Abstract
Flow impingement at arterial bifurcations causes high frictional force [or wall shear stress (WSS)], and flow acceleration and deceleration in the branches create positive and negative streamwise gradients in WSS (WSSG), respectively. Intracranial aneurysms tend to form in regions with high WSS and positive WSSG. However, little is known about the responses of endothelial cells (ECs) to either positive or negative WSSG under high WSS conditions. We used cDNA microarrays to profile gene expression in cultured ECs exposed to positive or negative WSSG for 24 h in a flow chamber where WSS varied between 3.5 and 28.4 Pa. Gene ontology and biological pathway analysis indicated that positive WSSG favored proliferation, apoptosis, and extracellular matrix processing while decreasing expression of proinflammatory genes. To determine if similar responses occur in vivo, we examined EC proliferation and expression of the matrix metalloproteinase ADAMTS1 under high WSS and WSSG created at the basilar terminus of rabbits after bilateral carotid ligation. Precise hemodynamic conditions were determined by computational fluid dynamic simulations from three-dimensional angiography and mapped on immunofluorescence staining for the proliferation marker Ki-67 and ADAMTS1. Both proliferation and ADAMTS1 were significantly higher in ECs under positive WSSG than in adjacent regions of negative WSSG. Our results indicate that WSSG elicits distinct EC gene expression profiles and particular biological pathways including increased cell proliferation and matrix processing. Such EC responses may be important in understanding the mechanisms of intracranial aneurysm initiation at regions of high WSS and positive WSSG.
Keywords: high wall shear stress, microarray, intracranial aneurysm initiation, vascular remodeling, spatial gradient
as the innermost lining of the blood vessel wall, endothelial cells (ECs) are the signal transduction interface between wall shear stress (WSS) exerted on the lining by flowing blood and the responses of underlying tissue. It is well known that endothelial structure, function, and gene expression are modulated by normal WSS (1.5–2.5 Pa) and that ECs respond differently when exposed to relatively low WSS (<0.4 Pa), and this effect is believed to play a role in atherosclerosis (21). However, endothelial responses under considerably higher WSS (>10 Pa) are not well characterized. Such high WSS occurs in arteries feeding arteriovenous fistulas (37, 42), in stenosed vessels (33, 34), in collateral arteries secondary to blockage (36, 50), and at arterial bifurcations, where high WSS predisposes intracranial vessels to aneurysm formation (24, 26).
There is mounting evidence that ECs are also sensitive to spatial differences in WSS over their luminal surface, specifically to streamwise gradients in WSS [WSS gradient (WSSG)]. The effects of WSSG under low WSS have been examined in vitro in flow recirculation zones, which in vivo are prone to atherogenesis (10, 56). Under low WSS and WSSG, ECs show increased permeability (31), migration (5, 46), proliferation (46), and activation of the transcription factors NF-κB, Egr-1, c-Jun, and c-FOS (28). Spatial gradients also occur in regions of very high WSS in vivo. WSSG with high WSS occurs chronically near the apices of intracranial bifurcations that are prone to aneurysm formation (2, 18), and computational modeling of patient-specific and idealized stenoses shows a combination of high WSS and WSSG near the throat of the stenosis (33, 34). The behavior of ECs exposed to WSSG when WSS is particularly high is not well defined.
Recently, the combination of high WSS and WSSG has been highlighted as a trigger for intracranial aneurysm initiation (24, 26). Both in the human disease and in animal models, aneurysms tend to develop near the apices of bifurcations in the cerebral vasculature. When flow impinges at a bifurcation, flow accelerates along the flank of the apex and then decelerates downstream, creating regions of positive and negative spatial WSSG, respectively (24, 26). Studies of surgically created bifurcations in dogs (24, 52) and of the basilar terminus in rabbits (23, 26) revealed that increased flow induces aneurysm-like destructive remodeling of the vessel wall in a well-defined microenvironment near the bifurcation apex. Specifically, internal elastic lamina degradation and smooth muscle cell loss occur under accelerating flow, where WSS is high and the spatial gradient in WSS is positive. Downstream, flow decelerates, creating negative WSSG under comparably high WSS; however, aneurysmal remodeling does not occur in the negative WSSG regions. Understanding how ECs respond to these hemodynamic conditions, especially their responses to positive and negative WSSG, may reveal novel mechanisms for regulating normal vascular function at bifurcations and its departure from homeostasis during aneurysm formation.
We previously reported that high WSS (10 Pa) compared with normal WSS (2 Pa), in the absence of a spatial gradient, induces a unique gene expression profile in ECs (8). However, intracranial aneurysms do not typically form in straight arteries even when WSS is high. They do form at bifurcations in regions of high WSS that are also under positive WSSG but not in adjacent regions of similar WSS but negative WSSG. Therefore, we hypothesize that ECs have unique responses to positive WSSG and their response to negative WSSG is considerably different. In the present study we obtain comprehensive gene expression profiles of confluent EC monolayers subjected to positive WSSG vs. negative WSSG in vitro and use this information to begin understanding differential responses in vivo, specifically at an intracranial bifurcation where aneurysmal development has been documented to occur in response to hemodynamic manipulation (9, 23, 26).
MATERIALS AND METHODS
Cell Culture
Experiments were performed using bovine aortic ECs that were cultured as previously described (25, 44). These cells maintain endothelial properties in culture as indicated by formation of monolayers with selective permeability; binding and uptake of low-density lipoprotein; tube formation on Matrigel; angiogenic migration in collagen gels; and positive staining for the endothelial markers, endothelial nitric oxide synthase, platelet endothelial cell adhesion molecule 1 (PECAM-1), VEGF receptor, and factor VIII. For exposure to flow, cells were seeded onto sterilized uncoated Fisherbrand Microscope Cover Glass (Fisher Scientific) and allowed to grow to confluence for 5 days. Twenty-four hours before exposing cells to flow, the culture medium was replaced with culture medium containing 5% FBS and supplemented with Dextran70 to increase the viscosity of the media to 3.5 cP at 37°C, equivalent to that of blood. This medium was used in all flow experiments.
Shear Stress Exposure
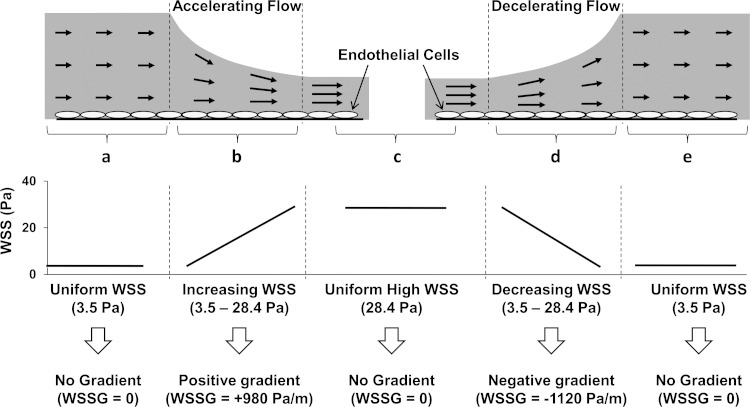
A flow chamber (Fig. 1) was used to impose high levels of shear stress and positive and negative WSSG on cultured ECs in time-matched experiments as previously described (7). In brief, flow entered between two parallel surfaces (Fig. 1a) that converged down over a 25-mm distance (Fig. 1b) to a narrower parallel section (Fig. 1c) and then diverged back over a 25-mm distance (Fig. 1d) to the original height (Fig. 1e). When media are pumped through the chamber, WSS in section a is 3.5 Pa (with zero gradient), representing WSS at the high end of baseline WSS levels. In Fig. 1b, where the height converges, WSS increased from 3.5 to 30 Pa and created a 16.2-mm length of constant positive WSSG (+980 Pa/m). In Fig. 1c, WSS is high at 28.4 Pa with zero gradient. In Fig. 1d, where the height diverges, WSS decreased from 28.4 to 1.0 Pa and created a 14.2-mm length of constant negative WSSG (−1,120 Pa/m). In Fig. 1e, the WSS recovers to the original WSS of 3.5 Pa.
Fig. 1.
Modified schematic of the flow chamber originally described in (7). a: Flow enters between 2 parallel surfaces with uniform height to produce a wall shear stress (WSS) of 3.5 Pa with zero gradient. b: Flow accelerates as the channel tapers to a narrower height, creating positive WSS gradient (WSSG; +980 Pa/m). c: Flow enters 2 more parallel surfaces to produce WSS of 28.4 Pa with zero gradient. d: Flow decelerates as the channel height increases, creating negative WSSG (−1,120 Pa/m). e: Flow recovers the original height to again produce uniform WSS of 3.5 Pa with zero gradient.
The flow chamber was connected to a medium-circulating flow loop as previously described (7, 25). Separate cover glasses containing EC monolayers were placed in the different sections of the chamber that experience the four different flow conditions represented in Fig. 1: positive WSSG, negative WSSG, 3.5 Pa with zero gradient, and 28.4 Pa with zero gradient. Note that the 3.5-Pa with-no-gradient condition occurs twice (Fig. 1, a and e); cells from these two regions were combined for analysis. After 24 h of flow exposure, cell attachment to the cover glasses was confirmed by viewing the cells under phase contrast at ×10 magnification. Total RNA was then extracted separately from each cover glass of cells using an RNeasy Mini kit (Qiagen) with an on-column genomic DNA elimination treatment. A total of 3 independent flow experiments were performed following this method, creating 12 samples for microarray analysis. The concentration and purity of each RNA sample were determined using a NanoDrop Spectrophotometer (Thermo Scientific, MA), and integrity was verified using an Agilent Bioanalyzer 2100 (Agilent Technologies, CA).
It should be noted that pressure loss across the chamber results in hydrostatic pressure being ∼15 mmHg higher in the converging segment than in the diverging segment (7). Stretching forces can modulate EC function and gene expression, and cells subjected to different pressure could be stretched to different degrees. To minimize such effects, ECs were maintained on a rigid substratum (glass coverslips) clamped against the solid backing of the chamber wall, and pump pulsation was dampened by three fluid capacitors in the flow loop so that there was no detectable pulsatile deformation of the cells or chamber. In a separate set of flow experiments (n = 3), the effects of hydrostatic pressure were assessed using RNA isolated from the two 3.5-Pa, no-gradient segments of the chamber (Fig. 1, a and e). For these experiments, RNA samples were not combined.
Microarray Analysis
Microarray processing of samples was conducted at the University at Buffalo Next-Generation Sequencing and Expression Analysis Core Facility located at the New York State Center of Excellence in Bioinformatics and Life Sciences. Briefly, RNA was transcribed to cDNA and biotin-labeled using GeneChip 3′ IVT Express Kit (Affymetrix). Each of the 12 samples was hybridized to its own GeneChip Bovine Genome Arrays (Affymetrix) containing 24,072 probe sets. Hybridized arrays were stained with streptavidin phycoerythrin conjugate and scanned using Affymetrix GeneChip Scanner and software. The raw data have been deposited into NCBI Gene under accession number GSE37127.
All analyses of microarray data were performed in R 2.13.0/Bioconductor (Gentleman 2004) with the full analysis code available on request (fjsim@buffalo.edu). Preprocessing and normalization were conducted on the Affymetrix bovine CEL files using RMA (13), and informative probe sets were determined using Factor Analysis for Robust Microarray Summarization (FARMS v1.4.1; Ref. 45). The FARMS analysis identified 7,194 probe sets, or ∼30%, that were classified as informative and were used in all further analyses. Sample-to-sample data exploration was performed using unsupervised hierarchical clustering and principle component analysis. Each group of samples was found to be clearly distinct from one another using both approaches.
Differential gene expression analysis was performed using a linear model (including all 12 samples) and employing an empirical Bayes method for calculation of statistical significance (Bioconductor, limma package; Ref. 39). Following fitting of this linear model, we identified probe sets whose expression was significantly enriched or depleted between (1) positive WSSG and negative WSSG and (2) high WSS and baseline WSS by at least twofold and was statistically significant following 5% false discovery rate (FDR) adjustment of P values. Power analyses performed using the SSPA package (51) revealed predicted powers of 41 and 59% for the two comparisons, respectively. Furthermore, we were able to determine the effect of WSSG on each gene expression by comparing positive WSSG to zero gradient (either uniform 3.5 or 28.4 Pa) and negative WSSG to the two zero gradient samples.
Pathway-Based Functional Analysis
Analyses were performed as previously described (8). In summary, the genes identified as differentially expressed between positive and negative WSSG were subdivided into four functional categories (ligand, receptor, extracellular matrix and adhesion, and enzyme) using Gene Ontology (GO)-based molecular function annotations to identify biological relationships between genes. To identify pathways of interest, hypergeometric tests were performed on both GO biological process annotations and Kyoto Encyclopedia of Gene and Genomes (KEGG) pathways. The topGO package was used to identify overrepresented GO terms using the “elim” algorithm and Fisher statistics (with 3 genes and P < 0.01) (1), and the GOstats package (P < 0.05) was used to test KEGG pathways. Roc.area (22) was used to perform receiver operating characteristic (ROC)-based area-under-curve analysis and to determine significance of GO categories (P < 0.05).
Quantitative PCR
cDNA was synthesized from samples using QuantiTect reverse transcriptase kit (Qiagen) following the manufacturer's instructions. Quantitative (q)PCR was performed with primers specific to the 36 genes listed in Table 1 and 18SrRNA. Primers were designed using Primer 3 software (MIT Whitehead and Howard Hughes Medical Institute, http://frodo.wi.mit.edu/primer3/primer3_code.html) and manufactured by Invitrogen (Grand Island, NY). The MyiQ Single-Color and CFX Connect Real-time PCR Detection Systems (Bio-Rad) were implemented to detect PCR products using SYBR Green Supermix (Bio-Rad). We used qPCR reaction conditions as described previously (15). A melting-curve analysis was performed to confirm the absence of primer dimers and other nonspecific PCR products. Samples were normalized based on 18SrRNA expression, and fold changes were determined using the 2 −ΔΔCt method (20). Primer sequences are shown in Supplemental Table S1 (Supplemental Material for this article is available online the Am J Physiol Cell Physiol website).
Table 1.
Gene expression profile of ECs under positive WSSG relative to negative WSSG*
| Symbol | Description | Expression Ratio (log2) | Adjusted P Value |
|---|---|---|---|
| ECM and Adhesion | |||
| ADAMTS1 | ADAM metallopeptidase with thrombospondin type 1 motif, 1 | +1.00 | 0.018 |
| VCAM1 | Vascular cell adhesion molecule 1 | −1.39 | 0.012 |
| BMP4 | Bone morphogenetic protein 4 | −1.21 | 0.029 |
| WISP2 | WNT1 inducible signaling pathway protein 2 | −1.11 | 0.016 |
| THBS1 | Thrombospondin 1 | −1.09 | 0.021 |
| Ligands | |||
| CXCL5 | Chemokine (C-X-C motif) ligand 5 | −1.77 | 0.049 |
| CCL26 | Chemokine (C-C motif) ligand 26 | −1.75 | 0.018 |
| CCL2 | Chemokine (C-C motif) ligand 2 | −1.59 | 0.009 |
| CSF2 | Colony stimulating factor 2 (granulocyte-macrophage) | −1.56 | 0.013 |
| TTR | Transthyretin | −1.34 | 0.018 |
| CSF1 | Colony stimulating factor 1 (macrophage) | −1.08 | 0.029 |
| Receptors | |||
| PROCR | Protein C receptor, endothelial | +1.64 | 0.016 |
| EMR3 | EGF-like module-containing mucin-like receptor 3 | +1.53 | 0.039 |
| PTPRR | Protein tyrosine phosphatase, receptor type, R | +1.07 | 0.033 |
| RTP4 | Receptor (chemosensory) transporter protein 4 | −1.08 | 0.031 |
| Enzymes | |||
| NCEH1 | Neutral cholesterol ester hydrolase 1 | +1.22 | 0.007 |
| DCK | Deoxycytidine kinase | +1.03 | 0.021 |
| TOP2A | Topoisomerase (DNA) II alpha 170 kDa | +1.00 | 0.018 |
| DHRS3 | Dehydrogenase/reductase (SDR family) member 3 | −1.07 | 0.016 |
| Other | |||
| CKAP2 | Cytoskeleton associated protein 2 | +1.29 | 0.033 |
| SMC2 | Structural maintenance of chromosomes 2 | +1.16 | 0.014 |
| HSPH1 | Heat shock 105 kDa/110 kDa protein 1 | +1.15 | 0.039 |
| SMC4 | Structural maintenance of chromosomes 4 | +1.11 | 0.018 |
| CENPF | Centromere protein F, 350/400 kDa (mitosin) | +1.07 | 0.047 |
| GAS2 | Growth arrest-specific 2 | −1.34 | 0.033 |
| FABP4 | Fatty acid binding protein 4, adipocyte | −1.26 | 0.023 |
| TAGLN | Transgelin | −1.24 | 0.021 |
| FBXO32 | F-box protein 32 | −1.22 | 0.015 |
| RPRM | Reprimo, TP53 dependent G2 arrest mediator candidate | −1.20 | 0.012 |
| PDLIM4 | PDZ and LIM domain 4 | −1.20 | 0.012 |
| LOC539374 | Similar to Family with sequence similarity 43, member A | −1.19 | 0.016 |
| NOV | Nephroblastoma overexpressed | −1.16 | 0.042 |
| FABP5 | Fatty acid binding protein 5 (psoriasis-associated) | −1.14 | 0.031 |
| CD14 | CD14 molecule | −1.12 | 0.046 |
| H4 | Histone H4 | −1.05 | 0.019 |
| PRUNE2 | Prune homolog 2 (Drosophila) | −1.02 | 0.027 |
Significantly enriched or depleted genes were identified between endothelial cells (ECs) exposed to positive wall shear stress gradient (WSSG) and negative WSSG and subdivided into functional categories based on Gene Ontology (GO)-based molecular function annotations. ECM, extracellular matrix.
Animal Surgeries
Five adult female New Zealand White rabbits underwent bilateral common carotid artery ligation to increase flow at the basilar terminus bifurcation as previously described (26). Three rabbits underwent sham surgery in which the carotid arteries were exposed but not ligated. Rabbits were euthanized by intravenous administration of 100 mg/kg of sodium pentabarbitol 5 days after the ligation (n = 5) or sham surgery. All care and procedures were performed in accordance with institutional guidelines as approved by the University at Buffalo Institutional Animal Care and Use Committee.
Flow Simulation and Mapping Hemodynamics Onto Histology
Before euthanasia, the in vivo bifurcation geometry was imaged with high-resolution three-dimensional rotational digital-subtraction cerebral angiography using a Toshiba Infinix VS-I system. Computational fluid dynamics analysis was performed on the three-dimensional image of the basilar terminus bifurcation to obtain the 5-day postligation flow fields, using as the inlet boundary condition a blood velocity of 0.867m/s, which is the average velocity in the rabbit basilar artery as measured using transcranial Doppler sonography 5 days after ligation (n = 12 rabbits). As previously described (26, 48), computational fluid dynamics analysis was conducted for each bifurcation and the luminal WSS and WSSG were mapped onto histological images. WSS was calculated from the flow velocity field, and the WSSG was calculated along the flow streamline. These hemodynamic distributions were virtually sectioned, superimposed, and morphed onto a photomicrograph of the corresponding histological section, thus allowing identification of the specific hemodynamic environment in which particular cell or molecular changes occurred.
Immunofluorescence
After animals were euthanized, their cerebrovasculature was perfused with phosphate-buffered saline via the vertebral arteries and pressure fixed at 150 mmHg with 10% formalin for 30 min. The entire brain was then removed and placed in 10% formalin for 24 h. The basilar terminus bifurcation was excised, embedded in paraffin, and sectioned longitudinally into 4-um thick sections. Adjacent slides were used for Van Gieson staining to detect internal elastic lamina and immunostaining for molecular and cellular changes. For immunofluorescent staining, sections were deparaffinized, rehydrated, and boiled in 10 mM citric buffer (pH 6.0) for antigen retrieval. Sections were incubated with goat anti-human ADAMTS1 (R&D Systems, Minneapolis, MN) and mouse anti-human Ki-67 (Dako) followed by either a donkey anti-goat or a bovine anti-mouse Dylight 649-conjugated secondary (Jackson ImmunoResearch, PA), respectively. Sections were costained with a mouse monoclonal antibody to PECAM-1 (a gift from Dr. Peter Newman of the Medical College of Wisconsin) and visualized with donkey anti-mouse rhodamine-conjugated secondary to aid in identifying the EC layer. Sections were mounted with medium containing DAPI for staining cell nuclei. Fluorescent images were taken at ×20 using a Zeiss Axio Imager Z1 microscope.
To minimize variability, immunostaining of Ki-67 and ADAMTS1 was performed at the same time for all tissue samples, and all images were acquired at the same time and captured under the same magnification, exposure, and illumination. Quantitation of images was performed using the National Institutes of Health ImageJ software. First, the background of each image was determined by calculating the mean intensity of an empty field in the image. The respective background intensity was then subtracted from each corresponding Ki-67 or ADAMTS1 image. ECs in each analyzed field were then identified based on the corresponding images of nuclei (DAPI) and an EC marker (PECAM-1). ECs that appeared to lie within the internal elastic lamina (due to sectioning artifact) were excluded from analysis to preclude counting internal elastic lamina autofluorescence. Next, the ECs were manually traced in the ADAMTS1 and Ki-67 images to segment them from the adjacent medial layer. The mean intensity of ADAMTS1 in the EC layer was then determined and averaged across samples. For Ki-67, the number of positively stained nuclei were counted and normalized to the total number of ECs present in either the positive WSSG or negative WSSG region. A paired t-test was used to determine if a statistically significant difference (P ≤ 0.05) in mean intensity existed between ECs under positive vs. negative WSSG.
RESULTS
WSSG Induces Differential Gene Expression
To form an initial understanding of the effect of spatial gradient on ECs, we used Affymetrix GeneChip microarrays to identify the gene expression profile of ECs exposed to gradient flow conditions for 24 h. A total of three independent flow experiments were conducted, each with time-matched samples from positive WSSG and negative WSSG. Using a linear model approach, we identified differentially expressed genes as those having at least twofold change in expression between compared samples and were statistically significantly regulated using an adjusted FDR P value of 5%.
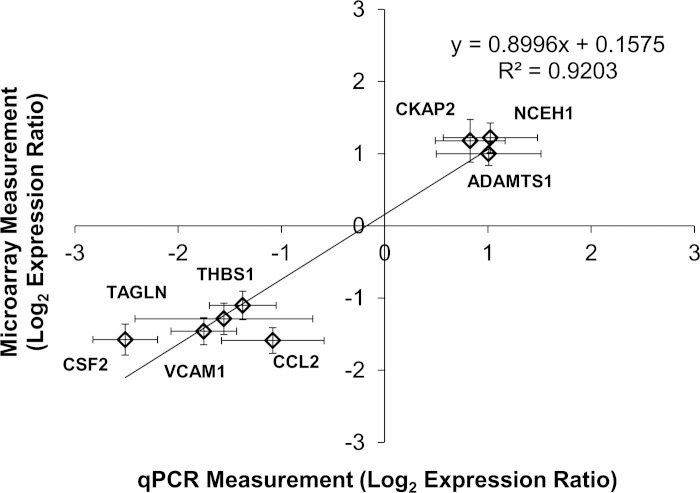
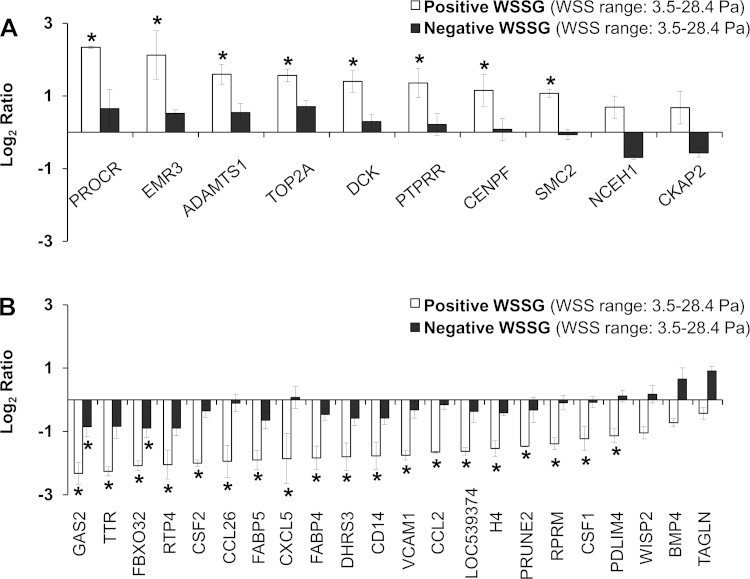
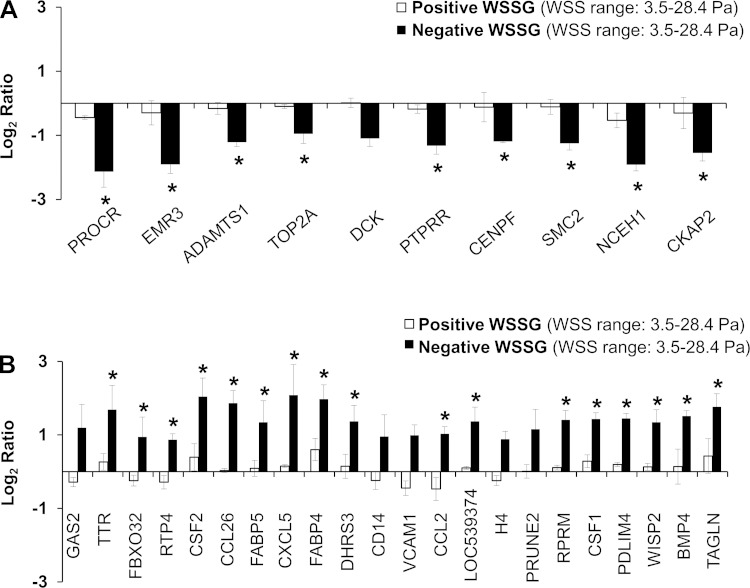
We determined the effect of WSSG on ECs by comparing cells exposed to accelerating flow with those exposed to decelerating flow (Fig. 1). In the accelerating region (Fig. 1b), cells experience a uniform positive WSSG of +980 Pa/m; cells in the decelerating region (Fig. 1d) experience the same range of WSS, but a different gradient: uniform negative WSSG of −1,120 Pa/m (7). Microarray comparison identified 36 genes that were differentially expressed between positive WSSG and negative WSSG (Table 1). Of these genes, 12 were more highly expressed in positive WSSG, while 24 were more highly expressed in negative WSSG. To confirm the differences in gene expression observed in the microarrays, quantitative real-time PCR was performed on eight of the differentially expressed genes. In agreement with the microarrays, qPCR showed upregulation of NCEH1, CKAP2, and ADAMTS1 and downregulation of CCL2, THBS1, TAGLN, VCAM1, and CSF2 in positive WSSG relative to negative WSSG (Fig. 2). Furthermore, very close agreement was found in the expression ratios between the array and qPCR results (R2 = 0.9203; P = 0.0002). Thus qPCR confirmed the accuracy of the microarray data in all cases examined, supporting the validity of the overall genomic dataset.
Fig. 2.
Quantitative (q)PCR validation of microarray results. The log2 expression ratios between positive WSSG and negative WSSG were measured by qPCR analysis for 8 selected genes and compared with log2 expression ratios obtained by microarray analysis. Each diamond represents data for 1 gene, as indicated. Line represents the least squares fit to the data (correlation coefficient R2 = 0.9203; P < 0.0002 by linear regression). Error bars are means ± SE; n = 3.
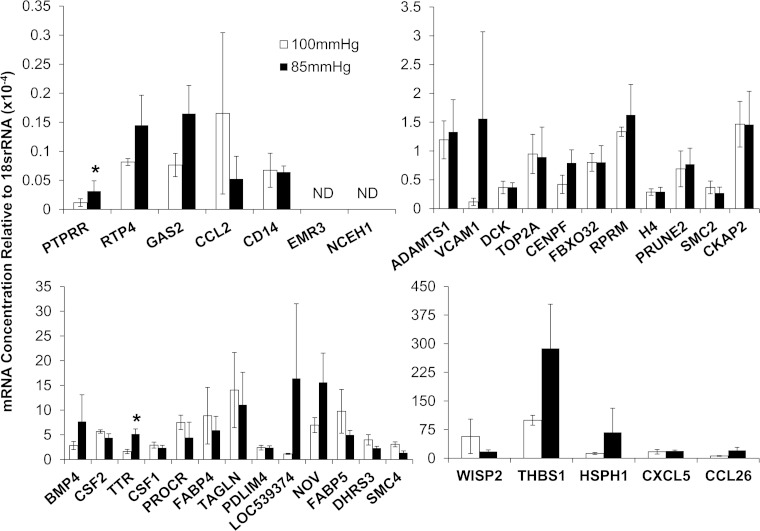
Because there was a small pressure drop across the flow chamber (7), we tested whether hydrostatic pressure could account for the observed differences in gene expression. We compared gene expression in the entry channel of the chamber with the exit channel, both of which have uniform WSS of 3.5 Pa (Fig. 1, sections a and e), but between which there is a pressure difference of 15 mmHg. qPCR revealed that only 2 of the 36 genes were significantly different (TTR, P = 0.004; and PTPRR, P = 0.005) between the two sections experiencing equal magnitudes of WSS but different pressures (Fig. 3). Two genes (NCEH1 and EMR3) were not expressed at detectable levels in either section. Thus, for at least 32 of the 36 genes that were differentially expressed between positive and negative WSSG, the pressure difference did not account for the changes in gene expression.
Fig. 3.
Endothelial cell (EC) gene expression of cells experiencing uniform WSS of 3.5 Pa at 2 different hydrostatic pressures, 100 mmHg (open bars) and 85 mmHg (solid bars), in the 2 uniform segments at the entrance and exit of the flow chamber (sections a and e, respectively). Gene expression was assayed with qPCR, and only 2 genes (TTR and PTPRR) had statistically significant differences in expression (*P < 0.05, paired t-test,). Error bars are means ± SE; n = 3. ND, transcripts expressed at nondetectable levels.
Positive WSSG Affects Endothelial Functions Related to Vascular Remodeling
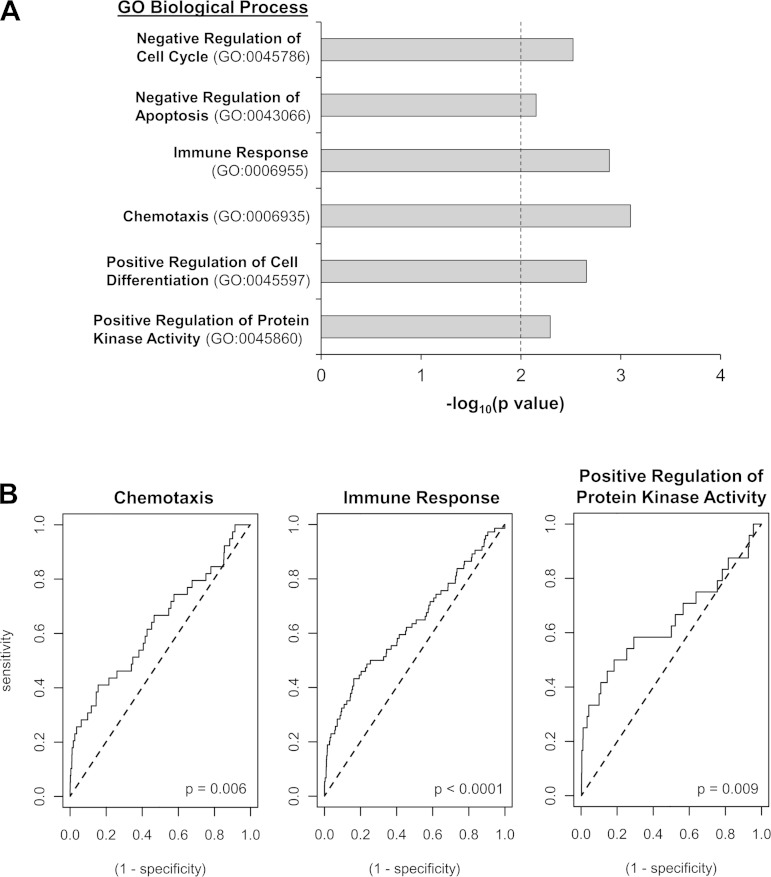
To better understand the potential consequences of WSSG on endothelial biology, we performed data mining analyses to identify key biological functions affected by WSSG. First, differentially expressed genes between positive and negative WSSG were classified according to GO-based molecular functions (Table 1). Second, we conducted an unbiased pathways analysis for overrepresented pathways in both GO (Fig. 4A) and KEGG database (Table 2). Third, we used ROC analysis (Fig. 4B) to determine if there was significant regulation of the above-identified GO pathways when all genes in the pathway were considered. These analyses suggested that positive WSSG promotes proliferation, apoptosis, and extracellular matrix processing while suppressing an inflammatory response.
Fig. 4.
Gene Ontology (GO)-based biological processes that are regulated by positive WSSG relative to negative WSSG. A: differentially expressed genes were analyzed for enriched GO biological processes; i.e., processes represented by a minimum of 3 genes and P < 0.01 cutoff (dotted line). The level of significance for each GO category is represented as the −log10 (P value), and each overrepresented category contains 3–5 downregulated genes. B: relative depletion/enrichment of gene expression across the entire pathway for each of the GO categories as determined using receiver operating characteristic (ROC) analysis. Three of the identified GO pathways were highly significantly downregulated by positive WSSG (P < 0.05, Wilcoxon rank sum test). Deflection of the line above the “line of identity” (dotted) indicates downregulation and the sharp vertical leading edge indicates that several of the most highly downregulated genes were annotated for that term.
Table 2.
Pathways downregulated by positive WSSG relative to negative WSSG
| KEGG Pathway* | Number of Downregulated Genes | Adjusted P Value |
|---|---|---|
| map04062: Chemokine signaling pathway | 4 | 0.0097 |
| map04060: Cytokine-cytokine receptor interaction | 3 | 0.0027 |
| map04640: Hematopoietic cell lineage | 3 | 0.0002 |
| map03320: PPAR signaling pathway | 2 | 0.0094 |
| map04350: TGF-β signaling pathway | 2 | 0.0213 |
| map05144: Malaria | 3 | 0.0004 |
| map05356:Rheumatoid arthritis | 4 | 0.0001 |
| map05146: Amoebiasis | 2 | 0.0354 |
Kyoto Encyclopedia of Gene and Genomes (KEGG) pathways identified from the set of differentially expressed genes between positive and negative WSSG-treated cells. Hypergeometric testing of KEGG pathways was performed using the GOstats package (minimum 2 genes included with P < 0.05).
Regarding proliferation, genes downregulated by positive WSSG were significantly enriched in the “negative regulation of cell cycle” GO category (Fig. 4A; P = 0.006). Specifically, positive WSSG downregulated RPRM, which mediates G2 arrest, and BMP4, which inhibits proliferation (3, 14). In addition, positive WSSG upregulated several genes involved in cell division (Table 1; CKAP2, SMC2, TOP2A, and CENPF) (11, 12, 32, 35). Thus positive WSSG may promote EC proliferation.
“Negative regulation of apoptosis” was also represented in the GO-based biological process analysis (Fig. 4A; P = 0.007). Specifically, genes that protect against apoptosis, CCL2, CSF2, BMP4, and THBS1 (19, 30, 40, 54), were downregulated. Decreased expression under positive WSSG of genes that inhibit apoptosis could increase the propensity for ECs to be apoptotic.
Extracellular matrix appeared in the GO-based molecular function analysis (Table 1; ADAMTS1 and THBS1). Positive WSSG upregulated ADAMTS1, an extracellular matrix protease, while downregulating TAGLN (Table 1), a repressor of collagenase (matrix metalloproteinase-9) expression (29), which would further potentiate matrix degradation.
Both GO and KEGG analyses indicated that positive WSSG affects inflammation. Positive WSSG downregulated several genes related to “immune response” (Fig. 4A; P = 0.0002) and “chemotaxis” (P = 0.0002). These two categories were also significantly affected by positive WSSG (Fig. 4B; P < 0.0001 and P = 0.006, respectively) when expression changes were considered for each gene in the pathways indentified in Fig. 4A. KEGG pathway analysis further indicated the downregulation of inflammation pathways in positive WSSG vs. negative WSSG (Table 2).
Endothelial Responses to WSSG In Vivo
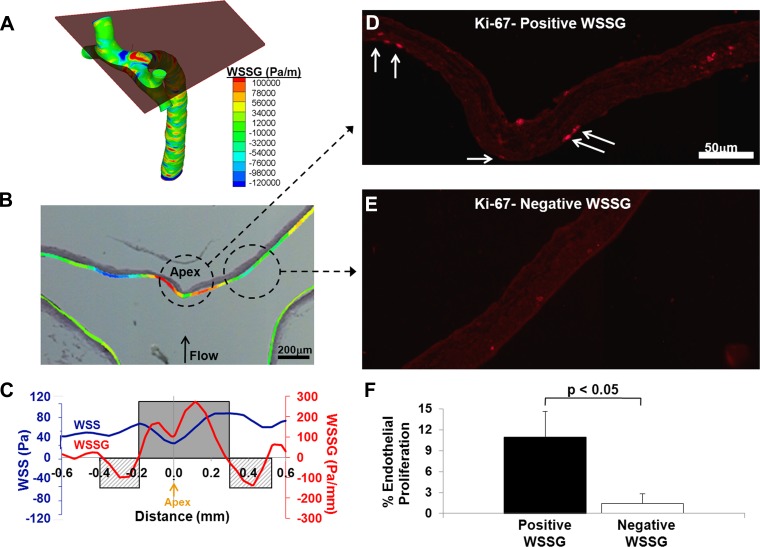
To test if ECs respond similarly to WSSG in vivo, we examined two of the WSSG-specific responses identified in the GO analysis (EC proliferation and expression of ADAMTS1) in the endothelium at the basilar terminus of rabbits. Neither Ki-67, a maker of cell proliferation, nor ADAMTS1 staining was detectable at quantifiable levels in sham-operated animals (data not shown). Therefore, these proteins were evaluated under conditions of exaggerated WSS and WSSG. Ligation of the carotid arteries increases WSS and WSSG at the basilar terminus bifurcation and leads to aneurysmal remodeling specifically in the flow acceleration zone where WSS is high and the gradient is positive (26). Five rabbits underwent carotid ligation, and the hemodynamics at their basilar bifurcations were mapped as described in the materials and methods and superimposed onto histology of the vessels (Fig. 5, A and B). Figure 5C shows WSS and WSSG at one representative bifurcation. There is a local minimum in WSS at the apex, where flow impingement occurs, and increasing WSS on either side, as flow accelerates away from the impingement. WSS maxima flank the apex, with WSS decreasing distally toward arterial baseline levels in the branches. This creates regions of positive WSSG between the minimum and maxima and negative WSSG distal to the WSS maximum, consistent with previous observations (26, 48).
Fig. 5.
EC proliferation in the rabbit basilar terminus is colocalized with positive WSSG. A: 3-dimensional WSSG distribution in the basilar bifurcation from computational fluid dynamics for a rabbit 5 days after bilateral carotid artery ligation. The plane equivalent to the plane of sectioning for the histology shown in B–E is indicated. B: overlay of the WSSG distribution from the plane in A on the image of the corresponding histological section stained with Van Gieson stain. C: plot of WSS (blue) and WSSG (red) vs. distance from the apex along the luminal surface in the cutting plane used for B–E. Regions of positive WSSG (shaded) and negative WSSG (cross-hatched) are indicated. D: representative immunofluorescent staining for Ki-67 in a region under positive WSSG, 5 days after carotid ligation. Ki-67 staining of ECs is seen along the luminal surface (arrows). E: representative staining from the negative WSSG in the same animal. F: percentage of proliferating ECs under positive and negative WSSG was scored in 5 animals. Bars indicate the average percentage ± SE; the percentage was significantly different between positive and negative WSSG (paired t-test, P < 0.05).
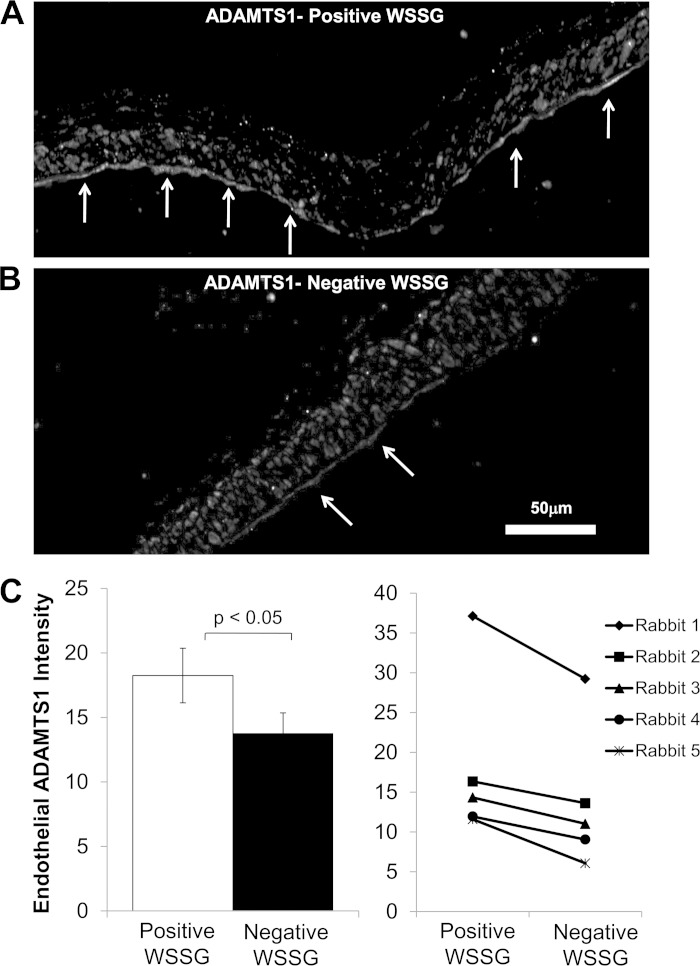
Ki-67 staining at the basilar terminus 5 days after bilateral common carotid artery ligation is shown in Fig. 5, D and E. The percentage of proliferating ECs was significantly higher (Fig. 5F; P < 0.05) in regions with positive WSSG (Fig. 5D) compared with downstream regions where WSSG was negative (Fig. 5E). Immunofluorescence staining for ADAMTS1 shows that this protein was widely distributed in the intima, media, and adventitia across the entire the bifurcation (Fig. 6, A and B). However, the section of the wall exposed to positive WSSG displayed brighter ADAMTS1 staining in the endothelial layer (Fig. 6A). In all rabbits examined, ADAMTS1 staining in ECs appeared stronger in positive WSSG regions than downstream ECs in negative WSSG regions. To confirm this subjective assessment, fluorescence intensity was quantified as described in the materials and methods. Although variability among the measured intensities for different animals was high, ECs in positive WSSG regions were brighter than in negative WSSG in every case (Fig. 6C), and the mean intensities over five experiments were significantly different between positive and negative WSSG regions (P < 0.05). These results are consistent with the bioinformatics analysis of EC responses in vitro, which indicated increased proliferation and higher expression of the ADAMTS1 gene under positive WSSG vs. negative WSSG.
Fig. 6.
Endothelial ADAMTS1 protein is increased in the positive WSSG region compared with the negative WSS region at the basilar terminus. The basilar terminus of rabbits 5 days after carotid artery ligation was stained for ADAMTS1 by indirect immunofluorescence, and regions of positive WSSG and negative WSSG were determined by mapping computational fluid dynamics onto histology as in Fig. 7. A: under positive WSSG, the endothelial layer stained strongly for ADAMTS1 (arrows). ADAMTS1 staining was also present in many cells in the media. B: under negative WSSG, ADAMTS1 staining was weak in ECs and the other cells of the vessel. C: fluorescence intensity specifically within ECs was measured as described in the materials and methods for each of the 5 animals. Average mean EC intensity was higher in positive WSSG than in negative WSSG regions. Bars represent mean intensity ± SE from 5 animals. Intensities were significantly different between positive WSSG and negative WSSG (P < 0.05, paired t-test).
High WSS Alone Affects Gene Expression
Because cells in the gradient regions experience different WSS depending on their position in the gradient, we also asked how higher WSS alone, in the absence of a spatial gradient, affected gene expression. Gene expression was compared between the two zero- gradient WSS values, 3.5 to 28.4 Pa (Fig. 1; a and e vs. c). Comparing gene expression of cells exposed to 28.4 Pa WSS with cells under 3.5 Pa WSS, we identified 155 genes that responded to WSS in the absence of gradient, with 86 genes upregulated and 69 downregulated. In a previous study, we demonstrated differential gene expression between ECs experiencing 10 and 2 Pa (8). Thirty percent of the differentially expressed genes in the previous comparison were also regulated by the much higher WSS of 28.4 vs. 3.5 Pa examined in the current study. A complete list of the genes that were differentially expressed between 28.4 and 3.5 Pa is provided in the Supplemental Materials (Supplemental Table S2).
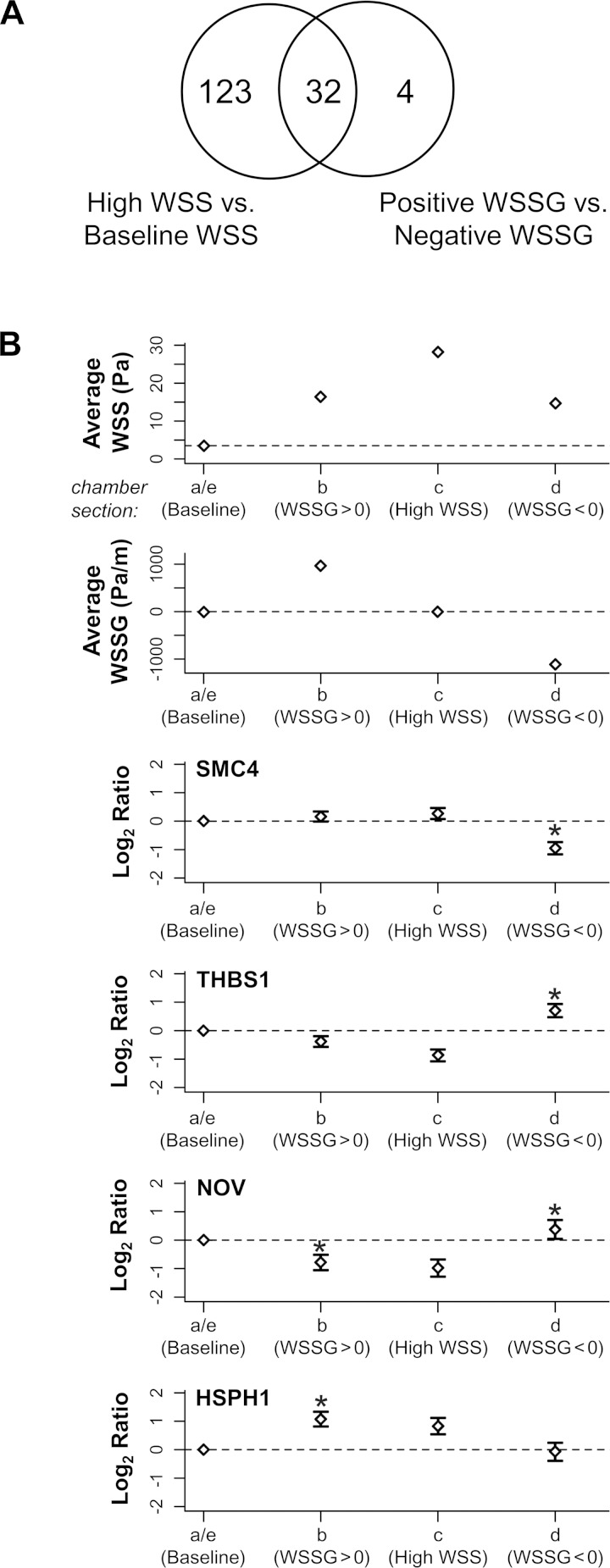
To elucidate how the presence of spatial shear stress gradient interacts with the regulatory effects of WSS, we cross-checked the genes that were differentially expressed between positive WSSG and negative WSSG against the list of genes that were modulated by spatially uniform high WSS (28.4 vs. 3.5 Pa). We found that 32 of the 36 gradient-sensitive genes were also affected by the magnitude of WSS alone (Fig. 7A). Of these 32 overlapping genes, 10 were upregulated by high WSS and 22 were downregulated.
Fig. 7.
Expression of 4 WSSG-sensitive genes that are relatively insensitive to WSS in the absence of gradient. A: Venn diagram shows the number of genes overlapping between 2 comparisons: high WSS of 28.4 Pa vs. baseline WSS of 3.5 Pa and positive WSSG vs. negative WSSG. Four genes, SMC4, HSPH1, THBS1, and NOV, are differentially expressed between positive and negative WSSG but are not significantly different (2-fold difference in expression intensities at 5% FDR) between high WSS and baseline WSS. B: expression ratios in positive WSSG (chamber, b), high WSS (c), and negative WSSG (d) were each compared with baseline WSS with WSSG = 0 (a and e; baseline levels are indicated across each panel by a dotted line). Negative WSSG significantly downregulated SMC4 and upregulated THBS1 and NOV compared with baseline WSS. The direction in which expression of SMC4, THBS1, and NOV was altered by negative WSSG was opposite to the change effected by high WSS. Positive WSSG significantly downregulated NOV and upregulated HSPH1 relative to baseline WSS, although their expression under positive WSSG was not significantly different between positive WSSG or high WSS. Each bar represents the mean intensity ± SE from 3 biological replicates. *Indicates ≥2-fold difference in expression at 5% FDR from baseline WSS.
Four of the genes differentially expressed in positive vs. negative WSSG were not significantly affected by WSS (i.e., they did not exhibit ≥2-fold change at a 5% FDR; Fig. 7A). Specifically, SMC4, THBS1, NOV, and HSPH1 displayed log fold-changes of 0.27 (P = 0.16), 0.−87 (P = 0.001), −0.90 (P = 0.01), and 0.83 (P = 0.01), respectively, when subjected to 28.4 vs. baseline WSS of 3.5 Pa. However, each of these four genes did exhibit a significant response to either positive or negative WSSG compared with the zero-gradient, baseline condition (Fig. 7B). For example, negative WSSG decreased expression of SMC4 compared with 3.5 Pa and increased it for THBS1 and NOV (Fig. 7B). Although, THBS1 and NOV were downregulated by almost two-fold by high WSS compared with baseline (log2 fold changes close to −1), exposure to negative WSSG upregulated these two genes. Positive WSSG also increased expression of HSPH1 and NOV relative to 3.5 Pa. These results suggest that ECs can sense positive and negative WSSG in addition to WSS. Differential gene expression between positive and negative WSSG could arise because one gradient stimulates or inhibits expression or by a combination of effects from both gradients.
Interaction Between WSSG and WSS
We next explored how WSSG interacted with WSS by comparing expression of the 32 overlapping genes under gradient conditions against uniform WSS conditions. Because cells under shear stress gradient experience a range of WSS values, there is no single WSS to use as a reference. Therefore, we compared gene expression under positive and negative WSSG against the two zero-gradient references, baseline WSS of 3.5 Pa (Fig. 8) at the low-end, and uniform WSS of 28.4 Pa (Fig. 9) at the high end.
Fig. 8.
Gene expression is affected differently by positive WSSG and negative WSSG relative to WSS = 3.5 Pa and zero gradient. A: expression under positive WSSG (open bars) and negative WSSG (solid bars) was compared with baseline WSS (3.5 Pa) with WSSG = 0 for all 10 WSSG-sensitive genes that were also upregulated by high WSS. Note that expression under positive WSSG was significantly higher for 8 of the 10 genes, but expression in negative WSSG was not higher than baseline WSS for any of the 10 genes. B: same comparisons were made for the 22 WSSG-sensitive genes that were downregulated by high WSS. Expression under positive WSSG was lower compared with baseline WSS. In contrast, expression under negative WSSG was not significantly different from the baseline for all of the genes except GAS2 and FBXO32. Each bar represents the mean intensity ± SE from 3 flow experiments. *Expression difference relative to the baseline WSS of 3.5 Pa was ≥2-fold difference at 5% FDR.
Fig. 9.
Gene expression is affected differently by positive WSSG and negative WSSG relative to WSS = 28.4 Pa and zero gradient. A: expression under negative WSSG (solid bars) and positive WSSG (open bars) was compared with high WSS (28.4 Pa) with WSSG = 0 for all 10 WSSG-sensitive genes that were also upregulated by high WSS. Note that expression under negative WSSG was significantly different from expression in high WSS for all genes except 1, DCK, yet expression in positive WSSG was not significantly higher than high WSS for any of the 10 genes. B: same comparisons were made for the 22 WSSG-sensitive genes that were downregulated by high WSS. Expression under negative WSSG was significantly different from the high WSS for all of the genes except GAS2, CD14, VCAM1, H4, and PRUNE2. In contrast, expression under positive WSSG was not significantly different from expression in high WSS for any of the 22 genes. Each bar represents the mean intensity ± SE from 3 flow experiments. *Expression difference relative to high WSS of 28.4 Pa was ≥2-fold at 5% FDR.
Negative WSSG tends to antagonize the effects of WSS.
For comparison with the low-end WSS of 3.5 Pa (Fig. 1, a and e), we first considered the 10 WSSG-sensitive genes that were upregulated by high WSS without gradient (Fig. 8A) and found that all 10 genes were upregulated by the positive WSSG (open bars), with 8 out of 10 exhibiting ≥ 2 fold-change (5% FDR). This upregulation was expected, given that all WSS values in the positive gradient regions (Fig. 1b) were >3.5 Pa. However, for cells under the same range of WSS, but with a negative gradient (Fig. 1d), genes were not consistently upregulated, with none showing significantly different expression from 3.5 Pa (Fig. 8A, solid bars). This suggests that negative WSSG counteracted the tendency for these genes to be upregulated by higher WSS.
Analogous behavior was observed for the 22 WSSG-sensitive genes that were downregulated by high WSS (Fig. 8B): 19 of them had lower expression under positive WSSG (with WSS = 3.5–28.4 Pa) compared with 3.5 Pa (open bars). This downregulation was again expected, since all WSS values in the gradient section were >3.5 Pa. However, only 2 genes (GAS2 and FBXO32) were significantly downregulated under negative WSSG (solid bars), while the remaining 20 genes were note significantly downregulated. Thus negative WSSG appeared to suppress the downregulation of these genes by high WSS.
Taken together, these observations suggest that negative WSSG may antagonize the effect of high WSS on gene expression.
Positive WSSG tends to augment the effects of WSS.
For comparison with the high-end WSS, a complementary effect was observed (Fig. 9). For the genes that were upregulated by high WSS alone, expression levels for 9 out of 10 were significantly lower under negative WSSG, where WSS ranged from 3.5–28.4 Pa, than in uniform high-end WSS of 28.4 Pa (Fig. 9A, solid bars). This is expected, given that all WSS values in the negative gradient region were <28.4 Pa (Fig. 1, c and d), thus lowering the expression for genes upregulated by high WSS. However, expression by cells under positive WSSG of the same WSS range of 3.5–28.4 Pa was not significantly different from the expression of high WSS alone, for any of the 10 genes (Fig. 9A, open bars). This suggests that positive WSSG increased the expression of these genes to levels equivalent to the high WSS condition alone, even though the cells in the gradient were exposed to lower WSS.
For the 22 genes that were downregulated by uniform 28.4 Pa WSS alone, expression of all but 5 was significantly higher in the negative WSSG region than in 28.4 Pa WSS with zero gradient (Fig. 9B, solid bars). This indicates that WSS between 3.5 and 28.4 Pa did not downregulate expression as much as the uniform high WSS of 28.4 Pa. On the other hand, under positive WSSG (Fig. 9B, open bars) none of the 22 genes was significantly lower than under high WSS alone. This suggests that positive gradient downregulated these genes to levels equivalent to the high-end WSS condition even though the cells in the gradient were exposed to lower WSS.
Taken together, these observations suggest that positive WSSG may augment the effect of high WSS on gene expression.
DISCUSSION
This study demonstrates that ECs discriminate between accelerating and decelerating flow, as indicated by the differential gene expression profiles under positive and negative WSSG. Such hemodynamics occur in vivo at stenoses and bifurcation apices, locations that are prone to highly localized vascular pathologies (6). Our examination of gene expression under flow identified relatively few differentially expressed genes influenced by WSSG. Although sample size was limiting, power analysis revealed that we were able to identify 41% of all differentially expressed genes, suggesting that only a total of 85–90 genes would be regulated by WSSG. This relatively small number suggests that the response of ECs to WSSG is restricted to specific cellular processes and signaling cascades. Specifically, bioinformatic analyses indicate that positive WSSG can induce gene expression that could promote matrix remodeling, proliferative, apoptotic and anti-inflammatory functions in ECs.
Matrix Remodeling by Positive WSSG and High WSS
Induction of matrix remodeling by positive WSSG and high WSS could contribute to intracranial aneurysm formation (24, 26, 52) and vulnerable plaque rupture (6). Aneurysm formation is an active remodeling process characterized by loss of the internal elastic lamina, media layer thinning, and bulge formation, which requires degradation of extracellular matrix (16, 43). Similarly, rupture of a vulnerable plaque requires degradation of extracellular matrix in the fibrous cap (38). Both pathologies localize to high WSS/positive WSSG microenvironments (24, 26, 33, 34). In the current study, endothelial expression of ADAMTS1 was increased under positive WSSG compared with negative WSSG in vitro. Furthermore, quantitative image analysis permitted assessment of endothelial ADAMTS1 in vivo in a rabbit model of hemodynamically induced aneurysm initiation (9, 15, 23, 26), where the precise localization and small size of the affected tissues preclude measurement by Western blotting or qPCR. By comparing regions of positive vs. negative WSSG within the same tissue sections, we observed increased endothelial ADAMTS1 protein during aneurysm formation specifically in a zone of positive WSSG where aneurysms preferentially initiate. ADAMTS1 protein degrades extracellular matrix proteins including aggrecan, versican and thrombospondin, and thus flow-induced ADAMTS1 might be an important initiator of aneurysm formation.
Upregulation of Proliferation and Apoptosis by WSSG
Gene expression analysis suggests that positive WSSG favors EC proliferation. Proliferation could be important for maintaining EC numbers under the chronic mechanical insult of high WSS and WSSG at bifurcations. Wright (55) found that endothelial mitosis in guinea pig aortas is greatest near bifurcations and proposed that proliferation around bifurcations is a source of replacement cells as ECs turn over through natural damage and senescence. Sho et al. (36) found that high flow not only induces EC proliferation, but that bromodeoxyuridine-labeled ECs disproportionately accumulate in the distal portion of arteries subjected to elevated flow, suggesting that high flow (and thus high WSS) causes ECs to migrate in the direction of flow. Syzmanski et al. (44) also found downstream migration of ECs when exposed to positive WSSG under high WSS conditions in vitro. Thus ECs proliferating in the positive WSSG region at bifurcation apices might migrate downstream to replenish cells in the arterial tree.
In addition to upregulating genes associated with proliferation, positive WSSG also modulated genes to favor apoptosis. ECs exhibit both increased terminal transferase-mediated dUTP nick end-labeling staining and bromodeoxyuridine incorporation under positive WSSG in vitro (7) and we observed apoptotic cells in the periapical region of basilar bifurcations during aneurysm initiation (15), although those cells were not definitively identified as ECs. Under positive WSSG, increased proliferation and apoptosis would increase EC turnover, which could contribute to aneurysm initiation by causing transient gaps in the endothelium or diverting ECs from producing signals that maintain quiescence in underlying smooth muscle. Furthermore, high levels of turnover coupled with matrix remodeling might weaken the arterial wall and make it more susceptible to mechanical damage.
Inflammation Response in ECs under WSSG
We previously reported that high WSS of 10 Pa in the absence of gradient induces anti-inflammatory responses in ECs (8), and similar results were found at 28.4 Pa in the current study. Furthermore, the current study found that gradient also affects anti-inflammatory genes: positive WSSG appears to augment the effects of high WSS, whereas negative WSSG antagonizes them. The in vivo implications of anti-inflammatory effects under positive WSSG are unclear. On one hand, suppressing inflammatory infiltration could prevent unnecessary matrix degradation in response to minor mechanical damage, which might be common at bifurcations, where WSS is typically always higher than straight arterial segments. On the other hand, inhibiting inflammatory responses may suppress desirable repair mechanisms (27, 41). Notably, during the early stages of hemodynamic-induced aneurysm initiation at the rabbit basilar terminus, no macrophages or neutrophils were found in the damaged region, despite increased matrix metalloproteinase expression and obvious matrix degradation (15). Downregulation of endothelial cytokines might be suppressing the attraction and attachment of inflammatory cells that normally accompanies injury.
EC Sensing of WSSG
Although the ability of endothelial cells to respond to spatial gradient in WSS is gaining more attention, it is currently unknown how the gradient is “felt” by the endothelium. One hypothesis is that because adjacent cells in a gradient experience different WSS, flow acceleration (positive WSSG) creates a stretching force within the luminal surface (6, 17, 52), whereas deceleration (negative WSSG) creates compression. These forces may modulate responses to WSS by adding to or subtracting from tension on the cytoskeleton and cell adhesions arising from WSS dragging the luminal surface (6, 7). Added stretch would explain our observation that positive WSSG may augment many responses to high WSS alone. Similarly, compression by negative WSSG would explain its apparent antagonism of high-WSS responses. WSSG similarly augments/antagonizes the effects of high WSS on EC proliferation, apoptosis, and alignment to flow (7).
However, we also found at least four genes that responded to WSSG while not being significantly affected by WSS alone, i.e., differences between samples did not reach our threshold criterion of a twofold change in expression with an adjusted 5% FDR. This suggests that WSSG may trigger a different mechanism. A critical difference between WSSG and WSS is that WSSG creates force differentials that would be felt within the plane interfacing with flow, whereas uniform WSS drags the entire interface, so its force is felt across the endothelium (between the dragged surface and the sheet's attachments). Thus we speculate that WSSG might act on stretch-activated ion channels or molecules in apical junctions. A +980 Pa/m gradient acting along the long dimension of a sheet of 10 μm × 30 μm ECs produces ∼9 pN shear force between two successive cells, which is in a range capable of changing protein conformation (17). Candidates include the junctional protein PECAM-1, which is stretched by uniform WSS to expose a regulatory phosphorylation site (49), and gap- and tight-junction proteins, Cx43 and ZO-1, which are disrupted in ECs under spatial gradients in low WSS conditions (4, 47). WSSG sensing mechanisms is an unexplored area of EC biology that requires further study.
Effects of High WSS Alone
We previously examined EC gene expression under high WSS without gradient, comparing 10 and 2 Pa (8). Interestingly, only 30% of those genes were differentially expressed between 28.4 and 3.5 Pa in the current study. This suggests that ECs do not simply respond to WSS proportionately, with larger responses at higher WSS magnitudes. Instead, entirely different responses might be induced in different WSS regimes. Both we (8) and White et al. (53) reported that high WSS induces expression patterns that are quite distinct from baseline WSS, which in turn affects ECs very differently from below-normal WSS. Thus different magnitudes of WSS turn different responses on or off, and important thresholds may exist in vivo that distinguishes among trophic and protective flow, repair and remodeling environments, and pathology-inducing conditions.
Future Considerations
Although blood flow is pulsatile in vivo, we deliberately performed our in vitro experiments under steady flow conditions to isolate the effect of spatial WSSG without the confounding influence of temporal changes or possible pulsation-induced mechanical stretching. It should be noted that pulsatile flow would create a transient temporal gradient in WSS with each pulsation, and this, in turn, would create short-lived spatial gradients as each pulse passes. We do not know if such additional hemodynamic forces would accentuate, mask, or have no interaction with the effects of our static gradients on EC gene expression. Nonetheless, at least two of the responses identified under static WSSG in vitro, EC proliferation and increased ADAMTS1, also occurred in vivo. In future studies we wish to examine the response of ECs to spatial gradients under more physiological flow profiles.
In conclusion, ECs respond to positive and negative WSSG at high WSS, expressing distinct gene profiles. Positive WSSG promotes gene expression patterns suggestive of proliferative, apoptotic, matrix remodeling, and anti-inflammatory responses in cultured ECs, and at least some of these responses occur under positive WSSG/high WSS conditions in vivo. The responses of ECs to this hemodynamic environment provide insight into the mechanism of aneurysm initiation and may also play a role in atherosclerotic plaque rupture and normal endothelial homeostasis at arterial bifurcations.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01-NS-064592 (to H. Meng) and the American Society for Quality Biomedical Division Dr. Richard J. Schlesinger Grant (to J. Dolan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.D., H.M., and J.K. conception and design of research; J.M.D. performed experiments; J.M.D. and F.J.S. analyzed data; J.M.D., H.M., F.J.S., and J.K. interpreted results of experiments; J.M.D. prepared figures; J.M.D. drafted manuscript; J.M.D., H.M., F.J.S., and J.K. edited and revised manuscript; J.M.D., H.M., F.J.S., and J.K. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Jianping Xiang for teaching computational fluid dynamics and histology-hemodynamics comapping, Vincent Tutino for assistance with figures and Nicholas Liaw and Max Mandelbaum for conducting the animal surgeries and for stimulating discussions. We thank Dr. Song Liu for initial input and guidance in getting these studies off the ground. We also acknowledge Dr. Te-Chung Lee for use of laboratory equipment and the assistance of Wade Sigurdson and the Confocal Microscope and Flow Cytometry Core Facility at the University of Buffalo.
REFERENCES
- 1.Alexa A, Rahnenfuhrer J, Lengauer T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22: 1600–1607, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Alnaes MS, Isaksen J, Mardal KA, Romner B, Morgan MK, Ingebrigtsen T. Computation of hemodynamics in the circle of Willis. Stroke 38: 2500–2505, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Corriere MA, Rogers CM, Eliason JL, Faulk J, Kume T, Hogan BL, Guzman RJ. Endothelial Bmp4 is induced during arterial remodeling: effects on smooth muscle cell migration and proliferation. J Surg Res 145: 142–149, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DePaola N, Davies PF, Pritchard WF, Jr, Florez L, Harbeck N, Polacek DC. Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc Natl Acad Sci USA 96: 3154–3159, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DePaola N, Gimbrone MA, Jr, Davies PF, Dewey CF., Jr Vascular endothelium responds to fluid shear stress gradients. Arterioscler Thromb 12: 1254–1257, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Dolan JM, Kolega J, Meng H. High wall shear stress and spatial gradients in vascular pathology. Ann Biomed Eng 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolan JM, Meng H, Singh S, Paluch R, Kolega J. High fluid shear stress and spatial shear stress gradients affect endothelial proliferation, survival, and alignment. Ann Biomed Eng 39: 1620–1631, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolan JM, Sim FJ, Meng H, Kolega J. Endothelial cells express a unique transcriptional profile under very high wall shear stress known to induce expansive arterial remodeling. Am J Physiol Cell Physiol 302: C1109–C1118, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao L, Hoi Y, Swartz DD, Kolega J, Siddiqui A, Meng H. Nascent aneurysm formation at the basilar terminus induced by hemodynamics. Stroke 39: 2085–2090, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med 112: 1018–1031, 1988. [PubMed] [Google Scholar]
- 11.Harvey SH, Krien MJ, O'Connell MJ. Structural maintenance of chromosomes (SMC) proteins, a family of conserved ATPases. Genome Biol 3: REVIEWS3003, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt SV, Vergnolle MA, Hussein D, Wozniak MJ, Allan VJ, Taylor SS. Silencing Cenp-F weakens centromeric cohesion, prevents chromosome alignment and activates the spindle checkpoint. J Cell Sci 118: 4889–4900, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeffery TK, Upton PD, Trembath RC, Morrell NW. BMP4 inhibits proliferation and promotes myocyte differentiation of lung fibroblasts via Smad1 and JNK pathways. Am J Physiol Lung Cell Mol Physiol 288: L370–L378, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Kolega J, Gao L, Mandelbaum M, Mocco J, Siddiqui AH, Natarajan SK, Meng H. Cellular and molecular responses of the basilar terminus to hemodynamics during intracranial aneurysm initiation in a rabbit model. J Vasc Res 48: 429–442, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krex D, Schackert HK, Schackert G. Genesis of cerebral aneurysms–an update. Acta Neurochir (Wien) 143: 429–448; discussion 448–429, 2001. [DOI] [PubMed] [Google Scholar]
- 17.LaMack JA, Friedman MH. Individual and combined effects of shear stress magnitude and spatial gradient on endothelial cell gene expression. Am J Physiol Heart Circ Physiol 293: H2853–H2859, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Lindekleiv HM, Valen-Sendstad K, Morgan MK, Mardal KA, Faulder K, Magnus JH, Waterloo K, Romner B, Ingebrigtsen T. Sex differences in intracranial arterial bifurcations. Gend Med 7: 149–155, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Das AM, Seideman J, Griswold D, Afuh CN, Kobayashi T, Abe S, Fang Q, Hashimoto M, Kim H, Wang X, Shen L, Kawasaki S, Rennard SI. The CC chemokine ligand 2 (CCL2) mediates fibroblast survival through IL-6. Am J Respir Cell Mol Biol 37: 121–128, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282: 2035–2042, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Mason SJ, Graham NE. Areas beneath the relative operating characteristics (ROC) and relative operating levels (ROL) curves: Statistical significance and interpretation. Quart J Royal Meteorolog Soc 128: 2145–2166, 2002. [Google Scholar]
- 23.Meng H, Metaxa E, Gao L, Liaw N, Natarajan SK, Swartz DD, Siddiqui AH, Kolega J, Mocco J. Progressive aneurysm development following hemodynamic insult. J Neurosurg 114: 1095–1103, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Meng H, Wang Z, Hoi Y, Gao L, Metaxa E, Swartz DD, Kolega J. Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke 38: 1924–1931, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metaxa E, Meng H, Kaluvala SR, Szymanski MP, Paluch RA, Kolega J. Nitric oxide-dependent stimulation of endothelial cell proliferation by sustained high flow. Am J Physiol Heart Circ Physiol 295: H736–H742, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metaxa E, Tremmel M, Natarajan SK, Xiang J, Paluch RA, Mandelbaum M, Siddiqui AH, Kolega J, Mocco J, Meng H. Characterization of critical hemodynamics contributing to aneurysmal remodeling at the basilar terminus in a rabbit model. Stroke 41: 1774–1782, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol 36: 1031–1037, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Nagel T, Resnick N, Dewey CF, Jr, Gimbrone MA., Jr Vascular endothelial cells respond to spatial gradients in fluid shear stress by enhanced activation of transcription factors. Arterioscler Thromb Vasc Biol 19: 1825–1834, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Nair RR, Solway J, Boyd DD. Expression cloning identifies transgelin (SM22) as a novel repressor of 92-kDa type IV collagenase (MMP-9) expression. J Biol Chem 281: 26424–26436, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Pallero MA, Elzie CA, Chen J, Mosher DF, Murphy-Ullrich JE. Thrombospondin 1 binding to calreticulin-LRP1 signals resistance to anoikis. FASEB J 22: 3968–3979, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phelps JE, DePaola N. Spatial variations in endothelial barrier function in disturbed flows in vitro. Am J Physiol Heart Circ Physiol 278: H469–H476, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Roca J. Topoisomerase II: a fitted mechanism for the chromatin landscape. Nucleic Acids Res 37: 721–730, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schirmer CM, Malek AM. Computational fluid dynamic characterization of carotid bifurcation stenosis in patient-based geometries. Brain Behav 2: 42–52, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schirmer CM, Malek AM. Wall shear stress gradient analysis within an idealized stenosis using non-Newtonian flow. Neurosurgery 61: 853–863; discussion 863–854, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Seki A, Fang G. CKAP2 is a spindle-associated protein degraded by APC/C-Cdh1 during mitotic exit. J Biol Chem 282: 15103–15113, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Sho E, Komatsu M, Sho M, Nanjo H, Singh TM, Xu C, Masuda H, Zarins CK. High flow drives vascular endothelial cell proliferation during flow-induced arterial remodeling associated with the expression of vascular endothelial growth factor. Exp Mol Pathol 75: 1–11, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Shumacker HB., Jr Aneurysm development and degenerative changes in dilated artery proximal to arteriovenous fistula. Surg Gynecol Obstet 130: 636–640, 1970. [PubMed] [Google Scholar]
- 38.Slager CJ, Wentzel JJ, Gijsen FJ, Schuurbiers JC, van der Wal AC, van der Steen AF, Serruys PW. The role of shear stress in the generation of rupture-prone vulnerable plaques. Nat Clin Pract Cardiovasc Med 2: 401–407, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article3, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Southwood M, Jeffery TK, Yang X, Upton PD, Hall SM, Atkinson C, Haworth SG, Stewart S, Reynolds PN, Long L, Trembath RC, Morrell NW. Regulation of bone morphogenetic protein signalling in human pulmonary vascular development. J Pathol 214: 85–95, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Sporn MB, Roberts AB. Peptide growth factors and inflammation, tissue repair, and cancer. J Clin Invest 78: 329–332, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stehbens WE. Chronic changes in experimental saccular and fusiform aneurysms in rabbits. Arch Pathol Lab Med 105: 603–607, 1981. [PubMed] [Google Scholar]
- 43.Stehbens WE. Histopathology of cerebral aneurysms. Arch Neurol 8: 272–285, 1963. [DOI] [PubMed] [Google Scholar]
- 44.Szymanski MP, Metaxa E, Meng H, Kolega J. Endothelial cell layer subjected to impinging flow mimicking the apex of an arterial bifurcation. Ann Biomed Eng 36: 1681–1689, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talloen W, Clevert DA, Hochreiter S, Amaratunga D, Bijnens L, Kass S, Gohlmann HW. I/NI-calls for the exclusion of non-informative genes: a highly effective filtering tool for microarray data. Bioinformatics 23: 2897–2902, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Tardy Y, Resnick N, Nagel T, Gimbrone MA, Jr, Dewey CF., Jr Shear stress gradients remodel endothelial monolayers in vitro via a cell proliferation-migration-loss cycle. Arterioscler Thromb Vasc Biol 17: 3102–3106, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Thi MM, Kojima T, Cowin SC, Weinbaum S, Spray DC. Fluid shear stress remodels expression and function of junctional proteins in cultured bone cells. Am J Physiol Cell Physiol 284: C389–C403, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Tremmel M, Xiang J, Natarajan SK, Hopkins LN, Siddiqui AH, Levy EI, Meng H. Alteration of intra-aneurysmal hemodynamics for flow diversion using enterprise and vision stents. World Neurosurg 74: 306–315, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431, 2005. [DOI] [PubMed] [Google Scholar]
- 50.van Everdingen KJ, Klijn CJ, Kappelle LJ, Mali WP, van der Grond J. MRA flow quantification in patients with a symptomatic internal carotid artery occlusion. Dutch EC-IC Bypass Study Group. Stroke 28: 1595–1600, 1997. [DOI] [PubMed] [Google Scholar]
- 51.van Iterson M, t Hoen PA, Pedotti P, Hooiveld GJ, den Dunnen JT, van Ommen GJ, Boer JM, Menezes RX. Relative power and sample size analysis on gene expression profiling data. BMC Genomics 10: 439, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Kolega J, Hoi Y, Gao L, Swartz DD, Levy EI, Mocco J, Meng H. Molecular alterations associated with aneurysmal remodeling are localized in the high hemodynamic stress region of a created carotid bifurcation. Neurosurgery 65: 169–177; discussion 177–168, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White SJ, Hayes EM, Lehoux S, Jeremy JY, Horrevoets AJ, Newby AC. Characterization of the differential response of endothelial cells exposed to normal and elevated laminar shear stress. J Cell Physiol 226: 2841–2848, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams GT, Smith CA, Spooncer E, Dexter TM, Taylor DR. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature 343: 76–79, 1990. [DOI] [PubMed] [Google Scholar]
- 55.Wright HP. Endothelial mitosis around aortic branches in normal guinea pigs. Nature 220: 78–79, 1968. [DOI] [PubMed] [Google Scholar]
- 56.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res 53: 502–514, 1983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.