Abstract
The second messenger cyclic AMP (cAMP) plays a vital role in vascular physiology, including vasodilation of large blood vessels. We recently demonstrated cAMP activation of Epac-Rap1A and RhoA-Rho-associated kinase (ROCK)-F-actin signaling in arteriolar-derived smooth muscle cells increases expression and cell surface translocation of functional α2C-adrenoceptors (α2C-ARs) that mediate vasoconstriction in small blood vessels (arterioles). The Ras-related small GTPAse Rap1A increased expression of α2C-ARs and also increased translocation of perinuclear α2C-ARs to intracellular F-actin and to the plasma membrane. This study examined the mechanism of translocation to better understand the role of these newly discovered mediators of blood flow control, potentially activated in peripheral vascular disorders. We utilized a yeast two-hybrid screen with human microvascular smooth muscle cells (microVSM) cDNA library and the α2C-AR COOH terminus to identify a novel interaction with the actin cross-linker filamin-2. Yeast α-galactosidase assays, site-directed mutagenesis, and coimmunoprecipitation experiments in heterologous human embryonic kidney (HEK) 293 cells and in human microVSM demonstrated that α2C-ARs, but not α2A-AR subtype, interacted with filamin. In Rap1-stimulated human microVSM, α2C-ARs colocalized with filamin on intracellular filaments and at the plasma membrane. Small interfering RNA-mediated knockdown of filamin-2 inhibited Rap1-induced redistribution of α2C-ARs to the cell surface and inhibited receptor function. The studies suggest that cAMP-Rap1-Rho-ROCK signaling facilitates receptor translocation and function via phosphorylation of filamin-2 Ser2113. Together, these studies extend our previous findings to show that functional rescue of α2C-ARs is mediated through Rap1-filamin signaling. Perturbation of this signaling pathway may lead to alterations in α2C-AR trafficking and physiological function.
Keywords: GPCR, yeast two hybrid, filamin-2, filamin-2 phospho-Ser2113, yeast α-galactosidase assays, Rap1A
the g protein-coupled α2C-adrenoceptors (α2C-ARs) are stress receptors of the vascular sympathetic system (9). These receptors have a unique mechanism of regulation compared with the other two family members, the α2A-ARs and α2B-ARs. α2C-ARs are generally retained in intracellular Golgi compartment and are silent but can translocate to the cell surface under vascular stress, including cold exposure, and elicit a functional response leading to vasoconstriction (7, 8, 18, 20). It is therefore important to understand the mechanisms of receptor expression and trafficking for a clear understanding of α2C-AR physiology and its role in pathobiology.
We recently demonstrated a role of the Ras-related small GTPase Rap1A in enhancing translocation of α2C-ARs from the perinuclear compartment to the cell surface of microvascular smooth muscle cells (microVSM; Ref. 19). In human microVSM, increased levels of intracellular cAMP or exogenous addition of the cAMP analog 8-pCPT-2′-O-cAMP activated Rap1, leading to subsequent activation of RhoA, Rho-associated kinase (ROCK), and increase in F-actin (8, 19). This increased F-actin facilitated cell surface receptor translocation on actin filaments, which was disrupted by the actin destabilizing agents cytochalasin D or latrunculin A (19). These studies demonstrated that functional rescue of α2C-ARs is not restricted to cold exposure but can also occur at physiological temperatures (temperature-independent). The goal of the present study, therefore, was to identify the mechanism(s) involved. In this study, we used the α2C-AR carboxy terminus (last 20 amino acids) as bait in a yeast two-hybrid genetic screen and identified a novel interaction with the actin cross-linker filamin-2. The α2C-AR but not the α2A-AR subtype interacted with filamin. Our results also suggest that the phosphorylation of filamin-2 at Ser2113 by Rap1A-Rho-ROCK is necessary for receptor translocation.
These studies show that Rap1-filamin signaling regulates redistribution of α2C-ARs and show that functional rescue of α2C-ARs can occur at normal temperatures via this pathway. These findings suggest that loss-of-function genetic mutations in filamin may lead to reduced α2C-AR trafficking, affecting physiological function. Alternatively, gain-of-function due to activation of this signaling pathway during vascular stress may lead to increased α2C-AR biological activity and vascular reactivity. Together, these studies provide insights into the mechanism of G protein-coupled receptor (GPCR) trafficking.
EXPERIMENTAL PROCEDURES
DNA Constructs
Hemagglutinin-tagged receptors.
The constructs including murine amino-terminal hemagglutinin (HA)-tagged α2A- and α2C-ARs have been described in previous studies (10, 18).
Green fluorescent protein-tagged α2C-AR.
Plasmid DNA (10 ng) containing the entire coding region (1,386 bp) of human α2C-AR (pα2C4ENeo; Ref. 32) was used as template to PCR amplify (with high-fidelity Platinum Taq; Invitrogen, Carlsbad, CA) 15 bp of 5′-sequence and the coding region (excluding the stop codon). The primers used were as follows: forward primer: 5′-TCT CGA GCT CAA GCT TGC CCG CGG GAG GAC CAT GGC GTC CCC GGC GCT GGC G-3′ (HindIII restriction enzyme site in italics, 5′-sequence underlined, and part of coding region in bold), and reverse primer: 5′-GGC GAC CGG TGG ATC CTG CCT GAA GCC CCT TCT CCT C-3′ (BamHI restriction enzyme site in italics, part of coding region in bold).The PCR conditions were as follows: 94°C, 4 min (1 cycle), followed by 25 cycles (94°C, 1 min, 55°C, 45 s, 68°C, 2 min), and 1 cycle (68°C, 10 min). The amplification was optimized by using final concentrations of 2.5 mM Mg2+ and 2× enhancer solution for GC-rich sequences (Invitrogen).
The PCR product was column-purified (Concert Rapid PCR Purification System; GIBCO-BRL, Gaithersburg, MD), digested with HindIII and BamHI, gel purified, and ligated into the HindIII-BamHI sites of pEGFP-N1 vector (Clontech Laboratories, Mountain View, CA). This approach allowed the α2C-AR coding region to be fused in frame with enhanced green fluorescent protein (EGFP), with six amino acids of spacer region between the two genes. The fidelity of the PCR reaction was confirmed by DNA sequencing the entire α2C-AR region.
Rap1A.
Rap1A-63E (constitutively active; Rap1A-CA) plasmid DNA and the backbone plasmid (pcDNA) were kindly provided by Dr. Lawrence A. Quilliam, Indiana University School of Medicine, and have been previously described (8, 19, 28).
Adenovirus Constructs
The Rap1A-63E (constitutively active; Ad-Rap1A-CA) and vector-CMV backbone without insert (Ad-Control) were generated by the method described by He et al. (15) and optimized for microVSM delivery as previously described (9, 19). All experimental studies with adenovirus were performed according to National Institutes of Health Guidelines and Institutional Biological and Chemical Safety Committee guidelines and approved protocols.
Site-Directed Mutagenesis
Site-directed mutagenesis was performed using mutant oligonucleotide primers and double stranded DNA using the QuickChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). The five arginines (R454−458) in the COOH terminus of the α2C-AR were mutated to alanines (A454–458). The mutated construct (referred to as α2C-AR-1–5 MUT) generated by this approach was examined for any gross deletions by restriction enzyme digestions and agarose gel analyses, and the mutations were confirmed by DNA sequencing.
Antibodies
1) Anti-α2C-AR affinity purified rabbit polyclonal antibody. This antibody recognizes the carboxy terminus of α2C-ARs. The specificity of this antibody for Western blot analysis and imaging has been established in published studies. (2, 9, 19). This antibody recognizes the ∼70-kDa mature receptor form that translocates from the Golgi compartment to the cell surface (18).
2) Anti-human filamin (monoclonal, purified by protein A affinity chromatography, clone 1.BB.804) was purchased from US Biological Biochemicals and Biological Reagents (Swampscott, MA).
3) Anti-human and murine filamin-2 phospho-Ser2113-specific antibody (rabbit polyclonal, purified by affinity chromatography) was purchased from Invitrogen (Camarillo, CA).
4) Anti-β-actin was purchased from Sigma-Aldrich (clone AC-15).
5) Anti-HA.11 monoclonal antibody (clone 16B12) was purchased from Covance (Berkeley, CA).
6) Anti-aequorea victoria GFP affinity-purified monoclonal antibody (JL-8) was purchased from Clontech (Mountain View, CA).
7) Normal IgG (rabbit and mouse) used as negative control IgG for coimmunoprecipitation (Co-IP) studies was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
All secondary horseradish peroxidase- or alkaline phosphatase-labeled (anti-mouse and anti-rabbit) antibodies were purchased from GE Healthcare/Amersham (Pittsburgh, PA) or Jackson ImmunoResearch Laboratories (West Grove, PA).
Chemicals
1) Forskolin: this adenylyl cyclase activator, derived from Coleus forskohlii and ≥98% pure, was obtained in powder form from Sigma (St. Louis, MO). Working stock solutions of 10 mM were made by dissolving in DMSO solvent.
2) 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (abbreviated 8-pCPT-2′-O-Me-cAMP): 5-μmol aliquots of this membrane-permeant Epac-Rap activator were purchased from BIOLOG Life Science Institute (Bremen, Germany) and reconstituted in 50 μl of sterile distilled, deionized water to give 100 mM stocks.
3) Fasudil hydrochloride [1-(5-isoquinolinesulfonyl)-homopiperazine hydrochloride; Tocris Bioscience, Ellisville, MO]: fresh stocks (10 mM) of this ROCK inhibitor were prepared in sterile distilled, deionized water before each experiment.
Yeast Two-Hybrid Screen
The last 37 amino acids of α2C-AR, including part of transmembrane 7 and carboxy terminus (amino acids 426–462), or the last 20 amino acids (443–462), or the last 36 amino acids of α2A-AR (415–450) were amplified by PCR (Platinum Taq DNA polymerase, high-fidelity, Invitrogen) using primers containing restriction enzyme sites (NcoI, forward primer, and BamHI, reverse primer). The gel-purified PCR product was digested with NcoI-BamHI and inserted downstream in-frame with the Gal4 DNA-binding domain in the bait vector pGBKT7 (Clontech, Palo Alto, CA), also digested with NcoI-BamHI. The constructs were confirmed to be error-free and in-frame by DNA sequencing.
The MATCHMAKER library construction kit (Clontech Laboratories, Mountain View, CA) was used to generate a cDNA expression library using polyA mRNA isolated from serum-induced (10% FBS, 12 h) microVSM. (FastTrack 2.0 kit for isolation of mRNA; Invitrogen). Cloning of cDNA inserts in vector pGADT7 allowed expression as a fusion to GAL4 activation domain. Yeast two-hybrid screening was performed according to manufacturer's protocol (Clontech) using the last 20 amino acids of α2C-AR as bait. Approximately 1.04 × 105 clones were screened. As previously demonstrated by us these microVSM have inducible, plasma membrane-associated endogenous α2C-ARs (8, 9, 19), and harbor physiologically relevant protein-protein interactions with correct stoichiometry.
Yeast Two-Hybrid α-Galactosidase Assays
These assays were performed according to the manufacturer recommendations (Clontech) using filamin-2 fragment (aa 1979–2206), and α2A-AR (aa 415–450), α2C-AR [aa 426–462, wild type (WT)], or α2C-AR [aa 426–462, mutant (MUT)] fused in frame to the GAL4 DNA-binding domain. Positive controls included p53-SV40 T antigen. Selection was performed on media deficient in amino acids histidine, leucine, and tryptophan. Under these conditions, protein-protein interactions would lead to survival on selection media and to colony growth.
Human and Murine VSM Culture
Human arteriolar VSM cells explanted from dermal arterioles of healthy volunteers, and murine VSM derived from tail arteries of C57BL/6J WT mice (control) or mice deficient in the Ras-related GTPase Rap1A (Rap1A-null; referred to as microVSM) were used for the studies as previously described (9, 19). The studies performed on human tissue were approved by the Biomedical Sciences Institutional Review Board of Ohio State University, and the studies performed on murine tissue were approved by the Institutional Animal Care and Use Committees of the Ohio State University and the Research Institute at Nationwide Children's Hospital.
Amaxa nucleofections.
Amaxa nucleofections (Amaxa, Gaithersburg, MD) were performed for delivery of plasmid DNA to human microVSM as previously described (12, 19). Transfected cells were subsequently washed once with serum-depleted medium (0.5% Hams), quiesced in 0.5% Ham's for 48–72 h, followed by solvent (DMSO, H2O), forskolin (10 μM), or 8-pCPT-2′-O-Me-cAMP (100 μM) treatments for indicated times.
Co-IP and Immunoflurescence
Endogenous receptors.
Cells were lysed in IP buffer (50 mM Tris/7.5, 0.25% sodium deoxycholate, 1% NP40, 150 mM NaCl, 1 mM EDTA, plus fresh protease and phosphatase inhibitors), sonicated (3 × 10 s on ice), and centrifuged at 14,000 g, 10 min at 4°C. Protein estimations were performed on the supernatant, and 200–450 μg of total cell lysates were used to IP with antibodies (overnight at 4°C, with gentle rotation) against α2C-ARs (at 1:200 dilution) or against filamin (at 1:200 dilution). The target protein-antibody complex was pulled down with protein A/G agarose (Pierce Biotechnology, Rockford, IL) for 2 h at 4°C, and nonspecific binding was removed by three washes (1 ml each) with the same IP buffer. The beads were resuspended in sample buffer (62.5 mM Tris·HCl, pH 6.8, 2% SDS, 10% glycerol, 50 mM DTT or 5% β-mercaptoethanol, 0.01% bromophenol blue), incubated at 37°C for 1 h, and used for Western blot analysis. Similarly, IP experiments were performed with IgG (mouse or rabbit) alone as control for antibody specificity.
Tagged receptors.
Plasmids encoding HA- or GFP-tagged receptors were used. Amaxa nucleofected quiescent microVSM were treated with forskolin (10 μM) for 16 h before harvesting cells for Co-IP studies. IP was performed as described above for endogenous receptors using 26–80 μg of total lysates and antibodies against HA (1:200, gentle rotation overnight) or GFP (1:200, gentle rotation overnight).
Measurement of intracellular cAMP.
The intracellular cAMP levels in human microVSM were measured to assess endogenous α2C-AR cell surface association and function in the absence (solvent control) or presence of fasudil (3 μM), control nonsilencing RNA [non-small interfering (si)RNA, non-siRNA], or filamin-2 silencing RNA (FLN-2 siRNA). Cyclic AMP levels were quantitated using ELISA (Enzo Life Sciences, Farmingdale, NY) as previously described (19).
Human Embryonic Kidney 293 Studies
Cell culture and transient transfections.
Human embryonic kidney (HEK) 293 cells from American Type Culture Collection (Manassas, VA) were utilized. Cells were grown in MEM supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, 1.5 g/l sodium bicarbonate, and without antibiotics and were routinely kept in a 37°C incubator with 5% CO2. Transient cotransfections were performed as previously described (19).
Co-IP studies.
These studies utilized amino-terminal HA-tagged α2C-ARs, pcDNA (control empty vector), and Rap1A-CA. Co-IP was performed using the same buffer conditions described above for microVSM studies. The cell lysates were immunoprecipitated with HA and blotted with filamin. Similarly, IP experiments were performed with IgG (mouse) alone as control for antibody specificity.
Live-cell labeling studies.
The amino-terminal HA-tagged α2C-ARs were utilized for transient transfections and allowed quantitation of cell surface receptors by immunoprecipitation of the extracellular domain HA tag followed by Western blot analysis. The live-cell labeling was performed as previously described (19).
For studies examining the role of filamin-2 in receptor mobilization, HEK293 cells were cotransfected with siRNAs and plasmid DNA using Lipofectamine 2000 (Invitrogen), as previously described (2).
cAMP studies.
The intracellular cAMP levels in HEK293 cells were measured to assess transfected α2C-AR WT and α2C-AR-1–5 MUT cell surface association and function. Cyclic AMP levels were quantitated using 125I-radioimmunoassay kit (Biomedical Technologies, Stoughton, MA) as previously described (19).
Filmamin-2 siRNA Studies
siRNA duplexes (Custom HiPerformance silencing siRNA duplexes 1–5) targeting different regions of human filamin-2 were synthesized and purchased from Qiagen.
Duplex-1.
The duplex-1 targeting sequence was CCGGTACACCATCACCATCAA (gene bank accession no. AF146692, nucleotides 4824–4844, relative to the A of the translation start site, sense strand 3′-Alexa Fluor 647 labeled).
Duplex-2.
The duplex-2 targeting sequence was CACCTACACCATTACCATCAA (nt 3666–3686, relative to the A of the translation start site).
Duplex-3.
The duplex-3 targeting sequence was CACCTTCACTATTGTCACCAA (nt 5535–5555, relative to the A of the translation start site).
Duplex-4.
The duplex-4 targeting sequence was CCGCGCCAGCATGATGAACAA (overlapping the translation start site, 10 nt noncoding and 11 nt coding).
Duplex-5.
The duplex-5 targeting sequence was CAAGGTGGATATCACCTACGA (nt 3105–3125, relative to the A of the translation start site).
The nonsilencing siRNA duplex with target sequence AATTCTCCGAACGTGTCACGT that shows no known homology to mammalian genes using BLAST (and shows 16-base overlap with Thermotoga maritima bacteria) was used as control.
The siRNA duplexes were transfected in equimolar concentrations in human microVSM by electroporation (Amaxa nucleofection) or by magnetic nanoparticle-assisted delivery (IBA, St. Louis, MO). Various concentrations of duplex-1 and -2, ranging from 178 to 535 pmol were utilized in imaging studies. For cAMP studies, magnetic nanoparticle-assisted delivery of fluorescein-labeled dsRNA oligomer (BLOCK-iT, Invitrogen) was utilized to test various concentrations (0–100 pmol) to optimize delivery utilizing immunofluorescence microscopy. Using this approach we observed ∼100% transfection efficiency with no cytotoxicity. Knockdown of filamin-2 was optimized with duplex-2, -4, and -5 (equimolar amounts, combined concentration of 100 pmol) and showed 19 ± 3.8% inhibition compared with the equivalent concentration of control nonsilencing siRNA duplex (n = 3; P < 0.01, determined by Western blot analysis using cells harvested ∼65 h after siRNA delivery). This level of knockdown was sufficient to elicit a noticeable effect on receptor function.
Similarly, studies in HEK293 cells established the feasibility of using combination of silencing duplex-1, -3, and -4 (equimolar amounts, combined concentration of 480 pmol) to effectively knockdown filamin-2 (62% ± 10% inhibition compared with equivalent concentration of control nonsilencing siRNA duplex; n = 3; P < 0.05, determined by Western blot analysis).
Western Blot Analyses
Visualization of proteins of interest was performed as previously described (12, 19). Samples were separated on 10% SDS-PAGE for α2C-AR or 8% SDS-PAGE for filamin. Samples were incubated at 37°C for 2 h (for α2C-ARs) or at 95°C for 10 min (for filamin) before loading.
Microscopy
Immunofluorescence-confocal microscopy and quantitation of fluorescence.
This was performed on microVSM as described previously (19). The optical slices obtained by this approach allowed spatial visualization of intracellular and cell surface α2C-ARs (referred to as cell boundary, which includes surface and subsurface receptors). For fluorescence comparisons, the highest intensity observed was used as reference for each set of experiments and identical settings and conditions were used to capture and process all images. For assessing changes in mean fluorescence intensity, the region-of-interest tool (NIS-Elements AR Laboratory Image Analysis System; Nikon Instruments, Melville, NY) was used for quantitation for each set of experiments. The quantitation of mean fluorescence intensity of α2C-ARs at the cell boundary was assessed at four different regions of the cell boundary per cell.
α2C-AR-GFP live cell studies.
Nucleofected quiescent human microVSM on glass bottom culture dishes (MatTek, Ashland, MA) were treated with 8-pCPT-2′-O-Me-cAMP (100 μM, time, t = 0) and placed in a stage top incubator (Tokai Hit) with temperature controlled at 37°C and 5% CO2 flow. Images were collected at 10-min intervals for 9 h using the Nikon Eclipse Ti fluorescence microscope (×40 objective; NIS Elements Imaging Software). To adjust for drift and to automatically maintain focus on a point of interest during image collection, the Perfect Focus System was used (Nikon Instruments). Differential interference contrast images were also obtained with the same microscope.
Quantitative immunofluorescence assessment of surface receptors.
These experiments utilized amino-terminal HA-tagged α2C-ARs. Receptors on the cell surface will have the amino terminus in the extracellular region and be accessible to anti-HA antibody binding. This approach therefore allowed quantitative assessment of cell surface vs. subsurface receptors. Cells were cotransfected with HA-α2C-AR-1–5 MUT (3.5 μg) and pmaxGFP (0.5 μg; Amaxa nucleofection); or HA-α2C-AR WT (3.5 μg), pmaxGFP (0.5 μg), and 100 nM of control nonsilencing RNA; or HA-α2C-AR WT (3.5 μg), pmaxGFP (0.5 μg), and 100 nM of filamin-2 (FLN 2) siRNA 2, 4, and 5 in equimolar concentrations. Quiescent cells were treated with the Epac-Rap1 activator 8-pCPT-2′-O-Me-cAMP (100 μM) for the indicated time intervals and fixed in 3% paraformaldehyde. Nonpermeabilized human microVSM were stained with anti-HA (1:200 dilution) followed by secondary Alexa Fluor 568 goat anti-mouse IgG (1:200 dilution, red) and Hoechst nuclear stain (blue). Quantitation of mean fluorescence intensity of cell surface α2C-ARs in GFP-positive cells was assessed at four different regions of the cell boundary per cell using the region-of-interest tool (NIS-Elements AR Laboratory Image Analysis System).
Statistical Analysis
Statistical analysis of the data was performed by Student's t-test for either paired or unpaired observations. When more than two means were compared, either a one-way ANOVA with Bonferroni or Dunnett's post hoc test for multiple comparisons or a two-way ANOVA with Tukey-Kramer's post hoc test (GraphPad Prism, San Diego, CA) was used to identify differences among groups. Data are presented as means ± SE, where n equals the number of independent experiments or individual cells. Values were considered statistically different when P < 0.05.
RESULTS
Actin-Binding Protein Filamin-2 Interacts With α2C-ARs
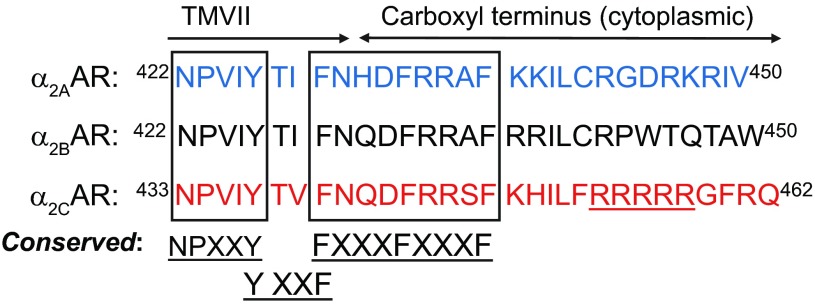
To identify potential α2C-AR interacting proteins involved in receptor translocation, a yeast two-hybrid screen was performed under stringent selection conditions on a cDNA expression library derived from human microVSM using the last 20 amino acids (part of the cytoplasmic carboxy terminus) of α2C-AR as bait. This region contained a unique α2C-AR nonconserved arginine-rich region (amino acids 454–458, R454–458) not present in α2A- or α2B-ARs (Fig. 1).
Fig. 1.
Alignment and comparison of cytoplasmic carboxy termini and part of transmembrane 7 (TMVII) of wild-type α2-adrenoceptor (α2-AR) subtypes. Conserved regions are shown. Note the nonconserved α2C-AR arginine-rich region (R454–458). Single letter amino acid codes are shown.
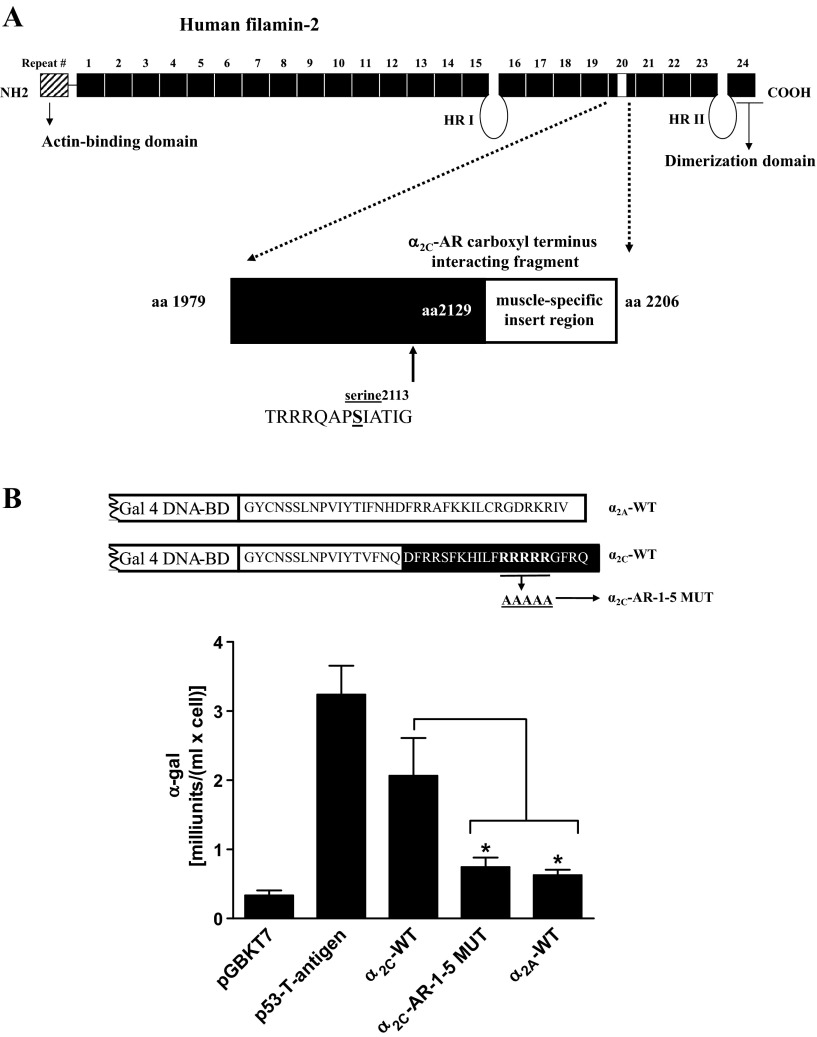
The results showed a novel interaction with a fragment of the muscle isoform of the actin cross-linking protein filamin, also known as filamin-2, filamin C, or gamma filamin (gene bank accession no. AF146692). The interacting clone included amino acids 1979–2128 of repeat 20, 78 amino acids of the muscle-specific insert (amino acids 2129–2206), and a conserved phosphorylation site Ser2113 (Fig. 2A). The screen also showed interactions with component of oligomeric Golgi complex (COG1) and α2B-mannosidase, consistent with endoplasmic reticulum-Golgi trafficking of α2C-ARs (6). For the purpose of this study, the novel α2C-AR-filamin interaction was further examined.
Fig. 2.
Filamin-2 smooth muscle isoform, a α2C-AR carboxy-terminal interacting protein. A: α2C-AR carboxy-terminal interacting region of human filamin-2 in microvascular smooth muscle cells (microVSM; gene bank accession no. AF 146692). This repeat 20 region includes 78 amino acids of muscle-specific insert region and the phosphorylation site Ser2113. Phosphorylation of this conserved Ser2113 has been shown to be necessary and sufficient for actin reorganization in non-VSM. HR, hinge region. B: quantitative yeast α-galactosidase assays were utilized to examine protein-protein interactions between filamin-2 fragment and α2A- and α2C-AR [wild-type (WT) or 1–5 mutant (MUT)] regions of interest. Gal 4 DNA-binding domain (DNA-BD) was fused to the last 36 amino acids of α2A-AR or the last 37 amino acids of α2C-AR, and expressed as fusion protein in the yeast strain AH109. Assays were performed on colonies selected on dropout selection medium-histidine/-leucine/-tryptophan. The α2C-AR WT or α2C-AR-1–5 MUT interaction with filamin-2 fragment was compared with α2A-AR WT and pGBKT7 backbone vector interactions with the same filamin-2 fragment. Similar results were observed when Gal4 DNA-BD was also fused to the last 20 amino acids of α2C-AR (amino acid residues in white). Positive control, p53-SV40 large T-antigen and negative control, lamin C (lam), and T antigen (undetectable, 0 on the x-axis). Data are presented as means ± SE (n = 6). *P < 0.05.
Arteriolar VSM express detectable levels of α2A-AR and α2C-AR subtypes (9). These subtypes were, therefore, further tested for filamin binding specificity. The yeast two-hybrid α-galactosidase assays were utilized to examine the role of α2-AR C-tail region in interaction with filamin-2 (amino-acids 1979–2206). These assays allow quantitation and relative comparison of the strength of protein-protein interactions. The α2A-AR WT, α2C-AR WT, and arginine to alanine mutations (α2C-AR-1–5 MUT) regions were tested for filamin-2 interaction using this approach (Fig. 2B).
These interactions with the filamin-2 fragment were compared with pGBKT7 backbone vector interaction with the same filamin-2 fragment. The tumor-suppressor gene p53 shows strong protein-protein association with the viral protein SV40 large T-antigen (21, 40). This p53 and SV-40 large T-antigen interaction was utilized as a positive control in these assays. The α2A-AR WT region showed basal activity comparable to pGBKT7 backbone vector interaction. The α2A-ARs are not regulated by cold temperature or by cAMP signaling and are ubiquitously expressed on the cell surface (9, 18). In sharp contrast, the α2C-AR WT region showed strong interaction with filamin-2 (Fig. 2B; similar results were observed when Gal4 DNA-BD was also fused to the last 20 amino acids of α2C-AR). Interestingly, α2C-AR-1–5 MUT showed significant loss of specific interaction between the two regions (∼3-fold loss compared with WT, P < 0.05; Fig. 2B). The activity of α2C-AR-1–5 MUT fragment was comparable to the control α2A-WT (P = NS).
Filamin and α2C-ARs Co-IP
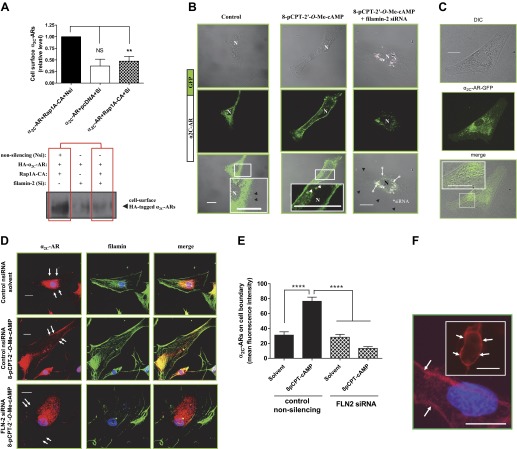
We tested the in vivo interaction between α2C-ARs and filamin using Co-IP assays. We previously demonstrated that Rap1A (constitutively active, Rap1A-CA) increased cell surface translocation of α2C-ARs in transiently transfected heterologous HEK293 cells (19). Experiments were therefore performed to examine the association of α2C-ARs and filamin in these cells. Transient cotransfections and Co-IP in HEK293 cells showed that Rap1A-CA increased α2C-AR-filamin interaction (Fig. 3A, 2.9 ± 0.7 increase compared with control vector; no changes in total levels of filamin were observed). Experiments were also performed in human microVSM to examine endogenous receptor-filamin interaction. In these cells increased intracellular cAMP activates both the A-kinase and Epac-Rap1 signaling pathways (8, 19). However, the A-kinase independent, cAMP-Epac-Rap1A-JNK-c-Jun pathway is coupled to transcriptionally increasing α2C-ARs (8, 12, 19). A novel analog of cAMP, 8-pCPT-2′-O-Me-cAMP, which selectively activates Epac-Rap1, or forskolin, the adenylyl cyclase activator, both increase expression of α2C-ARs [detectable induction at 9 h for 8-pCPT-2′-O-Me-cAMP (100 μM) or 16 h for forskolin (10 μM) (19)]. Co-IP experiments utilizing human microVSM demonstrated that endogenous α2C-ARs interact with endogenous filamin. We tested the microVSM under untreated (solvent control, 16 h) or treated conditions (forskolin, 10 μM, 16 h, or 8-pCPT-2′-O- cAMP, 100 μM, 16 h) to increase endogenous levels of α2C-ARs. Indeed, forskolin or 8-pCPT-2′-O-Me-cAMP increased the association between α2C-AR-filamin (3-fold for forskolin and 2-fold for 8-pCPT-2′-O-Me-cAMP, Fig. 3B). To verify microVSM specificity of α2C-AR-filamin interaction, cells were transiently transfected with tagged full-length α2C-AR WT or α2C-AR-1–5 MUT (GFP-or HA-tagged) and examined after 16 h of forskolin (10 μM) treatment. Immunoprecipitations were performed with antibodies to the tags and blotted to filamin. The WT α2C-ARs tagged either with GFP or HA coimmunoprecipitated with filamin. However, α2C-AR-1–5 MUT, like the control α2A-ARs, did not coimmunoprecipitate with filamin (Fig. 3C).
Fig. 3.
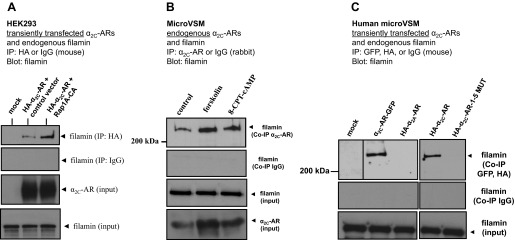
Filamin and α2C-ARs co-immunoprecipitate. A: α2C-AR-filamin interaction in transiently transfected human embryonic kidney (HEK) 293 cells. Cells were cotransfected with control vector or constitutively active Rap1A (Rap1A-CA). Coimmunoprecipitation (Co-IP) was performed with HA or control IgG (mouse) and blotted with filamin. Representative results are shown, including filamin and α2C-ARs (input). The α2C-AR-specific antibody recognizes the ∼70-kDa mature receptor form that translocates from the Golgi compartment to the cell surface. B: endogenous α2C-ARs interact with endogenous filamin in human microVSM. α2C-AR-filamin interaction under untreated (solvent control, 16 h) or treated conditions (forskolin, 10 μM, 16 h), or the selective Epac-Rap activator 8-pCPT-2′-O-Me-cAMP (8-CPT-cAMP; 100 μM, 16 h). Immunoprecipitations were performed with α2C-AR-specific antibody or control IgG (rabbit), and blotted with antibody to filamin. Representative results are shown, including filamin and α2C-ARs (input). C: α2C-AR-filamin interaction in transiently transfected human microVSM. Cells were transfected with various tagged full-length WT or mutated (1–5 MUT) α2C-ARs [green fluorescent protein (GFP) or hemagglutinin (HA)-tagged] and examined after 16 h of forskolin (10 μM) treatment. The α2A-ARs do not interact with filamin and do not Co-IP. Immunoprecipitations were performed with antibodies to the tags or control IgG (mouse) and blotted to filamin. The α2C-ARs tagged either with GFP or HA coimmunoprecipated with filamin. However, α2C-AR-1–5 MUT, like the control α2A-ARs, did not coimmunoprecipate with filamin. Expression of these constructs was confirmed by Western blotting or by imaging (not shown). Dividing line indicates noncontiguous lanes.
α2C-AR-1–5 MUT Localizes to the Cell Surface
The impact of the alanine mutations was assessed in HEK293 cells, which allow quantitation of cell-surface receptors and Gi-coupled function (18, 19). In these cells the WT α2C-ARs localize predominantly to the trans-Golgi network (18). In contrast to WT receptors, when the arginine-rich sequence of the carboxy terminal was mutated to alanines (R454–458 to A454–458, α2C-AR-1–5 MUT), the mutant receptor localized predominantly to the cell surface. The level of cell surface receptors was also quantitated by live cell labeling of receptor amino-terminal HA-tag, immunoprecipitation, and Western blot analysis. Although WT and α2C-AR-1–5 MUT transfectants exhibited similar levels of total α2C-AR expression, the level of α2C-AR-1–5 MUT at the cell surface was 3.0 ± 0.6 (means ± SE; n = 4; P < 0.05)-fold higher than that of the WT (Fig. 4A).
Fig. 4.
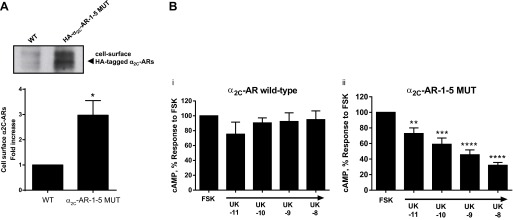
Effect of the R454–458 to A454–458 mutation (α2C-AR-1–5 MUT) on the localization (A) and function (B) of α2C-ARs in HEK293 cells. A: cells were transfected with either the WT or α2C-AR-1–5 MUT. In WT and mutant receptors, an HA tag is at the NH2 terminus and is therefore in the extracellular domain of cell surface receptors. Forty-eight hours after transfection, cells were either lysed and processed for total cellular expression of α2C-ARs or processed via live-cell labeling to detect cell surface receptors. Briefly, the cells were labeled with anti-HA antibody, and the cell-surface α2C-ARs subsequently were immunoprecipitated from cell lysates with protein-A/G-Sepharose beads and then subjected to SDS-PAGE followed by Western blotting. Data were normalized to the total α2C-ARs expression (not shown). Data are expressed as means ± SE (n = 4; P < 0.05). B: intracellular cyclic AMP was measured in transiently transfected HEK293 cells treated with the α2-AR agonist UK 14,304 (UK; 1 min, 10 pM-10 nM), followed by adenylyl cyclase stimulation by forskolin (FSK; 3 μM, 5 min). The data are corrected for the baseline value and are presented as a percentage of the response to FSK alone, and expressed as means ± SE; n = 3 independent experiments measured in duplicate; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The functional consequences of the R454–458 to A454–458 mutation were evaluated by the Gi-protein-mediated, α2C-AR-dependent inhibition of forskolin-stimulated intracellular cAMP accumulation. In WT α2C-AR-transfected cells, the α2-AR agonist UK 14,304 (concentration range: 10 pM-10 nM) did not significantly affect the intracellular accumulation of cAMP stimulated by forskolin (3 μM; Fig. 4Bi; 94.9 ± 11.8% with 10 nM UK 14,304 vs. forskolin alone; n = 3; P = NS), supporting intracellular retention of WT α2C-ARs. However, in HEK293 cells expressing α2C-AR-1–5 MUT, UK 14,304 (10 pM-10 nM) inhibited forskolin-stimulated cAMP accumulation in a concentration-dependent manner, showing highest inhibition at 10 nM (Fig. 4Bii; 32.03 ± 3.64% vs. forskolin alone; n = 3; P < 0.0001), supporting cell-surface association of α2C-AR-1–5 MUT, and showing that the mutations did not affect receptor coupling and function. There was no statistically significant difference in the effect of forskolin (3 μM) to increase cAMP accumulation between WT and α2C-AR-1–5 MUT transfected cells.
Filamin and α2C-ARs Colocalize
Immunofluorescence studies utilizing confocal microscopy supported Co-IP results and showed colocalization of endogenous α2C-ARs and filamin on trans-cytoplasmic filaments and showed increased α2C-ARs on cell boundary in 8-pCPT-2′-O-Me-cAMP-treated human microVSM (Fig. 5, A and B). Of note, the staining for α2C-ARs was punctate (vesicular), and these punctae appeared to be organized along the length of the filamin-decorated actin filaments (Fig. 5A, inset). These results were in agreement with our previous study that showed increased association of α2C-ARs with F-actin (19). Indeed, filamin colocalized with F-actin in 8-pCPT-2′-O-Me-cAMP-treated human microVSM (Fig. 5C), consistent with the role of filamin as an actin-crosslinking protein.
Fig. 5.
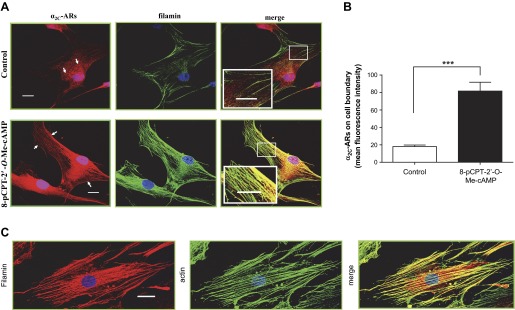
Colocalization of filamin and α2C-ARs in human microVSM. A: quiescent (control) microVSM were treated with the Epac-Rap1 activator 8-pCPT-2′-O-Me-cAMP (100 μM, 9 h), and cells were fixed and stained for endogenous α2C-ARs (Alexa Fluor-568, red), filamin (Alexa Fluor-488, green), and nuclei (Hoechst, blue). Similar results were observed with FSK (not shown). Scale bars = 20 μm. B: quantitation of mean fluorescence intensity of cell boundary α2C-ARs (which includes surface and subsurface receptors), assessed at 4 different regions of the cell boundary per cell. Data from 3–5 cells per condition are shown; ***P < 0.001. Control, quiescent microVSM with solvent or microVSM treated with the Epac-Rap1 activator 8-pCPT-2′-O-Me-cAMP. C: filamin-actin overlay in human microVSM treated with 8-pCPT-2′-O-Me-cAMP (100 μM, 9 h). Filamin Alexa Fluor-568 (red) and phalloidin Alexa Fluor-488 (green). Scale bar = 20 μm.
Time Course of α2C-AR-GFP Receptor Translocation
Previous studies showed that cooling leads to rapid (within 60 min upon exposure to 28°C) translocation of functional α2C-ARs to the cell surface (2, 3, 7, 18). Live-cell imaging experiments were therefore performed to examine the time course of receptor translocation at physiological 37°C upon stimulation of quiescent α2C-AR-GFP nucleofected human microVSM with the Epac-Rap1 activator 8-pCPT-2′-O-Me-cAMP (100 μM; t = 0 min; Fig. 6). Using this approach showed that translocation is a slower process and that the α2C-AR-GFP begins detectable translocation at ∼175 min after Epac-Rap1 stimulation. This process continues over time with increased receptor at the cell boundary (Fig. 6, t = 175 min to t = 555 min).
Fig. 6.
Time course of α2C-AR-GFP translocation. Live-cell imaging was performed on α2C-AR-GFP nucleofected quiescent human microVSM stimulated with 8-pCPT-2′-O-Me-cAMP (100 μM, t = 0 min) using fluorescence microscopy. Images were captured at 10-min intervals. Arrows point to the cell boundary and show increased α2C-AR-GFP translocation over time (t = 175 min to t = 555). Scale bar = 20 μm.
Filamin-2 and α2C-AR Translocation
Live-cell labeling of receptor amino-terminal HA-tag, immunoprecipitation, and Western blot analysis were performed to assess the role of filamin-2 in Rap1A-mediated α2C-AR cell-surface translocation in HEK293 cells.
Filamin-2 was knocked-down using siRNA. Indeed, filamin-2 knockdown inhibited Rap1A-mediated cell surface α2C-ARs (Fig. 7A; P < 0.01; n = 3). These results were consistent in human microVSM using immunofluorescence confocal microscopy and colocalization with GFP-tagged α2C-ARs and filamin-2 siRNA duplexes (one duplex having 3′-AlexaFluor-647 label). The α2C-AR-GFP localized to the perinuclear region in quiescent cells treated with solvent (control). However, in cells treated with 8-pCPT-2′-O-Me-cAMP (100 μM, 8–9 h, time showing increased receptor at the cell boundary using immunofluorescence and live-cell imaging; Figs. 5, A and B, and 6), α2C-AR-GFP was present on the cell boundary in addition to intracellular vesicular compartments; this intracellular vesicular compartment localization of α2C-AR-GFP was clearly observed in microVSM using differential interference contrast-fluorescence imaging (Fig. 7, B and C). In microVSM with siRNA filamin-2 duplexes, the α2C-AR-GFP cell boundary localization was not seen (Fig. 7B). Indeed, knockdown of filamin-2 significantly reduced endogenous α2C-ARs on the cell boundary (Fig. 7, D and E), consistent with α2C-AR-GFP studies.
Fig. 7.
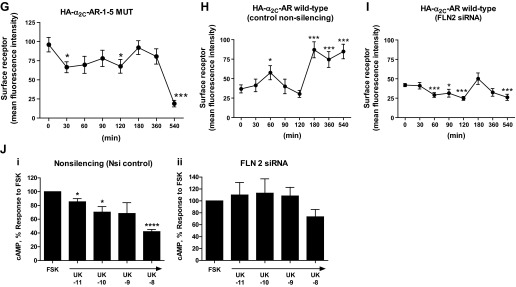
(Continued Caption)—Filamin-2 and α2C-AR translocation. A: quantitation of surface α2C-ARs. Live labeling, immunoprecipitation, and Western blot analysis to assess cell surface α2C-ARs after filamin-2 knockdown in transiently transfected HEK293 cells. Cells were co-transfected with small interfering (si)RNAs (nonsilencing or filamin-2 silencing), backbone plasmid DNA (pcDNA), plasmid DNA expressing amino-terminal HA-tagged α2C-AR and the constitutively activated form of Rap1A (Rap1A-CA). The outcome on cell surface α2C-ARs in the presence of control siRNA or in the presence of filamin-2 targeting siRNAs is highlighted by boxed regions in red. Nsi, nonsilencing siRNA; Si, silencing siRNA; Rap1A-CA, constitutively activated Rap1A. Data were normalized to the total α2C-ARs expression (not shown). **P < 0.01; NS, not significant. B: live-cell imaging of GFP-α2C-ARs in human microVSM: α2C-AR-GFP were found in intracellular vesicular structures that were delivered to the plasma membrane in response to Epac-Rap1 activation by 8-pCPT-2′-O-Me-cAMP (100 μM, 9–10 h). Note perinuclear α2C-AR-GFP localization in control (solvent-treated microVSM), and cell-surface α2C-AR-GFP localization in cells treated with 8-pCPT-2′-O-Me-cAMP. Live cell imaging of α2C-AR-GFP in microVSM in the presence of silencing siRNA to filamin-2 (includes 3′-Alexa Fluor-647 labeled siRNA; *arrows point to fluorescent siRNA; pink). Note perinuclear location of siRNA and absence of cell-surface receptors. Control, quiescent microVSM, or 8-pCPT-2′-O-Me-cAMP treated. Arrowheads (white) point to vesicles, and (black) point to cell boundary, visualized by transmission light microscopy. N, nucleus. Scale bars = 20 μm. C: differential interference contrast-fluorescence imaging of human microVSM transfected with α2C-AR-GFP. Tagged receptors can be seen in intracellular vesicular compartments. Scale bar = 20 μm. D: knockdown of filamin-2 (FLN-2) disrupts colocalization with α2C-ARs and reduces cell boundary α2C-ARs compared with control nonsilencing siRNA (nsiRNA). Arrows indicate cell boundary. Human microVSM (with nsiRNA or FLN-2 siRNA) were treated with H2O for 9 h (solvent-control) or with 8-pCPT-2′-O-Me-cAMP (100 μM, 9 h), and cells were fixed and stained for endogenous α2C-ARs (Alexa Fluor-568, red), filamin (Alexa Fluor-488, green), and nuclei (Hoechst, blue). Scale bars = 20 μm. E: quantitation of mean fluorescence intensity of cell boundary α2C-ARs, assessed at 4 different regions of the cell boundary per cell. Data from 12–13 cells per condition are shown, ****P < 0.0001. F: immunofluorescence assessment of surface receptors using amino-terminal HA-tagged α2C-ARs. In human microVSM nucleofected with HA-α2C-AR-1–5 MUT and pmaxGFP (quiescent, untreated cells), or HEK293 cells cotransfected with HA-α2C-AR-1–5 MUT and pmaxGFP (inset). Fixed, nonpermeabilized microVSM or HEK293 cells were stained with anti-HA mouse monoclonal antibody followed by secondary Alexa Fluor-568 goat anti-mouse IgG (red), and nuclear stain Hoechst (blue). Arrows point to surface immunofluorescence staining in GFP positive human microVSM and HEK 293 cells. Scale bars = 20 μm. G: relative cell surface HA-α2C-AR-1–5 MUT receptors (mean fluorescence intensity) in human microVSM treated with the Epac-Rap1 activator 8-pCPT-2′-O-Me-cAMP (100 μM, for various time intervals in minutes: 0, 30, 60, 90, 120, 180, 360, and 540). Quantitation of mean fluorescence intensity of cell surface α2C-ARs was assessed at 4 different regions of the cell boundary per cell. Data from 8–10 GFP positive cells per interval are shown, *P < 0.05, ***P < 0.001. H: relative cell surface HA-α2C-AR WT receptors (mean fluorescence intensity) in human microVSM treated with the Epac-Rap1 activator 8-pCPT-2′-O-Me-cAMP (100 μM, for various time intervals in minutes: 0, 30, 60, 90, 120, 180, 360, and 540), and control nonsilencing RNA. Quantitation of mean fluorescence intensity of cell surface α2C-ARs was assessed at four different regions of the cell boundary per cell. Data from 7–10 GFP-positive cells per interval are shown, *P < 0.05, ***P < 0.001. I: relative cell surface HA-α2C-AR WT receptors (mean fluorescence intensity) in human microVSM treated with the Epac-Rap1 activator 8-pCPT-2′-O-Me-cAMP (100 μM, for various time intervals in minutes: 0, 30, 60, 90, 120, 180, 360, and 540), and filamin-2 siRNA (FLN2). Quantitation of mean fluorescence intensity of cell surface α2C-ARs was assessed at four different regions of the cell boundary per cell. Data from 7–12 GFP positive cells per interval are shown, *P < 0.05, ***P < 0.001. J: effect of filamin-2 (FLN-2) knockdown on human microVSM α2C-AR function. Quiescent cells [48 h after delivery of control nonsilencing (i) or FLN-2 siRNA (ii)] were treated with 8-pCPT-2′-O-Me-cAMP (100 μM, 14 h). Receptor function was assessed by ability to inhibit forskolin (FSK) stimulated intracellular cyclic AMP accumulation in response to the α2-AR agonist UK 14,304 (UK, 10 pM - 10 nM). Basal level of cyclic AMP was elevated by FSK (5 μM, 5 min). Data are corrected for the baseline value and are presented as a percentage of the response to FSK alone and are expressed as means ± SE for n = 2–3 independent experiments measured in duplicate; *P < 0.05, ****P < 0.0001. The α2C-AR function in cells with FLN-2 siRNA is impaired, consistent with the role of F-actin-filamin in receptor translocation.
Experiments were also performed to assess cell surface-associated vs. subsurface receptors in human microVSM. These experiments utilized amino-terminal HA-tagged α2C-ARs. Receptors on the cell surface in nonpermeabilized cells will have the amino terminus in the extracellular region and be accessible to anti-HA antibody binding and immunofluorescence-microscopy analysis. This approach therefore allowed quantitative assessment of cell surface vs. subsurface receptors in transfected quiescent microVSM treated with the Epac-Rap1 activator 8-pCPT-2′-O-Me-cAMP over various time intervals (0–540 min). Studies with HA-α2C-AR-1–5 MUT in nonpermeabilized microVSM showed cell surface-association, in agreement with HEK 293 studies (Fig. 7F). Variable levels of surface receptors were noted upon stimulation of microVSM with 8-pCPT-2′-O-Me-cAMP (0–360 min; Fig. 7G). A significant drop in surface receptors, however, was observed upon prolonged treatment (540 min; Fig. 7G, mean fluorescence intensity of 18.92 ± 4.2 vs. 95.96 ± 9.5 at 0 min, P < 0.0001). In contrast, the levels of surface-associated HA-α2C-AR WT receptors, examined in the presence of control nonsilencing RNA, showed a slight, yet significant, and transient increase between 0 and 120 min (mean fluorescence intensity of 57.64 ± 9.05 at 60 vs. 36.8 ± 5.2 at 0 min, P < 0.05, not detected in α2C-AR-GFP live-cell imaging studies), followed by a significant and sustained increase at 180–540 min vs. 0 min (Fig. 7H, mean fluorescence intensity of 84.8 ± 9.34 at 540 min vs. 36.8 ± 5.2 at 0 min, P < 0.0001, detected similarly in α2C-AR-GFP live-cell imaging studies). These observations suggest increased cell surface translocation of intracellular receptors. This profile with HA-α2C-AR WT receptors, however, was not observed in cells with filamin-2 siRNA (Fig. 7I). In these cells a significant reduction in surface-associated receptors was observed with 8-pCPT-2′-O-Me-cAMP (P < 0.05 at 60–120 min, and 540 vs. 0 min; mean fluorescence intensity of 26.14 ± 3.9 at 540 min vs. 41.9 ± 2.0 at 0 min). These results were similar to the immunofluorescence imaging studies (Fig. 7, D and E) and suggest that the receptor does not translocate to the cell surface and remains intracellular.
Consistent with these observations, knockdown of filamin-2 impaired receptor function, supporting intracellular retention of α2C-ARs, assessed by ability of cell surface activated receptors to inhibit intracellular cAMP produced by adenylyl cyclase stimulation by forskolin (P = NS) at all concentrations of the α2-AR agonist UK 14,304 tested (10 pM-10 nM; Fig. 7Jii), compared with receptor function in the presence of control nonsilencing RNA in 8-pCPT-2′-O-Me-cAMP-treated human microVSM, supporting cell surface association of α2C-ARs (Fig. 7Ji; 73.33 ± 12.13% with 10 nM UK 14,304 vs. forskolin alone in filamin-2 knockdown, P = NS, compared with 42 ± 3.05% with 10 nM UK 14,304 vs. forskolin alone in control nonsilencing RNA, P < 0.0001).
Rap1A-Rho-ROCK and Filamin-2 Ser2113
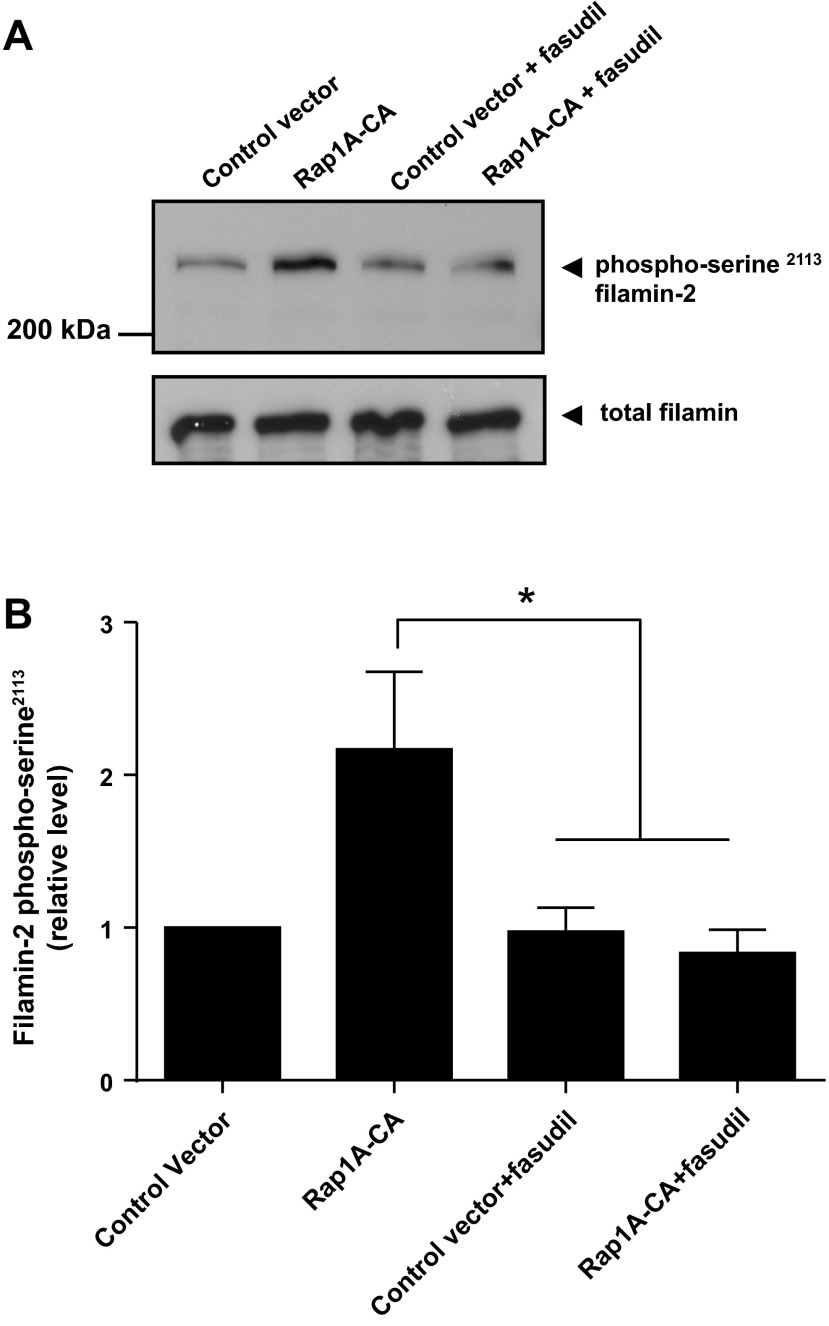
We recently demonstrated the role of Rap1A-Rho-ROCK signaling in α2C-AR translocation to the cell surface in transiently transfected HEK293 cells (19). The constitutively activated form of Rap1A (Rap1A-CA) increased cell surface receptors. This effect of Rap1A was inhibited by the ROCK inhibitor fasudil. Experiments were therefore performed to examine the role of Rap1A-Rho-ROCK in receptor translocation by filamin-2.
The filamin-2 interacting region identified in this study harbors a conserved serine at amino acid position 2113 (Ser2113; Fig. 2A). The phosphorylation of this filamin-2 Ser2113 has been shown to be necessary and sufficient for actin reorganization in non-VSM (41, 44). The role of Rap1A in this phosphorylation, however, was not previously examined. The studies therefore examined the effect of Rap1A-CA on filamin-2 Ser2113 phosphorylation status in the absence or presence of fasudil (3 μM) in HEK293 cells. Indeed, Rap1A-CA significantly increased Ser2113 phosphorylation, which was inhibited by fasudil (2.17 ± 0.51 with solvent control vs. 0.84 ± 0.15 with fasudil; n = 4–5; P < 0.05; Fig. 8, A and B).
Fig. 8.
Role of Rap1A-Rho-ROCK signaling in filamin-2 Ser2113 phosphorylation in transiently transfected HEK293 cells. A: impact of expression vector (control) or constitutively activated Rap1A (Rap1A-CA) on the phosphorylation status of filamin-2 Ser2113 in the absence (solvent control) or presence of the Rho-associated kinase (ROCK) inhibitor fasudil (3 μM) determined by Western blot analysis using phospho-Ser2113-specific antibody. B: graphical representation of data. Data were normalized to total filamin and presented as means ± SE (n = 4–5). *P < 0.05.
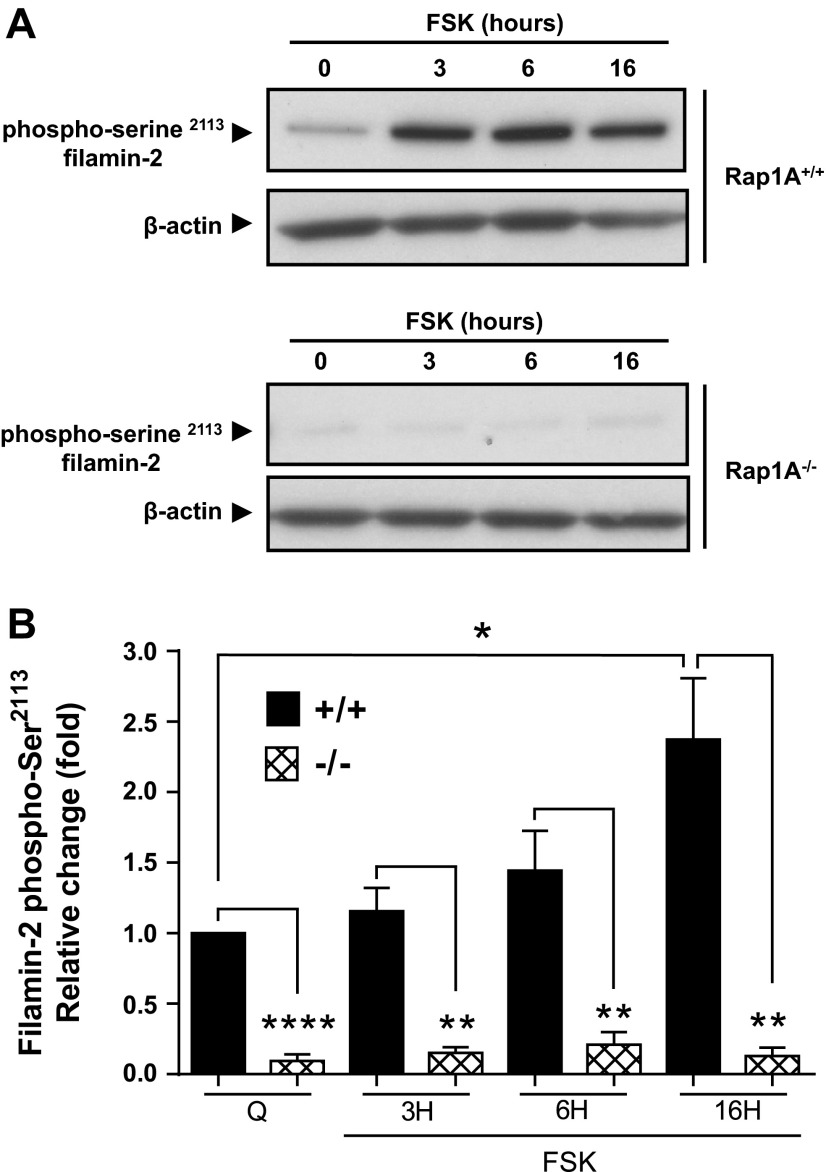
To directly examine the role of Rap1A in filamin-2 Ser2113 phosphorylation we utilized murine microVSM that were deficient in Rap1A (Rap1A−/− or Rap1A-null). We previously utilized these cells to show that Rap1A is necessary for α2C-AR expression and translocation (19). Rap1A-null microVSM showed significantly reduced receptor expression and lacked Rho-mediated F-actin formation compared with control WT cells. The expression and translocation could be rescued by introducing Rap1A-CA in the Rap1A-null background (19). Forskolin (10 μM, 0–16 h) increased filamin-2 Ser2113 phosphorylation, with highest induction at 16 h in control WT (Rap1A+/+) cells (Fig. 9, A and B). This effect of forskolin was absent in Rap1A-null cells, which showed significantly decreased levels of filamin-2 Ser2113 phosphorylation (Fig. 9, A and B). Indeed, rescue studies that involved introduction of Rap1A-CA in these cells showed increased filamin-2 Ser2113 phosphorylation (3.9 ± 0.35-fold increase compared with control vector; n = 4; P < 0.01). Similarly, introduction of Rap1A-CA in human microVSM showed increased filamin-2 Ser2113 phosphorylation (1.9 ± 0.17-fold increase compared with control vector; n = 8; P < 0.001). Together, these studies showed that Rap1A is necessary for filamin-2 Ser2113 phosphorylation in murine and human microVSM. Indeed, the regulation of α2C-AR expression and translocation is remarkably similar in these cells (19).
Fig. 9.
The small GTPase Rap1A is necessary for phosphorylation of filamin-2 Ser2113 (p-filamin-2). Time course (hours, H) of p-filamin-2 induction in murine C57BL6 WT (Rap1A+/+) or Rap1A-null (Rap1A−/−) microVSM upon treatment with the adenylyl cyclase activator forskolin (FSK, 10 μM). A: Western blot analysis examining the phosphorylation status of filamin-2 Ser2113 using phospho-Ser2113-specific antibody (β-actin was used as a loading control). B: graphical representation of time course of p-filamin-2 by FSK. Data are presented as means ± SE (n = 4). Q, quiescent. *P < 0.05, **P < 0.001, ****P < 0.0001.
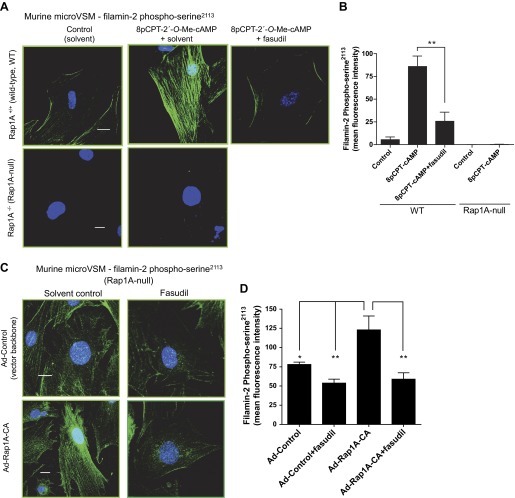
Imaging studies were performed to examine the spatial distribution of filamin-2 phospho-Ser2113 and to quantitate mean fluorescence intensity as a measure of phosphorylation status. In murine microVSM stimulated with the Epac-Rap1 activator 8p-CPT-2′-O-Me-cAMP (100 μM, 9 h), increased levels of filamin-2 phospho-Ser2113 were detected on filaments and on the cell boundary in WT cells (Fig. 10, A and B). This pattern of filamin-2 phospho-Ser2113 increase and distribution was not seen in Rap1A-null microVSM (Fig. 10, A and B). However, introduction of constitutively activated Rap1A (Rap1A-CA) in Rap1A-null microVSM showed a significant increase in filamin-2 phospho-Ser2113 intensity and distribution on filaments, which was inhibited by fasudil (Fig. 10, C and D). Similarly, in human microVSM stimulated with 8pCPT-2′-O-Me-cAMP (100 μM, 9 h), the increased intensity and filament associations were inhibited by fasudil (Fig. 10, E and F). Finally, the effect of fasudil on receptor function was tested in human microVSM (assessed by ability of cell surface activated receptors to inhibit intracellular cAMP produced by adenylyl cyclase stimulation by forskolin). Compared with solvent control (vehicle), the α2C-AR function was impaired in the presence of fasudil, consistent with the role of Rap1A-Rho-ROCK signaling in filamin-2 Ser2113 phosphorylation and intracellular retention of α2C-ARs (Fig. 10G; 54.25 ± 6.03% with 10 nM UK 14,304 vs. forskolin alone in vehicle control, P < 0.0001 (i), compared with (ii) 87.75 ± 10.08% with 10 nM UK 14,304 vs. forskolin alone in presence of fasudil, P = NS).
Fig. 10.
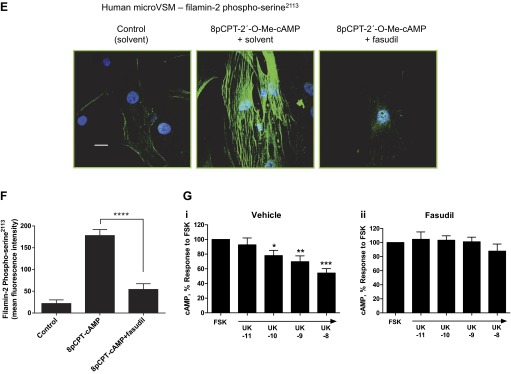
Rap1A-Rho-ROCK signaling and filamin-2 Ser2113 phosphorylation in microVSM. Spatial distribution and intensity of filamin-2 Ser2113 was examined by using phospho-Ser2113-specific antibody and confocal microscopy-fluorescence imaging. A: murine microVSM (WT) filamin-2 phospho-Ser2113 in the absence (solvent control) or presence of the ROCK inhibitor fasudil (3 μM, pretreated 30 min before and during stimulation) and in murine microVSM (Rap1A-null). Quiescent (control) microVSM were treated with the Epac-Rap1 activator 8-pCPT-2′-O-Me-cAMP (100 μM, 9 h), and cells were fixed and stained for endogenous filamin-2 phospho-Ser2113 (Alexa Fluor-488, green), and nuclei (Hoechst, blue). Scale bar = 20 μm. B: quantitation of the mean fluorescence intensity of filamin-2 phospho-Ser2113. Data from 8–9 cells per condition are shown. **P < 0.001. C: impact of introducing constitutively activated Rap1A (Rap1A-CA) on filamin-2 phospho-Ser2113 in Rap1A-null microVSM via adenovirus transductions (Ad-Control and Ad-Rap1A-CA) in the absence (solvent control) or presence of fasudil (3 μM, added ∼24 h before fixing). Scale bar = 20 μm. D: quantitation of the mean fluorescence intensity of filamin-2 phospho-Ser2113. Data from 7–9 cells per condition are shown. *P < 0.05 and **P < 0.001. E: human microVSM filamin-2 phospho-Ser2113 and ROCK. Quiescent (control) microVSM were treated with the Epac-Rap1 activator 8-pCPT-2′-O-Me-cAMP (100 μM, 9 h) in the absence (solvent control) or presence of the ROCK inhibitor fasudil (3 μM, pretreated 30 min before and during stimulation), and cells were fixed and stained for endogenous filamin-2 phospho-Ser2113 (Alexa Fluor-488, green), and nuclei (Hoechst, blue). Scale bar = 20 μm. F: quantitation of the mean fluorescence intensity of filamin-2 phospho-Ser2113. Data from 8–11 cells per condition are shown. ****P < 0.0001. G: receptor function in the (i) absence (solvent control, vehicle) or presence (ii) of the ROCK inhibitor fasudil (3 μM, pretreated 30 min before and during stimulation) was assessed by ability to inhibit forskolin (FSK) stimulated intracellular cyclic AMP accumulation in response to the α2-AR agonist UK 14,304 (UK, 10 pM - 10 nM). Basal level of cyclic AMP was elevated by FSK (5 μM, 5 min). Data are corrected for the baseline value and are presented as a percentage of the response to FSK alone and are expressed as means ± SE for n = 4 independent experiments measured in duplicate. *P < 0.05, **P < 0.001, ***P < 0.0001. The α2C-AR function is impaired in the presence of fasudil, consistent with the role of Rap1A-Rho-ROCK in filamin-2 Ser2113 phosphorylation.
DISCUSSION
In this study we demonstrate that the actin-binding protein filamin-2 associates with microVSM α2C-ARs and that this interaction plays a vital role in cell surface receptor translocation. We confirmed the specificity of this interaction by Co-IP and colocalization studies in human microVSM and by a live-cell labeling and immunoprecipation approach in heterologous HEK293 cells. These findings extend our previous observations that identified a role of F-actin in receptor translocation. Indeed, filamin colocalized with F-actin, and knockdown of filamin-2 impaired cell surface α2C-AR translocation and function. These studies show that interaction with filamin-actin is necessary for translocation and for proper function of microVSM α2C-ARs and provide a mechanism of receptor translocation by actin.
The filamin-2 interacting region identified in this study harbors a conserved serine at amino acid position 2113 (Ser2113). This serine is a physiologically important residue and phosphorylation of this residue plays a key role in actin reorganization in non-VSM (41, 44). In this study we demonstrate that phosphorylated filamin-2 phospho-Ser2113 is primarily associated with filaments and plasma membrane, consistent with previous studies in non-VSM. We further show that Rap1-Rho-ROCK signaling is necessary for the phosphorylation of filamin-2 Ser2113 in microVSM. Inhibition of this signaling by the ROCK inhibitor fasudil inhibited phosphorylation and filament distribution and impaired α2C-AR receptor function. Filamin, therefore, has a role in increased cell surface translocation of this GPCR in microVSM.
We previously demonstrated that the Ras-related small GTPase Rap1A, in addition to transcriptionally increasing α2C-AR expression, also enhances translocation of α2C-ARs to the cell surface. Our results suggested that α2C-AR expression and translocation were independent and involved two separate processes. The effect of Rap1 on translocation was mediated through the activation of Rho-ROCK signaling and the actin cytoskeleton (19). In human microVSM increased levels of intracellular cAMP or exogenous addition of the cAMP analog 8-p-CPT-2′-O-cAMP activated Rap1, leading to subsequent activation of RhoA and ROCK (Rho-ROCK) (8, 19). Rap1-Rho-ROCK increased F-actin and facilitated cell surface receptor translocation on actin filaments (19). This mechanism of regulation is not seen for α2A-ARs, which do not show protein-protein interaction with filamin-2, are not influenced by Rap1 signaling, and are ubiquitously expressed on the cell surface.
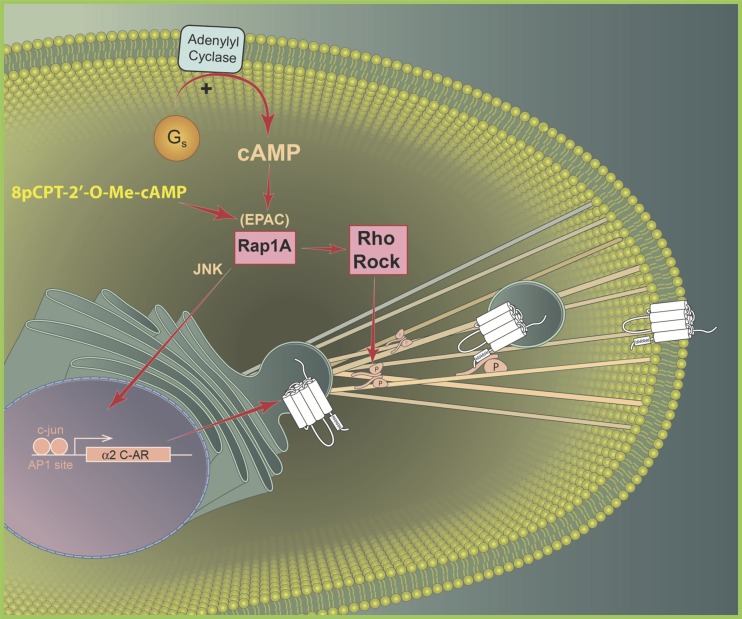
Together, our studies show that Rap1-Rho-ROCK-filamin-2 signaling regulates redistribution of functional α2C-ARs and explains the increased association of the receptor with filamin-2 upon Rap1 activation. This mechanism is summarized in Fig. 11.
Fig. 11.
Proposed intracellular signaling mechanism coupled to α2C-adrenoceptor (α2C-AR) expression and translocation in microVSM. Signaling molecules Rap1A and RhoA-ROCK implicated in α2C-AR expression and translocation in response to increased intracellular cAMP in human microVSM. According to this model activation of Rap1 by cAMP is sufficient for α2C-AR expression, loading to filaments, and translocation to the cell surface. Cell surface α2C-ARs elicit vasoconstriction to the endogenous agonist norepinephrine or the synthetic agonist UK-14,304. EPAC, exchange proteins directly activated by cyclic AMP; ROCK, RhoA kinase; JNK, c-Jun amino-terminal kinase; RRRRR, the arginine-rich region (R454–458) within the last 14 amino acids of α2C-AR carboxy terminus. Phosphorylation (P) of filamin-2 Ser2113 is necessary for receptor deployment to the cell surface. 8-pCPT-2′-O-Me-cAMP, a synthetic cAMP analog that selectively activates EPAC-Rap1 signaling.
The presence of putative motifs in close proximity in part of the transmembrane 7 and carboxy terminus of α2C-AR (last 30 amino acids; Fig. 1) suggests multiple roles in receptor mobilization. For example, the NPXXYXXF motif has an important structural role, and depending on the particular GPCR, may contribute to Golgi network targeting, cell surface targeting, and endocytosis-internalization signals. The FXXXFXXXF motif encodes an endoplasmic reticulum (ER) export signal facilitating cell surface expression (4, 37). The α2C-AR also contains a unique arginine-rich region (RRRRR). When fused to heterologous receptors, the α2C-AR (RRRRR) has a dominant-negative effect and functions as a potent ER-Golgi retention sequence, overriding ER export signals in K+ channels (27, 33). Mutating all five arginines to alanines released this effect and led to cell surface expression (33). Similar arginine-based motifs function as ER retention and retrieval signals in other receptors (5, 29, 36, 45, 46). In combination with the ER export motif in α2C-ARs, the arginine-rich region may mediate regulated delivery to the cell surface under certain physiological stimuli. For example, intracellular vesicle-mediated delivery coupled to cAMP-Rap1-Rho-ROCK-filamin-2 signaling seen in our studies. Indeed, mutation of all five arginines to alanines leads to loss of interaction with filamin-2 and to cell surface expression of functional receptors. Our studies, therefore, identify the last 14 amino acids of α2C-AR, harboring the (RRRRR) region, to be critical for filamin-2 interaction and regulated (vs. constitutive) delivery to the cell surface. In the unbound state, (RRRRR) may dominate over the adjacent conserved GPCR export signal FXXXFXXXF, thus retaining and accumulating mature intracellular α2C-ARs. The filamin-2-α2C-AR interaction links the actin cytoskeleton in mobilizing the receptor to the plasma membrane. Binding of filamin-2, an actin-binding protein, may unmask the export signal, releasing the receptor to the plasma membrane.
The findings in this study have broader implications for understanding biological mechanisms in cAMP signaling as well as thermoregulation. Divergent signaling pathways may converge on Rho-ROCK-filamin-2 to mediate receptor translocation and function. For example, the α2C-ARs have a known physiological role in human and murine cutaneous circulation response to cold temperatures (7, 17, 38). Moderate cooling to 28°C leads to increased biological activity of arteriolar α2C-ARs and to vasoconstriction. The mechanism identified includes cooling triggered release of mitochondrial reactive oxygen species, RhoA and ROCK activation, and cell surface translocation of functional α2C-AR (2, 3). This physiological response to cooling reduces loss of body heat and directs blood to vital organs to sustain a normal body core temperature (42). The findings in this study suggest cooling-triggered Rho-ROCK activation, phosphorylation of filamin-2 Ser2113, and α2C-AR cell surface translocation to be linked. Whether cooling mediates this effect on α2C-ARs through Epac-Rap1-Rho-ROCK pathway activation remains unknown. Further studies utilizing Rap1A-null microVSM will allow examination of this possibility. Altogether, the vital role of the α2C-AR COOH terminus in receptor translocation identified in this study suggests the physiological relevance of this region in thermoregulation. The α2C-ARs may have evolved to allow warm blooded, vs. cold-blooded, species to thermoregulate. Further studies are required, including phylogenetic analysis, which may shed light on the evolutionary significance of this region in mammals.
The Epac-Rap1A-Rho-ROCK-α2C-AR pathway identified in our studies may be activated at physiological temperature in certain individuals manifesting the peripheral vascular condition of cold feet and cold hands (30). Further, this pathway is activated by increased intracellular cAMP, triggered by by-products of inflammation (8), and may contribute to disease progression and severity in individuals with the peripheral vascular disorder of Raynaud's phenomenon. A deeper molecular understanding of α2C-AR-filamin-2 protein-protein interaction using computational modeling and prediction approaches (molecular docking studies) may enable testing of novel targeting approaches to disrupt this interaction and thereby receptor cell surface translocation. For example, decoy peptide or small molecule inhibitor interference, with potential therapeutic applications.
In non-VSM, filamin is necessary for proper biological activity of other GPCRs. For example, filamin is required for proper cell surface expression and function of dopamine D2 and D3 receptors, calcium-sensing receptors, metabotropic glutamate receptor type 7 (mGluR7), and calcitonin receptors, where filamin acts as a scaffolding protein to anchor receptor and signaling molecules and facilitates receptor stability and recycling from endosomes (1, 13, 16, 22–24, 34, 47). Other roles described for filamin include directing trafficking of the endoprotease furin in the trans-Golgi network/endosomal system and processing of furin substrates in the endocytic pathway (25), and a role in μ opioid receptor agonist-induced downregulation and desensitization (31).
Filamin is also known to play an important role in human development and disease. The muscle-specific subtype filamin-2 has a role in structural support and assembly of signaling complexes including sarcoglycan and contributes to skeletal muscle integrity and signaling complex necessary for muscle function. Filamin-2, therefore, plays a role in skeletal muscle dystrophy, including limb girdle muscular dystrophy (39). Indeed, the first disease-related mutation (W2710X, tryptophan to stop codon, repeat 24, dimerization domain, see Fig. 2A) was described in filamin-2 causing autosomal dominant myofibrillar myopathy (MFM, referred to as an autosomal dominant filaminopathy, a form of MFM; Ref. 43). This mutation contributes to a truncated protein that lacks the ability to dimerize correctly. This mutation, therefore, contributes to mislocalization, disruption of dimerization, and aggregate formation of filamin-2 in skeletal muscle fibers. The clinical and molecular features of this mutation resemble limb-girdle muscular dystrophy and MFM (43). Additional mutations in filamin-2 (referred to as filaminopathies, that belong to a subgroup of MFM) have since been identified and include 1) a frame shift deletion resulting in premature stop codon and nonsense-mediated mRNA decay, altogether leading to partial loss of filamin-2 protein (filamin-2 haploinsufficiency; causing distal myopathy; Ref. 14); 2) in frame 12 nucleotide deletion in repeat seven that removes four amino acids, leading to aggregation of filamin-2 (in MFM patients; Ref. 35); 3) deletion of six amino acids and insertion of two amino acids in frame in repeat seven, leading to protein accumulation (26); and 4) NH2-terminal missense mutations leading to increased association with F-actin and increased potential to aggregation (distal myopathy; Ref. 11). The in vivo effects of filamin-2 mutations on vascular α2C-AR function remain unknown. It is possible that loss in filamin-2 function may contribute to loss of α2C-AR receptor translocation to the membrane. Further work will need to be performed to test this possibility.
GRANTS
This study was supported by the Ohio State University Davis Heart and Lung Research Institute and The Research Institute at Nationwide Children's Hospital (Institutional support to M. A. Chotani), American Heart Association, Great Rivers Affiliate (Beginning Grant-in-Aid 0765204B, to M. A. Chotani), and National Heart, Lung and Blood Institute (NHLBI) Grant 1R21-HL-088087-01 (to M. A. Chotani). S. C. Jeyaraj was supported by NHLBI Institutional Training Grant T32-HL-098039-01 “Training in Pediatric and Adult Congenital Heart Disease,” and H. K. B. Motawea by a graduate training Joint Supervision Program supported by The Egyptian Cultural and Educational Bureau, Washington DC, and Egyptian Cultural and Educational Sector, Cairo, Egypt. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.K.B.M., S.C.J., A.A.A., N.A.F., and M.A.C. conception and design of research; H.K.B.M., S.C.J., A.H.E., S.M., N.T.U., and M.A.C. performed experiments; H.K.B.M., S.C.J., A.H.E., N.A.F., and M.A.C. analyzed data; H.K.B.M., S.C.J., N.A.F., and M.A.C. interpreted results of experiments; H.K.B.M., S.C.J., and M.A.C. prepared figures; H.K.B.M., S.C.J., and M.A.C. drafted manuscript; M.A.C. edited and revised manuscript; M.A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We sincerely thank Stephanie Cush for technical assistance during the making of the α2A-Gal4 construct, Dr. Orin L. Hemminger (Nikon Instruments) for technical assistance with imaging studies, and Anthony Baker, Center for Knowledge Management, The Ohio State University, for schematic drawing.
REFERENCES
- 1.Awata H, Huang C, Handlogten ME, Miller RT. Interaction of the calcium-sensing receptor and filamin, a potential scaffolding protein. J Biol Chem 276: 34871–34879, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of α2C-adrenoceptor translocation. Circ Res 94: 1367–1374, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol 289: H243–H250, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bermak JC, Li M, Bullock C, Zhou QY. Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat Cell Biol 3: 492–498, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Chang XB, Cui L, Hou YX, Jensen TJ, Aleksandrov AA, Mengos A, Riordan JR. Removal of multiple arginine-framed trafficking signals overcomes misprocessing of delta F508 CFTR present in most patients with cystic fibrosis. Mol Cell 4: 137–142, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Chotani MA, Flavahan NA. Intracellular α2C-adrenoceptors: storage depot, stunted development or signaling domain? Biochim Biophys Acta 1813: 1495–1503, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent α2C-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol 278: H1075–H1083, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Chotani MA, Mitra S, Eid AH, Han SA, Flavahan NA. Distinct cyclic AMP signaling pathways differentially regulate α2C-adrenoceptor expression: role in serum induction in human arteriolar smooth muscle cells. Am J Physiol Heart Circ Physiol 288: H69–H76, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Chotani MA, Mitra S, Su BY, Flavahan S, Eid AH, Clark KR, Montague CR, Paris H, Handy DE, Flavahan NA. Regulation of α2-adrenoceptors in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 286: H59–H67, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Daunt DA, Hurt C, Hein L, Kallio J, Feng F, Kobilka BK. Subtype-specific intracellular trafficking of α2-adrenergic receptors. Mol Pharmacol 51: 711–720, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Duff RM, Tay V, Hackman P, Ravenscroft G, McLean C, Kennedy P, Steinbach A, Schoffler W, van der Ven PF, Furst DO, Song J, Djinovic-Carugo K, Penttila S, Raheem O, Reardon K, Malandrini A, Gambelli S, Villanova M, Nowak KJ, Williams DR, Landers JE, Brown RH, Jr, Udd B, Laing NG. Mutations in the N-terminal actin-binding domain of filamin C cause a distal myopathy. Am J Hum Genet 88: 729–740, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eid AH, Chotani MA, Mitra S, Miller TJ, Flavahan NA. Cyclic AMP acts through Rap1 and JNK signaling to increase expression of cutaneous smooth muscle α2C-adrenoceptors. Am J Physiol Heart Circ Physiol 295: H266–H272, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enz R. The actin-binding protein Filamin-A interacts with the metabotropic glutamate receptor type 7. FEBS Lett 514: 184–188, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Guergueltcheva V, Peeters K, Baets J, Ceuterick-de Groote C, Martin JJ, Suls A, De Vriendt E, Mihaylova V, Chamova T, Almeida-Souza L, Ydens E, Tzekov C, Hadjidekov G, Gospodinova M, Storm K, Reyniers E, Bichev S, van der Ven PF, Furst DO, Mitev V, Lochmuller H, Timmerman V, Tournev I, De Jonghe P, Jordanova A. Distal myopathy with upper limb predominance caused by filamin C haploinsufficiency. Neurology 77: 2105–2114, 2011 [DOI] [PubMed] [Google Scholar]
- 15.He TC, Zhou S, DaCosta LT, Yu J, Kinzler K, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95: 2509–2514, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hjalm G, MacLeod RJ, Kifor O, Chattopadhyay N, Brown EM. Filamin-A binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase. J Biol Chem 276: 34880–34887, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Honda M, Suzuki M, Nakayama K, Ishikawa T. Role of α2C-adrenoceptors in the reduction of skin blood flow induced by local cooling in mice. Br J Pharmacol 152: 91–100, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeyaraj SC, Chotani MA, Mitra S, Gregg HE, Flavahan NA, Morrison KJ. Cooling evokes redistribution of α2C-adrenoceptors from Golgi to plasma membrane in transfected human embryonic kidney 293 cells. Mol Pharmacol 60: 1195–1200, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Jeyaraj SC, Unger NT, Eid AH, Mitra S, Paul El-Dahdah N., Quilliam LA, Flavahan NA, Chotani MA. Cyclic AMP-Rap1A signaling activates RhoA to induce α2C-adrenoceptor translocation to the cell surface of microvascular smooth muscle cells. Am J Physiol Cell Physiol 303: C499–C511, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krajnak K, Dong RG, Flavahan S, Welcome D, Flavahan NA. Acute vibration increases α2C-adrenergic smooth muscle constriction and alters thermosensitivity of cutaneous arteries. J Appl Physiol 100: 1230–1237, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Li B, Fields S. Identification of mutations in p53 that affect its binding to SV40 large T antigen by using the yeast two-hybrid system. FASEB J 7: 957–963, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Li M, Bermak JC, Wang ZW, Zhou QY. Modulation of dopamine D2 receptor signaling by actin-binding protein (ABP-280). Mol Pharmacol 57: 446–452, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Li M, Li C, Weingarten P, Bunzow JR, Grandy DK, Zhou QY. Association of dopamine D3 receptors with actin-binding protein 280 (ABP-280). Biochem Pharmacol 63: 859–863, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Lin R, Karpa K, Kabbani N, Goldman-Rakic P, Levenson R. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with filamin A. Proc Natl Acad Sci USA 98: 5258–5263, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu G, Thomas L, Warren RA, Enns CA, Cunningham CC, Hartwig JH, Thomas G. Cytoskeletal protein ABP-280 directs the intracellular trafficking of furin and modulates proprotein processing in the endocytic pathway. J Cell Biol 139: 1719–1733, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luan X, Hong D, Zhang W, Wang Z, Yuan Y. A novel heterozygous deletion-insertion mutation (2695–2712 del/GTTTGT ins) in exon 18 of the filamin C gene causes filaminopathy in a large Chinese family. Neuromuscul Disord 20: 390–396, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Ma D, Zerangue N, Lin YF, Collins A, Yu M, Jan YN, Jan LY. Role of ER export signals in controlling surface potassium channel numbers. Science 291: 316–319, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Maly FE, Quilliam LA, Dorseuil O, Der CJ, Bokoch GM. Activated or dominant inhibitory mutants of Rap1A decrease the oxidative burst of Epstein-Barr virus-transformed human B lymphocytes. J Biol Chem 269: 18743–18746, 1994 [PubMed] [Google Scholar]
- 29.Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABAB receptor heterodimerization. Neuron 27: 97–106, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Michel MC, Insel PA. Can you blame cold feet on Epac (and Rap1A)? Focus on “Cyclic AMP-Rap1A signaling activates RhoA to induce α2C-adrenoceptor translocation to the cell surface of microvascular smooth muscle cells”. Am J Physiol Cell Physiol 303: C488–C489, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Onoprishvili I, Andria ML, Kramer HK, Ancevska-Taneva N, Hiller JM, Simon EJ. Interaction between the μ opioid receptor and filamin A is involved in receptor regulation and trafficking. Mol Pharmacol 64: 1092–1100, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Schaak S, Devedjian JC, Paris H. Use of eukaryotic vectors for the expression of adrenergic receptors. Methods Mol Biol 126: 189–206, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Schwappach B, Zerangue N, Jan YN, Jan LY. Molecular basis for KATP assembly: transmembrane interactions mediate association of a K+ channel with an ABC transporter. Neuron 26: 155–167, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Seck T, Baron R, Horne WC. Binding of filamin to the C-terminal tail of the calcitonin receptor controls recycling. J Biol Chem 278: 10408–10416, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Shatunov A, Olive M, Odgerel Z, Stadelmann-Nessler C, Irlbacher K, van Landeghem F, Bayarsaikhan M, Lee HS, Goudeau B, Chinnery PF, Straub V, Hilton-Jones D, Damian MS, Kaminska A, Vicart P, Bushby K, Dalakas MC, Sambuughin N, Ferrer I, Goebel HH, Goldfarb LG. In-frame deletion in the seventh immunoglobulin-like repeat of filamin C in a family with myofibrillar myopathy. Eur J Hum Genet 17: 656–663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Standley S, Roche KW, McCallum J, Sans N, Wenthold RJ. PDZ domain suppression of an ER retention signal in NMDA receptor NR1 splice variants. Neuron 28: 887–898, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Tan CMBA, Nickols HH, Wang Q, Limbird LE. Membrane trafficking of G protein-coupled receptors. Annu Rev Pharmacol Toxicol 44: 559–609, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL. Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin. Am J Physiol Heart Circ Physiol 292: H1700–H1705, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Thompson TG, Chan YM, Hack AA, Brosius M, Rajala M, Lidov HG, McNally EM, Watkins S, Kunkel LM. Filamin 2 (FLN2): A muscle-specific sarcoglycan interacting protein. J Cell Biol 148: 115–126, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thukral SK, Blain GC, Chang KK, Fields S. Distinct residues of human p53 implicated in binding to DNA, simian virus 40 large T antigen, 53BP1, and 53BP2. Mol Cell Biol 14: 8315–8321, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, Kumar R. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol 4: 681–690, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Vanhoutte PM. Physical factors of regulation. In: Handbook of Physiology. The Cardiovascular System: Vascular Smooth Muscle. Bethesda, MD: American Physiological Society, 1980, sect 2, vol II, sect 2, p. 443–474 [Google Scholar]
- 43.Vorgerd M, van der Ven PF, Bruchertseifer V, Lowe T, Kley RA, Schroder R, Lochmuller H, Himmel M, Koehler K, Furst DO, Huebner A. A mutation in the dimerization domain of filamin C causes a novel type of autosomal dominant myofibrillar myopathy. Am J Hum Genet 77: 297–304, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woo MS, Ohta Y, Rabinovitz I, Stossel TP, Blenis J. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol Cell Biol 24: 3025–3035, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia H, Hornby ZD, Malenka RC. An ER retention signal explains differences in surface expression of NMDA and AMPA receptor subunits. Neuropharmacology 41: 714–723, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron 22: 537–548, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Zhang M, Breitwieser GE. High affinity interaction with filamin A protects against calcium-sensing receptor degradation. J Biol Chem 280: 11140–11146, 2005 [DOI] [PubMed] [Google Scholar]