Abstract
The goal of this study was to determine whether transforming growth factor-β1 (TGF-β1) affects epithelial cells lining the vas deferens, an organ that is universally affected in cystic fibrosis male patients. In PVD9902 cells, which are derived from porcine vas deferens epithelium, TGF-β1 exposure significantly reduced short-circuit current (Isc) stimulated by forskolin or a cell membrane-permeant cAMP analog, 8-pCPT-cAMP, suggesting that TGF-β1 affects targets of the cAMP signaling pathway. Electrophysiological results indicated that TGF-β1 reduces the magnitude of current inhibited by cystic fibrosis transmembrane conductance regulator (CFTR) channel blockers. Real-time RT-PCR revealed that TGF-β1 downregulates the abundance of mRNA coding for CFTR, while biotinylation and Western blot showed that TGF-β1 reduces both total CFTR and apical cell surface CFTR abundance. These results suggest that TGF-β1 causes a reduction in CFTR expression, which limits CFTR-mediated anion secretion. TGF-β1-associated attenuation of anion secretion was abrogated by SB431542, a TGF-β1 receptor I inhibitor. Signaling pathway studies showed that the effect of TGF-β1 on Isc was reduced by SB203580, an inhibitor of p38 mitogen-activated protein kinase (MAPK). TGF-β1 exposure also increased the amount of phospho-p38 MAPK substantially. In addition, anisomycin, a p38 MAPK activator, mimicked the effect of TGF-β1, which further suggests that TGF-β1 affects PVD9902 cells through a p38 MAPK pathway. These observations suggest that TGF-β1, via TGF-β1 receptor I and p38 MAPK signaling, reduces CFTR expression to impair CFTR-mediated anion secretion, which would likely compound the effects associated with mild CFTR mutations and ultimately would compromise male fertility.
Keywords: TGF-β1, vas deferens epithelial cells, male infertility, CFTR, p38 MAPK
transforming growth factor-β1 (TGF-β1), the predominant isoform of TGF-β, is a pleiotropic cytokine regulating cell proliferation and apoptosis, differentiation, inflammation, extracellular matrix synthesis, and developmental processes (6). TGF-β1 signals through binding to a cell surface type II serine/threonine kinase receptor, which then recruits and activates the type I receptor. Intracellular signal transduction is most commonly mediated through the canonical Smad pathway, although several noncanonical kinase-mediated pathways (phosphatidylinositol 3-kinase, Erk, JNK, and p38 MAPK) have been implicated (6, 11). Such components of the activated signaling cascades (whether Smads or protein kinases) translocate into the nucleus and alter gene expression to regulate multiple cellular functions.
Altered expression of TGF-β1 has been involved in fibrotic diseases including cystic fibrosis, a life-shortening disease affecting 1 in every 2,000–3,000 newborn Caucasians (7, 9). Cystic fibrosis affects many organ systems including the respiratory, gastrointestinal, musculoskeletal, and reproductive systems. It is associated with variations in the sequence of CFTR, the gene coding for the cystic fibrosis transmembrane conductance regulator (CFTR). CFTR mutations lead to the reduction or absence of functional CFTR protein in the apical membrane of epithelial cells throughout the body, which results in attenuated anion secretion across epithelial tissues. The clinical severity of cystic fibrosis is determined not only by the type of CFTR mutation(s) carried by the patient but also by genetic modifiers (7). Genotyping results suggest that cystic fibrosis patients with a TGF-β1 “high producer” genotype for codon 10 are associated with a more severe lung disease as well as a more rapid deterioration in lung function (1, 12). Additionally, measurement of plasma TGF-β1 levels suggests that higher concentrations of TGF-β1 are correlated with increased severity of lung disease in pediatric cystic fibrosis patients (16). All these observations indicate that elevated levels of circulating TGF-β1 lead to worse cystic fibrosis lung disease.
In cystic fibrosis male patients, vas deferens and epididymis are the most frequently affected tissue and these tissues undergo anatomical disruptions early in life. About 98% of male cystic fibrosis patients are infertile due to congenital bilateral absence of the vas deferens (CBAVD), a condition characterized by duct atresia and posttesticular azoospermia (10, 47). Besides a population of ∼30,000 cystic fibrosis patients in the United States and ∼70,000 worldwide, about 1 in every 25 Caucasians are carriers of disease-associated CFTR sequences. Cystic fibrosis carriers will not be diagnosed by the sweat test and do not have apparent cystic fibrosis symptomology, but they are associated with a higher frequency of infertility (27). Genotypic analysis revealed no apparent correlation between CBAVD in European populations and two distinct single nucleotide polymorphisms in the TGF-β1 gene (17). However, no study has focused on whether TGF-β1 affects vas deferens epithelium. It is possible that TGF-β1 attenuates vas deferens epithelial anion secretion in cystic fibrosis carriers who are associated with mild disease or with no typical symptomology and would promote cystic fibrosis-related pathology.
The objective of the current study was to determine whether TGF-β1 impairs ion transport across cultured vas deferens epithelial monolayers. PVD9902, a cell line that was derived from porcine vas deferens epithelium and recapitulates most ion transport characteristics of freshly isolated porcine vas deferens epithelial tissue, was used (5). Electrophysiological, molecular, and immunological assays were performed to evaluate the changes in PVD9902 cells when exposed to TGF-β1. Experiments were focused to elucidate the potential mechanisms by which TGF-β1 affects vas deferens tissue.
MATERIALS AND METHODS
Chemicals.
Forskolin (Coleus forskohlii) was obtained from Calbiochem (La Jolla, CA). GlyH-101 was obtained from Millipore (Billerica, MA). N-[4-methylphenylsulfonyl]-N′-[4-trifluoromethylphenyl] urea (DASU-02) was synthesized in the laboratory. Unless indicated otherwise, chemicals used were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture.
PVD9902 cells (5) were seeded onto plastic 25-cm2 cell culture flasks (Corning, Corning, NY) and grown in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated FBS (Atlanta Biologicals, Atlanta, GA) and 1% penicillin and streptomycin (Invitrogen) in a humidified 5% CO2 incubator at 37°C. Confluent cells were washed with PBS, lifted by trypsin-EDTA (0.25%; Invitrogen), seeded onto permeable supports (Snapwell or Transwell; Costar, Corning), and cultured for 14 days before experimental assay. Media were refreshed every other day, and monolayers were exposed to TGF-β1 (5 ng/ml; R&D Systems, Minneapolis, MN) in the basolateral compartments for the last 24 h before assay.
Electrophysiology.
PVD9902 monolayers were mounted in modified Ussing-style chambers (model DCV9; Navicyte, San Diego, CA), bathed symmetrically with Ringer solution (composition in mM: 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.83 K2HPO4, 1.2 MgCl2, and 1.2 CaCl2), maintained at 39°C and bubbled with 5% CO2-95% O2. After open circuit potential was recorded, monolayers were clamped to 0 mV, and a 5-s bipolar pulse was applied every 100 s using a voltage clamp (model 558C-5; University of Iowa, Dept. of Bioengineering, Iowa City, IA). Short circuit current (Isc), which represents transepithelial electrogenic ion transport, was monitored and recorded at 1 Hz with an Intel-based computer and MP100A-CE interface (v.3.2.6; BIOPAC Systems, Santa Barbara, CA). The change in current in response to the periodic voltage pulse was used to calculate transepithelial electrical resistance using Ohm's law (Rte = ΔV/ΔIsc). Test reagents were added to the apical and/or basolateral side(s) of each chamber as indicated. The magnitude of test reagent-stimulated Isc was determined by ΔIsc between the preaddition Isc value and the peak value within first 10 min after addition.
RNA isolation and real-time RT-PCR.
RNA was isolated from PVD9902 monolayers using an RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. RNA quantity was determined spectrophotometrically (ND 8000; NanoDrop Products, Wilmington, DE) while RNA quality was confirmed using microfluidics (Bioanalyzer; Agilent Technologies, Santa Clara, CA). Ten nanograms of RNA were used as template and RT-PCR was performed using the iScript One-Step RT-PCR Kit with SYBR Green (Bio-Rad, Hercules, CA) with specific primer pairs. The sequences of primer pairs were as follows: CFTR (forward) 5′-CCG GGC ACC ATT AAA GAA AAC-3′ and (reverse) 5′-GCC ATC AAT TTA CGA ACA CGG C-3′ (29); and 18S (forward) 5′-GAG GTT CGA AGA CGA TCA GA-3′ and (reverse) 5′-TCG CTC CAC CAA CTA AGA AC-3′ (49). Relative quantification of mRNA was conducted by a SmartCycler (Cepheid, Sunnyvale, CA) using the ΔΔCt method with 18S as the reference, in which ΔCt = Ct(Vehicle) − Ct(Treated), ΔΔCt = ΔCt(Target) − ΔCt(Reference); the change in copy number of target RNA relative to reference RNA=2ΔΔCt. The quality of RT-PCR products was validated by a single peak melt curve, which represents single PCR product, and by a single band of expected mobility in a 1.5% agarose DNA gel. The cDNA was visualized by inclusion of ethidium bromide (1 μg/ml), and the product size was determined by a 100-bp DNA ladder (Promega, Madison, WI).
Biotinylation and immunoblotting.
PVD9902 monolayers were homogenized and lysed with Laemmli sample buffer (2×) containing 4% SDS, Tris·HCl (125 mM), and protease inhibitors (Roche Diagnostics, Indianapolis, IN). Lysates were passed through 25-gauge needles repeatedly and then centrifuged at 14,000 g for 10 min. Supernatant protein concentrations were determined by measuring absorbance at 280 nm (A280). Collected supernatants were then mixed with bromophenol blue (0.01% wt/vol), glycerin (10%), and DTT (0.1 M), and the mixtures were incubated at 37°C for 2 h. Equal amounts of protein from each treatment were loaded to 8–16% Tris-HEPES-SDS gradient gels (Pierce, Rockford, IL). Following electrophoresis, proteins were transferred onto Immobilon-P transfer membrane (Millipore). Membranes were blocked with 5% nonfat dry milk (Bio-Rad) in PBS with 0.1% Tween-20 for 2 h and then probed with primary antibodies: anti-CFTR (Millipore, 05–583), anti-phospho-p38 MAPK (4511; Cell Signaling, Danvers, MA), and anti-actin (A2066; Sigma-Aldrich) overnight at 4°C; incubated in peroxidase-conjuated secondary antibodies (Pierce) for 1 h; and followed by development using chemiluminiscent substrates (Super Signal West Pico and Femto, Pierce). Images were obtained digitally (Kodak Image Station 4000 R; Kodak, Rochester, NY), and quantification was performed by measuring band signal intensity (UN-SCAN-IT Gel; Silk Scientific, Orem, UT).
For the detection of membrane proteins, filter-grown PVD9902 monolayers were washed and incubated with Ringer solution at 37°C for 30 min and then placed on ice. Biotinylation reagent containing sulfo-NHS-SS-biotin (400 μM; Pierce) in Ringer solution was added to the apical or basolateral compartment for 15 min. After biotin incubation, monolayers were rinsed and washed three times with Ringer solution containing 50 mM glycine to quench unreacted sulfo-NHS-SS-biotin groups. After quenched biotin was removed, PVD9902 monolayers were lysed and homogenized. Supernatants were incubated with immobilized neutravidin-agarose (Pierce) setting in micro-spin columns (Pierce) for 2 h at room temperature. No-bound proteins were collected as flow-through samples. Avidin gels were washed three times with lysis buffer and incubated with Laemmli buffer containing DTT (50 mM) to elute bound biotinylated proteins. Immunoblotting was completed as indicated previously to detect cell surface labeling.
Lactate dehydrogenase assay.
The activity of lactate dehydrogenase (LDH) released from damaged cells into the culture medium was measured using the LDH cytotoxicity detection kit (CytoTox-ONE; Promega, Madison, WI). PVD9902 cells were seeded at a density of 1 × 105 cells per well onto sterile 96-well opaque-walled tissue culture plates (Nalge Nunc International, Rochester, NY), cultured for 72 h, treated with vehicle or TGF-β1 (5 ng/ml) for 24 h, and incubated with CytoTox-ONE reagent according to the manufacturer's instructions. Lysis solution was added to cells in paired wells to determine maximal LDH release in “lysed” cells. The fluorescence values were recorded to determine LDH activity and to estimate the number of damaged cells.
Data analysis.
All numerical results are reported as means ± SE. Calculations, t-tests, and graphs were performed with Sigmaplot (v 10.0; SPSS, Richmond, CA) and Excel 2007 (Microsoft, Redmond, WA).
RESULTS
TGF-β1 impairs cAMP-stimulated anion secretion across PVD9902 monolayers.
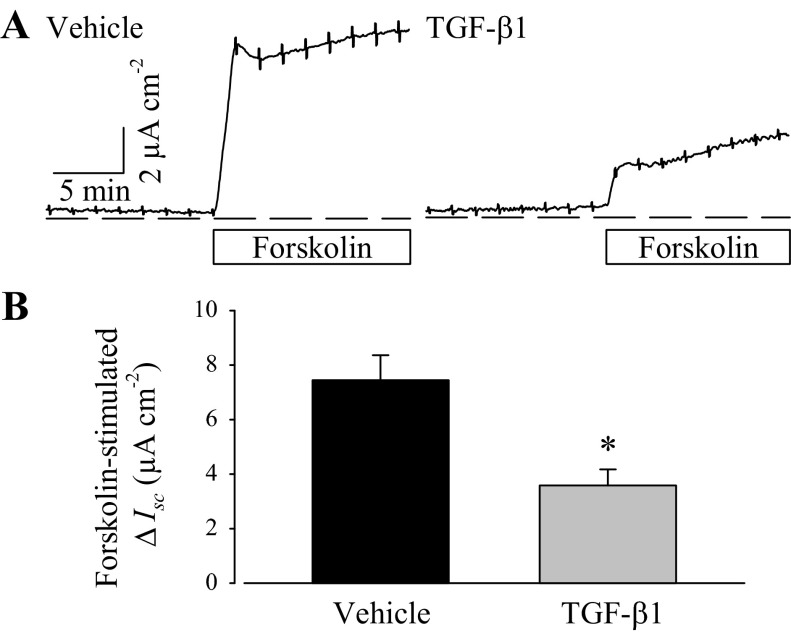
Paired vehicle- or TGF-β1-treated PVD9902 monolayers were mounted in modified Ussing-style chambers for measurements of Rte and Isc. Ussing chamber assays also included in-chamber pharmacological tests, and any acute responses were visualized and recorded in real time. Both vehicle and TGF-β1-treated PVD9902 cells formed electrically tight epithelial monolayers. TGF-β1 exposure was associated with lower Rte compared with paired monolayers exposed to vehicle (Table 1) although the difference did not reach statistical significance (P > 0.18). Forskolin (2 μM), an adenylyl cyclase activator that increases intracellular cAMP generation, was employed to stimulate cAMP-dependent anion secretion (26, 28). Typically, forskolin exposure elicited a transient peak in Isc followed by a sustained plateau, indicating ongoing anion secretion (Fig. 1). In TGF-β1-treated monolayers, the response to forskolin followed the same pattern but was reduced to less than half of the typical magnitude. The forskolin-induced increase in Isc was associated with decreased Rte, suggesting the activation of channels that contribute to anion secretion. In vehicle-treated PVD9902 monolayers, forskolin exposure induced a reduction of 6,096 ± 1,174 Ω·cm2 in Rte while in the TGF-β1-treated monolayer, the reduction in Rte was significantly less (1,799 ± 460 Ω·cm2).
Table 1.
Basal transepithelial electrical resistance of PVD9902 cells after different treatments
| Treatment | Basal Rte, Ω·cm2 |
|---|---|
| Vehicle | 8,184 ± 477 |
| TGF-β1 | 6,721 ± 803 |
| Vehicle + SB431542 | 3,923 ± 657 |
| TGF-β1 + SB431542 | 4,635 ± 783 |
| Vehicle + SB203580 | 6,731 ± 580 |
| TGF-β1 + SB203580 | 4,822 ± 683 |
| Anisomycin | 4,123 ± 1,026 |
Values are means ± SE. TGF-β1, tranforming growth factor-β1; Rte, transepithelial electrical resistance.
Fig. 1.
Transforming growth factor-β1 (TGF-β1) impairs forskolin-stimulated anion secretion across PVD9902 monolayers. A: representative Ussing chamber recordings of vehicle or TGF-β1-treated (5 ng/ml, 24 h) PVD9902 monolayers exposed to forskolin (2 μM). Dashed lines represent the reference point of zero net current, indicating no net ion flux. Bar indicates the duration of symmetrical forskolin exposure. B: summarized results of forskolin-stimulated anion secretion from 7 paired experiments. *P < 0.05, significant difference between vehicle and TGF-β1-treated monolayers.
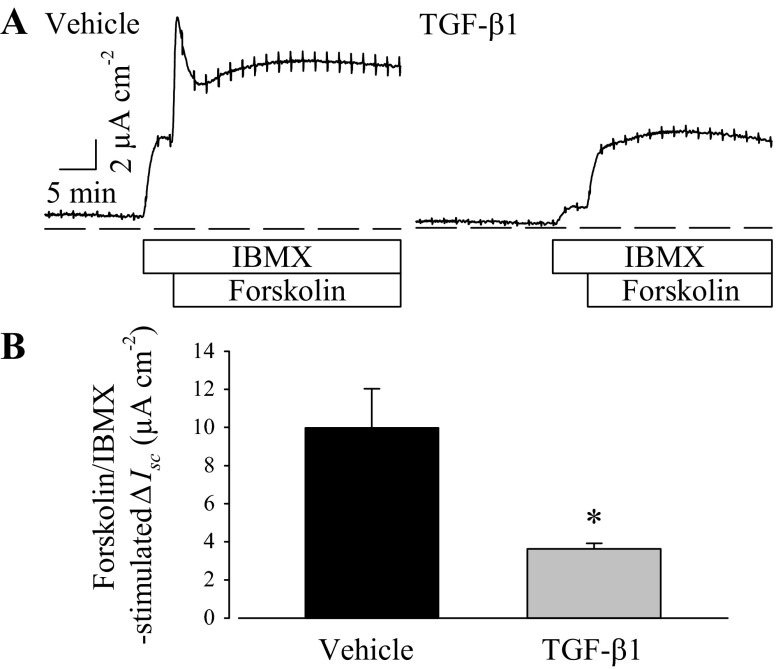
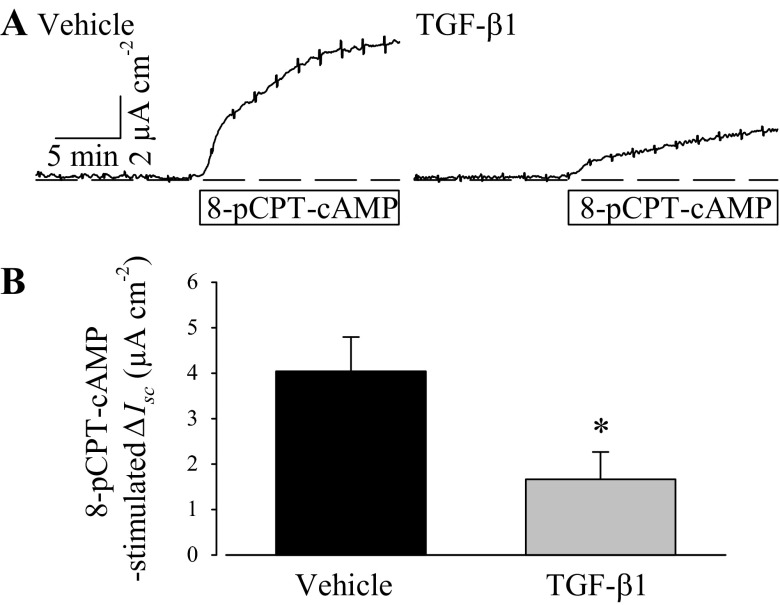
Isobutylmethylxanthine (IBMX; 100 μM), a nonselective cyclic nucleotide phosphodiesterase inhibitor, was added before and with forskolin during some experiments to preclude cAMP breakdown and maximize cytosolic cAMP accumulation. IBMX elicited an immediate increase in Isc, which suggests that the PVD9902 cells have ongoing cyclic nucleotide turnover at rest, and subsequent exposure to forskolin further increased anion secretion (Fig. 2). Although PVD9902 monolayers exposed to TGF-β1 responded to both IBMX and forskolin with an increase in Isc, the response to both was significantly muted, suggesting that reduced cytosolic cAMP concentration in cells exposed to TGF-β1 did not likely account for the differences in outcome. Results from experiments using a membrane-permeant cAMP analog, 8-pCPT-cAMP, to stimulate Isc showed that TGF-β1 also reduces these responses (Fig. 3). Taken together, these outcomes suggest that a downstream component(s) of the cAMP signaling pathway or an ion transport protein(s) is negatively impacted by TGF-β1 exposure.
Fig. 2.
TGF-β1 impairs anion secretion across PVD9902 monolayers stimulated by forskolin in the presence of isobutylmethylxanthine (IBMX). A: representative current recordings of vehicle or TGF-β1-treated (5 ng/ml, 24 h) PVD9902 monolayers exposed to IBMX (100 μM) followed by forskolin (2 μM) as indicated by the labeled bars. B: results summarized from 4 paired experiments comparing the responses to the combination of IBMX and forskolin. *P < 0.05, statistical difference from vehicle.
Fig. 3.
TGF-β1 impairs 8-pCPT-cAMP-stimulated anion secretion across PVD9902 monolayers. A: Ussing chamber traces of vehicle or TGF-β1-treated (5 ng/ml, 24 h) PVD9902 monolayers in response to 8-pCPT-cAMP (300 μM) are shown. B: summary of responses to 8-pCPT-cAMP from 5 paired experiments. *P < 0.05, statistical significance.
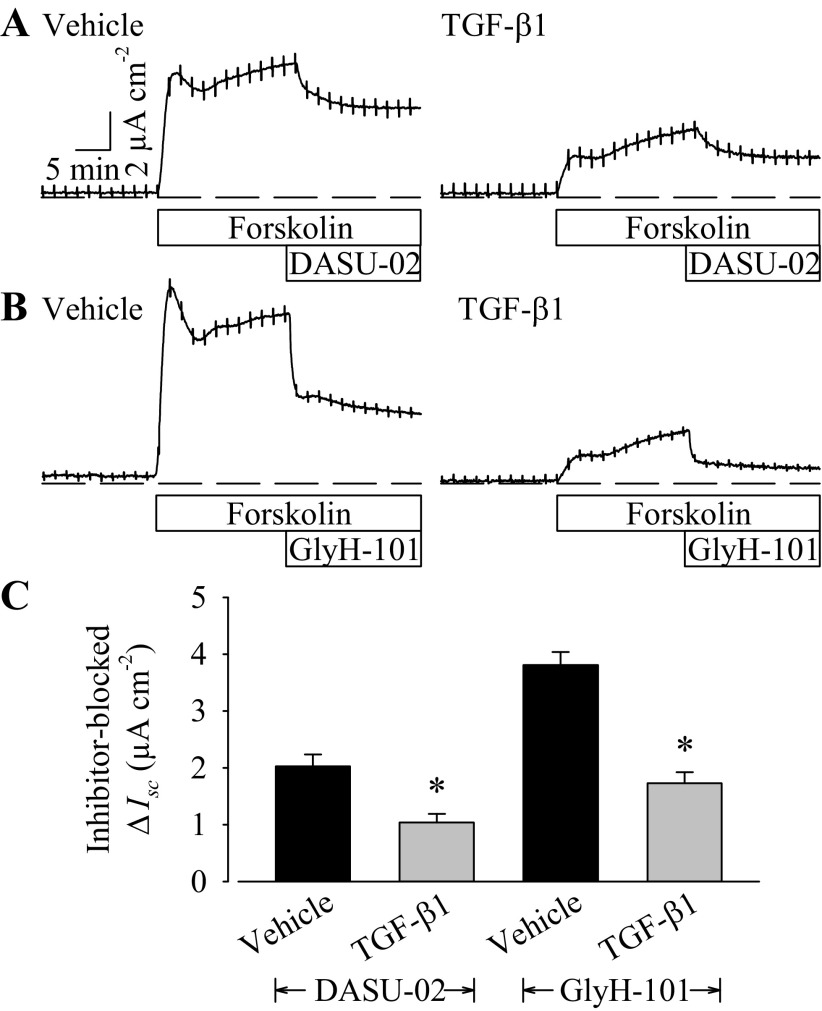
To test more directly for TGF-β1-induced changes CFTR-mediated anion secretion, cells were exposed to selective CFTR channel blockers after the sustained response to forskolin had been established. In both vehicle and TGF-β1-treated monolayers, DASU-02 (100 μM) inhibited ∼40% of forskolin-stimulated Isc (Fig. 4A). However, in vehicle-treated PVD9902 monolayers, the absolute value of Isc inhibited by DASU-02 was more than twofold of the Isc in TGF-β1-treated PVD9902 monolayers. Another CFTR selective blocker, GlyH-101, was also tested. Similar to the effect of DASU-02, GlyH-101 (10 μM) inhibited forskolin-stimulated Isc in both vehicle- and TGF-β1-treated monolayers. The magnitude of Isc blocked by GlyH-101 in TGF-β1-treated monolayers was much less than in monolayers exposed to vehicle alone (Fig. 4B). The attenuated effect of both DASU-02 and GlyH-101 on TGF-β1-treated monolayers, together with a less profound reduction in Rte stimulated by the activation of cAMP-mediated pathway (see Fig. 1A), suggests that TGF-β1 exposure reduces CFTR-mediated ion transport.
Fig. 4.
TGF-β1 reduces the effect of cystic fibrosis transmembrane conductance regulator (CFTR) inhibitors. Typical current recordings of vehicle or TGF-β1-treated (5 ng/ml, 24 h) PVD9902 monolayers exposed sequentially to forskolin (2 μM) followed by DASU-02 (A; 100 μM) or GlyH-101 (B; 10 μM). C: summarized results of CFTR inhibitor blocked anion secretion from 5–6 paired experiments. *P < 0.05, statistical difference from vehicle.
TGF-β1 downregulates CFTR mRNA expression.
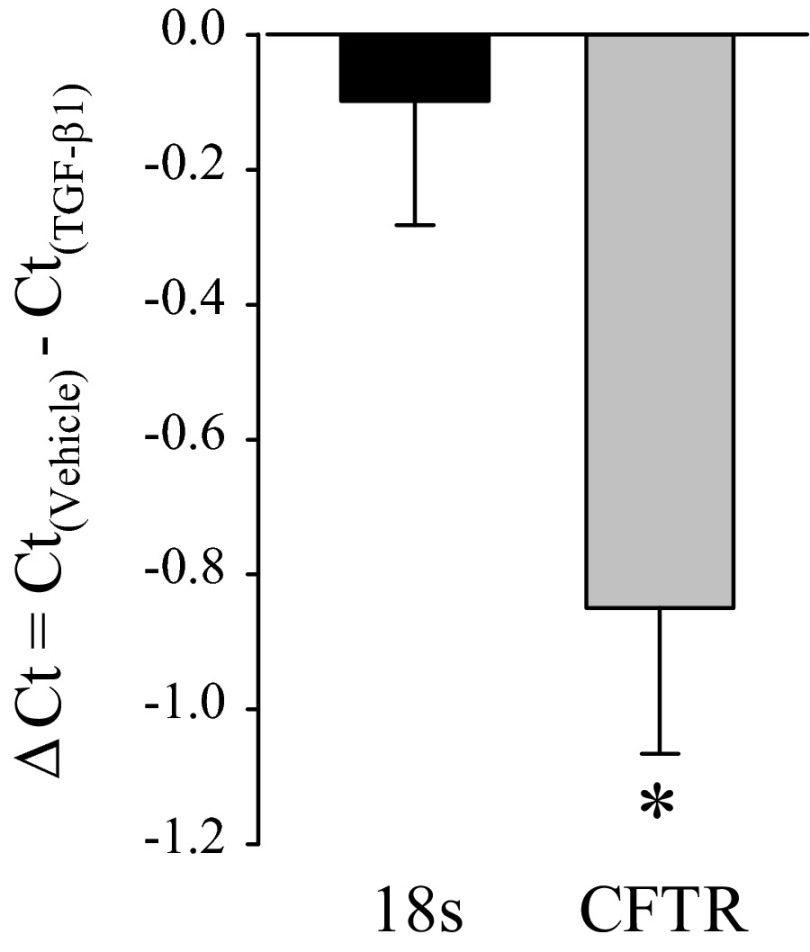
Real-time RT-PCR was performed using RNA isolated from five paired monolayers of different passage numbers to detect the relative abundance of mRNA coding for CFTR. The Ct value for CFTR in vehicle-treated PVD9902 cells was 28.9 ± 0.4 while the Ct value of CFTR in TGF-β1-treated cells was 29.8 ± 0.6. For the reference gene 18S, the Ct values in vehicle-treated and TGF-β1-treated cells were virtually equal, 12.7 ± 0.3 and 12.8 ± 0.3 for vehicle and TGF-β1 treatments, respectively (Fig. 5). With the use of the ΔΔCt method, the relative abundance of mRNA coding for CFTR in TGF-β1-treated cells was 60.2 ± 8.4% of its abundance in cells exposed to vehicle, indicating that TGF-β1 downregulates CFTR mRNA expression.
Fig. 5.
TGF-β1 downregulates CFTR mRNA abundance. Expression of mRNA coding for CFTR in PVD9902 monolayers exposed to vehicle or TGF-β1 (5 ng/ml, 24 h) was determined by real-time RT-PCR. Data are summarized from 5 paired experiments and are expressed as the ΔCt between vehicle-treated cells and TGF-β1-treated cells for the reference 18S and for CFTR. *P < 0.05, significant difference.
TGF-β1 downregulates CFTR protein expression.
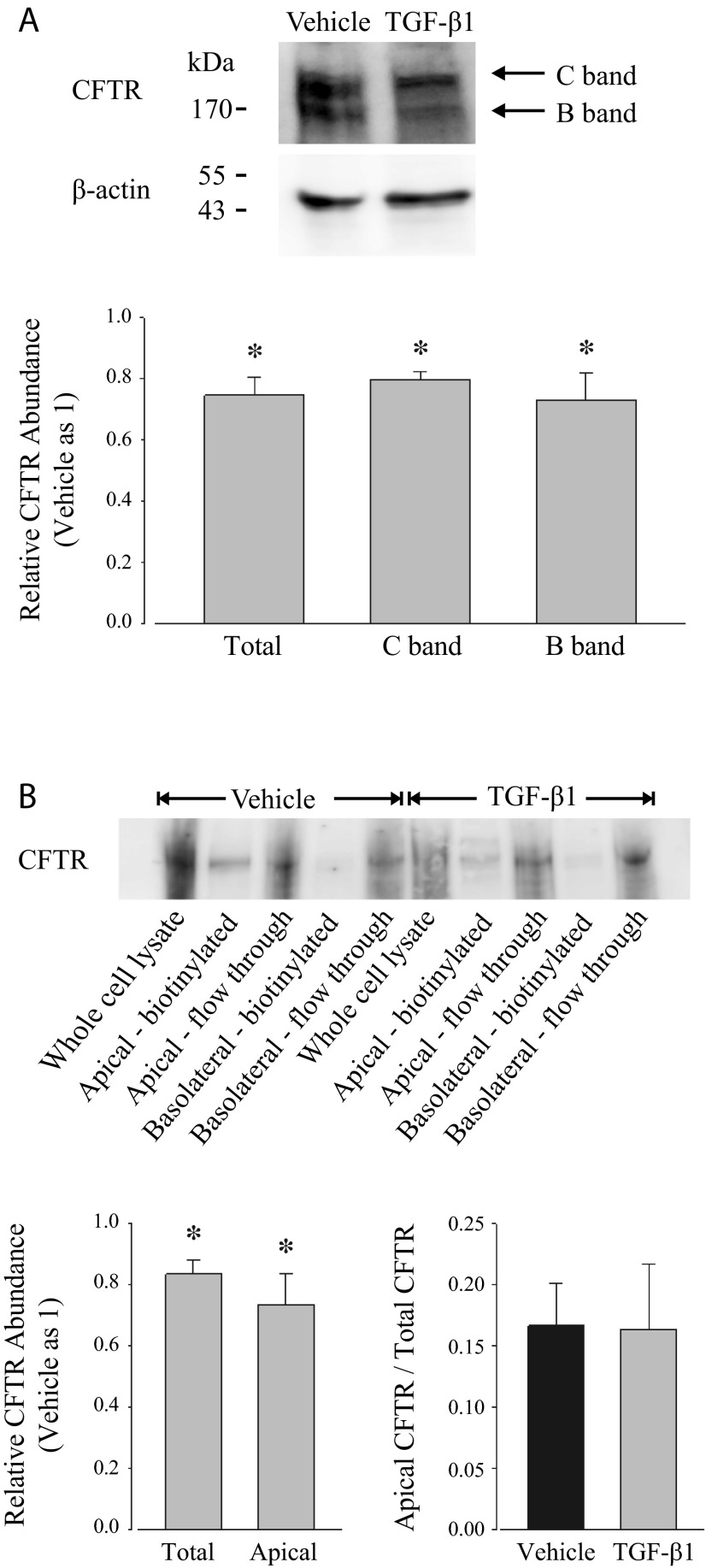
Given the electrophysiological evidence that TGF-β1 impairs anion secretion through CFTR as well as the RT-PCR outcomes that TGF-β1 downregulates the copy number of CFTR mRNA, Western blot analysis was performed to detect whether TGF-β1 affects the apparent protein expression of CFTR using an antibody that detects CFTR in primary adult and neonatal porcine vas deferens epithelial cells at the same mobility as in Calu-3 cells (35). Typically, CFTR is observed following electrophoresis in three different isoforms, namely A band, B band, and C band, that exhibit distinct mobilities due to the extent of posttranslational modification (32). A band represents nascent CFTR protein lacking glycosylation, B band represents CFTR with core glycosylation, and C band represents the mature fully glycosylated form. Both B-band and C-band isoforms were identified by Western blot in PVD9902 cells, whereas A band was not detected (Fig. 6A). CFTR protein expression was quantified by densitometry and then normalized to an internal loading control, β-actin. The relative abundance of CFTR following TGF-β1 exposure was then normalized to vehicle monolayers. Summarized immunoblotting results suggest that TGF-β1 exposure led to a significant reduction of the abundance of CFTR protein for both B-band and C-band isoforms (Fig. 6A).
Fig. 6.
TGF-β1 reduces CFTR abundance in cell lysates and in the apical cell surface. A: CFTR expression was assessed in whole cell lysates from PVD9902 monolayers exposed to vehicle or TGF-β1 (5 ng/ml, 24 h) by Western blots. Densitometric analysis was conducted to quantify signal intensity that could be attributed to total CFTR (C band and B band), fully glycosylated CFTR (C band), and core-glycosylated CFTR (B band). Data are expressed as relative abundance in CFTR normalized to β-actin with respect to vehicle. Data are summarized from 4 independent experiments. B: apical cell surface CFTR expression was determined by biotinylation and Western blots from PVD9902 monolayers exposed to vehicle or TGF-β1 (5 ng/ml, 24 h). Densitometric analysis was conducted to determine protein abundance of total CFTR and apical cell surface CFTR. Summarized relative CFTR abundance is from 6 independent experiments. *P < 0.05, significant reductions compared with vehicle.
Cell surface biotinylation together with Western blot analysis was applied to investigate whether TGF-β1 exposure also would decrease apical cell surface CFTR protein expression. PVD9902 monolayers were biotinylated from the apical compartment or from the basolateral compartment. All fractions were subjected to SDS-PAGE followed by Western blot analysis. Occludin, a tight junction protein that can be biotinylated from both apical and basolateral compartments, was used to verify unilateral biotin labeling of proteins in either the apical or basolateral membrane in each experiment (data not shown). Results show that CFTR is detected in apical biotinylated cell lysate and flow through but not in basolateral biotinylated cell lysate, which suggests that CFTR exists in the apical cell surface and exists intracellularly, but not in the basolateral cell surface (Fig. 6B). Similar to the outcomes in Fig. 6A, in the whole cell lysate of TGF-β1-treated PVD9902 cells, the expression of total CFTR was less than its expression in vehicle-treated cells. The absolute expression of apical cell surface CFTR in cells exposed to TGF-β1 was also less than its apical expression in cells exposed to vehicle alone. Additionally, the abundance of apical biotinylated CFTR was compared with the abundance of total CFTR in whole cell lysate to evaluate the percentage of apical cell surface CFTR expression. In both vehicle-treated cells and TGF-β1-treated cells, apical cell surface CFTR accounts for ∼16% of total CFTR, suggesting that TGF-β1 does not affect the relative expression of apical cell surface CFTR (Fig. 6B). Taken together, these outcomes suggest that TGF-β1 decreases the total amount of CFTR protein present in the cells as well as its amount in the apical membrane where it could contribute to anion secretion. Decreased expression could limit CFTR-mediated anion secretion, as was observed.
Receptor inhibitor abolishes TGF-β1-induced reduction in anion secretion.
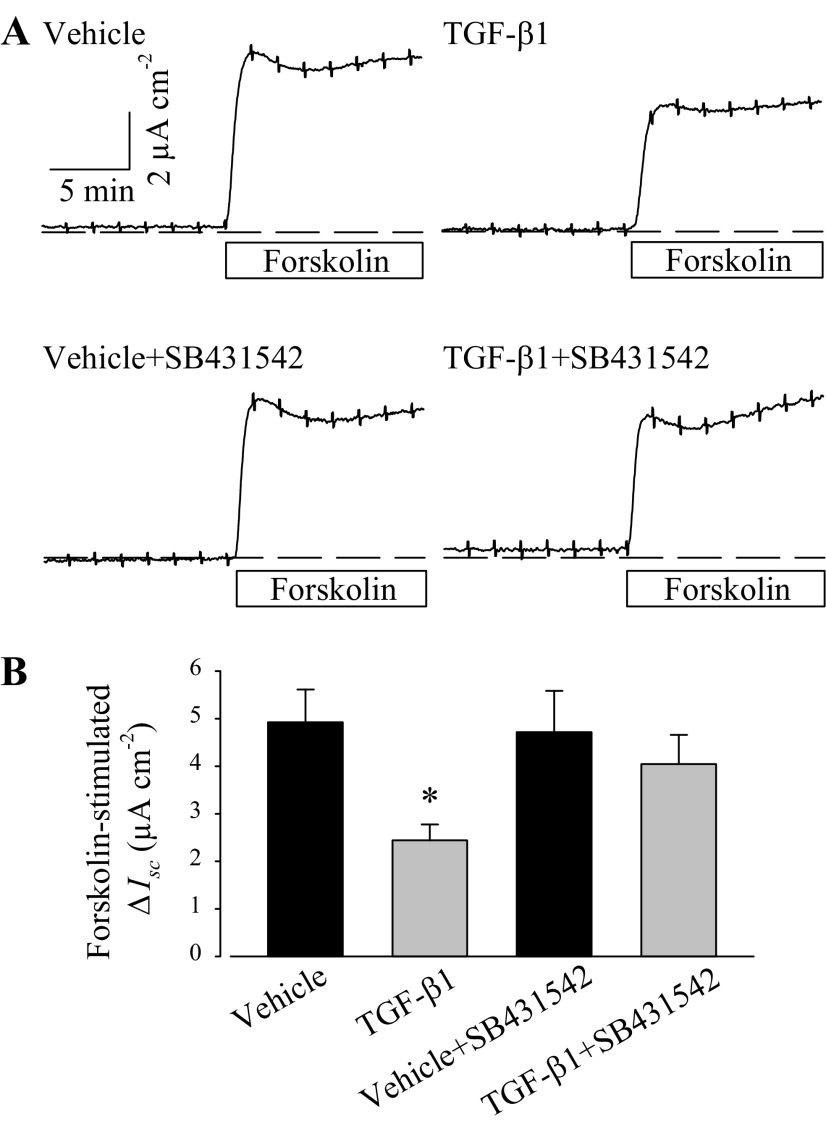
TGF-β1 activates transmembrane receptors to elicit cytosolic signaling and ultimately to affect cellular activity. SB431542 (10 μM), a selective TGF-β1 receptor I inhibitor (14, 25), was added into PVD9902 culture media 1 h before TGF-β1 exposure. After 24 h of TGF-β1 exposure, PVD9902 cells were mounted into modified Ussing chambers to measure forskolin-stimulated anion secretion. In PVD9902 monolayers treated with both TGF-β1 and SB431542, forskolin elicited a sharp increase in Isc that was significantly greater than monolayers exposed to TGF-β1 alone and was not different, statistically, from vehicle alone (Fig. 7). The outcome that the reduction of forskolin response by TGF-β1 can be abolished by SB431542 indicates that TGF-β1 receptor signaling pathway is necessary and sufficient to account for TGF-β1-suppressed anion secretion across PVD9902 monolayers.
Fig. 7.
A TGF-β receptor I inhibitor, SB431542, abolishes the TGF-β1-associated reduction in the response to forskolin. A: typical responses to forskolin (2 μM) in PVD9902 monolayers exposed to vehicle, TGF-β1 (5 ng/ml), SB431542 (10 μM), or TGF-β1 plus SB431542. B: summary of forskolin-stimulated anion secretion from 10–14 experiments. *P < 0.05, statistical difference from vehicle.
TGF-β1 activates the p38 MAPK signaling pathway in PVD9902 cells.
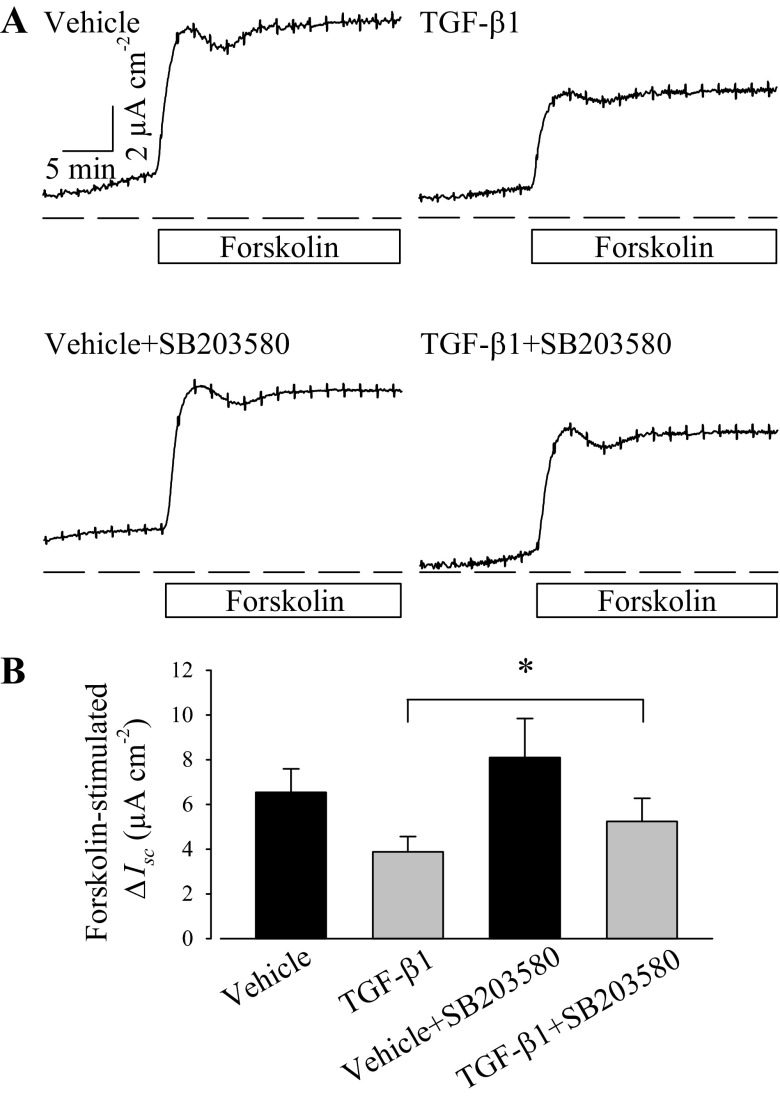
Given the observations that TGF-β1 activates its cognate receptor and attenuates CFTR-mediated anion secretion, downstream signaling cascades elicited by TGF-β1 were investigated using a pharmacological approach. PVD9902 cells were exposed to SB203580, a compound that inhibits p38 MAPK catalytic activity (15, 24). PVD9902 monolayers exposed to SB203580 (10 μM) exhibited a rapid response to forskolin that is not significantly different from monolayers exposed to vehicle along. However, monolayers exposed to TGF-β1 plus SB203580 exhibited a significantly higher response to forskolin than monolayers treated with TGF-β1 alone (Fig. 8). The observation that SB203580 attenuates the effect of TGF-β1 on forskolin-stimulated Isc provides evidence that p38 MAPK is required for the TGF-β1-induced change in ion transport.
Fig. 8.
A p38 MAPK inhibitor, SB203580, attenuates TGF-β1-impaired forskolin response. A: typical responses to forskolin (2 μM) in PVD9902 monolayers exposed to vehicle, TGF-β1 (5 ng/ml), SB203580 (10 μM), or TGF-β1 plus SB203580. B: responses to forskolin are summarized from 4–5 experiments. *P < 0.05, statistical significance.
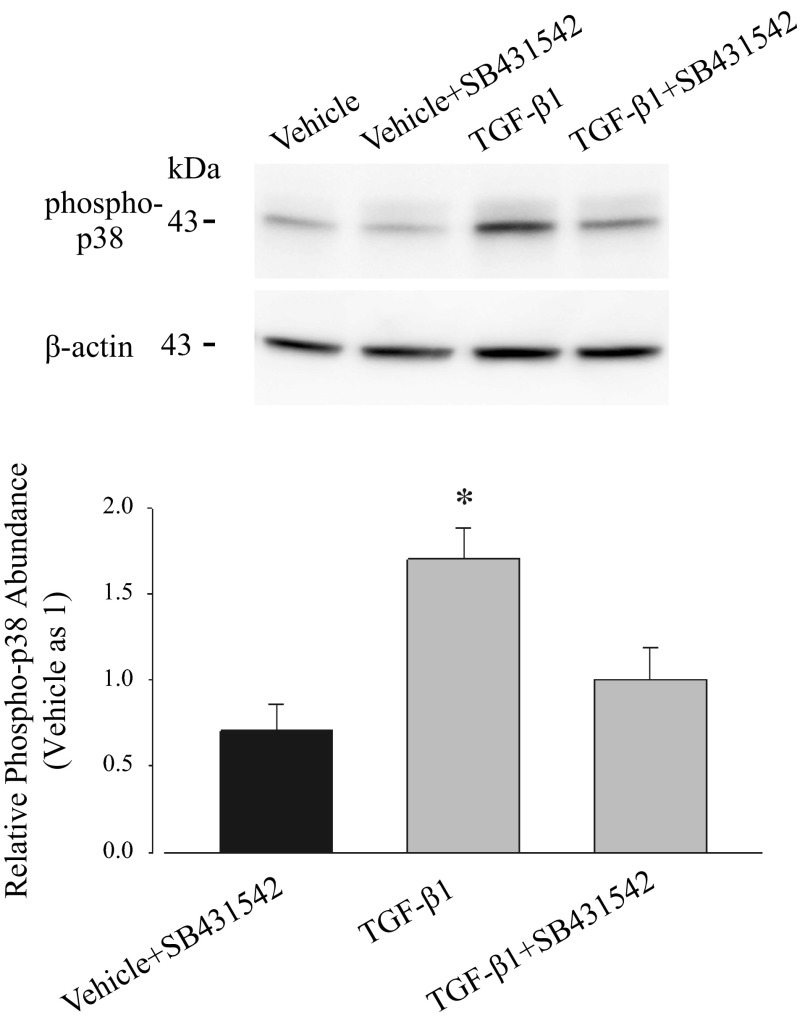
Western blot analysis was performed to detect phospho-p38 MAPK, which is thought to be the active form. Results presented in Fig. 9 demonstrate that phospho-p38 MAPK is present in all samples, regardless of TGF-β1 exposure. However, after normalization to β-actin as a loading control, the intensity of the phospho-p38 MAPK band was ∼1.7-fold in samples from cells exposed to TGF-β1 compared with cells exposed to vehicle. This increase in the relative abundance of phospho-p38 MAPK was prevented by the pretreatment of SB431542, suggesting that TGF-β1-induced activation of p38 MAPK signaling pathway is through the activation of TGF-β1 receptor. Together with ion transport results showing that the p38 MAPK inhibitor attenuates the effect of TGF-β1, these outcomes suggest quite strongly that TGF-β1, through the activation of its receptor, induces phosphorylation of p38 MAPK and thus activates the p38 MAPK signaling pathway.
Fig. 9.
TGF-β1 upregulates the abundance of phospho-p38. Expression of phospho-p38 MAPK was determined by immunoblots and measured by densitometric analysis using whole cell lysates isolated from PVD9902 monolayers treated with vehicle, SB431542 (10 μM), TGF-β1 (5 ng/ml), or TGF-β1 plus SB431542. The relative abundance of phospho-p38 under each treatment is normalized to β-actin with respect to vehicle. Summarized data are from 6–12 independent experiments. *P < 0.05, significant reductions compared with vehicle.
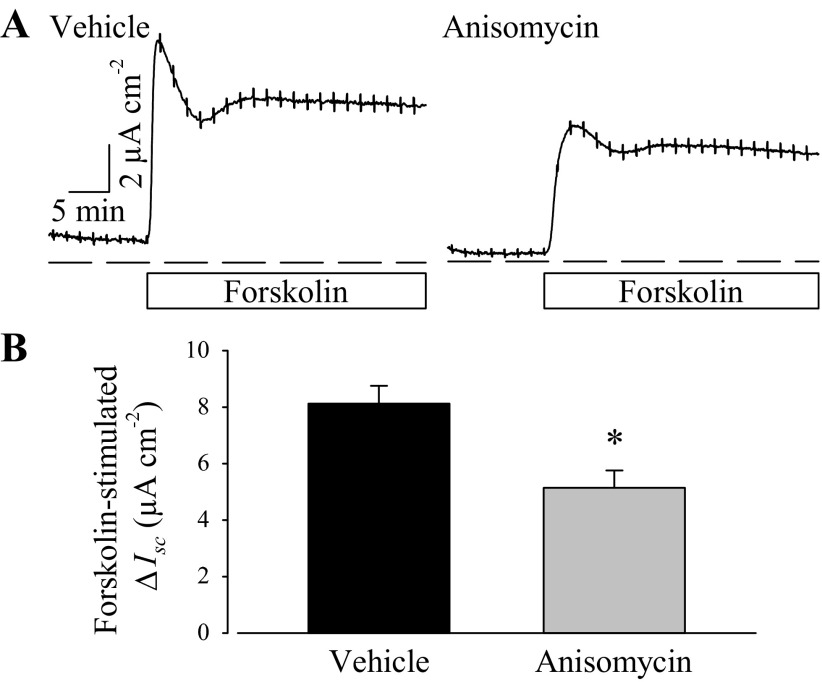
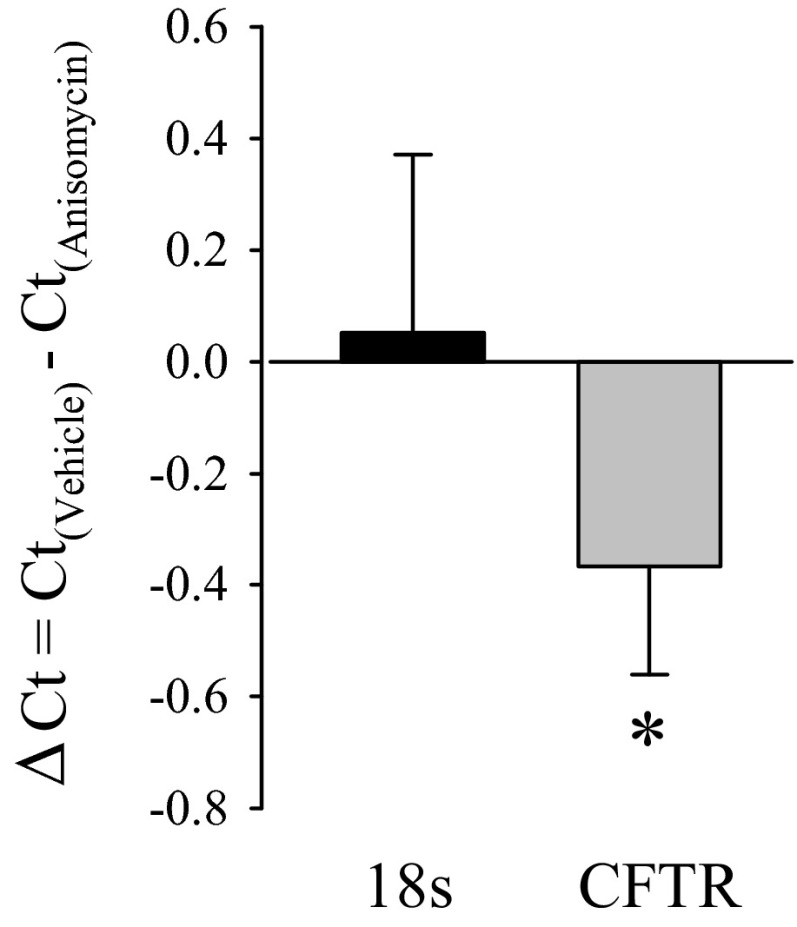
The role of the p38 MAPK signaling pathway in forskolin-stimulated anion secretion was also tested by anisomycin, an activator of the p38 MAPK pathway (3, 50). PVD9902 cells were exposed to vehicle or anisomycin (2 μg/ml) for 24 h followed by modified Ussing chamber experiments. Similar to the effect of TGF-β1, anisomycin exposure led to a significant reduction in forskolin-stimulated anion secretion compared with paired monolayers (Fig. 10). Real-time RT-PCR was also performed to identify the effect of anisomycin on CFTR mRNA abundance. The ΔCt value between vehicle and anisomycin-treated PVD9902 cells for CFTR was ∼−0.4 while the ΔCt value for 18S was ∼0.05, with a ΔΔCt value of −0.4 ± 0.2 (Fig. 11). These outcomes suggested that anisomycin exposure was associated with a 24.0 ± 7.7% reduction in mRNA coding for CFTR. These observations show that anisomycin mimics the effect of TGF-β1 on forskolin response and on CFTR mRNA abundance, which provide further evidence that the p38 MAPK signaling pathway is involved in the effect of TGF-β1 on PVD9902 monolayers.
Fig. 10.
A p38 MAPK activator, anisomycin, impairs forskolin-stimulated anion secretion across PVD9902 monolayers. A: representative Ussing chamber traces of vehicle or anisomycin-treated (2 μg/ml, 24 h) PVD9902 monolayers exposed to forskolin (2 μM). B: summarized results of forskolin-stimulated anion secretion from 11 paired experiments. *P < 0.05, statistical difference from vehicle.
Fig. 11.
Anisomycin downregulates CFTR mRNA abundance. CFTR mRNA abundance in PVD9902 monolayers exposed to vehicle or anisomycin (2 μg/ml, 24 h) was determined by real-time RT-PCR. Data are summarized from 4 paired experiments and are expressed as the ΔCt between vehicle-treated cells and anisomycin-treated cells for 18S and for CFTR. *P < 0.05, indicates significant difference.
TGF-β1 does not affect the viability of PVD9902 cells.
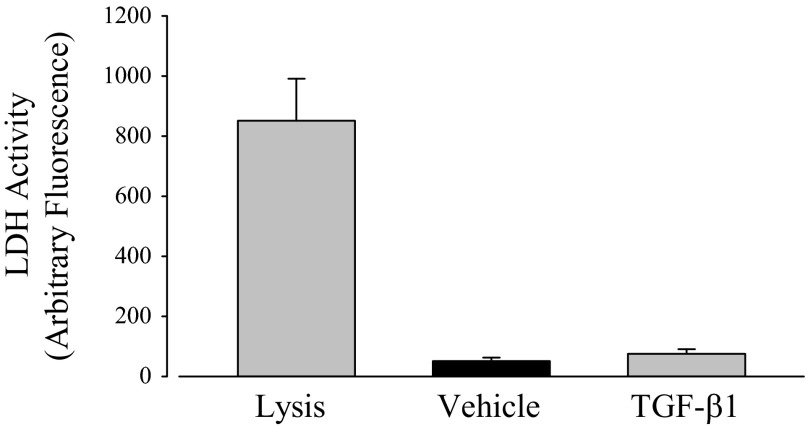
Another mechanism that can explain the reduction of anion secretion induced by TGF-β1 is that TGF-β1 leads to cell death and thus reduces the number of cells present in the monolayer. This mechanism, however, is unlikely based on the Rte measured at the outset of Ussing chamber experiments (Table 1). PVD9902 monolayers exposed to TGF-β1 exhibited a high basal Rte that was not different, statistically, from vehicle. Additional evidence was gathered by measuring LDH release to determine whether TGF-β1 compromises cell viability. A commercial kit in which florescence is proportional to LDH activity in the cell culture medium was used to measure the release of LDH from cells with damaged membranes. PVD9902 cells were cultured on 96-well plates to measure the leakage of LDH from cytoplasm into culture medium. Cells exposed to TGF-β1 showed an LDH release that was not different from vehicle alone while cells exposed to the lysis solution exhibited a remarkable release (Fig. 12). This outcome indicates that TGF-β1 has no measurable effect on cell viability and thus rules out the possibility that reduced anion secretion across PVD9902 monolayers is due to decreased cell numbers.
Fig. 12.
TGF-β1 has no detectable effect on lactate dehydrogenase (LDH) release. PVD9902 cells were grown on 96-well plates for 3 days and then exposed to vehicle or TGF-β1 (5 ng/ml, 24 h). Cells in paired wells were lysed to determine maximum LDH activity. LDH activity in the apical medium is proportional to fluorescence at 590 nm. Results are summarized from 6 experiments.
DISCUSSION
This study demonstrated that TGF-β1 exposure reduces the secretory response to a variety of test reagents that initiate signaling through the cAMP-mediated pathway, including forskolin, the combination of forskolin and IBMX, and 8-pCPT-cAMP. Real-time RT-PCR, immunoblots, and electrophysiological measurements showed that TGF-β1 reduces the relative copy number of mRNA coding for CFTR, that less CFTR is expressed, that less CFTR is present in the apical cell membrane, and that there is reduced anion secretion attributable to CFTR. Studies focusing on the cellular signal transduction mechanisms suggest that TGF-β1 binds to and activates TGF-β1 receptor I. Receptor activation is associated with an increase in the activity of the p38 MAPK signaling pathway as demonstrated by an increase in the phosphorylated form of the enzyme. Additional support for this scenario was drawn from experiments in which anisomycin mimicked the effect of TGF-β1 on CFTR expression to impaired anion transport across cultured vas deferens epithelial cells. These observations define a scenario by which elevated levels of TGF-β1 could heighten the symptomology of mild cystic fibrosis.
Previous studies suggested that TGF-β1 modulates epithelial ion transport by changing the amount, location, or activity of the apical CFTR channel, although none of them has studied the effect of TGF-β1 on vas deferens epithelia (18, 19, 38, 39). TGF-β1 exposure led to a significant reduction in the magnitude of the response to forskolin or a cAMP analog in colonic epithelia, T84 and HT-29 cells, and in kidney epithelia, Madin-Darby canine kidney cells, by changing the expression and distribution of CFTR (18, 19). A similar role of TGF-β1 to reduce cAMP-driven anion secretion and CFTR expression has been identified in lung and airway epithelial cells (38, 39). The present report demonstrates that TGF-β1 downregulates the gene expression of CFTR, its protein abundance, and especially the protein abundance of functional CFTR in the cell surface. A single CFTR channel has a conductance of ∼10 pS and a channel open probability of ∼0.4 when exposed to forskolin (40, 41, 44). If one employs a series of reasonable assumptions that are consistent with a previous report (e.g., PVD9902 cells exposed to forskolin have an apical membrane driving force of ∼4 mV, that there are 400 CFTR channels in apical membranes of typical cells, and that there are ∼106 cells per monolayer), forskolin exposure will induce an increase in Isc of ∼6.4 μA, which approximates the magnitude of our observations (Fig. 1). Furthermore, if one assumes apical, basolateral and paracellular resistances of 9,000, 8,000, and 15,000 Ω·cm2 in resting conditions, the calculated Rte would be ∼8,100 Ω·cm2, which is similar to what is observed for these cell monolayers. Activation of CFTR by forskolin would decrease apical resistance to ∼5,900 Ω·cm2 and Rte to ∼5,500 Ω·cm2. A 25% reduction in the number of channels would be expected to have no effect on basal Rte, but would reduce forskolin-stimulated Isc by 25% and would reduce the forskolin-induced change in Rte by a lesser amount, which again is consistent with the observations. These simple calculations support certain inferences. First, TGF-β1-attenuated forskolin response is explainable largely by invoking a single change in the cells, a reduced number of CFTR channels in the apical membranes. A change in the signaling cascade leading to channel activation, in the CFTR channel activation mechanism, in the channel inactivation mechanism, or in maximal channel open probability is not required. However, not all observations are fully explained by this mechanism. Notably, one expects a reduction in channel number to have no effect on basal Rte. Further, the full magnitudes of the shifts in Rte are not fully explained by the difference in channel expression. Initial sets of experiments were conducted to test for indications either of cell death or of epithelial to mesenchymal transition. In both cases the results were negative. TGF-β1 exposure does not alter the release of LDH, suggesting that TGF-β1 does not affect the viability of PVD9902 cells. Outcomes from Western blots showed that TGF-β1 exposure does not alter the expression of E-cadherin, an epithelial cell marker that typically is downregulated following exposure of epithelial cell types to TGF-β1, suggesting that TGF-β1 did not induce epithelial to mesenchymal transition in PVD9902 cells (data not shown). Clearly, additional experiments are required to address these observations further. Nonetheless, the preponderance of the observed effects can be explained by a TGF-β1-associated reduction in CFTR expression that requires activation of the TGF-β1 receptor I and the p38 MAPK pathway.
TGF-β1 has been associated with reproductive function and male fertility (20). Male TGF-β1 null mutant mice exhibited a reduced level of testosterone as well as a complete inability to mate with females although the weight and histology of male reproductive tissues, including penis, seminal vesicles, and testes, were normal (21). However, the effect of TGF-β1 on vas deferens, especially ion transport across vas deferens epithelia, is unknown. It is of critical importance since the concentration of TGF-β1 in seminal plasma is 85–238 ng/ml (31, 37), which is much higher than its concentration in plasma or any other bodily fluids (34). These observations, which demonstrate that TGF-β1 modulates ion transport across cultured vas deferens epithelial monolayers, extend our understanding of the physiological and/or pathological roles of TGF-β1 in vas deferens and in male fertility.
Vas deferens epithelia express numerous ion channels and transporters that are required to accomplish vectorial transport of salt and water (4, 5, 43). Among those channels and transporters, CFTR mediates the movement of bicarbonate as well as chloride and contributes to the adjustment of luminal volume and pH (2, 22, 45). Impaired CFTR-mediated anion secretion would diminish water movement, decrease bicarbonate secretion, and change vas deferens luminal pH. An acid environment is required for sperm to mature as they traverse the epididymis. However, an alkaline luminal pH is required to initiate motility and to induce fertilizing capacity of sperm cells (8, 30, 36). The critical role of CFTR in the male reproductive system has been demonstrated by the magnitude of infertility that is associated with CFTR mutations including the high frequency of CBAVD in both severely and mildly affected cystic fibrosis patients. Importantly, men with “mild” or hemizygous CFTR gene mutations that have intact vasa differentia often exhibit reduced sperm quality and/or male infertility (10, 23, 42, 48). Reduced CFTR function would be expected to result in reduced luminal fluid volume and reduced pH, which ultimately would have a negative impact on sperm maturation, activation, motility, and function (33, 46). Our observations that TGF-β1 signaling reduces CFTR expression and limits anion secretion suggests that individuals with an elevated level of TGF-β1 may exhibit aberrant ion transport across epithelia lining the vas deferens. This, by itself, could lead to reduced fertility. Moreover, in CF patients harboring mild or hemizygous CFTR mutations, greater TGF-β1 signaling may further compromise fertility.
TGF-β1 modulates ion transport by activating numerous intracellular signaling cascades in different types of epithelial cells. TGF-β1 inhibited fluid transport across ATII monolayers through a phosphatidylinositol 3-kinase-dependent mechanism (39). Sodium and fluid transport across ATII monolayers were decreased by TGF-β1 through an ERK1/2-dependent mechanism (13). Chloride secretion across T84 monolayers was also impaired through a p38 MAPK pathway (18). In PVD9902 cells, with the observation that TGF-β1 activates TGF-β receptor I, additional experiments were conducted to test for mediators of downstream TGF-β1 signaling. Results from initial experiments designed with this goal suggested p38 MAPK as a potential mediator. Therefore, subsequent experiments focused on a role for p38 MAPK in the transduction of TGF-β1 signaling in PVD9902 cells. By applying the selective p38 MAPK signaling pathway inhibitor SB203580, by measuring protein abundance of phosphorylated p38 MAPK, and by using the activator of the p38 MAPK signaling pathway anisomycin, to mimic the effect of TGF-β1, it was shown that TGF-β1 activates the p38 MAPK signaling pathway in PVD9902 monolayers. Another inhibitor of p38 MAPK, SKF86002, exhibited similar effects as SB203580 (data not shown). PVD9902 cells exposed to the combination of SKF86002 and TGF-β1 exhibited a higher response to forskolin than cells exposed to TGF-β1 only, suggesting the involvement of the p38 MAPK signaling pathway in the effect of TGF-β1.
In conclusion, the results show that PVD9902 cells exposed to TGF-β1 exhibit reduced cAMP-stimulated anion secretion. This effect was associated with the activation of TGF-β receptor I and the activation of the p38 MAPK signaling pathway. Reductions in mRNA coding for CFTR and CFTR protein expression were detected. These observations may have clinical implications because high levels of TGF-β1 in close association with vas deferens epithelial cells might induce aberrant ion transport, change the luminal environment, and reduce male fertility.
GRANTS
This article represents contribution number 14-032-J from the Kansas Agricultural Experiment Station. This work was supported by the National Institute of Child Health and Human Development Grant HD-058398 and by the COBRE Epithelial Function in Health and Disease Grant RR-017686.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.Y., F.P.-A., and B.D.S. conception and design of research; S.Y. performed experiments; S.Y. and B.D.S. analyzed data; S.Y., F.P.-A., and B.D.S. interpreted results of experiments; S.Y. prepared figures; S.Y. drafted manuscript; S.Y., F.P.-A., and B.D.S. edited and revised manuscript; F.P.-A. and B.D.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Lin-Hua “Florence” Wang and Qian Wang for technical assistance.
REFERENCES
- 1.Arkwright PD, Laurie S, Super M, Pravica V, Schwarz MJ, Webb AK, Hutchinson IV. TGF-beta(1) genotype and accelerated decline in lung function of patients with cystic fibrosis. Thorax 55: 459–462, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballard ST, Trout L, Bebok Z, Sorscher EJ, Crews A. CFTR involvement in chloride, bicarbonate, and liquid secretion by airway submucosal glands. Am J Physiol Lung Cell Mol Physiol 277: L694–L699, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Barros LF, Young M, Saklatvala J, Baldwin SA. Evidence of two mechanisms for the activation of the glucose transporter GLUT1 by anisomycin: p38(MAP kinase) activation and protein synthesis inhibition in mammalian cells. J Physiol 504: 517–525, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlin RW, Quesnell RR, Zheng L, Mitchell KE, Schultz BD. Functional and molecular evidence for Na+-HCO cotransporter in porcine vas deferens epithelia. Am J Physiol Cell Physiol 283: C1033–C1044, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Carlin RW, Sedlacek RL, Quesnell RR, Pierucci-Alves F, Grieger DM, Schultz BD. PVD9902, a porcine vas deferens epithelial cell line that exhibits neurotransmitter-stimulated anion secretion and expresses numerous HCO3− transporters. Am J Physiol Cell Physiol 290: C1560–C1571, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Chaudhury A, Howe PH. The tale of transforming growth factor-beta (TGFbeta) signaling: a soigne enigma. IUBMB Life 61: 929–939, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaco JM, Cutting GR. Update on gene modifiers in cystic fibrosis. Curr Opin Pulm Med 14: 559–566, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contri A, Gloria A, Robbe D, Valorz C, Wegher L, Carluccio A. Kinematic study on the effect of pH on bull sperm function. Anim Reprod Sci 136: 252–259, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Corvol H, Boelle PY, Brouard J, Knauer N, Chadelat K, Henrion-Caude A, Flamant C, Muselet-Charlier C, Boule M, Fauroux B, Vallet C, Feingold J, Ratjen F, Grasemann H, Clement A. Genetic variations in inflammatory mediators influence lung disease progression in cystic fibrosis. Pediatr Pulmonol 43: 1224–1232, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Cuppens H, Cassiman JJ. CFTR mutations and polymorphisms in male infertility. Int J Androl 27: 251–256, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425: 577–584, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Drumm ML, Konstan MW, Schluchter MD, Handler A, Pace R, Zou F, Zariwala M, Fargo D, Xu A, Dunn JM, Darrah RJ, Dorfman R, Sandford AJ, Corey M, Zielenski J, Durie P, Goddard K, Yankaskas JR, Wright FA, Knowles MR. Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med 353: 1443–1453, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Frank J, Roux J, Kawakatsu H, Su G, Dagenais A, Berthiaume Y, Howard M, Canessa CM, Fang X, Sheppard D, Matthay MA, Pittet JF. Transforming growth factor-beta1 decreases expression of the epithelial sodium channel alphaENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem 278: 43939–43950, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Halder SK, Beauchamp RD, Datta PK. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia 7: 509–521, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265: 808–811, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Harris WT, Muhlebach MS, Oster RA, Knowles MR, Clancy JP, Noah TL. Plasma TGF-beta(1) in pediatric cystic fibrosis: potential biomarker of lung disease and response to therapy. Pediatr Pulmonol 46: 688–695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Havasi V, Rowe SM, Kolettis PN, Dayangac D, Sahin A, Grangeia A, Carvalho F, Barros A, Sousa M, Bassas L, Casals T, Sorscher EJ. Association of cystic fibrosis genetic modifiers with congenital bilateral absence of the vas deferens. Fertil Steril 94: 2122–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howe K, Gauldie J, McKay DM. TGF-β effects on epithelial ion transport and barrier: reduced Cl− secretion blocked by a p38 MAPK inhibitor. Am J Physiol Cell Physiol 283: C1667–C1674, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Howe KL, Wang A, Hunter MM, Stanton BA, McKay DM. TGFbeta downregulation of the CFTR: a means to limit epithelial chloride secretion. Exp Cell Res 298: 473–484, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ingman WV, Robertson SA. The essential roles of TGFB1 in reproduction. Cytokine Growth Factor Rev 20: 233–239, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Ingman WV, Robertson SA. Transforming growth factor-beta1 null mutation causes infertility in male mice associated with testosterone deficiency and sexual dysfunction. Endocrinology 148: 4032–4043, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Ishiguro H, Yamamoto A, Nakakuki M, Yi L, Ishiguro M, Yamaguchi M, Kondo S, Mochimaru Y. Physiology and pathophysiology of bicarbonate secretion by pancreatic duct epithelium. Nagoya J Med Sci 74: 1–18, 2012 [PMC free article] [PubMed] [Google Scholar]
- 23.Jakubiczka S, Bettecken T, Stumm M, Nickel I, Musebeck J, Krebs P, Fischer C, Kleinstein J, Wieacker P. Frequency of CFTR gene mutations in males participating in an ICSI programme. Hum Reprod 14: 1833–1834, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Jiang MS, Adams JL, Lee JC. Pyridinylimidazole compound SB 203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochem Biophys Res Commun 263: 825–831, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, Martin W, Fornwald J, Lehr R, Harling J, Gaster L, Callahan JF, Olson BA. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol 62: 58–64, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Laurenza A, Sutkowski EM, Seamon KB. Forskolin: a specific stimulator of adenylyl cyclase or a diterpene with multiple sites of action? Trends Pharmacol Sci 10: 442–447, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Lewis-Jones DI, Gazvani MR, Mountford R. Cystic fibrosis in infertility: screening before assisted reproduction: opinion. Hum Reprod 15: 2415–2417, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Li C, Naren AP. Macromolecular complexes of cystic fibrosis transmembrane conductance regulator and its interacting partners. Pharmacol Ther 108: 208–223, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Li H, Ganta S, Fong P. Altered ion transport by thyroid epithelia from CFTR(−/−) pigs suggests mechanisms for hypothyroidism in cystic fibrosis. Exp Physiol 95: 1132–1144, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannowetz N, Wandernoth PM, Wennemuth G. Glucose is a pH-dependent motor for sperm beat frequency during early activation. PLoS One 7: e41030, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nocera M, Chu TM. Characterization of latent transforming growth factor-beta from human seminal plasma. Am J Reprod Immunol 33: 282–291, 1995 [DOI] [PubMed] [Google Scholar]
- 32.O'Riordan CR, Lachapelle AL, Marshall J, Higgins EA, Cheng SH. Characterization of the oligosaccharide structures associated with the cystic fibrosis transmembrane conductance regulator. Glycobiology 10: 1225–1233, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Okamura N, Tajima Y, Ishikawa H, Yoshii S, Koiso K, Sugita Y. Lowered levels of bicarbonate in seminal plasma cause the poor sperm motility in human infertile patients. Fertil Steril 45: 265–272, 1986 [DOI] [PubMed] [Google Scholar]
- 34.Oppenheim JJ, Feldmann M, Durum SK. Cytokine Reference: a Compendium of Cytokines and Other Mediators of Host Defense. San Diego, CA: Academic, 2001 [Google Scholar]
- 35.Pierucci-Alves F, Akoyev V, Stewart JC, 3rd, Wang LH, Janardhan KS, Schultz BD. Swine models of cystic fibrosis reveal male reproductive tract phenotype at birth. Biol Reprod 85: 442–451, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierucci-Alves F, Duncan CL, Lillich JD, Schultz BD. Porcine vas deferens luminal pH is acutely increased by systemic xylazine administration. Biol Reprod 82: 132–135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod 22: 2928–2935, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Pruliere-Escabasse V, Fanen P, Dazy AC, Lechapt-Zalcman E, Rideau D, Edelman A, Escudier E, Coste A. TGF-β1 downregulates CFTR expression and function in nasal polyps of nonCF patients. Am J Physiol Lung Cell Mol Physiol 288: L77–L83, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Roux J, Carles M, Koh H, Goolaerts A, Ganter MT, Chesebro BB, Howard M, Houseman BT, Finkbeiner W, Shokat KM, Paquet AC, Matthay MA, Pittet JF. Transforming growth factor beta1 inhibits cystic fibrosis transmembrane conductance regulator-dependent cAMP-stimulated alveolar epithelial fluid transport via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem 285: 4278–4290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schultz BD, Singh AK, Devor DC, Bridges RJ. Pharmacology of CFTR chloride channel activity. Physiol Rev 79: S109–144, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Schultz BD, Takahashi A, Liu C, Frizzell RA, Howard M. FLAG epitope positioned in an external loop preserves normal biophysical properties of CFTR. Am J Physiol Cell Physiol 273: C2080–C2089, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Schulz S, Jakubiczka S, Kropf S, Nickel I, Muschke P, Kleinstein J. Increased frequency of cystic fibrosis transmembrane conductance regulator gene mutations in infertile males. Fertil Steril 85: 135–138, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Sedlacek RL, Carlin RW, Singh AK, Schultz BD. Neurotransmitter-stimulated ion transport by cultured porcine vas deferens epithelium. Am J Physiol Renal Physiol 281: F557–F570, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Singh AK, Schultz BD, van Driessche W, Bridges RJ. Transepithelial fluctuation analysis of chloride secretion. J Cyst Fibros 3, Suppl 2: 127–132, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol 67: 377–409, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Tajima Y, Okamura N. The enhancing effects of anion channel blockers on sperm activation by bicarbonate. Biochim Biophys Acta 1034: 326–332, 1990 [DOI] [PubMed] [Google Scholar]
- 47.Taussig LM, Lobeck CC, di Sant'Agnese PA, Ackerman DR, Kattwinkel J. Fertility in males with cystic fibrosis. N Engl J Med 287: 586–589, 1972 [DOI] [PubMed] [Google Scholar]
- 48.van der Ven K, Messer L, van der Ven H, Jeyendran RS, Ober C. Cystic fibrosis mutation screening in healthy men with reduced sperm quality. Hum Reprod 11: 513–517, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Wangemann P, Itza EM, Albrecht B, Wu T, Jabba SV, Maganti RJ, Lee JH, Everett LA, Wall SM, Royaux IE, Green ED, Marcus DC. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med 2: 30, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem 280: 15649–15658, 2005 [DOI] [PubMed] [Google Scholar]