Abstract
Background
In patients with cancer, hepatocyte growth factor (HGF) is elevated and is a predictor of prognosis. We investigated whether serum HGF was a predictive marker for cancer death in a population of community-dwelling Japanese.
Methods
We studied 1492 apparently healthy Japanese adults who underwent health examinations in 1999. Those who reported a history of liver disease or malignancy on a baseline questionnaire were excluded, and plasma HGF was measured in the remaining 1470 participants, who were followed periodically for 10 years. Multivariate proportional hazards regression was used to estimate cancer mortality.
Results
A total of 169 participants died during follow-up (61 from cancer, 32 from cerebrocardiovascular disease, and 76 from other diseases). Mean HGF at baseline was significantly higher among decedents than among survivors (0.26 ± 0.11 vs 0.23 ± 0.09 ng/ml, respectively; P < 0.01). The Cox proportional hazards model showed that age, systolic blood pressure, HGF (hazard ratio, 1.27; 95% CI, 1.06–1.52; P = 0.009), albumin level, smoking status, and creatinine were independent predictors of all-cause death. Age, HGF (hazard ratio, 1.31; 95% CI, 1.04–1.65; P = 0.02), and total cholesterol were independent predictive markers for cancer death.
Conclusions
Serum HGF was a predictor of cancer death in an apparently healthy population of community-dwelling Japanese.
Key words: cytokine, prospective study, Seven Countries Study, mortality, cancer
Abstract
<背景>
肝細胞増殖因子(Hepatocyte growth factor;HGF)はがん患者においてその血中濃度が上昇し、予後予測因子のひとつである。我々は我が国における大規模な一般住民検診に於いて血清HGF濃度を測定し、将来のがん死亡の予知因子となりうるかどうかを検討した。
<方法>
1999年に行った検診受診者1492名を対象とした。ベースライン時に明らかな肝疾患の既往や最近のがんの既往のあるものを除外した1470名を10年間前向きに追跡した。
<結果>
追跡期間中に169名の死亡が確認された。そのうち61名が癌死亡、32名が心血管死亡、76名がその他の死亡であった。生死別に血清HGF濃度を比較したところ死亡者で有意に高値であった。(死亡者0.26±0.11、生存者0.23±0.09ng/ml,p<0.01)
またCoxの比例ハザードモデルを用いて全死亡との関連性について検討したところ年齢、収縮期血圧、HGF(ハザード比1.27;95%信頼区間1.06-1.52,p=0.009)、アルブミン、喫煙、クレアチニンが独立した全死亡の予測因子であった。さらに年齢、HGF(ハザード比1.31;95%信頼区間1.04-1.65,p=0.02)、総コレステロールは独立したがん死亡の予測因子であった。
<結論>
血清HGF濃度は我が国における健康な一般住民におけるがん死亡の予測因子として有用である可能性を示唆することができた。
INTRODUCTION
Hepatocyte growth factor (HGF) was discovered in 1984,1 purified and isolated in 1986,2,3 and first characterized as a strong mitogen for hepatocytes. It is produced in a number of organs and is now known to be a multifunctional factor with various biological activities.4–6 HGF was found to be elevated in serum from patients with liver disease and cancer, including those with malignancies of the breast,7 stomach,8,9 colorectum,10 and lung.11 Furthermore, HGF was inversely correlated with survival time in cancer patients,12 which indicates that it might be a prognostic marker in such patients.10,13 However, existing reports are limited to patients with diagnosed cancer. Thus, we examined whether serum HGF was a predictor of cancer death among apparently healthy Japanese living in the general community.
METHODS
A periodic epidemiologic survey was performed in 1999 in the small farming community of Tanushimaru, Japan. As reported previously, the demographic characteristics of the residents of this area are similar to those of the general Japanese population.14 A total of 1492 adults aged 40 years or older underwent health check-ups, and a questionnaire was used to ascertain their medical history (particularly cancer), use of alcohol, smoking, and current use of medications for hypertension, hyperlipidemia, and diabetes. Alcohol intake and smoking were classified as current habitual use or not. Use of medication for hypertension, hyperlipidemia, and diabetes was coded as dummy variables. Body mass index (BMI) was calculated from measurements of height and body weight. Blood pressure was measured twice with participants in the supine position. A second blood pressure reading was taken after 5 deep breaths, and the fifth-phase diastolic pressure was recorded and used in the analysis. Blood samples obtained from the antecubital vein were centrifuged and frozen. Using these samples, we measured serum glycosylated hemoglobin A1c (Japan Diabetes Society; HbA1c [JDS]), lipids (total cholesterol, high-density lipoprotein [HDL]-cholesterol, and triglycerides), blood urea nitrogen (BUN), creatinine, uric acid, albumin, C-reactive protein (CRP), and liver enzymes (alanine aminotransferase [ALT], aspartate aminotransferase [AST], and γ-glutamyl transpeptidase [γ-GTP]). Plasma HGF was measured by enzyme-linked immunosorbent assay (ELISA).15 Intra- and inter-assay coefficients of variation of HGF, as determined by a commercially available laboratory (Kyodo Igaku Laboratory, Fukuoka, Japan), were 1.0% and 3.0%, respectively. The details of HGF measurement have been previously described.16 Twenty-four participants who had a history of liver disease, lung disease, or cancer were excluded from the analysis. Ultimately, 1470 participants (595 men and 875 women) were enrolled.
The follow-up period was 10 years. Cause of death was determined on the basis of a review of obituaries, medical records, death certificates, and hospital charts, as well as interviews with primary care physicians, the families of the deceased, and other witnesses. Because many patients with cancer ultimately die from infection or other illnesses, great care was taken to identify underlying cause of death. The information was coded independently according to the rules of the Seven Countries Study17 and the World Health Organization’s 10th Revision of the International Statistical Classification of Diseases and Related Health Problems (WHO-ICD).18 Follow-up data through the end of March 2010 were analyzed. The follow-up rate was 92.9%.
This study was approved by the Ukiha Branch of the Japan Medical Association, by the local citizens’ committee of Tanushimaru, and by the Ethics Committee of Kurume University. All participants gave informed consent.
Statistical analysis
Results are presented as mean ± SD. Because of skewed distributions, natural logarithmic transformations were performed for CRP, triglycerides, and γ-GTP. These variables are shown in the original scale in the tables, after analysis using the log (natural)-transformed values. In Tables 1 and 2, the values for CRP, triglycerides, and γ-GTP are presented as geometric mean and range.
Table 1. Characteristics of participants by quartile of HGF.
| Variables | Quartile of HGF level | P for trenda | |||||||||||
|
| |||||||||||||
| Q1 (≤0.16 ng/ml) | Q2 (0.17–0.21 ng/ml) | Q3 (0.22–0.27 ng/ml) | Q4 (≥0.28 ng/ml) | ||||||||||
|
|
|
|
|
||||||||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | ||
| Total No. | 349 | 24 | 397 | 27 | 341 | 23 | 383 | 26 | |||||
| No. and % of deaths | 25 | 7 | 36 | 9 | 49 | 14 | 59 | 15 | 0.001 | ||||
| HGF, ng/ml | 0.14 (0.02) | 0.19 (0.01) | 0.24 (0.02) | 0.35 (0.08) | |||||||||
| Age, years | 62 (10) | 63 (11) | 63 (11) | 63 (11) | 0.397 | ||||||||
| Male sex | 93 | 27 | 154 | 39 | 155 | 45 | 193 | 50 | <0.0001 | ||||
| Body mass indexb | 22 (3) | 23 (3) | 23 (3) | 24 (3) | <0.0001 | ||||||||
| Systolic BP, mm Hg | 131 (21) | 132 (20) | 136 (21) | 136 (22) | 0.001 | ||||||||
| Diastolic BP, mm Hg | 78 (11) | 78 (11) | 80 (12) | 80 (12) | 0.034 | ||||||||
| HbA1c, % | 5.2 (0.7) | 5.2 (0.7) | 5.3 (0.7) | 5.3 (0.9) | 0.103 | ||||||||
| Blood urea nitrogen, mmol/l | 5.9 (1.5) | 5.7 (1.4) | 5.9 (1.5) | 5.8 (1.5) | 0.323 | ||||||||
| Creatinine, µmol/l | 73 (15) | 74 (14) | 77 (19) | 78 (17) | <0.0001 | ||||||||
| Uric acid, µmol/l | 268 (71) | 292 (83) | 303 (89) | 303 (83) | <0.0001 | ||||||||
| C-reactive proteinc, mg/dl | 0.17 | 0.18 | 0.19 | 0.23 | <0.0001 | ||||||||
| Range | 0.1–3.9 | 0.1–3.9 | 0.1–3.9 | 0.1–11.5 | |||||||||
| Albumin, g/l | 44 (2) | 44 (2) | 44 (3) | 44 (3) | 0.546 | ||||||||
| Total cholesterol, mmol/l | 5.3 (0.9) | 5.2 (0.9) | 5.3 (1.0) | 5.0 (0.9) | <0.001 | ||||||||
| HDL cholesterol, mmol/l | 1.5 (0.4) | 1.5 (0.3) | 1.5 (0.4) | 1.4 (0.4) | <0.0001 | ||||||||
| Triglyceridesc, mmol/l | 0.99 | 1.08 | 1.13 | 1.20 | <0.0001 | ||||||||
| Range | 0.33–7.29 | 0.33–10.9 | 0.41–13.5 | 0.32–14.5 | |||||||||
| AST, units/l | 27 (4) | 28 (4) | 28 (6) | 29 (6) | 0.010 | ||||||||
| ALT, units/l | 26 (3) | 26 (3) | 26 (3) | 27 (4) | 0.049 | ||||||||
| γ-GTPc, units/l | 15 | 18 | 20 | 23 | <0.0001 | ||||||||
| Range | 1–201 | 1–218 | 3–896 | 3–305 | |||||||||
| Smoking | 30 | 9 | 52 | 13 | 69 | 20 | 87 | 23 | <0.0001 | ||||
| Alcohol | 61 | 17 | 90 | 23 | 74 | 22 | 85 | 22 | 0.297 | ||||
| Antihypertensive medication | 51 | 15 | 72 | 18 | 71 | 21 | 89 | 23 | 0.022 | ||||
| Antihyperlipidemic medication | 18 | 5 | 21 | 5 | 19 | 6 | 14 | 4 | 0.619 | ||||
| Antidiabetic medication | 6 | 2 | 11 | 3 | 8 | 2 | 15 | 4 | 0.311 | ||||
Data are mean (SD), geometric mean, range, or percent.
Abbreviations: HGF, hepatocyte growth factor; SD, standard deviation; BP, blood pressure; HbA1c, glycosylated hemoglobin A1c; HDL, high-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ-glutamyl transpeptidase.
aCalculated from linear regression analysis for continuous variables and from logistic regression analysis for dichotomous variables.
bWeight (kg)/height (m)2.
cThese variables are shown in the original scale after analysis using log (natural)-transformed values.
Table 2. Characteristics of participants stratified by vital status.
| Variables | Survivors | Decedents | P value | ||||
|
|
|
||||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | ||
| Total No. | 1291 | 88 | 169 | 12 | |||
| HGF, ng/ml | 0.23 (0.09) | 0.26 (0.11) | 0.009 | ||||
| Age, years | 61 (10) | 72 (9) | <0.0001 | ||||
| Male sex | 487 | 38 | 103 | 61 | <0.0001 | ||
| Body mass indexa | 23 (3) | 22 (3) | 0.016 | ||||
| Systolic BP, mm Hg | 132 (20) | 144 (24) | <0.0001 | ||||
| Diastolic BP, mm Hg | 79 (11) | 81 (12) | 0.056 | ||||
| HbA1c, % | 5.2 (0.7) | 5.4 (1.2) | 0.033 | ||||
| Blood urea nitrogen, mmol/l | 5.7 (1.4) | 6.4 (1.9) | <0.0001 | ||||
| Creatinine, µmol/l | 74 (15) | 85 (22) | <0.0001 | ||||
| Uric acid, µmol/l | 286 (83) | 321 (89) | <0.0001 | ||||
| C-reactive proteinb, mg/dl | 0.19 | 0.22 | 0.0004 | ||||
| Range | 0.1–11.5 | 0.1–5.2 | |||||
| Albumin, g/l | 44 (2) | 42 (3) | <0.0001 | ||||
| Total cholesterol, mmol/l | 5.2 (0.9) | 4.9 (0.9) | <0.0001 | ||||
| HDL cholesterol, mmol/l | 1.5 (0.4) | 1.5 (0.4) | 0.485 | ||||
| Triglyceridesb, mmol/l | 1.10 | 1.09 | 0.837 | ||||
| Range | 0.32–14.4 | 0.34–4.73 | |||||
| AST, units/l | 28 (5) | 28 (6) | 0.485 | ||||
| ALT, units/l | 26 (4) | 26 (3) | 0.460 | ||||
| γ-GTPb, units/l | 18 | 22 | 0.043 | ||||
| Range | 1–310 | 3–896 | |||||
| Smoking | 187 | 15 | 47 | 28 | <0.0001 | ||
| Alcohol | 268 | 21 | 40 | 24 | 0.429 | ||
| Antihypertensive medication | 234 | 18 | 48 | 28 | 0.02 | ||
| Antihyperlipidemic medication | 60 | 5 | 12 | 7 | 0.228 | ||
| Antidiabetic medication | 32 | 2 | 8 | 5 | 0.148 | ||
Data are mean (SD), geometric mean, range, or percent.
Abbreviations: HGF, hepatocyte growth factor; SD, standard deviation; BP, blood pressure; HbA1c, glycosylated hemoglobin A1c; HDL, high density lipoprotein; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; γ-GTP, γ-glutamyl transpeptidase.
aWeight (kg)/height (m)2.
bThese variables are shown in the original scale after analysis using log (natural)-transformed values.
Mean HGF level was classified into quartiles as follows: ≤0.16 ng/ml, 0.17–0.21 ng/ml, 0.22–0.27 ng/ml, and ≥0.28 ng/ml. Analysis of variance was used to compare the means of variables, stratified by quartile of HGF levels. Differences between the 2 groups (survivors vs decedents) were assessed using the t test. The χ2 test was used to test differences between groups in categorical variables.
Multivariate proportional hazards regression was used to estimate the predictive HGF level for all-cause death and cancer death. We estimated hazard ratios (HRs) and their 95% CIs per 1-unit (approximately 1 SD) increase in the variable. Survival curves for all-cause death and cancer death for each HGF quartile were estimated with the Kaplan-Meier method and interpreted using the log-rank statistic. Statistical significance was defined as a P value less than 0.05. All statistical analyses were performed using SAS software (Release 9.2, SAS Institute, Cary, NC, USA).
RESULTS
Baseline characteristics associated with HGF
Participants were divided into quartiles according to HGF level. There was a significant positive association between HGF and mortality. In addition, HGF was significantly positively associated with male sex, BMI, systolic and diastolic blood pressures, creatinine, uric acid, CRP, triglycerides, AST, ALT, γ-GTP, smoking, and use of antihypertensive medication and significantly inversely associated with total cholesterol and HDL cholesterol (Table 1).
Cause of death and characteristics of decedents
We were able to ascertain cause of death for 92.9% of deaths. There were 169 (103 men and 66 women) deaths: 61 (36.1%) from cancer, 32 (18.9%) from cerebrocardiovascular disease, 27 (16.0%) from infection, and 49 (29.0%) from other causes.
Table 2 shows the characteristics of participants stratified by vital status. Factors positively associated with death were age, male sex, systolic blood pressure, HbA1c, BUN, creatinine, uric acid, CRP, γ-GTP, smoking, use of antihypertensive medication, and HGF, whereas BMI, albumin, and total cholesterol were inversely associated with death.
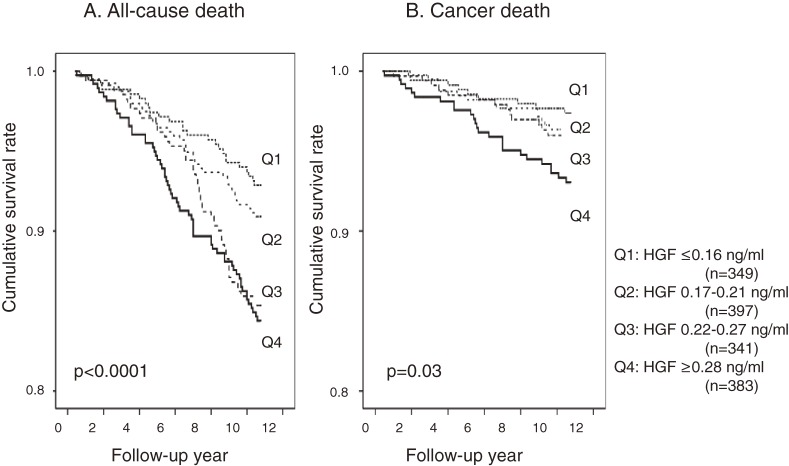
Regression coefficients for all-cause death in the univariate proportional hazards regression model are shown in Table 3. Age, male sex, systolic blood pressure, HbA1c, BUN, creatinine, uric acid, CRP, γ-GTP, smoking, antihypertensive medication, and HGF were significant positive predictors of all-cause death, whereas BMI, albumin, and total cholesterol were inversely associated with all-cause death. Figure 1A shows the cumulative survival curves for all-cause death stratified by HGF quartile in univariate analysis. The HRs by HGF quartile were 1.30 (95% CI, 0.77–2.22) for Q2, 2.18 (1.31–3.62) for Q3, and 2.41 (1.47–3.94) for Q4. Cancer death was positively associated with age, male sex, systolic blood pressure, HbA1c, BUN, creatinine, and smoking and inversely associated with albumin and total cholesterol (Table 4). Figure 1B shows the cumulative survival curves for cancer death stratified by HGF quartile in univariate analysis. Mortality increased in relation to HGF (log-rank test = 11.3; P < 0.001). The HRs by HGF quartile were 1.39 (95% CI, 0.60–3.26) for Q2, 1.39 (0.58–3.35) for Q3, and 2.72 (1.25–5.92) for Q4. For cancer death, mortality increased in relation to HGF (log rank test = 8.23; P = 0.03).
Table 3. Univariate regression coefficients for all-cause death from a Cox proportional hazards regression model.
| Variable (increment) | Beta | SE | Hazard ratio | 95% CI | P value |
| HGF (0.09 ng/ml) | 3.271 | 0.721 | 1.35 | 1.19, 1.54 | <0.0001 |
| Age (10 years) | 0.103 | 0.008 | 2.95 | 2.49, 3.49 | <0.0001 |
| Male sex | 0.835 | 0.185 | 2.31 | 1.60, 3.32 | <0.0001 |
| Body mass indexa (3 kg/m2) | −0.067 | 0.025 | 0.81 | 0.69, 0.95 | 0.009 |
| Systolic BP (20 mm Hg) | 0.021 | 0.003 | 1.57 | 1.38, 1.79 | <0.0001 |
| Diastolic BP (11 mm Hg) | 0.012 | 0.006 | 1.15 | 0.99, 1.34 | 0.060 |
| HbA1c (0.7%) | 0.210 | 0.069 | 1.18 | 1.06, 1.31 | 0.002 |
| Blood urea nitrogen (1.4 mmol/l) | 0.089 | 0.016 | 1.44 | 1.26, 1.65 | <0.0001 |
| Creatinine (16.4 µmol/l) | 1.452 | 0.168 | 1.30 | 1.22, 1.38 | <0.0001 |
| Uric acid (59.4 µmol/l) | 0.244 | 0.050 | 1.40 | 1.22, 1.61 | <0.0001 |
| C-reactive proteinb (1.8 times) | 0.328 | 0.104 | 1.39 | 1.13, 1.70 | 0.002 |
| Albumin (2 g/l) | −2.253 | 0.268 | 0.56 | 0.49, 0.64 | <0.0001 |
| Total cholesterol (0.87 mmol/l) | −0.011 | 0.002 | 0.68 | 0.58, 0.80 | <0.0001 |
| HDL cholesterol (0.36 mmol/l) | −0.003 | 0.005 | 0.99 | 0.82, 1.11 | 0.557 |
| Triglyceridesb (1.1 times) | −0.038 | 0.153 | 0.96 | 0.71, 1.30 | 0.804 |
| AST (5 units/l) | 0.011 | 0.014 | 1.06 | 0.91, 1.24 | 0.447 |
| ALT (3 units/l) | −0.020 | 0.027 | 0.93 | 0.77, 1.13 | 0.463 |
| γ-GTPb (2.2 times) | 0.224 | 0.102 | 1.25 | 1.03, 1.53 | 0.030 |
| Smoking | 0.785 | 0.172 | 2.19 | 1.57, 3.07 | <0.0001 |
| Alcohol | 0.172 | 0.181 | 1.19 | 0.83, 1.69 | 0.341 |
| Antihypertensive medication | 0.545 | 0.170 | 1.73 | 1.23, 2.41 | 0.001 |
| Antihyperlipidemic medication | 0.428 | 0.299 | 1.54 | 0.85, 2.76 | 0.152 |
| Antidiabetic medication | 0.592 | 0.362 | 1.81 | 0.89, 3.68 | 0.102 |
Abbreviations: HGF, hepatocyte growth factor; BP, blood pressure; HDL, high-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ-glutamyl transpeptidase.
aWeight (kg)/height (m)2.
bThese variables are shown in the original scale after analysis using log (natural)-transformed values. These values indicate hazard ratios per times increase.
Figure 1. (A) Kaplan-Meier survival curves for all-cause death, stratified by quartile of hepatocyte growth factor (HGF), after adjustment for age and sex. (B) Kaplan-Meier survival curves for cancer death, stratified by quartile of hepatocyte growth factor (HGF), after adjustment for age and sex.
Table 4. Univariate regression coefficients for cancer death from a Cox proportional hazards regression model.
| Dependent variable (increment) | Beta | SE | Hazard ratioc | 95% CI | P value |
| HGF (0.09 ng/ml) | 3.949 | 1.185 | 1.44 | 1.16, 1.78 | <0.001 |
| Age (10 years) | 0.072 | 0.013 | 2.14 | 1.62, 2.82 | <0.0001 |
| Male sex | 0.919 | 0.295 | 2.51 | 1.41, 4.47 | 0.001 |
| Body mass indexa (3 kg/m2) | −0.005 | 0.042 | 0.98 | 0.76, 1.27 | 0.894 |
| Systolic BP (20 mm Hg) | 0.013 | 0.005 | 1.31 | 1.04, 1.66 | 0.024 |
| Diastolic BP (11 mm Hg) | 0.009 | 0.011 | 1.11 | 0.87, 1.43 | 0.413 |
| HbA1c (0.7%) | 0.280 | 0.100 | 1.24 | 1.07, 1.45 | 0.005 |
| Blood urea nitrogen (1.4 mmol/l) | 0.058 | 0.029 | 1.27 | 1.01, 1.61 | 0.044 |
| Creatinine (16.4 µmol/l) | 1.042 | 0.409 | 2.84 | 1.27, 6.32 | 0.011 |
| Uric acid (59.4 µmol/l) | 0.101 | 0.090 | 1.15 | 0.90, 1.47 | 0.259 |
| C-reactive proteinb (1.8 times) | 0.320 | 0.174 | 1.38 | 0.98, 1.94 | 0.066 |
| Albumin (2 g/l) | −1.828 | 0.471 | 0.63 | 0.49, 0.79 | <0.001 |
| Total cholesterol (0.87 mmol/l) | −0.015 | 0.004 | 0.58 | 0.44, 0.76 | <0.0001 |
| HDL cholesterol (0.36 mmol/l) | −0.009 | 0.009 | 0.87 | 0.67, 1.14 | 0.327 |
| Triglyceridesb (1.1 times) | −0.019 | 0.257 | 0.98 | 0.59, 1.62 | 0.940 |
| AST (5 units/l) | −0.007 | 0.031 | 0.96 | 0.69, 1.33 | 0.813 |
| ALT (3 units/l) | −0.033 | 0.051 | 0.89 | 0.63, 1.26 | 0.508 |
| γ-GTPb (2.2 times) | 0.123 | 0.181 | 1.13 | 0.79, 1.61 | 0.496 |
| Smoking | 0.828 | 0.288 | 2.29 | 1.30, 4.03 | 0.004 |
| Alcohol | 0.422 | 0.287 | 1.53 | 0.87, 2.68 | 0.141 |
| Antihypertensive medication | −0.127 | 0.347 | 0.88 | 0.45, 1.74 | 0.714 |
| Antihyperlipidemic medication | 0.621 | 0.467 | 1.86 | 0.75, 4.66 | 0.183 |
| Antidiabetic medication | 0.951 | 0.517 | 2.59 | 0.94, 7.15 | 0.066 |
Abbreviations: SE, standard error; HGF, hepatocyte growth factor; BP, blood pressure, HDL, high-density lipoprotein, AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, γ-glutamyl transpeptidase.
aWeight (kg)/height (m)2.
bThese variables are shown in the original scale after analysis using log (natural)-transformed values. These values indicate hazard ratios per times increase.
cHazard ratio per 1-increment increase in variable.
Using the significant factors shown in Tables 3 and 4, we performed multivariate proportional hazards regression analysis (Tables 5 and 6). Age, systolic blood pressure, HGF, albumin (inversely), smoking, and creatinine remained significantly associated with all-cause death (Table 5): the HRs by HGF quartile after adjustment for confounding factors were 1.15 (95% CI, 0.68–1.93) for Q2, 1.49 (0.91–2.44) for Q3, and 1.77 (1.10–2.97) for Q4. For cancer death, age, HGF, and total cholesterol (inversely) remained significant (Table 6): after adjustment for confounding factors, the HRs by HGF quartile were 1.22 (95% CI, 0.53–2.83) for Q2, 1.19 (0.50–2.83) for Q3, and 2.21 (1.03–4.76) for Q4.
Table 5. Multivariate proportional hazards regression analysis of all-cause death.
| Dependent variable (increment) | Beta | SE | Hazard ratioc | 95% CI | P value |
| Age (10 years) | 0.088 | 0.013 | 2.53 | 1.90, 3.37 | <0.0001 |
| Systolic BP (20 mm Hg) | 0.013 | 0.005 | 1.32 | 1.08, 1.63 | 0.007 |
| HGF (0.09 ng/ml) | 2.593 | 1.006 | 1.27 | 1.06, 1.52 | 0.009 |
| Albumin (2 g/l) | −1.019 | 0.460 | 0.77 | 0.61, 0.97 | 0.026 |
| Smoking | 0.654 | 0.298 | 1.93 | 1.07, 3.45 | 0.028 |
| Creatinine (16.4 µmol/l) | 0.706 | 0.311 | 2.03 | 1.10, 5.73 | 0.033 |
| γ-GTPb (2.2 times) | 0.072 | 0.110 | 1.19 | 0.96, 1.48 | 0.109 |
| C-reactive proteinb (1.8 times) | 0.160 | 0.106 | 1.17 | 0.96, 1.44 | 0.128 |
| Hypertensive medication | −0.382 | 0.263 | 0.68 | 0.41, 1.14 | 0.146 |
| Blood urea nitrogen (1.4 mmol/l) | 0.038 | 0.027 | 1.17 | 0.94, 1.46 | 0.161 |
| HbA1c (0.7%) | 0.133 | 0.114 | 1.11 | 0.93, 1.32 | 0.242 |
| Total cholesterol (0.87 mmol/l) | −0.002 | 0.003 | 0.91 | 0.71, 1.17 | 0.455 |
| Uric acid (59.4 µmol/l) | 0.040 | 0.086 | 1.06 | 0.84, 1.34 | 0.639 |
| Male sex | 0.107 | 0.296 | 1.11 | 0.62, 1.99 | 0.716 |
| Body mass indexa (3 kg/m2) | 0.003 | 0.038 | 1.01 | 0.80, 1.28 | 0.924 |
Abbreviations: HGF, hepatocyte growth factor; SE, standard error; BP, blood pressure; γ-GTP, γ-glutamyl transpeptidase.
aWeight (kg)/height (m)2.
bThese variables are shown in the original scale after analysis using log (natural)-transformed values. These values indicate hazard ratios per times increase.
cHazard ratio per 1-increment increase in variable.
Table 6. Multivariate proportional hazards regression analysis of cancer death.
| Dependent variable (increment) | Beta | SE | Hazard ratioa | 95% CI | P value |
| Age (10 years) | 0.065 | 0.017 | 2.00 | 1.39, 2.87 | <0.001 |
| HGF (0.09 ng/ml) | 2.929 | 1.267 | 1.31 | 1.04, 1.65 | 0.020 |
| Total cholesterol (0.87 mmol/l) | −0.010 | 0.005 | 0.68 | 0.48, 0.97 | 0.034 |
| Blood urea nitrogen (1.4 mmol/l) | 0.059 | 0.034 | 1.28 | 0.97, 1.68 | 0.082 |
| HbA1c (0.7%) | 0.193 | 0.123 | 1.16 | 0.96, 1.41 | 0.116 |
| Male sex | 0.547 | 0.408 | 1.73 | 0.78, 3.85 | 0.180 |
| Albumin (2 g/l) | −0.787 | 0.630 | 0.82 | 0.59, 1.12 | 0.211 |
| Smoking | 0.472 | 0.383 | 1.60 | 0.76, 3.40 | 0.217 |
| Creatinine (16.4 µmol/l) | 0.506 | 0.567 | 1.66 | 0.55, 5.04 | 0.371 |
| Systolic BP (20 mm Hg) | 0.003 | 0.007 | 1.08 | 0.81, 1.45 | 0.608 |
Abbreviations: HGF, hepatocyte growth factor; SE, standard error; BP, blood pressure.
aHazard ratio per 1-increment increase in variable.
There was no significant association between HGF and cerebrocardiovascular death (data not shown).
DISCUSSION
We investigated whether plasma HGF predicted cancer death among a population of Japanese adults. Our results showed that plasma HGF level was predictive of both all-cause death and cancer death.
We made every effort in this prospective study to ascertain cause of death, and the death rates among this population were comparable to those of the Japanese general population.19 Multivariate proportional hazards regression analysis revealed that age, systolic blood pressure, HGF, albumin (inversely), smoking, and creatinine were independently associated with all-cause death (Table 5). Age, blood pressure, and smoking are known causes of cerebrocardiovascular death. Thus, it is feasible that these factors contributed to all-cause death in this population. However, the number of deaths from cerebrocardiovascular disease was too small to analyze, and we were thus unable to identify the factors responsible for cerebrocardiovascular death in this study. In addition to the above-mentioned factors, low albumin and HGF were significant predictors of all-cause death (Table 5). Low albumin may indicate poor nutritional status. The predictive power of HGF was comparable to that of systolic blood pressure (Table 5). At present, it is unclear why HGF was a significant predictor of all-cause death.
We investigated whether HGF was a predictor of cancer death in this apparently healthy population because cancer is the most common cause of death in this population and because HGF level was found to be a predictor of death in patients with cancer.8–13 Multivariate proportional hazards regression analysis revealed that age, HGF, and cholesterol (inversely) were predictors of cancer death. Although baseline HGF was associated with cholesterol (inversely) and some other factors, as shown in Table 1, the data in Table 6 show that HGF was a significant independent factor for cancer death, which indicates that HGF is a predictor for cancer death in apparently healthy community-dwelling Japanese adults.
The pathophysiologic mechanisms for the association between HGF and cancer death in this population were not investigated in this epidemiologic study. Because we excluded patients with history of cancer at baseline, it is natural to assume that the remaining study participants developed cancer during follow-up. To confirm this, we divided cancer deaths into those that occurred during the first 5 years of follow-up and those that occurred in the second 5 years of follow-up. There were 30 cancer deaths during the first 5 years and 31 during the second 5 years. As mentioned above, serum HGF is elevated in individuals with cancer.7–11 HGF is released from cancer cells11,12 and stimulates not only tumor cell invasion but also neovascularization as a potent endothelial growth factor.13,20–23 If participants who died within 5 years had subclinical cancer at baseline, they might have had higher baseline HGF levels. However, we found that baseline HGF was similar in the early and late study periods among participants who died of cancer. Thus, the increase in the risk of cancer death associated with high HGF was not due to the presence of subclinical cancer. It is possible that participants with high baseline HGF levels experienced rapid growth and spread of cancer once they developed cancer. However, this hypothesis cannot be confirmed in an observational study.
A strength of the present study is that it is the first prospective cohort study to show that HGF is a predictor of cancer death in apparently healthy adults. Moreover, the follow-up rate was high (92.9%).
A limitation of this study is that, because of the relatively small number of cancer deaths, we were unable to investigate the association between HGF and particular malignancies. Second, the cancer endpoint was not incidence but mortality. Third, the association between HGF and cancer death might be confounded by several factors. However, this association remained significant after adjustment for confounders.
In conclusion, serum HGF was a predictor of cancer death in an apparently healthy community-dwelling Japanese population. Our findings suggest that serum HGF may be a predictive marker for cancer death in the general population.
ONLINE ONLY MATERIALS
ACKNOWLEDGMENTS
We are grateful to the members of the Ukiha Branch of the Japan Medical Association, the elected officials and residents of Tanushimaru, and the team of cooperating physicians for their help in performing the health examinations. We also thank Professor Hiroki Inutsuka, Kurume University School of Nursing, for developing the SAS system.
Conflicts of interest: None declared.
REFERENCES
- 1.Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450–9 10.1016/0006-291X(84)91253-1 [DOI] [PubMed] [Google Scholar]
- 2.Nakamura T, Teramoto H, Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci USA. 1986;83:6489–93 10.1073/pnas.83.17.6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura T, Nawa K, Ichihara A, Kaise N, Nishino T. Purification and subunit structure of hepatocyte growth factor from rat platelets. FEBS Lett. 1987;224:311–6 10.1016/0014-5793(87)80475-1 [DOI] [PubMed] [Google Scholar]
- 4.Higashio K, Shima N, Goto M, Itagaki Y, Nagao M, Yasuda H, et al. . Identity of a tumor cytotoxic factor from human fibroblasts and hepatocyte growth factor. Biochem Biophys Res Commun. 1990;170:397–404 10.1016/0006-291X(90)91287-3 [DOI] [PubMed] [Google Scholar]
- 5.Stoker M, Gherardi E. Regulation of cell movement: the motogenic cytokines. Biochim Biophys Acta. 1991;1072:81–102 [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T Structure and function of hepatocyte growth factor. Prog Growth Factor Res. 1991;3:67–85 10.1016/0955-2235(91)90014-U [DOI] [PubMed] [Google Scholar]
- 7.Edakuni G, Sasatomi E, Satoh T, Tokunaga O, Miyazaki K. Expression of the hepatocyte growth factor/c-Met pathway is increased at the cancer front in breast carcinoma. Pathol Int. 2001;51:172–8 10.1046/j.1440-1827.2001.01182.x [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi T, Kitamura M, Arai K, Iwasaki Y, Yamamoto Y, Igari A, et al. . Increase in the circulating level of hepatocyte growth factor in gastric cancer patients. Br J Cancer. 1997;75:673–7 10.1038/bjc.1997.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niki M, Isozaki H, Toyoda M, Ishibashi T, Fujii K, Nomura E, et al. . Serum human hepatocyte growth factor (hHGF) is elevated in patients with metastatic gastric carcinoma. Hepatogastroenterology. 1999;46:568–73 [PubMed] [Google Scholar]
- 10.Toiyama Y, Miki C, Inoue Y, Okugawa Y, Tanaka K, Kusunoki M. Serum hepatocyte growth factor as a prognostic marker for stage II or III colorectal cancer patients. Int J Cancer. 2009;125:1657–62 10.1002/ijc.24554 [DOI] [PubMed] [Google Scholar]
- 11.Siegfried JM, Weissfeld LA, Luketich JD, Weyant RJ, Gubish CT, Landreneau RJ. The clinical significance of hepatocyte growth factor for non-small cell lung cancer. Ann Thorac Surg. 1998;66:1915–8 10.1016/S0003-4975(98)01165-5 [DOI] [PubMed] [Google Scholar]
- 12.Yamashita J, Ogawa M, Yamashita S, Nomura K, Kuramoto M, Saishoji T, et al. . Immunoreactive hepatocyte growth factor is a strong and independent predictor of recurrence and survival in human breast cancer. Cancer Res. 1994;54:1630–3 [PubMed] [Google Scholar]
- 13.Toi M, Taniguchi T, Ueno T, Asano M, Funata N, Sekiguchi K, et al. . Significance of circulating hepatocyte growth factor level as a prognostic indicator in primary breast cancer. Clin Cancer Res. 1998;4:659–64 [PubMed] [Google Scholar]
- 14.Hino A, Adachi H, Toyomasu K, Yoshida N, Enomoto M, Hiratsuka A, et al. . Very long chain N-3 fatty acids intake and carotid atherosclerosis: an epidemiological study evaluated by ultrasonography. Atherosclerosis. 2004;176:145–9 10.1016/j.atherosclerosis.2004.04.020 [DOI] [PubMed] [Google Scholar]
- 15.Yamada A, Matsumoto K, Iwanari H, Sekiguchi K, Kawata S, Matsuzawa Y, et al. . Rapid and sensitive enzyme-linked immunosorbent assay for measurement of HGF in rat and human tissues. Biomed Res. 1995;16:105–14 [Google Scholar]
- 16.Hiratsuka A, Adachi H, Fujiura Y, Yamagishi S, Hirai Y, Enomoto M, et al. . Strong association between serum hepatocyte growth factor and metabolic syndrome. J Clin Endocrinol Metab. 2005;90:2927–31 10.1210/jc.2004-1588 [DOI] [PubMed] [Google Scholar]
- 17.Koga Y, Hashimoto R, Adachi H, Tsuruta M, Tashiro T, Toshima H. Recent trends in cardiovascular disease and risk factors in the Seven Coutries Study. Lessons for science from the Seven Countries Study: A 35-year collaborative experience in cardiovascular disease epidemiology. Springer Verlag; 1994. p. 63–74. [Google Scholar]
- 18.International Statistical Classification of Diseases and Related Health Problems. 10th Revision. Geneva: World Health Organization; 2003. [Google Scholar]
- 19.Health and Welfare Statistics Association. Journal of Health and Welfare Statistics. 2009;56 (in Japanese). [Google Scholar]
- 20.Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol. 1995;129:1177–80 10.1083/jcb.129.5.1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuck AB, Park M, Sterns EE, Boag A, Elliott BE. Coexpression of hepatocyte growth factor and receptor (Met) in human breast carcinoma. Am J Pathol. 1996;148:225–32 [PMC free article] [PubMed] [Google Scholar]
- 22.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, et al. . Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–41 10.1083/jcb.119.3.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, et al. . Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci USA. 1993;90:1937–41 10.1073/pnas.90.5.1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.