Abstract
Obesity at conception and excess gestational weight gain pose significant risks for adverse health consequences in human offspring. This study evaluated the effects of reducing dietary intake of obese/overfed ewes beginning in early gestation on fetal development. Sixty days prior to conception, ewes were assigned to a control diet [CON: 100% of National Research Council (NRC) recommendations], a diet inducing maternal obesity (MO: 150% of NRC recommendations), or a maternal obesity intervention diet (MOI: 150% of NRC recommendations to day 28 of gestation, then 100% NRC) until necropsy at midgestation (day 75) or late (day 135) gestation. Fetal size and weight, as well as fetal organ weights, were greater (P < 0.05) at midgestation in MO ewes than those of CON and MOI ewes. By late gestation, whereas fetal size and weight did not differ among dietary groups, cardiac ventricular weights and wall thicknesses as well as liver and perirenal fat weights remained elevated in fetuses from MO ewes compared with those from CON and MOI ewes. MO ewes and fetuses exhibited elevated (P < 0.05) plasma concentrations of triglycerides, cholesterol, insulin, glucose, and cortisol at midgestation compared with CON and MOI ewes and fetuses. In late gestation, whereas plasma triglycerides and cholesterol, insulin, and cortisol remained elevated in MO vs. CON and MOI ewes and fetuses, glucose concentrations were elevated in both MO and MOI fetuses compared with CON fetuses, which was associated with elevated placental GLUT3 expression in both groups. These data are consistent with the concept that reducing maternal diet of obese/overfed ewes to requirements from early gestation can prevent subsequent alterations in fetal growth, adiposity, and glucose/insulin dynamics.
Keywords: maternal obesity, fetal growth and metabolism, dietary intervention
recent epidemiological data indicate that between 1993 and 2003, prepregnancy obesity increased 69% among women in the US (42). Indeed, more than two-thirds of women of childbearing age are reported to be overweight [body mass index (BMI) ranging from 24.9 to 29.9 kg/m2] or obese (BMI >30 kg/m2) (1, 13, 73). Maternal obesity during pregnancy increases the risk of several adverse health outcomes, including hypertension, gestational diabetes mellitus, premature delivery, macrosomia, and increased fetal mortality (13). Among overweight and obese women, the greatest risk of adverse infant outcomes is associated with increased prepregnancy BMI and is further impacted by gestational weight gain (15, 58). However, the long-term consequences may be even greater, because maternal obesity has been associated with an increased risk of insulin resistance, obesity, and type 2 diabetes in their children later in life (17). Furthermore, even mild impairment of glucose tolerance during pregnancy is a risk factor for the development of obesity and type 2 diabetes in children and adolescents (56, 63). Similarly, studies in the sheep indicate that fetuses exposed in utero to maternal overnutrition/obesity with a resultant increase in insulin resistance are hyperglycemic and hyperinsulinemic and have a high risk of becoming overweight or obese later in life (45, 51, 52).
Although it is difficult to alleviate maternal obesity once it has occurred, small lifestyle modifications during pregnancy that alter the intrauterine environment may produce substantial changes in health outcomes of the child (1). Although a significant number of dietary and lifestyle interventions in pregnancy have been conducted to reduce maternal gestational weight gain in overweight/obese women, those based on diet are the most effective and are associated with both reductions in maternal gestational weight gain and improved obstetric outcomes (69). We have developed and characterized a diet-based model of maternal obesity (MO) where ewes are fed an obesogenic diet at 150% of National Research Council (NRC) recommendations or a control (CON) diet at 100% of NRC recommendations from 60 days before conception through gestation. MO ewes gained an average of 65–70% in body weight compared with CON ewes who gained 15–20% in body weight during gestation (27, 72). Fetuses of MO ewes were macrosomic at midgestation (∼30% heavier than CON fetuses) and exhibited altered organ growth and development, increased adiposity, and increased plasma glucose and insulin levels (30). Furthermore, if MO offspring were fed ad libitum as adults, they exhibited markedly greater appetites, were more insulin resistant, and exhibited greater adiposity than CON offspring (45). We hypothesized that returning MO ewes to the CON diet in early gestation at a time that would approximate the time pregnant women are first aware of their pregnancy would at least in part prevent altered placental uptake of maternal nutrients as well as correct fetal metabolism and result in normal organ and tissue growth of MO fetuses.
MATERIALS AND METHODS
Animals.
All animal procedures were approved by the University of Wyoming Animal Care and Use Committee. Multiparous ewes (n = 12/treatment group) were fed either a highly palatable pelleted diet (88.5% dry matter, 17.4% crude protein, 93.9% in vitro dry matter digestibility) at 100 (CON) or 150% (MO) of NRC recommendations (53) from 60 days before conception (1st day of mating = day 0) or 150% of NRC recommendation from 60 days before conception to day 28 of gestation and then 100% NRC [MO intervention (MOI)] until necropsy on day 75 (0.5) or day 135 (0.9) of gestation. All ewes consumed 100% of their diet each day. Only ewes carrying twin fetuses were utilized in this study, and data only from the first fetus bled and recovered at necropsy are presented. All ewes were weighed at weekly intervals, and rations were adjusted for weight gain. A body condition score of 1 (emaciated) to 9 (obese) was assigned independently by two trained individuals at weekly intervals at the time of weighing. Body condition score is highly related to carcass lipids and can be used to estimate energy reserves available to ewes (61).
Dual energy X-ray absorptiometry.
The use of dual-energy X-ray absorptiometry (DEXA; Prodigy 8743; GE Lunar, Madison, WI) to determine total percent body fat in sheep has been described previously in detail (27, 30) and fully validated for use in the sheep (49, 55). Briefly, DEXA scanning was performed in a subset of 18 ewes (6 from each dietary group) at three different time points: 1) immediately prior to diet initiation, 2) at midgestation and, 3) at late gestation. Ewes were deprived of food and water for ∼24 h to prevent emesis and subsequent aspiration of gastric material while under sedation and were sedated with ketamine (22.2 mg/kg body wt) immediately prior to the scan. The whole body scan mode was used for all animals, and scan times were ∼3 min. A single, blinded, and experienced investigator performed all DEXA scans and quantified percent body fat. DEXA was calibrated, and quality assurance tests were performed daily prior to measurement and according to the manufacturer's specifications and programmed acceptable limits.
Blood and tissue collection.
Maternal and fetal blood samples and placental and fetal tissues were obtained at necropsy. Immediately before necropsy each ewe was weighed, and a 10-ml sample of blood was collected via jugular venipuncture into a chilled heparinized tube (sodium heparin, 143 USP units; Becton Dickinson, Franklin Lakes, NJ) and centrifuged at 2,000 g for 10 min for plasma collection. Plasma was frozen at −80°C until being utilized for hormone analyses. Ewes were sedated with ketamine (10 mg/kg) and maintained under isofluorane inhalation anesthesia (∼2.5%). Immediately following laparotomy, umbilical vein plasma was collected and stored as described for maternal blood. Ewes and fetuses were euthanized by exsanguination while still under general anesthesia, and the gravid uterus was quickly removed. Fetal body weight (BW), empty BW, crown rump length (CRL), and thoracic and abdominal circumferences were recorded for all fetuses. Fetal tissues, including the heart, kidneys, adrenals, liver, and perirenal fat depots, were dissected and weights recorded. Fetal hearts were dissected to record weights of right ventricular (RV) and left ventricular (LV) free wall. A total weight was calculated for paired organs (kidneys, adrenals, and perirenal fat depots).
Intravenous glucose tolerance test.
Glucose tolerance was studied in a subset of ewes from each treatment group (n = 4) at days 73 and 133 of gestation, as described previously (27). Briefly, ewes were weighed and fasted for a 12-h period, and jugular veins were catheterized (AbboCath, 14 gauge × 5.5 in. long; Abbott Laboratories, Chicago, IL) under local anesthesia using aseptic procedures. They were allowed 6 h to recover before the intravenous glucose tolerance test (IVGTT) was initiated. Jugular venous blood samples (3 ml) were drawn and placed into chilled heparinized tubes for subsequent plasma collection. Samples were collected at 15 and 0 min before administration of a glucose bolus at 0.25 g/kg BW for 20 s (50% dextrose solution; Vedco, St. Joseph, MO) and at 2, 5, 10, 15, 30, 60, and 120 min after glucose infusion via the catheter for glucose and insulin measurement. Catheters were carefully flushed with saline following the glucose infusion and between each blood sampling. Plasma was collected and stored at −80°C until subsequent analysis.
Biochemical assays.
Plasma glucose was measured in triplicate by colorimetric methods following the addition of a glucose hexokinase reagent (Liquid Glucose Hexokinase Reagent Set; Pointe Scientific, Canton, MI) using 96-well plates, as described previously (26). Mean intra-assay coefficient of variation (CV) was 1.5%, and interassay CV was 4.0%. Plasma triglyceride and cholesterol concentrations were determined by colorimetric procedures using commercial kits (Pointe Scientific). Mean intra-assay CV was 2.1 and 1.9% and inter-assay CV 3.1 and 2.0% for triglycerides and cholesterol, respectively. Plasma insulin was measured in duplicate by commercial insulin radioimmunoassay (RIA) kit (Siemens Healthcare Diagnostics, Deerfield, IL). Intra- and interassay CVs for insulin were 7.6 and 14.9%, respectively. Plasma cortisol was determined in duplicate, as described previously (23), using a commercial cortisol RIA kit with a sensitivity of 0.5 μg/dl (Siemens Healthcare Diagnostics). Both the intra- and interassay CVs for cortisol were <4.5%.
Western blotting.
Western blot analyses were conducted by procedures previously published from our laboratory (75). Briefly, individual frozen samples of cotyledon tissue were pulverized in liquid nitrogen, and 100 mg of tissue was homogenized in a polytron homogenizer (Kinematica, Bohemia, NY) with 300–500 μl of ice-cold lysis buffer (137 mM NaCl, 50 mM Tris·HCl, 2% SDS, 1% Triton-100 solution, 10% glycerol, 2.5 mM EDTA, 1 mM CaCl2, 1 mM MgCl2, 2 mM Na3VO4, 100 mM NaF, and 1% protease inhibitor cocktail, pH 7.4; Sigma, St. Louis, MO). Homogenates were then centrifuged, and an aliquot of each lysate was used for protein quantification using a NanoDrop 2000C spectrophotometer (Thermo Scientific, Wilmington, DE). Homogenates were mixed with different volumes of 2× standard SDS sample loading buffer containing 5% β-mercaptoethanol to make the final concentration of each sample equal. Fifty micrograms of protein extractions was separated by 10% SDS-PAGE gels and transferred to nitrocellulose membranes. Following transfer, membranes were blocked with 5% nonfat milk powder in Tris-buffered saline-0.01% Tween 20 (50 mM Tris·HCl, pH 7.6, 150 mM NaCl, and 0.05% Tween 20) for 1 h. The membranes were incubated in the following primary antibodies: anti-glucose transporter 1 (anti-GLUT1; cat. no. 14683) purchased from Abcam (Cambridge, MA), anti-glucose transporter 3 (anti-GLUT3; cat. no. sc-31838), fatty acid transport protein 1 (anti-FATP1; cat. no. sc-25541), fatty acid transport protein 4 (anti-FATP4; cat. no. sc-25670), and cluster of differentiation 36 (anti-CD36; cat. no. sc-9154) purchased from Santa Cruz Biotechnology (Dallas, TX), diluted in 5% (wt/vol) nonfat milk, at 1:500, 1:300, 1:200, 1:500, and 1:200, respectively, overnight at 4°C. Membranes were incubated in secondary antibodies at 1:3,000–1:5,000 concentrations diluted in 2% (wt/vol) nonfat milk for 60 min at room temperature. The membranes were then visualized using ECL Western blotting detection reagents and exposed to film. The density of bands was quantified by using an Imager Scanner II (Amersham Biosciences, Piscataway, NJ) and ImageQuant TL software (Amersham Biosciences). The target protein band density was normalized according to the density of β-actin content (anti-β-actin, cat. no. 4790; Cell Signaling Technology, Danvers, MA).
Statistical analysis.
Data were analyzed as repeated measurements using the MIXED procedure of SAS (SAS Institute, Cary, NC), with treatment and time and their interaction in the model statement. Areas under the curves (AUC) for glucose and insulin during IVGTT along with the baseline concentrations of both glucose and insulin were analyzed using the general linear model procedure of SAS with treatment in the model statement. Maternal body fat data were analyzed using the MIXED procedure of SAS. Maternal and fetal glucose, insulin, cortisol, and fetal morphometric data were also analyzed by the MIXED procedures of SAS with treatment and offspring sex and their interaction as the main effects. A “by day” statement was used to separate midgestation and late gestation data. Offspring sex effects were found to be nonsignificant for all variables investigated, and therefore, they were removed from the final models. Data are presented as least square means ± SE, and differences are considered significant at P ≤ 0.05, with a tendency at P ≤ 0.10.
RESULTS
Fetal morphometrics and organ characteristics.
At the midgestation necropsy, all fetal morphometric measurements as well as selected organ weights were increased (P < 0.01) in MO fetuses compared with fetuses of MOI and CON ewes (Table 1). Specifically, MO fetuses had greater BW, empty BW, CRL, and thoracic and abdominal circumferences (P < 0.01) than MOI or CON fetuses. Weights of the LV, RV, kidneys, pancreas, liver, and perirenal fat were also greater (P < 0.001) in day 75 fetuses from MO ewes compared with those from MOI and CON ewes (Table 2). Additionally, LV and RV thickness was greater (P < 0.05) in fetuses from MO ewes than CON and MOI fetuses, which were similar (Table 2).
Table 1.
Fetal body measurements in CON, MO, and MOI dietary groups at mid- and late gestation
|
Day 75 |
Day 135 |
|||||
|---|---|---|---|---|---|---|
| Item | CON | MO | MOI | CON | MO | MOI |
| Fetal body wt, g | 224 ± 5a | 248 ± 5b | 231.2 ± 5a | 4082 ± 219a | 4549 ± 230a | 4114 ± 173a |
| Empty body wt, g | 171 ± 4a | 187 ± 3b | 174.1 ± 4a | 3,103 ± 173a | 3,305 ± 181a | 3,207 ± 137a |
| CRL, cm | 20.1 ± 0.2a | 21.2 ± 0.2b | 20.5 ± 0.2a | 56.21 ± 0.7a | 57.36 ± 0.7a | 57.0 ± 0.5a |
| Thoracic circum., cm | 13.05 ± 0.11a | 13.88 ± 0.11b | 13.13 ± 0.12a | 33.5 ± 0.61a | 34.54 ± 0.62a | 34.10 ± 0.48a |
| Abdominal circum., cm | 11.92 ± 0.10a | 12.96 ± 0.10b | 11.91 ± 0.11a | 33.63 ± 0.85a | 34.89 ± 0.89a | 32.55 ± 0.67a |
Data are presented as means ± SE; n = 6 fetuses. CON, control; MO, maternal obese; MOI, maternal obese intervention; CRL, crown rump length; circum., circumference.
Means ± SE within a gestational age and measurement with different superscripted letters differ (P < 0.05).
Table 2.
Selected fetal organ measurements in CON, MO, and MOI dietary groups at mid- and late gestation
|
Day 75 |
Day 135 |
|||||
|---|---|---|---|---|---|---|
| Item | CON | MO | MOI | Con | MO | MOI |
| Right ventricle wt, g | 0.50 ± 0.03a | 0.64 ± 0.03b | 0.56 ± 0.03a | 7.77 ± 0.55a | 8.87 ± 0.57a | 7.72 ± 0.43a |
| RV thickness, cm | 1.62 ± 0.05a | 2.46 ± 0.05b | 1.67 ± 0.05a | 4.97 ± 0.18a | 6.07 ± 0.19b | 4.77 ± 0.14a |
| Left ventricle wt, g | 0.82 ± 0.03a | 0.99 ± 0.04b | 0.84 ± 0.04a | 10.84 ± 0.72a,b | 12.64 ± 0.72b | 10.17 ± 0.61a |
| LV thickness, cm | 2.44 ± 0.06a | 3.48 ± 0.06b | 2.60 ± 0.06a | 6.74 ± 0.23a | 8.38 ± 0.24b | 6.34 ± 0.18a |
| Total kidney wt, g | 2.47 ± 0.11a | 3.09 ± 0.11b | 2.59 ± 0.12a | 21.57 ± 0.97a | 23.89 ± 1.02a | 21.24 ± 0.77a |
| Pancreas weight, g | 0.32 ± 0.03a | 0.45 ± 0.03b | 0.36 ± 0.03a | 3.80 ± 0.32a | 3.10 ± 0.34a | 3.20 ± 0.28a |
| Liver weight, g | 14.53 ± 0.52a | 17.27 ± 0.52b | 15.96 ± 0.55a | 102.38 ± 6.89a,b | 119.54 ± 7.23b | 98.30 ± 5.46a |
| Total perirenal fat wt, g | 1.02 ± 0.08a | 1.36 ± 0.08b | 0.95 ± 0.08a | 24.20 ± 1.31a | 31.40 ± 1.37b | 21.81 ± 1.04a |
Data are presented as means ± SE; n = 6 fetuses. RV, right ventricular; LV, left ventricular.
Means ± SE within a gestational age and measurement with different superscripted letters differ (P < 0.05).
At the late gestation necropsy at day 135, total cotyledonary and placentomal weights were similar across groups, averaging 371.5 ± 12.70 and 459.8 ± 12.7 g, respectively. Furthermore, fetal BW, empty BW, CRL, and thoracic and abdominal circumferences were similar among treatment groups (Table 1). In contrast, RV and LV wall thickness and perirenal fat weight remained markedly greater (P < 0.0001) in fetuses from MO ewes than MOI and CON ewes, which were similar (Table 2). Furthermore, whereas LV weights of fetuses from MOI and CON ewes were similar, the weights of the LV from fetuses of MOI ewes were less (P < 0.05) than that of fetuses from MO ewes.
Ewe body weight, percent body fat, glucose, and insulin dynamics.
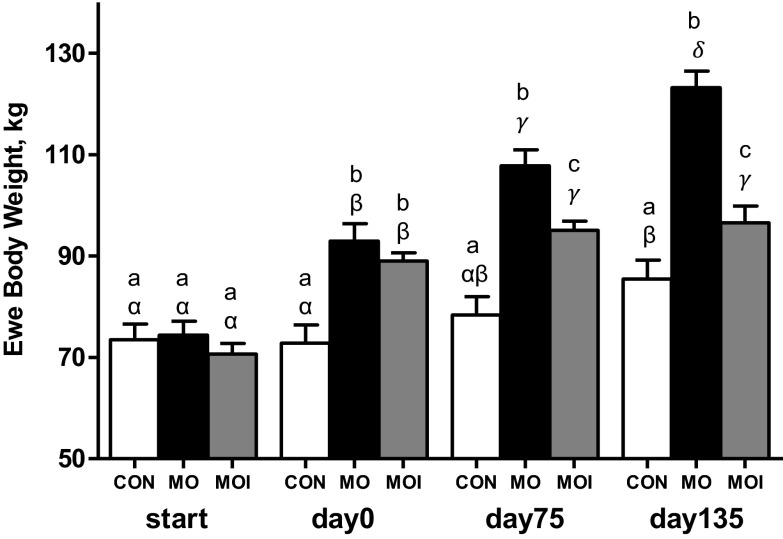
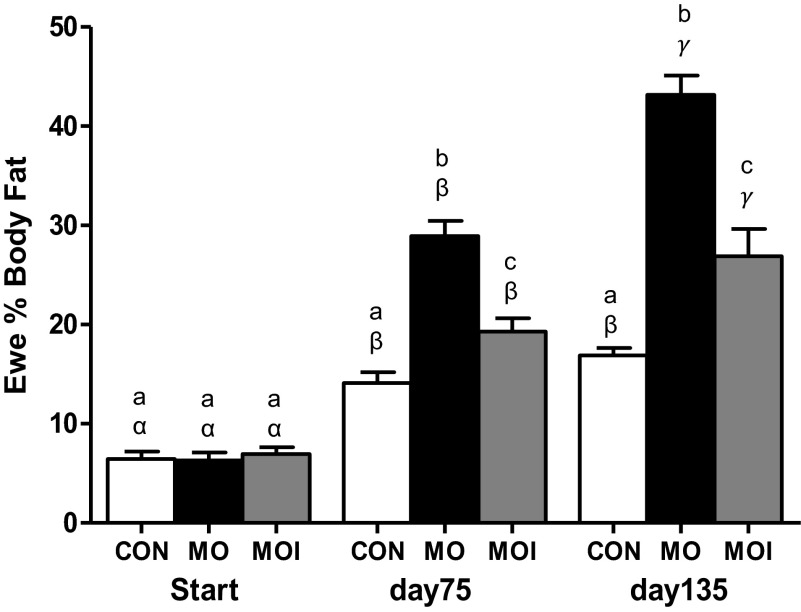
Body weights of CON, MO, and MOI ewes were similar at the start of the experimental diets (Fig. 1). By conception, weights of MO and MOI ewes were similar and greater (P < 0.01) than CON ewes, although by mid- and late gestation, the weights of all three groups differed significantly (P < 0.05; CON < MOI < MO). CON ewe body weights remained similar through day 75 of gestation before increasing (P < 0.01) by day 135 in association with the increase in gravid uterine weight. Body weights of MO ewes increased progressively from diet initiation until day 135 of gestation, whereas body weights of MOI ewes only increased from diet initiation through day 75 of gestation and then remained constant through day 135. As with ewe body weight, the percent body fat of CON, MO, and MOI ewes did not differ at diet initiation (Fig. 2). By midgestation and late gestation, however, percent body fat of all three groups differed significantly (P < 0.01, CON < MOI < MO). Body fat percentage of CON ewes increased (P < 0.01) from diet initiation to day 75 of gestation and then remained relatively constant through day 135. In contrast, percent body fat increased (P < 0.01) progressively from diet initiation through day 135 of gestation in both MO and MOI ewes.
Fig. 1.
Ewe body weights for control (CON; open bars), maternal obese (MO; black bars), and maternal obese intervention (MOI; gray bars) groups at diet initiation 60 days before breeding (start; n = 12/group), at conception (day 0; n = 12/group), at midgestation (day 75; n = 12/group), and at late gestation (day 135; n = 6/group). a,b,cMeans ± SE within a time point with different letters differ (P < 0.01); α,β,γ,δmeans ± SE within a dietary group across all time points with different Greek letters differ (P < 0.01).
Fig. 2.
Ewe %body fat determined by dual-energy X-ray absorptiometry for CON (open bars), MO (black bars), and MOI (gray bars) groups at diet initiation 60 days before breeding (start; n = 6/group), at midgestation (day 75; n = 6/group), and at late gestation (day 135; n = 6/group). a,b,cMeans ± SE within a time point with different letters differ (P < 0.01); α,β,γmeans ± SE within a dietary group across all time points with different Greek letters differ (P < 0.01).
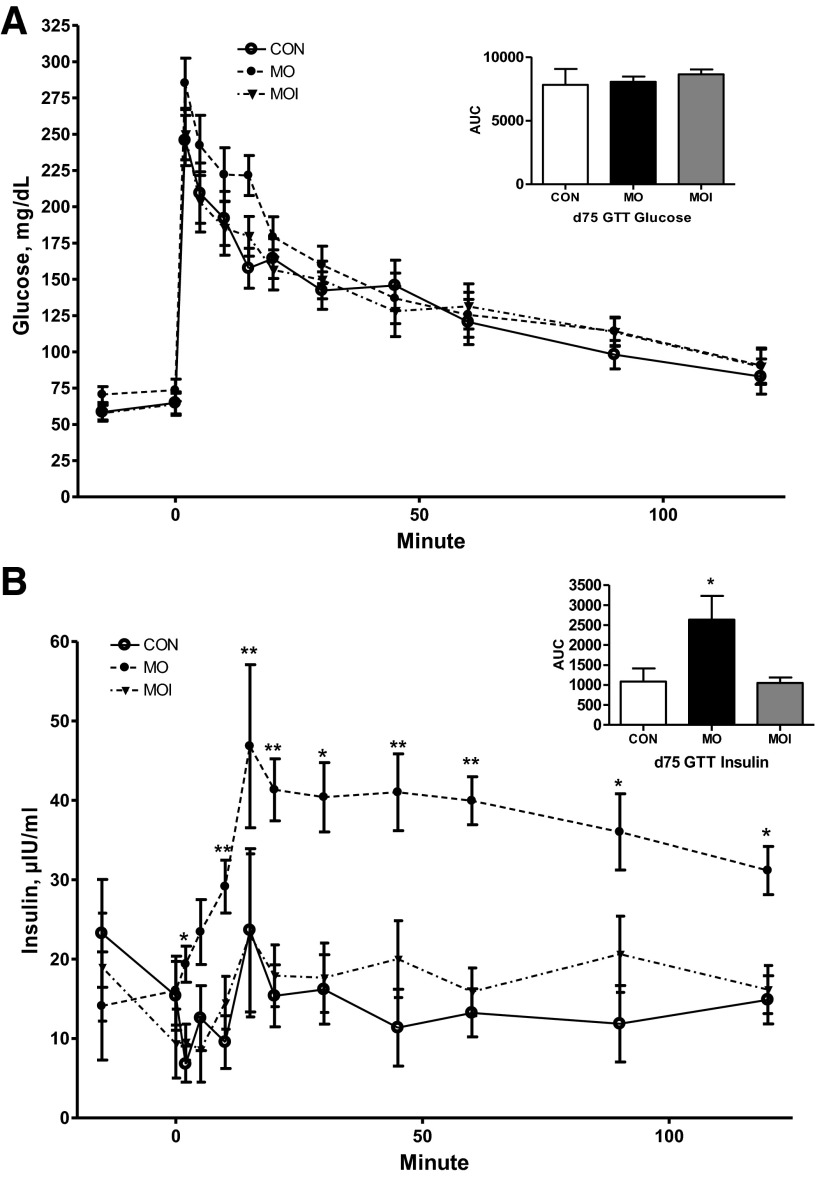
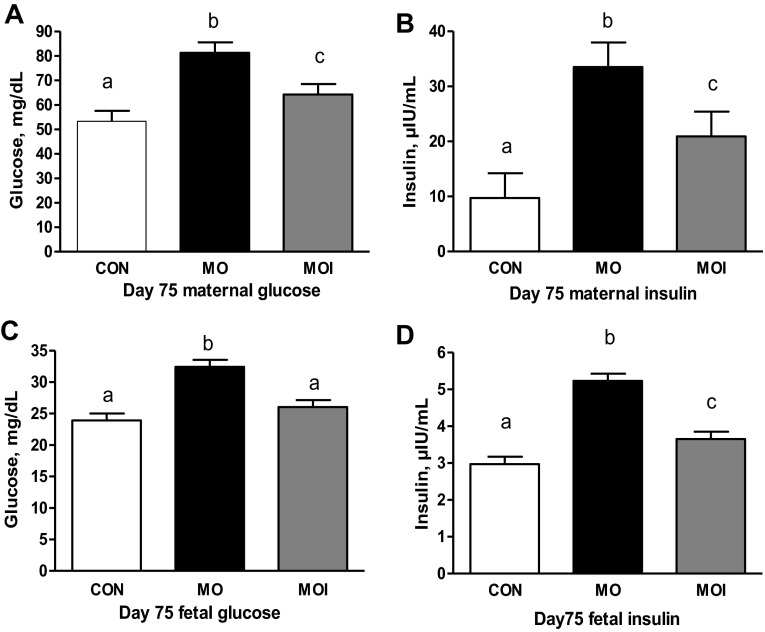
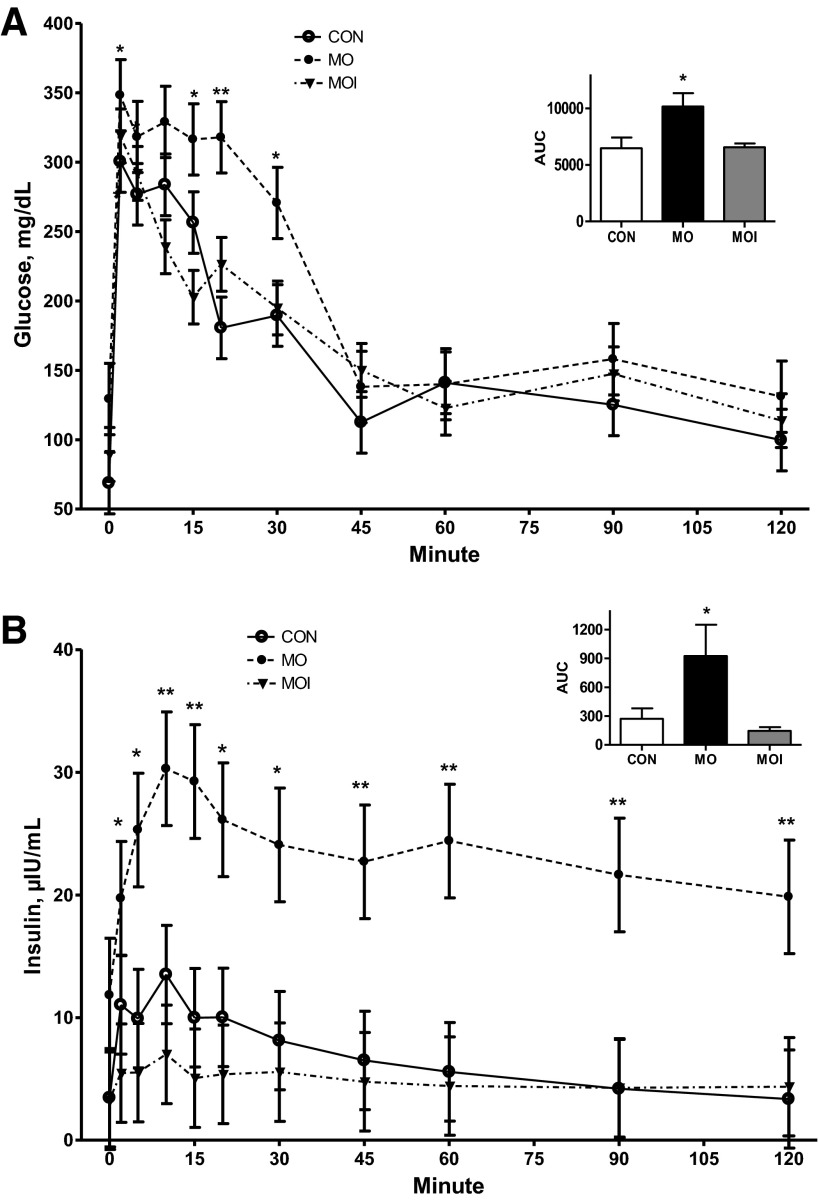
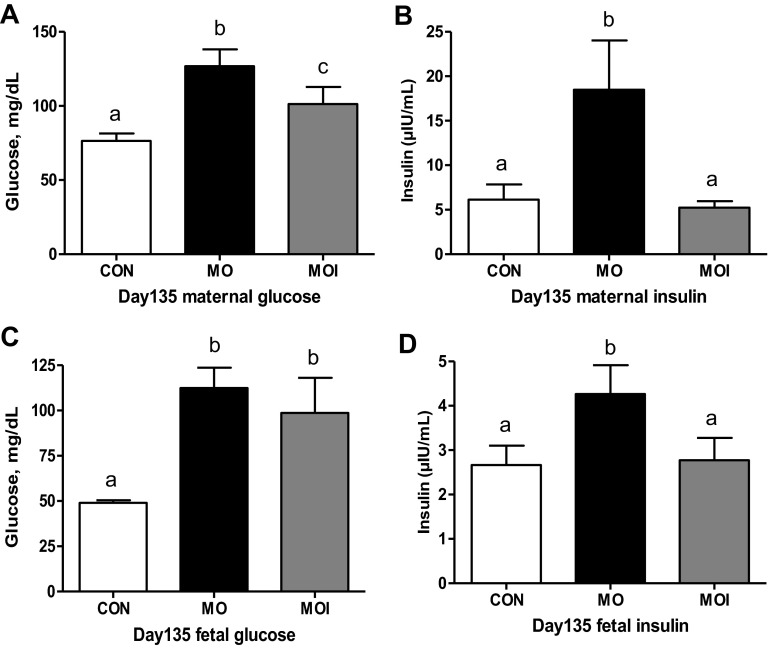
There was no effect of treatment on glucose clearance rate (glucose AUC) in ewes during the midgestation IVGTT (Fig. 3A). In contrast, MO ewes exhibited a markedly greater (P < 0.05) insulin release in response to glucose infusion than CON or MOI ewes, which did not differ (Fig. 3B). Furthermore, insulin concentrations of MO ewes continued to be elevated over those of CON and MOI ewes throughout the IVGTT. Baseline concentrations of glucose and insulin in maternal plasma at the day 75 necropsy differed across treatment groups CON < MOI < MO (P < 0.05; Fig. 4, A and B). Concentrations of glucose in fetal plasma were elevated (P < 0.01; Fig. 4C) in the MO group over that in the blood of MOI and CON fetuses, which were similar. However, fetal plasma insulin exhibited a pattern similar to those observed in their dams (P < 0.05, CON < MOI < MO; Fig. 4D).
Fig. 3.
Plasma glucose (A) and insulin (B) dynamics of CON (n = 4; ○), MO (n = 4; ●), and MOI (n = 4; ▼) during an intravenous glucose tolerance test (IVGTT) at midgestation. Area under the curve (AUC) is shown as a bar graph at right for each dietary group. Means ± SE; differences are noted compared with CON group; *P < 0.05; **P < 0.0001.
Fig. 4.
Maternal plasma glucose (n = 6; A), maternal plasma insulin (n = 6; B), fetal plasma glucose (n = 6; C), and fetal plasma insulin levels (n = 6; D) of CON (open bars), MO (black bars), and MOI (gray bars) dietary groups at midgestation. a,b,cMeans ± SE within a measurement with different letters differ (maternal, P < 0.05; fetal, P < 0.01).
Following glucose infusion during the late gestation IVGTT, plasma glucose concentrations were elevated in all ewes from 0 to 30 min after glucose challenge (P < 0.05) and then returned to baseline levels within 45 min (Fig. 5A). Concentrations of glucose, however, were higher in MO ewes than CON or MOI ewes during this time interval, as reflected by the increase (P < 0.05) in the AUC. Insulin dynamics followed a pattern similar to glucose, with peak plasma concentrations observed in all ewes ∼10 min after glucose infusion. Insulin concentrations remained markedly elevated in MO vs. MOI and CON ewes throughout the entire test period, as reflected by the increase (P < 0.01) in the AUC (Fig. 5B). At the day 135 necropsy, concentrations of glucose and insulin in maternal plasma were greater (P < 0.05) in MO compared with MOI and CON ewes, which did not differ (Fig. 6, A and B). Fetal plasma insulin was also greater (P < 0.05) in MO than in MOI and CON fetuses (Fig. 6D). Plasma glucose concentrations in MOI fetuses were similar to that of MO fetuses, and both were higher (P < 0.05) than observed in CON fetuses (Fig. 6C).
Fig. 5.
Plasma glucose (A) and insulin dynamics (B) of CON (n = 4; ○), MO (n = 4; ●), and MOI (n = 4; ▼) during an IVGTT at late gestation. AUC is shown as a bar graph at right for each dietary group. Means ± SE differences are noted compared with CON group. *P < 0.05; **P < 0.0001.
Fig. 6.
Maternal plasma glucose (n = 6/group; A), maternal plasma insulin (n = 6/group; B), fetal plasma glucose (n = 6/group; C), and fetal plasma insulin levels (n = 6/group; D) in CON (open bars), MO (black bars), and MOI (gray bars) dietary groups in late gestation. a,b,cMeans ± SE within a measurement with different letters differ (P < 0.05).
Maternal and fetal cortisol profile.
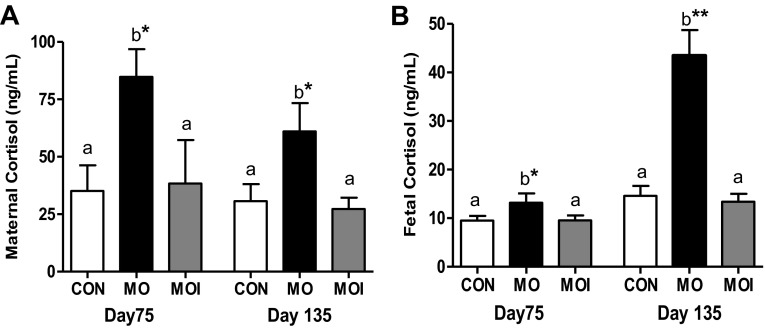
Greater (P < 0.05) concentrations of cortisol were observed in maternal plasma of MO ewes on both day 75 and day 135 compared with MOI and CON ewes (Fig. 7A). Similarly, fetal plasma cortisol was also greater (P < 0.05) in MO than MOI and CON fetuses during both gestational periods (Fig. 7B). However, in late gestation, there was a markedly greater difference in plasma cortisol in MO fetuses compared with both MOI and CON fetuses (P < 0.001).
Fig. 7.
Baseline cortisol levels at midgestation (day 75; n = 6) and late gestation (day 135; n = 6) in maternal (A) and fetal plasma (B) for CON (open bars), MO (black bars), and MOI (gray bars) dietary groups. a,bMeans ± SE within animal type and stage of gestation with different letters differ; *P < 0.05; **P < 0.0001.
Maternal and fetal triglyceride and cholesterol concentrations.
Blood concentrations of triglycerides and cholesterol were similar in CON, MOI, and MO ewes on day 75 and day 135 of gestation and were unchanged from mid- to late gestation. In contrast, at both gestational ages, blood concentrations of triglycerides and cholesterol were markedly elevated (P < 0.01) in fetuses from MO ewes compared with fetuses from MOI and CON ewes, which were similar (Table 3). Furthermore, concentrations of triglycerides declined ∼50% (P < 0.05) in fetal blood across all three dietary groups from days 75 to 135 (Table 3), whereas fetal cholesterol concentrations remained unchanged from mid- to late gestation.
Table 3.
Triglyceride and cholesterol levels in fetal and maternal plasma of CON, MO, and MOI dietary groups at mid- and late gestation
| Triglycerides, mg/dl |
Cholesterol, mg/dl |
|||||
|---|---|---|---|---|---|---|
| CON | MO | MOI | CON | MO | MOI | |
| Midgestation (day 75) | ||||||
| Maternal | 34.1 ± 3.2a | 28.7 ± 3.2a | 33.9 ± 3.2a | 37.4 ± 3.7a | 46.5 ± 3.7a | 44.8 ± 3.7a |
| Fetal | 31.6 ± 1.6a* | 42.0 ± 1.6b* | 34.7 ± 1.6a* | 24.1 ± 1.8a | 33.4 ± 1.8b | 27.1 ± 2.0a |
| Late gestation (day 135) | ||||||
| Maternal | 30.6 ± 3.5a | 32.5 ± 3.5a | 33.4 ± 3.9a | 45.4 ± 4.1a | 53.5 ± 4.1a | 44.4 ± 4.1a |
| Fetal | 16.2 ± 1.9a* | 23.1 ± 1.8b* | 15.9 ± 1.9a* | 22.7 ± 2.3a | 36.3 ± 2.1b | 22.8 ± 2.3a |
Values are means ± SE; n = 6.
Means ± SE within a gestational age and animal type with different superscripted letters differ (P < 0.05);
means ± SE differ (P < 0.0001) by stage of gestation within animal type.
GLUT and FATP protein expression in cotyledon tissue.
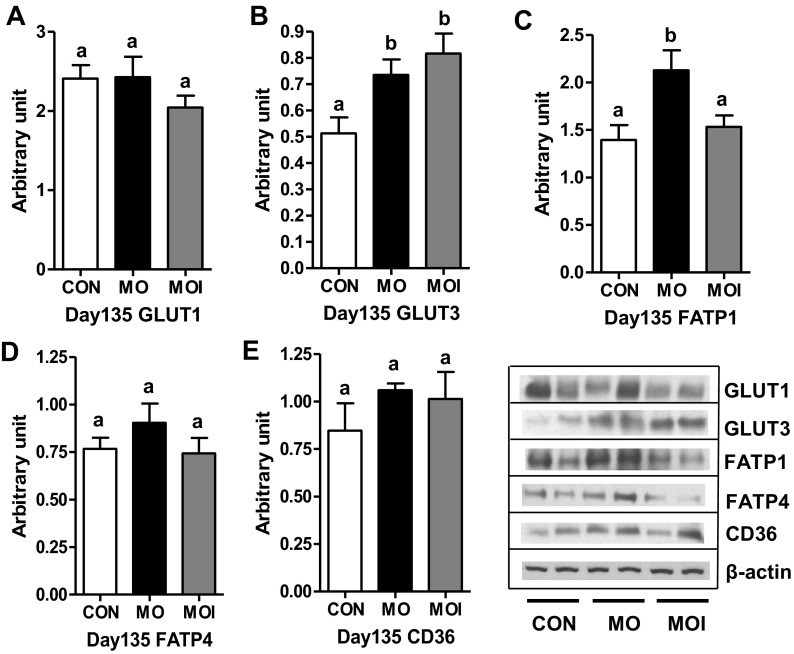
Whereas cotyledonary GLUT1 expression was similar across dietary groups, GLUT3 protein expression of both MO and MOI groups was increased (P < 0.05) compared with CON fetuses on day 135 of gestation (Fig. 8, A and B). Cotyledonary expression of FATP4 and CD36 proteins was similar across dietary groups, whereas FATP1 was elevated (P < 0.05) in MO vs. CON and MOI groups, which were similar (Fig. 8, C–E).
Fig. 8.
Protein expression of glucose transporter 1 (GLUT1), glucose transporter 3 (GLUT3), fatty acid transport protein 1 (FATP1), fatty acid transport protein 4 (FATP4), and cluster of differentiation 36 (CD36) in cotyledonary tissue for CON (open bars), MO (black bars), and MOI (gray bars) dietary groups at late gestation (day 135). a,bMeans ± SE with different letters differ. P < 0.05.
DISCUSSION
To our knowledge, these data demonstrate for the first time in obese/overnourished ewes, a large precocial species, that a dietary reduction to requirements beginning in early gestation can return fetal growth, adiposity, and organ development to CON levels at both midgestation and late gestation. The sheep has been used extensively as an appropriate animal model to study the impacts of overnutrition/obesity to provide data for translation to the human fetus, as it exhibits many similarities with humans (one important feature that sheep and humans share is their readiness to overeat). Furthermore, both the sheep and human give birth to well-developed precocial offspring, exhibit the same newborn-to-maternal weight ratios, and exhibit the same temporal pattern of fetal tissue and organ development throughout pregnancy (7). Furthermore, the fetal sheep has a metabolism similar to the human fetus and is dependent on glucose for its major source of energy (4–6, 20, 22, 36, 44). As reported previously (27), MO ewes experiencing the dietary regimen as described in the present study exhibited markedly higher body weights and percent body fat, had elevated blood concentrations of glucose and insulin, and were more insulin resistant at both midgestation and late gestation than ewes fed to requirements. Interestingly, although MOI ewes in this study exhibited intermediate body weights and body fat percentages between MO and CON ewes throughout gestation, their baseline glucose and insulin concentrations, as well as insulin resistance, were similar to CON ewes. Most of the increase in percent body fat observed during pregnancy in this ovine model can be attributed largely to intra-abdominal fat accumulation, as reported previously in a study from our group (30). One mechanism by which pregnancy may contribute to future disease risk is via adverse metabolic effects mediated by excess deposition of visceral fat (33, 43). Visceral fat is not an inert storage depot; rather, it is a metabolically active endocrine tissue whose clinical effects remain largely uncharacterized (30, 33). Visceral adipose tissue is thought to differ from subcutaneous fat in the production of adipocytokines that may regulate insulin sensitivity and ultimately influence the development of type 2 diabetes (3). Indeed, our MO ewes exhibited markedly greater insulin response to glucose challenge at both mid- and late gestation IVGTT and elevated plasma glucose and insulin at necropsy in both gestational periods compared with CON and MOI ewes, indicating the development of an increased level of insulin resistance that was corrected by nutritional intervention in MOI ewes.
We did not evaluate adipose tissue type (white vs. brown) in the fetuses in this study. However, the mid- to late gestation transition of adipocytes in the perirenal depot was recently well characterized by Pope et al. (57). This depot represents ∼75% of the total adipose tissue in the fetal/neonatal sheep (2). Pope et al. (57) reported that, on day 80 of gestation, adipocytes exhibited a dense cellular structure and high mitotic rate, as evidenced by high expression of Ki-67, a marker of proliferating cells, without the key marker protein of brown fat UCP1. By day 140, as the depot increased in size, both white and brown adipocytes were clearly visible with significant UCP1 abundance. After birth, there was a notable reduction in the numbers of white adipocytes and maximal UCP1 abundance. One could speculate that day 135 MO fetuses may have greater numbers of brown adipocytes than CON and MOI fetuses, as cortisol is elevated in their blood at both midgestation and late gestation, and UCP1 is markedly increased in perirenal adipose tissue of fetal sheep by cortisol infusion and gestational age (50, 68).
The two notable maternal conditions predisposing human offspring to increased size and/or adiposity at birth are maternal overweight/obesity and maternal diabetes, both of which are marked by hyperinsulinemia and hyperglycemia (13, 27, 43, 62). Insulin is a fetal growth factor and has been shown to stimulate fetal growth rate in the sheep independent of differences in nutrient load (28, 36). In addition, insulin has been reported to increase placental glucose transporter capacity and system A (neutral amino acid transport) activity (25). Furthermore, Silverman et al. (63) reported a significant positive correlation between fetal pancreatic insulin secretion and obesity in offspring of diabetic mothers during adolescence. The baseline glucose concentration in the blood of MOI fetuses was still elevated despite the reduction in maternal glucose levels at late gestation. As discussed previously, glucose is the major energy substrate for fetal and placental metabolism in all mammalian species studied to date (35a). Placental glucose transport is accomplished by facilitated diffusion, and in the sheep, this is accomplished by two major GLUT isoforms, GLUT1 and GLUT3 (19, 24). Although placental mRNA and protein expression of both of these isoforms increases with gestational age, the increase in GLUT3 is far greater (16, 24). It has been reported that GLUT3 is localized to the apical maternal facing surface of the trophoblast cell layer that forms the fetomaternal interface, whereas GLUT1 is localized predominantly at the basolateral surfaces (18). Because of these differences in localization patterns, it was suggested by these authors that GLUT3, with a lower Km, readily takes up maternal glucose, whereas basolateral GLUT1, with a higher Km, facilitates its intracellular transport and exit. Thus the greater protein expression of GLUT3 observed in cotyledonary tissue of MO and MOI vs. CON in the present study suggests increased uptake of maternal glucose for delivery to the fetal compartment and may have resulted in the observed increase in glucose levels in fetal blood in these dietary groups.
Unlike many tissues, adipose tissue has the potential for unlimited growth. A direct influence of fetal adipocyte hyperplasia and/or hypertrophy might be anticipated by maternal overnutrition, since glucose is the primary metabolic precursor for lipid synthesis, and direct fetal glucose infusion in sheep is accompanied by a parallel increase in fat mass (46, 65). The importance of maternal glucose in fetal lipid synthesis is supported by the observed increase in perirenal fat weights of MO fetuses related to elevated blood glucose but not triglyceride concentrations in MO vs. MOI and CON ewes across both gestational stages. Cholesterol is an essential component for placental and fetal development, and the fetus synthesizes a significant amount of cholesterol (73). The marked decrease in circulating fetal triglycerides but not maternal triglycerides from mid- to late gestation in this study may result from decreased placental uptake from maternal blood. FATPs and FAT/CD36 (long-chain fatty acid acyl-coenzyme A synthetases) are integral membrane proteins that are important for cellular uptake of long-chain fatty acids (14, 41). We have reported previously that placental FATP1, FATP4, and CD36 mRNA expression are elevated with maternal obesity in the sheep in association with elevated fetal blood cholesterol and triglyceride concentrations (75). In agreement with these data, MO fetuses in the present study exhibited increased fetal blood levels of triglycerides and cholesterol compared with CON fetuses. In contrast, MOI fetuses exhibited blood cholesterol and triglyceride concentrations similar to those of CON fetuses in association with reduced placental FATP1 protein expression. Stahl et al. (66) reported that insulin-induced FATP1 translocation, but not FATP4 or CD36 translocation, coincides with increased long-chain fatty acid uptake in fat, and fatty acid uptake is completely abolished in adipocytes of FATP1-knockout mice. In contrast, neither FATP4 nor CD36 showed a similar dynamic localization in the same cells (66). This led them to conclude that insulin can regulate the uptake of long-chain fatty acids by tissues via FATP1 activation, determining the tissue distribution of dietary lipids. These data are consistent with the concept that the early pregnancy reduction of dietary intake of overfed/obese ewes to requirements reduced the transport of the maternal lipids into the fetal compartment in MOI ewes by reducing maternal and fetal blood insulin concentrations, downregulating placental FATP1 expression.
As reported previously (48), fetuses of MO ewes at midgestation were larger and heavier than CON fetuses, whereas on day 135, fetuses of MO ewes were similar in size and weight to fetuses from CON ewes. Evidence suggests that the reduced trajectory of fetal growth in MO ewes after midgestation may result from decreased placental vascularity (48). Research conducted in a well-characterized adolescent sheep model of acute excess nutrition demonstrated a 43% reduction in uterine blood flow on day 88 of gestation compared with controls (71). Furthermore, by late gestation in this model, both uterine and umbilical volume blood flows were markedly reduced (69). This reduction in placental flow may result from reduction in angiogenic factor expression and vascularity that we and others have observed in sheep placentae at midgestation through late gestation in response to diet-induced obesity (48, 59). These growth factors are vital for proliferation of placental capillaries through branching of new capillaries from preexisting vessels, a process crucial for proliferation of the placental capillary beds (8). Cortisol, which is elevated in the blood of MO fetuses at mid- and late gestation, may be partially responsible for this decreased placental vascularity, as Jensen et al. (39) reported that infusion of cortisol into the ovine fetus resulted in decreased blood flow through the cotyledonary (placental) zone of their placentomes. It is known that placental vascularity is positively related to blood flow through the fetal-maternal interface and with nutrient and oxygen delivery to the developing fetus (9, 64). Similarly, Frias et al. (29) reported that primates exhibiting obesity in response to being fed a high-fat diet during pregnancy exhibited evidence of decreased blood flow to the fetal side of the placenta coupled with histopathological evidence of decreased placental perfusion. Although primate and sheep placentae differ in many structural and functional aspects (11, 60), these data suggest a link between diet-induced maternal obesity, decreased uterine blood flow, and reduced placental perfusion, with potential impacts on fetal growth and development. In this study, fetuses from MOI ewes were similar in size and weight to CON ewes at both stages of gestation, suggesting a normal growth trajectory, implying normal placental vascularity and function.
As with fetal size and weight, fetal organs were also affected by maternal obesity at midgestation. Fetal ventricular free wall weights and thicknesses, as well as weights of the kidneys, pancreas, and liver, were increased significantly at midgestation in MO fetuses compared with fetuses from CON and MOI ewes, which were similar. Perhaps more importantly, whereas fetal weights of MO ewes had returned to that of CON and MOI ewes by day 135, left ventricular weights and right and left ventricular thicknesses remained greater, suggesting a permanent alteration in heart development in this dietary group. Altered fetal nutritional status, especially during the first two trimesters of pregnancy, has been shown to enhance susceptibility to cardiovascular, metabolic, and endocrine diseases during postnatal life (10, 31, 32). Several studies have demonstrated that elevated maternal glucocorticoids decrease the rate of fetal growth (47, 54, 65) and alter fetal cardiovascular function (38). Additionally, infusion of fetal sheep with cortisol from days 115 to 130 has been shown to reduce fetal growth rate and increase heart weight and ventricular wall thickness in association with increased arterial blood pressure (39). Thus, the elevated cortisol in both maternal and fetal blood of MO but not MOI or CON ewes from days 75 through 135 of gestation may slow fetal growth and alter heart growth trajectory and function. These data confirm previous reports by Dong et al. (23) and George et al. (30), using the same maternal obesity paradigm, who reported that both right and left ventricular weights were increased in fetuses from overfed vs. control ewes throughout gestation. Furthermore, we have demonstrated previously that MO results in greater fetal left ventricular connective tissue accumulation associated with an upregulated TGFβ/p38 signaling pathway in late gestation (37), which has been linked to myocardial stiffening and loss of myocardial compliance (40). Additionally, using the same cohort of sheep, inflammation was detected in the fetal myocardium of MO ewes, as evidenced by enhanced phosphorylation of JNK, which correlated with impaired contractile function of fetal myocardia in response to high workload stress (72). Thus, the return of fetal cardiac ventricular weights and thicknesses of MOI ewes to that of fetuses from CON ewes by late gestation suggest a recovery of normal cardiac growth trajectory in this group. We are currently evaluating the impacts of MOI on returning the composition, biochemistry, and function of the fetal heart to control levels.
Although the source of the increased cortisol in both mother and fetus is most likely to be the pituitary adrenal axis, we have previously demonstrated increased local cortisol production in fetal baboon perirenal fat accompanied by increased expression of 11β-hydroxysteroid dehydrogenase 1 that converts inactive cortisone to cortisol (34). Therefore, it is possible that the increased cortisol in the ewe and fetal lamb in the presence of increased adipose tissue results from local adipose tissue production of cortisol. If this is so, one could postulate a positive feed-forward system where increased circulating cortisol in the setting of maternal obesity induces expression of 11β-hydroxysteroid dehydrogenase 1 in adipose tissues, thus producing feed-forward of cortisol generation and expression of glucocorticoid-activated genes that regulate adipogenesis and thus further cortisol production.
These data demonstrate for the first time in a large precocial species that reducing the maternal diet of obese/overnourished ewes to requirements in early gestation prevents resultant alterations in fetal growth, adiposity, and glucose/insulin dynamics throughout gestation. The timing of the dietary reduction utilized in this study is based on previous studies in our laboratory demonstrating that maternal undernutrition (50% global undernutrition) starting on day 28 of gestation in the ewe resulted in fetal intrauterine growth restriction by midgestation (30), and the resultant offspring exhibited significant metabolic and cardiovascular disturbances (i.e., they exhibited hyperphagia, increased insulin resistance, and adiposity and were hypertensive) as adults (45). It was thus predicted that a dietary intervention where obesogenic diets were reduced from 150 to 100% of NRC requirements beginning on day 28 of gestation would be early enough to correct the negative impacts of maternal overnutrition/obesity on the fetus and resulting offspring. Day 28 of pregnancy in the sheep is equivalent to approximately day 50 in human pregnancy, which is about the time when women confirm that they are pregnant and early enough for a doctor to provide overweight/obese women with a corrective dietary regimen. Given the relative importance of maternal metabolic parameters on offspring development in humans and the similarities between our ovine MOI model data and those from obese pregnancies in humans, we suggest that our model could potentially lead to a better understanding of the specific control mechanisms involved.
GRANTS
This work was supported by the Division of Research Resources, National Institutes of Health (INBRE-P20-RR-016474).
DISCLOSURES
The authors declare that they have no competing interests, financial or otherwise.
AUTHOR CONTRIBUTIONS
N.T., N.M.L., and S.P.F. contributed to the conception and design of the research; N.T., J.F.O., N.M.L., D.R.S., and S.P.F. performed the experiments; N.T. and J.F.O. analyzed the data; N.T., J.F.O., and S.P.F. interpreted the results of the experiments; N.T. prepared the figures; N.T., J.F.O., and S.P.F. drafted the manuscript; N.T., J.F.O., N.M.L., D.R.S., P.W.N., and S.P.F. approved the final version of the manuscript; S.P.F. edited and revised the manuscript.
ACKNOWLEDGMENTS
We thank Christopher Dorozynski of the University of Wyoming Department of Kinesiology and Health for providing DEXA evaluations and the students at the Center for the Study of Fetal Programming for their assistance in animal care and data collection on the farm. We also thank Adam Uthlaut and Robert Cordery-Cotter for animal care and management.
REFERENCES
- 1.Adamo KB, Ferraro ZM, Brett KE. Can we modify the intrauterine environment to halt the intergenerational cycle of obesity? Int J Environ Res Public Health 9: 1263–1307, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander G, Bell AW. Quantity and calculated oxygen consumption during summit metabolism of brown adipose tissue in newborn lambs. Biol Neonate 26: 214–220, 1975 [DOI] [PubMed] [Google Scholar]
- 3.Altomonte J, Harbaran S, Richter A, Dong H. Fat depot-specific expression of adiponectin is impaired in Zucker fatty rats. Metabolism 52: 958–963, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Anderson MS, Flowers-Ziegler J, Das UG, Hay WW, Jr, Devaskar SU. Glucose transporter protein responses to selective hyperglycemia or hyperinsulinemia in fetal sheep. Am J Physiol Regul Integr Comp Physiol 281: R1545–R1552, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Anderson MS, He J, Flowers-Ziegler J, Devaskar SU, Hay WW., Jr Effects of selective hyperglycemia and hyperinsulinemia on glucose transporters in fetal ovine skeletal muscle. Am J Physiol Regul Integr Comp Physiol 281: R1256–R1263, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Anderson MS, Thamotharan M, Kao D, Devaskar SU, Qiao L, Friedman JE. Effects of acute hyperinsulinemia on insulin signal transduction and glucose transporters in ovine fetal skeletal muscle. Am J Physiol Regul Integr Comp Physiol 288: R473–R481, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Anthony RV, Scheaffer AN, Wright CD, Regnault TR. Ruminant models of prenatal growth restriction. Reprod Suppl 61: 183–194, 2003 [PubMed] [Google Scholar]
- 8.Aron EA, Anthony RV. Angiogenesis. Fetal and Neonatal Physiology (3rd ed.). Philadelphia, PA: W. B. Saunders, 2003 [Google Scholar]
- 9.Bassingthwaighte JB, Goresky CA. Modeling in the analysis of solute and water exchange in the microvasculature. In: Handbook of Physiology: The Cardiovascular System. Microcirculation. Bethesda, MD: Am. Physiol. Soc., 1984, sect. 2, vol. IV, pt. 1, chapt. 13, p. 549–626 [Google Scholar]
- 10.Buckley AJ, Jaquiery AL, Harding JE. Nutritional programming of adult disease. Cell Tissue Res 322: 73–79, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Carter AM. Animal models of human placentation—a review. Placenta 28: S41–S47, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Catalano PM, Tyzbir ED, Allen SR, McBean JH, McAuliffe TL. Evaluation of fetal growth by estimation of neonatal body composition. Obstet Gynecol 79: 46–50, 1992 [PubMed] [Google Scholar]
- 13.Chu SY, Kim SY, Bish CL. Prepregnancy obesity prevalence in the United States, 2004–2005. Matern Child Health J 13: 614–620, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Coburn CT, Hajri T, Ibrahimi A, Abumrad NA. Role of CD36 in membrane transport and utilization of long-chain fatty acids by different tissues. J Mol Neurosci 16: 117–121, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Crane JM, White J, Murphy P, Burrage L, Hutchens D. The effect of gestational weight gain by body mass index on maternal and neonatal outcomes. J Obstet Gynaecol Can 31: 28–35, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Currie MJ, Bassett NS, Gluckman PD. Ovine glucose transporter-1 and -3 cDNA partial sequences and developmental gene expression in the placenta. Placenta 18: 393–401, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Dabelea D, Hanson RL, Lindsey RS, Pettitt DJ, Impesatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to obesity conveys risks for type 2 diabetes: a study of discordant sib ships. Diabetes 49: 2208–2211, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Das UG, Jing H, Ehrhardt RA, Hay WW, Jr, Devaskar SU. Time-dependent physiological regulation of ovine placental GLUT-3 glucose transporter protein. Am J Physiol Regul Integr Comp Physiol 279: R2252–R2261, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Das UG, Sadiq HF, Soares MJ, Hay WW, Jr, Devaskar SU. Time-dependent physiological regulation of rodent and ovine placental glucose transporter (GLUT-1) protein. Am J Physiol Regul Integr Comp Physiol 274: R339–R347, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Das UG, Schroeder RE, Hay WW, Devaskar SU. Time-dependent and tissue-specific effects of circulating glucose on fetal ovine glucose transporters. Am J Physiol Regul Integr Comp Physiol 276: R809–R817, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Di Cianni G, Miccoli R, Volpe L, Lencioni C, Ghio A, Giovannitti MG, Cuccuru I, Pellegrini G, Chatzianagnostou K, Boldrini A, Del Prato S. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med 22: 21–25 2005 [DOI] [PubMed] [Google Scholar]
- 22.DiGiacomo JE, Hay WW. Effect of hypoinsulinemia and hyperglycemia on fetal glucose utilization. Am J Physiol Endocrinol Metab 259: E506–E512, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Dong F, Ford SP, Nijland MJ, Nathanielsz PW, Ren J. Influence of maternal undernutrition and overfeeding on cardiac ciliary neurotrophic factor receptor and ventricular size in fetal sheep. J Nutr Biochem 19: 409–414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehrhardt RA, Bell AW. Developmental increases in glucose transporter concentration in the sheep placenta. Am J Physiol Regul Integr Comp Physiol 273: R1132–R1141, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Ericsson A, Hamark B, Jansson N, Johansson BR, Powell TL, Jansson T. Hormonal regulation of glucose and system A amino acid transport in first trimester placental villous fragments. Am J Physiol Regul Integr Comp Physiol 288: R656–R662, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Ford SP, Hess BW, Schwope MM, Nijland MJ, Gilbert JS, Vonnahme KA, Means WJ, Han H, Nathanielsz PW. Maternal undernutrition during early gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J Anim Sci 85: 1285–1294, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Ford SP, Zhang L, Zhu M, Miller MM, Smith DT, Hess BW, Moss GE, Nathanielsz PW, Nijland MJ. Maternal obesity accelerates fetal pancreatic β-cell but not α-cell development in sheep: prenatal consequences. Am J Physiol Regul Integr Comp Physiol 297: R835–R843, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowden AL, Hay WW. The effects of pancreatectomy on the rates of glucose utilization, oxidation and production in the sheep fetus. Q J Exp Physiol 73: 973–984, 1988 [DOI] [PubMed] [Google Scholar]
- 29.Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, Grove KL. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 152: 2456–2464, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George LA, Uthlaut AB, Long NM, Zhang L, Ma Y, Smith DT, Nathanielsz PW, Ford SP. Different levels of overnutrition and weight gain during pregnancy have differential effects on fetal growth and organ development. Reprod Biol Endocrinol 8: 75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr 71: 1344S–1352S, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Godfrey K, Robinson S. Maternal nutrition, placental growth and fetal programming. Proc Nutr Soc 57: 105–111, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Gunderson EP, Sternfeld B, Wellons MF, Whitmer RA, Chiang V, Quesenberry CP, Lewis CE, Sidney S. Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity (Silver Spring) 16: 1078–1084, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo, Li C, Myatt L, Nathanielsz PW, Sun K. Sexually dimorphic effects of maternal nutrient reduction on expression of genes regulating cortisol metabolism in fetal baboon adipose and liver tissues. Diabetes 62: 1175–1185, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammami M, Walters JC, Hockman EM, Koo WW. Disproportionate alterations in body composition of large for gestational age neonates. J Pediatr 138: 817–821, 2001 [DOI] [PubMed] [Google Scholar]
- 35a.Hay WW., Jr.Placental-fetal glucose exchange and fetal glucose metabolism. Trans Am Clin Climatol Assoc 117: 321–339; discussion 339–340, 2006 [PMC free article] [PubMed] [Google Scholar]
- 36.Hay WW, DiGiacomo JE, Meznarich HK, Hirst K, Zerbe G. Effects of glucose and insulin on fetal glucose oxidation and oxygen consumption. Am J Physiol Endocrinol Metab 256: E704–E713, 1989 [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Yan X, Zhao JX, McCormick RJ, Ford SP, Nathanielsz PW, Ren J, Du M. Maternal obesity induces fibrosis in fetal myocardium of sheep. Am J Physiol Endocrinol Metab 299: E968–E975, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen EC, Gallaher BW, Breier BH, Harding JE. The effect of a chronic maternal cortisol infusion on the late-gestation fetal sheep. J Endocrinol 174: 27–36, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Jensen E, Wood CE, Keller-Wood M. Chronic alterations in ovine maternal corticosteroid levels influence uterine blood flow and placental and fetal growth. Am J Physiol Regul Integr Comp Physiol 288: R54–R61, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Kai H, Kuwahara F, Tokuda K, Imaizuma T. Diastolic dysfunction in hypertensive hearts: roles of perivascular inflammation and reactive myocardial fibrosis. Hypertens Res 28: 483–490, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Kazantzis M, Stahl A. Fatty acid transport proteins, implications in physiology and disease. Biochim Biophys Acta 1821: 852–857, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity (Silver Spring) 15: 986–993, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Lemieux S, Prud'homme D, Nadeau A, Tremblay A, Bouchard C, Després JP. Seven-year changes in body fat and visceral adipose tissue in women. Association with indexes of plasma glucose-insulin homeostasis. Diabetes Care 19: 983–991, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Limesand SW, Rozance PJ, Smith D, Hay WW., Jr Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 293: E1716–E1725, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Long NM, George LA, Uthlaut AB, Smith DT, Nijland MJ, Nathanielsz PW, Ford SP. Maternal obesity and increased nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring. J Anim Sci 88: 3546–3553, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Long NM, Rule DC, Zhu MJ, Nathanielsz PW, Ford SP. Maternal obesity upregulates fatty acid and glucose transporters and increases expression of enzymes mediating fatty acid biosynthesis in fetal adipose tissue depots. J Anim Sci 90: 2201–2210, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Long NM, Shasa DR, Ford SP, Nathanielsz PW. Growth and insulin dynamics in two generations of female offspring of mothers receiving a single course of synthetic glucocorticoids. Am J Obstet Gynecol 207: e1–e8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma Y, Zhu MJ, Zhang L, Hein SM, Nathanielsz PW, Ford SP. Maternal obesity and overnutrition alter fetal growth rate and cotyledonary vascularity and angiogenic factor expression in the ewe. Am J Physiol Regul Integr Comp Physiol 299: R249–R258, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mercier J, Pomar C, Marcoux M, Goulet F, Thériault M, Castonguay FW. The use of dual-energy X-ray absorptiometry to estimate the dissected composition of lamb carcasses. Meat Sci 73: 249–257, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Mostyn A, Pearce S, Budge H, Elmes M, Forhead AJ, Fowden AL, Stephenson, Symonds ME. Influence of cortisol on adipose tissue development in the fetal sheep during late gestation. J Endocrinol 176: 23–30, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Muhlhausler BS, Adam CL, Findlay PA, Duffield JA, McMillen IC. Increased maternal nutrition alters development of the appetite-regulating network in the brain. FASEB J 20: 1257–1259, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Muhlhausler BS, Duffield JA, McMillen IC. Increased maternal nutrition increases leptin expression in perirenal and subcutaneous adipose tissue in the postnatal lamb. Endocrinology 148: 6157–6163, 2007 [DOI] [PubMed] [Google Scholar]
- 53.National Research Council Nutrient Requirements of Sheep. Washington, DC: National Academies, 2007 [Google Scholar]
- 54.Newnham JP, Evans SF, Godfrey M, Huang W, Ikegami M, Jobe A. Maternal, but not fetal, administration of corticosteroids restricts fetal growth. J Matern Fetal Med 8: 81–87, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Pearce KL, Ferguson M, Gardner G, Smith N, Greef J, Pethick DW. Dual X-ray absorptiometry accurately predicts carcass composition from live sheep and chemical composition of live and dead sheep. Meat Sci 81: 285–293, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Plagemann A, Harder T, Kohlhoff R, Rhode W, Dorner G. Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int J Obes Relat Metab Disord 21: 451–456, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Pope M, Budge H, Symonds ME. The developmental transition of ovine adipose tissue through early life. Acta Physiol (Oxf). In press [DOI] [PubMed] [Google Scholar]
- 58.Ramachenderan J, Bradford J, McLean M. Maternal obesity and pregnancy complications: a review. Aust N Z J Obstet Gynaecol 48: 228–235, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Redmer DA, Luther JS, Milne JS, Aitken RP, Johnson ML, Borowicz PP, Borowicz MA, Reynolds LP, Wallace JM. Fetoplacental growth and vascular development in overnourished adolescent sheep at day 50, 90 and 130 of gestation. Reproduction 137: 749–757, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Reynolds LP, Borowitz PP, Vonnahme KA, Johnson ML, Grazul-Bilska AT, Wallace JM, Caton JS, Redmer DA. Animal models of placental angiogenesis. Placenta 26: 689–708, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Sanson DW, West TR, Tatman WR, Riley ML, Judkins MB, Moss GE. Relationship of body composition of mature ewes with condition score and body weight. J Anim Sci 71: 1112–1116, 1993 [DOI] [PubMed] [Google Scholar]
- 62.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol 195: 1100–1103, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. Diabetes Care 21: B142–B149, 1998 [PubMed] [Google Scholar]
- 64.Smith JJ, Kampine JP. Circulatory Physiology—The Essentials (3rd ed.). Baltimore, MD: Williams & Wilkins, 1990 [Google Scholar]
- 65.Sloboda DM, Newnham JP, Challis JR. Effects of repeated maternal betamethasone administration on growth and hypothalamic-pituitary-adrenal function in the ovine fetus at term. J Endocrinol 165: 79–91, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Stahl A, Evans JG, Pattel S, Hirsch D, Lodish HF. Insulin causes fatty acid transport translocation and enhanced fatty acid uptake in adipocytes. Dev Cell 2: 277–488, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Stevens D, Alexander G, Bell AW. Effect of prolonged glucose infusion into fetal sheep on body growth, fat deposition and gestation length. J Dev Physiol 13: 277–281, 1990 [PubMed] [Google Scholar]
- 68.Symonds ME, Pope M, Sharkey D, Budge H. Adipose tissue and fetal programming. Diabetologia 55: 1597–1606, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, Kunz R, Mol BW, Coomarasamy A, Khan KS. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ 344: e2088, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wallace JM, Bourke DA, Aitken RP, Leitch N, Hay WW., Jr Blood flows and nutrient uptakes in growth-restricted pregnancies induced by overnourishing adolescent sheep. Am J Physiol Regul Integr Comp Physiol 282: R1027–R1036, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Wallace JM, Milne JS, Matsuzaki M, Aitken RP. Serial measurement of uterine blood flow from mid to late gestation in growth restricted pregnancies induced by overnourishing adolescent sheep dams. Placenta 29: 718–724, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Wang J, Ma H, Tong C, Zhang M, Lawlis GB, Li Y, Zang M, Ren J, Nijland MJ, Ford SP, Nathanielsz PW, Li J. Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart. FASEB J 24: 2066–2076, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woollett LA. Review: Transport of maternal cholesterol to the fetal circulation. Placenta 25: S218–S221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yogev Y, Catalano PM. Pregnancy and obesity. Obstet Gynecol Clin North Am 36: 285–300, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Zhu MJ, Ma Y, Long NM, Du M, Ford SP. Maternal obesity markedly increases placental fatty acid transporter expression and fetal blood triglycerides at midgestation in the ewe. Am J Physiol Regul Integr Comp Physiol 299: R1224–R1231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]