Abstract
OBJECTIVE:
To investigate the antifibrotic effects of crocetin in scleroderma fibroblasts and in sclerotic mice.
METHODS:
Skin fibroblasts that were isolated from three systemic scleroderma (SSc) patients and three healthy subjects were treated with crocetin (0.1, 1 or 10 μM). Cell proliferation was measured with an MTT assay. Alpha-smooth muscle actin was detected via an immunohistochemical method. Alpha 1 (I) procollagen (COL1A1), alpha 1 (III) procollagen (COL3A1), matrix metalloproteinase (MMP)-1 and tissue inhibitor of matrix metalloproteinase (TIMP)-1 mRNA levels were measured using real-time PCR. SSc mice were established by the subcutaneous injection of bleomycin. Crocetin (50 mg/kg/d) was injected intraperitoneally for 14 days. Dermal thickness and lung fibrosis were assessed with Masson's trichrome staining. Plasma ET-1 was detected with an enzyme-linked immunosorbent assay (ELISA). Skin and lung ET-1 and COL1A1 mRNA levels were measured via real-time PCR.
RESULTS:
Crocetin inhibited the proliferation of SSc and normal fibroblasts, an effect that increased with crocetin concentration and incubation time. Crocetin decreased the expression of α-SMA and the levels of mRNA for COL1A1, COL3A1 and matrix metalloproteinase-1, while crocetin increased TIMP-1 mRNA levels in both SSc and normal fibroblasts. Skin and lung fibrosis was induced, and the levels of ET-1 in the plasma, skin and lungs were elevated in bleomycin-injected mice. Crocetin alleviated the thickening of the dermis and lung fibrosis; decreased COL1A1 mRNA levels in the skin and lung; and simultaneously decreased ET-1 concentrations in the plasma and ET-1 mRNA levels in the skin and lungs of the bleomycin-induced sclerotic mice, especially during the early phase (weeks 1-3).
CONCLUSION:
Crocetin inhibits cell proliferation, differentiation and collagen production in SSc fibroblasts. Crocetin alleviates skin and lung fibrosis in a bleomycin-induced SSc mouse model, in part due to a reduction in ET-1.
Keywords: Crocetin, Fibroblasts, Systemic scleroderma, Collagen, Fibrosis
INTRODUCTION
Systemic scleroderma (SSc) is a complex, chronic connective tissue disease that has three cardinal clinical features: the excessive deposition of extracellular matrix (ECM), vascular damage and inflammation/autoimmunity (1). Despite an unclear pathogenesis, SSc is characterized by the pathologic remodeling of the connective tissues in the skin and internal organs, which is due to the overproduction of ECM, especially the production of collagen by fibroblasts (2). Therefore, many of the landmark in vitro SSc studies were based on the study of cultured fibroblasts. Regarding in vivo research, the bleomycin-induced experimental sclerotic mouse is a good model for studying the prevention or treatment of fibrosis and is the most frequently used model (3). However, there is currently still no cure for SSc and little possibility of modifying or reversing the fibrosis of the skin and internal organs.
Saffron, a spice and food colorant present in the dry stigmas of the plant Crocus sativus L., has been used as an herbal remedy for various ailments, including cancer, in the ancient Arabian, Indian and Chinese cultures. Crocetin, an important carotenoid constituent of saffron, has shown significant potential as an anti-tumor agent in animal models and cell culture systems (4). This unique carotenoid contains a short carbon chain length (C20 apocarotenoid) and carboxyl groups at both ends of the carbon chain (5). Additionally, crocetin exhibits other pharmacological actions, including the inhibition of retinal ischemic damage in mice (6) and neuroprotection in conjunction with selenium in cognitive impairment (7). More intriguingly, recent research has revealed that crocetin reverses cardiac hypertrophy in vivo and inhibits the collagen synthesis that is stimulated by angiotensin (Ang) II in cardiac fibroblasts (8). Because the pathogenic processes of SSc and cardiac hypertrophy share many similar features, we became interested in whether crocetin has an effect on SSc.
In this study, we investigated the possible antifibrotic effects of crocetin in vitro in fibroblasts isolated from patients with SSc and in vivo in bleomycin-induced sclerotic mice. This study contributes to the potential use of crocetin as a treatment for fibrosis in SSc patients.
MATERIALS AND METHODS
Cell culture and crocetin treatment
Skin biopsies were obtained from three patients who met the American College of Rheumatology criteria for SSc(9) (with a less than 1-year duration of the disease) and were not undergoing treatment and from three age- and sex-matched healthy subjects. Full-thickness 7-mm biopsies that were 50 mm2 in size were excised from the forearm lesions of the patients with SSc and from the healthy controls. Primary cultures of the skin fibroblasts were established using the method introduced by Zhu et al. (10). The fibroblasts were serially passaged in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) (PAA, Pasching, Austria) in an incubator at 37°C with 5% CO2. The cells were used for the crocetin administration experiments at passages 3 to 6 at early confluence. The study protocol was reviewed and approved by the research ethics committee of Zhongshan Hospital, and informed consent was obtained from all of the participants. In the following assays, after reaching 90% confluency, the fibroblasts were synchronized by serum starvation for 24 h. The fibroblasts were then treated for 24 h in serum-free culture medium (except for the MTT assay) with crocetin (0.1, 1 or 10 μM, ChromaDex, USA). The crocetin was dissolved in dimethylsulfoxide (DMSO, Sigma, USA) for all of the in vitro studies. The above concentrations are commonly employed in in vitro studies (8) and caused no apparent cell toxicity in our preliminary assay. Untreated cells in the same medium were used as controls.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
For the MTT assay, the fibroblasts were seeded into 96-well microplates (2×103 cells/well) in triplicate. After incubation with crocetin (0.1, 1 or 10 μM) in DMEM containing 2% FBS for 1, 3 or 6 days (with a change of the culture media on the fourth day), an MTT solution (Amresco, USA) at a final concentration of 0.5 g/L was added, and the clones were further incubated at 37°C for 4 h. The cells were then solubilized in 1 ml of 10% DMSO (Sigma, USA) for 10 min. Finally, the absorbance at 570 nm was measured with a microplate reader (Bio-Rad, USA).
Immunohistochemistry
To measure α-smooth muscle actin (α-SMA), sterile microscope coverslips (Fisher, USA) were placed into the individual wells of 24-well cell culture plates, and the fibroblasts from the SSc patients or the healthy controls were incubated on the coverslips at a concentration of 1×105 cells/cm2. After reaching 90% cell confluency, the cells were treated with crocetin. The cell-containing cover slips were blocked with 5% BSA and then incubated overnight at 4°C with an α-SMA mouse monoclonal antibody (Boster, China). The visualization of antibody staining was performed according to the manufacturer's instructions for the 3,3'-Diaminobenzidine (DAB) Horseradish Peroxidase Color Development Kit (Boster, China). The staining wasrepeated for each sample at least three times, and photomicrographs were obtained with a microscope (Olympus, Japan). At minimum, 30 cells in several microscopic fields in each slide at ×400 magnification were scored by two independent examiners. The ratio of positive cells/total number of cells counted was calculated.
Detection of COL1A1, COL3A1, MMP-1 and TIMP-1 mRNA levels by real-time PCR
After incubation with crocetin (0.1, 1 or 10 μM) for 24 h, total RNA from the fibroblasts was isolated with TRIzol (Invitrogen, USA) and was reverse-transcribed to generate cDNA. COL1A1, COL3A1, MMP-1 and TIMP-1 mRNA levels in the fibroblasts were detected via real-time RT-PCR, which was performed using a SYBR Green Master Mix (TaKaRa, Japan) at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 20 s. GAPDH was used as an internal control. The primers that were used are shown in Table 1. A relative quantification was performed using the 2-ΔΔCt method (11,12). The experiments were performed in triplicate and were repeated twice.
Table 1.
List of primers used for real-time PCR.
| Sample/control gene | Primer Sequence (5'-3') | |
| Homo MMP-1 | TGAAAAGCGGAGAAATAGTGG | |
| GAGGACAAACTGAGCCACATC | ||
| Homo TIMP-1 | ATACTTCCACAGGTCCCACAAC | |
| GGATGGATAAACAGGGAAACAC | ||
| Homo COL1A1 | CCTGGAAAGAATGGAGATGATG | |
| ATCCAAACCACTGAAACCTCTG | ||
| Homo COL3A1 | GCTGGCTACTTCTCGCTCTG | |
| TCCGCATAGGACTGACCAAG | ||
| Homo GAPDH | GGTGAAGGTCGGTGTGAACG | |
| CTCGCTCCTGGAAGATGGTG | ||
| Mus ET-1 | GACCAGACACCGTCCTCTTC | |
| TGGAAAGTCACGAACAGCAG | ||
| Mus COL1A1 | GGTCTTGGTGGTTTTGTATTCG | |
| AACAGTCGCTTCACCTACAGC | ||
| Mus GAPDH | CCCCTTCATTGACCTCAACTAC | |
| GAGTCCTTCCAGGATACCAAAG | ||
Induction of dermal and lung sclerosis in mice and the administration of crocetin
Female C3H/He mice, aged 6 weeks and weighing 20 to 25 g, were obtained from Vitalriver Laboratory Animal Center (Beijing, China) and were maintained under pathogen-free conditions. The animal protocol was approved by the Committee of Animal Care and Use of Fudan University. Bleomycin powder (Nihon Kayaku, Tokyo, Japan) was dissolved in phosphate-buffered saline (PBS; 0.01 M, pH 7.4) at a concentration of 0.2 g/L. A crocetin suspension was prepared using a 0.5% carboxymethylcellulose (CMC) solution for the animal experiments. For the SSc mouse model, 100 μl of filter-sterilized bleomycin or PBS was subcutaneously injected into the shaved backs of the mice daily for 3 weeks with a 27-gauge needle. Simultaneously, either vehicle (CMC) or crocetin (50 mg/kg/d) was injected intraperitoneally for 14 days into the mice that were injected subcutaneously with bleomycin. The concentration of crocetin used in these experiments had previously exhibited maximal efficacy in suppressing cardiac hypertrophy without apparent cell toxicity in mice (8). At the end of week 1 (W1), W2, W3, W5 and W7 after bleomycin/PBS injection, the mice (six per group per time point) were euthanized by CO2 asphyxiation, and peripheral blood plasma samples were taken by cardiac puncture and centrifuged at 4°C. The back skins and lungs were collected for histological examination or were snap-frozen in liquid nitrogen.
Histological evaluation of skin and lung tissues
Formalin-fixed skin and lung tissues were paraffin-embedded and stained with hematoxylin and eosin and Masson's trichrome stain. The sections were examined using a Leica DFC 280 light microscope. Five randomly selected sites in two Masson's trichrome-stained skin or lung sections from each mouse were examined at ×100 magnification. Using a Leica Q Win Plus Image Analysis System (Leica Micros Imaging Solutions Ltd., Cambridge, UK), the dermal thickness (measured from the epidermal–dermal junction to the dermal–fat junction) was determined. The percentage of lung tissue fibrosis was evaluated by counting the number of pixels corresponding to the stained collagen areas.
Plasma endothelin-1 (ET-1) concentration detected with an enzyme linked immunosorbent assay (ELISA)
Peripheral blood plasma ET-1 concentrations were measured according to the manufacturer's protocol with an ELISA kit (USCN, Wuhan, China). The absorbance at 570 nm was measured using a microplate reader (Bio-Rad, USA). The experiments were performed in triplicate and were repeated twice.
Skin and lung ET-1 and COL1A1 mRNA expression detected by real-time PCR
The skin and lung ET-1 and COL1A1 mRNA levels in the mice were detected via real-time PCR as described above. The primer pairs that were used are shown in Table 1. The experiments were performed in triplicate and were repeated twice.
Statistics
All of the statistical analyses were performed using SPSS 13.0 software. The quantitative data were expressed as the means ± SEM. The quantitative variables were compared using the two-sample Student's t-test or a one-way ANOVA. Statistical significance was defined as p<0.05.
RESULTS
Inhibitory effect of crocetin on fibroblast proliferation
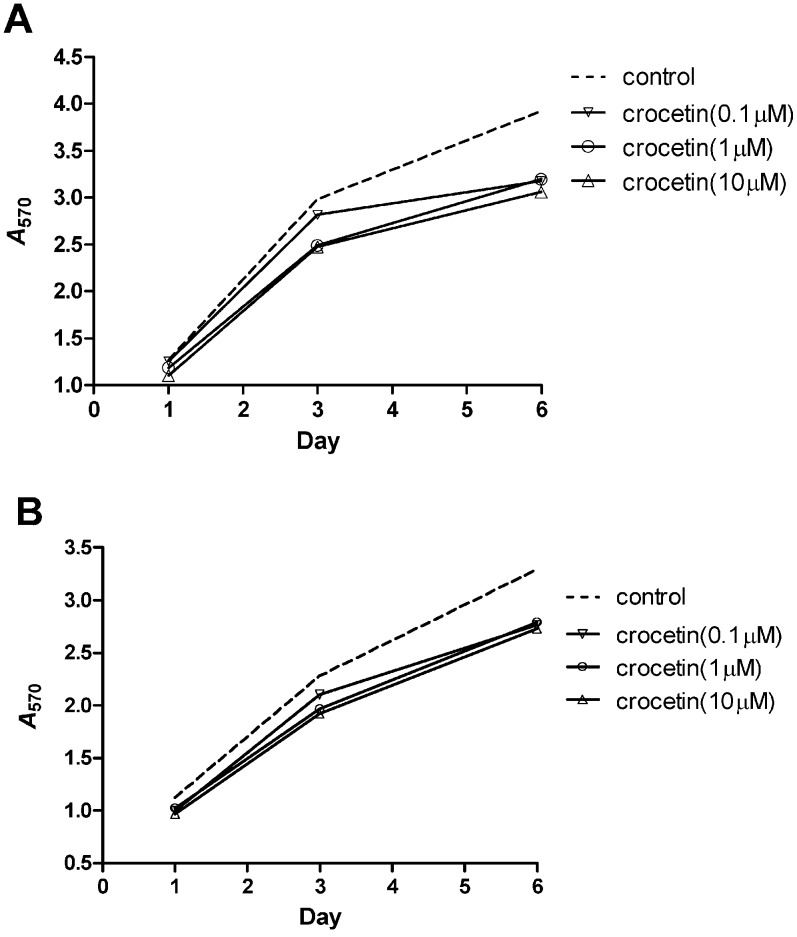
On D1, D3 and D6, crocetin inhibited fibroblast proliferation derived from both SSc patients and healthy individuals compared to the untreated control fibroblasts. Moreover, this inhibitory effect increased with concentration and incubation time (Figures 1A and 1B).
Figure 1.
Inhibitory effect of crocetin on the proliferation of fibroblasts derived from SSc patients and healthy individuals. Fibroblast proliferation was detected with the MTT assay, and absorbance at 570 nm was measured. (A) Proliferation of SSc fibroblasts. (B) Proliferation of normal fibroblasts.
Effect of crocetin on COL1A1, COL3A1, MMP-1 and TIMP-1 mRNA levels in fibroblasts
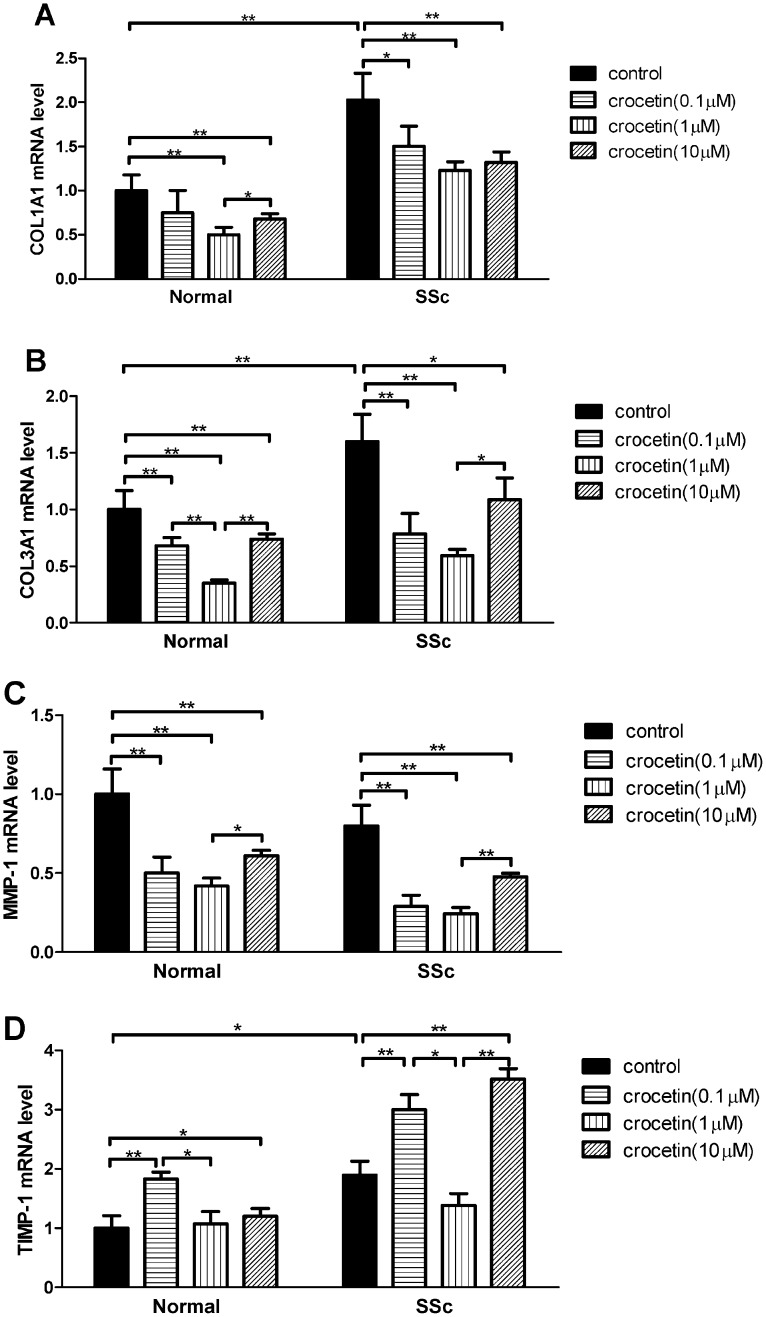
Compared to normal fibroblasts, the levels of COL1A1, COL3A1 and TIMP-1 mRNAs in the SSc fibroblasts increased significantly (p<0.05 or p<0.01), while there was no difference in MMP-1 mRNA levels between the SSc and normal fibroblasts (p>0.05). Crocetin decreased COL1A1 and COL3A1 mRNA levels in SSc and normal fibroblasts (p<0.05 or p<0.01) in comparison with the untreated controls (Figure 2A and 2B). Crocetin also decreased MMP-1 mRNA levels in SSc and normal fibroblasts (p<0.05 or p<0.01, Figure 2C). In contrast, crocetin increased TIMP-1 mRNA levels in the fibroblasts at concentrations of 0.1 and 10 μM (p<0.05 or p<0.01, Figure 2D) with no significant effect on TIMP-1 mRNA levels in the fibroblasts at a concentration of 1 μM. However, among the three concentrations used, crocetin at a concentration of 1 μM resulted in the most significant inhibitory effect on the expression of COL1A1, COL3A1, and MMP-1 mRNA levels.
Figure 2.
Effect of crocetin on COL1A1, COL3A1, MMP-1 and TIMP-1 mRNA expression in SSc and normal fibroblasts. mRNA expression was detected using quantitative reverse transcription–polymerase chain reaction, and relative quantification was performed using the 2-ΔΔCt method. The experiments were performed in triplicate and repeated twice. (A) COL1A1; (B) COL3A1; (C) MMP-1; (D) TIMP-1. * p<0.05, ** p<0.01.
Inhibitory effect of crocetin on α-SMA expression in SSc and normal fibroblasts
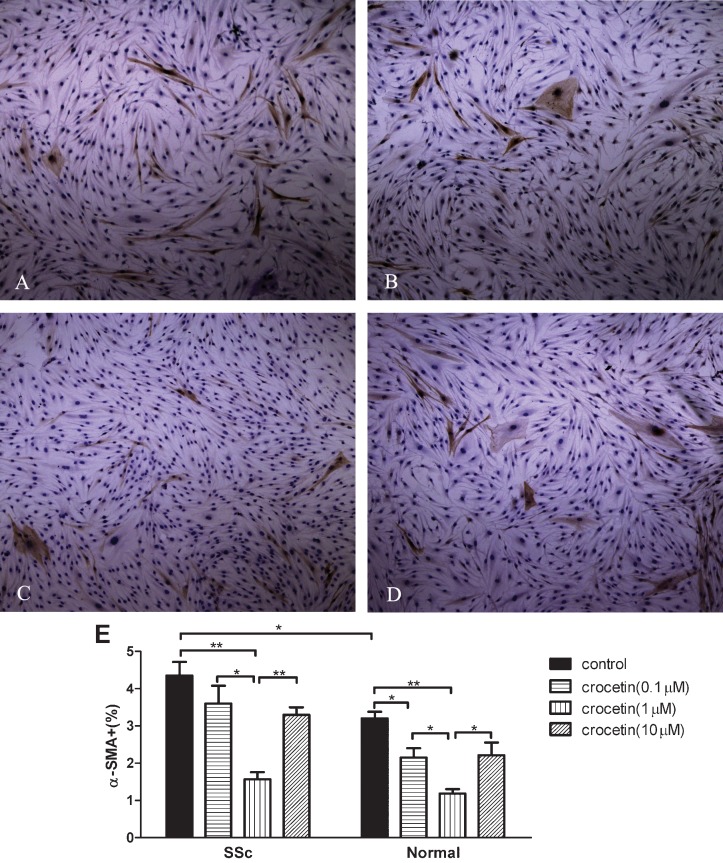
α-SMA expression increased in SSc fibroblasts compared to normal fibroblasts (p<0.05). All tested concentrations of crocetin decreased α-SMA expression in both SSc and normal fibroblasts (p<0.05 or p<0.01, Figure 3A-3D). Crocetin at a concentration of 1 μM significantly decreased α-SMA expression in SSc and normal fibroblasts compared to the other two concentrations tested (p<0.05 or p<0.01, Figure 3E).
Figure 3.
Inhibitory effect of crocetin on the expression of α-smooth muscle actin (α-SMA) in SSc and normal fibroblasts. (A-D) α-SMA expression in normal fibroblasts. Original magnification ×100. (α-SMA expression in SSc fibroblasts is not shown because the appearance is similar to normal cells). (E) α-SMA positive cells/total number of cells counted (%). * p<0.05, ** p<0.01.
Antifibrotic effect of crocetin in bleomycin-induced sclerotic mice
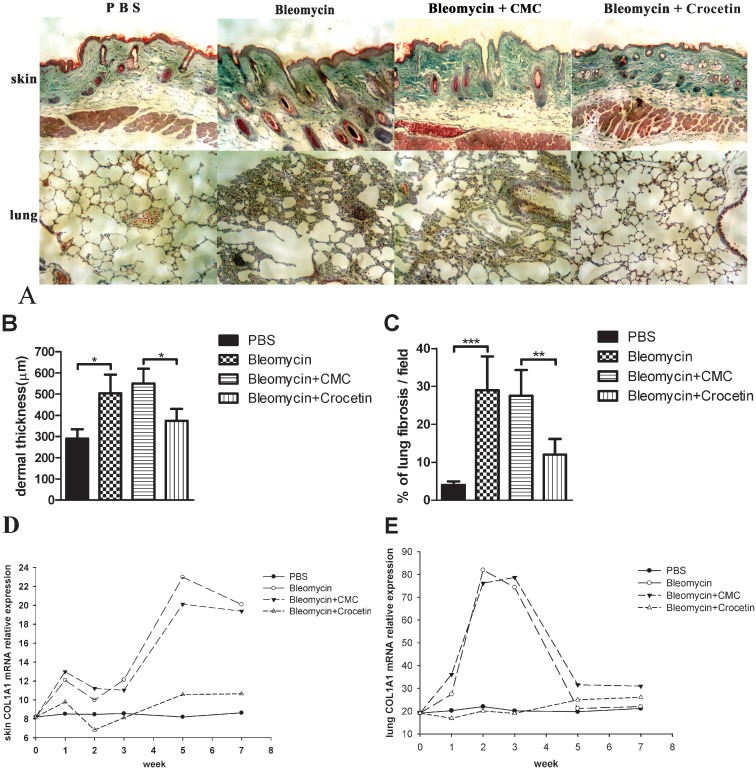
The histological examination of the skin and lung tissue from the mice is shown in Figure 4A. The dermal thicknesses and percentages of lung fibrosis in the bleomycin-injected and the bleomycin+CMC-injected mice were significantly higher than in the PBS- and bleomycin+crocetin-injected mice, respectively (p<0.05, Figure 4B and 4C). The COL1A1 mRNA levels in the skin and lungs from the mice at different time points are shown in Figure 4D and Figure 4E. The bleomycin-injected mice had higher skin COL1A1 mRNA levels than the PBS-injected mice, a difference that increased with time. The bleomycin+crocetin-injected mice had lower skin COL1A1 mRNA levels than the bleomycin+CMC-injected mice. The bleomycin-injected mice had higher lung COL1A1 mRNA levels than the PBS-injected mice, and the bleomycin+CMC-injected mice had higher lung COL1A1 mRNA levels than the crocetin-injected mice at the end of W1, W2 and W3.
Figure 4.
Histological evaluation of mouse skin and lung tissues. (A) Representative pathologic findings from the skin and lung tissue from the mice at W3 after beginning the bleomycin or crocetin treatment; ×100, Masson's trichrome staining. (B) Dermal thickness (measured from the epidermal–dermal junction to the dermal–fat junction). (C) Percentage of lung fibrosis (evaluated by counting the numbers of pixels corresponding to stained collagen areas). (D) Skin COL1A1 mRNA levels; (E) Lung COL1A1 mRNA levels.
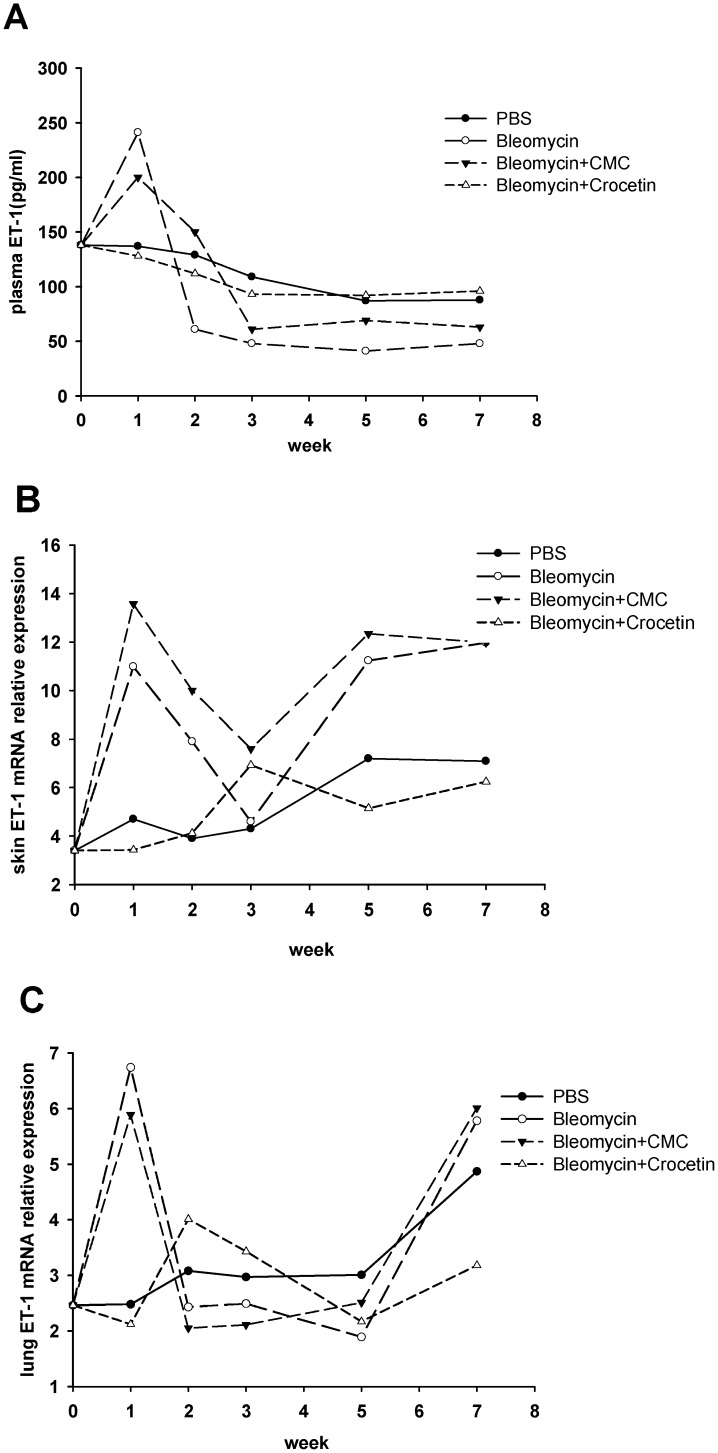
Plasma ET-1 levels and ET-1 mRNA levels in mouse skin and lungs
At W1, the bleomycin-injected mice had significantly higher plasma ET-1 levels than the PBS-injected mice, and the bleomycin+crocetin-injected mice had significantly lower plasma ET-1 levels than the bleomycin+CMC-injected mice (Figure 5A). Afterward, the differences between the bleomycin- and PBS-injected mice and between the bleomycin+CMC-injected and bleomycin+crocetin-injected mice were not significant. The bleomycin-injected mice had significantly higher skin ET-1 mRNA levels than the PBS-injected mice after W1, W5 and W7. The bleomycin+crocetin-injected mice had significantly lower skin ET-1 mRNA levels than the bleomycin+CMC-injected mice (Figure 5B). Afterward, the differences between the bleomycin-injected and the PBS-injected mice and between the bleomycin+CMC-injected and the bleomycin+crocetin-injected mice were not significant. The bleomycin-injected mice had higher lung ET-1 levels than the PBS-injected-mice at W1. Afterward, the difference between the bleomycin-injected and the PBS-injected mice was not significant. The bleomycin+crocetin-injected mice had lower lung ET-1 levels than the bleomycin+CMC-injected mice at the end of the W1 (Figure 5C).
Figure 5.
Plasma, skin and lung ET-1 levels in mice. (A) Plasma ET-1 levels; (B) skin ET-1 mRNA levels; (C) lung ET-1 mRNA levels. Plasma ET-1 levels were detected using an ELISA. ET-1 mRNA expression was detected via a quantitative reverse transcription-polymerase chain reaction, and relative quantification was performed using the 2-ΔΔCt method.
DISCUSSION
The most prominent clinical features of SSc are caused by the excessive deposition of ECM components, especially type I and type III collagen, in the skin and involved organs, such as the lung. Type I collagen is composed of two alpha 1 (I) chains and one alpha 2 (I) chain that are expressed coordinately in human fibroblasts (13,14). Type III collagen is composed of two alpha 1 (III) chains(13). The upregulated COL1A1 and COL3A1 genes in SSc fibroblasts play important roles in the pathogenesis of SSc. MMP-1 promotes the degradation of both type I and type III collagen, and MMP-1 activity is inhibited by TIMP-1. Therefore, the balance of MMP-1 and TIMP-1 maintains the homeostasis of tissue damage and repair (15). Another marker of the activated SSc fibroblast is the expression of α-SMA. SSc is associated with the differentiation of fibroblasts into myofibroblasts, which is characterized by the expression of α-SMA contractile filaments. Myofibroblasts present some activation features, including high levels of ECM gene expression, during normal reparative or pathological fibrotic processes (16).
In vitro, we observed an inhibitory effect of crocetin on the proliferation of both the SSc and normal fibroblasts, an effect that increased with concentration and time. We also observed crocetin-induced inhibition of α-SMA expression in SSc fibroblasts. In addition, crocetin significantly decreased COL1A1 and COL3A1 mRNA levels in both SSc and normal fibroblasts. These findings indicate that crocetin inhibits overproliferation, the overproduction of collagen and the differentiation of fibroblasts, whether the fibroblasts are derived from patients with SSc or from healthy individuals. In addition, we noted that MMP-1 mRNA levels in the fibroblasts decreased, while TIMP-1 mRNA levels increased after treatment with crocetin. This observation suggests that crocetin may have dual effects on the metabolism of collagen in fibroblasts, which involve inhibiting both the production and the degradation of collagen. Among the three concentrations of crocetin used in nearly all of the in vitro assays, crocetin at 1 μM had the most significant effect. This result indicates that 1 μM is possibly one of the most suitable concentrations for studying the effect of crocetin on cultured fibroblasts.
ET-1, an important endogenous peptide hormone that potently promotes vasculopathy, inflammation and fibrosis, is one of the most important pathogenic endogenous peptide hormones in the pathogenesis of SSc(17). Furthermore, elevated levels of circulating ET-1 in patients with SSc have been observed (18,19). Therefore, elevated ET-1 may be an indicator for disease activity in SSc. Our in vivo study demonstrated that plasma ET-1 levels and ET-1 mRNA levels in the skin and lungs of bleomycin-induced sclerotic mice were significantly higher than the levels found in the PBS-injected mice at an early phase (1-3 weeks), which is similar to previous results(20,21). Crocetin significantly reversed skin thickening, lung fibrosis and COL1A1 mRNA levels in the skin and lungs of bleomycin-induced sclerotic mice, especially within the early phase (1-3 weeks). Simultaneously, crocetin decreased plasma ET-1 levels and ET-1 mRNA levels in the skin and lungs of bleomycin-induced sclerotic mice, especially within the early phase (1-3 weeks). Therefore, we speculate that in addition to the direct antifibrotic effect, the capacity of crocetin to alleviate bleomycin-induced fibrosis may be caused, at least in part, by the reduction of ET-1 in the plasma and fibrotic tissues of these mice.
Furthermore, some cytokines, such as interleukin-6 (IL-6) and transforming growth factor β (TGF-β), are highly involved in the pathogenesis of scleroderma (22). Crocetin has been found to attenuate the expression of IL-6 in mice (23), but whether this effect can be blocked by the addition of IL-6 remains unknown. Nevertheless, little is known regarding the effect of crocetin on other crucial cytokines, such as TGF-β. These observations indicate the necessity to further investigate the potential effect of crocetin on the profibrotic cytokines in cultured SSc fibroblasts in vitro or in the SSc experimental model in vivo.
In conclusion, our findings provide new evidence that crocetin inhibits the overproliferation and differentiation of SSc fibroblasts. In addition, crocetin may possess the dual effects of inhibiting both the overproduction and the degradation of collagen in fibroblasts. Furthermore, crocetin reversed the skin and lung fibrosis that occurs in a bleomycin-induced SSc mouse model, which may be due, at least in part, to the reduction of ET-1 in the plasma and fibrotic tissues of these mice. This study highlights the potential use of crocetin as a new drug for the treatment of fibrosis in patients with SSc.
ACKNOWLEDGMENTS
We thank doctors Di Gao, Ji Yang and Xiaojing Xing for providing reagents and information for the immunohistochemical staining. This project was supported by the National Natural Science Foundation of China (30901291).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117(3):557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharyya S, Wei J, Varga J. Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat Rev Rheumatol. 2012;8:42–54. doi: 10.1038/nrrheum.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avouac J, Elhai M, Allanore Y. Experimental models of dermal fibrosis and systemic sclerosis. Joint Bone Spine. 2013;80(1):23–8. doi: 10.1016/j.jbspin.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Gutheil WG, Reed G, Ray A, Anant S, Dhar A. Crocetin: an agent derived from saffron for prevention and therapy for cancer. Curr Pharm Biotechnol. 2012;13(1):173–9. doi: 10.2174/138920112798868566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiang M, Qian ZY, Zhou CH, Liu J, Li WN. Crocetin inhibits leukocyte adherence to vascular endothelial cells induced by AGEs. J Ethnopharmacol. 2006;107(1):25–31. doi: 10.1016/j.jep.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Ishizuka F, Shimazawa M, Umigai N, Ogishima H, Nakamura S, Tsuruma K, et al. Crocetin, a carotenoid derivative, inhibits retinal ischemic damage in mice. Eur J Pharmacol. 2013;703(1-3):1–10. doi: 10.1016/j.ejphar.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Khan MB, Hoda MN, Ishrat T, Ahmad S, Moshahid Khan M, Ahmad A, et al. Neuroprotective efficacy of Nardostachys jatamansi and crocetin in conjunction with selenium in cognitive impairment. Neurol Sci. 2012;33(5):1011–20. doi: 10.1007/s10072-011-0880-1. [DOI] [PubMed] [Google Scholar]
- 8.Cai J, Yi FF, Bian ZY, Shen DF, Yang L, Yan L, et al. Crocetin protects against cardiac hypertrophy by blocking MEK-ERK1/2 signalling pathway. J Cell Mol Med. 2009;13(5):909–25. doi: 10.1111/j.1582-4934.2008.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masi AT RG, Medsger TA, Jr, et al. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23(5):581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, Gao D, Yang J, Li M. Characterization of the phenotype of high collagen-producing fibroblast clones in systemic sclerosis, using a new modified limiting-dilution method. Clin Exp Dermatol. 2012;37(4):395–403. doi: 10.1111/j.1365-2230.2011.04254.x. [DOI] [PubMed] [Google Scholar]
- 11.Shi R, Chiang VL. Facile means for quantifying microRNA expression by real-time PCR. Biotechniques. 2005;39(4):519–25. doi: 10.2144/000112010. [DOI] [PubMed] [Google Scholar]
- 12.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 13.Hulmes DJ. Building collagen molecules, fibrils, and suprafibrillar structures. J Struct Biol. 2002;137(1-2):2–10. doi: 10.1006/jsbi.2002.4450. [DOI] [PubMed] [Google Scholar]
- 14.Hata R. Transfection of normal human skin fibroblasts with human alpha 1(I) and alpha 2(I) collagen gene constructs and evidence for their coordinate expression. Cell Biol Int. 1995;19(9):735–41. doi: 10.1006/cbir.1995.1124. [DOI] [PubMed] [Google Scholar]
- 15.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5(8):2145–54. [PubMed] [Google Scholar]
- 16.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13(1):7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 17.Leask A. The role of endothelin-1 signaling in the fibrosis observed in systemic sclerosis. Pharmacol Res. 2011;63(6):502–3. doi: 10.1016/j.phrs.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Kuryliszyn-Moskal A, Klimiuk PA, Sierakowski S. Soluble adhesion molecules (sVCAM-1, sE-selectin), vascular endothelial growth factor (VEGF) and endothelin-1 in patients with systemic sclerosis: relationship to organ systemic involvement. Clin Rheumatol. 2005;24(2):111–6. doi: 10.1007/s10067-004-0987-3. [DOI] [PubMed] [Google Scholar]
- 19.Peterlana D, Puccetti A, Caramaschi P, Biasi D, Beri R, Simeoni S, et al. Endothelin-1 serum levels correlate with MCP-1 but not with homocysteine plasma concentration in patients with systemic sclerosis. Scand J Rheumatol. 2006;35(2):133–7. doi: 10.1080/03009740500385584. [DOI] [PubMed] [Google Scholar]
- 20.Mutsaers SE, Foster ML, Chambers RC, Laurent GJ, McAnulty RJ. Increased endothelin-1 and its localization during the development of bleomycin-induced pulmonary fibrosis in rats. Am J Respir Cell Mol Biol. 1998;18(5):611–9. doi: 10.1165/ajrcmb.18.5.2898. [DOI] [PubMed] [Google Scholar]
- 21.Onat AM, Turkbeyler IH, Pehlivan Y, Demir T, Kaplan DS, Taysi S, et al. The efficiency of a urotensin II antagonist in an experimental lung fibrosis model. Inflammation. 2012;35(3):1138–43. doi: 10.1007/s10753-011-9421-6. [DOI] [PubMed] [Google Scholar]
- 22.Rose NR, Leskovsek N. Scleroderma: immunopathogenesis and treatment. Immunol Today. 1998;19(11):499–501. doi: 10.1016/s0167-5699(98)01322-x. [DOI] [PubMed] [Google Scholar]
- 23.Yang R, Yang L, Shen X, Cheng W, Zhao B, Ali KH, et al. Suppression of NF-kappaB pathway by crocetin contributes to attenuation of lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol. 2012;674(2-3):391–6. doi: 10.1016/j.ejphar.2011.08.029. [DOI] [PubMed] [Google Scholar]