Abstract
Mice deficient for general control nondepressible-2 (Gcn2) either globally or specifically in the liver display reduced capacity to maintain glucose homeostasis during fasting, suggesting the hypothesis that GCN2 may regulate gluconeogenesis (GNG), which normally plays a key role maintaining peripheral glucose homeostasis. Gcn2-deficient mice exhibit normal insulin sensitivity and plasma insulin but show reduced GNG when administered pyruvate, a gluconeogenic substrate. The basal expression of phosphoenolpyruvate carboxykinase, a rate-limiting enzyme in GNG, is abnormally elevated in Gcn2 knockout (KO) mice in the fed state but fails to be further induced during fasting. The level of tricarboxylic acid cycle intermediates, including malate and oxaloacetate, and the NADH-to-NAD+ ratio are perturbed in the liver of Gcn2 KO mice either in the fed or fasted state, which may directly impinge upon GNG. Additionally, the expression of the CCAAT enhancer-binding protein-β (C/EBPβ) in the liver fails to be induced in Gcn2 KO mice after 24 h fasting, and the liver-specific Cebpβ KO mice show reduced fasting GNG similar to that seen in Gcn2-deficient mice. Our study demonstrates that GCN2 is important in maintaining GNG in the liver, which is likely to be mediated through regulation of C/EBPβ.
Keywords: general control nondepressible-2, gluconeogenesis, glucose homeostasis
the liver plays a central role in maintaining glucose homeostasis, particularly during fasting, when the liver converts glycerol or amino acids into glucose by the glycogenolytic and gluconeogenic pathways. Hepatic gluconeogenesis (GNG) is primarily regulated by the hormonal actions of insulin, glucagon, and cortisol (1, 14, 29, 30). Glucagon, which is secreted by the endocrine pancreas under low glucose conditions, stimulates a cAMP-signaling pathway in the liver to activate cAMP response element-binding protein (CREB), which in turn induces the expression of downstream enzymes of the GNG pathway. Insulin acts to suppress GNG during the fed state, but as insulin levels decline during fasting this repression is released. In addition to being the primary site of glucose production via release of glycogen stores and synthesis of glucose by GNG, the liver is a major source of amino acids during short-term fasting. Proteasomal-dependent degradation of hepatic proteins generates amino acids (38), which are then used to generate new proteins and glucose in the liver.
The eukaryotic initiation factor 2α (eIF2α) translational control pathway has been implicated in regulating hepatic glucose production through genetic analysis of mice containing mutations in the regulatory phosphorylation site of eIF2α (31). We have investigated the potential role of the general control nondepressible-2 (GCN2) eIF2α kinase in regulating the fasting response in the liver. GCN2 was initially discovered in yeast as the key sensor of amino acid deprivation (12, 17, 22, 39), which triggers amino acid biosynthesis through the translational control of GCN4, a transcriptional activator of amino acid synthesis genes. In mammals, GCN2 is expressed in most tissues but predominately in the liver, skeletal muscle, and the brain (33, 41). GCN2 is required for fetal development. Fewer Gcn2 knockout (KO) mouse pups are born or survive when their mothers are deprived of essential amino acids during pregnancy (41). Leucine deprivation in adult Gcn2 KO mice leads to severe fatty liver and enhanced loss of adipose tissue (15), suggesting that GCN2 may also play a broader role in regulating the fasting response. In addition, the CCAAT enhancer-binding protein β (C/EBPβ) was previously shown to regulate GNG during fasting (3, 10, 19) and also regulates the response to amino acid limitation (7, 26, 37) in conjunction with GCN2 (15). In this study, we investigated the regulatory role of GCN2 and C/EBPβ in GNG using mice with global or hepatic specific knockout mutations in the GCN2 or C/EBPβ genes.
MATERIALS AND METHODS
Animals, diets, and experiments.
Wild-type C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Gcn2 KO mice with C57BL/6J background were generated as previously described (28). Liver-specific Gcn2 knockout (LiGcn2 KO) mice were generated by crossing Albumin-Cre transgenic mice to a floxed allele of Gcn2 (41). Liver-specific Cebpβ knockout mice (LiCebpβ KO) were generated by crossing Albumin-Cre transgenic mice to a floxed allele of Cebpβ (34) provided by Dr. Esta Sterneck (National Cancer Institute). All mice were maintained on a 12:12-h light-dark cycle and were provided free access to 22% (kcal/100 kcal) fat rodent chow (5020; LabDiet, St. Louis, MO) and tap water before the experiments. Male mice around 6 mo old (body weight: 40 ± 5 g) were used exclusively unless otherwise indicated. Metabolic and biochemical measurements for fed (ad libitum) mice were performed on blood or organs sampled at 3 h after light cycle (ca. 10:00 A.M.). For the glucose tolerance test (GTT), mice were fasted overnight (14 h) and injected intraperitoneally with glucose solution (2 g/kg). For the insulin tolerance test (ITT), mice were fasted for 4 h and injected intraperitoneally with human insulin (0.75 U/kg; Eli Lilly, Indianapolis, IN). For pyruvate or glycerol loading to determine GNG, mice were fasted for 24 h and injected intraperitoneally with 1.5 g/kg sodium pyruvate or 1.5 g/kg glycerol, respectively. The GTT, ITT, pyruvate, and glycerol GNG experiments were conducted after methods previously described for measurement of these metabolic parameters in mice (25, 42). Blood glucose was measured from tail blood using a OneTouch Ultra glucometer (LifeScan, Milpitas, CA). All animal experiments were approved by the Pennsylvania State University Institutional Animal Care and Use Committee.
Serum and liver metabolites measurement.
Mice were killed by CO2 euthanization following the recommended procedure of the American Veterinary Medical Association (AVMA Euthanasia Panel Report) and the Office of Laboratory Animal Welfare of the National Institutes of Health. Although these procedures minimize stress associated with CO2 euthanization, some of the metabolic parameters measured may be impacted. Plasma was obtained from blood samples collected with Microvette CB 300LH (SARSTEDT, Nümbrecht, Germany). Posteuthanization, tissues were resected, snap-frozen in liquid nitrogen, and stored at −80°C. Plasma insulin and leptin were measured using the mouse insulin/leptin assay kit (MSD, Rockville, MD). Liver malate, oxaloacetate (OAA), lactate, and pyruvate contents were measured with assay kits from BioVision (Milpitas, CA). cAMP was measured with a cAMP Direct Immunoassay Kit (Abcam, Cambridge, MA). All assays were performed according to the manufacturer's instructions. Liver glycogen was extracted and hydrolyzed with amyloglucosidase (Sigma, St. Louis, MO) as previously described (28), and glucose release was evaluated by glucose assay reagent (Sigma).
Primary hepatocyte isolation.
Primary hepatocytes were isolated from livers of neonatal mice as previously described (11, 21). Briefly, the whole liver was taken and cut into 1- to 2-mm small pieces in Hanks' balanced salt solution buffer and shaken at 37°C for 5 min in HBSS buffer with 5 mM EDTA and then for 20 min in HBSS buffer containing 2.5 mg/ml collagenase type I (Sigma), 0.1 mg/ml deoxyribonuclease I, type IV (Sigma), and 5 mM CaCl2. The resulting cell suspension was poured through the nylon mesh and centrifuged at 250 g for 2 min. The cell pellet was washed two times with DMEM with 10% FBS and then resuspended in M199 medium with Earle salts (Invitrogen, Grand Island, NY), supplemented with 10% FBS, 2.0 mM l-glutamine, 20 ng/ml human epidermal growth factor (BD Biosciences, San Jose, CA), 50 nM bovine insulin (Sigma), and antibiotics. Cells were seeded in BioCoat Cellware, collagen I-coated 12-well plates (BD Biosciences, San Jose, CA) at a density of 5 × 104 cells/well in 1 ml M199 medium for 1–2 days before the experiments.
Glucose production assay.
Glucose production assay was performed on primary hepatocytes as previously described (18). The M199 medium was replaced with 500 μl glucose production buffer consisting of glucose-free DMEM (pH 7.4), without phenol red (Invitrogen), supplemented with 20 mmol/l sodium lactate, 2 mmol/l sodium pyruvate, and with a combination of 500 nM dexamethasone and 0.1 mM 8-(4-chloro-phenylthio)-cAMP (Sigma) for stimulation of glucose production. After 4 h of incubation, an aliquot of supernatant was sampled, and glucose concentration was measured with the Glucose Assay Reagent (Sigma). Glucose production was expressed as the amount of glucose (μg) produced in 1 ml of medium.
RNA isolation and relative quantitative RT-PCR.
RNA was extracted with the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA) from tissues or cell cultures. RNA was quantitated by the Quant-It RiboGreen RNA Assay Kit (Invitrogen, Eugene, OR). RNA (1 μg) was used for reverse transcription with qScriptTM cDNA supermix (Quanta, Gaithersburg, MD) to generate cDNA in a 20-μl reaction volume. Quantitative RT-PCR was performed with the qPCR core kit for SYBR Green I (Eurogentec, Fremont, CA) by the 7000 Sequence detection system (Applied Biosystems, Foster City, CA). Gapdh was coamplified and used as an internal normalization of all other gene mRNAs. No significant differences were seen in Gapdh mRNA levels between Gcn2 or Cebpβ genotypes. Primers used for real-time PCR are listed in Table 1.
Table 1.
Primers used for real-time PCR
| Forward | Reverse | |
|---|---|---|
| Gapdh | GTCGGTGTGAACGGATTTGG | GACTCCACGACATACTCAGC* |
| Cebpb | AAGAGCCGCGACAAGGC | GTCAGCTCCAGCACCTTGTG |
| Ppargc1a | TCTGGGGTCAGAGGAAGAGAG | GTCAACAGCAAAAGCCACAA |
| Pepck | GTGCTGGAGTGGATGTTCGG | CTGGCTGATTCTCTGTTTCAGG |
| G6pc | TCTTGTGGTTGGGATACTGG | GCAATGCCTGACAAGACTC |
| Pcx | AATGTCCGGCGTCTGGAGTA | ACGCACGAAACACTCGGAT |
| Me1 | GACCCGCATCTCAACAAGGAC | AGATACCTGTCGAAGTCAGAGTT |
Cebp, CCAAT enhancer-binding protein; Ppar, peroxisome proliferator-activated receptor; Pepck, phosphoenolpyruvate carboxykinase; G6pc, glucose 6-phosphate; Pcx, pyruvate carboxylase; Me1, malic enzyme 1.
All primer sequences are listed from 5′ to 3′.
Western blot analysis.
Whole liver lysates were extracted with RIPA buffer containing protease inhibitor cocktail (Sigma) and phosphatase inhibitor cocktail 1, 2 (Sigma). Primary antibodies for phosphoenolpyruvate carboxykinase (PEPCK), C/EBPβ, CREB-1, phospho-CREB-1 (Ser129) (Santa Cruz Biotech, Santa Cruz, CA), eIF2α and eIF2α(pS52) (Invitrogen, Camarillo, CA), and α-tubulin (Sigma) were used.
Data analysis.
All data are expressed as means ± SE, including numbers of mice as per strain-time. The two-tailed Student's test was used to evaluate statistical differences between paired samples, and two-way ANOVA with replication was performed to analyze measurements obtained by time course.
RESULTS
Phosphorylation of eIF2α by GCN2 in the liver is induced by fasting. Mice were subjected to short-term or long-term fasting, and liver samples were assessed by Western blot for phosphorylation status of Ser51 of eIF2α, the regulatory phosphorylation site and target of the eIF2α kinases. A linear increase in phosphorylated eIF2α was observed across a 24-h fasting period (Fig. 1A). The induction of eIF2α phosphorylation in the liver is seen in both Gcn2 KO and wild-type mice over a 72-h period but is substantially lower at each time point in Gcn2 KO mice, suggesting that fasting-induced eIF2α phosphorylation is largely, but not exclusively, dependent upon GCN2, particularly at the 72-h time point (Fig. 1B).
Fig. 1.
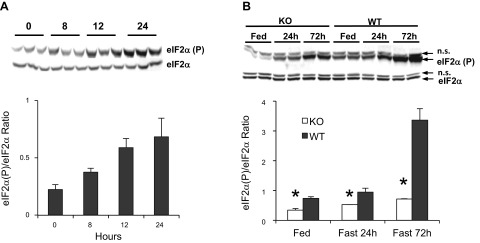
Phosphorylation of translation factor eukaryotic initiation factor 2α (eIF2α) is induced in the liver during fasting and is dependent upon general control nondepressible-2 (GCN2). A: phosphorylated and total eIF2α protein in liver lysates from fed wild-type mice and after 8, 12, and 24 h of fasting (time point 0:3 h after light cycle). Top, Western blot; bottom, ratio of phosphorylated to total eIF2α protein. B: immunoblot of phosphorylated and total eIF2α protein in liver lysates from fed Gcn2 knockout (KO) and wild-type (WT) mice and after 24 and 72 h of fasting. Top, Western blot; bottom, ratio of phosphorylated to total eIF2α protein (mean ± SE, n = 3; *P < 0.001, KO vs. WT by 2-way ANOVA for Fed, Fast 24 h and Fast 72 h comparisons; ns, nonspecific band).
GNG is impaired in Gcn2 KO mice during starvation, without impacting glucose tolerance. Gcn2 KO mice show relatively low blood glucose at 24 and 48 h after fasting (Fig. 2A), suggesting deficient glucose production or an alteration in glucose tolerance or insulin sensitivity. Liver glycogen content was not significantly altered in either the fed or fasted state in Gcn2 KO mice (Fig. 2B), indicating the glycogen storage in the fed state and glycogenolysis in the fasted state is normal. In contrast, Gcn2 KO mice exhibit reduced glucose production from exogenous pyruvate, a glucogenic substrate (Fig. 2C), implicating a deficiency in GNG. However, no genotypic differences were seen in GNG when glycerol was administered to fasted mice (Fig. 2D), suggesting that the GNG deficiency in Gcn2 KO mice lies within the early part of the GNG pathway before the generation of glycerol 3-phosphophate. Insulin levels, insulin sensitivity, and glucose tolerance were all normal in Gcn2 KO, suggesting that reduced fasting glucose levels and deficient GNG were not caused by changes in serum insulin levels or insulin sensitivity of peripheral tissues (Fig. 3, A–C). Although Gcn2 KO mice exhibited normal insulin sensitivity in the initial glucose clearance seen in the ITT test (Fig. 3B), the glucose recovery phase, which is dependent upon GNG, was retarded.
Fig. 2.
Fasting serum glucose levels and gluconeogenesis are impaired in Gcn2 KO mice. A: blood glucose of fed (0) WT and Gcn2 KO mice and after 8, 12, and 24 h of fasting (mean ± SE, n ≥ 24/strain-time; P = 0.004, KO vs. WT by 2-way ANOVA). B: liver glycogen content of fed and 24-h-fasted mice of indicated genotypes (mean ± SE, n = 8). C: blood glucose levels as a function of time after ip injection of sodium pyruvate in mice of indicated genotypes (mean ± SE, n ≥ 12; P < 0.001, KO vs. WT by 2-way ANOVA). D: blood glucose levels as a function of time after ip injection of glycerol in mice of indicated genotypes (mean ± SE, n = 6; no significant difference, KO vs. WT by 2-way ANOVA).
Fig. 3.
GCN2-deficient mice exhibit normal insulin, glucose clearance, and insulin sensitivity. A: plasma insulin levels in the fed and 24-h-fasted mice of indicated genotypes (mean ± SE, n ≥ 24). B: blood glucose levels as a function of time (expressed as a percentage of the starting level, KO starting blood glucose value: 168 ± 8 mg/dl; WT starting value: 150 ± 3 mg/dl) after ip injection of insulin in mice of indicated genotypes (mean ± SE, n ≥ 6; P < 0.001, KO vs. WT by 2-way ANOVA). C: blood glucose levels as a function of time after ip injection of glucose in mice of indicated genotypes (mean ± SE, n ≥ 12; no significant difference, KO vs. WT by 2-way ANOVA).
GCN2 deficiency leads to imbalanced energy homeostasis.
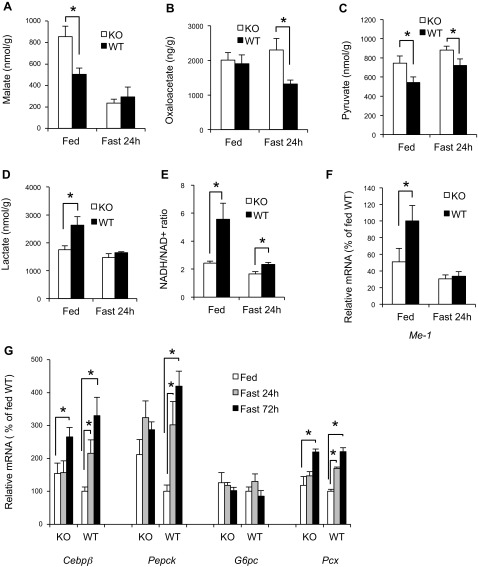
We measured the levels of key tricarboxylic acid (TCA) cycle intermediates and other metabolites that are known to influence hepatic GNG. We found a 69% increase in malate in the liver of Gcn2 KO mice in the fed state (Fig. 4A) but no difference in the fasted mice, whereas OAA levels were normal in fed Gcn2 KO mice but 74% higher than wild type in the fasted state (Fig. 4B). The pyruvate and lactate content also exhibited significant differences between genotypes in either the fasted or fed state (Fig. 4, C and D). The ratio of lactate to pyruvate, a surrogate measurement of the NADH-to-NAD+ ratio, was less than half of normal (Fig. 4E), indicating that Gcn2 KO mice may have altered energy homeostasis, although ATP levels were not different (data not shown). The mRNA encoding cytosolic malic enzyme-1, which is important for both production of NADPH and for regulating pyruvate cycling (5), was also found to be expressed at a lower level in the fed state in Gcn2 KO mice (Fig. 4F). In addition, the gene expression of gluconeogenic regulatory proteins, including Pepck and Cebpβ mRNAs, was both abnormally high in Gcn2 KO mice and failed to be induced after 24 h fasting (Fig. 4G). PEPCK protein levels also exhibited high basal expression and an absence of induction in the liver of Gcn2 KO mice (Fig. 5A). GNG and Pepck expression is induced in part by cAMP, which in turn stimulates the phosphorylation of CREB. We found that cAMP and phospho-CREB levels were significantly reduced in fasted Gcn2 KO mice (Fig. 5, B and C), and this may partially explain the lack of PEPCK induction.
Fig. 4.
Tricarboxylic acid cycle intermediates, reducing equivalents, and gene expression are perturbed in the liver of Gcn2 KO mice. A: liver malate content in fed and 24-h-fasted mice of indicated genotypes (mean ± SE, n = 8; *P < 0.05 as bracket indicated). B: liver oxaloacetate content in fed and 24-h-fasted mice of indicated genotypes (mean ± SE, n = 8; *P < 0.05 as indicated). C: liver pyruvate content in fed and 24-h-fasted mice of indicated genotypes (mean ± SE, n ≥ 8; *P < 0.05 as indicated). D: liver lactate content in fed and 24-h-fasted mice of indicated genotypes (mean ± SE, n ≥ 8, *P < 0.05 as indicated). E: NADH-to-NAD+ ratio (calculated from ratio of lactate/pyruvate level) in livers of fed and 24-h-fasted mice of indicated genotypes (mean ± SE, n ≥ 8; *P < 0.05 as indicated). F: liver malic enzyme-1 (Me1) mRNA in fed and 24-h-fasted mice of indicated genotypes (normalized to fed WT mice, mean ± SE, n = 8; *P < 0.05 as indicated). G: expression of Cebpβ, phosphoenolpyruvate carboxykinase (Pepck), G6pc, pyruvate carboxylase (Pcx), and Pdk4 mRNAs in livers of fed and 24-h- and 72-h-fasted mice of indicated genotypes (normalized to fed WT mice, mean ± SE, n = 8; *P < 0.05 as indicated).
Fig. 5.
PEPCK, cAMP, and phospho-cAMP response element-binding protein (CREB) levels are altered in the liver of Gcn2 KO mice. A: Western blotting of PEPCK protein in liver lysates from fed and 72-h-fasted Gcn2 KO and WT mice. Top, Western blot; bottom, PEPCK protein relative to tubulin (mean ± SE, n = 3; *P < 0.05 as indicated). B: cAMP level in livers of 24-h-fasted mice of indicated genotypes. cAMP levels are expressed as pmol/g of liver (mean ± SE, n = 4; *P < 0.05, Gcn2 KO vs. WT). C: CREB and phospho (p)-CREB protein levels in the liver of fed and 72-h-fasted Gcn2 KO and WT mice. Left, Western blot; right, ratio of phosphorylated CREB to total CREB protein (mean ± SE, n = 3; *P < 0.05 as indicated).
GCN2 and C/EBPβ expression in the liver regulates GNG. To determine if the metabolic differences seen in Gcn2 KO mice are due to functions of GCN2 in the liver, liver-specific Gcn2 KO mice (LiGcn2 KO) were generated by crossing the Albumin-Cre transgenic deletor strain to a floxed allele of Gcn2. We estimated the recombination deletion efficiency to be 69% in the liver of LiGcn2 KO mice. LiGcn2 KO exhibited normal fasting glucose, but similar to global Gcn2 KO mice GNG was impaired as demonstrated by significantly lower glucose synthesis from pyruvate administration (Fig. 6A). The expression of Pepck and G6pc mRNA was normal in LiGcn2 KO mice in the fed and fasted states, whereas basal expression of Cebpβ was abnormally high and failed to be further induced by fasting (Fig. 6B).
Fig. 6.
Liver-specific Gcn2 KO (LiGcn2 KO) mice and Gcn2-deficient primary hepatocytes display misregulation of gluconeogenesis. A: blood glucose levels as a function of time after ip injection of sodium pyruvate in LiGcn2 KO and WT mice after 24 h fasting (mean ± SE, n ≥ 6; P = 0.003, KO vs. WT by 2-way ANOVA). B: expression of Cebpβ, G6pc, and Pepck mRNAs in livers of LiGcn2 KO and WT mice after 24 h fasting (normalized to fed WT mice, mean ± SE, n = 4; *P < 0.05 as indicated). C: glucose production of primary Gcn2 KO and WT hepatocytes in glucose production medium supplemented with sodium pyruvate and cAMP or control medium (mean ± SE, n ≥ 4; *P < 0.05 as indicated). D: expression of Pepck, G6pc, Pgc1α, and Cebpβ mRNAs in primary Gcn2 KO and WT hepatocytes in glucose production assay (normalized to control group, mean ± SE, n = 4; *P < 0.05 as indicated). E: expression of CEBPβ mRNA in livers of fed and 24-h-fasted WT and Gcn2 KO mice (normalized to fed WT mice, mean ± SE, n = 8; *P < 0.05 as indicated). F: C/EBPβ (LAP) protein from liver lysates of fed and 24-h-fasted mice of indicated genotypes. Top, Western blot; bottom, CEBPβ protein relative to tubulin (mean ± SE, n = 3; *P < 0.05 as indicated).
To directly assess the GCN2-dependent regulation of GNG in the liver, hepatocytes were isolated from neonatal Gcn2 KO and wild-type mice, and glucose production assays were performed. The Gcn2 KO primary hepatocytes exhibited a modest reduction in glucose production from pyruvate substrate stimulated by cAMP (Fig. 6C). The expression of gluconeogenic genes was examined in these primary hepatocytes, and the induction of Pepck, glucose-6-phosphatase (G6pase), and Cebpβ by cAMP and pyruvate was diminished in Gcn2 KO hepatocytes (Fig. 6D). Overall, these data support the hypothesis that reduced GNG in fasting Gcn2 KO mice is largely due to a GCN2 deficiency in the liver, and specifically in hepatocytes.
C/EBPβ is a likely candidate for mediating GCN2-dependent regulation of GNG because it was previously shown that the eIF2α pathway regulates C/EBPβ expression (6), and mice deficient for C/EBPβ exhibit impaired GNG (3, 10, 19). We found that C/EBPβ mRNA and protein failed to be induced in the liver of Gcn2 KO mice (Fig. 6, E and F). To further probe the hepatic function of C/EBPβ, liver-specific Cebpβ KO (LiCebpβ KO) mice were generated using a floxed Cebpβ mouse (34) crossed to the Albumin-Cre deletor strain. The ablation of Cebpβ expression in LiCebpβ KO mice was evident in a 72-h fasting experiment where Cebpβ mRNA was virtually undetectable in contrast to robust expression in the wild type (Fig. 7A). The LiCebpβ KO mice showed normal random fed blood glucose levels, but blood glucose levels were significantly reduced after the 24-h fasting period (Fig. 7B). Glucose production from exogenous pyruvate was significantly reduced in LiCebpβ KO mice (Fig. 7C) similar to Gcn2-deficient mice. LiCebpβ KO mice exhibited altered gene expression profiles in the fed and/or fasted state for Pepck and G6pc (Fig. 7D). However, glucose clearance was normal in LiCebpβ KO mice, as assessed by GTT (Fig. 7E), suggesting that the perturbance in glucose homeostasis is unrelated to the action of insulin.
Fig. 7.
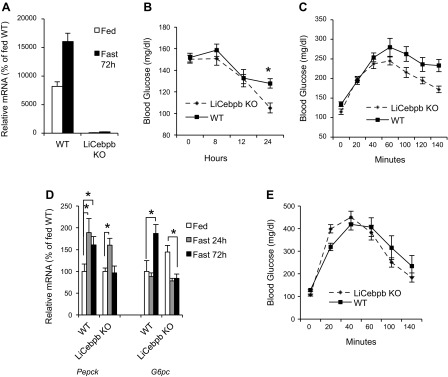
Hepatic deficiency of C/EBPβ results in dysfunctions of gluconeogenesis. A: Cebpβ mRNA levels in fed and 72-h-fasted LiCebpβ KO and WT mice. B: blood glucose levels of fed (0) and 8-, 12-, and 24-h-fasted LiCebpβ KO and WT mice (mean ± SE, n ≥ 12; *P < 0.05, Gcn2 KO vs. WT at 24 h after fasting). C: blood glucose levels as a function of time after ip injection of sodium pyruvate in mice of indicated genotypes (mean ± SE, n ≥ 6; P < 0.001, KO vs. WT by 2-way ANOVA). D: expression of Pepck and G6pc mRNAs in livers of mice of indicated genotypes (normalized to fed WT mice, mean ± SE, n = 4; *P < 0.05 as indicated). E: blood glucose levels as a function of time after ip injection of glucose in mice of indicated genotypes (mean ± SE, n ≥ 6; no significant difference, LiCebpb KO vs. WT by 2-way ANOVA).
DISCUSSION
Hepatic glucose production in mice bearing a mutation in the regulatory phosphorylation site of the translation initiation factor eIF2α is insufficient to sustain perinatal/neonatal life, and pups die of hypoglycemia within a few hours after birth, leading to the speculation that one or more of the four known eIF2α kinases were likely to regulate hepatic glucose production (31). Although Gcn2 KO mice exhibit normal viability following birth, we found that Gcn2-deficient adult mice do indeed exhibit reduced glucose production when they are fasted or recovering from exogenous insulin administration. Phosphorylation of eIF2α at the regulatory Ser51 site exhibits a linear increase in the liver over a 24-h fasting period, and we showed that GCN2 was primarily responsible for this induction although other eIF2α kinases likely contributed as well. Gcn2-deficient mice administered pyruvate, a glucogenic substrate, exhibited reduced conversion of pyruvate to glucose, strongly supporting the hypothesis GNG is reduced. Parallel experiments using glycerol as the glucogenic substrate suggested that reduced GNG in Gcn2-deficient mice is the result of an alteration in the GNG pathway between pyruvate and glycerol 3-phosphate.
Repressed GNG in Gcn2 KO mice is largely a deficiency of GCN2 in the liver and hepatocytes, since we discovered that liver-specific Gcn2 KO mice and isolated hepatocytes exhibit impairment in glucose production and GNG. However, we observed a smaller genotype difference in pyruvate-derived GNG and no difference in fasting glucose in LiGcn2 KO, suggesting that either incomplete deletion of Gcn2 in the liver of these mice or extra hepatic sources of GNG contribute to the larger genotype difference in GNG seen in global Gcn2 KO mice. In particular, the kidney and intestine have been shown to conduct GNG (23), and these organs may exhibit different glucogenic substrate preferences compared with the liver (35).
Burgess and coworkers have shown that a deficiency of PEPCK in the liver results in an imbalance in the TCA cycle (4). Similarly, we found elevated malate and OAA in Gcn2-deficient mice, consistent with diminished cataplerosis. However, the amounts of PEPCK mRNA and protein were not reduced in Gcn2 KO mice; instead the basal level of PEPCK was elevated to a level equivalent to fasted mice and did not show a further induction in the fasted state. In addition, we found that Gcn2-deficient mice also exhibited an apparent decrease in the NADH-to-NAD+ ratio (as measured by the ratio of lactate/pyruvate), indicating a reduction in the energy status. Because energy in the form of ATP, GTP, and NADH is required for GNG, we speculate that the decrease in NADH/NAD+ may impede GNG in Gcn2 KO mice and lead to the observed back up of TCA cycle intermediates that supply substrates for GNG. The use of the lactate-to-pyruvate ratio to estimate the NADH-to-NAD+ ratio assumes that equilibrium conditions occur in the examined tissue (36), which we have not directly assessed. Thus these estimates may not accurately reflect the true in vivo levels of NADH and NAD+. Nonetheless, we believe that they suggest possible differences in cellular redox states. Malic enzyme-1 gene expression was substantially reduced in the fed state of Gcn2 KO mice, which may contribute to the excess malate, and the imbalance in reducing equivalents. In addition to genotypic differences in metabolic intermediates in the fasted, we also observed significant differences in the fed state, suggesting that the repression of GNG in Gcn2 KO mice in the fasted state may be the result of preexisting metabolic imbalances before fasting. To further examine the role of metabolite differences in Gcn2 KO mice, it will be necessary to assess the level of these metabolites in the cytosol and mitochondria separately.
GNG in mice globally deficient for the C/EBPβ transcription factor was previously shown to be impaired (3, 9, 10, 19). C/EBPβ is normally induced upon fasting and is required for the induction of Pepck mRNA (8, 13, 27). We found that Gcn2-deficient mice have elevated basal expression of Cebpβ mRNA, but the C/EBPβ protein and mRNA failed to be induced in response to fasting. This pattern is remarkably similar to that seen in PEPCK expression in Gcn2 KO mice, suggesting that the well-known regulation of PEPCK by C/EBPβ during fasting is GCN2-dependent. We show herein that ablating C/EBPβ or GCN2 specifically in the liver leads to a reduction in GNG, supporting the hypothesis that the expression and action of GCN2 and C/EBPβ in the liver modulates GNG. However, GCN2 in skeletal muscle may also play a role, since this tissue is a major source of the gluconeogenic substrates pyruvate and lactate, which are imbalanced in Gcn2 KO mice. Leucine deprivation in Gcn2 KO mice results in a loss of skeletal muscle (2), suggesting that GCN2 may also act in skeletal muscle to regulate metabolic homeostasis during nutrient deprivation.
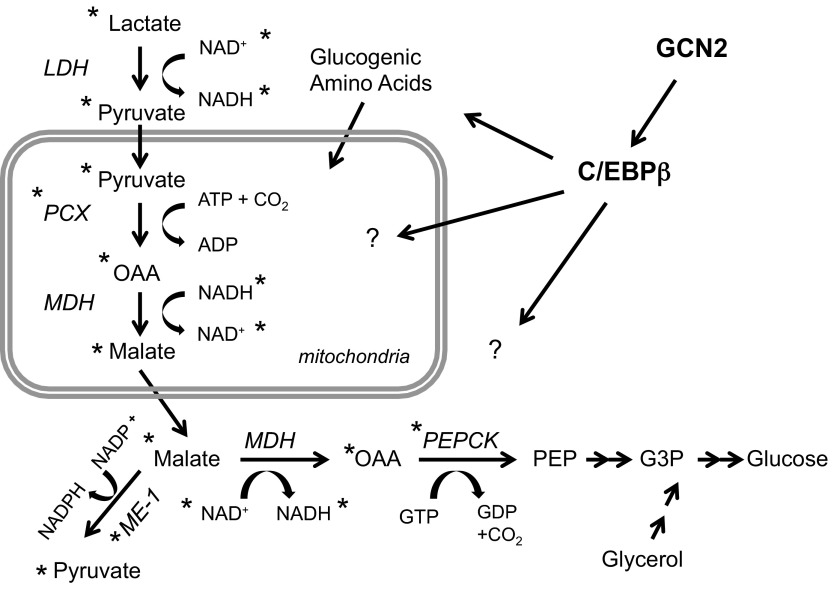
Our studies suggest that the function of GCN2 and C/EBPβ in regulating GNG lies within the initial steps of the GNG pathway between pyruvate and glycerol 3-phosphate (Fig. 8), since several intermediates and the expression of key enzymes that regulate these steps are perturbed in GCN2- and C/EBPβ-deficient hepatocytes. The conversion of OAA to phosphoenolpyruvate (PEP) by PEPCK is often cited as the key regulatory step in GNG, and we discovered alterations in PEPCK mRNA and/or protein expression and OAA levels in mice deficient for GCN2 and/or C/EBPβ. Specifically, we found that PEPCK mRNA and protein in GCN2-deficient mice were derepressed in the fed state and failed to be further induced upon fasting. The simplest prediction from these results would be elevated hepatic glucose output in the fed state with no further increase upon fasting. However, we observed euglycemia in the fed state but hypoglycemia and poor response to glucogenic substrates during fasting. We speculate that, although the metabolic perturbation seen in the fed state did not significantly impact glucose homeostasis, they set the stage for impaired hepatic glucose output during fasting. In addition, we think it is likely that GCN2 and C/EBPβ regulate more than just a single step in the GNG pathway, since we observed multiple alterations in both the fed and fasted state, some of which cannot be readily explained by altering only PEPCK expression. We also discovered reduced levels of cAMP and phospho-CREB in fasted Gcn2 KO mice, which, in addition to negatively impacting the induction of PEPCK and G6Pase, may disrupt the normal energy and metabolite balance of hepatocytes necessary for GNG. However, this interpretation is confounded by discordance in the metabolic state at which we see the reduced levels of cAMP and phospho-CREB (fasted state) vs. the elevation of PEPCK at the fed state and lack of further induction during fasting.
Fig. 8.
Proposed model for regulation of hepatic gluconeogenesis by GCN2 and CEBPβ. *Metabolites or enzymes (protein and/or mRNA) that are significantly different between GCN2 KO and WT mice in the fed and/or fasted state. Although the observed differences do not suggest a precise mechanism of action, the widespread differences imply that GCN2 and C/EBPβ may regulate multiple nodes in glucose and amino acid metabolism.
We speculate that the reduction in cAMP and phospho-CREB is due to an alteration of glucagon signaling. Pancreatic and serum glucagon levels are not significantly different in Gcn2 KO mice (unpublished data), suggesting that reduced glucagon signaling is autonomous to hepatocytes and downstream of the glucagon receptor. We have not detected diminished gene expression of the other key players in glucagon signaling, including adenylate cyclase, TORC1, and PKA, but have not excluded possible differences in their activities.
The expression of C/EBPβ, particularly at the translation initiation level, has been shown to be regulated by the eIF2α pathway (6, 40). The two major isoforms of C/EBPβ, C/EBPβ liver activator protein (LAP) and C/EBPβ liver inhibitor protein (LIP), are encoded by a single mRNA species but initiated by two different translation start codons (24). The LIP and LAP C/EBPβ isoforms were previously shown to be differentially regulated by eIF2α phosphorylation (6). However, in our study, we found that both the mRNA and protein levels of C/EBPβ were differentially regulated as a function of Gcn2 with equal impact on the relative expression of LAP and LIP, suggesting that GCN2-dependent regulation is mediated by transcription of C/EBPβ rather than by translational control.
GCN2 is activated by uncharged tRNAs that increase inversely proportional to amino acid levels, and hence GCN2 is the sensor of amino acid deprivation. How activation by amino acid deprivation is physiologically connected to regulation of GNG is unknown. We speculate that GCN2 and C/EBPβ may regulate glucogenic amino acids that are converted to TCA intermediates followed by cataplerosis to PEP (Fig. 8). During the first 24 h of fasting mice lose up to 40% of their liver mass, which is in part due to proteasome degradation that releases amino acids that can be redeployed for synthesis of essential proteins or used for GNG (32, 38). GCN2 and C/EBPβ are indeed known to regulate the response to amino acid deprivation (7, 15, 26, 37). GCN2 in the piriform cortex of the brain also regulates aversive feeding behavior to diets lacking essential amino acids (16, 20). Therefore, a growing body of evidence suggests that GCN2 plays multiple roles of regulating metabolic responses to nutritional changes that entail the action of GCN2 in the liver and/or central nervous system.
GRANTS
This projected was supported by funds provided by Ajinomoto 3ARP and National Institutes of Health Grants GM-056857 and DK-088140 to D. R. Cavener.
DISCLOSURES
The authors declare no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
Author contributions: X.X. and D.R.C. conception and design of research; X.X., J.H., and D.R.C. performed experiments; X.X., J.H., B.C.M., and D.R.C. analyzed data; X.X., J.H., B.C.M., and D.R.C. interpreted results of experiments; X.X. and D.R.C. prepared figures; X.X. and D.R.C. drafted manuscript; X.X., J.H., B.C.M., and D.R.C. edited and revised manuscript; X.X., J.H., B.C.M., and D.R.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ajin Wang for assistance in mouse colony management. We thank Dr. Esta Sterneck, NCI, for providing the C/EBPβ floxed mouse strain.
REFERENCES
- 1.Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol 12: 141–151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, Wek RC. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem 279: 36553–36561, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Arizmendi C, Liu S, Croniger C, Poli V, Friedman JE. The transcription factor CCAAT/enhancer-binding protein beta regulates gluconeogenesis and phosphoenolpyruvate carboxykinase (GTP) gene transcription during diabetes. J Biol Chem 274: 13033–13040, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Burgess SC, Hausler N, Merritt M, Jeffrey FM, Storey C, Milde A, Koshy S, Lindner J, Magnuson MA, Malloy CR, Sherry AD. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J Biol Chem 279: 48941–48949, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Burgess SC, Iizuka K, Jeoung NH, Harris RA, Kashiwaya Y, Veech RL, Kitazume T, Uyeda K. Carbohydrate-response element-binding protein deletion alters substrate utilization producing an energy-deficient liver. J Biol Chem 283: 1670–1678, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Calkhoven CF, Muller C, Leutz A. Translational control of C/EBPalpha and C/EBPbeta isoform expression. Genes Dev 14: 1920–1932, 2000 [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C, Dudenhausen E, Chen H, Pan YX, Gjymishka A, Kilberg MS. Amino-acid limitation induces transcription from the human C/EBPbeta gene via an enhancer activity located downstream of the protein coding sequence. Biochem J 391: 649–658, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croniger C, Leahy P, Reshef L, Hanson RW. C/EBP and the control of phosphoenolpyruvate carboxykinase gene transcription in the liver. J Biol Chem 273: 31629–31632, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Croniger C, Trus M, Lysek-Stupp K, Cohen H, Liu Y, Darlington GJ, Poli V, Hanson RW, Reshef L. Role of the isoforms of CCAAT/enhancer-binding protein in the initiation of phosphoenolpyruvate carboxykinase (GTP) gene transcription at birth. J Biol Chem 272: 26306–26312, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Croniger CM, Millward C, Yang J, Kawai Y, Arinze IJ, Liu S, Harada-Shiba M, Chakravarty K, Friedman JE, Poli V, Hanson RW. Mice with a deletion in the gene for CCAAT/enhancer-binding protein beta have an attenuated response to cAMP and impaired carbohydrate metabolism. J Biol Chem 276: 629–638, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Curran TR, Jr, Bahner RI, Jr, Oh W, Gruppuso PA. Mitogen-independent DNA synthesis by fetal rat hepatocytes in primary culture. Exp Cell Res 209: 53–57, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 68: 585–596, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Gadbut AP, Riccardi D, Wu L, Hebert SC, Galper JB. Specificity of coupling of muscarinic receptor isoforms to a novel chick inward-rectifying acetylcholine-sensitive K+ channel. J Biol Chem 271: 6398–6402, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Giaccari A, Morviducci L, Pastore L, Zorretta D, Sbraccia P, Maroccia E, Buongiorno A, Tamburrano G. Relative contribution of glycogenolysis and gluconeogenesis to hepatic glucose production in control and diabetic rats. A re-examination in the presence of euglycaemia. Diabetologia 41: 307–314, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Guo F, Cavener DR. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab 5: 103–114, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, Gietzen DW. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 307: 1776–1778, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Hinnebusch AG. A hierarchy of trans-acting factors modulates translation of an activator of amino acid biosynthetic genes in Saccharomyces cerevisiae. Mol Cell Biol 5: 2349–2360, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu NC, Lin WJ, Kim E, Collins LL, Lin HY, Yu IC, Sparks JD, Chen LM, Lee YF, Chang C. Loss of TR4 orphan nuclear receptor reduces phosphoenolpyruvate carboxykinase-mediated gluconeogenesis. Diabetes 56: 2901–2909, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Croniger C, Arizmendi C, Harada-Shiba M, Ren J, Poli V, Hanson RW, Friedman JE. Hypoglycemia and impaired hepatic glucose production in mice with a deletion of the C/EBPbeta gene. J Clin Invest 103: 207–213, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurin AC, Jousse C, Averous J, Parry L, Bruhat A, Cherasse Y, Zeng H, Zhang Y, Harding HP, Ron D, Fafournoux P. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab 1: 273–277, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Mizushima Y, Ishikawa M, Yoshizaki T. Absence of bicarbonate abolishes the glycogenic effect of cortisol in cultured fetal rat hepatocytes. Biochim Biophys Acta 721: 87–93, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Mueller PP, Hinnebusch AG. Multiple upstream AUG codons mediate translational control of GCN4. Cell 45: 201–207, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Mutel E, Gautier-Stein A, Abdul-Wahed A, Amigo-Correig M, Zitoun C, Stefanutti A, Houberdon I, Tourette JA, Mithieux G, Rajas F. Control of blood glucose in the absence of hepatic glucose production during prolonged fasting in mice: induction of renal and intestinal gluconeogenesis by glucagon. Diabetes 60: 3121–3131, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ossipow V, Descombes P, Schibler U. CCAAT/enhancer-binding protein mRNA is translated into multiple proteins with different transcription activation potentials. Proc Natl Acad Sci USA 90: 8219–8223, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab 7: 520–532, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palii SS, Kays CE, Deval C, Bruhat A, Fafournoux P, Kilberg MS. Specificity of amino acid regulated gene expression: analysis of genes subjected to either complete or single amino acid deprivation. Amino Acids 37: 79–88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park EA, Gurney AL, Nizielski SE, Hakimi P, Cao Z, Moorman A, Hanson RW. Relative roles of CCAAT/enhancer-binding protein beta and cAMP regulatory element-binding protein in controlling transcription of the gene for phosphoenolpyruvate carboxykinase (GTP). J Biol Chem 268: 613–619, 1993 [PubMed] [Google Scholar]
- 28.Passonneau JV, Lauderdale VR. A comparison of three methods of glycogen measurement in tissues. Anal Biochem 60: 405–412, 1974 [DOI] [PubMed] [Google Scholar]
- 29.Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol 54: 885–909, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab 13, Suppl 1: 118–125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7: 1165–1176, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Sokolovic M, Sokolovic A, Wehkamp D, Ver Loren van Themaat E, de Waart DR, Gilhuijs-Pederson LA, Nikolsky Y, van Kampen AH, Hakvoort TB, Lamers WH. The transcriptomic signature of fasting murine liver (Abstract). BMC Genomics 9: 528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sood R, Porter AC, Olsen DA, Cavener DR, Wek RC. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2alpha. Genetics 154: 787–801, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterneck E, Zhu S, Ramirez A, Jorcano JL, Smart RC. Conditional ablation of C/EBP beta demonstrates its keratinocyte-specific requirement for cell survival and mouse skin tumorigenesis. Oncogene 25: 1272–1276, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stumvoll M, Meyer C, Perriello G, Kreider M, Welle S, Gerich J. Human kidney and liver gluconeogenesis: evidence for organ substrate selectivity. Am J Physiol Endocrinol Metab 274: E817–E826, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Sun F, Dai C, Xie J, Hu X. Biochemical issues in estimation of cytosolic free NAD/NADH ratio. PLoS One 7: e34525, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thiaville MM, Dudenhausen EE, Zhong C, Pan YX, Kilberg MS. Deprivation of protein or amino acid induces C/EBPbeta synthesis and binding to amino acid response elements, but its action is not an absolute requirement for enhanced transcription. Biochem J 410: 473–484, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vabulas RM, Hartl FU. Protein synthesis upon acute nutrient restriction relies on proteasome function. Science 310: 1960–1963, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Wek RC, Jackson BM, Hinnebusch AG. Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc Natl Acad Sci USA 86: 4579–4583, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wethmar K, Begay V, Smink JJ, Zaragoza K, Wiesenthal V, Dorken B, Calkhoven CF, Leutz A. C/EBPbetaDeltauORF mice–a genetic model for uORF-mediated translational control in mammals. Genes Dev 24: 15–20, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR, Jefferson LS, Cavener DR. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol 22: 6681–6688, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab 4: 491–497, 2006 [DOI] [PubMed] [Google Scholar]