Abstract
Large-conductance, Ca2+-activated K+ channels, commonly referred to as BK channels, have a major role in flow-induced K+ secretion in the distal nephron. With-no-lysine kinase 4 (WNK4) is a serine-threonine kinase expressed in the distal nephron that inhibits ROMK activity and renal K+ secretion. WNK4 mutations have been described in individuals with familial hyperkalemic hypertension (FHHt), a Mendelian disorder characterized by low-renin hypertension and hyperkalemia. As BK channels also have an important role in renal K+ secretion, we examined whether they are regulated by WNK4 in a manner similar to ROMK. BK channel activity was inhibited in a rabbit intercalated cell line transfected with WNK4 or a WNK4 mutant found in individuals with FHHt. Coexpression of an epitope-tagged BK α-subunit with WNK4 or the WNK4 mutant in HEK293 cells reduced BK α-subunit plasma membrane and whole cell expression. A region within WNK4 encompassing the autoinhibitory domain and a coiled coil domain was required for WNK4 to inhibit BK α-subunit expression. The relative fraction of BK α-subunit that was ubiquitinated was significantly increased in cells expressing WNK4, compared with controls. Our results suggest that WNK4 inhibits BK channel activity, in part, by increasing channel degradation through an ubiquitin-dependent pathway. Based on these results, we propose that WNK4 provides a cellular mechanism for the coordinated regulation of two key secretory K+ channels in the distal nephron, ROMK and BK.
Keywords: BK channel, familial hyperkalemic hypertension, Maxi-K, With-no-lysine kinase 4, WNK4
extracellular potassium concentration ([K+]) must be maintained within a fairly narrow range, as large deviations may result in cardiac arrhythmias as well as neuromuscular disorders. The kidney has a major role in maintaining K+ balance and a relatively constant extracellular [K+] by matching rates of renal K+ excretion with that of K+ intake. Renal K+ excretion largely reflects K+ secretion into the tubular lumen by cells lining the aldosterone-sensitive distal nephron (ASDN). This segment is composed of two morphologically distinct cell types, principal cells (PCs) and intercalated cells (ICs) (8). PCs have a key role in facilitating K+ secretion (24). Recent studies suggest that ICs also have a role in K+ secretion (23, 31, 42, 43).
Several distinct K+ channels involved in renal K+ secretion are expressed in the apical membrane of the connecting tubule (CNT) and cortical collecting duct (CCD) (5). These include the small-conductance K+ channel ROMK (Kir1.1) and the Ca2+-activated large-conductance K+ channel (BK; 5, 11). ROMK channels have a high open probability and constitute the main pathway for K+ secretion in the CNT and CCD under basal conditions (6, 10). BK channels are expressed in both PCs and ICs where they have a primary role in flow-activated K+ secretion (30, 34, 42, 43). Individuals with Bartter's syndrome due to loss of function ROMK mutations as well as mice that lack ROMK expression do not exhibit hyperkalemia, presumably due to excretion of K+ via BK channels (19, 32). It is still not clear which cell type (PC, IC, or both) mediates BK channel-dependent K+ secretion in the CCD (18, 23, 25, 27, 29). When dietary K+ intake is high, the functional activities of BK and ROMK channels are enhanced (23, 26, 30, 38).
With-no-lysine kinase 4 (WNK4) has been identified as an important regulator of K+ secretion. Recent studies have shown that wild-type WNK4 decreases ROMK surface expression by stimulating clathrin-dependent endocytosis of the channel (16, 17). Individuals with familial hyperkalemic hypertension (FHHt) have decreased renal K+ secretion. FHHt-causing WNK4 mutations result in an enhanced inhibition of ROMK, when compared with wild-type WNK4, and are associated with a further decreased ROMK surface density and impaired K+ secretion (16).
WNK4 is expressed in the ASDN of the kidney (15, 41), although its specific cellular localization within the ASDN (i.e., PCs vs. ICs) has not been defined. Recent studies suggested that WNK4 alters the activity of BK channels (47, 48). Given the important role of WNK kinases in modulating renal K+ secretion, we examined whether WNK4 and an FHHt-associated WNK4 mutant have a role in modulating BK channel activity, whether changes in channel activity are associated with changes in channel expression at the plasma membrane, and whether WNK4 alters the extent of BK α-subunit ubiquitination.
MATERIALS AND METHODS
Molecular biology.
The BK α-subunit plasmid pCMV-Myc-QEERL encoding NH2-terminal Myc-tagged QEERL isoform of Slo1 was generously provided by Dr. Stuart Dryer (Univ. of Houston, Houston, TX). Mouse WNK4 was cloned in a bicistronic vector (pIRES-hrGFP II, Clontech) encoding a humanized recombinant green fluorescent protein (GFP). COOH-terminal truncations of WNK4 were generated by insertion of a stop codon at positions 445, 585, and 809.
C-RIC cell culture and transient transfection.
Rabbit intercalated cells (C-RIC) were obtained from Dr. Qais Al-Awqati's laboratory courtesy of Dr. Soundarapandian Vijayakumar (3, 36). C-RIC cells were cultured with 5% CO2 at 32°C in DMEM-F-12 (1:1) medium (Invitrogen) supplemented with 10% fetal calf serum, 5% penicillin-streptomycin (Invitrogen), 1 mM glutamine (Sigma), 55 μM hydrocortisone (Sigma), 5 μg/l insulin (Sigma), 5 μg/l transferrin (Sigma), 5 ng/l sodium selenite (Sigma), and 15 μg/l epidermal growth factor (Sigma), as previously described (1, 3). Transient transfections with the bicistronic vector encoding GFP and either mouse WNK4 or the WNK4 Q562E mutant were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's recommendations. Cells transfected with the vector expressing GFP alone served as controls.
Patch-clamp studies.
Transiently transfected C-RIC cells grown on 8-mm diameter round glass coverslips were transferred to a chamber mounted on the stage of an Olympus inverted microscope equipped with a mercury lamp to detect GFP-expressing cells. Whole cell patch recordings from C-RIC cells were obtained at room temperature with the perforated patch technique with amphotericin B (Sigma) in the patch pipette. The patch pipettes were drawn with a PP-81 puller (Narishige). The bath solution was composed of (in mM) 138 NaCl, 5 KCl, 0.5 MgCl2, 1.5 CaCl2, 2 EGTA, and 10 HEPES, pH 7.4. The free Ca2+ concentration was 400 nM. The pipette solution was composed of (in mM) 138 KCl, 4 MgCl2, 0.955 CaCl2, 1 EGTA, and 5 HEPES (pH 7.2). The free Ca2+ concentration was 6 μM. Amphotericin B was added in the patch pipette to a final concentration of 120 μg/ml. For current recordings, the membrane potential was initially held at −80 mV. Whole cell currents were evoked by 0.2-s, 10-mV depolarizing steps from −80 to +100 mV with a PC-ONE patch-clamp amplifier (Dagan, Minneapolis, MN). Channel currents were acquired with pClamp 8.02 (Axon Instruments, Union City, CA) and recorded to a hard drive of a PC computer. Currents were low-pass filtered at 1 KHz and digitized with an Axon interface (Digidata 1322A). Data were analyzed using the pClamp software system 8.02 (Axon Instruments). Capacitance was estimated with pClamp 8.02. Charybdotoxin (CHTX) (Santa Cruz) and iberiotoxin (IBTX) (Alomone) were used at concentrations of 100 nM and 50 nM, respectively. Whole cell currents measured at a pipette potential of +80 mV are reported in the figures.
Single-channel recordings using a cell-attached configuration were obtained at room temperature in C-RIC cells. Currents were low-pass filtered at 1 kHz. Data were digitized with an Axon interface and stored on the hard drive of a PC computer. pClamp software system 8.02 was used to analyze the data. The bath solution for cell-attached patches was composed of (in mM) 138 NaCl, 5 KCl, 0.5 MgCl2, 1.5 CaCl2, 2 EGTA, and 10 HEPES (pH 7.4). The pipette solution was composed of (in mM) 138 KCl, 4 MgCl2, 0.955 CaCl2, 1 EGTA, and 5 HEPES (pH 7.2).
BK whole cell and surface expression.
HEK293 cells were plated at 50% confluency on polylysine-coated plastic (30-mm well dish of a 6-well Costar Cluster, Corning, NY) the day before transfection with plasmids and Lipofectamine 2000. Cells were transfected with the BK α-subunit and either mouse WNK4, the WNK4 Q562E mutant, or an empty vector (GFP alone). Two days after transfection, cells were washed 4 times with ice-cold phosphate-buffered saline (PBS, 137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.46 mM KH2PO4, pH 7.4) for 5 min, and surface proteins were labeled with 1 mg/ml EZ-Link sulfo-NHS-SS-biotin (Thermo Scientific) for 30 min in biotinylation buffer (137 mM NaCl, 1 mM CaCl2, 15 mM Na borate, pH 9.0) on ice. Free biotin reagent was then quenched by washing cells 3 times for 3 min with ice-cold DMEM-F-12 (1:1) media containing 5% fetal calf serum, followed by 2 washes with ice-cold PBS. To extract proteins, cells were incubated for 20 min at room temperature on a rotating shaker with 250 μl of detergent buffer [50 mM Tris·HCl, pH 8, 4 mg/ml deoxycholate, 1% Nonidet P-40, Protease Inhibitor Cocktail Set III (EMD Bioscience)]. Cell debris was removed by centrifugation at 20,800 g for 10 min at 4°C. The supernatant was recovered, 10% was aliquoted for whole cell immunoblotting, and 80% to precipitate surface (i.e., biotin labeled) proteins (14).
To assess BK α-subunit surface expression, biotinylated proteins were precipitated with 30 μl of streptavidin-conjugated agarose in a 1.5-ml microfuge tube. Samples were incubated overnight with end-over-end rotation at 4°C and then washed once with 1% Triton X-100 in HEPES Buffered Saline (HBS, 150 mM NaCl, 10 mM HEPES, pH 7.4), once with HBS supplemented with 0.1% SDS, and once with HBS. To elute biotinylated proteins, beads were incubated for 30 min at 50°C with Laemmli sample buffer (Bio-Rad, Hercules, CA) containing 50 mM dithiothreitol (DTT). Proteins were then separated by SDS-PAGE and transferred to a nitrocellulose membrane. Immunoblotting was performed with an anti-myc antibody conjugated to horseradish peroxidase at a 1:500 dilution (Roche), or a mouse anti-β actin antibody at a 1:2,000 dilution (Sigma-Aldrich) and goat anti-mouse second antibody conjugated to horseradish peroxidase at a 1:5,000 dilution (KPL). Bands were visualized using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences) and quantified with a Bio-Rad Versadoc (Bio-Rad) and Bio-Rad Quantity One software (22).
To assess whole cell BK α-subunit and actin expression, cell lysates were diluted in Laemmli sample buffer with 50 mM DTT, heated for 30 min at 50°C, subjected to SDS-PAGE and immunoblotting with either an anti-myc antibody (to detect BK α-subunit), or mouse anti-β actin antibody and goat anti-mouse second antibody conjugated to horseradish peroxidase. Bands were visualized and quantified as described above. The percentage of biotinylated (i.e., cell surface) and whole cell BK α-subunit in each sample was normalized to whole cell actin.
Detection of ubiquitinated BK α-subunit.
Whole cell lysates obtained as indicated above were incubated overnight by end-over-end rotation at 4°C with a monoclonal anti-myc antibody derived from a hybridoma (gift from Dr. Ora Weisz) and protein-G Sepharose. Beads were washed once with 1% Triton X-100 in HBS, once with HBS supplemented with 0.1% SDS, and once with HBS. To elute proteins, beads pelleted by centrifugation were incubated for 30 min at 50°C with Laemmli sample buffer containing 50 mM DTT. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. To detect ubiquitinated proteins, the nitrocellulose membrane was sandwiched between cellulose chromatography paper and then boiled in water for 20–40 min as previously described by Tran and Brodsky (35). Immunoblotting was performed with an anti-ubiquitin antibody at a 1:500 dilution (Santa Cruz Biotechnology) and a secondary antibody conjugated to peroxidase. Bands were visualized using Western Lightning Chemiluminescence Reagent Plus as indicated above.
Statistical analyses.
Data are presented as means ± SE, and significance was determined using Student's t-test, Mann-Whitney test, or one-way ANOVA followed by Tukey's multiple comparison test. P < 0.05 was considered significant, and n indicates the number of independent experiments. Electrophysiological data were collected from rabbit intercalated cells at different passage numbers. Each data point was obtained from a separated coverslip.
RESULTS
BK channels are expressed in C-RIC intercalated cells.
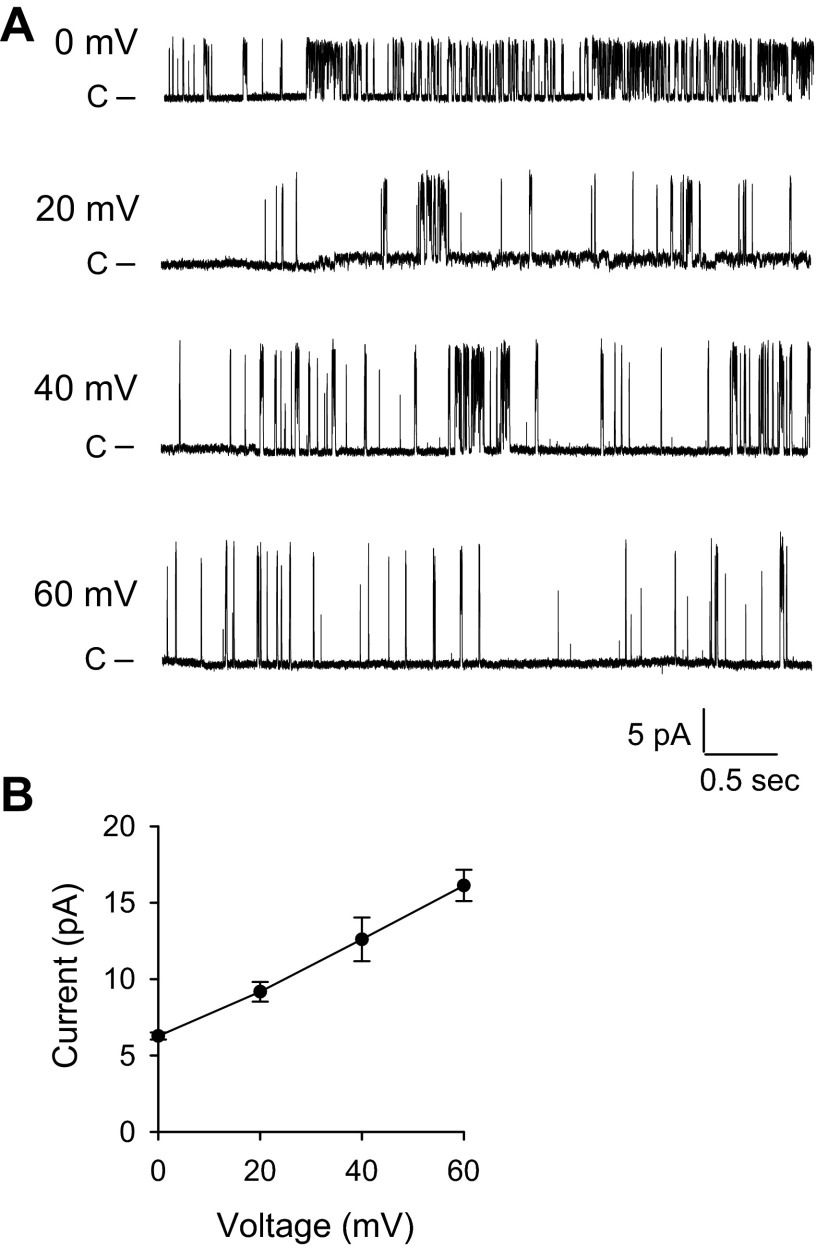
Functional BK channels have been observed in PCs and ICs in the ASDN (12, 37, 46). Patch-clamp and immunolocalization studies suggest that levels of BK expression are greater in ICs than PCs (23, 25, 43). We therefore examined whether BK channels are expressed in an intercalated cell line (C-RIC) (3, 36). We previously used RT-PCR to amplify BK α- and β-subunits from rabbit CCDs (4). We amplified the predicted products from reverse transcribed mRNA isolated from C-RIC cells, seeded on permeable supports at a high density (106 cells/cm2) and grown for 14 days at 40°C, using specific rabbit BK α-, β2-, and β4-subunit primers that we previously used [data not shown (23, 26, 30, 38)]. When C-RIC cells were grown on coverslips, we observed a large-conductance K+-permeable channel with a single-channel conductance of ∼165 pS, consistent with the biophysical characteristics of BK channels (28) (Fig. 1). To confirm that the 165 pS channel is indeed BK, we examined channel activity with an inside-out patch-clamp configuration, where IBTX, a selective BK channel inhibitor, was either present or absent in the patch pipette. While the large-conductance channel was readily detected in 9 patches from total 28 patches, no channel activity was detected in 30 patches containing IBTX in the patch pipette. At the whole cell level, a component of the K+ conductance was blocked by 100 nM CHTX, a known inhibitor of BK channels [data not shown (9)]. As CHTX may block other K+ channels, we used IBTX (50 nM) to define the BK component of the whole cell K+ conductance [Fig. 2 (7, 43)]. These observations provide functional evidence of the presence of BK channels in C-RIC cells.
Fig. 1.
Large-conductance K+ channels are expressed in C-RIC cells. A: representative single-channel recordings obtained with the cell-attached configuration from C-RIC cells bathed in a NaCl solution (see materials and methods). The pipette contained a KCl solution. Applied potentials are indicated at the left of each trace. B: current-voltage relationship of the large-conductance K+ channels (n = 4–5).
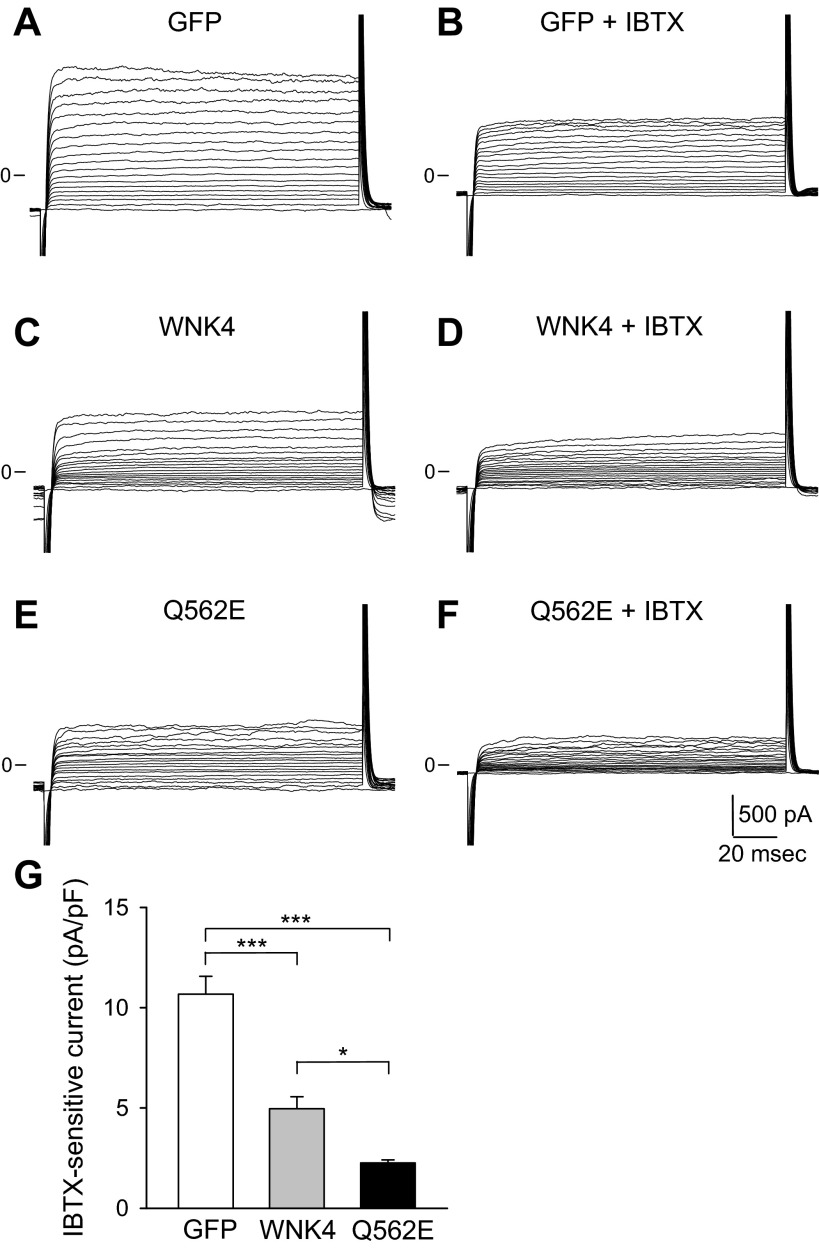
Fig. 2.
With-no-lysine kinase 4 (WNK4) and the WNK4 Q562E mutant inhibit IBTX-sensitive whole cell currents in C-RIC cells. Experiments were performed with a whole cell patch mode, as described in materials and methods. The initial holding potential was −80 mV. Currents were measured with voltage steps from −80 mV to +100 mV (10-mV increments). A and B: representative recordings showing the effect of 50 nM IBTX on green fluorescent protein (GFP)-transfected C-RIC cells. C and D: representative recordings showing the effect of IBTX on WNK4 transfected C-RIC cells. E and F: representative recordings showing the effect of IBTX on WNK4 Q562E-transfected C-RIC cells. G: summary of the effects of WNK4 and WNK4 Q562E on IBTX-sensitive whole cell currents measured at +80 mV (n = 6 for GFP, n = 8 for WNK4, n = 6 for WNK4 Q562E; *P < 0.05 and ***P < 0.001, ANOVA followed by Tukey's multiple comparison test).
WNK4 and WNK4 Q562E inhibit BK channels.
To determine whether WNK kinases affect BK channel activity, C-RIC cells were transfected with a bicistronic vector encoding WNK4 and GFP. As a control, cells were transfected with the vector encoding GFP alone. Whole cell patch clamp was used to measure K+ currents in GFP-positive cells. Representative whole cell K+ current traces at varying holding potentials in cells transfected with the bicistronic vector encoding GFP alone, wild-type WNK4 or the FHHt-associated WNK4 Q562E mutant are shown in Fig. 2. IBTX-sensitive (i.e., BK-mediated) whole cell currents were significantly reduced in cells expressing wild-type WNK4 compared with controls. A further decrease in IBTX-mediated whole cell currents was observed in cells expressing the WNK4 mutant Q562E. Whole IBTX-sensitive currents in cells transfected with GFP alone (control), WNK4 or WNK4 Q562E are summarized in Fig. 2G. IBTX-sensitive currents were 10.7 ± 0.9 pA/pF in controls (GFP), 5.0 ± 0.6 pA/pF in cells expressing WNK4, and 2.3 ± 0.2 pA/pF in cells expressing WNK4 Q562E (n = 6–8; P < 0.001, control vs. WNK4 or WNK4 Q562E; P < 0.05, WNK4 vs. WNK4 Q562E). Consistent with previous studies (47, 48), our data show that BK channel activity is dramatically reduced in C-RIC cells overexpressing WNK4. The overexpression of the FHHt-associated Q562E mutant further enhanced the inhibitory effect of WNK4.
WNK4 and WNK4 Q562E reduce BK α-subunit surface expression.
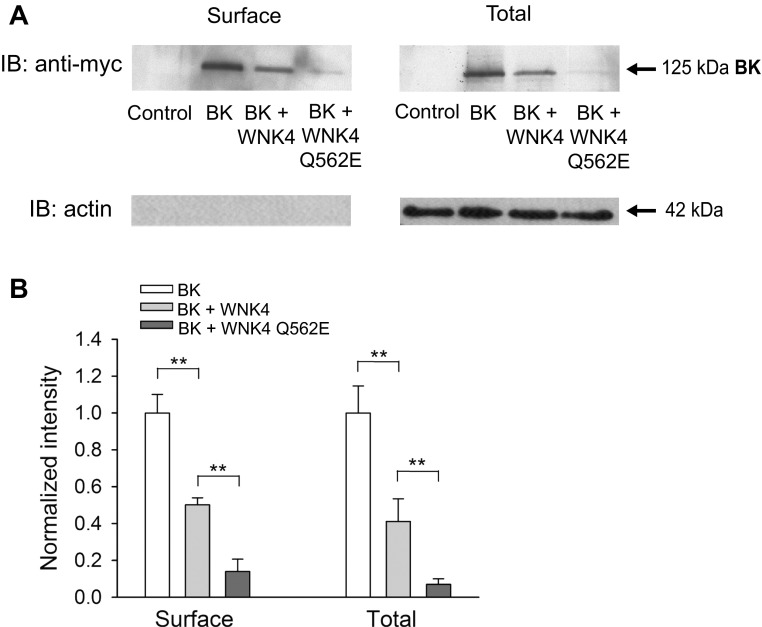
Previous studies established that WNK4 inhibits ROMK by reducing levels of channel expression at the cell surface (16). We examined whether WNK4 inhibits BK channels by a similar mechanism. Our attempts to examine surface expression of endogenous BK channels in C-RIC cells by labeling surface proteins with biotin, and probing streptavidin precipitates for the BK α-subunit were not successful. We therefore elected to examine BK α-subunit surface expression using a heterologous expression system, where HEK293 cells were transfected with a myc epitope-tagged BK α-subunit. HEK293 cells were transfected with 1) GFP alone (control), 2) BK α-subunit and GFP, 3) BK α-subunit and wild-type WNK4, or 4) BK α-subunit and WNK4 Q562E. Cell surface proteins were labeled with biotin and precipitated with streptavidin; immunoblots were probed with an anti-myc antibody to detect BK α-subunits (Fig. 3). BK α-subunit surface expression was significantly greater in cells transfected with the α-subunit alone, than in cells cotransfected with WNK4. The relative surface expression of the BK α-subunit (normalized to whole cell actin) was 1.00 ± 0.10 (BK alone) vs. 0.50 ± 0.04 (BK + WNK4) (n = 3, P < 0.01, BK alone vs. BK + WNK4). Coexpression of the WNK4 Q562E further reduced relative BK α-subunit surface expression to 0.14 ± 0.07 (n = 3, P < 0.01, BK + WNK4 vs. BK + WNK4 Q562E) (Fig. 3). Whole cell BK α-subunit protein expression also was reduced in cells coexpressing WNK4. Relative whole cell α-subunit expression (normalized by actin) was 1.00 ± 0.15 (BK alone) vs. 0.41 ± 0.12 (BK + WNK4) (n = 3, P < 0.01, BK alone vs. BK + WNK4). Coexpression of WNK4 Q562E further reduced BK α-subunit relative whole cell expression to 0.07 ± 0.03 (n = 3, P < 0.01, BK + WNK4 vs. BK + WNK4 Q562E) (Fig. 3). Since the expression of BK α-subunit in HEK cells is under the control of the CMV promoter, the reduction in BK expression observed in cells overexpressing WNK4 likely represents a posttranslational event. Our data suggest that WNK4 reduces BK α-subunit surface and whole cell expression and that this effect is enhanced by the FHHt-associated WNK4 mutant Q562E.
Fig. 3.
WNK4 and the WNK4 Q562E mutant reduce large-conductance, Ca2+-activated K+ (BK) channel α-subunit cell surface and whole cell expression. A: HEK293 cells were transfected with GFP alone (control), myc-tagged BK α-subunit, or myc-tagged BK α-subunit with either wild-type (WT) or mutant (Q562E) WNK4. Following surface biotinylation, surface proteins were recovered with streptavidin-conjugated beads and then subjected to immunoblotting (IB) with an anti-myc antibody to detect BK α-subunits or with an anti-actin antibody as a control. In addition, whole cell lysates were subjected to IB with an anti-myc antibody to detect BK α-subunit or with an anti-actin antibody. Results are representative of three experiments. B: summary of analyses of BK α-subunit cell surface and whole cell expression. Immunoblots were quantified as described in materials and methods. Relative BK α-subunit cell surface and whole cell expression are shown, normalized to whole cell actin levels (n = 3, **P < 0.01, ANOVA followed by Tukey's multiple comparison test).
Specific WNK4 domains are required to reduce BK α-subunit expression.
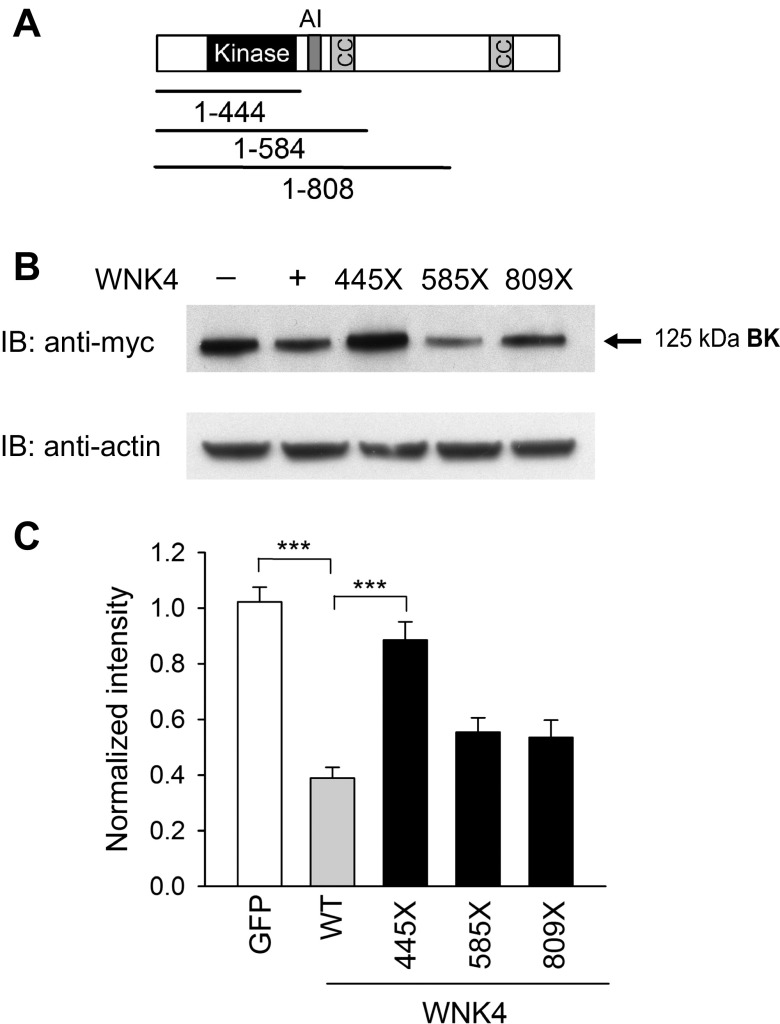
WNK kinases contain a kinase domain, an autoinhibitory domain with two essential phenylalanine residues, and coiled coil domains (Fig. 4A). Zhang and colleagues (48) previously showed that WNK4-dependent BK degradation requires its kinase activity. To identify domains within WNK4 necessary for BK degradation, we truncated the COOH terminus of WNK4 at positions 445, 585, and 809. The construct 1–444 contains the NH2 terminus and kinase domain, the construct 1–584 includes also the autoinhibitory domain and the first coiled coil domain, and the construct 1–808 has an additional component of the COOH terminus of WNK4 (Fig. 4A). Wild-type and truncated WNK4 constructs were cotransfected with BK α-subunits in HEK293 cells, and whole cell channel expression was determined as described above. BK α-subunit whole cell expression was similar in HEK293 cells expressing GFP alone (control) and WNK4 1–444, which lacked the autoinhibitory and coiled coil domains. Inclusion of residues in the tract 445–584 reduced BK α-subunit whole cell expression, similar to that observed with wild-type WNK4. These results suggest that the region containing the autoinhibitory domain and first coiled coil domain is required for the WNK4-dependent reduction in BK α-subunit expression. Interestingly, the FHHt-associated WNK4 mutation Q562E is located near the first coiled coil domain of the kinase.
Fig. 4.
Identification of WNK4 domains that contribute to BK α-subunit degradation. A: schematic representation of WNK4 domains. Approximate location is shown of kinase domain (black), autoinhibitory domain (dark gray), and coiled coil (cc) domains (gray). B: effect of WNK4 truncations on BK α-subunit whole cell expression. HEK293 cells were transfected with myc-tagged BK α-subunit and GFP (control), or myc-tagged BK α-subunit with either WT or truncated WNK4 constructs. Whole cell lysates were subjected to IB with an anti-myc antibody to detect BK α-subunit or with an anti-actin antibody. Representative immunoblots are shown. C: summary of relative BK α-subunit whole cell expression normalized to whole cell actin levels (n = 5–6, ***P < 0.001, ANOVA followed by Tukey's multiple comparison test). Immunoblots were quantified as described in materials and methods.
WNK4 enhances polyubiquitination of the BK α-subunit.
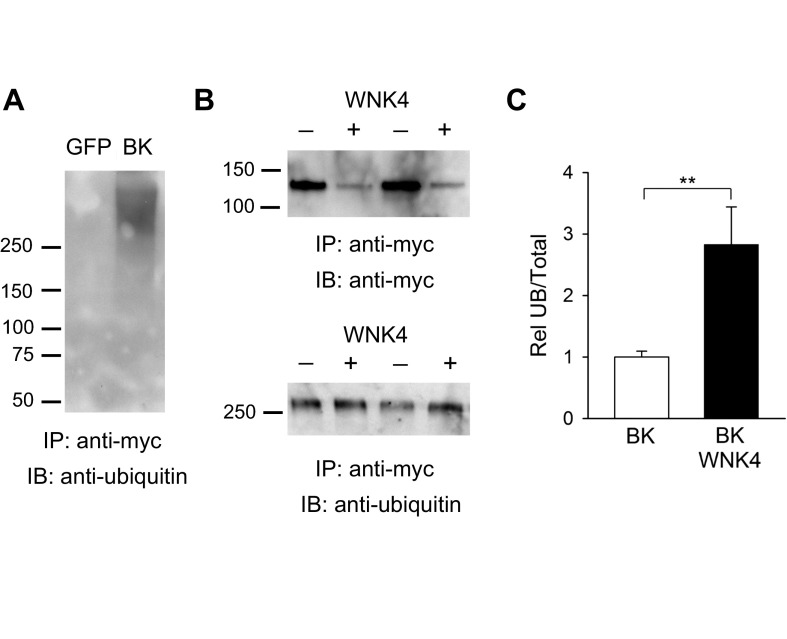
Our data suggest that WNK4 reduces BK α-subunit expression by enhancing channel degradation. Ubiquitination is a posttranslational modification that targets proteins for retrieval from plasma membranes, and for proteasomal or lysosomal degradation (13). Within the biosynthetic pathway, ubiquitinated membrane proteins are selected in endosomes for lysosomal targeting (40). To determine whether WNK4 enhances ubiquitination of the channel, we estimated the relative amount of ubiquitinated BK α-subunits in controls and cells expressing WNK4. BK α-subunits were immunoprecipitated with anti-myc antibody, separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with either an anti-ubiquitin or an anti-myc antibody. The ubiquitinated form of the BK α-subunit has a molecular mass > 250 kDa, consistent with BK α-subunit polyubiquitination (Fig. 5A). Although WNK4 reduced the total cellular pool of BK α-subunit, the amount of the ubiquitinated BK α-subunit was similar in controls and cells overexpressing WNK4 (Fig. 5B). Consequently, when normalized by whole cell BK α-subunit expression, the amount of ubiquitinated BK α-subunit increased by 1.8-fold in cells expressing WNK4 compared with controls (n = 6, P < 0.01, control vs. WNK4; Fig. 5C). These results suggest that WNK4 facilitates BK α-subunit ubiquitination.
Fig. 5.
WNK4 augments BK α-subunit ubiquitination. A: the BK α-subunit is polyubiquitinated. HEK293 cells were transfected with GFP or a myc-tagged BK α-subunit. Proteins were immunoprecipitated (IP) from whole cell lysates with an anti-myc antibody. IB was performed with an anti-ubiquitin antibody to detect ubiquitinated BK α-subunit as described in materials and methods. B: WNK4 augments BK α-subunit ubiquitination. HEK293 cells were transfected with myc-tagged BK α-subunit with or without wild-type WNK4. BK α-subunit was immunoprecipitated from whole cell lysates with an anti-myc antibody. IB was performed with an anti-myc antibody to detect BK α-subunit or with an anti-ubiquitin antibody to detect ubiquitinated BK α-subunit as indicated in A. Representative blots from experiments conducted in duplicate are shown. C: summary of analyses of BK α-subunit ubiquitination. Immunoblots were quantified as described in materials and methods. The fraction of ubiquitinated BK α-subunit normalized to the total whole cell BK α-subunit expression (Rel UB/Total) is shown (n = 6, **P < 0.01, Mann-Whitney test).
DISCUSSION
The renal excretion of K+ is primarily mediated by K+ secretion in the ASDN. ROMK channels have an important role in this process. ROMK expression is increased in response to aldosterone and increases in dietary K+ intake (12, 37, 46). However, these channels are not the only conduit for K+ secretion in the ASDN. Large-conductance, Ca2+-activated K+ channels, commonly referred to as BK channels, have been shown to have a major role in flow-induced K+ secretion (30, 42, 43). Expression of BK channels is enhanced by increases in dietary K+ intake, but not in response to aldosterone (4). WNK kinases have an important role in the adaptive response of ROMK channels to changes in dietary K+ intake (17, 39). We addressed the question of whether BK channels are regulated by WNK4.
We confirmed the presence of functional IBTX-sensitive K+ channels (i.e., BK channels) in an immortalized rabbit intercalated cell line (C-RIC) and found that mouse WNK4 inhibits functional BK channel expression in these cells. Furthermore, the FHHt-associated WNK4 mutant Q562E exhibited an enhanced inhibitory effect. In agreement with previous reports, WNK4 downregulated BK α-subunit whole cell and surface expression in HEK293 cells (47, 48). The FHHt-associated WNK4 mutant Q562E resulted in a further decrease in BK α-subunit whole cell and surface expression.
It is notable that FHHt-associated mutations of WNK kinases inhibit ROMK channels and are associated with hyperkalemia (16). In contrast, loss-of-function ROMK mutations found in patients with Bartter's syndrome are associated with hypokalemia (32). While these differences in renal K+ handling may reflect differences in tubular flow rates in the ASDN in Bartter's syndrome versus FHHt, our data suggest that an enhanced inhibition of BK channels with FHHt-associated WNK4 mutations also contributes to reductions of renal K+ secretion in this disorder.
Studies from two independent groups reported that overexpression of WNK4 reduced the whole cell and surface expression of BK α-subunit in HEK293 cells (47, 48). Zhuang et al. (48) observed that the effect of WNK4 on BK α-subunit expression was kinase dependent and sensitive to inhibitors of lysosomal degradation. These effects were not altered by the expression of a dominant-negative dynamin (K44A), suggesting that WNK4 enhances the routing of BK α-subunits to lysosomes in a dynamin-independent manner. Quite the opposite, Yue et al. (47) showed that the overexpression of the dominant-negative dynamin (K44A) or the treatment of HEK293 cells with the cell-permeable dynamin inhibitor dynasore largely abolished the inhibitory effect of WNK4 on BK channels. We observed that the ubiquitinated fraction of the BK α-subunit was significantly increased in cells expressing WNK4 compared with controls, consistent with the hypothesis that WNK4 targets the channel for degradation through an ubiquitin-dependent lysosomal pathway. It remains to be determined whether WNK4 enhances BK α-subunit delivery to lysosomes via a mechanism that involves dynamin-dependent endocytic retrieval from the plasma membrane, or alternatively by redirecting BK α-subunit anterograde trafficking to lysosomes. In this regard, it is notable that recent work suggests that WNK4 suppresses the forward trafficking of the NaCl cotransporter (NCC) from the trans-Golgi network, diverting the transporter from the biosynthetic pathway to the endolysosomal pathway for degradation (2, 33).
WNK4-mediated degradation of BK α-subunit requires its kinase activity (48). We generated a series of truncations in WNK4 to define additional domains required for BK α-subunit degradation. Expression of a construct bearing the NH2 terminus and kinase domain of WNK4 (residues 1–444) did not alter the cellular pool of the BK α-subunits when compared with control cells. Addition of the auto-inhibitory and first coiled coil domain to the NH2 terminus and kinase domain (residues 1–584) reduced total BK α-subunit expression to levels similar to that observed with wild-type WNK4, suggesting that the tract 445–584 encompasses sites that are critical for WNK4-dependent regulation of BK channels. Interestingly, the FHHt-associated WNK4 mutation Q562E maps within this 139 residue tract.
Our results suggest that the Q562E WNK4 mutant augments the inhibitory effect of this kinase on BK channel activity as well as on BK α-subunit whole cell and cell surface expression. Our results differ from a recent study that examined BK α-subunit abundance in a WNK4 D561A knock-in mouse model of FHHt. These mice develop thiazide-sensitive hypertension and hyperkalemia, in association with a modest increase in BK α-subunit expression (45). The authors of this study suggested that that this effect was in response to hyperkalemia. It should be noted, however, that this conclusion was based on immunoblot analyses of whole kidney homogenates, which contain several different cell types that express the BK channel, including smooth muscle, mesangium, podocytes, and thick ascending limb (20, 21, 42). Variations in BK α-subunit expression at these different sites within the kidney will contribute to its levels assessed by whole kidney immunoblots. An assessment of BK channel expression and activity in the ASDN was not provided. Future efforts in these mice and other models of FHHt should be directed at assessing the effect of WNK4 mutations on BK channel expression in defined cell types in the kidney.
FHHt-associated WNK4 mutants appear to have opposing effects on NCC and BK trafficking. While these mutations relieve WNK4 suppression of NCC surface expression and protein abundance (2, 44), we observed an enhanced suppression of BK channel activity and surface expression. These divergent effects may reflect differences in the regulated routing of NCC and BK channels to lysosomes. Thus, it will be important elucidate mechanisms by which WNK4 as well as FHHt associated-WNK4 mutants influence the trafficking itinerary of BK channels.
Coupled with the observations of other investigators (16, 33, 48), our results support a model in which WNK4 influences K+ homeostasis through multiple mechanisms. Apical K+ secretion, via both ROMK- and BK-dependent mechanisms, is suppressed by WNK4, effects that are augmented with mutations causing FHHt. Similarly, the same FHHt mutation partially relieves the inhibitory effect of WNK4 on NCC, and presumably enhances electroneutral NaCl reabsorption in the distal convoluted tubule (44). This would be expected to impair distal Na+ delivery and K+ secretion.
In summary, our observations suggest that WNK4 enhances the ubiquitination of the BK α-subunit and reduces its cell surface and whole cell expression, consequently inhibiting BK channel activity. An FHHt-associated WNK4 mutant augments these inhibitory effects. Our findings suggest a mechanism by which WNK kinases participate in the renal regulation of K+ homeostasis.
GRANTS
This work was supported by grants from the National Institutes of Health, including R01 DK-038470, R01 DK-084184, R37 DK-051391, T32 DK-061296, and P30 DK0-79307 (Pittsburgh Center for Kidney Research, Univ. of Pittsburgh).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Z.W., A.R.S., L.M.S., N.M.P.-S., M.D.C., and T.R.K. conception and design of research; Z.W. and M.D.C. performed experiments; Z.W., M.D.C., and T.R.K. analyzed data; Z.W., A.R.S., L.M.S., N.M.P.-S., M.D.C., and T.R.K. interpreted results of experiments; Z.W. and M.D.C. prepared figures; Z.W., M.D.C., and T.R.K. drafted manuscript; Z.W., A.R.S., L.M.S., N.M.P.-S., M.D.C., and T.R.K. edited and revised manuscript; Z.W., A.R.S., L.M.S., N.M.P.-S., M.D.C., and T.R.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Soundarapandian Vijayakumar and Qais Al-Awqati for providing the rabbit intercalated cells. We thank Dr. Stuart E. Dryer for providing the NH2-terminal Myc-tagged BK α-subunit cDNA.
REFERENCES
- 1.Alzamora R, Thali RF, Gong F, Smolak C, Li H, Baty CJ, Bertrand CA, Auchli Y, Brunisholz RA, Neumann D, Hallows KR, Pastor-Soler NM. PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells. J Biol Chem 285: 24676–24685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai H, Cebotaru V, Wang YH, Zhang XM, Cebotaru L, Guggino SE, Guggino WB. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int 69: 2162–2170, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Edwards JC, van Adelsberg J, Rater M, Herzlinger D, Lebowitz J, al-Awqati Q. Conditional immortalization of bicarbonate-secreting intercalated cells from rabbit. Am J Physiol Cell Physiol 263: C521–C529, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Estilo G, Liu W, Pastor-Soler N, Mitchell P, Carattino MD, Kleyman TR, Satlin LM. Effect of aldosterone on BK channel expression in mammalian cortical collecting duct. Am J Physiol Renal Physiol 295: F780–F788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frindt G, Palmer LG. Apical potassium channels in the rat connecting tubule. Am J Physiol Renal Physiol 287: F1030–F1037, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Frindt G, Palmer LG. Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F143–F151, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem 265: 11083–11090, 1990 [PubMed] [Google Scholar]
- 8.Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol Renal Physiol 274: F817–F833, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Gimenez-Gallego G, Navia MA, Reuben JP, Katz GM, Kaczorowski GJ, Garcia ML. Purification, sequence, and model structure of charybdotoxin, a potent selective inhibitor of calcium-activated potassium channels. Proc Natl Acad Sci USA 85: 3329–3333, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray DA, Frindt G, Palmer LG. Quantification of K+ secretion through apical low-conductance K channels in the CCD. Am J Physiol Renal Physiol 289: F117–F126, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev 85: 319–371, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebert SC, Wang WH. Structure and function of the low conductance KATP channel, ROMK. Wien Klin Wochenschr 109: 471–476, 1997 [PubMed] [Google Scholar]
- 13.Hochstrasser M. Origin and function of ubiquitin-like proteins. Nature 458: 422–429, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem 278: 37073–37082, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Kahle KT, Wilson FH, Lalioti M, Toka H, Qin H, Lifton RP. WNK kinases: molecular regulators of integrated epithelial ion transport. Curr Opin Nephrol Hypertens 13: 557–562, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35: 372–376, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA 103: 1615–1620, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li D, Wang Z, Sun P, Jin Y, Lin DH, Hebert SC, Giebisch G, Wang WH. Inhibition of MAPK stimulates the Ca2+-dependent big-conductance K channels in cortical collecting duct. Proc Natl Acad Sci USA 103: 19569–19574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenz JN, Baird NR, Judd LM, Noonan WT, Andringa A, Doetschman T, Manning PA, Liu LH, Miller ML, Shull GE. Impaired renal NaCl absorption in mice lacking the ROMK potassium channel, a model for type II Bartter's syndrome. J Biol Chem 277: 37871–37880, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Ma R, Pluznick JL, Sansom SC. Ion channels in mesangial cells: function, malfunction, or fiction. Physiology (Bethesda) 20: 102–111, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Morita T, Hanaoka K, Morales MM, Montrose-Rafizadeh C, Guggino WB. Cloning and characterization of maxi K+ channel α-subunit in rabbit kidney. Am J Physiol Renal Physiol 273: F615–F624, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Mueller GM, Maarouf AB, Kinlough CL, Sheng N, Kashlan OB, Okumura S, Luthy S, Kleyman TR, Hughey RP. Cys palmitoylation of the beta subunit modulates gating of the epithelial sodium channel. J Biol Chem 285: 30453–30462, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am J Physiol Renal Physiol 289: F922–F932, 2005 [DOI] [PubMed] [Google Scholar]
- 24.O'Neil RG, Hayhurst RA. Functional differentiation of cell types of cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 248: F449–F453, 1985 [DOI] [PubMed] [Google Scholar]
- 25.Palmer LG, Frindt G. High-conductance K channels in intercalated cells of the rat distal nephron. Am J Physiol Renal Physiol 292: F966–F973, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol Renal Physiol 277: F805–F812, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-β1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol 288: F846–F854, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7: 921–931, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Sansom SC, O'Neil RG. Effects of mineralocorticoids on transport properties of cortical collecting duct basolateral membrane. Am J Physiol Renal Fluid Electrolyte Physiol 251: F743–F757, 1986 [DOI] [PubMed] [Google Scholar]
- 30.Satlin LM. Developmental regulation of expression of renal potassium secretory channels. Curr Opin Nephrol Hypertens 13: 445–450, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Satlin LM, Carattino MD, Liu W, Kleyman TR. Regulation of cation transport in the distal nephron by mechanical forces. Am J Physiol Renal Physiol 291: F923–F931, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP. Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14: 152–156, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Subramanya AR, Liu J, Ellison DH, Wade JB, Welling PA. WNK4 diverts the thiazide-sensitive NaCl cotransporter to the lysosome and stimulates AP-3 interaction. J Biol Chem 284: 18471–18480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taniguchi J, Imai M. Flow-dependent activation of maxi K+ channels in apical membrane of rabbit connecting tubule. J Membr Biol 164: 35–45, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Tran JR, Brodsky JL. Assays to measure ER-associated degradation in yeast. Methods Mol Biol 832: 505–518, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Adelsberg J, Edwards JC, Takito J, Kiss B, al-Awqati Q. An induced extracellular matrix protein reverses the polarity of band 3 in intercalated epithelial cells. Cell 76: 1053–1061, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Wald H, Garty H, Palmer LG, Popovtzer MM. Differential regulation of ROMK expression in kidney cortex and medulla by aldosterone and potassium. Am J Physiol Renal Physiol 275: F239–F245, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Wang WH, Schwab A, Giebisch G. Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 259: F494–F502, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Welling PA, Chang YP, Delpire E, Wade JB. Multigene kinase network, kidney transport, and salt in essential hypertension. Kidney Int 77: 1063–1069, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol 8: 355–368, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Woda CB, Miyawaki N, Ramalakshmi S, Ramkumar M, Rojas R, Zavilowitz B, Kleyman TR, Satlin LM. Ontogeny of flow-stimulated potassium secretion in rabbit cortical collecting duct: functional and molecular aspects. Am J Physiol Renal Physiol 285: F629–F639, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab 5: 331–344, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Yoo D, Kim BY, Campo C, Nance L, King A, Maouyo D, Welling PA. Cell surface expression of the ROMK (Kir 1.1) channel is regulated by the aldosterone-induced kinase, SGK-1, and protein kinase A. J Biol Chem 278: 23066–23075, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Yue P, Zhang C, Lin DH, Sun P, Wang WH. WNK4 inhibits Ca-activated big-conductance potassium channels (BK) via mitogen-activated protein kinase-dependent pathway. Biochim Biophys Acta 1833: 2101–2110, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhuang J, Zhang X, Wang D, Li J, Zhou B, Shi Z, Gu D, Denson DD, Eaton DC, Cai H. Wnk4 kinase inhibits Maxi K channel activity by a kinase-dependent mechanism. Am J Physiol Renal Physiol 301: F410–F419, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]