Abstract
Slc4a11, a member of the solute linked cotransporter 4 family that is comprised predominantly of bicarbonate transporters, was described as an electrogenic 2Na+-B(OH)4− (borate) cotransporter and a Na+-2OH− cotransporter. The goal of the current study was to confirm and/or clarify the function of SLC4A11. In HEK293 cells transfected with SLC4A11 we tested if SLC4A11 is a: 1) Na+-HCO3− cotransporter, 2) Na+-OH−(H+) transporter, and/or 3) Na+-B(OH)4− cotransporter. CO2/HCO3− perfusion yielded no significant differences in rate or extent of pHi changes or Na+ flux in SLC4A11-transfected compared with control cells. Similarly, in CO2/HCO3−, acidification on removal of Na+ and alkalinization on Na+ add back were not significantly different between control and transfected indicating that SLC4A11 does not have Na+-HCO3− cotransport activity. In the absence of CO2/HCO3−, SLC4A11-transfected cells showed higher resting intracelllular Na+ concentration ([Na+]i; 25 vs. 17 mM), increased NH4+-induced acidification and increased acid recovery rate (160%) after an NH4 pulse. Na+ efflux and influx were faster (80%) following Na+ removal and add back, respectively, indicative of Na+-OH−(H+) transport by SLC4A11. The increased alkalinization recovery was confirmed in NHE-deficient PS120 cells demonstrating that SLC4A11 is a bonafide Na+-OH−(H+) transporter and not an activator of NHEs. SLC4A11-mediated H+ efflux is inhibited by 5-(N-ethyl-N-isopropyl) amiloride (EIPA; EC50: 0.1 μM). The presence of 10 mM borate did not alter dpHi/dt or ΔpH during a Na+-free pulse in SLC4A11-transfected cells. In summary our results show that SLC4A11 is not a bicarbonate or borate-linked transporter but has significant EIPA-sensitive Na+-OH−(H+) and NH4+ permeability.
Keywords: SLC4A11, pH, Na+ permeability, bicarbonate, borate
slc4a11 was cloned based on its homology to known bicarbonate transporters of the SLC4 family and for that reason was named bicarbonate transporter 1 (BTR1; Ref. 21). The SLC4 family of transporters consists of three functional groups: 1) electroneutral Na+-independent Cl−/HCO3− exchangers, 2) electrogenic or electroneutral Na+-HCO3− cotransporters, and 3) electroneutral Na+-driven Cl−/HCO3− exchangers. Interestingly, SLC4A11 has low identity (14–20%) with other SLC4 members. Phylogenetic analysis indicates that it does not cluster strongly with any of the three SLC4 functional groups (23). Lastly, SLC4A11 is postulated to be the mammalian homologue of the Arabidopsis thaliana borate transporter BOR1 (20).
Mutations in SLC4A11 are associated with hearing deficits and poor vision (8). Recessive congenital hereditary endothelial dystrophy (CHED2; Ref. 13), corneal dystrophy and perceptive deafness (Harboyan syndrome; Ref. 8), and dominant late-onset Fuchs endothelial corneal dystrophy (FECD; Ref. 25) are the only known phenotypes associated with SLC4A11 mutations. In the cornea, these diseases are ultimately associated with corneal endothelial cell apoptosis leading to edema and impaired vision (14). The function of SLC4A11 in the corneal endothelium is unknown. However, corneal endothelial function is highly dependent on the presence of bicarbonate, bicarbonate transport, pHi regulation (3), suggesting that any putative bicarbonate transport or Na+-OH− cotransport activity by SLC4A11 is important. A possible role for borate in the cornea, however, is unknown.
In a previous report using SLC4A11-transfected HEK293 cells, SLC4A11 was described as an electrogenic 2Na+-B(OH)4− cotransporter and an electrogenic Na+-2OH− cotransporter (or Na+ and OH− permeable channel) when borate is absent from the media (20). The same report suggested a lack of bicarbonate transport by SLC4A11, but this was not explicitly tested. The evidence for borate as a cotransported substrate rested on data showing cell acidification upon Na+ removal that was faster in the presence of borate. The interpretation of these results is confounded, however, because the sodium substitute used, N-methyl-d-glucamine (NMDG+), is an excellent chelator of borate (27).
To provide direct comparison with previous work (20), we transfected SLC4A11 in HEK293 cells and examined whether SLC4A11 has 1) Na+-HCO3− cotransport activity, 2) Na+-OH−(H+) transport activity, or 3) Na+-B(OH)4− cotransport activity. HEK293 is a good model cell line for studying bicarbonate transport, as expression of bicarbonate transporters is low (1–2, 29) and endogenous expression of SLC4A11 is low (20). In addition, we tested Na+-OH−(H+) transport activity in NHE-deficient PS120 cells to differentiate SLC4A11 as a bonafide transporter or activator of NHEs (15). Our results indicate that SLC4A11 1) has 5-(N-ethyl-N-isopropyl) amiloride (EIPA)-sensitive Na+-OH−(H+) permeability, 2) has NH4+ permeability, 3) does not transport Na+-HCO3−, and 4) does not transport Na+-B(OH)4−.
MATERIALS AND METHODS
Cell culture.
HEK293 (CRL-1573; ATCC, Manassas, CA) cells were cultured in DMEM, 5% fetal bovine serum-heat inactivated, 1% antibiotic/antimycotic (100 U/ml penicillin and 0.25 μ/ml fungizone) and gassed with 5% CO2-95% air at 37°C, and the medium was changed every 2 days. NHE-deficient PS120 Chinese hamster fibroblasts were generously provided by Mark Donowitz and cultured with the same medium used for HEK293.
Transient transfection.
Human wild type SLC4A11 was cloned as an NH2-terminal HA-tagged construct in the phCMV2 vector (Gene Therapy Systems, San Diego, CA) as described previously (24). HEK293 cells grown on 10 cm2 6-well plates or onto 25-mm round glass coverslips (poly-l-lysine-coated) at 50% confluence were transfected with 2.5 μg of HA-SLC4A11-phCMV2 or 1.6 μg of empty vector phCMV2 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). All assays unless indicated otherwise were performed at 48 h posttransfection.
Stable transfection.
To generate stable transfectants in PS120 cells, the cells were transfected and selection with Geneticin (G418, 1 mg/ml) was started 2 days later. The Geneticin medium was replenished every 2 days until no live cells were observed in untransfected control wells. Monoclonal cells transfected with SLC4A11 or empty vector were obtained by single cell dilution cloning. Clones with high expression of SLC4A11 as tested by Western blot were selected for the study.
RNA isolation and RT-PCR.
Total RNA was isolated from HEK293 (RNeasy kit; Qiagen, Valencia, CA). Reverse transcription was carried out (High Capacity RNA-to-cDNA kit; Applied Biosystems) with 200 ng RNA in a 20-μl reaction. PCR was performed following the manufacturer's protocol (Accuprime Taq DNA Polymerase; Invitrogen, Carlsbad, CA) using primers specific primers. The primers sequences used here are as follows: pNBCF: ATGGAGGAT GAAGCTGTCCTGGACAGAGGG; pNBCR: TCAGACATCAGGGTGGCAATGGCTCTGCC; kNBCF: ATGTCCACTGAAAATGTGGAAGGGAAGCCC; kNBCR: GTCAGACATCAAGGTGGCGATGGCTCTTCC; NHE1F: GACTACACACACGTGCGCACCCC; NHE1R: TCCAGGATGATGGGCGGCAGCAGGAAGAGGAA; NHE2F: GAAGATGTTTGTGGACATTGGGG; NHE2R: CGTCTGAGCTGCTGCTATTGC; NHE3F: AGAAGCGGAGAAACAGCAG; NHE3R: GGAGAAAACACAGGGTTGTC; NHE4F: AAGAATATCCGCTACCTCTCCTA; NHE4R: CTGTGTAGGCTCTTCATTGGTAT; NHE5F: CATCTGCTTCACCAAGAGCA; NHE5R: ACGAGCCACAAAGATGATCC; β-actin F: GCAAAGACCTGTACGCCAAC; and β-actin R: AGTACTTGCGCTCAGGAGGA.
Immunoblotting.
HEK293 cells were washed twice in PBS and then lysed in RIPA buffer (50 mM Tris base, 150 mM NaCl, 0.5% deoxycholic acid-sodium salt, 2% SDS, and 1% NP40, pH 7.5, protease inhibitor cocktail) and sonicated (Branson 250, Danbury, CT) on ice. This was followed by centrifugation at 10,000 g for 15 min to pellet cell debris. The supernatant was collected, and an aliquot was taken for protein concentration measurement (Pierce BCA Protein Assay; Thermo Scientific, Rockford, IL). Then, 1× Laemmli sample buffer was added to 30 μg of protein and the mixture was heated at 65°C for 5 min before being resolved by SDS-PAGE and transferred to PVDF membranes (Bio-Rad, Hercules, CA). Blots were then probed overnight at 4°C with SLC4A11 rabbit polyclonal antibody raised against the COOH-terminal region of the protein: IIEAKYLDVMDAEH (1:1,000; Covance, Richmond, CA), mouse monoclonal anti-HA (1:2,000, 16B12, Covance), or rabbit polyclonal NBC1 (pBNC) antibody (1:2,000, AB-3212; Chemicon, Millipore, Temecula, CA). Secondary horseradish peroxidase-conjugated antibody (1:5,000) was incubated for 1 h at room temperature. Bound antibody was detected by enhanced chemiluminescence (Supersignal West Pico Chemiluminescent Substrate; Pierce).
Cell surface processing assay.
To assess the membrane surface expression of recombinant HA-SLC4A11 and endogenous SLC4A11, primary amines exposed to the cell surface were biotinylated with cell-impermeable reagent sulfo-NHS-SS-biotin (SNSB; kit 89881; Pierce Biotechnology, Rockford, IL) with minor modifications. Briefly, HEK293 cells grown in T75 flaks were transfected with 19 μg of HA-SLC4A11-phCMV2 or 11.9 μg of empty vector phCMV2 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Two days posttransfection, cells were washed with PBS. Then, cells were incubated with PBS containing 0.1 mg/ml SNSB at 4°C for 30 min. The biotinylation was stopped with Quenching solution containing excess of primary amines. Cells were detached from the flask using a cell scrapper and washed with TBS. Cells were lysed in 500 μl of lysis buffer supplemented with proteinase inhibitors. The lysate was maintained on ice for 30 min with periodic vortexing and sonication to improve protein solubilization. This was followed by centrifugation at 10,000 g for 15 min to pellet cell debris. The clarified supernatant was recovered. The supernatant was diluted 1:5 in lysis buffer. Diluted supernatants (250 μl) were stored for subsequent SDS-PAGE analysis (total protein fraction). Another volume of 250 μl of diluted supernatant was applied to a column containing 100 μl of Neutroavidin agarose, and the mix was incubated 1 h at room temperature in a rotator. Samples were centrifugated for 1 min at 1,000 g, and the flow through was collected for SDS-PAGE analysis (unbound protein fraction). The Neutroavidin agarose resin was washed three times in wash buffer. Proteins were eluted from the resin by adding 100 ul of SDS-PAGE sample buffer containing 50 mM DTT and incubating 1 h at room temperature in a rotator. The eluted protein was recovered by centrifugation (bound protein fraction). Equal amounts (1 μl) of total and unbound protein fractions were analyzed by SDS-PAGE. For the bound protein fraction, 40 μl were analyzed. The specificity of the assay was tested with cells not treated with SNSB. Immunoblot was performed as described above.
Immunofluorescence.
HEK293 cells grown on 22 × 22 poly-l-lysine-coated were transiently transfected as described above. Cells were washed in PBSCM (PBS containing 1 mM CaCl2 and 1 mM MgCl2) and fixed for 20 min at room temperature with 3.5% (wt/vol) paraformaldehyde in PBSCM. After three washes with PBSCM, autofluorescence was quenched by incubating two times for 5 min with 50 mM NH4Cl in PBSCM. Then coverslips were washed three times in PBSCM. Cells were permeabilized for 30 min at room temperature in PBSCM containing 0.1% (vol/vol) of Triton X-100 and then washed two times in PBSCM. The samples were blocked for 1 h at room temperature with fluorescence dilution buffer (FDB; 5% normal goat serum, 5% FBS, and 2% BSA in PBSCM). The coverslips were incubated with anti-HA (1:200, 16B12; Covance) for 1 h at room temperature in a humidified chamber. After three washes in PBSCM, coverslips were incubated for 1 h in a dark humidified chamber with a 1:200 dilution of Alexa Fluor 488-conjugated goat anti-mouse IgG. Coverslips were washed, counterstained with DAPI, and mounted with anti-fade medium (Prolong; Molecular Probes, Eugene, OR) according to the manufacturer's instructions. Fluorescence was observed with a standard epifluorescence microscope equipped with a cooled CCD camera.
Measurement of pHi.
HEK293 cells were cultured onto 25-mm glass coverslips and incubated with the pH-sensitive fluorescent dye 2′7′-bis(2carboxyethel)-5(6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM; 10 μM) in bicarbonate-free (BF) Ringer at room temperature for 30 min. The composition of the standard BF Ringer used throughout this study was the following (in mM): 150 Na+, 4 K+, 0.6 Mg2+, 1.4 Ca2+, 118 Cl−, 1 HPO42−, 10 HEPES, 28.5 gluconate−, and 5 glucose. Bicarbonate-rich (BR) Ringer solution was prepared by equimolar substitution of sodium gluconate with NaHCO3−. Ringer solutions were equilibrated with air (BF) or 5% CO2 (BR) and pH was adjusted to 7.50 with NaOH at 37°C. Osmolarity of all solutions was adjusted to 295–300 mosM by adding sucrose. EIPA was obtained from Sigma-Aldrich (St. Louis, MO). BCECF-AM was purchased from Molecular Probes (Eugene, OR). Dye-loaded cells were then kept in BF Ringer solution for at least 30 min before use. Fluorescence was excited alternately at 495 ± 10 and 440 ± 10 nm. Fluorescence emission (520–550 nm) ratios (F495/F440) obtained at 1 Hz were calibrated against pHi by the high-K+-nigericin technique as described previously (4). Coverslips were placed in a single-sided perfusion chamber as described previously (6). The assembled chambers were placed on a watered-jacketed (37°C) brass collar held on the stage of an inverted microscope (Eclipse TE200; Nikon, Tokyo, Japan) and viewed with a long working distance (2 mm) water-immersion objective (×40; Nikon). The chamber was connected by tubing (Phar-Med; Saint-Gobain Performance Plastics, Paris, France) to hanging syringes that contained Ringer solution in a polymethyl methacrylate (Plexiglas; Altuglas, Philadelphia, PA) warming box (37°C). The flow of the perfusate (∼0.5 ml/min) was achieved by gravity. An eight-way valve was used to select the desired perfusate for the chamber.
Measurement of intracellular sodium.
The intracellular sodium concentration was measured as previously described (5). The sodium-sensitive dye SBFI-AM was dissolved first in DMSO to 10 mmol/l. Then, the SBFI 10 mmol/l stock was mixed 1:1 with a 20% (wt/vol) solution of Pluronic F-127 and added to bicarbonate-free Ringer to a final concentration of 10 μmol/l. The coverslips were incubated with this solution for 30 min at room temperature, washed in BF for 30 min, and then loaded into the microscope perfusion chamber. Fluorescence was excited alternately at 340 ± 11 and 380 ± 13 nm. The excitation light was passed to the cells via a 400-nm dichroic reflector. Emitted light >450 nm was collected and measured by photon-counting photomultiplier tube as previously described (5). Fluorescence emission (340–380 nm) ratios (F340/F380) obtained at 1 Hz were calibrated against Na+i by exposing the cells to solutions containing different concentrations of Na+ in presence of the ionophores 5 μM gramicidin D, 5 μM nigericin, and 5 μM monensin as described previously (5).
Sequence analysis of SLC4A11.
Reference human amino acid sequences for SLC4, NHE, and amiloride-sensitive Na+ transporters families were obtained from NCBI. Amino acid sequences were aligned with ClustalW2 program (http://ebi.ac.uk/Tools/msa/clustalw2). The alignment was loaded in Jalview 2.7 software (http://jalview.org) to generate the phylogenetic tree showing evolutionary relationship between the proteins. The method used for calculation was neighbor joining using Blosum62.
For sequence comparison on the first and second hinge loop of SLC4 members, the sequences were aligned using Jalview 2.7 in the regions described by Damkier et al. (7).
Amino acid sequence alignment between BOR1 and SLC4 family was performed using BlastP (http://blast.ncbi.nlm.nih.gov).
Statistical analysis.
Data analysis is presented as mean values ± SE. Paired or unpaired t-test was used for statistical analysis, and P < 0.05 was considered significant. EIPA dose-response data were fitted to four-parameter EC50 curves using PSI-Plot software. The correlation was >0.8 for all the curves.
RESULTS
Suitability of HEK293 as a model to test HCO3− transport.
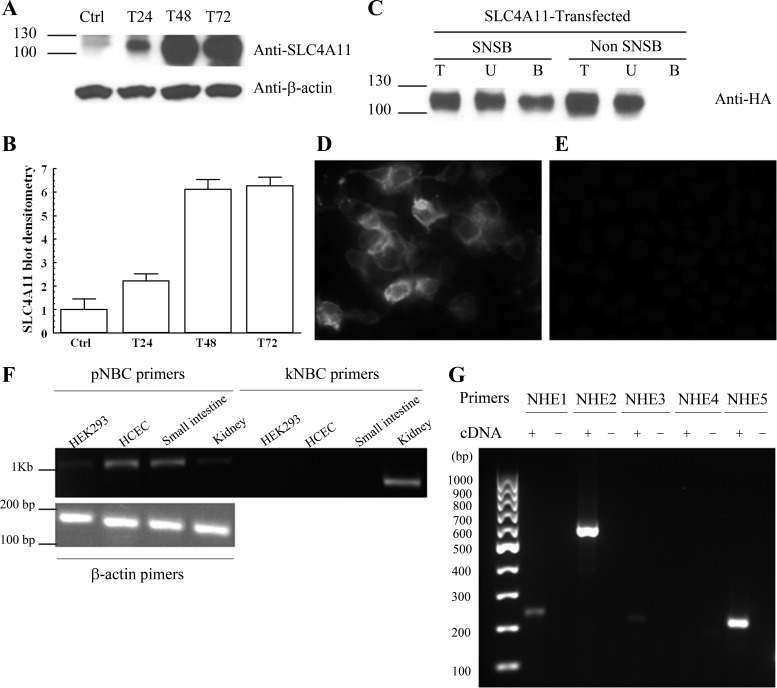
The goal of the present study was to examine whether SLC4A11 has 1) Na+-HCO3− cotransport activity, 2) Na+-OH−(H+) transport activity, or 3) Na+-B(OH)4− cotransport activity. We used HEK293 cells so that direct comparisons with the only other functional study of SLC4A11 can be made (20). Moreover, this cell type has been successfully used to characterize variants of the sodium bicarbonate cotransporter, pNBC and kNBC, by overexpression (1–2, 29) as endogenous expression in HEK293 cells is low (Fig. 1F). Figure 1A shows that HEK293 cells transfected with phCMV2-HA-SLC4A11 express high levels of the recombinant protein as detected using anti-SLC4A11 antibody. SLC4A11 was detected as a 120-kDa band using either anti-SLC4A11 or anti-HA antibodies (Fig. 1, A and C). The levels of SLC4A11 are approximately six times higher in transfected cells compared with untransfected (Fig. 1, A and B). The recombinant HA-SLC4A11 is localized to the membrane as evident by membrane protein isolation (Fig. 1C) and immunofluorescence (Fig. 1, D and E).
Fig. 1.
SLC4A11 expression and localization. A: expression of the recombinant protein in HEK 293 cells transfected with phCMV2-HA-SLC4A11 at 24, 48, and 72 h. Detection was performed with anti-SLC4A11 antibody. SLC4A11 is detected as a 120-kDa band. B: average band density (n = 3). C: sulfo-NHS-S-S-Biotin (SNSB) Membrane preparation of SLC4A11-transfected cells. Detection was performed with anti-HA antibody. Total protein fraction (T), unbound protein fraction (U), and bound eluted membrane protein fraction (B). The presence of the protein in the eluted fraction indicates that the protein is localized in the membrane. D: immunohistochemistry using anti-HA in SLC4A11-transfected HEK293 shows membrane localization of the recombinant protein. E: immunohistochemistry using anti-HA in empty vector-transfected [control (Ctrl)] HEK293 cells. F: RT-PCR for pNBC and kNBC bicarbonate transporters in HEK293 cells and positive controls. G: RT-PCR for NHE1–5 in HEK293 cells.
SLC4A11 is not a bicarbonate transporter.
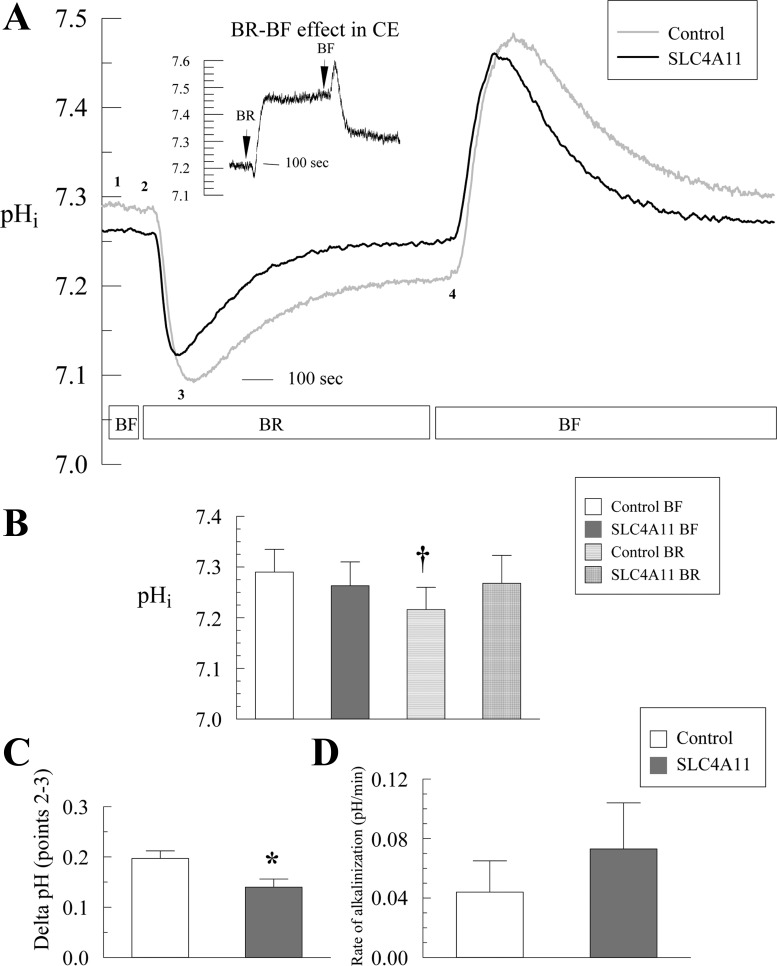
To test if SLC4A11 is a bicarbonate transporter, the cells were perfused in CO2/HCO3−-rich Ringer (BR). Net bicarbonate influx, e.g., electroneutral Na+-HCO3− cotransport or 1Na+-2HCO3− cotransport (NBCe1B), would show a robust alkalinization following the initial CO2-induced acidification and a higher steady-state pHi in BR relative to BF as observed in bovine corneal endothelial cells (Fig. 2A inset). In contrast net bicarbonate efflux, e.g., a 1Na- 3HCO3− cotransporter (NBCe1A) would show a net acidification in BR and lower steady-state pHi (28). In control HEK cells addition of BR induced a fast CO2 acidification of 0.197 ± 0.015 pH units (Fig. 2, A and C, points 2–3) followed by a slow alkalinization of 0.044 ± 0.007 pHi/min (Fig. 2, A and D, points 3–4), reaching a steady-state pHi slightly lower than in bicarbonate free Ringer (Fig. 2, A and B). Relative to corneal endothelial cells, known to have robust 1Na+-2HCO3− cotransport (see Fig. 2A, inset) (4), this result indicates little or no bicarbonate transport by HEK293 cells consistent with previous reports (1–2) and very low expression of pNBC (NBCe1B, see Fig. 1F) in these cells.
Fig. 2.
Effect of bicarbonate on pHi in SLC4A11-transfected HEK293. A: cells were loaded with BCECF-AM and pHi changes were analyzed in bicarbonate-free (BF) and bicarbonate-rich Ringer (BR). Traces represent the average of 12 experiments. Inset: pHi changes in bovine corneal endothelial cells (BCE). B: steady-state pHi in BF and BR conditions. C: delta pHi due to CO2-induced acidification (points 2–3 in A). D: initial maximum rate of alkalinization recovery was obtained by linear regression on the first 30 s of change. †P < 0.05, significantly different to paired BF condition. Labels 1–4 are explained on the text.
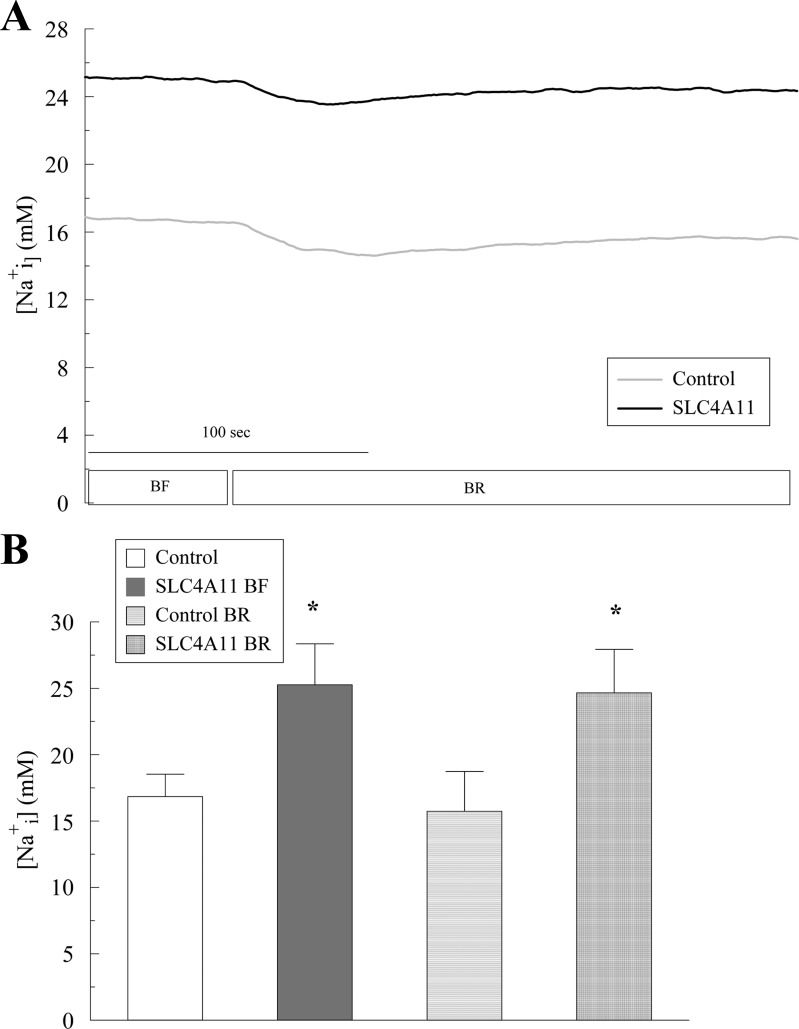
If SLC4A11 is a Na+-nHCO3− cotransporter, then CO2-induced acidification, the subsequent rate of pHi change, and the steady-state pHi in BR should be significantly different from control. In SLC4A11-transfected cells, the only significant differences from control were less CO2-induced acidification (0.14 vs. 0.20 in control; Fig. 2, A and C, points 2–3) and less difference in pHi between BR and BF (Fig. 2, A and B). The alkalinization rate (Fig. 2A, points 3–4) and the resting pHi were not significantly different from control (Fig. 2, A, B, and D). Less CO2-induced acidification suggests greater capacity to remove acid. These results indicate some additional proton efflux (or base influx) capacity in the transfected cells but not what would be expected for a Na+-dependent HCO3− cotransporter. Consistent with the lack of Na+-linked HCO3− transport, Fig. 3 shows that introducing BR Ringer produces small changes in SBFI fluorescence ratio in control and SLC4A11-transfected cells, which can be totally explained by the small pH sensitivity of SBFI (9). In SLC4A11-transfected cells, Na+i levels in BF and BR are not significantly different, which argues against Na+-dependent HCO3− transport capability by SLC4A11. Interestingly, steady-state [Na+]i in BF and in BR is significantly higher in SLC4A11-transfected cells relative to control cells indicating that SLC4A11 has Na+-transport capability (Fig. 3, A and B).
Fig. 3.
Effect of bicarbonate on intracellular Na+ concentration ([Na+]i) in SLC4A11-transfected HEK293. A: [Na+]i in response to BR addition. Traces represent the average of 6 experiments. B: average [Na+]i at the steady state in BF and BR conditions. *P < 0.05, significantly different from control.
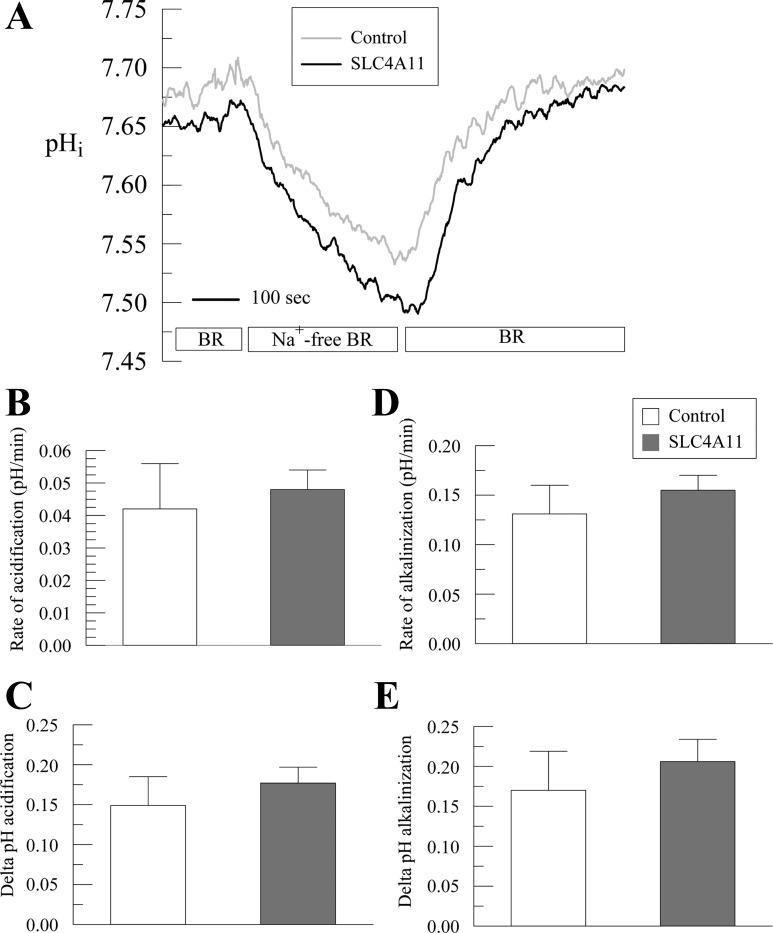
The hypothesis that SLC4A11 has Na+-dependent HCO3− cotransporter activity was further tested in the Na+-efflux mode. In control cells, a Na+-free pulse in BR produces a moderate rate of acidification (0.042 ± 0.014 pHi/min) of 0.149 ± 0.036 pH units (Fig. 4, A–C). In SLC4A11-transfected cells, the acidification rate in BR (0.048 ± 0.006 pHi/min, P = 0.741) and the extent are similar (0.177 ± 0.020 pH units; P = 0.529) compared with control cells indicating that increased SLC4A11 membrane expression does not provide Na+-dependent HCO3− cotransport in the Na+-efflux mode (Fig. 4, A–C). Similarly the extent and rate of alkalinization upon Na+-addition in BR (Na+-influx mode) in SLC4A11 transfected are not different from control cells (control: 0.131 ± 0.029 pHi/min vs. SLC4A11: 0.155 ± 0.015 pHi/min; P = 0.477; Fig. 4, A, D, and E). These results combined with those shown in Figs. 2&3 suggest that SLC4A11 does not transport Na+-nHCO3− either in Na+-influx or Na+-efflux modes.
Fig. 4.
Effect of sodium-free BR perfusion on pHi in SLC4A11-transfected HEK293. A: pHi responses to Na free perfusion in BR for SLC4A11 or empty vector (control)-transfected HEK293 cells. Traces represent the average of 6 experiments. B: summary of rates of acidification (Na+-free). C: extent of acidification. D: rate of alkalinization (Na+ add back). E: extent of alkalinization.
SLC4A11, a Na+-OH− transporter or equivalent.
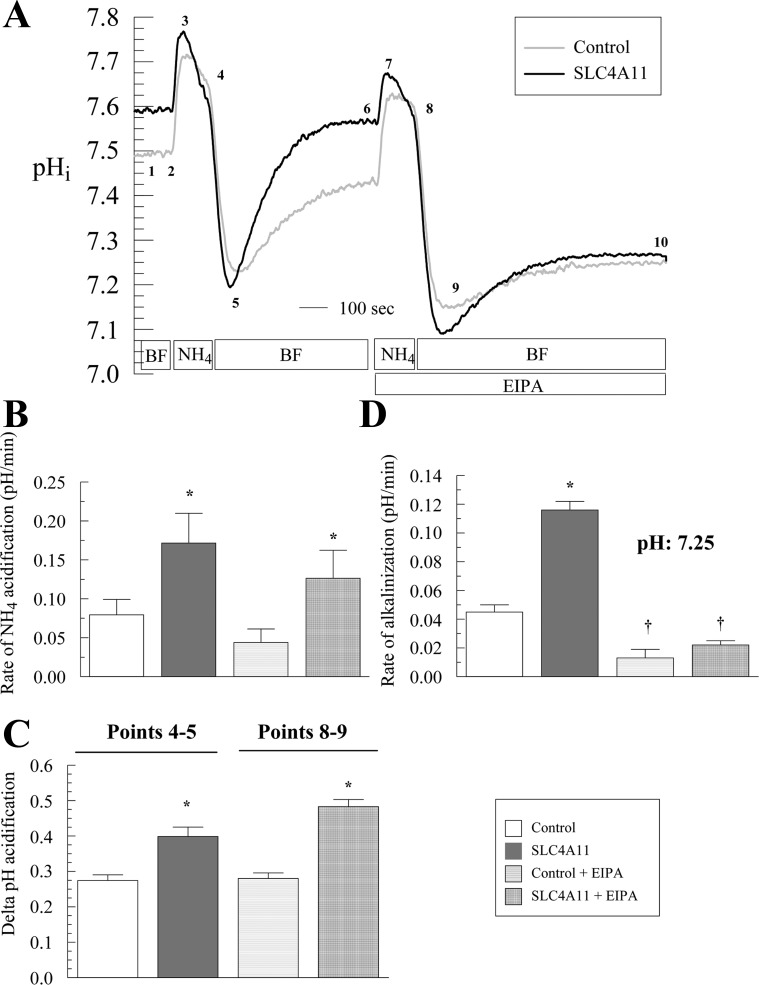
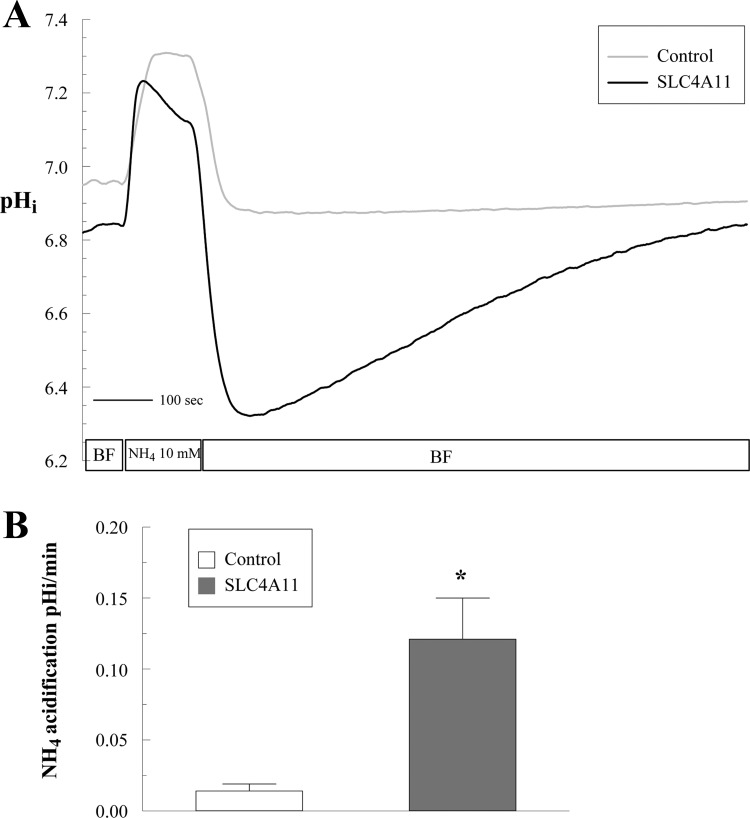
Less acidification upon addition of CO2/HCO3− (initial CO2 acid load) in SLC4A11-transfected cells may be explained by a Na+-OH− or Na+-H+ transport mechanism as previously described by Park et al. (20) for SLC4A11. To test this hypothesis, an acid load was imposed by addition of 10 mM NH4Cl for 2 min in the absence of bicarbonate and the recovery from acid load was measured.
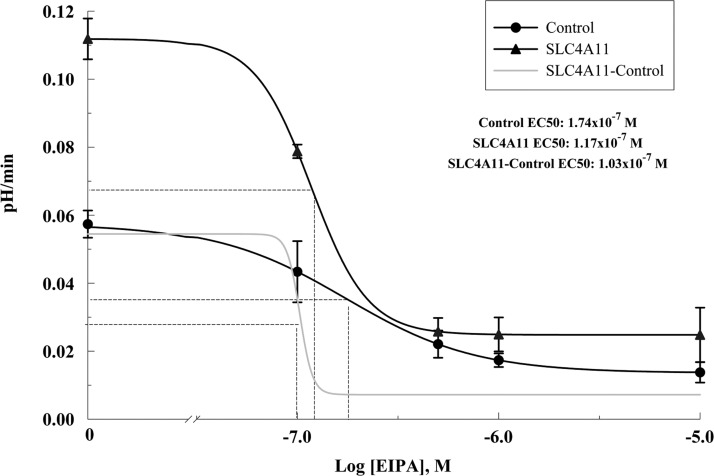
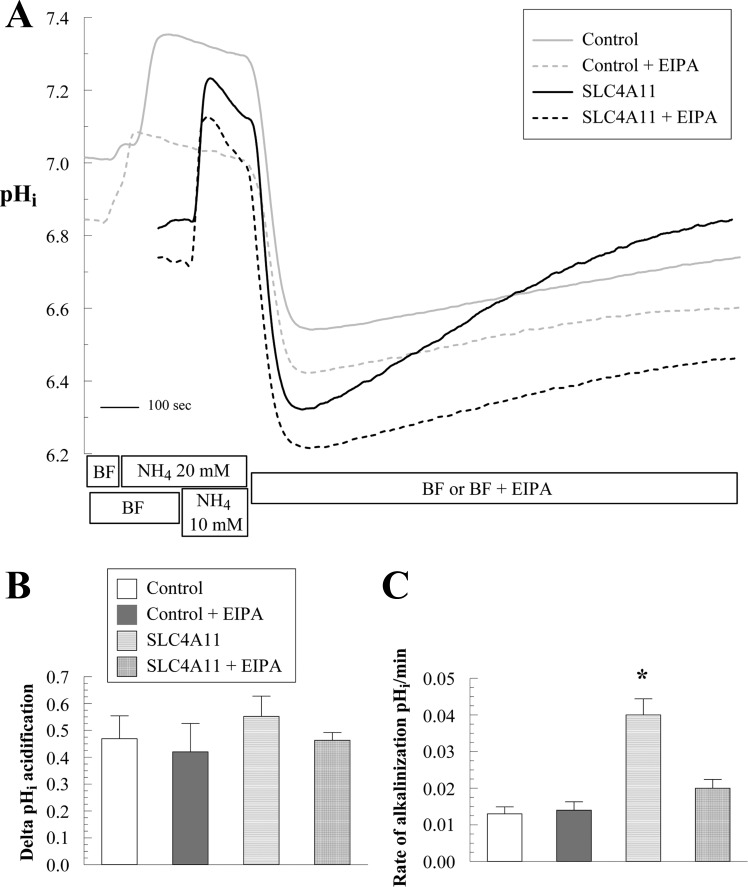
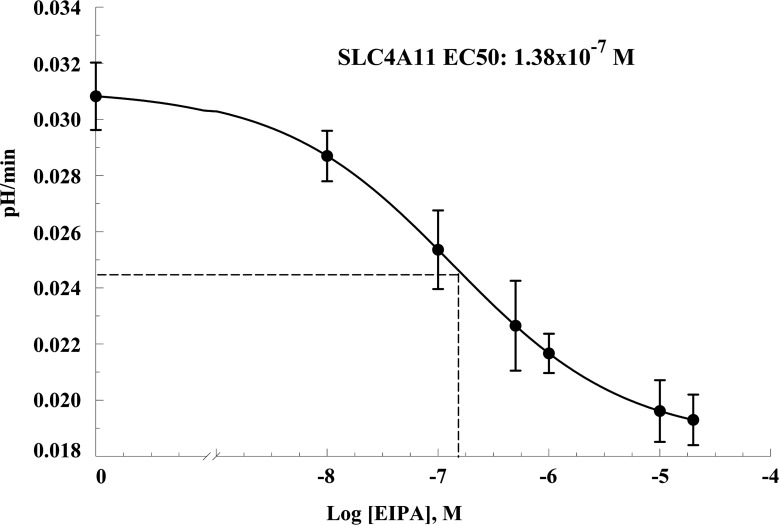
In control cells NH4Cl induces a fast alkalinization (diffusive NH3 influx; Fig. 5A, points 2–3) followed by a slow acidification (NH4+ influx; Fig. 5, A and B, points 3–4). Removal of NH4Cl produces a fast acidification (Fig. 5, A and C, points 4–5), followed by a slow alkalinization or recovery from the acid load (Fig. 5, A and D, points 5–6; values are shown in Fig. 5, B–D). In SLC4A11-transfected cells, the following differences from control were found: faster acidification in the presence of NH4Cl (Fig. 5, A and B, points 3–4) and greater extent of acidification upon NH4Cl removal (Fig. 5, A and C, points 4–5), together suggesting increased NH4+ permeability and increased rate of recovery from the acid load (2.6-fold increase at pHi 7.25; Fig. 5, A and D, points 5–6). These differences are summarized in Fig. 5, B–D, and suggest that SLC4A11 is acting as a Na+-OH− transporter or a Na+/H+-like exchanger. To differentiate this Na+-dependent proton efflux from endogenous NHEs (Na+/H+ exchangers), we repeated the ammonium pulse in the presence of the amiloride analog EIPA, a known inhibitor of NHEs. Figure 5, A and D, points 9–10, shows that 1 μM EIPA almost totally blocked acid recovery in control HEK cells (71% inhibition). Surprisingly, this was true for transfected cells as well (81% inhibition). All of the additional acid efflux capacity in transfected cells was inhibited by EIPA. To estimate the affinity of SLC4A11 to EIPA, dose-response studies were performed in SLC4A11-transfected and control cells. Control cells have an EC50 of 0.174 μM (Fig. 6) that may reflect the combined EIPA sensitivity of the isoforms NHE1, 2, 3 and 5 and low SLC4A11 detected in HEK293 cells (Fig. 1, A and G). The SLC4A11 specific EC50 is 0.103 μM (Fig. 6). This value is intermediate between the reported EC50 of NHE1 (0.015 μM) and NHE3 (2.4 μM; Ref. 19). A previous study (20) used 0.1 μM EIPA to block endogenous NHE activity in HEK293 cells and suggested that SLC4A11 was not inhibited by EIPA. In contrast, our data indicates that SLC4A11-mediated acid recovery is sensitive to EIPA. Additionally, the putative NH4+ transport associated with SLC4A11 is slowed 30% by EIPA but was not significant (Fig. 5, A and B).
Fig. 5.
Recovery from acid load in SLC4A11-transfected cells. A: alkalinization, acidification, and recovery from acid load in SLC4A11 or empty vector (control)-transfected HEK293 cells following exposure (2 min) and removal of 10 mM NH4Cl in BF Ringer. Traces represent the average of 7 experiments. B: NH4+-induced acidification was calculated by linear regression of the first 20 s of pH change. C: delta pHi following NH4+ removal. D: rate of alkalinization recovery was calculated by linear regression on the first 30 s of pH change corresponding to pH: 7.25 in BF. Rate of alkalinization recovery in presence of 5-(N-ethyl-N-isopropyl) amiloride (EIPA; 1 μM) was calculated by linear regression at matched pH: 7.25 and does not represent in this case the initial rate. *P < 0.05, significantly different from control. †P < 0.05, significantly different in the presence of EIPA. Labels 1–10 are explained in the text.
Fig. 6.
EIPA dose-response analysis of SLC4A11-transfected HEK293 cells. Recovery from NH4+ pulse was measured in SLC4A11 and empty vector (control)-transfected HEK293 cells in the presence of 0, 0.1, 0.5, 1, and 10 μM EIPA. Each point represents the average of 6 experiments. Data were fitted to a 4-parameter EC50 function.
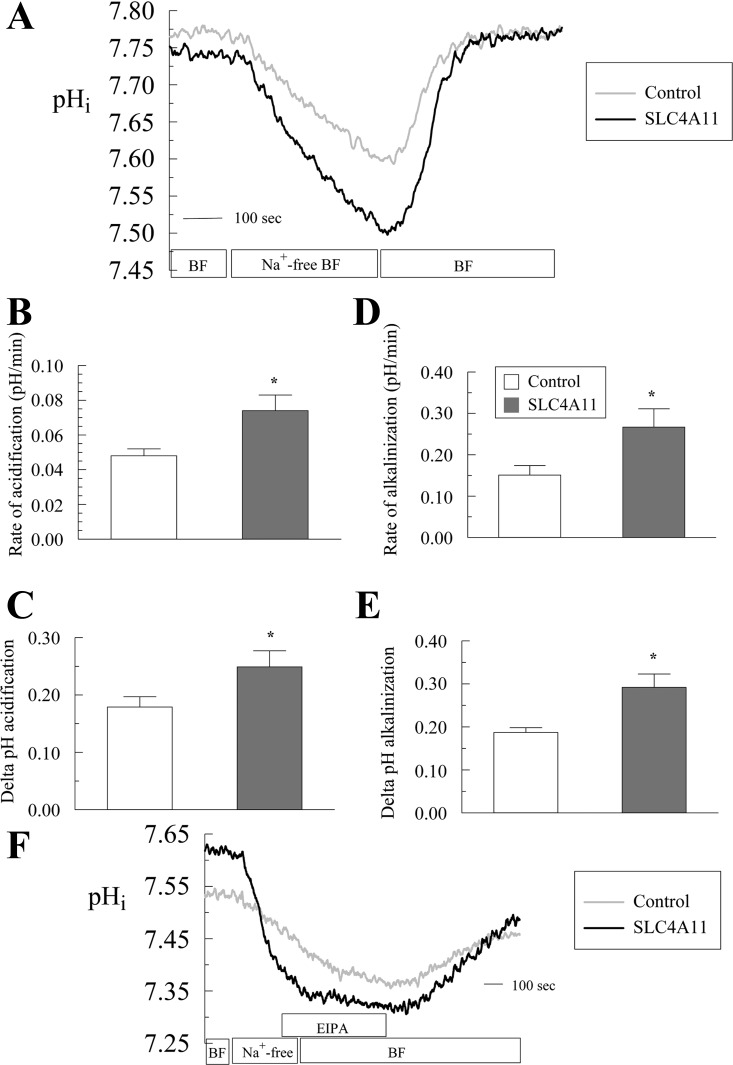
SLC4A11 activity was also tested in the Na+-efflux mode. Figure 7 shows that in control cells, a Na+-free pulse in BF Ringer induced a moderate acidification (0.048 ± 0.004 pHi/min) of 0.179 ± 0.018 pH units. In SLC4A11-transfected cells, the acidification is faster (0.074 ± 0.009 pHi/min; P = 0.039) and of greater extent (0.249 ± 0.028 pH units; P = 0.045) (Fig. 7, A–C). Similarly the extent and rate of alkalinization recovery upon Na+-addition in BF is higher in SLC4A11-transfected cells (control: 0.151 ± 0.023 pHi/min vs. SLC4A11: 0.267 ± 0.044 pHi/min, P = 0.036; Fig. 7, A, D, and E).
Fig. 7.
Effect of Na+-free BF perfusion on pHi in SLC4A11-transfected and control HEK293. A: pHi decrease in response to 5 min of Na+-free Ringer. Traces represent the average of 6 experiments. B: acidification rate on Na+ removal. C: extent of acidification. D: alkalinization rate on Na+ add back. E: extent of pHi change on Na+ add back. *P < 0.05. F: effect of EIPA (1 μM) on pHi recovery following Na+-free acidification in SLC4A11 or empty vector (control) HEK293-transfected cells. Traces represent the average of 6 experiments.
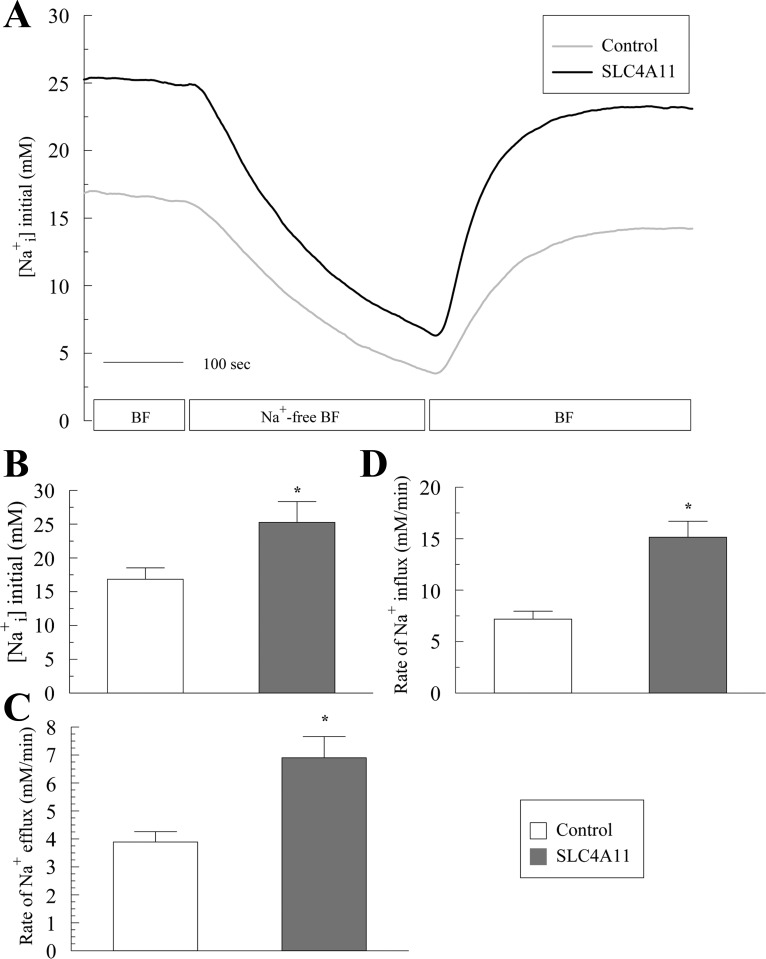
The recovery from acid load (Fig. 5) indicated that SLC4A11 driven Na+-OH− transport or Na+/H+ exchange is sensitive to EIPA. The EIPA sensitivity of SLC4A11 activity was also tested in the Na+-influx mode after a Na+-free pulse. Figure 7F shows that in control cells, a Na+-free pulse in BF induces moderate acidification. Upon Na+ addition in BF there is no alkalinization recovery as long as EIPA (1 μM) is present in the media, consistent with NHE-mediated Na+/H+ exchange being responsible for the recovery in control cells. In SLC4A11-transfected cells, a Na+-free pulse in BF produced a greater and faster acidification (Fig. 7F) as previously observed in Fig. 7A. However, there is no alkalinization recovery upon Na+ addition in the presence of EIPA, indicating that SLC4A11-mediated alkalinization recovery is EIPA sensitive. Lastly, the increased acidification and alkalinization response in SLC4A11-transfected cells is accompanied by increased Na+ fluxes (Fig. 8, A–D). These results are consistent with SLC4A11 acting as a Na+-OH− transporter or Na+/H+ exchanger.
Fig. 8.
Sodium fluxes following a 5-min sodium free pulse in BF in SLC4A11-transfected and control HEK293. A: [Na+]i in response to Na+ removal and add back in BF. Traces represent the average of 8 experiments. B: resting [Na+]i in bicarbonate-free Ringer. C: Na+ efflux rates. D: Na+ influx rates. *P < 0.05.
A possible caveat in the interpretation of results in HEK293 cells is that the observed increase in recovery from acid load is not mediated by SLC4A11 directly but through activation of NHEs or other mechanisms. To address this issue NHE-deficient PS120 cells were stable transfected with SLC4A11 or empty vector. PS120 fibroblasts do not express any NHE isoform and have no Na+-dependent recovery from acid load. A pulse of 10 mM NH4 for 2 min induces significant acidification in the presence of NH4+ in SLC4A11-transfected PS120 cells, but negligible acidification in control cells (Fig. 9, A and B) consistent with increased NH4 permeability seen in HEK-transfected cells (Fig. 5). The NH4+ transport is evidenced by the high acidification after NH4+ removal in SLC4A11-transfected PS120 cells (Fig. 9A). To acidify control cells to the same extent a higher dose and time of incubation with NH4 is required (Fig. 10, A and B). Figure 10 shows that under these conditions the alkalinization recovery is significantly higher in SLC4A11-transfected PS120 (Fig. 10, A and C) indicating that SLC4A11 is a bonafide Na+-OH−(H+) transporter and not an activator of endogenous NHEs. Consistent with an NHE-deficient cell line 10 μM EIPA had no effect on recovery from acid load in control PS120 cells, whereas SLC4A11-dependent recovery from acid load is completely inhibited with 10 μM EIPA (Fig. 10, A and C). Figure 11 shows that the EC50 for EIPA is 0.138 μM in PS120 cells, a value similar to the EC50 of 0.103 μM found in HEK293 cells (Fig. 6).
Fig. 9.
Ammonium pulse and acidification in control and SLC4A11-transfected PS120 cells. A: pHi responses during and following brief perfusion with 10 mM NH4Cl. Traces represent the average of 6 experiments. B: average ± SE of rate of acidification in presence of NH4+. *P < 0.05.
Fig. 10.
Recovery from acid load in control and SLC4A11-transfected PS120 cells. The duration of the ammonium pulse was adjusted to provide more similar acidification in control and-transfected PS120 cells. A: alkalinization, acidification, and recovery from acid load in SLC4A11 or empty vector (control)-transfected PS120 cells following exposure and removal of NH4Cl in BF Ringer in presence or absence of EIPA 10 μM. SLC4A11-transfected cells were acidified by incubation for 2 min with 10 mM NH4Cl. To acidify control cells to the same extent, 4 min incubation with 20 mM NH4Cl was required. Traces represent the average of 8 experiments. B: average ± SE of ΔpHi of acidification after NH4 removal. C: rate of rate of alkalinization recovery. *P < 0.05.
Fig. 11.
EIPA dose-response analysis of SLC4A11-transfected PS120 cells. Recovery from NH4+ pulse was measured in SLC4A11-transfected PS120 cells in the presence of 0, 0.01, 0.1, 0.5, 1, 10, and 20 μM EIPA. Each point represents the average of 6 experiments. Data were fitted to a 4-parameter EC50 function.
SLC4A11 is not a Na+-B(OH)4− cotransporter.
In a previous report, it was concluded that SLC4A11 is a Na+-B(OH)4− cotransporter. The key finding was that the rate of acidification when perfused with sodium free Ringer was faster in the presence of borate in SLC4A11-transfected cells (20). However, in this report [Park et al. (20)], NMDG+ was used to replace Na+. This is problematic since NMDG+ chelates borate (27).
We replicated the experiment using NMDG+ to replace Na+. In contrast to Park et al. (20), Fig. 12 shows that there was no increase in Na+-free-induced acidification in the presence of borate in either control or in SLC4A11-transfected HEK293 cells. During the preparation of borate buffers we noticed that NMDG+-containing borate solutions are 2 pH units lower than solutions without NMDG+, consistent with NMDG+ chelating borate but not boric acid. This indicates that the concentration of borate and boric acid is lower in NMDG+ solutions with respect to Na+ containing solutions. This is confirmed by the fact there is a fast acidification when a switching from NMDG+-borate to Na+-borate (Fig. 12A) in control and SLC4A11-transfected cells. A failure to carefully monitor pH in NMDG+-borate solutions may explain the strong acidification responses observed by Park et al. (2).
Fig. 12.
NMDG-based Na+-free Ringer to test Borate transport. Absence of borate dependent proton flux in SLC4A11-transfected and control HEK293 cells. A: pHi responses to Na+-free pulse in the presence or absence of 10 mM borate. Traces represent the average of 6 experiments. B: average ± SE of rate of acidification with and without borate.
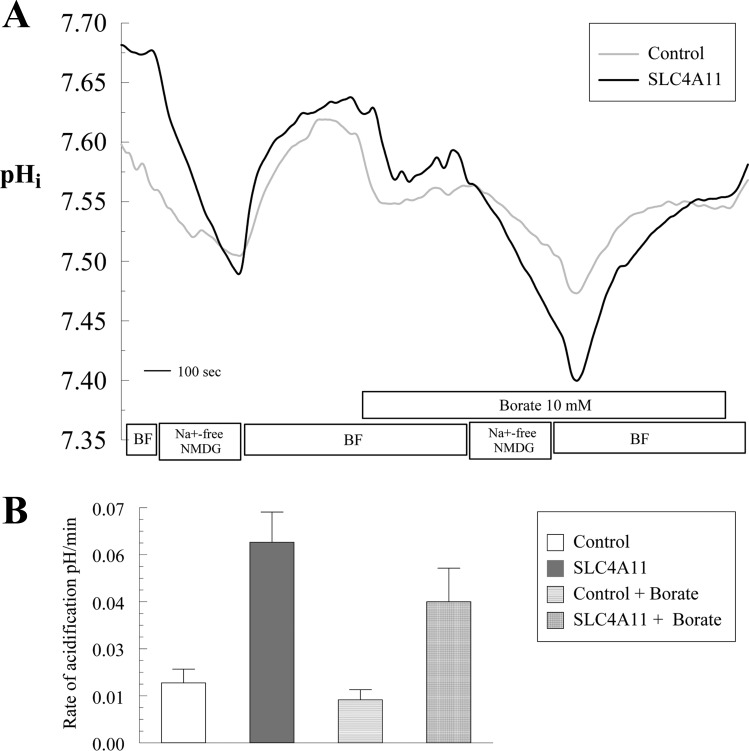
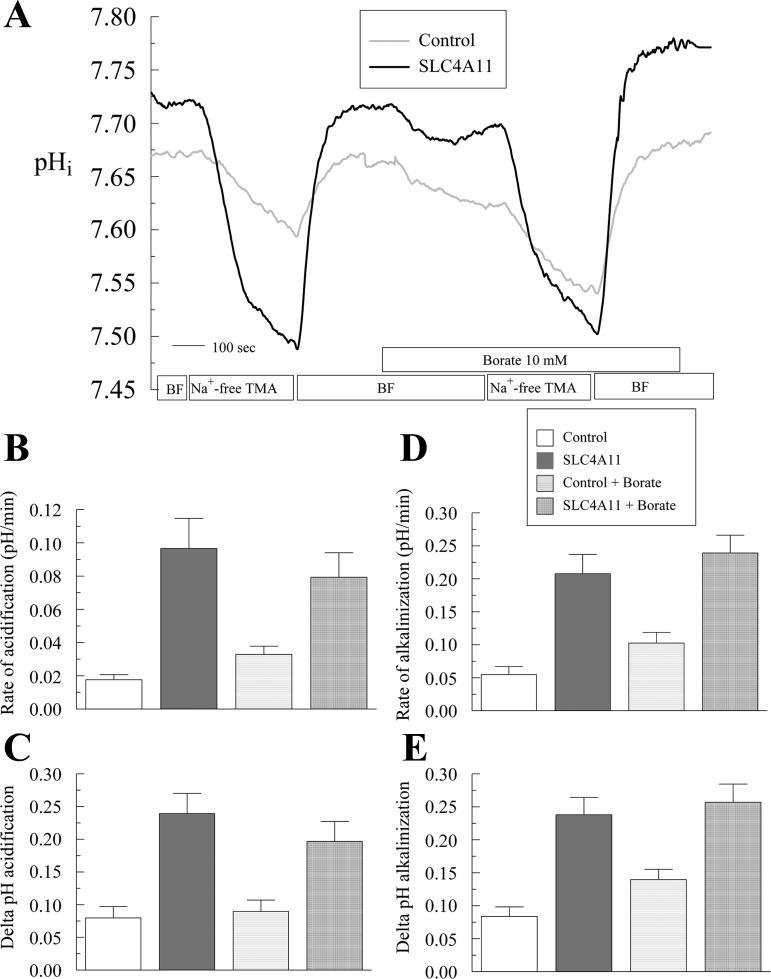
We repeated this experiment using tetra-methyl-ammonium (TMA) to replace Na+. Borate addition to TMA-containing solutions caused similar solution acidification as with sodium-containing solutions, indicating that TMA is not chelating borate. Figure 13, A–C, shows that SLC4A11-transfected cells have faster and higher acidification upon Na+-free pulse with respect to control cells as observed previously (Fig. 7). Upon Na+ readdition the alkalinization recovery is higher in SLC4A11-transfected cells (Fig. 13, A, D, and E). Perfusion with 10 mM borate produces a small acidification in both control and transfected cells due the presence of boric acid. However, the rate and extent of Na+-free-induced acidification were not changed in SLC4A11-transfected cells, inconsistent with Na+-B(OH)4− transport by SLC4A11.
Fig. 13.
TMA-based Na+-free Ringer to test borate transport. Absence of borate dependent proton flux in SLC4A11-transfected and control HEK293 cells. A: pHi responses to Na+-free pulse in the presence or absence of 10 mM borate. Traces represent the average of 7 experiments. B: average ± SE of rate of acidification with and without borate. C: extent of acidification. D: rate of alkalinization on Na+ add back. E: extent of alkalinization on Na+ add back.
Using BlastP we performed amino acid alignments and found that the identity between Arabidopsis thaliana boron transporter BOR1 and SLC4A11 (30%) is essentially the same as that between BOR1 and other members of the SLC4 family (26–30%).
DISCUSSION
The goal of this study was to further clarify the function of SLC4A11 by examining the potential Na+-dependent HCO3−, OH−(H+), or B(OH)4− permeability. Following the approach of Park et al. (20), we overexpressed SLC4A11 in HEK293 cells because of the low levels of endogenous SLC4A11 (Fig. 1A) and bicarbonate transporters (Fig. 1F). Our results suggested Na+/H+ exchanger-like activity so we also stably overexpressed SLC4A11 in NHE-deficient PS120 cells to rule out possible NHE activation. Our results indicate that SLC4A11 does not transport HCO3− or borate but is an EIPA-sensitive Na+-OH− or H+ transporter.
We tested SLC4A11 for Na+-HCO3− cotransport activity because of its membership in the SLC4 family of transporters (21). Moreover, since mutations in SLC4A11 are associated with corneal endothelial dystrophy and corneal endothelial function is dependent on bicarbonate transport, it was important to determine if this gene codes for a bicarbonate transporter. Previous work indicated that HCO3− transport was not affected by incubating cells in Cl−-free medium and was not inhibited by 0.5 mM DIDS (20); however, Na+ dependency was not tested. Here we show that in SLC4A11-transfected cells no net alkalinization or acidification and no differences in Na+ flux with respect to control cells were observed when cells were exposed to CO2/HCO3− (Figs. 2 and 3). As well, no significant changes in rate of pHi change were observed upon a Na+-free pulse in CO2/HCO3−-rich Ringer (Fig. 4) compared with control cells, indicating that SLC4A11 does not provide Na+-dependent HCO3− cotransport. These findings are consistent with our recent report in bovine primary corneal endothelial cells where siRNA knockdown of SLC4A11 expression had no effect on pHi or [Na+] when exposed to BR or a Na+-free pulse in BR (12).
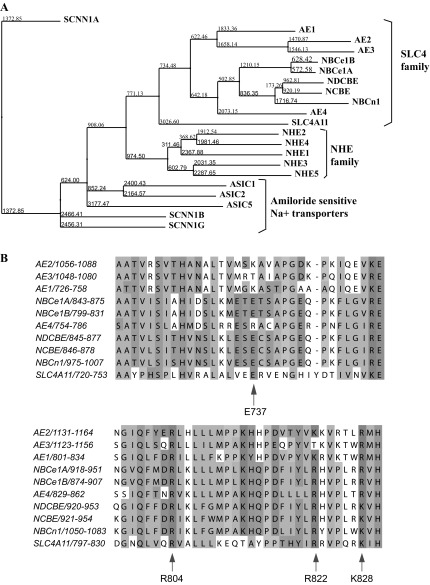
These results are not surprising since the percent identity with HCO3− transporters (14–20%) is quite low. Phylogenetic analysis indicates that SLC4A11 does not strongly cluster with any of the three SLC4 subfamilies and represents a separate branch within the SLC4 family with an early evolutionary divergence and possibly a different function (Fig. 14A and Ref. 23).
Fig. 14.
Sequence analysis of SLC4A11. A: phylogenetic tree showing relationship between SLC4, NHE, and Amiloride-sensitive Na+ transporter families of ion transporters. Sequences were aligned with ClustalW2 program and the alignment was loaded in Jalview 2.7 software (http://jalview.org) to generate the phylogenetic tree showing evolutionary relationship between the proteins. Numbers at the nodes and the lengths of the lines indicate relative evolutionary distance. B: SLC4 family alignment of the first hinge loop (top) and the second hinge loop (bottom). This 1st hinge loop is located in the EL5 of SLC4A11 between TM9 and TM10. The 1st hinge loop is postulated to have sodium attracting amino acids. E737 (Glutamine, net negative charge) is conserved in all members of the SLC4 family that transport sodium and also in SLC4A11. The 2nd hinge loop is located in the TM12 domain of SLC4A11. This region it is postulated to have anion attracting motifs. R804, R822, and K828 (arginine and lysine, net positive charge) are conserved in SLC4A11.
The second potential property, Na+- dependent OH−(H+) transport by SLC4A11, was tested by acidifying cells with NH4+ or Na+-free pulses in CO2/HCO3−-free Ringer. In both cases, a significantly higher alkalinization recovery rate (Figs. 5 and 7) was observed in SLC4A11-transfected. This alkalinization recovery is accompanied by an increased Na+ flux in SLC4A11-transfected (Fig. 8) consistent with a Na+- dependent OH−(H+) transport. SLC4A11-mediated alkalinization recovery was sensitive to EIPA (Figs. 5, 6, and 7). SLC4A11 is a true Na+-OH−(H+) transporter and not an activator of endogenous NHEs because SLC4A11-transfected NHE-deficient PS120 cells have enhanced EIPA-sensitive recovery from acid load when acidified to the same extent as control cells (Fig. 10). The SCL4A11 EC50 for EIPA is virtually the same ∼0.1 μm when studied in SLC4A11-transfected HEK293 or PS120 (Figs. 6 and 11). These results are consistent with a recent report showing that reduced SLC4A11 expression in bovine corneal endothelial cells produced a 30% decrease in recovery from acid load following NH4 pulse and 43% decreased acidification upon a Na+-free (12).
Acid extruding activity and sensitivity to EIPA are classic properties of Na+/H+ exchangers (NHEs). However, in the study of Park et al. (20), a model of SLC4A11 acting as an electrogenic 1Na+-2OH− cotransporter rather than a Na+/H+ exchanger was favored as spontaneous pHi changes and Na+ fluxes were observed upon membrane depolarization (high [K+] solutions), consistent with an electrogenic transporter (20). However, in that study high [K+] produced Na+ efflux instead of the expected Na+-influx that would be consistent with a transporter of 1Na+-2OH− stoichiometry (20). The interpretation of these data was that SLC4A11 functions as a Na+ and OH− permeable channel. This interpretation is problematic because the high [K+] solutions used had progressively lower [Na+] as well. Using the dpHi/dt and Na flux data shown in Figs. 7D and 8D, respectively (control fluxes were subtracted from SLC4A11 fluxes), and an intrinsic buffering capacity of ∼27 mM/pH (determined from small ammonium pulses in Na+-free Ringer), we calculate a ratio of ∼2.4 Na+ per OH−(H+), which is more in line with whole cell patch clamp experiments that estimated at least 2 Na+ being transported per anion (20). Further electrophysiology studies are needed to clarify the stoichiometry of SLC4A11 transport.
Whereas our results are consistent with Park et al. (20) in that SLC4A11 transfection confers increased Na+-dependent OH− or H+ flux, Park et al. described EIPA insensitivity at concentrations as high as 10 μM. We observed complete inhibition at 1 μM EIPA using the same experimental design (Na+-free pulse in BF; Fig. 7F) and during recovery from an NH4Cl pulse (Figs. 5, 6, and 11). It is possible that SLC4A11 retained amiloride sensitivity because it represents an early divergent branch of the SLC4 family and it shares common ancestors with NHEs and amiloride-sensitive Na+ channels (Fig. 14A). To further characterize SLC4A11 function and compare with NHEs, studies of sensitivity to amiloride, HOE 694, and harmaline will be needed; however, this is beyond the scope of this initial study.
Our results clearly indicate that SLC4A11 transfection produces additional Na+ permeability (Figs. 3 and 8). According to Damkier et al. (7), there is a hinge loop region in SLC4 family members that contains sodium attracting amino acids. For example, E737 (glutamine, net negative charge) is conserved only in SLC4 members that transport sodium and is present in SLC4A11 (Fig. 14B). Damkier et al. (7) also postulates the presence of a second hinge loop in the SLC4 family that contains anion attracting amino acids. R804, R822, and K828 (arginine and lysine, net positive charge) anion attracting amino acids are conserved in SLC4A11 (Fig. 14B). This conservation may support the hypothesis of Na+-OH− transport rather than Na+/H+ exchange by SLC4A11.
Unexpectedly we found that SLC4A11 provides additional NH4+ permeability as shown by faster acidification in the presence of NH4+ and a greater extent of acidification after NH4+ removal (Figs. 5 and 9), consistent with greater NH4+ uptake. Other transporters such as NHEs, amiloride-sensitive Na+ channels, and NKCC2 have the capacity to transport cations such as NH4+ (10, 17, 26). Further studies are needed to address the significance of SLC4A11-mediated NH4+ permeability.
Lastly, we tested the third hypothesis as proposed by Park et al. that SLC4A11 has Na+-B(OH)4− cotransport activity. The notion of potential borate transport for SLC4A11 arose because of its homology to the Arabidopsis thaliana boron transporter BOR1 (20). However, we found that BOR1 has a similar identity for all members of the SLC4 family (26–30%). We tested for borate transport by applying a Na+-free pulse in the presence or absence of borate, using NMDG+ to replace Na+ as performed previously by Park et al. (20). In contrast, we did not find any acceleration of the acidification response in SLC4A11-transfected cells in the presence of borate with respect to the absence of borate (Fig. 12). The use of NMDG+ to replace Na+ in a borate containing solution is problematic since NMDG+ chelates borate (27) but not boric acid, acidifying the solution. NMDG+-borate solutions have less free borate and boric acid than Na+-borate solutions as evidenced by a fast acidification when a switching from NMDG+-borate to Na+-borate (Fig. 12A) in control and SLC4A11-transfected cells. The strong acidification responses reported by Park in NMDG+-borate solutions may be explained by a failure to monitor the solution pH after boric acid addition.
We repeated the experiment using TMA+ to replace Na+ and obtained a similar result: no increased Na+-free-induced acidification or alkalinization upon Na+ added back in the presence of borate in SLC4A11-transfected cells (Fig. 13), indicating that SLC4A11 is not a Na+-B(OH)4− cotransporter. Our results are consistent with our recent report in bovine corneal endothelial cells where no evidence of Na+-B(OH)4− cotransport was found in endogenously expressing control cells or cells overexpressing SLC4A11 (12).
There are two reports describing phenotypes of Slc4a11-knockout mice. In one model, only deafness was observed, no corneal phenotype (16). In the second model, deafness along with corneal endothelial dystrophy and polyuria was found (11). In this Slc4a11-knockout model, the corneal thickness is increased and the stroma has higher concentrations of sodium and chloride (11). These results suggest that SLC4A11 function is crucial for corneal endothelial pump activity. Furthermore, the renal function of these mice shows higher daily urinary volume excretion and higher daily urinary excretion of Na+, Cl−, and K+ (11). These observations support possible Na+-absorption capacity by SLC4A11.
In humans and mice, SLC4A11 is the most highly expressed SLC4 family member in the corneal endothelium (22). Given the pHi regulatory activity demonstrated here, SLC4A11 could add significant cellular buffering capacity that would facilitate the removal of lactic acid from the highly glycolytic cornea. Loss of SLC4A11 function in the corneal endothelium could thereby slow the removal of lactate from the cornea, which will produce corneal edema (18).
In conclusion, we found that SLC4A11 transports Na+-OH−(or Na+-H+) and NH4+ but not Na+-HCO3− or Na+-B(OH)4−. Additional studies will be needed to establish the electrogenicity, and cotransporter or channel-like nature of the Na+-OH−(H+) permeability of SLC4A11.
GRANTS
This work was supported by National Eye Institute Grants EY-008834 (to J. A. Bonanno) and Core Facility P30-EY-019008. This study was also supported by Grants NO-07/1/35/19/520 from the Biomedical Research Council, Singapore (to E. N. Vithana).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.G.O., S.S.J., E.N.V., and J.A.B. conception and design of research; D.G.O. and W.Z. performed experiments; D.G.O. analyzed data; D.G.O., S.S.J., and J.A.B. interpreted results of experiments; D.G.O. prepared figures; D.G.O. drafted manuscript; D.G.O., S.S.J., E.N.V., and J.A.B. edited and revised manuscript; D.G.O., W.Z., E.N.V., and J.A.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Michelle E. Brown and John S. Sowinski for technical assistance.
REFERENCES
- 1.Amlal H, Wang Z, Burnham C, Soleimani M. Functional characterization of a cloned human kidney Na+:HCO3− cotransporter. J Biol Chem 273: 16810–16815, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Bachmann O, Franke K, Yu H, Reiderer B, Li HC, Soleimani M, Manns MP, Seidler U. cAMP-dependent and cholinergic regulation of the electrogenic intestinal/pancreatic Na+/HCO3− cotransporter pNBC1 in human embryonic kidney (HEK293) cells. BMC Cell Biol 9: 70, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonanno JA. Molecular mechanisms underlying the corneal endothelial pump. Exp Eye Res 95: 2–7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonanno JA, Giasson C. Intracellular pH regulation in fresh and cultured bovine corneal endothelium. I. Na+/H+ exchange in the absence and presence of HCO3−. Invest Ophthalmol Vis Sci 33: 3058–3067, 1992 [PubMed] [Google Scholar]
- 5.Bonanno JA, Giasson C. Intracellular pH regulation in fresh and cultured bovine corneal endothelium. II. Na+:HCO3− cotransport and Cl−/HCO3− exchange. Invest Ophthalmol Vis Sci 33: 3068–3079, 1992 [PubMed] [Google Scholar]
- 6.Bonanno JA, Yi G, Kang XJ, Srinivas SP. Reevaluation of Cl−/HCO3− exchange in cultured bovine corneal endothelial cells. Invest Ophthalmol Vis Sci 39: 2713–2722, 1998 [PubMed] [Google Scholar]
- 7.Damkier HH, Aalkjaer C, Praetorius J. Na+-dependent HCO3− import by the slc4a10 gene product involves Cl− export. J Biol Chem 285: 26998–27007, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desir JA. Congenital hereditary endothelial dystrophy with progressive sensineural deafness (Harboyan syndrome). Orphanet J Rare Dis 3: 28–36, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diarra A, Sheldon C, Church J. In situ calibration and [H+] sensitivity of the fluorescent Na+ indicator SBFI. Am J Physiol Renal Physiol 280: F1623–F1633, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Good DW. Ammonium transport by the thick ascending limb of Henle's loop. Annu Rev Physiol 56: 623–647, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Groger N, Frohlich H, Maier H, Olbrich A, Kostin S, Braun T, Boettger T. SLC4A11 prevents osmotic imbalance leading to corneal endothelial dystrophy, deafness and polyuria. J Biol Chem 285: 15567–14474, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jalimarada SS, Ogando DG, Zhang W, Vithana EN, Bonanno JA. Ion transport function of SLC4A11 in corneal endothelium. Invest Ophthalmol Vis Sci 54: 4330–4340, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiao XS, Garg P, Ramamurthy B, Vemuganti GK, Gangopadhyay N, Hejtmancik JF, Kannabiran C. Autosomal recessive corneal endothelial distrophy (CHED2) is associated with mutations in SLC4A11. J Med Genet 44: 64–68, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klintworth GK. Corneal dystrophies. Orphanet J Rare Diseases 4: 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine SA, Montrose HM, Ming Tse C, Donowitz M. Kinetics and regulation or three cloned mammalian Na+/H+ exchangers stably expressed in a fibroblast cell line. J Biol Chem 268: 25527–25535, 1993 [PubMed] [Google Scholar]
- 16.Lopez IA, Rosenblatt MI, Kim C, Galbraith GC, Jones SM, Kao L, Newman D, Liu W, Yeh S, Pushkin A, Abuladze N, Kurtz I. Slc4a11 gene disruption in mice. Cellular targets of sensorineural abnormalities. J Biol Chem 284: 26882–26896, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL. Ammonium interaction with the epithelial sodium channel. Am J Physiol Renal Physiol 281: R493–R502, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Nguyen TT, Bonanno JA. Lactate-H+ transport is a significant component of the in vivo corneal endothelial pump. Invest Ophthalmol Vis Sci 53: 2020–2029, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlowski J. Heterologous expression and functional properties of amiloride high affinity (NHE-1) and low afinity (NHE-3) isoforms of the rat Na/H exchanger. J Biol Chem 268: 16369–16377, 1993 [PubMed] [Google Scholar]
- 20.Park M, Li Q, Shcheynikov N, Zeng W, Muallem S. NaBC1 is a ubiquitous electrogenic Na+-coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol Cell 16: 331–341, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Parker MD, Ourmozdi EP, Tanner MJA. Human BTR1, a new bicarbonate transporter superfamily member and human AE4 from kidney. Biochem Biophys Res Commun 282: 1103–1109, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Shei W, Liu J, Aung T, Vithana EN. Differential expression of the Slc4 bicarbonate transporter family in murine corneal endothelium and cell culture. Mol Vis 24: 1096–1106, 2013 [PMC free article] [PubMed] [Google Scholar]
- 23.Vilas GL, Morgan PE, Loganathan SK, Quon A, Casey JR. A biochemical framework for SLC4A11, the plasma membrane protein defective in corneal dystrophies. Biochemistry 50: 2157–2169, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Vithana EN, Morgan P, Sundaresan P, Ebenezer ND, Tan DTH, Mohamed MD, Anand S, Khine KO, Venkataraman D, Yong VHK, Salto-Tellez M, Venkatraman A, Guo K, Hemadevi B, Srinivasan M, Prajna V, Khine M, Casey JR, Inglehearn CF, Aung T. Mutations in sodium-borate cotransporter SLC4A11 cause recessive congenital hereditary dystrophy (CHED2). Nat Genetics 38: 755–757, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Vithana EN, Morgan PE, Ramprasad V, Tan DTH, Yong VH, Venkataraman D, Venkatraman A, Yam GH, Nagasamy S, Law RW, Rajagopal R, Pang CP, Kumaramanickevel G, Casey JR, Aung T. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum Mol Genet 17: 656–666, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Weiner ID, Verlander JW. Role of NH3 and NH4+ transporters in renal acid-base transport. Am J Physiol Renal Physiol 300: F11–F23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshimura K, Miyazaki Y, Ota F, Matsuoka S, Sakashita H. Complexation of boric acid with the N-methyl-d-glucamine group in solution and in crosslinked plymer. J Chem Soc Faraday Transactions 94: 683–689, 1998 [Google Scholar]
- 28.Yoshitomi K, Burckhardt BC, Fromter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflügers Arch 405: 360–366, 1985 [DOI] [PubMed] [Google Scholar]
- 29.Zhu Q, Azimov RK, L, Newman D, Liu W, Abuladze N, Pushkin A, Kurtz I. NBCe1-A transmembrane segment 1 lines the ion translocation pathway. J Biol Chem 284: 8918–8929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]