pulmonary arterial hypertension (PAH) is a rare human disease displaying a very poor prognosis of survival. The chronic increase in pulmonary vascular resistance associated with PAH eventually leads to right ventricular hypertrophy and failure, and ultimately death. It is well accepted that the increase in pulmonary arterial resistance to blood flow in PAH primarily involves a partial or complete occlusion of small resistance vessels. Arterial occlusion in PAH is the combined product of increased smooth muscle contractility and enhanced sensitivity to vasoconstrictors, reduction in lumen diameter produced by arterial wall thickening, and increased thrombosis. We still have a poor understanding of the etiology of PAH (1).
Agonist-Mediated Pulmonary Arterial Tone
The pulmonary arterial circulation is a high-capacitance low-pressure circulatory system that offers little resistance to blood flow under physiological conditions (1). Pulmonary arterial smooth muscle cells (PASMCs) normally exhibit very little myogenic tone in response to pressure and rather contract when exposed to neurotransmitters and circulating or local humoral factors such as serotonin (5-HT), endothelin-1, or others, which binds to their respective G protein-coupled receptors. Activation of such receptors stimulates phospholipase C (PLC) or, in some cases, other enzymes such as phospholipase D, which break down the membrane phospholipid phosphatidylinositol bisphosphate or PIP2 into inositol 1,4,5-trisphosphate (InsP3) and diacylglycerol (DAG), two important second messengers respectively releasing Ca2+ from internal stores and stimulating the enzyme protein kinase C (PKC). Stimulation of the PLC pathway elevates intracellular Ca2+ concentration ([Ca2+]i) by multiple mechanisms, including Ca2+ release from InsP3-sensitive stores, Ca2+ influx through voltage-gated L-type Ca2+ channels triggered by membrane depolarization (Fig. 1A), and voltage-independent Ca2+ entry evoked by receptor-operated channels (ROCs) and store depletion (so-called store-operated Ca2+ entry or SOCE) (not shown). Intracellular Ca2+ binds to calmodulin, which then activates myosin light chain kinase leading to light chain phosphorylation, the critical step triggering acto-myosin bridge cycling and contraction (1).
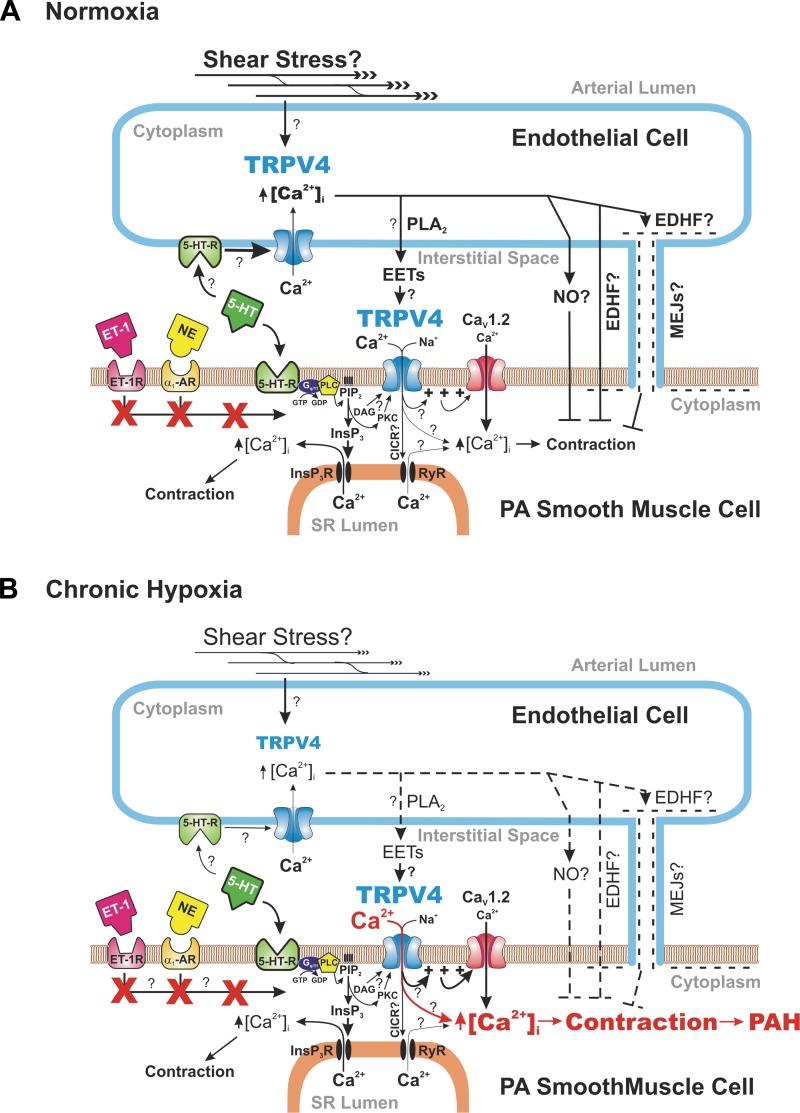
Fig. 1.
Unique coupling between G protein-coupled serotonin receptors and TRPV4 channels in determining pulmonary arterial tone under physiological conditions and chronic hypoxia. The two panels illustrate how serotonin (5-HT) binds to 5-HT receptors (5-HT-R) in both pulmonary arterial endothelial and smooth muscle cells and regulate intracellular Ca2+ concentration ([Ca2+]i) and smooth muscle contraction under physiological conditions in mice exposed to normal oxygen tension levels (A) or following chronic hypoxia (B). Diagram in A highlights the possibility that cation influx through TRPV4 in smooth muscle cells in healthy pulmonary arteries (PA) promotes membrane depolarization and subsequent activation of L-type Ca2+ channels evoked by signal transduction through 5-HT receptors, and that this effect may be largely attenuated by endothelium-derived relaxing factors triggered by activation of similar receptors on endothelial cells. In contrast, Ca2+ entry through TRPV4 in smooth muscle cells may be enhanced (highlighted in red) and the role of endothelial cell signaling attenuated (highlighted by dashed lines) in PA from chronic hypoxic animals, and contributing to the well-documented enhanced sensitivity of PA to agonists in pulmonary arterial hypertension (PAH). Both panels also highlight (three bolded red X's) that the coupling between 5-HT receptors and TRPV4 is not shared by endothelin-1 (ET-1R) and α1-adrenergic receptors (α1-AR). Nomenclature: PLA2, phospholipase A2; EETs, epoxyeicosatrienoic acids; EDHF, endothelium-derived hyperpolarizing factor; NO, nitric oxide; MEJs, myo-endothelial junctions; NE, norepinephrine; Gq/11, G protein type q/11; PLC, phospholipase C; GDP, guanosine diphosphate; GTP, guanosine triphosphate; PIP2, phosphatidylinositol bisphosphate; InsP3, inositol 1,4,5-trisphosphate; InsP3R; InsP3 receptor; DAG, diacylglycerol; PKC, protein kinase C; CICR, Ca2+-induced Ca2+ release; RyR, ryanodine receptor; SR, sarcoplasmic reticulum; CaV1.2, L-type Ca2+ channel.
Potential Role of TRPV4 in Pulmonary Hypertension
PASMCs from distal resistance arteries contract in response to hypoxia, a physiological process referred to as hypoxic pulmonary vasoconstriction [HPV;(8)]. It is thought that HPV serves to redirect blood flow from poorly ventilated alveoli to those exposed to normal oxygen tension levels, thereby optimizing blood oxygenation. Sustained pulmonary hypertension also occurs in response to chronic hypoxia (CH), a situation that stimulates functional and structural remodeling of distal pulmonary arteries that resembles some of the changes observed in PAH patients. Significant progress has been made over the past two decades in identifying the ion channels participating in the control of membrane potential, [Ca2+]i, and tone mediated by vasoactive agents, HPV, and CH. Seminal work from several groups has provided convincing evidence for an important role of voltage-gated K+ channels [KV1.5, and others; (1)], L-type Ca2+ channels [CaV1.2; (4)], Ca2+-activated Cl− channels [TMEM16A or anoctamin-1; (3, 7)], and several members of the transient receptor potential canonical [TRPC1, TRPC3, and TRPC6; (1)] subfamily of ion channels in the increased sensitivity to agonists in PASMCs from PAH patients and animal models of pulmonary hypertension.
In this issue of American Journal of Physiology-Cell Physiology, Xia et al. (9) present new data showing that the fourth member of the vallinoid subfamily of TRP channels, TRPV4, is involved in the contraction of small pulmonary arteries from normoxic mice to 5-HT (Fig. 1A) and that the channel's contribution is elevated in CH-induced pulmonary hypertension (Fig. 1B). TRPV4 is a widely expressed nonselective cation channel permeable to Ca2+ that is activated by a wide range of physical and chemical stimuli including shear stress, hypoosmotic stress, heat, acidity, PKC-activating and nonactivating phorbol esters, and epoxyeicosatrienoic acids (EETs) produced by cytochrome p450 epoxygenase enzymes from arachidonic acid liberated from the plasma membrane by Ca2+-dependent phospholipase A2 (6). A previous study from the same group recently showed that of all members of the melastatin-related (TRPM) and TRPV subfamilies of TRP channels, TRPV4 was the only channel to be upregulated in PASMCs from chronic hypoxic rats (10). That report also revealed that the increased expression of TRPV4 occurred early following the onset of CH and was associated with increased myogenic tone in small pressurized pulmonary arteries. The current study (9) extended this initial discovery by examining the contribution of TRPV4 to agonist-mediated contractions and their alteration in CH. Pharmacological agents targeting TRPV4 and TRPV4 knockout (KO) mice were used to determine the role of this channel in the responses of PASMCs, and pulmonary arteries with or without endothelium. A role for TRPV4 in 5-HT-induced contraction in preparations from normoxic mice was revealed by removal of the endothelium and appeared as a small but consistent rightward shift of the dose-response curve in TRPV4 KO relative to wild-type mice, or in the presence of the selective TRPV4 antagonist HC-067047 vs. vehicle in wild-type mice. A logical interpretation of these results is that TRPV4 channels are expressed in both endothelial and smooth muscle cells and the contractile effects of elevation of [Ca2+]i in smooth muscle cells following activation of TRPV4 channels may be antagonized by elevations in endothelial cell [Ca2+]i (2, 5), which results in the production of endothelial-derived relaxing factors such as nitric oxide (NO) and possibly endothelium-dependent hyperpolarization elicited by a diffusible factor (e.g., HETEs, K+) or through direct coupling between endothelial and smooth muscle cells via myo-endothelial junctions (MEJs), to ultimately oppose smooth muscle contraction (Fig. 1A). In contrast to 5-HT, the contraction of pulmonary arteries from wild-type mice elicited by endothelin-1 (ET-1) or the α-adrenergic agonist phenylephrine (PE) was independent of TRPV4 signaling, despite their respective receptors acting, like the 5-HT receptor, via PLC (Fig. 1A). The authors justifiably hypothesized that these distinct receptors and their respective G protein may be compartmentalized in the cell membrane so that only 5-HT receptors are in sufficient proximity to TRPV4 channels to elicit signaling.
An important finding of the study by Xia et al. (9) is the observation of a significant elevation in maximal tone elicited by 5-HT in pulmonary arteries from CH wild-type mice, which was greatly attenuated in TRPV4 KO mice, or by pharmacological inhibition of TRPV4 in wild-type mice (Fig. 1B). This effect was also observed in arteries without endothelium, suggesting that the contraction involving TRPV4 channels present in smooth muscle overwhelmed the vasorelaxation response caused by endothelial cell TRPV4 activity, or that the endothelium was damaged by chronic hypoxia, which is well documented (1). These results suggest that TRPV4 may play a significant excitatory role in the well-known increased sensitivity to agonists of distal pulmonary arteries in CH and other animal models of pulmonary hypertension, as well as human PAH (Fig. 1B).
Future Directions
There still remain many unanswered questions concerning TRPV4 signaling in the pulmonary arterial circulation. While Xia et al. (9) convincingly show that the signal transduction pathways linked to ET-1 and PE did not appear to couple to TRPV4, this conclusion was derived from results obtained in pulmonary arteries from wild-type mice. Does this hold true for pulmonary arteries from CH mice? Does the expression profile and cellular and subcellular distribution of TRPV4 change in pulmonary hypertension? Is the functional role of TRPV4 similar in pulmonary arteries from normoxic mice versus mice exposed to CH? What are the endogenous activators of TRPV4 (EETs? DAG? PKC?) during 5-HT signaling in pulmonary arteries from normoxic and CH mice, the 5-HT receptor subtype(s) involved in these responses, and potential role played by stretch and flow-mediated shear stress? Is Ca2+ entry through TRPV4 playing a significant role in smooth muscle cell hypertrophy and hyperplasia, contributing to the thickening of the wall of pulmonary arteries in pulmonary hypertension? Importantly, are TRPV4 channels also participating in vasomotor tone of human pulmonary arteries and is their expression and function altered in human PAH (1)? Despite these and other questions, the work by Xia et al. (9) unveils a new player in the control of the pulmonary arterial circulation in health and disease. This discovery will undoubtedly lead to new exciting basic and clinical research in the near future, examining its relative contribution and interaction with other signaling partners recently identified to modulate smooth muscle tone in pulmonary hypertension.
GRANTS
This work was supported, in part, by National Heart, Lung, and Blood Institute Grant R01 HL-091905 and by a Monfort Excellence Award (Monfort Family Foundation; to S. Earley).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.E. and N.L. edited and revised the manuscript; S.E. and N.L. approved final version of the manuscript; N.L. prepared the figure; N.L. drafted the manuscript.
REFERENCES
- 1.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation 121: 2045–2066, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Forrest AS, Joyce TC, Huebner ML, Ayon RJ, Wiwchar M, Joyce J, Freitas N, Davis AJ, Ye L, Duan DD, Singer CA, Valencik ML, Greenwood IA, Leblanc N. Increased TMEM16A-encoded calcium-activated chloride channel activity is associated with pulmonary hypertension. Am J Physiol Cell Physiol 303: C1229–C1243, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirenallur SD, Haworth ST, Leming JT, Chang J, Hernandez G, Gordon JB, Rusch NJ. Upregulation of vascular calcium channels in neonatal piglets with hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L915–L924, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol 26: 1495–1502, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Liedtke W. Role of TRPV ion channels in sensory transduction of osmotic stimuli in mammals. Exp Physiol 92: 507–512, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Sun H, Xia Y, Paudel O, Yang XR, Sham JS. Chronic hypoxia-induced upregulation of Ca2+-activated Cl− channel in pulmonary arterial myocytes: a mechanism contributing to enhanced vasoreactivity. J Physiol 590: 3507–3521, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev 92: 367–520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia Y, Fu Z, Hu J, Huang C, Paudel O, Cai S, Liedtke W, Sham JS. TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am J Physiol Cell Physiol (June 5, 2013). 10.1152/ajpcell.00099.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang XR, Lin AH, Hughes JM, Flavahan NA, Cao YN, Liedtke W, Sham JS. Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302: L555–L568, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]