Abstract
Background
We assessed the impact of parental history of stroke on stroke mortality, as well as the effect modification between lifestyle and stroke mortality, among Japanese.
Methods
In this community-based, prospective cohort study, 22 763 men and 30 928 women aged 40 to 79 years with no history of cardiovascular disease or cancer at baseline (1988–1990) were followed through 2008. We examined the association between parental history of stroke and stroke mortality and estimated the impact of the combination of lifestyle and parental history on stroke mortality in offspring.
Results
During a mean follow-up period of 15.9 years, there were 1502 stroke deaths. In both sexes, participants with a parental history of stroke had a higher risk of stroke mortality as compared with those without such a history. The respective multivariable hazard ratio (95% CI) and population attributable fraction were 1.28 (1.10–1.49) and 5.4% in men, 1.22 (1.04–1.43) and 4.3% in women, and 1.25 (1.12–1.40) and 4.8% in all participants, for offspring with a maternal and/or paternal history of stroke. There was an inverse association between healthy-lifestyle score and stroke mortality, irrespective of parental history of stroke. The overall multivariable hazard ratio for the highest (6–8) versus the lowest (0–3) score categories was 0.56 (95% CI, 0.43–0.72) for participants with a maternal and/or paternal history of stroke and 0.44 (0.36–0.53) for those without such a history.
Conclusions
Parental history of stroke was associated with stroke mortality in offspring. The inverse association between healthy lifestyle behaviors and stroke mortality, regardless of parental history, suggests that lifestyle modification is beneficial, even among individuals with a parental history of stroke.
Key words: parental history, stroke, lifestyle, cohort study
INTRODUCTION
There is substantial evidence of a relationship between a family history of coronary heart disease in parents and increased risk of coronary heart disease in their offspring.1,2 However, the evidence of such an association with stroke is limited, and the results of studies have been inconsistent.3–5 Current guidelines for assessment of cardiovascular risk6 recommend consideration of a parental history of early-onset cardiovascular disease (onset age <55 years in fathers or <65 years in mothers) as a risk factor for incident cardiovascular disease in their offspring.6,7
Well-established risk factors for stroke include hypertension, diabetes mellitus, and obesity, which are largely hereditary.8–12 Although lifestyle behaviors such as diet, exercise, and smoking can also be hereditary, they are largely influenced by family situations in which both parents and offspring exhibit similar lifestyle behaviors. We previously reported an inverse association between the extent of healthy lifestyle behaviors and mortality from stroke, coronary heart disease, and total cardiovascular disease in the general Japanese population.13 It is uncertain, however, whether this association is modified by parental history of stroke. Hence, knowledge of an association between healthy lifestyle behaviors and stroke mortality, stratified by parental history of stroke, would be important for motivating individuals with a parental history of stroke to improve their lifestyle behaviors and reduce their stroke risk.
In this large, prospective cohort study of samples of Japanese men and women from the general population, we examined the association of parental history of stroke with stroke-related mortality risk in their offspring and determined whether the association between lifestyle behaviors and stroke mortality was modified by parental history.
METHODS
Study population
The Japan Collaborative Cohort Study (JACC Study) was conducted between 1988 and 1990 and enrolled adults living in 45 areas of Japan. The sampling methods and details of the JACC study are described elsewhere.13–17 Participants were recruited mainly at the time of health check-ups, using a self-administered questionnaire that asked about lifestyle behaviors and parental medical history of stroke. The response rate to the questionnaire was 83%. We followed 110 792 adults (46 465 men and 64 327 women) aged 40 to 79 years at baseline. Of these individuals, 26 826 (11 121 men and 15 705 women) were excluded due to the absence of information on parental history of cardiovascular disease, 3837 (1747 men and 2090 women) were excluded due to a positive history of stroke, coronary heart disease, or cancer, and 30 275 (12 581 men and 17 694 women) were excluded due to the absence of information necessary for calculating healthy-lifestyle scores. Data from 53 691 (22 763 men and 30 928 women) were ultimately included in the analysis. There were no important differences between individuals who had complete information on parental history and those who did hot.
Mortality surveillance
Date and cause of death were confirmed by reviewing all death certificates in each study area, with the permission of the Director-General of the Prime Minister’s Office, up to the end of 31 December 2008, except for 11 areas in which follow-up was terminated at the end of 1999 (4 areas) or 2003 (4 areas). Stroke mortality was determined according to the International Classification of Diseases, 10th revision (I60 to I69). A total of 11 099 subjects were censored because of death, and 2863 subjects were censored due to departure or exclusion from the study community. The mean follow-up period was 15.9 years. The study design was approved by the Ethical Board of Nagoya University School of Medicine, the University of Tsukuba, and Osaka University.
Statistical analysis
Parental history of stroke was classified as maternal, paternal, or maternal and/or paternal. We first calculated sex-specific mean age, age-adjusted mean healthy-lifestyle score, and prevalence of stroke risk factors for participants with and without a paternal and/or maternal history of stroke using analysis of covariance for mean values and a logistic regression model for prevalence. We used Cox proportional hazards models to calculate hazard ratios with 95% CIs to determine sex-specific age-adjusted and multivariable-adjusted associations between parental history and stroke mortality associated with non-maternal, non-paternal, and no history of paternal stroke. We constructed 2 multivariable hazard models. The covariates for model 1 were baseline age (in years), healthy-lifestyle score (discussed below; 0–3, 4, 5, 6–8 points), perceived mental stress (low, moderate, high), level of education (<13, 13–15, 16–18, ≥19 years), regular employment (yes, no), and sex (in the analysis of all participants). Covariates for model 2 were those for model 1 plus hypertension and history of diabetes mellitus. Population attributable fractions (PAFs) were calculated with the standard method,18 using multivariable hazard ratios (model 1) with adjustment for history of hypertension and history of diabetes mellitus, as these histories are regarded as contributory intermediate factors in the causal relationship. To examine whether the magnitudes of associations between parental history and stroke mortality differed with regard to maternal or paternal history of stroke, we used an interaction term for maternal and paternal histories. We then repeated the analysis of all participants after stratification by age category (40–59 vs 60–79 years).
In addition, we calculated sex-specific multivariable hazard ratios and 95% CIs for stroke mortality for each healthy-lifestyle score category—with the worst score category of 0 to 3 points as the reference category—stratified by parental history of stroke status for the comparison of stroke mortality with regard to family history of stroke. We previously reported the details of the components of the healthy-lifestyle score.13 In summary, participants scored 1 point for each of the following healthy lifestyle behaviors: consumption of at least 1 serving of fruit per day, at least 1 serving of fish per day, regular consumption of milk, habitual exercise or walking (exercise ≥5 hours per week and/or walking ≥1 hour per day), body mass index (BMI) of 21 to 25 kg/m2, ethanol intake less than 46.0 g per day, no smoking habit, and sleep duration of 5.5 to 7.4 hours per day. Total score ranged from 0 to 8, and scores were grouped into 4 categories (0–3, 4, 5, 6–8 points) for analysis. We combined scores of 0 through 3 and those of 6 through 8 to keep the number of participants roughly balanced in each category. Covariates for multivariable analyses were baseline age (in years), perceived mental stress (low, moderate, high), level of education (<13, 13–15, 16–18, ≥19 years), and regular employment (yes, no). We again repeated the analysis of all participants after stratification by age category (40–59 vs 60–79). To illustrate the magnitude of associations between healthy lifestyle and stroke mortality between participants with and without a parental history of stroke, we repeated the analysis after stratification by parental history of stroke, with the category of worst lifestyle score (0–3) plus positive parental history of stroke as the reference category. Seven HRs were calculated as 3 score categories with a positive family history and 4 score categories with a negative family history. We used the statistical analysis software (SAS) program Ver. 9.13 (SAS Institute Inc., Cary, NC, USA) for all statistical analyses. All probability values for statistical tests were 2-tailed, and values of P less than 0.05 were regarded as statistically significant.
RESULTS
The mean age of participants was 56.5 years in men and 56.8 years in women, mean BMI was 22.7 in men and 22.9 in women, and a history of stroke in 1 or both parents was noted in 24.6% of men and 23.9% of women. During 855 758 person-years of follow-up (353 588 in men and 502 170 in women), there were 1502 deaths due to stroke (774 in men and 728 in women).
Table 1 shows sex-specific mean age, age-adjusted mean healthy-lifestyle scores, and prevalences of stroke risk factors at baseline among participants with and without a maternal and/or paternal history of stroke. As compared with participants without a parental history of stroke, both men and women with a parental history of stroke were likelier to be older, have a higher healthy-lifestyle score, and have a history of hypertension. They were also likelier to consume milk on an almost daily basis and to habitually exercise or walk. Men were likelier to be nonsmokers and heavy drinkers, and women were likelier to consume at least 1 serving of fish per day and were less likely to have graduated from college or university.
Table 1. Mean age, age-adjusted mean healthy-lifestyle score, and prevalences of stroke risk factors in subjects with and without a parental history of stroke.
| Maternal and/or paternal history of stroke |
P-value | ||||
| − | + | ||||
| Men | |||||
| No. at risk | 17 170 | 5593 | |||
| Age, years | 56.1 | 58.0 | <0.001 | ||
| Healthy-lifestyle score, points | 3.95 | 4.00 | 0.05 | ||
| Fruit ≥1/daya, % | 46.2 | 46.2 | 1.00 | ||
| Fish ≥1/daya, % | 30.6 | 31.6 | 0.16 | ||
| Milk almost dailya, % | 38.6 | 41.0 | 0.002 | ||
| Habitual exercise or walkinga, % | 65.4 | 67.2 | 0.01 | ||
| BMI 21–25 kg/m2 a, % | 51.3 | 52.2 | 0.20 | ||
| Ethanol intake <46.0 g/daya, % | 69.9 | 66.8 | <0.001 | ||
| Nonsmokera, % | 46.3 | 48.6 | 0.003 | ||
| Sleep 5.5–7.4 hours/daya, % | 46.8 | 46.0 | 0.33 | ||
| High perceived mental stress, % | 23.5 | 24 | 0.44 | ||
| History of hypertension, % | 18.0 | 26.1 | <0.001 | ||
| History of diabetes mellitus, % | 5.8 | 5.8 | 0.92 | ||
| College or higher education, % | 18.1 | 18.5 | 0.45 | ||
| Regular employment, % | 75.7 | 76.5 | 0.20 | ||
| Women | |||||
| No. at risk | 23 541 | 7387 | |||
| Age, years | 56.3 | 58.4 | <0.001 | ||
| Healthy-lifestyle score, points | 5.06 | 5.13 | <0.001 | ||
| Fruit ≥1/daya, % | 60.7 | 61.2 | 0.39 | ||
| Fish ≥1/daya, % | 32.0 | 35.0 | <0.001 | ||
| Milk almost dailya, % | 44.9 | 45.6 | 0.31 | ||
| Habitual exercise or walkinga, % | 66.7 | 67.2 | 0.40 | ||
| BMI 21–25 kg/m2 a, % | 47.5 | 48.4 | 0.21 | ||
| Ethanol intake <46.0 g/daya, % | 99.3 | 99.3 | 0.92 | ||
| Nonsmokera, % | 95.6 | 96.1 | 0.09 | ||
| Sleep 5.5–7.4 hours/daya, % | 59.7 | 59.9 | 0.83 | ||
| High perceived mental stress, % | 20.1 | 20.8 | 0.21 | ||
| History of hypertension, % | 19.4 | 27.6 | <0.001 | ||
| History of diabetes mellitus, % | 3.2 | 3.5 | 0.26 | ||
| College or higher education, % | 10.8 | 9.4 | 0.001 | ||
| Regular employment, % | 33.6 | 34.5 | 0.20 | ||
aComponent of healthy-lifestyle score (0–8).
Table 2 shows sex-specific age-adjusted and multivariable hazard ratios with 95% CIs and PAFs for mortality from stroke by parental history of stroke. Both men and women with a parental history of stroke had a higher age-adjusted risk of stroke mortality than did those without a parental history of stroke. Hazard ratios tended to be higher among those with a maternal versus a paternal stroke history; however, this difference was not statistically significant (P = 0.49). In model 1, the overall multivariable hazard ratio (95% CI) was 1.33 (1.16–1.52) for maternal stroke history, 1.16 (1.03–1.32) for paternal stroke history, and 1.25 (1.12–1.40) for maternal and/or paternal history of stroke. The hazard ratios were lower in model 2, which was further adjusted for history of hypertension and diabetes mellitus.
Table 2. Sex-specific age-adjusted and multivariable hazard ratios (HRs), 95% CIs, and population attributable fractions (PAFs) for mortality from stroke, stratified by parental history of stroke.
| Men | Women | Total | |||
| Person-years | 353 588 | 502 170 | 855 758 | ||
| Positive maternal history | |||||
| No. | 130 | 125 | 255 | ||
| Age-adjusted HR (95% CI) | 1.24 (1.03–1.50) | 1.37 (1.13–1.66) | 1.31 (1.15–1.50) | ||
| Multivariable HR1 (95% CI)a | 1.26 (1.05–1.53) | 1.40 (1.15–1.70) | 1.33 (1.16–1.52) | ||
| Multivariable HR2 (95% CI)b | 1.17 (0.97–1.42) | 1.28 (1.06–1.56) | 1.21 (1.06–1.39) | ||
| PAF (%)c | 2.4 | 3.2 | 2.8 | ||
| Positive paternal history | |||||
| No. | 158 | 148 | 306 | ||
| Age-adjusted HR (95% CI) | 1.16 (0.97–1.38) | 1.17 (0.98–1.40) | 1.17 (1.03–1.32) | ||
| Multivariable HR1 (95% CI)a | 1.17 (0.99–1.40) | 1.15 (0.96–1.37) | 1.16 (1.03–1.32) | ||
| Multivariable HR2 (95% CI)b | 1.09 (0.91–1.30) | 1.08 (0.90–1.30) | 1.08 (0.95–1.23) | ||
| PAF (%)c | 2.5 | 2.1 | 2.3 | ||
| Positive maternal and/or paternal history | |||||
| No. | 244 | 221 | 465 | ||
| Age-adjusted HR (95% CI) | 1.24 (1.07–1.45) | 1.22 (1.04–1.43) | 1.24 (1.11–1.38) | ||
| Multivariable HR1 (95% CI)a | 1.28 (1.10–1.49) | 1.22 (1.04–1.43) | 1.25 (1.12–1.40) | ||
| Multivariable HR2 (95% CI)b | 1.18 (1.02–1.38) | 1.13 (0.97–1.33) | 1.16 (1.04–1.29) | ||
| PAF (%)c | 5.4 | 4.3 | 4.8 | ||
aMultivariable HR1: adjusted for age, sex (for all subjects), healthy-lifestyle score, perceived mental stress, educational level, and regular employment.
bMultivariable HR2: adjusted for the above variables plus history of hypertension and history of diabetes mellitus.
cPAFs were calculated with the multivariable HR1.
The overall PAF for stroke mortality, 4.8%, was higher among participants with a maternal and/or paternal history of stroke than among those with only a maternal or only a paternal history.
We analyzed parental history and stroke mortality stratified by age category (not shown in table). Participants aged 40 to 59 years had higher age-adjusted and multivariable hazard ratios of stroke mortality for both maternal and paternal history of stroke than did those aged 60 to 79 years. In model 1, the respective multivariable hazard ratios (age 40–59 years vs 60–79 years) were 1.47 (95% CI, 1.05–2.04) versus 1.29 (1.11–1.50) for positive maternal history, 1.65 (1.25–2.18) versus 1.07 (0.93–1.24) for positive paternal history, and 1.63 (1.26–2.09) versus 1.18 (1.04–1.33) for positive maternal and/or paternal history of stroke. The respective PAFs for mortality from stroke were 3.0% versus 3.2% for positive maternal history, 6.0% versus 1.2% for positive paternal history, and 8.3% versus 4.3% for positive maternal and/or parental history.
Table 3 shows multivariable hazard ratios of mortality from stroke for each healthy-lifestyle score category, stratified by parental history of stroke with the 0 to 3 score (worst score) category as the reference. Overall, the risk of stroke mortality decreased with an increase in healthy-lifestyle score. The inverse association between healthy-lifestyle score and stroke mortality did not vary by parental history of stroke in either sex, with the exception of paternal stroke history and stroke mortality in women. Overall, the respective hazard ratio (95% CI) for stroke for the highest healthy-lifestyle score (6–8) versus the worst score (0–3) category was 0.50 (0.35–0.71) with the presence of a maternal history of stroke and 0.47 (0.40–0.55) without such a history, 0.55 (0.40–0.76) with the presence of paternal history of stroke and 0.46 (0.38–0.54) without such a history, and 0.55 (0.43–0.72) with the presence of maternal and/or paternal history of stroke and 0.44 (0.36–0.53) without such a history. These results did not materially change when history of hypertension and diabetes mellitus were included in the multivariable model (model 2; not shown in table).
Table 3. Sex-specific multivariable hazard ratios (HRs) and 95% CIs for mortality from stroke, stratified by parental history of stroke.
| Parental history | Healthy-lifestyle score (points) | |||||||
| 0–3 | 4 | 5 | 6–8 |
P-value for trend |
||||
| Men | ||||||||
| Maternal | ||||||||
| Positive Person-years | 15 075 | 9452 | 7628 | 7936 | ||||
| No. | 58 | 34 | 19 | 19 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.87 (0.57–1.34) | 0.57 (0.34–0.97) | 0.58 (0.34–0.99) | 0.01 | |||
| Negative Person-years | 120 483 | 78 259 | 62 970 | 51 785 | ||||
| No. | 293 | 158 | 115 | 78 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.77 (0.63–0.93) | 0.65 (0.52–0.81) | 0.52 (0.41–0.67) | <0.001 | |||
| Paternal | ||||||||
| Positive Person-years | 22 594 | 13 904 | 11 992 | 10 892 | ||||
| No. | 76 | 38 | 21 | 23 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.75 (0.51–1.11) | 0.43 (0.26–0.70) | 0.52 (0.32–0.85) | 0.001 | |||
| Negative Person-years | 112 963 | 73 807 | 58 606 | 48 829 | ||||
| No. | 275 | 154 | 113 | 74 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.79 (0.65–0.97) | 0.70 (0.56–0.88) | 0.53 (0.41–0.69) | <0.001 | |||
| Paternal and/or maternal | ||||||||
| Positive Person-years | 32 028 | 20 307 | 17 074 | 16 215 | ||||
| No. | 106 | 64 | 36 | 38 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.90 (0.66–1.23) | 0.55 (0.37–0.81) | 0.61 (0.42–0.89) | 0.001 | |||
| Negative Person-years | 103 529 | 67 404 | 53 525 | 43 506 | ||||
| No. | 245 | 128 | 98 | 59 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.74 (0.59–0.91) | 0.67 (0.53–0.86) | 0.49 (0.37–0.66) | <0.001 | |||
| Women | ||||||||
| Maternal | ||||||||
| Positive Person-years | 6879 | 11 602 | 15 198 | 22 547 | ||||
| No. | 26 | 33 | 29 | 37 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.85 (0.50–1.43) | 0.58 (0.34–1.00) | 0.58 (0.34–0.98) | 0.03 | |||
| Negative Person-years | 56 220 | 91 578 | 121 010 | 177 136 | ||||
| No. | 141 | 146 | 161 | 155 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.79 (0.62–0.99) | 0.79 (0.63–1.00) | 0.65 (0.51–0.82) | 0.001 | |||
| Paternal | ||||||||
| Positive Person-years | 10 575 | 17 183 | 21 096 | 32 243 | ||||
| No. | 23 | 41 | 37 | 47 | ||||
| Multivariable HR (95% CI) | 1.00 | 1.22 (0.73–2.04) | 1.04 (0.61–1.77) | 1.00 (0.59–1.69) | 0.76 | |||
| Negative Person-years | 52 523 | 85 998 | 115 112 | 167 440 | ||||
| No. | 144 | 138 | 153 | 145 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.73 (0.57–0.92) | 0.71 (0.57–0.90) | 0.57 (0.45–0.73) | <0.001 | |||
| Paternal and/or maternal | ||||||||
| Positive Person-years | 14 811 | 24 574 | 31 392 | 48 044 | ||||
| No. | 40 | 59 | 53 | 69 | ||||
| Multivariable HR (95% CI) | 1.00 | 1.03 (0.68–1.54) | 0.81 (0.53–1.23) | 0.80 (0.53–1.21) | 0.19 | |||
| Negative Person-years | 48 287 | 78 606 | 104 817 | 151 639 | ||||
| No. | 127 | 120 | 137 | 123 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.72 (0.56–0.93) | 0.74 (0.58–0.95) | 0.58 (0.44–0.75) | 0.001 | |||
| Total | ||||||||
| Maternal | ||||||||
| Positive Person-years | 21 953 | 21 054 | 22 826 | 30 493 | ||||
| No. | 84 | 67 | 48 | 56 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.79 (0.57–1.09) | 0.52 (0.37–0.75) | 0.50 (0.35–0.71) | <0.001 | |||
| Negative Person-years | 176 702 | 169 837 | 183 981 | 228 921 | ||||
| No. | 434 | 304 | 276 | 233 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.70 (0.60–0.81) | 0.62 (0.53–0.72) | 0.47 (0.40–0.55) | <0.001 | |||
| Paternal | ||||||||
| Positive Person-years | 33 169 | 31 087 | 33 088 | 43 135 | ||||
| No. | 99 | 79 | 58 | 70 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.78 (0.58–1.06) | 0.55 (0.40–0.77) | 0.55 (0.40–0.76) | <0.001 | |||
| Negative Person-years | 165 486 | 159 805 | 173 719 | 216 269 | ||||
| No. | 419 | 292 | 266 | 219 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.70 (0.60–0.81) | 0.62 (0.53–0.72) | 0.46 (0.38–0.54) | <0.001 | |||
| Paternal and/or maternal | ||||||||
| Positive Person-years | 46 839 | 44 881 | 48 466 | 64 258 | ||||
| No. | 146 | 123 | 89 | 107 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.83 (0.65–1.06) | 0.57 (0.43–0.74) | 0.55 (0.43–0.72) | <0.001 | |||
| Negative Person-years | 151 816 | 146 010 | 158 342 | 195 146 | ||||
| No. | 372 | 248 | 235 | 182 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.67 (0.57–0.79) | 0.62 (0.52–0.73) | 0.44 (0.36–0.53) | <0.001 | |||
Multivariable adjustment: age, sex (for all subjects), perceived mental stress, educational level, and regular employment.
We examined age-specific associations between healthy-lifestyle score and mortality from stroke, stratified by parental history of stroke (Table 4). The inverse associations between healthy-lifestyle score and stroke mortality were stronger in the younger age group than in the older one, but they did not vary by parental history of stroke in either age group.
Table 4. Age-specific multivariable hazard ratios (HRs) and 95% CIs for mortality from stroke, stratified by parental history of stroke.
| Parental history | Healthy-lifestyle score (points) | |||||||
| 0–3 | 4 | 5 | 6–8 |
P-value for trend |
||||
| Age 40–59 years | ||||||||
| Maternal | ||||||||
| Positive | 11 833 | 11 257 | 11 864 | 16 701 | ||||
| No. | 18 | 11 | 5 | 8 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.63 (0.30–1.36) | 0.27 (0.10–0.74) | 0.30 (0.13–0.72) | 0.002 | |||
| Negative Person-years | 114 253 | 108 263 | 117 929 | 154 643 | ||||
| No. | 84 | 54 | 48 | 45 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.65 (0.46–0.92) | 0.54 (0.37–0.78) | 0.38 (0.26–0.55) | <0.001 | |||
| Paternal | ||||||||
| Positive Person-years | 19 497 | 17 564 | 18 863 | 26 583 | ||||
| No. | 27 | 13 | 8 | 19 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.49 (0.25–0.95) | 0.28 (0.12–0.62) | 0.43 (0.23–0.81) | 0.005 | |||
| Negative Person-years | 106 590 | 101 957 | 110 931 | 144 760 | ||||
| No. | 75 | 52 | 45 | 34 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.71 (0.50–1.01) | 0.58 (0.39–0.84) | 0.33 (0.22–0.50) | <0.001 | |||
| Paternal and/or maternal | ||||||||
| Positive Person-years | 26 996 | 25 521 | 26 809 | 38 207 | ||||
| No. | 39 | 22 | 10 | 21 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.56 (0.33–0.95) | 0.24 (0.12–0.49) | 0.34 (0.19–0.59) | <0.001 | |||
| Negative Person-years | 99 090 | 94 000 | 102 984 | 133 136 | ||||
| No. | 63 | 43 | 43 | 32 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.70 (0.48–1.04) | 0.66 (0.44–0.97) | 0.37 (0.24–0.58) | <0.001 | |||
| Age 60–79 years | ||||||||
| Maternal | ||||||||
| Positive Person-years | 10 120 | 9797 | 10 962 | 13 782 | ||||
| No. | 66 | 56 | 43 | 48 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.84 (0.58–1.20) | 0.59 (0.40–0.88) | 0.56 (0.38–0.83) | 0.001 | |||
| Negative Person-years | 62 449 | 61 574 | 66 052 | 74 279 | ||||
| No. | 350 | 250 | 228 | 188 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.71 (0.60–0.84) | 0.63 (0.54–0.75) | 0.49 (0.41–0.59) | <0.001 | |||
| Paternal | ||||||||
| Positive Person-years | 13 673 | 13 523 | 14 226 | 16 553 | ||||
| No. | 72 | 66 | 50 | 51 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.89 (0.64–1.25) | 0.65 (0.45–0.95) | 0.58 (0.40–0.85) | 0.002 | |||
| Negative Person-years | 58 897 | 57 848 | 62 788 | 71 509 | ||||
| No. | 344 | 240 | 221 | 185 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.70 (0.59–0.82) | 0.63 (0.53–0.74) | 0.49 (0.41–0.59) | <0.001 | |||
| Paternal and/or maternal | ||||||||
| Positive Person-years | 19 843 | 19 360 | 21 657 | 26 051 | ||||
| No. | 107 | 101 | 79 | 86 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.93 (0.71–1.22) | 0.69 (0.51–0.93) | 0.64 (0.48–0.86) | 0.001 | |||
| Negative Person-years | 52 726 | 52 010 | 55 357 | 62 010 | ||||
| No. | 309 | 205 | 192 | 150 | ||||
| Multivariable HR (95% CI) | 1.00 | 0.66 (0.55–0.79) | 0.61 (0.51–0.73) | 0.46 (0.37–0.56) | <0.001 | |||
Multivariable adjustment: age, sex (for all subjects), perceived mental stress, educational level, and regular employment.
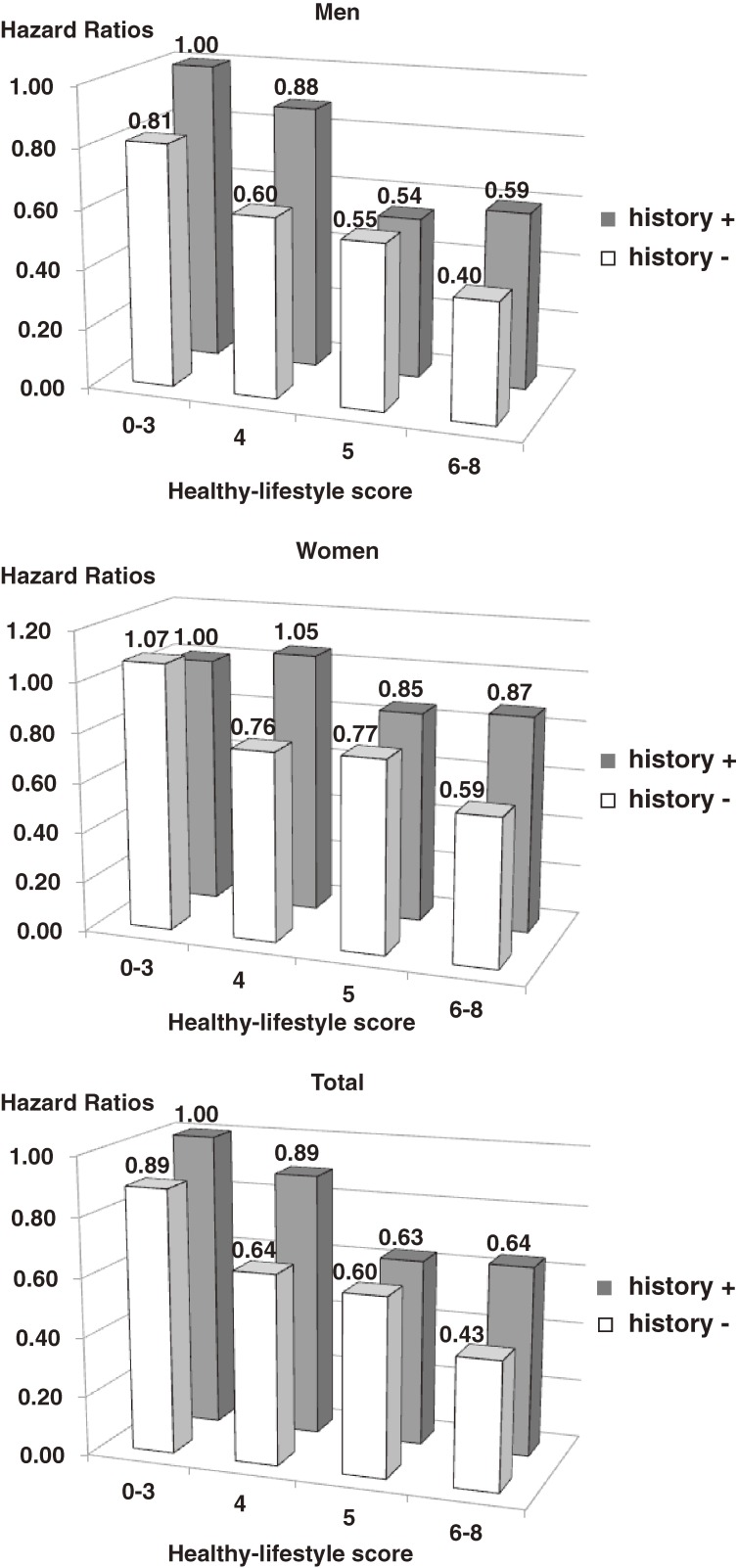
The Figure shows the multivariable hazard ratios of stroke mortality for each healthy-lifestyle score category among men and women with and without a parental history of stroke. Participants with a healthy-lifestyle score of 0 to 3 (worst score) and a maternal and/or paternal history of stroke were used as the reference. Among participants with and without a parental history of stroke, the hazard ratio decreased as healthy-lifestyle score increased. The hazard ratio for stroke mortality was generally higher among participants with a parental history of stroke than in those without such a history. The trends were virtually identical in men and women.
Figure. Multivariable hazard ratios for stroke mortality for each healthy-lifestyle score category for men, women, and all participants with and without a parental history of stroke, using those with a healthy-lifestyle score of 0–3 and a maternal and/or paternal history of stroke as the reference category.
DISCUSSION
In a prospective large cohort study of 53 691 Japanese men and women aged 40 to 79 years, we observed a 16% to 40% increase in stroke mortality risk in participants who had a parental history of stroke. We also found that the inverse relationship observed between healthy lifestyle behaviors and mortality was similar in both men and women and stronger in younger than in older adults, regardless of parental history of stroke, with the exception of paternal stroke history and stroke mortality in women.
A number of previous studies reported an association between parental history and risk of cardiovascular disease and stroke in offspring. In the Framingham Offspring Study, which followed 2302 men and women (28–62 years) for up to 8 years, Lloyd-Jones et al reported multivariate odds ratios (95% CI) for cardiovascular disease of 2.0 (1.2–3.1) in men and 1.7 (0.9–3.1) in women, among participants with at least 1 parent with early-onset cardiovascular disease (onset age <55 years in father, <65 years in mother), and 1.5 (0.9–2.4) in men and 1.1 (0.6–2.1) in women, among those at least 1 parent with non–early-onset cardiovascular disease, including ischemic stroke.7 In addition, on the basis of data from 149 896 person-years that were obtained from 14 371 Finnish men and women aged 25 to 64 years, the multivariable hazard ratio (95% CIs) for stroke associated with any parental history of stroke was 1.9 (1.2–2.9) in men and 1.8 (1.2–2.8) in women.3 Another study of 13 775 individuals aged 45 to 64 years from the atherosclerosis risk in communities (ARIC) cohort reported a multivariable odds ratio (95% CI) for subclinical stroke (cerebral infarct >3 mm) of 1.6 (1.2–2.2) in men and women combined; however, the multivariable hazard ratio for developing clinical stroke was 1.1 (0.8–1.4), which was not significant.4 Kadota et al showed no association between parental history of stroke and stroke mortality in a 19-year follow-up study of 8 037 randomly selected Japanese adults (age, ≥30 years) from the general population of NIPPON DATA 80.5
Our study is the first to show a positive association between parental history of stroke and stroke-related mortality among their offspring in a Japanese population. As compared with 2 previous studies that examined this association,3,4 the magnitude of the present association is smaller. One reason for this discrepancy is the difference in respective outcomes, ie, incidence and mortality, between the previous studies and the present study. Stroke mortality may be affected by survival factors, which can dilute associations between parental history and disease. In addition, the association of parental history of stroke and offspring stroke mortality was stronger among younger persons than older ones, which was consistent with the findings of the Framingham heart study.7
The hazard ratios of stroke mortality associated with parental history of stroke became smaller when we added history of hypertension and history of diabetes to the model. This finding supports the hypothesis that hypertension and diabetes mellitus are partial mediators of the association between parental history of stroke and stroke mortality in offspring.
The association between maternal stroke history and stroke mortality tended to be stronger than the association between paternal stroke history and stroke mortality in both men and women, although the difference was not statistically significant. As compared with fathers, mothers are believed to share a greater number of lifestyle behaviors with their offspring, and that might be a reason for our result.
In the present study, the impact of parental history on stroke mortality was much lower than that of lifestyle behaviors. The PAF for stroke mortality for maternal and/or paternal history of stroke was 5.4% in men and 4.3% in women. In our previous study, the PAF for stroke mortality in adults who did not have 7 to 8 healthy lifestyle behaviors was 45.0% in men and 43.4% in women.13
The effect modification of parental history of stroke on the association between healthy lifestyle behaviors and stroke mortality has not been studied. Our study showed that the association of healthy lifestyle behaviors and stroke mortality was similar in men and women and that younger persons had a stronger inverse association than did older ones. Neither of the associations was influenced by parental history of stroke. There was, however, an exception to this result, ie, a lack of a significant association between healthy-lifestyle score and stroke mortality in women with a paternal stroke history. The reasons for this discrepancy are unknown, but it might be a chance phenomenon due in part to the small number of participants in the reference group, namely, those with a paternal history of stroke and the worst healthy-lifestyle score (0–3).
The strengths of our study are as follows: (1) it was a large-scale, cohort-based study that enrolled participants from all over Japan, with over 1500 deaths reported during long-term follow-up, (2) multiple lifestyle variables were collected at baseline, and (3) adjustments were made for multiple potential confounding variables. These advantages allowed us to estimate the impact of parental history of stroke on stroke-related mortality in offspring, and further allowed us to assess the potential modifying effect of parental history of stroke on the association between healthy lifestyle behaviors and stroke mortality in offspring.
The study did have some limitations that warrant mention. First, we did not have information on the age at which parents had a stroke. The absence of this information meant we were unable to investigate the relationship between parental history of early-onset stroke and stroke-related mortality in offspring. Second, baseline data on lifestyle were only obtained at the initial assessment. It is possible that lifestyle changes occurred during the follow-up period. Consequently, nondifferential measurement errors would have attenuated the observed associations, when the actual/real associations might have been stronger. We used stroke mortality rather than stroke incidence as an endpoint; thus, stroke onset during follow-up might have induced lifestyle changes and consequently influenced mortality risk in some individuals. Finally, there might be a concern regarding selection bias due to the exclusion of 57 101 participants who consented to the study but lacked the necessary information to be included in the analysis. However, the exclusion of these individuals was unlikely to influence these relationships, as their baseline characteristics were similar to those of the rest of the cohort.
Conclusions
In conclusion, a parental history of stroke was associated with an increased risk of stroke mortality among a Japanese population. The association tended to be stronger among study participants who had a maternal versus a paternal history of stroke. The inverse association between lifestyle behaviors and stroke mortality in offspring was similar in men and women and was stronger for younger persons than older ones, regardless of parental history of stroke. However, there was no significant association between healthy life scores and stroke mortality rates in women with a paternal stroke history. These results confirm the need to motivate individuals to modify their lifestyle to prevent stroke, particularly when their parents have a history of stroke.
ACKNOWLEDGMENTS
The JACC Study (Japan Collaborative Cohort Study) was supported by grants-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture of Japan (Monbusho); 61010076, 62010074, 63010074, 1010068, 2151065, 3151064, 4151063, 5151069, 6279102, 11181101, 17015022, 18014011, and 20014026. This research was also supported by Grant-in-Aid from the Ministry of Health, Labor, and Welfare, Health and Labor Sciences research grants, Japan (Research on Health Services: H17-Kenkou-007, Comprehensive Research on Cardiovascular Disease and Life-Related Disease: H18-Junkankitou[Seishuu]-Ippan-012, Comprehensive Research on Cardiovascular Disease and Life-Related Disease: H19-Junkankitou [Seishuu]-Ippan-012, Comprehensive Research on Cardiovascular and Life-Style Related Diseases: H20-Junkan kitou [Seishuu]-Ippan-013 and Comprehensive Research on Cardiovascular and Life-Style Related Diseases: H23-Junkankitou [Seishuu]-Ippan-005.)
The authors sincerely express their appreciation to Dr. Kunio Aoki, Professor Emeritus, Nagoya University School of Medicine and the former chairman of the JACC Study, and Dr. Haruo Sugano, former director of the Cancer Institute, Tokyo, who greatly contributed to the initiation of the JACC Study, as well as Dr. Yoshiyuki Ohno, Professor Emeritus, Nagoya University School of Medicine, who was the past chairman of the study. The authors also wish to thank Dr. Tomoyuki Kitagawa, Cancer Institute of the Japanese Foundation for Cancer Research and the former chairman of Grant-in-Aid for Scientific Research on the Priority Area of Cancer and Dr. Kazuo Tajima, Aichi Cancer Center Research Institute and the former chairman of Grant-in-Aid for Scientific Research on the Priority Area of Cancer Epidemiology for their full support toward this study.
Conflicts of interest: None declared.
Study investigators
Dr. Akiko Tamakoshi (present chairperson of the study group), Aichi Medical University School of Medicine; Drs. Mitsuru Mori & Fumio Sakauchi, Sapporo Medical University School of Medicine; Dr. Yutaka Motohashi, Akita University School of Medicine; Dr. Ichiro Tsuji, Tohoku University Graduate School of Medicine; Dr. Yosikazu Nakamura, Jichi Medical School; Dr. Hiroyasu Iso, Osaka University Graduate School of Medicine; Dr. Haruo Mikami, Chiba Cancer Center; Dr. Michiko Kurosawa, Juntendo University School of Medicine; Dr. Naohito Tanabe, University of Niigata Prefecture; Dr. Koji Tamakoshi, Nagoya University School of Health Sciences; Dr. Kenji Wakai, Nagoya University Graduate School of Medicine; Dr. Shinkan Tokudome, National Institute of Health and Nutrition; Drs. Koji Suzuki & Shuji Hashimoto, Fujita Health University School of Health Sciences; Dr. Shogo Kikuchi, Aichi Medical University School of Medicine; Dr. Yasuhiko Wada, Faculty of Human Life and Environmental Science, Kochi Women’s University; Dr. Takashi Kawamura, Kyoto University Health Service; Dr. Yoshiyuki Watanabe, Kyoto Prefectural University of Medicine Graduate School of Medical Science; Dr. Kotaro Ozasa, Radiation Effects Research Foundation; Dr. Tsuneharu Miki, Kyoto Prefectural University of Medicine, Graduate School of Medical Science; Chigusa Date, University of Hyogo; Dr. Kiyomi Sakata, Iwate Medical University; Dr. Yoichi Kurozawa, Tottori University Faculty of Medicine; Dr. Takesumi Yoshimura, University of Occupational and Environmental Health; Dr. Yoshihisa Fujino, University of Occupational and Environmental Health; Dr. Naoyuki Okamoto, Kanagawa Cancer Center; Dr. Hideo Shio, Moriyama Municipal Hospital; Dr. Akio Yamamoto, Hyogo Prefectural Institute of Public Health and Consumer Sciences; Dr. Masahiko Ando, Nagoya University Hospital; Dr. Yoshiharu Hoshiyama, Yokohama Soei University School of Nursing; and Dr. Akira Shibata, Kurume University Institute of Health and Sports Science.
REFERENCES
- 1.Li R, Bensen JT, Hutchinson RG, Province MA, Hertz-Picciotto I, Sprafka JM, et al. Family risk score of coronary heart disease (CHD) as a predictor of CHD: The atherosclerosis risk in communities (ARIC) study and the NHLBI family heart study. Genet Epidemiol. 2000;18:236–50 [DOI] [PubMed] [Google Scholar]
- 2.Ciruzzi M, Schargrodsky H, Rozlosnik J, Pramparo P, Delmonte H, Rudich V, et al. Frequency of family history of acute myocardial infarction in patients with acute myocardial infarction. Argentine FRICAS (Factores de Riesgo Coronario en America del Sur) Investigators. Am J Cardiol. 1997;80:122–7 [DOI] [PubMed] [Google Scholar]
- 3.Jousilahti P, Rastenyte D, Tuomilehto J, Sarti C, Vartiainen E. Parental history of cardiovascular disease and risk of stroke. A prospective follow-up of 14371 middle-aged men and women in Finland. Stroke. 1997;28:1361–6 10.1161/01.STR.28.7.1361 [DOI] [PubMed] [Google Scholar]
- 4.Morrison AC, Fornage M, Liao D, Boerwinkle E. Parental history of stroke predicts subclinical but not clinical stroke: The Atherosclerosis Risk in Communities Study. Stroke. 2000;31:2098–102 10.1161/01.STR.31.9.2098 [DOI] [PubMed] [Google Scholar]
- 5.Kadota A, Okamura T, Hozawa A, Kadowaki T, Murakami Y, Hayakawa T, et al. Relationships between family histories of stroke and of hypertension and stroke mortality: NIPPON DATA80, 1980–1999. Hypertens Res. 2008;31:1525–31 10.1291/hypres.31.1525 [DOI] [PubMed] [Google Scholar]
- 6.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103 10.1016/j.jacc.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 7.Lloyd-Jones DM, Nam BH, D’Agostino RB Sr, Levy D, Murabito JM, Wang TJ, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: A prospective study of parents and offspring. JAMA. 2004;291:2204–11 10.1001/jama.291.18.2204 [DOI] [PubMed] [Google Scholar]
- 8.Shih PA, O’Connor DT. Hereditary determinants of human hypertension: Strategies in the setting of genetic complexity. Hypertension. 2008;51:1456–64 10.1161/HYPERTENSIONAHA.107.090480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss LA, Pan L, Abney M, Ober C. The sex-specific genetic architecture of quantitative traits in humans. Nat Genet. 2006;38:218–22 10.1038/ng1726 [DOI] [PubMed] [Google Scholar]
- 10.Harrison TA, Hindorff LA, Kim H, Wines RC, Bowen DJ, McGrath BB, et al. Family history of diabetes as a potential public health tool. Am J Prev Med. 2003;24:152–9 10.1016/S0749-3797(02)00588-3 [DOI] [PubMed] [Google Scholar]
- 11.Coady SA, Jaquish CE, Fabsitz RR, Larson MG, Cupples LA, Myers RH. Genetic variability of adult body mass index: A longitudinal assessment in framingham families. Obes Res. 2002;10:675–81 10.1038/oby.2002.91 [DOI] [PubMed] [Google Scholar]
- 12.Munafò M, Clark T, Johnstone E, Murphy M, Walton R. The genetic basis for smoking behavior: A systematic review and meta-analysis. Nicotine Tob Res. 2004;6:583–97 10.1080/14622200410001734030 [DOI] [PubMed] [Google Scholar]
- 13.Eguchi E, Iso H, Tanabe N, Wada Y, Yatsuya H, Kikuchi S, et al. Healthy lifestyle behaviors and cardiovascular mortality among Japanese men and women: The Japan collaborative cohort study. Eur Heart J. 2012;33:467–77 10.1093/eurheartj/ehr429 [DOI] [PubMed] [Google Scholar]
- 14.Ohno Y, Tamakoshi A; JACC Study Group . Japan collaborative cohort study for evaluation of cancer risk sponsored by monbusho (JACC study). J Epidemiol. 2001;11:144–50 10.2188/jea.11.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwai N, Hisamichi S, Hayakawa N, Inaba Y, Nagaoka T, Sugimori H, et al. Validity and reliability of single-item questions about physical activity. J Epidemiol. 2001;11:211–8 10.2188/jea.11.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Date C, Fukui M, Yamamoto A, Wakai K, Ozeki A, Motohashi Y, et al. Reproducibility and validity of a self-administered food frequency questionnaire used in the JACC study. J Epidemiol. 2005;15Suppl 1:S9–23 10.2188/jea.15.S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamakoshi A, Yoshimura T, Inaba Y, Ito Y, Watanabe Y, Fukuda K, et al. Profile of the JACC study. J Epidemiol. 2005;15Suppl 1:S4–8 10.2188/jea.15.S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miettinen OS Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol. 1974;99:325–32 [DOI] [PubMed] [Google Scholar]