Abstract
Background
Intracellular folate hemostasis depends on the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene. Because 5,10-MTHFR 677TT homozygosity and tobacco smoking are associated with low folate status, we tested the hypothesis that smoking in mothers with 5,10-MTHFR C677T or A1298C polymorphisms would be independently associated with lower birth weight among their offspring.
Methods
We assessed 1784 native Japanese mother-child pairs drawn from the ongoing birth cohort of The Hokkaido Study on Environment and Children’s Health. Data (demographic information, hospital birth records, and biological specimens) were extracted from recruitments that took place during the period from February 2003 to March 2006. Maternal serum folate were assayed by chemiluminescent immunoassay, and genotyping of 5,10-MTHFR C677T/A1298C polymorphisms was done using a TaqMan allelic discrimination assay.
Results
The prevalence of folate deficiency (<6.8 nmol/L) was 0.3%. The 5,10-MTHFR 677CT genotype was independently associated with an increase of 36.40 g (95% CI: 2.60 to 70.30, P = 0.035) in mean infant birth weight and an increase of 90.70 g (95% CI: 6.00 to 175.50, P = 0.036) among male infants of nonsmokers. Female infants of 677TT homozygous passive smokers were 99.00 g (95% CI: −190.26 to −7.56, P = 0.034) lighter. The birth weight of the offspring of smokers with 5,10-MTHFR 1298AA homozygosity was lower by 107.00 g (95% CI: −180.00 to −33.90, P = 0.004).
Conclusions
The results suggest that, in this population, maternal 5,10-MTHFR C677T polymorphism, but not the 5,10-MTHFR A1298C variant, is independently associated with improvement in infant birth weight, especially among nonsmokers. However, 5,10-MTHFR 1298AA might be associated with folate impairment and could interact with tobacco smoke to further decrease birth weight.
Key words: birth weight, tobacco smoking, MTHFR SNPs, folate, Japan
INTRODUCTION
Intracellular folate hemostasis depends on the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene, which is located at position 36 on the short arm of chromosome 1. This gene codes for the enzyme MTHFR, which catalyses the irreversible conversion of 5,10-MTHFR to 5-metyltetrahydrofolate, a substrate for methylation of homocysteine to methionine. Thus far, 14 rare mutations in MTHFR have been described, but the 2 most common single nucleotide polymorphisms (SNPs) are 5,10-MTHFR C677T (dbSNP ID: rs1801133)—a missense mutation in exon 4, characterized by an alanine to valine substitution on codon 222—and 5,10-MTHFR A1298C (dbSNP ID: rs1801131)—a point mutation in exon 7 characterized by a glutamate to alanine substitution on codon 429.1 5,10-MTHFR C677T is located in the catalytic N-terminal domain of the enzyme, while 5,10-MTHFR A1298C is located in the regulatory domain of the enzyme.2
Biochemically, the 5,10-MTHFR C677T polymorphism is associated with thermolability and reduced enzyme activity. The metabolic consequences are folate deficiency and mild hyperhomocysteinemia, a risk factor for thrombotic vascular diseases. It has been suggested that oxidative stress, platelet aggregation, and endothelial cell dysfunction contribute to the vasculotoxicity of homocysteine, and 5,10-MTHFR polymorphisms have been widely investigated in relation to a spectrum of several disease outcomes. Specifically, several studies have identified maternal 5,10-MTHFR C677T polymorphisms as obstetric genetic risk factors for spina bifida, placenta-related vasculopathies, spontaneous fetal loss, preterm delivery (PTD), low birth weight (LBW), small for gestational age (SGA), neurodevelopmental delays, and other congenital anomalies.3–15 However, several other investigators have found no such associations.16–25 This confusion might be explained by the fact that phenotypic expression of this genetic trait depends on folate status and other environmental factors that vary by geographic region and race.
Although the functional consequences of the 5,10-MTHFR A1298C variant are not well known, it is a risk factor for neural tube defects,26,27 and compound heterozygosity (5,10-MTHFR 677CT/1298AC) has been reported to have a biochemical profile similar to that of 677TT homozygosity.1,28
Maternal smoking during pregnancy is an established risk factor for intrauterine growth retardation (IUGR), SGA, PTD, and other adverse pregnancy outcomes.29 More recently, smoking has been associated with nutritional deficiencies, including folate deficiency.30–32 LBW secondary to IUGR or PTD remains a public health concern because it increases the risk of morbidity and mortality throughout life.
In Japan, there have been genetic association studies of the relation between folate and cardiovascular pathologies, cancers, Helicobacter pylori infection, and periodontal diseases. However, only a few such studies have investigated obstetric events, and none has considered infant birth size.3,6,17,33–35 We therefore tested the hypothesis that maternal smoking in the presence of the 5,10-MTHFR C677T or A1298C polymorphisms independently reduces birth weight.
METHODS
Study design and participants
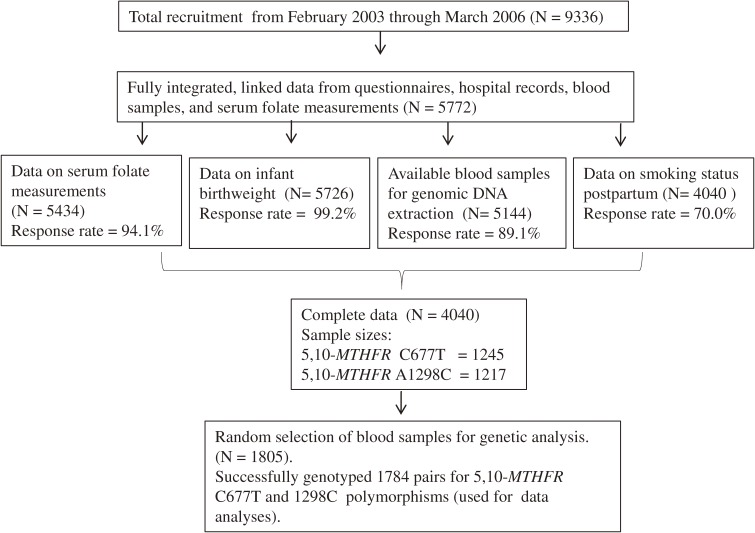
The study participants were native Japanese mother-child pairs drawn from an ongoing birth cohort: The Hokkaido Study on Environment and Children’s Health. This ongoing cohort started in February 2003, and the details of the study have been previously described.36 Briefly, all indigenous Japanese women who reserved antenatal care at any of 37 participating hospitals within Hokkaido during their first trimester of pregnancy were considered eligible. Health care personnel introduced the study, after which each potential participant was given an invitation that included a consent form, baseline questionnaire, and self-addressed envelopes for return of the signed consent forms and completed questionnaires. The participants were recruited between February 2003 and March 2006. Only participants with linked and integrated data (5772; 61.8%) were included in the baseline population of this study. The response rate for each variable was at least 70.0% from various sources. Based on the population allele frequencies of the 5,10-MTHFR C677T37 and A1298C38 polymorphic variants specific to Japanese and the prevalence of tobacco smoking during pregnancy,39 minimum sample sizes were calculated by using genetic software.40 We randomly selected 1805 extracted genomic DNAs, attempted to discriminate the alleles of 5,10-MTHFR polymorphisms, and successfully genotyped 1784, which were ultimately used in the data analysis (Figure 1). The Institutional Ethical Board for Human Gene and Genome Studies of Hokkaido University Graduate School of Medicine approved the study protocol.
Figure 1. Study selection flow chart.
Methods of data collection
Data were acquired from baseline self-administered questionnaires, hospital records of infant births, and postpartum self-administered questionnaires. Baseline information included biodata, lifestyle habits, drugs (including use of nutritional supplements), and gynecologic and obstetric histories. Infant birth records from hospitals had information about birth weight, gestational age at delivery, sex, obstetric events during index pregnancy, and congenital anomalies, among other information. Postpartum questionnaires collected information on infant anthropometric parameters, active or passive tobacco smoking during the index pregnancy, and whether the index pregnancy was eventful. Each variable in the dataset had a response rate of at least 70.0%, although the item on smoking status decreased from 99.0% at baseline to 70.0% after pregnancy. Whole-blood specimens were collected during the first trimester for serum folate assays; subsequent collections of whole blood specimens were stored at −80°C for genetic analyses.
Folate assay
Serum folate was assayed by a commercial laboratory (SRL, Inc. Tokyo, Japan) using an automated competitive protein binding (CPB) chemiluminescent enzyme immunoassay (CLEIA) technique according to the manufacturer’s protocols. This type of assay has an intra- and inter-assay imprecision of 10.0% or less and has become common in in vitro studies, as it is less costly, faster, and convenient. In addition, the need for smaller samples is advantageous in large scale epidemiologic studies.41 The specific assay method for this study was the ADVIA Centaur technique, which has a coefficient of variation between 4.0% to 4.3%.42 Analyses were conducted in batches, which were scheduled with regard to recruitment period and laboratory procedure.
Selection and genotyping of single nucleotide polymorphisms (SNPs)
We chose the 2 most common SNPs of this gene, namely C677T and A1298C, which have minor allele frequencies (MAF) of 35.2%37 and 19.0%,38 respectively, among Japanese. Genomic DNAs were extracted using a Maxwell 16 Instrument (Promega Corporation, WI, USA). DNA amplifications were performed in batches on 96-well micro-amp reaction plates using validated TaqMan probes for MTHFR C677T and A1298C (assay IDs: C_1202883_20 and C_850486_20), respectively, on a Gene Amp 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA) with an end-point allelic discrimination (AD) assay on a 7300/7500 Real-time PCR System43 (Applied Biosystems, Foster City, CA, USA). We randomly selected 95 samples (5.0% of the successfully genotyped samples) and repeated genotyping to check for genotyping quality. The results were 100% concordant.
Definition of variables
Environmental exposures
Overall smoking status was classified into 3 categories using both self-reported active smoking and passive exposure to environmental tobacco smoke (ETS) at home. Nonsmokers had no history of active smoking or exposure to ETS at home. Nonsmokers and quitters with ETS exposure were classified as the passive smoking group, while smokers consisted of active smokers irrespective of ETS exposure status. Quitters with no ETS exposure during the first trimester had mean infant birth weights similar to those of nonsmokers; hence, they were added to the nonsmoking group. Mothers who quit during the second or third trimesters were added to the active smoking group.
Genetic exposures
The 5,10-MTHFR C677T and A1298C genotypes were categorized as dominant homozygous, heterozygous, and recessive homozygous genotypes (677CC, 677CT, and 677TT; and 1298AA, 1298AC, and 1298CC, respectively).
Statistical analyses
Univariate ANOVA with multiple comparison tests was performed to assess the main effects of maternal 5,10-MTHFR C677T and A1298C polymorphisms and smoking on serum folate levels, while ANCOVA was used to investigate the interactive association between smoking and 5,10-MTHFR C677T and A1298C polymorphisms in relation to folate status and infant birth weight. Serum folate was log-transformed before the analyses and back-transformed after the analyses. Known major predictors of infant birth weight (infant sex, gestational age at delivery, maternal age, maternal prepregnancy weight, maternal height, parity, and alcohol intake during pregnancy) were adjusted for in the multivariate regression analyses. Use of a folic acid supplement, which was highly correlated with serum folate levels, was also included as a covariate. Smoking status was adjusted for when we assessed the predictive power of each SNP on birth weight. Categorical covariates were dichotomized to fit the regression equation. Subgroups with few participants (ie, subgroups for the 1298CC genotype) were excluded from the regression analyses. We used the codominant genetic model and per-allele approach. Our preliminary analyses revealed that mean serum folate was highest for the MTHFR 1298AC genotype. Because adequate folate status is an integral part of our hypothesis, we decided that it was biologically plausible to set 1298AC as the reference category in the regression analyses. Predictors were entered simultaneously into the equation. Assessment of the 5,10-MTHFR C677T and A1298C genotypes for deviation from the Hardy-Weinberg equilibrium, and other evaluations of data quality, were conducted using Haploview version 4.2 software.44 All other analyses were performed using SPSS version 16.00 for Windows (SPSS Inc., Chicago, IL, USA). The level of statistical significance was set at less than 0.05.
RESULTS
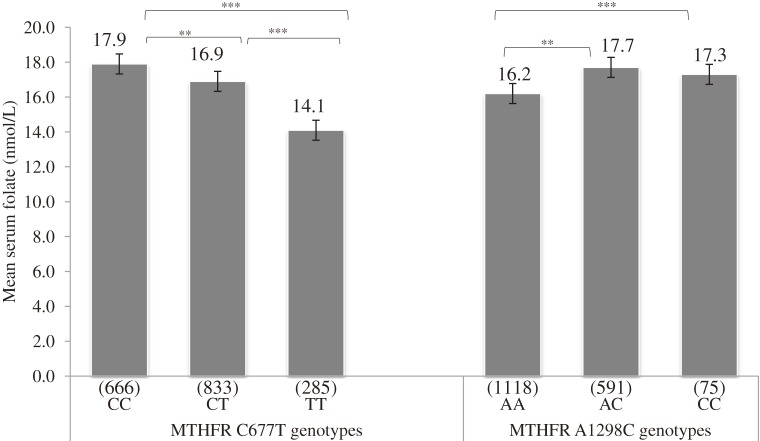
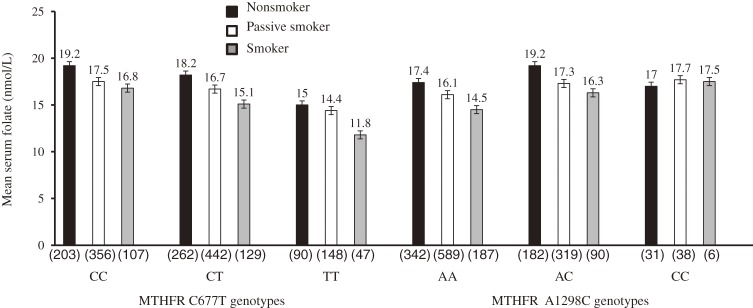
The maternal mean serum folate (SD) level was 16.4 (1.5) nmol/L. The prevalence of folate deficiency (<6.8 nmol/L) was 0.3%; most (73.0%) mothers had adequate folate status (≥13.6 nmol/L). The prevalence of folic acid supplementation was 10.0%. Mean infant birth weight (SD) was 3040 (374) g. The prevalence of active smoking during pregnancy was 15.9%, while that of passive smoking was 53.0%. The distributions of the 5,10-MTHFR C677T and A1298C genotypes did not deviate from the Hardy-Weinberg equilibrium (P = 0.546 and 0.909, respectively). The frequencies of MTHFR 677CC, 677CT, and 677TT were 37.3%, 46.7%, and 16.0%, respectively, while those of MTHFR 1298AA, 1298AC, and 1298CC were 62.7%, 33.1%, and 4.2%, respectively. A strong LD (D′ = 0.943) between MTHFR C677T and A1298C was also observed, and the minor allele frequencies were 0.392 and 0.205, respectively (Table 1). These findings were similar to those of previous studies of Japanese populations.6,37,38,45–47 We used 2-way analysis of variance to assess the main effects of 5,10-MTHFR on maternal mean serum folate concentration. Carrying the T allele was associated with a decrease in mean serum folate level, and the lowest level (14.1 nmol/L) was observed in the 677TT homozygous group. Tukey’s honestly significant differences (HSD) of 1.0 nmol/L (P = 0.008) and 3.8 nmol/L (P < 0.001) were observed between 677CC versus 677CT and between 677CC versus 677TT, respectively. In contrast, carrying the 1298C allele was associated with higher mean serum folate levels. A Tukey’s HSD of 1.5 nmol/L (P < 0.001) was observed between 1298AA versus 1298AC (Figure 2). Mean serum folate levels in the analysis of covariance were generally lower among smokers for all 5,10-MTHFR C677T genotypes, and the lowest level was found among 677TT homozygotes (11.8 nmol/L, Pinteraction < 0.001). With regard to 5,10-MTHFR A1298C genotypes, the lowest mean folate level was observed among smokers with the 1298AA homozygous genotype, (Pinteraction < 0.001; Figure 3).
Table 1. Characteristics of 1784 mother-child pairs.
| Characteristic | n (%) |
| Maternal age (years) | 30.0 (4.3)e |
| Maternal height (cm) | 158.0 (5.1)e |
| Prepregnancy weight (kg) | 53.0 (9.3)e |
| Maternal serum folate (nmol/L) | 16.4 (1.5)e |
| Gestational age at delivery (weeks) | 38.9 (1.3)e |
| Infant birth weight (g) | 3040 (374)e |
| Infant sex | |
| Male | 873 (48.9) |
| Female | 911 (51.1) |
| Parity | |
| Nulliparous | 391 (21.9) |
| Parous | 1393 (78.1) |
| Alcohol intake during pregnancy | |
| No | 1499 (84.0) |
| Yes | 285 (16.0) |
| Tobacco smoking during pregnancy | |
| Nonsmoker | 555 (31.1) |
| Passive smoker | 946 (53.0) |
| Smoker | 283 (15.9) |
| Folic acid supplementation | |
| No | 1601 (89.7) |
| Yes | 183 (10.3) |
| Maternal MTHFR C677T genotypea,b | |
| CC | 666 (37.3) |
| CT | 833 (46.7) |
| TT | 285 (16.0) |
| CT/TT | 1118 (62.7) |
| Maternal MTHFR A1298C genotypec,d | |
| AA | 1118 (62.7) |
| AC | 591 (33.1) |
| CC | 75 (4.2) |
| AC/CC | 666 (37.3) |
aHWE = Hardy-Weinberg equilibrium P = 0.5463
bMAF = Minor allele frequency = 0.392
cHWE = Hardy-Weinberg equilibrium P = 0.9091
dMAF = Minor allele frequency = 0.205
eMean (SD) MTHFR = Methylenetetrahydrofolate reductase gene
Figure 2. Maternal mean serum folate levels across MTHFR C677T and A1298C genotypes. **P < 0.01, ***P < 0.001 Univariate analysis with Tukey’s honestly significant differences test. Values in parentheses are counts in each group.
Figure 3. Maternal mean serum folate levels across MTHFR C677T and A1298C genotypes according to smoking status. ANCOVA Pinteraction < 0.001. For MTHFR C677T and A1298C. Values in parentheses are counts in each group.
We initially explored the role of maternal serum folate during the first trimester as a predictor of infant birth weight and found no significant linear association. After stratification by folate status, low folate status (<13.6 nmol/L) was associated with a nonsignificant reduction in birth weight. After stratification by birth weight status, low folate status was associated with a 34.00 g (P = 0.045) reduction in mean birth weight of infants in the normal birth weight group (data not shown).
To investigate whether the maternal 5,10-MTHFR C677T and A1298C polymorphisms were independently associated with birth weight, we conducted a multiple regression analysis with adjustments for known major predictors of birth weight. Among infants of 677CT heterozygous mothers, adjusted mean birth weight was highest (3061 g) for 5,10-MTHFR C677T. The 677CT genotype was associated with a 36.40 g increase in mean infant birth weight (95% CI: 2.60 to 70.30, P = 0.035). Carrying the 677T allele was associated with a marginally significant 27.00 g increase in infant birth weight (95% CI: −3.76 to −59.47, P = 0.084).
Polymorphism in 5,10-MTHFR A1298C or carrying the 1298C allele was not significantly independently associated with birth weight, although the adjusted mean infant birth weight was highest (3048 g) in the 1298AC heterozygous group. The adjusted mean birth weight of infants of active tobacco smokers was lowest (2978 g) and was 85.00 g (95% CI: 133.30 to −36.80, P = 0.001) less than that of children born to nonsmokers (Table 2).
Table 2. Association of maternal 5,10-MTHFR C677T, A1298C genotypes and tobacco smoking with infant birth weight (N = 1784).
| Maternal 5,10-MTHFR polymorphisms/smoking status |
n | Adjusted mean birth weight (SE) g |
Adjusted Δ B (SE) [95% CI] g |
Ptrendc |
| aMTHFR C677T genotype | ||||
| CC | 666 | 3024.70 (14.51) | Reference | |
| CT | 833 | 3061.13 (12.98) | 36.40 (17.30) [2.60, 70.30]* | |
| TT | 285 | 3015.15 (22.75) | 4.00 (23.50) [−42.20, 50.10] | 0.07 |
| aMTHFR C677T allele | ||||
| C | 2165 | 3044.97 (9.68) | Reference | |
| T | 1403 | 3049.52 (11.29) | 27.86 (16.12) [−3.76, 59.47]† | |
| aMTHFR A1298C genotype | ||||
| AA | 1118 | 3036.58 (11.17) | Reference | |
| AC | 591 | 3048.71 (15.67) | 14.47 (16.78) [−18.45, 47.38] | |
| CC | 75 | 3028.59 (44.69) | −27.65 (39.36) [−104.85, 49.55] | 0.49 |
| aMTHFR A1298C allele | ||||
| A | 2827 | 3040.77 (9.1) | Reference | |
| C | 741 | 3046.43 (14.78) | 9.74 (16.16) [−21.95, 41.43] | |
| bSmoking status | ||||
| Nonsmoker | 555 | 3062.81 (14.11) | Reference | |
| Passive smoker | 946 | 3046.08 (10.70) | −14.40 (17.90) [−49.50, 20.60] | |
| Smoker | 283 | 2978.29 (19.70) | −85.01 (24.60) [−133.30, −36.80]** | 0.001 |
**P < 0.01. *P < 0.05. †P < 0.1. Δ (Change in mean birth weight) B (Unstandardized coefficients) SE (standard error). (CI) confidence interval. (MTHFR) Methylenetetrahydrofolate reductase. Grams (g). aMultiple linear regression adjusted for gestational age, infant sex, maternal age, prepregnancy weight, height, parity, smoking during pregnancy, alcohol intake during pregnancy, and folic acid supplement use. bMultiple linear regression adjusted for gestational age, infant sex, maternal age, prepregnancy weight, height, parity, and folic acid supplement intake. cPolynomial univariate analysis.
We stratified mothers by tobacco smoking status. Among nonsmokers, male infants of 677CT genotype mothers were 90.00 g (95% CI: −2.11 to 182.50, P = 0.05) heavier than the infants in the reference category, which was marginally statistically significant, while among passive smokers, female infants of 677TT homozygous mothers were 99.00 g (95% CI: −190.26 to −7.56, P = 0.03) lighter than reference. None of the minor-allele genotypes among smokers showed any significant effect on infant birth weight as compared with those in the major-allele genotypes (data not shown). Per-allele analyses revealed that carrying the 677T allele was associated with a 68.00 g (95% CI: −121.74 to −15.27, P = 0.012, Ptrend = 0.003) reduction in mean birth weight among infants of smokers and an 89.00 g (95% CI: −168.89 to −9.56, P = 0.028, Ptrend = 0.018) reduction among female infants (Table 3a). Furthermore, smoking in mothers carrying the 1298A allele was associated with a 92.00 g (95% CI: −144.46 to −40.96, P < 0.001, Ptrend = 0.091) reduction in mean birth weight. Males were lighter by 79.00 g (95% CI −150.73 to −8.58, P = 0.028, Ptrend = 0.228), while females were lighter by 107.00 g (95% CI: −182.78 to −31.54, P = 0.006, Ptrend = 0.112; Table 3b).
Table 3a. Association of maternal 5,10-MTHFR C677T polymorphism and tobacco smoking with infant birth weight (N = 1784).
| 5,10-MTHFR | Smoking status | Overall (N = 1784) | Males (N = 873) | Females (N = 911) | ||||||
| n | Adjusted Mean birth weight (SE) g |
Adjusted Δ B (SE) [95% CI] g |
n | Adjusted Mean birth weight (SE) g |
Adjusted Δ B (SE) [95% CI] g |
n | Adjusted Mean birth weight (SE) g |
Adjusted Δ B (SE) [95% CI] g |
||
| C677T genotype | ||||||||||
| CC | Nonsmoker | 203 | 3008.84 (25.84) |
Reference | 93 | 3080.01 (37.75) |
Reference | 110 | 2949.95 (34.58) |
Reference |
| Passive smoker | 356 | 3035.93 (19.85) |
9.5 (29.3) [−47.9, 66.9] |
187 | 3037.8 (27.16) |
−39.6 (40.5) [−119.2, 39.9] |
169 | 3033.88 (29.16) |
59.3 (41.6) [−22.3, 140.8] |
|
| Smoker | 107 | 3017.29 (37.52) |
−38.7 (39.8) [−116.8, 39.4] |
52 | 3109.23 (52.51) |
−37.7 (55.8) [−147.3, 71.8] |
55 | 2928.76 (51.18) |
55.3 (56.5) [−166.2, 55.6] |
|
| CT | Nonsmoker | 262 | 3092.43 (24.44) |
60.0 (31.0) [−0.9, 120.9]† |
133 | 3162.77 (35.43) |
90.7 (43.20) [6.0, 175.50]* |
129 | 3020.99 (32.59) |
34.1 (44.1) [−52.4, 120.6] |
| Passive smoker | 442 | 3068.18 (17.15) |
47.5 (28.2) [−7.9, 102.9] |
198 | 3114.72 (24.29) |
21.2 (40.3) [−57.8, 100.3] |
244 | 3030.45 (23.78) |
62.1 (39.1) [−14.6, 138.8] |
|
| Smoker | 129 | 2973.99 (32.43) |
−53.1 (37.8) [−127.2, 21.0] |
70 | 3041.21 (35.16) |
−39.8 (51.1) [−140.0, 60.4] |
59 | 2894.24 (55.93) |
−63.5 (55.2) [−171.9, 44.9] |
|
| TT | Nonsmoker | 90 | 3087.77 (38.76) |
59.1 (42.0) [−23.3, 141.4] |
43 | 3123.57 (54.59) |
15.1 (58.8) [−100.6, 130.5] |
47 | 3055.09 (55.04) |
91.7 (59.0) [−24.0, 207.4] |
| Passive smoker | 148 | 2984.38 (31.57) |
−14.4 (36.1) [−85.2, 56.3] |
74 | 3045.41 (45.87) |
11.1 (50.1) [−87.2, 109.4] |
74 | 2922.5 (42.44) |
−39.7 (51.1) [−140.0, 60.6] |
|
| Smoker | 47 | 2974.11 (58.61) |
−49.1 (53.9) [−154.8, 56.7] |
23 | 3067.13 (73.23) |
−53.7 (74.9) [−200.7, 93.3] |
24 | 2884.96 (88.5) |
−48.3 (76.7) [−198.8, 102.2] |
|
| Pinteraction | 0.03 | 0.02 | 0.14 | |||||||
| C677T alleled | ||||||||||
| C | Nonsmoker | 465 | 3059.64 (15.39) |
Reference | 226 | 3137.62 (21.58) |
Reference | 239 | 2987.47 (21.95) |
Reference |
| Passive smoker | 798 | 3053.06 (11.64) |
−13.37 (19.69) [−51.98, 25.25] |
385 | 3075.26 (56.28) |
−60.94 (26.60) [−113.15, −8.73]* |
413 | 3030.17 (16.61) |
35.80 (29.20) [−21.51, 93.11] |
|
| Smoker | 236 | 2979.64 (21.57) |
−63.45 (33.22) [−128.61, 1.71]† |
122 | 3046.22 (29.14) |
−57.52 (46.52) [−148.82, 33.78] |
114 | 2909.50 (32.18) |
−78.85 (47.97) [−173.00, 15.30] |
|
| Pinteraction | 0.45 | 0.75 | 0.06 | |||||||
| T | Nonsmoker | 352 | 3084.10 (17.66) |
Reference | 176 | 3159.40 (24.30) |
Reference | 176 | 3015.04 (25.68) |
Reference |
| Passive smoker | 590 | 3055.28 (13.57) |
−9.93 (17.12) [−23.65, 43.52] |
272 | 3103.07 (19.34) |
12.38 (24.16) [−35.03, 59.79] |
318 | 3009.48 (19.08) |
7.75 (24.40) [−40.15, 55.64] |
|
| Smoker | 176 | 2975.05 (24.89) |
−68.50 (27.14) [−121.74, −15.27]* |
93 | 3042.31 (33.20) |
−48.48 (36.51) [−120.13, 23.18] |
83 | 2909.60 (37.38) |
−89.22 (40.59) [−168.89, −9.56]* |
|
| Pinteraction | 0.34 | 0.48 | 0.46 | |||||||
***P < 0.001. *P < 0.05. †P < 0.1. Δ (Change in mean birth weight), B (Unstandardized coefficient) SE (Standard error). CI (Confidence interval), g (Grams). aMultiple linear regression adjusted for infant sex, gestational age at delivery, maternal age, maternal height, prepregnancy weight, parity, alcohol intake during pregnancy, and folic acid supplement intake. bMultiple linear regression adjusted for gestational age at delivery, maternal age, maternal height, prepregnancy weight, parity, alcohol intake during pregnancy, and folic acid supplement intake. cExcluded from the regression analyses. dPtrend by smoking status (C allele = 0.021, Males = 0.045, Females = 0.040 and T allele = 0.003, males = 0.073, Females = 0.018).
Table 3b. Association of maternal 5,10-MTHFR A1298C polymorphism and tobacco smoking with infant birth weight (N = 1784).
| 5,10-MTHFR | Smoking status | Overall (N = 1784) | Males (N = 873) | Females (N = 911) | ||||||
| n | Adjusted Mean birth weight (SE) g | Adjusted Δ B (SE) [95% CI] g |
n | Adjusted Mean birth weight (SE) g | Adjusted Δ B (SE) [95% CI] g |
n | Adjusted Mean birth weight (SE) g | Adjusted Δ B (SE) [95% CI] g |
||
| A1298C genotype | ||||||||||
| AA | Nonsmoker | 342 | 3071.9 (20.47) |
Reference | 169 | 3145.63 (28.99) |
Reference | 173 | 3000.74 (27.94) |
Reference |
| Passive smoker | 589 | 3036.45 (15.32) |
−29.04 (22.36) [−72.91, 14.82] |
266 | 3069.39 (23.03) |
−59.35 (31.34) [−120.86, 2.16] |
323 | 3009.36 (20.43) |
−3.07 (32.08) [−66.03, 59.90] |
|
| Smoker | 187 | 2973.15 (26.53) |
−106.59 (30.12) [−165.67, −47.52]*** |
94 | 3045.5 (31.69) |
−104.19 (41.36) [−185.37, −23.03]* |
93 | 2900.02 (41.47) |
−113.45 (44.05) [−199.91, −26.99]* |
|
| AC | Nonsmoker | 182 | 3058.19 (29.74) |
−1.66 (30.08) [−60.66, 57.34] |
83 | 3110.86 (45.78) |
13.56 (42.70) [−70.26, 97.37] |
99 | 3015.1 (38.68) |
−13.88 (42.68) [−97.64, 69.89] |
| Passive smoker | 319 | 3049.36 (20.36) |
−15.20 (25.59) [−65.39, 34.98] |
173 | 3066.73 (25.49) |
−51.98 (34.39) [−119.47, 15.51] |
146 | 3028.88 (32.66) |
21.20 (38.41) [−54.19, 96.59] |
|
| Smoker | 90 | 3027.25 (42.41) |
−57.04 (39.11) [−133.74, 19.66] | 46 | 3120.85 (51.65) |
−59.18 (53.47) [−164.14, 45.78] |
44 | 2927.12 (65.41) |
−64.21 (58.00) [−178.05, 49.62] |
|
| CC | Nonsmoker | 31 | 2959.81 (55.2) |
−120.92 (61.81) [−242.16, 0.32]† |
17 | 3037.65 (79.96) |
−107.17 (81.49) [−267.12, 52.77] |
14 | 2865.29 (69.02) |
−142.72 (94.43) [−328.05, 42.61] |
| Passive smoker | 38 | 3092.97 (65.72) |
13.12 (61.81) [−97.08, 123.31] |
20 | 3158.9 (98.97) |
14.55 (75.56) [−133.75, 162.84] |
18 | 3019.72 (84.17) |
6.64 (84.21) [−158.64, 171.91] |
|
| Smoker | 6c | 2976.17 (247.92) |
— | 5c | 3054.6 (288.05) |
— | 1c | 2584 | — | |
| Pinteraction | 0.04 | 0.03 | 0.22 | |||||||
| A1298C alleled | ||||||||||
| A | Nonsmoker | 524 | 3069.05 (14.52) |
Reference | 252 | 3140.25 (20.45) |
Reference | 272 | 3004.92 (20.68) |
Reference |
| Passive smoker | 908 | 3045.91 (10.93) |
−15.55 (17.17) [−49.23, 18.12] |
439 | 3075.06 (15.24) |
−35.44 (24.30) [−83.13, 12.26] |
469 | 3016.12 (15.67) |
−6.65 (24.40) [−45.24, 50.54] |
|
| Smoker | 277 | 2978.20 (19.91) |
−92.71 (26.38) [−144.46, −40.96]*** |
140 | 3043.18 (27.23) |
−79.65 (36.21) [−150.73, −8.58]* |
137 | 2912.57 (29.35) |
−107.16 (38.53) [−182.78, −31.54]** |
|
| Pinteraction | 0.15 | 0.22 | 0.47 | |||||||
| C | Nonsmoker | 213 | 3049.58 (22.66) |
Reference | 100 | 3124.26 (32.36) |
Reference | 113 | 2980.20 (31.83) |
Reference |
| Passive smoker | 357 | 3056.17 (17.48) |
−18.49 (19.73) [−20.21, 57.20] |
193 | 3084.82 (23.06) |
−4.28 (26.42) [−56.13, 47.58] |
164 | 3031.86 (26.57) |
41.38 (29.65) [−16.81, 99.57] |
|
| Smoker | 97 | 3009.71 (33.78) |
−29.12 (34.99) [−97.75, 39.50] |
51 | 3070.73 (44.90) |
−19.37 (47.02) [−111.66, 72.92] |
45 | 2942.56 (51.53) |
−46.16 (52.88) [−149.95, 57.64] |
|
| Pinteraction | 0.37 | 0.72 | 0.51 | |||||||
***P < 0.001. *P < 0.05. †P < 0.1. Δ (Change in mean birth weight). B (Unstandardized coefficient) SE (Standard error). CI (Confidence interval), g (Grams). aMultiple linear regression adjusted for infant sex, gestational age at delivery, maternal age, maternal height, prepregnancy weight, parity, alcohol intake during pregnancy, and folic acid supplement intake. bMultiple linear regression adjusted for gestational age at delivery, maternal age, maternal height, prepregnancy weight, parity, alcohol intake during pregnancy, and folic acid supplement intake. cExcluded from the regression analyses. dPtrend by smoking status (A allele = 0.091, Males = 0.228, Females = 0.112 and C allele = 0.752, males = 0.828, Females = 0.271).
In cross-classification interactive analyses, infants born to nonsmokers with 5,10-MTHFR 677CT genotypes had the highest mean birth weight (3092 g); male newborns were 90.70 g (95% CI: 6.00 to 175.50, P = 0.036) heavier than the male infants of nonsmoking 677CC mothers, (Pinteraction = 0.020; Table 3). The 5,10-MTHFR 1298AA genotype was associated with a 107.00-g (95% CI: −165.67 to −47.52, P < 0.001) decrease in mean infant birth weight among smokers. Stratification by infant sex did not yield obvious differences in birth weight, Pinteraction = 0.040; Table 4). When 1298AC was set as the reference category, the 5,10-MTHFR 1298AA genotype was associated with a 107.00-g (95% CI, −180.00 to −33.90, P = 0.004) decrease in mean infant birth weight in smokers; the effect was more obvious in male infants (117.00 g; 95% CI: −218.60 to −14.70, P = 0.025; data not shown).
DISCUSSION
Among nonsmokers, we found an association of maternal 5,10-MTHFR 677CT heterozygosity with higher infant birth weight, while 5,10-MTHFR 677TT homozygosity was associated with lower birth weight among female infants of passive tobacco smokers. In addition, among smokers, 5,10-MTHFR 1298AA homozygosity was associated with low folate status and lower birth weight. To our knowledge, this is the first study to report such findings for a Japanese population.
Maternal 5,10-MTHFR C677T, MTHFR A1298C and serum folate status
Our results showed an association between the 5,10-MTHFR 677T allele and low folate status, which agrees with the findings of earlier reports.6,46 677TT homozygosity was associated with low folate status, and values were much lower among active and passive smokers, which suggests independent and combined effects of tobacco smoke and 5,10-MTHFR C677T polymorphism on folate status.
In contrast, the 5,10-MTHFR 1298C allele was associated with higher serum folate levels, while the 1298AA genotype was associated with lower folate levels. Although the metabolic and clinical functions of this SNP have not been fully characterized, it is currently being studied by a number of investigators. In a recent study of Koreans, mean plasma homocysteine was higher among 1298AA homozygotes as compared with those carrying the 1298C allele.48 Because serum folate is inversely correlated with plasma homocysteine, we inferred that our study population might have a similar plasma homocysteine distribution across genotypes. A report from Portugal noted that 1298AC heterozygosity was associated with a high plasma folate level and that the level was lowest among 1298CC homozygotes,49 which is similar to the findings of the present study. Our findings contradict those of a study of a Dutch population, in which MTHFR A1298C alone was not associated with any biochemical abnormalities except when in combination with MTHFR C677T, specifically compound heterozygosity 5,10-MTHFR 677CT/1298AC.28 The fact that our findings were similar to those from a report on a Korean population is genetically plausible because racial, geographic, and nutritional disparities might account for differences in the functional characteristics of 5,10-MTHFR SNPs.50,51
Effects of maternal 5,10-MTHFR A1298C polymorphism and tobacco smoke on infant birth weight
Smokers carrying 1298A alleles delivered infants with lower mean birth weights, especially female infants; however, these results must be interpreted with caution because alleles do not act in isolation. The effect of the maternal 5,10-MTHFR 1298AA genotype in reducing the birth weight of infants delivered by tobacco smokers might be due to low folate status associated with the 1298AA genotype. Perhaps some essential folate-dependent cellular processes were compromised. Cells that lack folate have been observed to accumulate in the S-phase of the cell cycle. Such cells have higher uracil misincorporation and DNA damage,52 which might have a role in the impairment of fetal growth. Moreover, chronic deficits in extracellular and intracellular folate due to the effects of tobacco smoke might have been severe enough to inflict nutritional stress. In our study, we could not examine the role of the 1298CC genotype among smokers because of its low frequency. However, previous reports observed that the maternal 1298CC genotype was associated with a greater reduction in the risk of low birth weight as compared with the 1298AA genotype.21 The 1298CC homozygous genotype was also reported to be protective against IUGR in Canadians.53 Hyperhomocysteinemia might have increased the risk of placental vasculopathy via oxidative stress, endothelial cell dysfunction, and/or coagulopathies leading to feto-placental hypoperfusion.5
Adequate serum vitamin B12 status has been shown to decrease total plasma homocysteine levels in Japanese.54 However, among smokers, the possible coexistence of nutritional deficiencies, including vitamin B12 deficiency, might have compromised the methylation of homocysteine to methionine, resulting in impaired fetal growth. The folate level was probably not adequate to silence the phenotypic expression of 1298AA among smokers. Higher exogenous folate may be needed to correct deficits and maintain ideal levels for optimal fetal growth.
With regard to the 5,10-MTHFR gene structure, the A1298C variant is located on the regulatory C-terminal domain, which contains protein retention signals that prevent delivery of proteins to the secretory pathway. It is possible that allosteric inhibitory interplay in the s-adenosyl methionine (SAM) and s-adenosyl homocysteine (SAH) cycle is involved in the functional behavior of this SNP in relation to folate status and mediation of fetal growth. Recently, the 5,10-MTHFR A1298C polymorphism was found to be associated with increased folate levels in red blood cells, in an inverse relationship with 5,10-MTHFR C677T polymorphism,55 which suggests that both SNPs have different functional characteristics with regard to phenotypic expression.
Due to limited evidence on the functional role of the 5,10-MTHFR A1298C polymorphism, especially among Japanese, further research is needed to verify this observation and elucidate the biological mechanisms associated with this SNP.
Effects of the maternal 5,10-MTHFR C677T polymorphism and tobacco smoke on infant birth weight
The 5,10-MTHFR 677T allele is associated with low folate and high homocysteine levels. In this study, the 677T allele was associated with lower birth weight in offspring of active smokers. The 677CT genotype was protective against low birth weight, especially among male offspring, but only in the absence of active or passive tobacco smoke. This might be due to the presence of higher mean serum folate levels among nonsmokers. In a Korean population, the 677T allele had a weak protective association with lung carcinoma.56 The protective effect of adequate folate status might be mediated by the stabilization of flavin-adenine-dinucleotide (FAD) binding at the catalytic domain.2 The birth weight of female infants of 677TT homozygous passive smokers was significantly lower than that of female infants born to passive smokers of similar genotypes. Male fetuses were favored, probably because pregnant mothers carrying male fetuses have a higher nutritional intake than those with female fetuses.57 Poorly understood phenomena on fetal sex-specific signals have been implicated in fetal growth, especially in response to glucocorticoid activity that might modify the fetal response to stress.58
Study strengths and limitations
The participants were indigenous Japanese; hence, we overcame issues of population stratification in genetic association studies. Overall, this study might be limited by selection bias, as we utilized integrated data from only 61% of the total recruitment during the study period. However, because we randomly selected the final study population based on known pooled population frequencies of the genetic factors and tobacco smoking among pregnant women, we believe that this study does not substantially differ from one with a higher participation rate (ie, ≥70%). The sample size of the study was adequate to detect gene-environment interactions; however, multiple comparisons and small sub-group sample sizes might have affected the study power. There could be misclassification bias from self-reported tobacco smoking; however, a previous study reported very low misclassification bias among Japanese women.59 Therefore, the findings of the present study are likely to be reliable. Nevertheless, this does not diminish the importance of using biomarkers like cotinine. The findings might have been confounded by other B vitamins, which were not studied, or by other unidentified sources common to cohort study designs. Finally, this study was hospital-based; therefore, our findings should not be generalized. Further studies of other factors in the folate-homocysteine pathway should prove interesting.
Public health implications
Analysis of the 5,10-MTHFR A1298C polymorphism shows that the frequency of the 1298AA genotype is greater than 60.0% in the Japanese population.38 Its association with low folate status is thus a considerable public health concern. With the recent increasing prevalence of smoking among young Japanese women, particularly in Hokkaido,60 maternofetal morbidity and mortality might also increase. Smoking cessation and targeted use of folic acid supplements could therefore prove to be very important public health tools in this population.
Conclusions
Our findings suggest that the maternal 5,10-MTHFR C677T polymorphism is independently associated with higher infant birth weight, especially among nonsmokers, while the 5,10-MTHFR A1298C variant is not independently associated with birth weight. In addition, the 5,10-MTHFR 1298AA polymorphism might be associated with folate impairment and could interact with tobacco smoke to further decrease birth weight.
ACKNOWLEDGMENTS
We would like to express our appreciation to all study participants and members of The Hokkaido study on Environment and Children’s Health. We also acknowledge Sharon Hanley for her enormous help in the translation of the original questionnaires. We express our profound gratitude to all personnel in the hospitals and clinics that collaborated with the study: Keiai Hospital, Endo Kikyo Maternity Clinic, Shiroishi Hospital, Memuro Municipal Hospital, Aoba Ladies Clinic, Obihiro-Kyokai Hospital, Akiyama Memorial Hospital, Sapporo Medical University Hospital, Hokkaido University Hospital, Kitami Red Cross Hospital, Hoyukai Sapporo Hospital, Gorinbashi Hospital, Hashimoto Clinic, Asahikawa Medical College Hospital, Hakodate Central General Hospital, Ohji General Hospital, Nakashibetsu Municipal Hospital, Sapporo Tokushukai Hospital, Asahikawa Red Cross Hospital, Wakkanai City Hospital, Kushiro Rosai Hospital, Sapporo-Kosei General Hospital, Shibetsu City General Hospital, Nikko Memorial Hospital, Sapporo City General Hospital, Kohnan Hospital, Hakodate City Hospital, Hokkaido Monbetsu Hospital, Tenshi Hospital, Hakodate Goryoukaku Hospital, Nakamura Hospital, Kin-ikyo Sapporo Hospital, Kitami Lady’s Clinic, Engaru-Kosei General Hospital, Kushiro Red Cross Hospital, Nayoro City General Hospital, and Obihiro-Kosei General Hospital.
Funding: This study was funded by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Health, Labour and Welfare; the Ministry of Education, Culture, Sports, Science and Technology, and the Japan Society for the Promotion of Science.
Conflicts of interest: None declared.
REFERENCES
- 1.Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64:169–72 10.1006/mgme.1998.2714 [DOI] [PubMed] [Google Scholar]
- 2.Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci USA. 2002;99:5606–11 10.1073/pnas.062066299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohda S, Arinami T, Hamada H, Yamada N, Hamaguchi H, Kubo T. Methylenetetrahydrofolate reductase polymorphism and pre-eclampsia. J Med Genet. 1997;34:525–6 10.1136/jmg.34.6.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isotalo PA, Wells GA, Donnelly JG. Neonatal and fetal methylenetetrahydrofolate reductase genetic polymorphisms: an examination of C677T and A1298C mutations. Am J Hum Genet. 2000;67:986–90 10.1086/303082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Molen EF, Arends GE, Nelen WL, van der Put NJ, Heil SG, Eskes TK, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene as a new risk factor for placental vasculopathy. Am J Obstet Gynecol. 2000;182:1258–63 10.1067/mob.2000.105199 [DOI] [PubMed] [Google Scholar]
- 6.Murakami S, Matsubara N, Saitoh M, Miyakaw S, Shoji M, Kubo T. The relation between plasma homocysteine concentration and methylenetetrahydrofolate reductase gene polymorphism in pregnant women. J Obstet Gynaecol Res. 2001;27:349–52 10.1111/j.1447-0756.2001.tb01284.x [DOI] [PubMed] [Google Scholar]
- 7.Hefler L, Jirecek S, Heim K, Grimm C, Antensteiner G, Zeillinger R, et al. Genetic polymorphisms associated with thrombophilia and vascular disease in women with unexplained late intrauterine fetal death: a multicenter study. J Soc Gynecol Investig. 2004;11:42–4 10.1016/j.jsgi.2003.07.008 [DOI] [PubMed] [Google Scholar]
- 8.Valdez LL, Quintero A, Garcia E, Olivares N, Celis A, Rivas F Jr, et al. Thrombophilic polymorphisms in preterm delivery. Blood Cells Mol Dis. 2004;33:51–6 10.1016/j.bcmd.2004.04.011 [DOI] [PubMed] [Google Scholar]
- 9.Johnson WG, Scholl TO, Spychala JR, Buyske S, Stenroos ES, Chen X. Common dihydrofolate reductase 19-base pair deletion allele: a novel risk factor for preterm delivery. Am J Clin Nutr. 2005;81:664–8 [DOI] [PubMed] [Google Scholar]
- 10.Relton CL, Pearce MS, Burn J, Parker L. An investigation of folate-related genetic factors in the determination of birthweight. Paediatr Perinat Epidemiol. 2005;19:360–7 10.1111/j.1365-3016.2005.00662.x [DOI] [PubMed] [Google Scholar]
- 11.Mtiraoui N, Zammiti W, Ghazouani L, Braham NJ, Saidi S, Finan RR, et al. Methylenetetrahydrofolate reductase C677T and A1298C polymorphism and changes in homocysteine concentrations in women with idiopathic recurrent pregnancy losses. Reproduction. 2006;131:395–401 10.1530/rep.1.00815 [DOI] [PubMed] [Google Scholar]
- 12.Tamura T, Picciano MF. Folate and human reproduction. Am J Clin Nutr. 2006;83:993–1016 [DOI] [PubMed] [Google Scholar]
- 13.Stonek F, Hafner E, Philipp K, Hefler LA, Bentz EK, Tempfer CB. Methylenetetrahydrofolate reductase C677T polymorphism and pregnancy complications. Obstet Gynecol. 2007;110:363–8 10.1097/01.AOG.0000270122.13198.6f [DOI] [PubMed] [Google Scholar]
- 14.Ozbek N, Ataç FB, Verdi H, Cetintaş S, Gürakan B, Haberal A. Relationship between small-for-gestational age births and maternal thrombophilic mutations. Thromb Res. 2008;122:175–8 10.1016/j.thromres.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 15.Pilsner JR, Hu H, Wright RO, Kordas K, Ettinger AS, Sánchez BN, et al. Maternal MTHFR genotype and haplotype predict deficits in early cognitive development in a lead-exposed birth cohort in Mexico City. Am J Clin Nutr. 2010;92:226–34 10.3945/ajcn.2009.28839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foka ZJ, Lambropoulos AF, Saravelos H, Karas GB, Karavida A, Agorastos T, et al. Factor V Leiden and prothrombin G20210A mutations, but not methylenetetrahydrofolate reductase C677T, are associated with recurrent miscarriages. Hum Reprod. 2000;15:458–62 10.1093/humrep/15.2.458 [DOI] [PubMed] [Google Scholar]
- 17.Kobashi G, Yamada H, Asano T, Nagano S, Hata A, Kishi R, et al. Absence of association between a common mutation in the methylenetetrahydrofolate reductase gene and preeclampsia in Japanese women. Am J Med Genet. 2000;93:122–5 [DOI] [PubMed] [Google Scholar]
- 18.Gebhardt GS, Scholtz CL, Hillermann R, Odendaal HJ. Combined heterozygosity for methylenetetrahydrofolate reductase (MTHFR) mutations C677T and A1298C is associated with abruptio placentae but not with intrauterine growth restriction. Eur J Obstet Gynecol Reprod Biol. 2001;97:174–7 10.1016/S0301-2115(00)00540-6 [DOI] [PubMed] [Google Scholar]
- 19.McCowan LM, Craigie S, Taylor RS, Ward C, McLintock C, North RA. Inherited thrombophilias are not increased in “idiopathic” small-for-gestational-age pregnancies. Am J Obstet Gynecol. 2003;188:981–5 10.1067/mob.2003.218 [DOI] [PubMed] [Google Scholar]
- 20.Chen DF, Hu YH, Yang F, Wu BY, Chen L, Fang ZA, et al. Mother’s and child’s methylenetetrahydrofolate reductase C677T polymorphism is associated with preterm delivery and low birth weight. Beijing Da Xue Xue Bao. 2004;36(3):248–53 [PubMed] [Google Scholar]
- 21.Nurk E, Tell GS, Refsum H, Ueland PM, Vollset SE. Associations between maternal methylenetetrahydrofolate reductase polymorphisms and adverse outcomes of pregnancy: the Hordaland Homocysteine Study. Am J Med. 2004;117:26–31 10.1016/j.amjmed.2004.01.019 [DOI] [PubMed] [Google Scholar]
- 22.Resch B, Gallistl S, Kutschera J, Mannhalter C, Muntean W, Mueller WD. Thrombophilic polymorphisms—factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations—and preterm birth. Wien Klin Wochenschr. 2004;116:622–6 10.1007/s00508-004-0223-9 [DOI] [PubMed] [Google Scholar]
- 23.Also-Rallo E, Lopez-Quesada E, Urreizti R, Vilaseca MA, Lailla JM, Balcells S, et al. Polymorphisms of genes involved in homocysteine metabolism in preeclampsia and in uncomplicated pregnancies. Eur J Obstet Gynecol Reprod Biol. 2005;120:45–52 10.1016/j.ejogrb.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 24.Engel SM, Olshan AF, Siega-Riz AM, Savitz DA, Chanock SJ. Polymorphisms in folate metabolizing genes and risk for spontaneous preterm and small-for-gestational age birth. Am J Obstet Gynecol 2006;195:1231.e1–11 10.1016/j.ajog.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 25.Ananth CV, Peltier MR, De Marco C, Elsasser DA, Getahun D, Rozen R, et al. ; New Jersey-Placental Abruption Study Investigators . Associations between 2 polymorphisms in the methylenetetrahydrofolate reductase gene and placental abruption. Am J Obstet Gynecol 2007;197:385.e1–7 10.1016/j.ajog.2007.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter B, Stegmann K, Röper B, Böddeker I, Ngo ET, Koch MC. Interaction of folate and homocysteine pathway genotypes evaluated in susceptibility to neural tube defects (NTD) in a German population. J Hum Genet. 2001;46(3):105–9 10.1007/s100380170096 [DOI] [PubMed] [Google Scholar]
- 27.Chango A, Fillon-Emery N, Mircher C, Bléhaut H, Lambert D, Herbeth B, et al. No association between common polymorphisms in genes of folate and homocysteine metabolism and the risk of Down’s syndrome among French mothers. Br J Nutr. 2005;94:166–9 10.1079/BJN20051490 [DOI] [PubMed] [Google Scholar]
- 28.van der Put NM, Gabreëls F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet. 1998;62:1044–51 10.1086/301825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi K, Matsuda Y, Kawamichi Y, Shiozaki A, Saito S. Smoking during pregnancy increases risks of various obstetric complications: a case-cohort study of the Japan Perinatal Registry Network database. J Epidemiol. 2011;21(1):61–6 Epub 2010 Nov 13 10.2188/jea.JE20100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown KS, Kluijtmans LA, Young IS, Murray L, McMaster D, Woodside JV, et al. The 5,10-methylenetetrahydrofolate reductase C677T polymorphism interacts with smoking to increase homocysteine. Atherosclerosis. 2004;174:315–22 10.1016/j.atherosclerosis.2004.01.023 [DOI] [PubMed] [Google Scholar]
- 31.Ozerol E, Ozerol I, Gökdeniz R, Temel I, Akyol O. Effects of smoking on serum concentrations of total homocysteine, folate, Vitamain B12, and Nitric Oxide in Pregnancy: A preliminary study. Fetal Diagn Ther. 2004;19:145–8 10.1159/000075139 [DOI] [PubMed] [Google Scholar]
- 32.Jauniaux E, Johns J, Gulbis B, Spasic-Boskovic O, Burton GJ. Transfer of folic acid inside the first-trimester gestational sac and the effect of maternal smoking. Am J Obstet Gynecol 2007;197:58.e1–6 10.1016/j.ajog.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 33.Takamura N, Kondoh T, Ohgi S, Arisawa K, Mine M, Yamashita S, et al. Abnormal folic acid-homocysteine metabolism as maternal risk factors for Down syndrome in Japan. Eur J Nutr. 2004;43:285–7 10.1007/s00394-004-0472-4 [DOI] [PubMed] [Google Scholar]
- 34.Kobashi G, Kato EH, Morikawa M, Shimada S, Ohta K, Fujimoto S, et al. MTHFR C677T Polymorphism and factor V Leiden mutation are not associated with recurrent spontaneous abortion of unexplained etiology in Japanese women. Semin Thromb Hemost. 2005;31:266–71 10.1055/s-2005-872430 [DOI] [PubMed] [Google Scholar]
- 35.Kobashi G Genetic and environmental factors associated with the development of hypertension in pregnancy. J Epidemiol. 2006;16:1–8 10.2188/jea.16.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kishi R, Sasaki S, Yoshioka E, Yuasa M, Sata F, Saijo Y, et al. Cohort profile: the Hokkaido study on environment and children’s health in Japan. Int J Epidemiol. 2011;40:611–8 Epub 2010 May 26 [DOI] [PubMed] [Google Scholar]
- 37.Botto LD, Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol. 2000;151:862–77 [DOI] [PubMed] [Google Scholar]
- 38.Robien K, Ulrich CM. 5,10-Methylenetetrahydrofolate reductase polymorphisms and leukemia risk: a HuGE minireview. Am J Epidemiol. 2003;157:571–82 10.1093/aje/kwg024 [DOI] [PubMed] [Google Scholar]
- 39.Sasaki S, Kondo T, Sata F, Saijo Y, Katoh S, Nakajima S, et al. Maternal smoking during pregnancy and genetic polymorphisms in the Ah receptor, CYP1A1 and GSTM1 affect infant birth size in Japanese subjects. Mol Hum Reprod. 2006;12(2):77–83 10.1093/molehr/gal013 [DOI] [PubMed] [Google Scholar]
- 40.Gauderman WJ, Morrison JM. QUANTO 1.1: A computer program for power and sample size calculations for genetic-epidemiology studies, 2006. Available from: http://hydra.usc.edu/gxe
- 41.Bock JL The New Era of Automated Immunoassay. Am J Clin Pathol. 2000;113:628–46 10.1309/DUDM-3Y6L-3R1L-QP15 [DOI] [PubMed] [Google Scholar]
- 42.Sujaku K, Ogiwara T, Kawasaki Y. Determination of vitamin B12 and folate by ADVIA Centaur. J Anal Bio-Sci. 2006;29:235–42(in Japanese) [Google Scholar]
- 43.Ranade K, Chang MS, Ting CT, Pei D, Hsiao CF, Olivier M, et al. High-throughput genotyping with single nucleotide polymorphisms. Genome Res. 2001;11:1262–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;76:887–93 10.1086/429864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuo K, Suzuki R, Hamajima N, Ogura M, Kagami Y, Taji H, et al. Association between polymorphisms of folate- and methionine-metabolizing enzymes and susceptibility to malignant lymphoma. Blood. 2001;97(10):3205–9 10.1182/blood.V97.10.3205 [DOI] [PubMed] [Google Scholar]
- 46.Lwin H, Yokoyama T, Date C, Yoshiike N, Kokubo Y, Tanaka H. Are the associations between life-style related factors and plasma total homocysteine concentration different according to polymorphism of 5,10-methylenetetrahydrofolate reductase gene (C677T MTHFR)? A cross-sectional study in a Japanese rural population. J Epidemiol. 2002;12:126–35 10.2188/jea.12.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin G, Kono S, Toyomura K, Hagiwara T, Nagano J, Mizoue T, et al. Methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and colorectal cancer: the Fukuoka Colorectal Cancer Study. Cancer Sci. 2004;95:908–13 10.1111/j.1349-7006.2004.tb02201.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim NK, Choi YK, Kang MS, Choi DH, Cha SH, An MO, et al. Influence of combined methylenetetrahydrofolate reductase (MTHFR) and thymidylate synthase enhancer region (TSER) polymorphisms to plasma homocysteine levels in Korean patients with recurrent spontaneous abortion. Thromb Res. 2006;117:653–8 10.1016/j.thromres.2005.05.025 [DOI] [PubMed] [Google Scholar]
- 49.Castro R, Rivera I, Ravasco P, Jakobs C, Blom HJ, Camilo ME, et al. 5,10-Methylenetetrahydrofolate reductase 677C→T and 1298A→C mutations are genetic determinants of elevated homocysteine. QJM. 2003;96:297–303 10.1093/qjmed/hcg039 [DOI] [PubMed] [Google Scholar]
- 50.Ueland PM, Refsum H, Beresford SA, Vollset SE. The controversy over homocysteine and cardiovascular risk. Am J Clin Nutr. 2000;72:324–32 [DOI] [PubMed] [Google Scholar]
- 51.Jhaveri MS, Wagner C, Trepel JB. Impact of extracellular folate levels on global gene expression. Mol Pharmacol. 2001;60:1288–95 [DOI] [PubMed] [Google Scholar]
- 52.Courtemanche C, Elson-Schwab I, Mashiyama ST, Kerry N, Ames BN. Folate deficiency inhibits the proliferation of primary human CD8+ T lymphocytes in vitro. J Immunol. 2004;173:3186–92 [DOI] [PubMed] [Google Scholar]
- 53.Infante-Rivard C, Rivard GE, Yotov WV, Génin E, Guiguet M, Weinberg C, et al. Absence of association of thrombophilia polymorphisms with intrauterine growth restriction. N Engl J Med. 2002;347:19–25 10.1056/NEJM200207043470105 [DOI] [PubMed] [Google Scholar]
- 54.Moriyama Y, Okamura T, Kajinami K, Iso H, Inazu A, Kawashiri M, et al. Effects of serum B vitamins on elevated plasma homocysteine levels associated with the mutation of methylenetetrahydrofolate reductase gene in Japanese. Atherosclerosis. 2002;164:321–8 10.1016/S0021-9150(02)00105-3 [DOI] [PubMed] [Google Scholar]
- 55.Parle-McDermott A, Mills JL, Molloy AM, Carroll N, Kirke PN, Cox C, et al. The MTHFR 1298CC and 677TT genotypes have opposite associations with red cell folate levels. Mol Genet Metab. 2006;88:290–4 10.1016/j.ymgme.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 56.Cui LH, Shin MH, Kim HN, Song HR, Piao JM, Kweon SS, et al. Methylenetetrahydrofolate reductase C677T polymorphism in patients with lung cancer in a Korean population. BMC Med Genet. 2011. Feb 22;12:28 10.1186/1471-2350-12-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tamimi RM, Lagiou P, Mucci LA, Hsieh CC, Adami HO, Trichopoulos D. Average energy intake among pregnant women carrying a boy compared with a girl. BMJ. 2003;326:1245–6 10.1136/bmj.326.7401.1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: the role of the mother, placenta and fetus. Endocr Rev. 2006;27:141–69 10.1210/er.2005-0011 [DOI] [PubMed] [Google Scholar]
- 59.Tsutsumi A, Kagawa J, Yamano Y, Nakadate T, Shimizu S. Relation between Cotinine in the Urine and Indices Based on Self-Declared Smoking Habits. Environ Health Prev Med. 2002;6:240–7 10.1007/BF02897976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smoking Rate Highest in Hokkaido: A survey by the Japan Tobacco Inc. 2009. Available from: http://e.nikkei.com/e/fr/tnks/Nni20100331D30HH717.htm