Abstract
One workweek of mild sleep restriction adversely impacts sleepiness, performance, and proinflammatory cytokines. Many individuals try to overcome these adverse effects by extending their sleep on weekends. To assess whether extended recovery sleep reverses the effects of mild sleep restriction on sleepiness/alertness, inflammation, and stress hormones, 30 healthy young men and women (mean age ± SD, 24.7 ± 3.5 yr; mean body mass index ± SD, 23.6 ± 2.4 kg/m2) participated in a sleep laboratory experiment of 13 nights [4 baseline nights (8 h/night), followed by 6 sleep restriction nights (6 h/night) and 3 recovery nights (10 h/night)]. Twenty-four-hour profiles of circulating IL-6 and cortisol, objective and subjective daytime sleepiness (Multiple Sleep Latency Test and Stanford Sleepiness Scale), and performance (Psychomotor Vigilance Task) were assessed on days 4 (baseline), 10 (after 1 wk of sleep restriction), and 13 (after 2 nights of recovery sleep). Serial 24-h IL-6 plasma levels increased significantly during sleep restriction and returned to baseline after recovery sleep. Serial 24-h cortisol levels during restriction did not change compared with baseline, but after recovery they were significantly lower. Subjective and objective sleepiness increased significantly after restriction and returned to baseline after recovery. In contrast, performance deteriorated significantly after restriction and did not improve after recovery. Extended recovery sleep over the weekend reverses the impact of one work week of mild sleep restriction on daytime sleepiness, fatigue, and IL-6 levels, reduces cortisol levels, but does not correct performance deficits. The long-term effects of a repeated sleep restriction/sleep recovery weekly cycle in humans remain unknown.
Keywords: recovery sleep, sleep restriction, alertness, cortisol, Il-6
in modern societies, increasing work demands and lifestyle changes have resulted in adults sleeping considerably less than the seven hours per night considered to be the average sleep time necessary to sustain optimal daytime functioning (8, 23). Experimental studies in healthy young adults have consistently demonstrated that chronic sleep restriction results in a number of abnormal physiological changes, including increased inflammatory markers (15, 22, 39) and impaired blood glucose regulation (33), which may be the mechanisms through which chronic sleep curtailment may affect health and longevity. A recent U.S. National Sleep Foundation survey showed that about 25% of the population do not get enough sleep during the weekdays due to the work demands, whereas about 40% sleep longer during the weekend trying to “catch up” for the shorter weekdays' sleep duration (23). Although it is commonly believed that sleep loss accumulated during the week can be compensated for by extending sleep over the weekend, it is not known whether recovery sleep adequately reverses the adverse effects of sleep loss. Most studies on the effects of recovery sleep after short-term sleep restriction are focused on psychomotor performance, which appears not to be fully restored by one, two, or three nights of recovery sleep (3, 4, 5, 11). Previous sleep restriction/recovery studies that included both genders have not found significant gender differences in terms of sleep architecture or psychomotor performance responses to the insufficient sleep (4). However, other studies have shown that women may be more resilient to the effects of sleep loss given that one week of mild sleep restriction resulted in elevation of the proinflammatory cytokine TNFα only in men (39). Furthermore, women have been shown to be more resilient than men to the sleep-disturbing effects of blood drawing procedures (6).
A two-hour afternoon nap after one night of total sleep loss decreases sleepiness, increases performance, and causes beneficial changes in cortisol and IL-6 secretion (37). However, no study to date has assessed the effects of extended sleep following a week of mild sleep restriction on around-the-clock hormone and cytokine secretory profiles in parallel with sleepiness and performance. The aim of this study was to assess objectively the effects of extended “weekend” recovery sleep following “one work week” of mild sleep restriction on sleepiness/alertness, inflammation, and stress hormones.
MATERIALS AND METHODS
Subjects
Thirty normal sleepers (16 men and 14 women), 18–34 yr of age (mean ± SD, 24.4 ± 3.6 and 25.1 ± 3.3 yr for men and women, respectively) with a body mass index (BMI) of 23.7 ± 2.5 and 23.5 ± 2.0 kg/m2 for men and women, respectively, were recruited from the community of the greater Harrisburg area and from the medical and technical staff and students of the Milton Hershey Medical Center. They were healthy, physically active but not excessively so, had no sleep complaints or circadian abnormalities, were not taking any medications, and were screened in the sleep laboratory for sleep-disordered breathing, nocturnal myoclonus, or other primary sleep disorders. Also, a battery of clinical tests (including a complete cell blood count, urinalysis, thyroid indexes, electrocardiogram, and urine screen for drug use) was negative for abnormal findings.
Protocol
Each subject participated in a sleep laboratory experiment that lasted 13 days (Table 1). Adequate sleep time and regular sleep schedules (i.e., habitual betime between 2200 and 2300, habitual sleep duration between 7.5 and 8 h of sleep per night, and no exposure to sleep restriction/recovery sleep pattern) were verified with a sleep log and actigraphy for 2 wk prior to the study while the subjects were screened for drug use. During the first 4 consecutive nights (baseline nights), the subjects were allowed to sleep for 8 h (2230–0630). During the subsequent 6 nights (sleep restriction nights), the subjects were awakened 2 h earlier (0430). During the next 3 nights (recovery nights), they were allowed to sleep for 10 h (2230–0830). We chose a 5-day restriction period and a 2-night recovery period to mimic the weeklong work schedule and rest period of a typical worker. Blood sampling was performed for 24 h every hour three times: 1) on the 4th day (the last day of the baseline period), 2) the 10th day following completion of 5-day sleep restriction, and 3) the 13th day following completion of 2 recovery nights. Start time of blood sampling at baseline and restriction was 0800 and for recovery was 0900. Subjects spent the days of blood sampling, i.e., the 4th, the 10th, and the 13th nights of the study, in the laboratory, where they were ambulatory and allowed to watch television, play computer games, go to the bathroom, etc., whereas they were dismissed from the laboratory to their daily activities during the rest of the study days, i.e., following nights 1–3, 5–9, and 11–12, respectively. They were instructed not to nap, to stay on the same diet including no use of caffeinated beverages, and not to deviate from their normal daily routines, including exercise throughout the experiment, which was documented by the daily log that subjects kept throughout the study. Their compliance was assessed by the sleep lab technicians using a daily activities questionnaire following admission to the sleep lab on nights 1–3, 5–9, and 11–12, respectively. To assess compliance of the subjects with the no-nap instructions, the subjects were monitored with actigraphy attached to the wrist of the nondominant hand throughout the experiment. We obtained sleep profiles that did not include sleep measures from the blood drawing nights. In the analysis of the sleep architecture, we calculated baseline sleep by averaging nights 2 and 3, nights 8 and 9 for the restriction period, and nights 11 and 12 for the recovery period, respectively. In 11 of 14 female subjects, all three periods of blood sampling took place during the follicular phase, as evidenced by progesterone levels that were obtained on the 4th, 10th, and 13th days of the experiment. In three female subjects, the last blood sampling period, i.e., the 13th day (following 2 recovery nights), took place in the luteal phase during the recovery period, as evidenced by progesterone levels >3.3 ng/ml. However, the pattern of changes of sleep architecture and levels of IL-6 and cortisol during the recovery of these three women were similar to the rest of them and were included in the analysis. An indwelling catheter was inserted in the antecubital vein about 30 min before the first blood sample. The catheter was kept patent with small amounts of heparin (the total amount of heparin did not exceed 800 U/24-h period). Hourly IL-6 and cortisol samples were assayed. During the days of blood sampling, objective assessment of daytime alertness/sleepiness was assessed with a standard Multiple Sleep Latency Test (MSLT) procedure six times during the day, whereas performance was assessed with Psychomotor Vigilance Task (PVT) every 2 h. Furthermore, subjective levels of sleepiness were measured with the Stanford Sleepiness Scale (SSS) hourly, during the days of blood drawing. Also, subjects were instructed not to change their diet, and their three daily meals were at about 0830, 1300, and 1800. The protocol was approved by the Institutional Review Board of the Pennsylvania State College of Medicine, and each subject signed a written consent form.
Table 1.
Sleep restriction/recovery protocol in young men and women
| Baseline, 8 h | Restriction, 6 h | Recovery, 10 h | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep laboratory | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| PSG | x | x | x | x | x | x | x | x | x | x | x | x | x |
| MSLT | x | x | x | ||||||||||
| SSS | x | x | x | ||||||||||
| PVT | x | x | x | ||||||||||
| 24-h Blood sampling | x | x | x | ||||||||||
PSG, polysomnography; MSLT, Multiple Sleep Latency Test; SSS, Stanford Sleepiness Scale; PVT, Psychomotor Vigilance Task.
Assessment of Nighttime Sleep
Sleep laboratory recordings were carried out in a sound-attenuated, light- and temperature-controlled room, which had a comfortable bedroom-like atmosphere. Each subject was monitored with EEG, electrooculogram, and electromyogram recordings continuously for 8 h (2230–0630) during the first 4 nights, for 6 h (2230–0430) during the subsequent 6 nights, and for 10 h (2230–0830) during the remaining 3 nights. The sleep schedule in the laboratory at baseline was similar to the subjects' normal sleep schedule. Specifically, the subjects' habitual times to go to bed and rise in the morning were no different than an hour from their sleep schedule in the laboratory. Also, total sleep duration 2 wk prior to the study, based on sleep log and actigraphy, was 7.9 ± 0.1 h and 7.2 ± 0.1 h, respectively. The polysomnographic data recording was accomplished through Grass-Telefactor Gamma Sleep Recording and Analysis Software v. 4.2. The sleep records were subsequently visually scored independently of knowledge of the experimental conditions according to standardized criteria (26). Sleep parameters were assessed from the sleep recordings as previously described (39).
Assessment of Daytime Sleepiness and Performance
MSLT.
At baseline (4th day) and restriction (10th day), the subjects' objective levels of sleepiness were evaluated six times following standard MSLT procedures, i.e., 0900, 1200, 1500, 1700, 1900, and 2100, whereas at recovery (13th day), MSLT was performed at 1000, 1200, 1500, 1700, 1900, and 2100 (28, 37, 39). Onset of sleep was defined as attaining any sleep stage for a duration of one epoch (30 s) or longer (28). The MSLT was terminated 20 min after lights-out if there had been no sleep or after two consecutive epochs of stage 2 (28).
SSS.
At baseline (4th day) and restriction (10th day), subjective levels of sleepiness were assessed every hour starting at 0800, whereas at recovery (13th day), sleepiness was assessed starting at 0900 using SSS (17, 37).
PVT.
At baseline (4th day) and restriction (10th day), the subjects' behavioral performance was evaluated from 0800 to 2200 every 2 h using PVT (11), while at recovery (13th day), PVT was performed every 2 h from 1000 to 2200. Each PVT trial lasted for 10 min, and each subject had three practice trials within an hour the night before the next day's testing.
Hormone and Cytokine Assays
Cortisol levels were measured by specific radioimmunoassay techniques, and IL-6 was measured by ELISA (R&D Systems, Minneapolis, MN), as previously described (39). The lower limit of detection for cortisol and IL-6 were 0.7 μg/dl and 0.094 pg/ml, respectively.
Statistical Analysis
To assess the effect of 1 wk of mild sleep restriction and 2 days of recovery sleep on cortisol and IL-6 values, linear mixed effects models were used for the repeated-measures analyses. Group (baseline, restriction, and recovery), time (as categorical variable), gender, and their interactions were included as fixed effects in the model. Due to the study design, i.e., unequal time periods between the three phases of the study, the specified covariance structure was Spatial Power (SP). Similar analyses using linear mixed effect modeling were performed to assess the effects of sleep restriction and subsequent recovery sleep on MSLT, SSS, and PVT values. Because IL-6 plasma values are mildly affected by the blood drawing technique, i.e., IL-6 values are higher at the end of the 24-h blood draw than at the beginning (16, 38), we proceeded with a “detrended” analysis as previously described (36).
Data are presented as means ± SE for the purpose of group comparisons, except for demographic and anthropometric data (age and BMI), for which we used SD to describe variance.
RESULTS
Sleep at Baseline, After 1 wk of Mild Sleep Restriction, and After 2 Nights of Recovery Sleep
At the end of mild sleep restriction, subjects demonstrated significantly shorter sleep latency (SL), a marginally significantly increased percentage of total sleep time (%ST), and significantly decreased percentage of wake time after sleep onset (%WTASO) (Table 2). In terms of sleep stages, subjects demonstrated increased slow-wave sleep percentage (%SWS) and spent less time in stage 1 sleep (Table 2). These changes were reversed during recovery sleep.
Table 2.
Nighttime sleep at baseline, restriction, and recovery in 30 normal sleepers, young men and women
| n = 30 | B (nts 2–3) | SR (nts 8–9) | R (nts 11–12) | B vs. SR | SR vs. R | B vs. R |
|---|---|---|---|---|---|---|
| SL, min | 18.5 ± 1.9 | 13.3 ± 1.4 | 15.6 ± 1.8 | 0.03 | 0.31 | 0.28 |
| %ST | 91.8 ± 0.8 | 93.5 ± 0.5 | 91.5 ± 0.7 | 0.07 | 0.02 | 0.77 |
| %WTASO | 5.3 ± 0.5 | 3.7 ± 0.5 | 6.5 ± 0.5 | 0.03 | <0.01 | 0.13 |
| %Stage 1 | 7.2 ± 0.7 | 5.3 ± 0.7 | 7.5 ± 0.7 | 0.03 | <0.01 | 0.68 |
| %Stage 2 | 51.5 ± 1.4 | 49.7 ± 1.4 | 51.1 ± 1.4 | 0.28 | 0.31 | 0.82 |
| %Stage 3 | 5.9 ± 0.5 | 7.4 ± 0.5 | 4.8 ± 0.5 | 0.02 | <0.01 | 0.13 |
| %Stage 4 | 12.1 ± 1.4 | 16.5 ± 1.4 | 10.5 ± 1.4 | <0.01 | <0.01 | 0.38 |
| %SWS | 17.9 ± 1.4 | 23.9 ± 1.4 | 15.4 ± 1.4 | <0.01 | <0.01 | 0.13 |
| SWS, min | 78.8 ± 6.3 | 81.3 ± 6.3 | 84.4 ± 6.3 | 0.69 | 0.54 | 0.46 |
| %REM | 23.4 ± 0.8 | 21.2 ± 0.8 | 25.7 ± 0.8 | 0.05 | <0.01 | 0.02 |
| REM, min | 102.9 ± 3.7 | 72.5 ± 3.7 | 142.9 ± 3.7 | <0.01 | <0.01 | <0.01 |
| REM latency, min | 80.9 ± 3.6 | 73.9 ± 3.6 | 71.8 ± 3.6 | 0.17 | 0.69 | 0.08 |
Data present means ± SE. B, baseline, 8 h; SR, sleep restriction, 6 h; R, recovery, 10 h; SL, sleep latency; WTASO, wake time after sleep onset; ST, sleep time; SWS, slow wave sleep; REM, rapid eye movements.
Absolute amount of SWS was preserved during both the restriction and recovery periods, whereas both percentage and absolute amount of REM sleep decreased during restriction and increased significantly during recovery (Table 2). There were no changes in REM latency throughout the study. Also, at baseline, women had significantly lower amounts of stage 1 (P < 0.05) and stage 2 (P < 0.05) sleep and greater amounts of SWS than men in terms of both percentage and absolute amount (P < 0.01). Interestingly, the responses of SL, %ST, and %REM to sleep restriction were significant or marginally significant in women (baseline minus restriction: 7.79 ± 3.46, P = 0.03; −2.65 ± 1.40, P = 0.06; 2.95 ± 1.56, P = 0.06, respectively), but not in men, who, however, showed similar nonsignificant trends (baseline minus restriction: 2.67 ± 3.23, P = 0.41; −0.85 ± 1.31, P = 0.52; 1.44 ± 1.46 P = 0.33, respectively).
Daytime Sleepiness and Performance
Objective sleepiness: MSLT.
There was no significant group by gender (P = 0.75) nor gender by time interaction (P = 0.66) effect; thus, data were analyzed for the entire group.
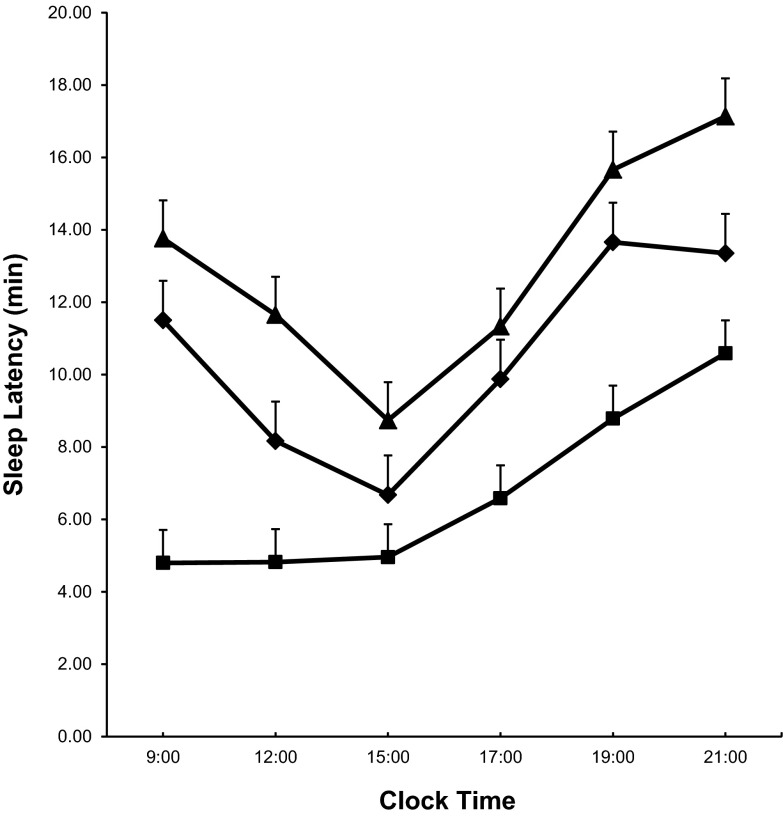
After 1 wk of modest sleep loss, the average daily SL decreased compared with baseline [difference of the mean SL (baseline minus restriction): 3.78 ± 0.91 min, P < 0.01; Fig. 1], with the strongest responses observed at 0900 and 1900 h, when sleep latency decreased by 6.70 ± 1.42 min and 4.87 ± 1.42 min, respectively (both P < 0.01). After 2 recovery nights, the average daily SL increased compared with restriction [difference of the mean SL (restriction minus recovery): 6.29 ± 0.95 min, P < 0.01]. Interestingly, the average daily SL was longer after 2 recovery nights compared with baseline [difference of the mean SL (baseline minus recovery): −2.50 ± 0.90 min, P < 0.01]. Of note also is that, during both baseline and recovery, the shortest SL was observed at 1500, whereas after sleep restriction it was observed at 0900.
Fig. 1.
Multiple sleep latency test mean values at baseline (♦), restriction (■), and recovery (▲).
Subjective sleepiness: SSS.
Neither group by gender nor gender by time interaction effects were observed for SSS. Subjective sleepiness increased after sleep restriction compared with baseline level [difference for the SSS (baseline minus restriction): −0.96 ± 0.18, P < 0.01], whereas following recovery sleep it improved even more and was significantly lower than baseline level [difference for the SSS (baseline minus recovery): 0.24 ± 0.12, P = 0.04].
Performance: PVT.
Neither group by gender nor gender by time or group by time interaction effects were observed for any of the PVT variables. A significant deterioration of performance in number of lapses, median RT, and fastest 10% RTs per trial was observed after 1 wk of mild sleep restriction compared with baseline (Table 3). No improvement was observed in any of the variables examined after recovery compared with restriction (Table 3).
Table 3.
Summary of performance variables at baseline, restriction, and recovery in young men and women
| Baseline | Restriction | Recovery | B vs. SR | SR vs. R | B vs. R | |
|---|---|---|---|---|---|---|
| PVT lapses totala | 2.9 ± 0.4 | 3.96 ± 0.6 | 4.3 ± 0.6 | 0.08 | 0.69 | 0.04 |
| PVT fastest 10% (ms) | 181.9 ± 2.7 | 190.9 ± 3.6 | 189.8 ± 4.8 | 0.03 | 0.83 | 0.13 |
| PVT slowest 10% (s)b | 2.5 ± 0.1 | 2.2 ± 0.2 | 2.2 ± 0.2 | 0.21 | 0.70 | 0.11 |
| PVT median RT (ms) | 231.5 ± 5.6 | 251.4 ± 10.1 | 253.2 ± 15.8 | 0.07 | 0.92 | 0.18 |
Data present means ± SE.
Conducted on transformed lapses frequency [√x + √ (x + 1)].
Conducted on transformed data 1/RT.
SWS and neurobehavioral parameters: gender effects.
Increased baseline SWS was associated with a lesser degree of subjective sleepiness after restriction (r = −0.57, P = 0.03) and greater improvement after recovery (r = 0.54, P = 0.04) in women, but not in men. Similarly, increased baseline SWS tended to be associated with a lesser deterioration in median reaction time (RT) after restriction (r = −0.44, P = 0.12) and greater improvement after recovery (r = 0.57, P = 0.07) in women, but not in men. No association between baseline SWS and change in objective sleepiness (MSLT) was found after restriction and recovery, respectively.
Effects of Sleep Restriction and Recovery Sleep on 24-h Hormonal/Cytokine Secretory Pattern
24-h Cortisol secretion.
There was no significant gender effect (P = 0.47), neither significant group by gender (P = 0.98) nor gender by time interaction (P = 0.63) effect; thus, data were analyzed for the entire group.
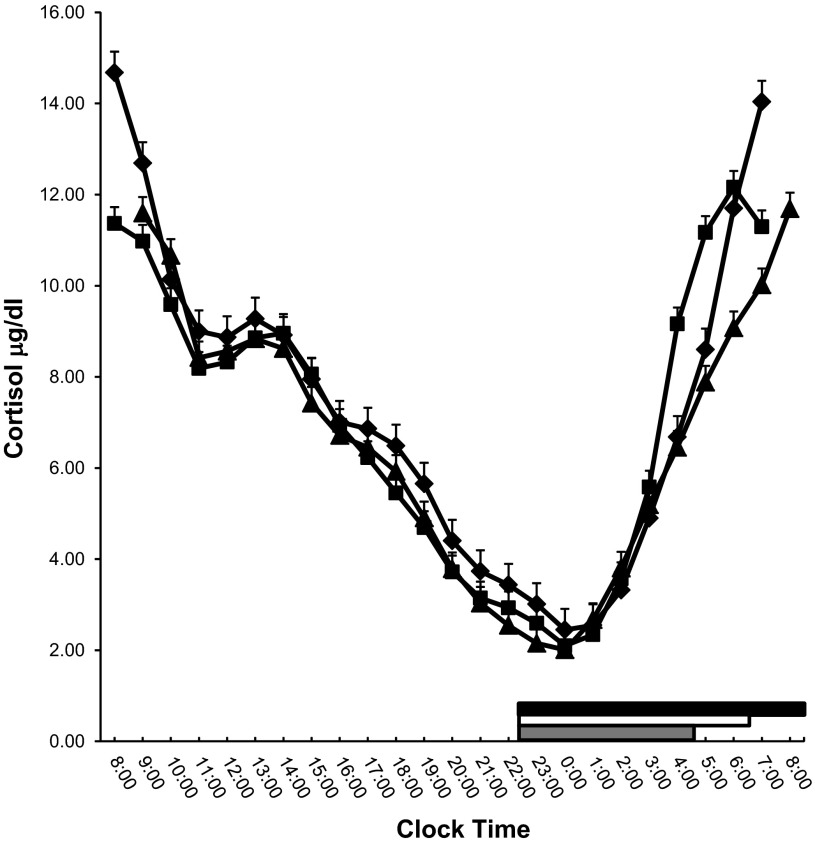
Sleep restriction was not associated with a change in 24-h cortisol levels (baseline minus restriction, 0.37 ± 0.35 μg/dl, P = 0.32). Furthermore, after recovery, cortisol levels were significantly lower compared with baseline (restriction minus recovery, 0.37 ± 0.31 μg/dl, P = 0.23; baseline minus recovery, 0.75 ± 0.37 μg/dl, P = 0.04). Additionally, there was a significant effect in terms of the circadian secretory pattern of the hormone, as indicated by the significant time, and group by time interaction effects (both P < 0.01). Specifically, at baseline and after recovery, the morning peak cortisol secretion was observed between 0700 and 8000, whereas after sleep restriction it moved 2 h earlier, at 0600 (Fig. 2). Also, the peak plasma cortisol concentration was significantly lower after sleep restriction than at baseline (baseline minus restriction, 2.52 ± 0.55 μg/dl, P < 0.01) and after recovery sleep than at baseline (baseline minus recovery, 2.98 ± 0.55 μg/dl, P < 0.01), while no difference in cortisol nadir time occurrence or mean values among the three phases was observed (data not shown).
Fig. 2.
Serial 24-h cortisol values at baseline (♦), restriction (■), and recovery (▲). Thick white, gray, and black lines on the abscissa indicate the nighttime sleep recording period at baseline, restriction, and recovery, respectively.
24-h IL-6 secretion.
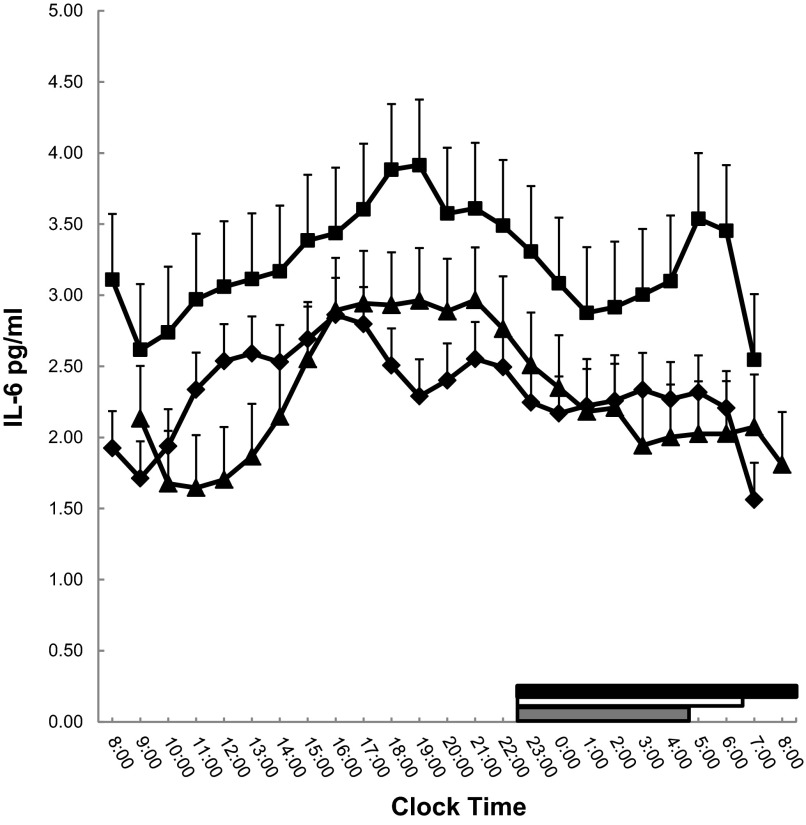
There was no significant gender effect (P = 0.12), neither significant group by gender (P = 0.65) nor gender by time interaction (P = 0.18); thus, data were analyzed for the entire group (Fig. 3). Sleep restriction was associated with a significant overall increase in 24-h secretion of IL-6 (baseline minus restriction, −0.90 ± 0.41 pg/ml, P = 0.03), whereas after recovery IL-6 levels decreased significantly and returned to baseline levels (restriction minus recovery, 0.93 ± 0.45 pg/ml, P = 0.04; baseline minus recovery, 0.02 ± 0.34 pg/ml, P = 0.94).
Fig. 3.
Serial 24-h IL-6 values at baseline (♦), restriction (■), and recovery (▲). Thick white, gray, and black lines on the abscissa indicate the nighttime sleep recording period at baseline, restriction, and recovery, respectively.
DISCUSSION
This study is the first to evaluate in a comprehensive way the effects of extended recovery sleep following mild sleep restriction on 24-h secretion of hormones/cytokines in parallel with sleepiness and performance in young men and women. It appears that 2 days of extended recovery sleep over the weekend reverses the impact of one work week of mild sleep curtailment on daytime sleepiness, fatigue, and IL-6 levels and reduced cortisol levels. However, 2 recovery nights were not sufficient to improve performance, suggesting that complete performance recovery following one work week of sleep restriction may require more than 2 days of extended sleep.
A week of mild sleep restriction in this study resulted in a significant rise of 24-h IL-6 plasma levels compared with baseline. Previous studies have shown a significant increase of plasma IL-6 levels following both mild (6 h of sleep per night for 1 wk) (39) and moderate-to-severe (4 h of sleep per night for 12 days) curtailment of sleep (15) and increased monocyte production of IL-6 (20) and levels of IL-6 mRNA (19) in the morning following sleep restriction. The increase of IL-6, a marker of systemic inflammation, has been associated with insulin resistance, cardiovascular events, and osteoporosis (29, 41). Our study shows that 2 days of extended recovery sleep reverses the adverse effects of short-term sleep loss on the secretion of IL-6, as it is marked by a significant drop in its 24-h plasma levels following 2 days of 10 h of sleep per night compared with the restriction period. A previous study that failed to detect beneficial effects of recovery sleep on the increase of IL-6 production by activated peripheral blood mononuclear cells following sleep restriction may be explained by methodological differences, such as single blood draw and 8 h of sleep during the recovery nights (35) versus 24-h hourly blood drawings and 10 h of sleep per night during the recovery period in this study.
This study shows that recovery sleep slightly reduces cortisol levels compared with baseline. These findings are in agreement with the many studies (40), including the effect of 2-h daytime nap following one night of total sleep loss (37), which have shown a mild but significant inhibitory effect of sleep on hypothalamic-pituitary-adrenal (HPA) axis activity. These cumulative findings suggest that sleep, through its inhibitory effect on the HPA axis may serve as an “antistress” antidote in individuals that face the multiple stressors of the modern world. The lack of activation of the HPA axis following 1 wk of mild sleep restriction is consistent with most of the studies that showed no change or decrease in cortisol secretion after a less stressful total or partial sleep deprivation (14, 24, 25, 31, 37, 39, 42, 44), in contrast to the effect of a more stressful sleep deprivation paradigm that may lead to increased cortisol levels (21, 32).
An interesting finding of our study is that daytime sleepiness, as measured with MSLT, following recovery sleep improved compared with baseline levels, suggesting that the young, healthy, normal sleepers in our study may have been chronically sleep deprived (8). A large epidemiologic study has shown an increased prevalence of EDS in ages younger than 30 yr compared with older groups (7), suggesting voluntary sleep restriction as one of the contributing factors of increased sleepiness in this age group. However, our participants' habitual TST, as evidenced by 2 wk of actigraphy prior to the study, was in the range of 7–9 h and did not differ from their polysomnographically measured baseline sleep duration while their baseline MSLT values were within the normal range. This suggest that group of young subjects in our study did not suffer from chronic sleep debt.
Another noteworthy finding is that 2 nights of extended sleep did not result in performance improvement as measured by PVT, suggesting that 2 nights of extended recovery sleep may not be sufficient to overcome behavioral alertness deficits resulting from mild sleep restriction. Our findings are consistent with several recent experiments reporting incomplete recovery of performance deficits even after 2 or 3 nights of regular or extended sleep post mild to severe restriction of sleep (2, 4, 5, 34). This may have important implications for people with safety-critical professions, such as health care workers, as well as transportation system employees (drivers, pilots, etc.). Furthermore, the observed dissociation of the effects of extended recovery sleep or daytime naps following sleep loss on MSLT vs. PVT may indicate that, although they correlate significantly, they may be measuring different central nervous system functions (34, 37, 39).
In this study, women had higher amounts of SWS and REM sleep at baseline than men, which is consistent with previous findings, both experimental (6) and epidemiologic (6, 27), showing that women have better quality (more SWS and REM sleep) and quantity of sleep (total sleep time). Furthermore, the amount of SWS at baseline was associated with a lesser degree of subjective sleepiness at restriction and greater improvement after recovery. Also, SWS at baseline tended to correlate with lesser deterioration and better improvement in median reaction time in women only. These results are also consistent with previous findings that women appear more resilient to the sleep-disturbing effects of the blood drawing procedures (6) and suffer less performance decrements after a night of sleep loss (43). These potential gender differences should be explored further in future studies.
The observed changes of sleep restriction and recovery in sleep architecture are consistent with previous findings (1, 5, 10, 34, 39). During restricted sleep, as reported previously, subjects fell asleep faster and spent more time asleep (39). Also, SWS was conserved throughout the experimental period, whereas REM sleep decreased during sleep restriction and increased during recovery sleep. The fact that restricted sleep led to significant hormonal/cytokine changes and neurobehavioral decrements, despite preservation of deep sleep (SWS), argues against the position that sleep other than SWS is optional and unnecessary, for example Stage 2 or REM sleep (18, 39). Finally, that SWS remained stable throughout the experiment, whereas REM sleep decreased during sleep restriction and then was compensated for during the recovery period, suggests a stronger biological drive to retain deep sleep than REM sleep (9).
One of the limitations of this study was the lack of a control group not exposed to restriction/recovery intervention to determine whether there was a time effect on the studied variables. However, in our study subjects served as their own controls, which reduced between-subjects variability. Additionally, the consistency of our results of the effects of restriction/recovery sleep on performance variables with the findings of several experiments that included control groups (4, 5, 34) suggests that the observed performance deterioration after restriction as well as no improvement after recovery were unlikely to be due to study environment/procedures or test repetition. Also, our study included healthy young men and women subjects with no primary sleep disorders. Future experiments should test whether these findings can be generalized to other age groups of healthy subjects (i.e., middle-aged or older adults). Furthermore, our study cannot address an important question, i.e., what the impact is of repeated cycles of sleep restriction/recovery associated with an intermittent pattern of adverse physiological changes, such as intermittent low-grade inflammation, on health and performance. For example, does the human body retain its ability to clear the system from potentially harmful influences, i.e., IL-6, after it has been challenged repeatedly? Also, could this chronic, intermittent, low inflammation lead to adverse health consequences in a way similar to the effect of intermittent activation of the stress system on the development of psychiatric problems, i.e., chronic recurrent major depression through kindling mechanisms? These critical questions emphasize the need for future prolonged naturalistic field studies in humans. Notably, recent experiments in animals showed that repeated sleep restriction/recovery cycles resulted in energy deficiency as shown by hyperphagia and significant weight loss and decreased plasma corticosterone and leptin concentrations as a consequence of diminished adiposity (12, 13). Some of these changes were still present even after a 4-mo-long recuperation period, suggesting that repeated sleep restriction/recovery cycles may have lasting effects, which in turn may increase the likelihood of various diseases (13).
In summary, 2 days of extended sleep over the weekend reverses the impact of one work week of mild sleep restriction on daytime sleepiness, fatigue, and IL-6 levels and reduces the levels of the stress hormone cortisol. However, it does not improve performance, suggesting that complete performance recovery may require more than just 2 days. Future experimental studies are needed to examine the long-term effects of a repeated sleep restriction/recovery weekly cycle in humans.
GRANTS
This study was partially funded by the National Institutes of Health Grant R0 HL-64415.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.P., M.B., M.T., D.S., and Z.S. performed experiments; S.P., I.K., and M.L.S. analyzed data; S.P., A.N.V., E.O.B., and G.P.C. interpreted results of experiments; S.P. prepared figures; S.P. drafted manuscript; S.P., M.B., A.N.V., I.K., E.O.B., and G.P.C. edited and revised manuscript; S.P., M.B., A.N.V., I.K., M.L.S., M.T., D.S., Z.S., E.O.B., and G.P.C. approved final version of manuscript; A.N.V. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. Hung-Mo Lin, Health Evidence and Policy at Mount Sinai for contributing to the Spatial Power analysis. We thank our research coordinator, Carrie Criley for help with recruitment and study coordination.
REFERENCES
- 1.Akerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep 32: 217–222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axelsson J, Kecklund G, Akerstedt T, Donofrio P, Lekander M, Ingre M. Sleepiness and performance in response to repeated sleep restriction and subsequent recovery during semi-laboratory conditions. Chronobiol Int 25: 297–308, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Balkin TJ, Rupp T, Picchioni D, Wesensten NJ. Sleep loss and sleepiness: current issues. Chest 134: 653–660, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Banks S, Van Dongen HP, Maislin G, Dinges DF. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep 33: 1013–1026, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res 12: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Bixler EO, Papaliaga MN, Vgontzas AN, Lin HM, Pejovic S, Karataraki M, Vela-Bueno A, Chrousos GP. Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J Sleep Res 18: 221–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab 90: 4510–4515, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bonnet MH, Arand DL. We are chronically sleep deprived. Sleep 18: 908–911, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms 14: 557–568, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Brunner DP, Dijk DJ, Borbély AA. Repeated partial sleep deprivation progressively changes in EEG during sleep and wakefulness. Sleep 16: 100–113, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep 20: 267–77, 1997 [PubMed] [Google Scholar]
- 12.Everson CA, Szabo A. Recurrent restriction of sleep and inadequate recuperation induce both adaptive changes and pathological outcomes. Am J Physiol Regul Integr Comp Physiol 297: R1430–R1440, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everson CA, Szabo A. Repeated exposure to severely limited sleep results in distinctive and persistent physiological imbalances in rats. PLoS One 6: e22987, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faraut B, Boudjeltia KZ, Dyzma M, Rousseau A, David E, Stenuit P, Franck T, Van Antwerpen P, Vanhaeverbeek M, Kerkhofs M. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav Immun 25: 16–24, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep 30: 1145–1152, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haack M, Kraus T, Schuld A, Dalal M, Koethe D, Pollmächer T. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinology 27: 921–931, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology 10: 431–436, 1973 [DOI] [PubMed] [Google Scholar]
- 18.Horne J. Why We Sleep. New York: Oxford Univ. Press, 1988 [Google Scholar]
- 19.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun 24: 54–57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med 166: 1756–1762, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep 20: 865–870, 1997 [PubMed] [Google Scholar]
- 22.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 43: 678–683, 2004 [DOI] [PubMed] [Google Scholar]
- 23.National Sleep Foundation Sleep in America. Poll, 2010 [Google Scholar]
- 24.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 94: 3242–3250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav 99: 651–656, 2010 [DOI] [PubMed] [Google Scholar]
- 26.RechtschaffenAKales A. Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: UCLA Brain Information Services, 1968 [Google Scholar]
- 27.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med 164: 406–418, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Richardson GS, Carskadon MA, Flagg W, van den Hoed J, Dement WC, Mitler MM. Excessive daytime sleepiness in man: multiple sleep latency measurement in narcoleptic and control subjects. Electroencephalogr Clin Neurophysiol 45: 621–627, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101: 1767–1772, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Rupp TL, Wesensten NJ, Bliese PD, Balkin TJ. Banking sleep: realization of benefits during subsequent sleep restriction and recovery. Sleep 32: 311–321, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Lehnert H, Born J, Schultes B. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep 34: 371–377, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiegel K, Leproult R, L'hermite-Balériaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 89: 5762–5771, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 354: 1435–1439, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26: 117–126, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Van Leeuwen WM, Lehto M, Karisola P, Lindholm H, Luukkonen R, Sallinen M, Härmä M, Porkka-Heiskanen T, Alenius H. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One 4: e4589, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Collins B, Basta M, Pejovic S, Chrousos GP. Selective effects of CPAP on sleep apnoea-associated manifestations. Eur J Clin Invest 38: 585–595, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bixler EO, Basta M, Fang J, Sarrigiannidis A, Chrousos GP. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab 292: E253–E261, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation 12: 131–140, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 89: 2119–2126, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am 31: 15–36, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab 85: 1151–1158, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Vgontzas AN, Mastorakos G, Bixler EO, Kales A, Gold PW, Chrousos GP. Sleep deprivation effects on the activity of the hypothalamic-pituitary-adrenal and growth axes: potential clinical implications. Clin Endocrinol (Oxf) 51: 205–215, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Ware JC, Risser MR, Manser T, Karlson KH., Jr Medical resident driving simulator performance following a night on call. Behav Sleep 4: 1–12, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Wu H, Zhao Z, Stone WS, Huang L, Zhuang J, He B, Zhang P, Li Y. Effects of sleep restriction periods on serum cortisol levels in healthy men. Brain Res Bull 77: 241–245, 2008 [DOI] [PubMed] [Google Scholar]