, overt cardiac failure, or cardiogenic shock that would preclude the safe administration of a topical beta-blocker; or a severe medical or psychiatric condition were also excluded from the study.
A prospective, randomized, single-blind, multicenter, parallel group, interventional study was conducted between March 2009 and September 2010 at the Federal University of São Paulo (UNIFESP) in São Paulo, Brazil.
The participants were allocated to each treatment group following the sequence of a randomization table. The randomization table was generated using software (Stata, version 11, College Station, Texas, USA) and a block size of three. The participants were enrolled and assigned to each group by the study coordinator.
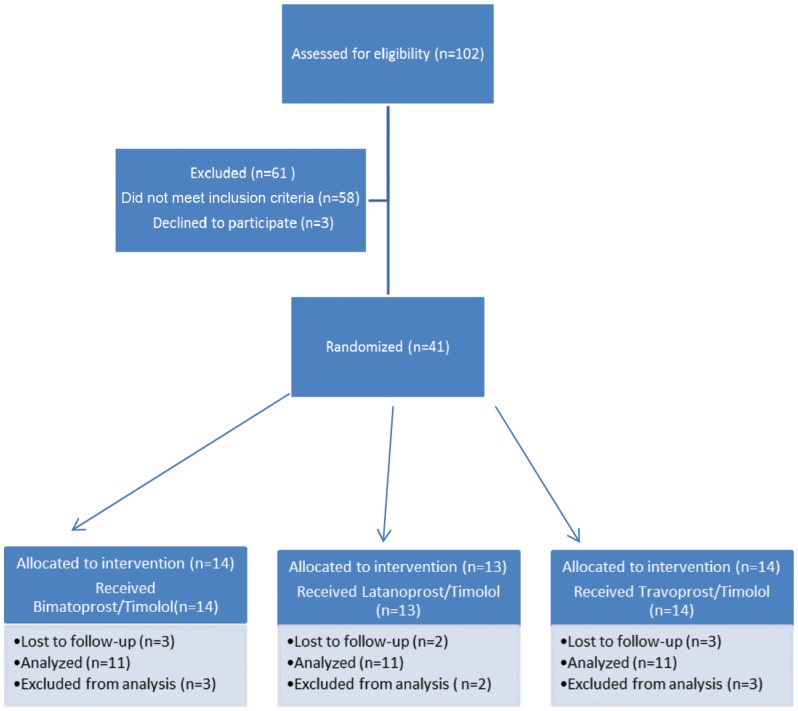
After the sample size was calculated, 33 patients were selected (11 patients were randomly distributed into three different groups according to medication regimen) by two examiners (HR and NL) and were allocated using permuted-block randomization (block size = 3; allocation rate 1:1:1) into the following three groups, independently of age, sex or residence: latanoprost+timolol (LT); bimatoprost+timolol (BT); or travoprost+timolol (TT) (Figure 1).
Figure 1.
Flow diagram of the progress through the phases of this parallel, randomized trial of three groups.
The medications were administered once daily in the evening for 12 weeks in selected patients in all of the groups.
The clinical data collected included the patients' demographic data (age, sex and ethnicity). All of the patients underwent routine ophthalmological examinations prior to and after three months of treatment. The ocular surface evaluation included biomicroscopic examination of the lids, conjunctiva, cornea and tear film. The diagnostic tests included Schirmer's test with anesthesia, Lissamine green vital staining, tear film break-up time (TBUT) and impression cytology. The OSDI questionnaire was also applied. After the ocular surface evaluation, intraocular pressure (IOP) was measured with the Goldmann applanation tonometer.
The patients were examined at two centers (Graefe Institute of Ophthalmology and Brasilia Base Hospital), following instructions provided by the Cornea and External Disease Service in the Department of Ophthalmology, UNIFESP. All of the IOP measurements were obtained at the same time (8 a.m.). The rooms where the examinations were performed had neither air conditioning nor windows, and the air humidity and temperature were controlled with specific equipment. Prior to the examination, the patients rested for 20 minutes with the door to the room closed. The tests were performed by two researchers (HR and NL) and were analyzed at the Ocular Surface Advanced Center (CASO) by two blinded investigators (JB and PANF).
Those investigators who assessed the primary outcomes of the study were blinded to the allocation status of the participants. The statistician who performed the data analysis was blinded to all information.
Dry eye was defined as a TBUT score of <5 seconds (2% fluorescein, Ophthalmos, São Paulo, SP, Brazil), <5 mm wetting in the Schirmer's test (Schirmer strips, Ophthalmos, São Paulo, SP, Brazil) with topical anesthesia (0.5% proxymetacaine chlorohydrate, Anestalcon®, Alcon Laboratórios do Brasil, São Paulo, SP, Brazil) and corneal and conjunctival staining with 1% Lissamine green (Ophthalmos, São Paulo, SP, Brazil) ≥3 on the van Bijsterveld scale (0 to 9).
Impression cytology
After the ocular surface evaluation, all of the patients were subjected to impression cytology (IC) sampling by two researchers (HR and NL). Following topical anesthesia, IC specimens were collected (HAWP 304, Millipore, Bedford, Massachusetts, USA) from an exposed area of the bulbar conjunctiva (temporal region) and an unexposed area of the conjunctiva (superior region) adjacent to the corneal limbus. All of the strips were processed for periodic acid Schiff and Gill's hematoxylin staining. Glass slides mounted with Entellan (Merck, Darmstadt, Germany) were examined with a blinded procedure under light microscopy by an experienced professional (JNB). For quality control, only IC specimens were included with at least one third of the filter surface covered by visible epithelial cells. The conjunctiva samples were evaluated according to established techniques for the following parameters: cellularity; cell-to-cell contact; nuclear-to-cytoplasmic (N/C) ratio; nuclear chromatin; goblet cell density; keratinization; and distribution of inflammatory cells. A score of 0 to 3 was assigned to each of these features: 0 - normal findings; 1 - borderline features; and 2 and 3 - abnormal features (29,30). The total score for each sample was classified as A (normal; total score 0-3), B (borderline; total score 4-6) or C (abnormal; total score >6). The goblet cell densities were considered normal when the cells were abundant, borderline when there was a slightly or moderately reduced number of cells and abnormal when there was a distinct reduction in the number of cells (the presence of one or no goblet cells).
Immunocytochemistry
Other conjunctival impression cytology samples were obtained from the same areas as the first samples, using Biopore membranes (Millicell-CM 0.4 μm PICM 012550, Millipore Corp, Bedford, Massachusetts, USA), and were immunostained with monoclonal antibodies to HLA-DR and IL-6. The samples with cells covering more than 80% of the membrane area or samples covering between 40% and 80% (where the cells were confluent and present in a discrete area) were considered suitable for immunocytological analysis. Samples with cellularity of less than 40% were considered unsuitable. The number of cells positive for HLA-DR and IL-6 and the total number of cells in five adjacent microscopic high-power fields (40X) were counted by the two masked observers (JNB and PANF). The results for each phenotype were expressed as a percentage of the total number of cells and were compared pre- and post-treatment.
Statistical analysis
The sample size calculation was based on a pilot study performed at one of the study sites. Two principal outcomes, the Schirmer's test and impression cytology, were selected for this purpose. To detect a difference of 3 mm (SD 2 mm) with the Schirmer's test at two points in time (pre- and post-treatment), a sample size of 10 was necessary to obtain a power of 80% at the 5% significance level. This sample size (N = 10) would provide a power of 94% to detect a difference of 0.4 cells (SD 0.2 cells) in the mean number of cells in the impression cytology test. Therefore, a sample size of 11 per treatment group was used in this study.
The Kolmogorov-Smirnov (K-S) test was used to determine whether the continuous variables had a normal distribution. One-way ANOVA with a significance level of 5% was used to compare the continuous variables, and the Kruskal-Wallis test was used to compare the continuous variables with a non-Gaussian distribution. When statistically significant differences were found, the data were further analyzed using post hoc comparisons with Fisher's exact test (for comparisons of up to three groups) or the Tukey-Kramer test (comparisons of more than three groups) for p-value correction. The calculations were performed with StatView statistical software (SAS Institute Inc., Cary, North Carolina, USA). The level of statistical significance was set at p = 0.05.
Ethics
The study was approved by the UNIFESP Medical Ethics Committee (reference no. 0954/06) and was registered with an internationally accredited site (UTN U1111-1129-2872; RBR-7mmp6k- www.ensaiosclinicos.gov.br) in accordance with the guidelines set forth in the Declaration of Helsinki. All of the patients provided informed consent. The research was funded by the Federal University of São Paulo/FAPESP.
RESULTS
The results of the statistical analysis were unaffected by the demographic data (age, sex and ethnicity) (Table 1).
Table 1.
Demographic data: age, sex and ethnicity.
| TT group | BT group | LT group | p-value | |
| Age (mean±sd) | 61.9±6.91 | 60.5±4.61 | 63.65±5.12 | p>0.05 |
| Ethnicity | ||||
| White caucasian | 3 | 4 | 3 | p>0.05 |
| African american | 1 | 1 | 1 | p>0.05 |
| Hispanic | 7 | 5 | 6 | p>0.05 |
| Asian | 0 | 1 | 1 | p>0.05 |
| Sex (male:female) | (4:7) | (5:6) | (6:5) | p>0.05 |
* There were significant differences between African American and Asian patients, compared with Caucasians and Hispanics, but not between the African American and Asian groups or between the Caucasian and Hispanic groups, respectively.
All three combinations (LT, BT and TT) produced a statistically significant reduction in IOP (p = 0.0001). The mean IOP for the TT group was 24.72±1.03 mm Hg (95% CI 22.66-26.78) prior to treatment and 14.00±0.44 mm Hg (95% CI 13.12-14.88 mm Hg) after treatment; the corresponding figures for the BT group were 22.32±5.58 mm Hg (95% CI 11.16-33.48) and 12.10±2.96 mm Hg (95% CI 6.18-18.02), respectively, and for the LT group, they were 20.32±4.99 mm Hg (95% CI 10.34-30.3) and 11.59±3.11 mm Hg, respectively (95% CI 5.37-17.81).
The ocular surface evaluations revealed a significant decrease in the Schirmer's test values for the patients treated with all of the drugs: the mean value for the TT group was 8.95±0.21 mm (95% CI 8.53-9.37) prior to treatment and 7.18±0.73 mm (95% CI 5.72-8.64) after treatment (p = 0.0001); the corresponding figures for the LT group were 13.77±4.72 mm (95% CI 4.33-23.21) and 9.09±3.65 mm (95% CI 1.79-16.39) (p = 0.0007), respectively, and they were 12.45±3.85 mm (95% CI 4.75-20.15) and 9.95±3.68 mm (95% CI 2.59-17.31) (p = 0.0333), respectively, for the BT group.
The TBUT decreased significantly in the TT and LT groups. The mean values for the former were 11.95±1.49 seconds (95% CI 8.97-14.93) prior to treatment and 9.54±0.85 seconds (95% CI 7.84-11.24) after treatment (p<0.0001), and for the latter, the values were 13.86±3.18 seconds (95% CI 7.5-20.22) and 11.68±3.38 seconds (95% CI 4.92-18.44), respectively (p = 0.0025). For the BT group, the difference was not statistically significant (p = 0.45).
Scores for Lissamine green staining increased significantly in patients treated with TT (mean score of 2.36±0.65 [95% CI 1.06-3.66] prior to treatment and 6±0.01 [95% CI 5.98-6.02] after treatment [p<0.0001]) and with BT (mean score of 2±1.48 [95% CI 0.94-4.96] prior to treatment and 3.27±1.45 [95% CI 0.37-6.17] after treatment [p = 0.0007]). For the LT group, this difference was not statistically significant (p = 0.22).
The OSDI scores increased in all of the groups, but the difference was only significantly different in the TT group (mean score 33.74±6.88 [95% CI 19.98-47.5] prior to treatment and 39.94±3.92 [95% CI 32.1-47.78] after treatment [p = 0.02]). All of the groups could be classified as having mild to moderate dry eye on the OSDI scale.
Table 2 summarizes the clinical ocular surface data for the three groups.
Table 2.
Clinical data prior to and three months after treatment with a fixed combination of travoprost 0.004%/timolol 0.5%, bimatoprost 0.03%/timolol 0.5% or latanoprost 0.005%/timolol 0.5% in naïve patients; n = 33.
| Travatan/Timolol | Bimatoprost/Timolol | Latanoprost/Timolol | |||||||
| pre-treatment (mean±SD) | post-treatment (mean±SD) | p-value | pre-treatment (mean±SD) | post-treatment (mean±SD) | p-value | pre-treatment (mean±SD) | post-treatment (mean±SD) | p-value | |
| Schirmer | 8.95±0.21 | 7.18±0.73 | 0.00 | 12.45±3.85 | 9.95±3.68 | 0.03 | 13.77±4.72 | 9.09±3.65 | 0.00 |
| TBUT | 11.95±1.49 | 9.54±0.85 | 0.00 | 11.90±3.22 | 11.18±3.21 | 0.45 | 13.86±3.18 | 11.68±3.38 | 0.00 |
| LGT | 2.36±0.65 | 6.00 | <0.001 | 2.00±1.48 | 3.27±1.45 | 0.00 | 0.86±1.12 | 1.41±1.71 | 0.22 |
| OSDI | 33.74±6.88 | 39.94±3.92 | 0.02 | 29.76±15.93 | 30.30±17.17 | 0.94 | 8.06±7.10 | 11.16±11.59 | 0.47 |
| HLA-DR | 39.50±70.56 | 88.77±116.28 | 0.02 | 126.86±149.20 | 95.79±108.01 | 0.26 | 176.79±185.95 | 172.95±167.09 | 0.92 |
| IL-6 | 54.48±91.70 | 150.52±171.31 | 0.00 | 93.97±124.17 | 120.59±130.13 | 0.33 | 171.75±126.28 | 198.16±128.65 | 0.33 |
| IOP | 24.72±1.03 | 14.00±0.44 | 0.00 | 22.32±5.58 | 12.10±2.96 | 0.00 | 20.32±4.99 | 11.59±3.11 | 0.00 |
Impression cytology
Although the total score (histological classification) was worse for all of the groups after treatment, this change was not statistically significant. However, when specific IC parameters were considered, an increase in cellularity could be observed in all of the groups: TT - 0.04±0.30 cells (95% CI -0.56-0.64) prior to treatment and 0.386±0.58 cells (95% CI -0.774-1.546) after treatment (p = 0.0008); BT - 0.27±0.66 (95% CI -1.05-1.59) and 0.70±0.82 cells (95% CI -0.94-2.34) prior to and after treatment, respectively (p = 0.008); and LT - 0.182±0.54 (95% CI -1.498-1.862) and 0.545±0.85 cells (95% CI -1.155-2.245) prior to and after treatment, respectively (p = 0.0022).
The TT group had 0.29±0.63 goblet cells (95% CI -0.97-1.55) prior to treatment and 0.86±0.93 cells (95% CI -1.0-2.72) after treatment (p = 0.012). For the BT group, the corresponding figures were 0.57±0.873 (95% CI -1.176-2.316) and 0.93±0.873 (95% CI -0.816-2.676) (p = 0.054) and 0.182±0.54 (95% CI -0.898-1.262) and 0.545±0.85 (95% CI -1.155-2.245) (p = 0.0186) for the LT group, respectively.
The mean cell-to-cell contact was 0.136±0.40 (95% CI -0.664-0.936) in the TT group prior to treatment and 0.59±0.73 (95% CI -0.87-2.05) after treatment (p = 0.0005). For the BT group, the mean values were 0.27±0.54 (95% CI -0.81-1.35) and 0.41±0.58 (95% CI -0.75-1.57) prior to and after treatment (p = 0.26), respectively, and for the LT group, the corresponding values were 0.16±0.43 (95% CI -0.7-1.02) and 0.38±0.66 (95% CI -0.94-1.7), respectively (p = 0.07).
There were no changes in inflammatory cells, keratinization or N/C ratio (Table 3).
Table 3.
Impression cytology scores. Several parameters were analyzed, but significant differences after three months of treatment were only found in: (a) cell-to-cell contact in the TT group; (b) cellularity in all of the groups; and (c) number of goblet cells in the TT and LT groups. NC: not counted.
| Travoprost+Timolol | Bimatoprost+Timolol | Latanoprost+Timolol | |||||||
| Pre | Post | p-value | Pre | Post | p-value | Pre | Post | p-value | |
| Keratinization | NC | NC | NC | NC | NC | NC | |||
| Cell-to-cell Contact (a) | 0.136 | 0.591 | 0.0005 | 0.273 | 0.409 | 0.26 | 0.159 | 0.381 | 0.067 |
| Inflammatory cells | NC | NC | NC | NC | NC | NC | |||
| Cellularity (b) | 0.045 | 0.386 | 0.0008 | 0.273 | 0.705 | 0.008 | 0.114 | 0.545 | 0.0022 |
| Goblet cells (C) | 0.295 | 0.864 | 0.0012 | 0.568 | 0.932 | 0.0541 | 0.182 | 0.545 | 0.0186 |
| N/C ratio | NC | NC | NC | NC | NC | NC | |||
Immunocytochemistry
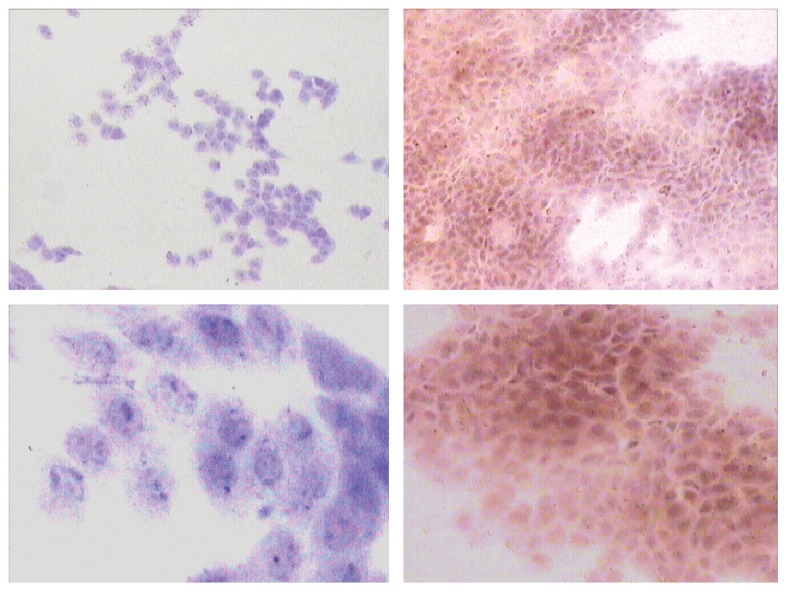
While TT induced a statistically significant increase in HLA-DR expression (the mean count of cells positive for HLA-DR in the TT group was 39.5±70.56 [95% CI -101.62-180.62] prior to treatment and 88.77±116.28 [95% CI -143.79-321.33] after treatment (p = 0.0184) [Figure 2]), BT and LT showed a decrease in HLA-DR expression that was not statistically significant for either group (BT, p = 0.26; LT, p = 0.92).
Figure 2.
HLA-DR expression. On the left: images of the travatan+timolol group pre-treatment, with cells negative for HLA. On the right: the same group after treatment, at 100× (on the top) and 400× (on the bottom) magnification, respectively.
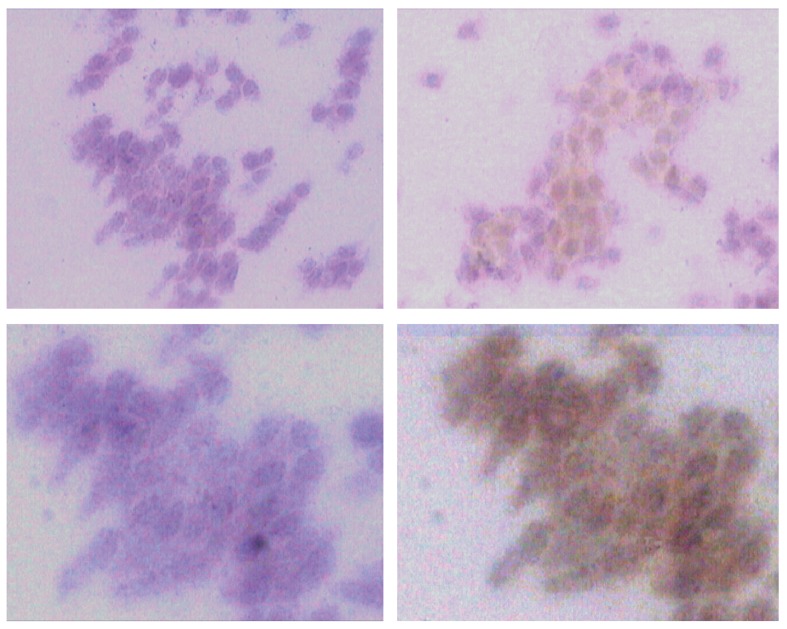
Similarly, TT induced a significant increase in IL-6-positive cells (Figure 3): the mean count was 58.48±91.70 (95% CI -124.92-241.88) prior to treatment and 150.52±171.31 (95% CI -192.1-493.14) after treatment (p = 0.0023). BT and LT also showed an increase in HLA-DR expression, but this increase was not statistically significant for either group (BT, p = 0.33; LT, p = 0.33).
Figure 3.
IL-6 expression. Top images: on the left, the bimatoprost+timolol group pre-treatment, with cells negative for IL-6. On the right: the same group after treatment, with 200× magnification. The figures on the bottom show the same images as above, with 400× magnification. Note the increase in positive cells after treatment (images on the right).
DISCUSSION
All three medications produced a statistically significant reduction in IOP, with no difference among them. Our results were similar to those described by Centofanti et al. regarding fixed combinations (14).
Impaired tear film production associated with glaucoma treatment has been described in many articles (8,10).
Strong evidence provided by previous clinical and experimental studies has indicated that the chronic use of antiglaucoma drugs might induce ocular surface changes, causing discomfort at instillation, conjunctival inflammation, tear film instability, subconjunctival fibrosis, apoptosis of conjunctival epithelial cells and corneal surface changes (15-17).
These changes could result in a greater risk of failure when patients undergo antiglaucoma surgery, particularly trabeculectomy, as a result of postoperative fibrosis (9,12,13).
In the present study, we observed that all three drugs induced a subclinical inflammatory reaction. This reaction was only detected via immunocytochemistry, which revealed cells positive for inflammatory markers, such as IL-6 and HLA-DR. No increase in inflammatory cells was detected via IC.
Subclinical inflammation associated with the use of latanoprost has been reported by other authors who, using histopathology and immunohistochemical markers (HLA-DR, IL-6 and IL-8), have described a strong correlation between dry eye and inflammatory cells in the conjunctival epithelium and substantia propria (15,17,18).
Immunocytochemistry after three months of treatment did not reveal any changes in total score. However, we observed changes in other related parameters, such as cellularity, number of goblet cells and cell-to-cell contact. The increase in cellularity without an inflammatory reaction was likely related to reactive hyperplasia of the conjunctival epithelial cells in response to the toxic effects of the drugs.
Authors such as Guenoun et al. have correlated the increase in goblet cells induced by PG analogues with a possible protective effect against BAK-induced toxicity to the ocular surface (19). In a previous study, we also observed an increase in the number of goblet cells in groups treated with PG analogues (latanoprost, travoprost and bimatoprost), but this increase was not sustained over six months (15,18,20). This effect has been controversial; hyperplasia in goblet cells seems to represent a protective mechanism in the initial phase of chronic aggression to the ocular surface, as observed in allergies or responses to pollution, but it is followed by a decrease in the number of these cells if the aggressive factor becomes chronic (21,22).
Changes in cell-to-cell contact result in a loss of “gap junctions” and in edema, which are reflected in the epithelial architecture and which lead to keratopathy. These pathological changes in the ocular surface could partly explain the occurrence of dry eye symptoms in patients using hypotensive drugs.
Herrera et al. reported that prolonged use (longer than six months) of 0.5% timolol maleate might lead to a higher incidence of dry eye, with lower TBUT values and Schirmer's test scores (7). Several reports have also demonstrated that timolol could cause a decrease in the number of goblet cells and keratoconjunctivitis sicca (7,8,13). Reductions in TBUT values and Schirmer's test scores have been described in many studies investigating the effects of the length of glaucoma treatment, the number of medications used and preservative concentrations (16,23,24).
In the present study, conjunctival function and tear film stability were worse after treatment, indicating deterioration of the ocular surface.
More recently, some authors have used the OSDI (Ocular Surface Disease Index) questionnaire, a useful tool for analyzing symptoms of dry eye, to measure symptoms in glaucoma patients; the lower the OSDI score is, the less toxic the medication is (24,26). In our study, OSDI scores also showed an increase, with most patients having mild or moderate dry eye, confirming the involvement of the lacrimal functional unit and the ocular surface. Preservative-free medications have been associated with lower scores, and improvements in scores have been reported for patients who switched to this type of medication (25,26).
One of the limitations in our study was related to ethnicity. The numbers of Asian and African American individuals distributed among the groups were considerably fewer than those of Caucasian and Hispanic individuals; this could have been a source of bias. Additionally, it should be noted that, in our study, a large variation was observed in patients treated with travoprost+timolol. Although the results were considered “normal,” this regimen group began with somewhat worse ocular surface conditions, compared with the other groups. There was a trend that was not statistically significant toward a poorer ocular surface, irrespective of sex or age, at the beginning of the study. This finding occurred by chance and was likely related to the randomization method (permuted-block) used to ensure sample balance, but it could lead to selection bias in a non-masked study. This trend was only noticed after the investigation was completed. For this reason and because of the short study period, we excluded comparisons among the three drugs.
The number of patients remaining after all of the exclusion criteria were applied was somewhat modest (n = 11 per group) for a multicentric study, but it was substantial for a two-center investigation. Thus, we assert that our results can be extrapolated and are applicable for general ophthalmic clinics, particularly for glaucoma and cornea specialists, as well as for large multicenter studies.
In summary, this study demonstrated that there were significant changes in the ocular surface after exposure to FCs for a short period (three months), despite the ease of administration (once daily), and reduced exposure to preservatives (one drop versus three if the medications were administered individually). However, it cannot be concluded from the present study whether any particular drug induced greater changes in the ocular surface.
ACKNOWLEDGMENTS
This study was supported by the Federal University of São Paulo and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Gordon MO, Beiser JA, Brandt JD. The ocular hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002 Jun;120(6):714–20. doi: 10.1001/archopht.120.6.714. discussion 829-30. [DOI] [PubMed] [Google Scholar]
- 2.Ritch R, Shields B, Krupin T. The Glaucomas. Second Edition. Vol. 3. St Louis, EUA: Mosby; 1996. Pharmacology; pp. 1375–489. [Google Scholar]
- 3.Stewart WC, Kolker AE, Stewart JA, Leech J, Jackson AL. Conjunctival hyperemia in healthy subjects after short-term dosing with latanoprost, bimatoprost, and travoprost. Am J Ophthalmol. 2003;135(3):314–20. doi: 10.1016/s0002-9394(02)01980-3. [DOI] [PubMed] [Google Scholar]
- 4.Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124(4):544–7. doi: 10.1016/s0002-9394(14)70870-0. [DOI] [PubMed] [Google Scholar]
- 5.Kook MS, Lee K. Increased eyelid pigmentation associated with use of latanoprost. Am J Ophhtalmol. 2000;129(6):804–6. doi: 10.1016/s0002-9394(00)00402-5. [DOI] [PubMed] [Google Scholar]
- 6.Arcieri ES, Santana A, Rocha FN, Guapo GL, Costa VP. Blood-aqueous barrier changes after the use of prostaglandin analogues in patients with pseudophakia and aphakia: a 6-month randomized trial. Arch Ophthalmol. 2005;123(2):186–92. doi: 10.1001/archopht.123.2.186. [DOI] [PubMed] [Google Scholar]
- 7.Herreras JM, Pastor JC, Calonge M, Asensio VM. Ocular surface alteration after long-term treatment with antiglaucomatous drug. Ophthalmology. 1992;99(7):1082–8. doi: 10.1016/s0161-6420(92)31847-0. [DOI] [PubMed] [Google Scholar]
- 8.Nuzzi R, Finazzo C, Cerruti A. Adverse effects of topical antiglaucomatous medications on the conjunctiva and the lacrymal. Int Ophthalmol. 1998;22(1):31–5. doi: 10.1023/a:1006051725115. [DOI] [PubMed] [Google Scholar]
- 9.Broadway DC, Grierson I, ÒBrien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. I- The conjunctival cell profile. Arch Ophthalmol. 1994;112(11):1437–45. doi: 10.1001/archopht.1994.01090230051020. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen J, Aaen K, Theodorsen F, Kessing SV, Prause JU. Short-term effect of latanoprost and timolol eye drops on tear fluid and the ocular surface in patients with primary open-angle glaucoma and ocular hypertension. Acta Ophthalmol Scand. 2000;78(1):37–44. doi: 10.1034/j.1600-0420.2000.078001037.x. [DOI] [PubMed] [Google Scholar]
- 11.Brandt JD, Wittpenn JR, Katz J, Steinmann WN, Spaeth GL. Conjunctival impression cytology in patients with glaucoma using long-term topical medication. Am J Ophthalmol. 1991;112(3):297–301. doi: 10.1016/s0002-9394(14)76730-3. [DOI] [PubMed] [Google Scholar]
- 12.Mietz H, Niesen U, Krieglstein GK. Effect of latanoprost and timolol on the histopathology of the rabbit conjunctiva. Invest Ophthalmol Vis Sci. 2001;42(3):679–87. [PubMed] [Google Scholar]
- 13.Broadway DC, Grierson I, ÒBrien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112(11):1446–54. doi: 10.1001/archopht.1994.01090230060021. [DOI] [PubMed] [Google Scholar]
- 14.Centofanti M, Oddone F, Gandolfi S, Hommer A, Boehm A, Tanga L, et al. Comparison of Travoprost and Bimatoprost plus timolol fixed combinations in open-angle glaucoma patients previously treated with latanoprost plus timolol fixed combination. American Journal of Ophthalmology. 2010;150(4):575–80. doi: 10.1016/j.ajo.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Russ HH, Costa VP, Montiani-Ferreira F, Valgas SR, Correa-Neto MA, Strobel EVL, et al. Conjunctival changes induced by prostaglandin analogues and timolol maleate: a histomorphometric study. Arq Bras Oftalmol. 2007;70(5):910–17. doi: 10.1590/s0004-27492007000600005. [DOI] [PubMed] [Google Scholar]
- 16.Baffa LP, Ricardo JRS, Dias AC, Modulo CM, Braz AM, Paula JS, et al. Tear film and ocular surface alterations in chronic users of antiglaucoma medications. Arq Bras Oftalmol. 2008;71(1):18–21. doi: 10.1590/s0004-27492008000100004. [DOI] [PubMed] [Google Scholar]
- 17.Mietz H, Esser JM, Welsandt G, Kociok N, Hueber A, Esser P, et al. Invest Ophthalmol Vis Sci. 2003;44(12):5182–8. doi: 10.1167/iovs.02-0462. [DOI] [PubMed] [Google Scholar]
- 18.Bensoussan L, Blondin C, Baudouin C, Hamard P, Sabeh Afaki G, Creuzot-Garcher C, et al. Flow cytometric analysis of HLA-DR, IL-6 and IL-8 expression by conjunctival epithelial cells from patients with prolonged topical antiglaucoma treatments. J Fr Ophtalmol. 2003;26(8):782–9. [PubMed] [Google Scholar]
- 19.Guenoun JM, Baudouin C, Rat P, Pauly A, Warnet JM, Brignole-Baudouin F. In vitro study of inflammatory potential and toxicity profile of latanoprost, travoprost, and bimatoprost in conjunctiva –derived epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(7):2444–50. doi: 10.1167/iovs.04-1331. [DOI] [PubMed] [Google Scholar]
- 20.Moreno M, Villena A, Cabarga C, Sanchez-Font E, Garcia Campos J. Impression cytology of the conjunctival epithelium after antiglaucomatous treatment with latanoprost. Eur J Ophthalmol. 2003;13(6):553–9. doi: 10.1177/112067210301300608. [DOI] [PubMed] [Google Scholar]
- 21.Novaes P, Saldiva PHN, Matsuda M, Macchioni M, Rangel MP, Kara-José N, et al. Ambient Levels of Air Pollution Induce Goblet-Cell Hyperplasia in Human Conjunctival Epithelium. Environ Health Perspect. 2007;115(12):1753–6. doi: 10.1289/ehp.10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baudouin C, Hamard P, Liang H, Creuzot-Garcher C, Bensoussan L, Brignole F. Conjunctival epithelial cell expression of interleukins and inflammatory markers in glaucoma patients treated over the long term. Ophthalmology. 2004;111(12):2186–92. doi: 10.1016/j.ophtha.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Cvenkel B, Ihan A. Ocular Surface Changes Induced by Topical Antiglaucoma Monotherapy. Ophthalmologica. 2002;216(3):175–9. doi: 10.1159/000059624. [DOI] [PubMed] [Google Scholar]
- 24.Arici MK, Arici DS, Topalkara A, Guler C. Adverse effects of topical antiglaucoma drugs on the ocular surface. Clin Experiment Ophthalmol. 2000;28(2):113–7. doi: 10.1046/j.1442-9071.2000.00237.x. [DOI] [PubMed] [Google Scholar]
- 25.Blondin C, Hamard P, Cholley B, Haeffner-Cavaillon N, Baudouin C. In vitro effects of preserved or preservative-free antiglaucoma medications on human complement system. Curr Eye Res. 2003;27(4):253–9. doi: 10.1076/ceyr.27.4.253.16603. [DOI] [PubMed] [Google Scholar]
- 26.Uusitalo H, Chen E, Pfeiffer N, Brignole-Baudouin F, Kaarniranta K, Leino M, et al. Switching from a preserved to a preservative-free prostaglandin preparation in topical glaucoma medication. Acta Ophthalmol. 2010;88(3):329–36. doi: 10.1111/j.1755-3768.2010.01907.x. [DOI] [PubMed] [Google Scholar]