Abstract
Objectives. To identify the instruments that have been used to measure health-related quality of life (HRQOL) in gout and assess their clinimetric properties, determine the distribution of HRQOL in gout and identify factors associated with poor HRQOL.
Methods. Medline, CINAHL, EMBASE and PsycINFO were searched from inception to October 2012. Search terms pertained to gout, health or functional status, clinimetric properties and HRQOL. Study data extraction and quality assessment were performed by two independent reviewers.
Results. From 474 identified studies, 22 met the inclusion criteria. Health Assessment Questionnaire Disability Index (HAQ-DI) and Short Form 36 (SF-36) were most frequently used and highest rated due to robust construct and concurrent validity, despite high floor and ceiling effects. The Gout Impact Scale had good content validity. Gout had a greater impact on physical HRQOL compared to other domains. Both gout-specific features (attack frequency and intensity, intercritical pain and number of joints involved) and comorbid disease were associated with poor HRQOL. Evidence for objective features such as tophi and serum uric acid was less robust. Limitations of existing studies include cross-sectional design, recruitment from specialist clinic settings and frequent use of generic instruments.
Conclusion. Most studies have used the generic HAQ-DI and SF-36. Gout-specific characteristics and comorbidities contribute to poor HRQOL. There is a need for a cohort study in primary care (where most patients with gout are treated) to determine which factors predict changes in HRQOL over time. This will enable those at risk of deterioration to be identified and better targeted for treatment.
Keywords: gout, health-related quality of life, clinimetrics
Introduction
Gout is the most prevalent inflammatory arthritis, affecting 1.4% of adults in Europe [1]. Health-related quality of life (HRQOL) may be adversely influenced by the excruciating pain, chronic arthropathy, associated co-morbidities (renal and cardiovascular disease, metabolic syndrome and OA) and frequent suboptimal management in gout [2]. The UK Department of Health and the Outcome Measures in Rheumatology Clinical Trials (OMERACT) group have identified HRQOL as a key component of patient outcome assessment alongside the more traditional markers such as survival rates, symptoms and cost of resources [3, 4]. HRQOL can be measured using generic instruments, which allow HRQOL to be compared between different disease states, or by disease-specific instruments, which account for the specific facets of individual diseases [5]. Recent interest in HRQOL in gout patients has resulted in the development of a disease-specific measure, the Gout Assessment Questionnaire 1.0 [6], which was subsequently revised, resulting in the Gout Assessment Questionnaire 2.0 and its subscale, the Gout Impact Scale (GIS) [7]. The aims of this systematic review were to (i) describe which instruments have been used to measure HRQOL in gout in existing studies, (ii) describe the clinimetric properties of these instruments, (iii) describe the distribution of HRQOL in gout and (iv) identify which factors associate with poor HRQOL in gout.
Methods
Search strategy
A systematic search was undertaken using the following databases from inception to October 2012: Medline, EMBASE, CINAHL, PsycINFO and Cochrane database of systematic reviews. The search aimed to identify studies of self-reported HRQOL in gout as well as those evaluating the clinimetric (measurement) properties of instruments used to assess HRQOL in gout patients. Clinimetrics is defined as a methodological discipline focused on measurement issues [8, 9]. The clinimetric properties of an instrument describe the quality of its clinical measurements, e.g. validity, reliability and responsiveness. Search terms included gout, health or functional status and HRQOL. These domains were combined with filters for measurement properties, such as elicitation method (scale, measure and questionnaire) and measure of scientific quality (psychometrics, validity, responsiveness, reliability) [10].
To increase the recall of the search results, all terms were typed as synonyms and free text and mapped to a thesaurus. Truncated terms and wildcards were used specific to each database.
Eligibility criteria
The following inclusion criteria were applied: (i) adults aged >18 years with gout, (ii) assessment of HRQOL or evaluation of the clinimetric properties of one or more instruments and (iii) publication in English. Both primary care and secondary care studies were included. Publications without empirical data (such as commentaries, editorials and reviews), randomized controlled trials deemed to be non-representative of a typical population with gout and articles not available as full text were excluded.
Study selection
Titles and abstracts of identified articles were independently reviewed against the criteria above by two reviewers (PC, LC). Articles that could not be excluded based on title and abstract screening alone were included for full-text review, carried out independently by the same two reviewers. Further exclusions were made based on re-application of the inclusion and exclusion criteria. The references of all full-text papers were examined for relevant studies. Disagreements at all stages were arbitrated through consensus meetings.
Data extraction
The following data were extracted: study design (length and method of recruitment, inclusion and exclusion criteria, controls), participants (sample size, geographic location, setting, mean age, gender, ethnicity, method of gout diagnosis), study response rate or attrition, methods of measurement (follow-up, statistical analysis), HRQOL scores and factors associated with poor HRQOL. The Consensus-based Standards for the Selection of Health Measurement Instruments (COSMIN) checklist was used to extract data on the clinimetric properties of questionnaires [11].
Methodological assessment
The quality of the following clinimetric properties of HRQOL instruments was assessed against a modified version of the quality criteria for measurement properties by Terwee et al. [12]—validity (content, known group, floor or ceiling effects, construct and concurrent), reliability (internal consistency and test–retest) and responsiveness. Qualitative studies were assessed against the criteria set by the Critical Appraisal Skills Programme (CASP) [13]. Cohort studies were assessed against the standards set by the Newcastle Ottawa Scale (NOS) for assessing the quality of non-randomized studies [14]. Assessment of the methodological quality of cross-sectional studies included modified components such as the baseline associates of HRQOL, response rate and a measure of association between poor HRQOL in gout compared with controls, in addition to the NOS quality assessment scale.
Results
Study selection
A total of 761 potentially relevant articles were identified: 474 articles were included in title and abstract screening after removal of duplicated papers. After full-text review of the remaining 24 articles as well as 5 articles identified from reference lists, 22 articles met the inclusion criteria. Reasons for exclusion are described in Fig. 1. Included studies are summarized in Table 1.
Fig. 1.
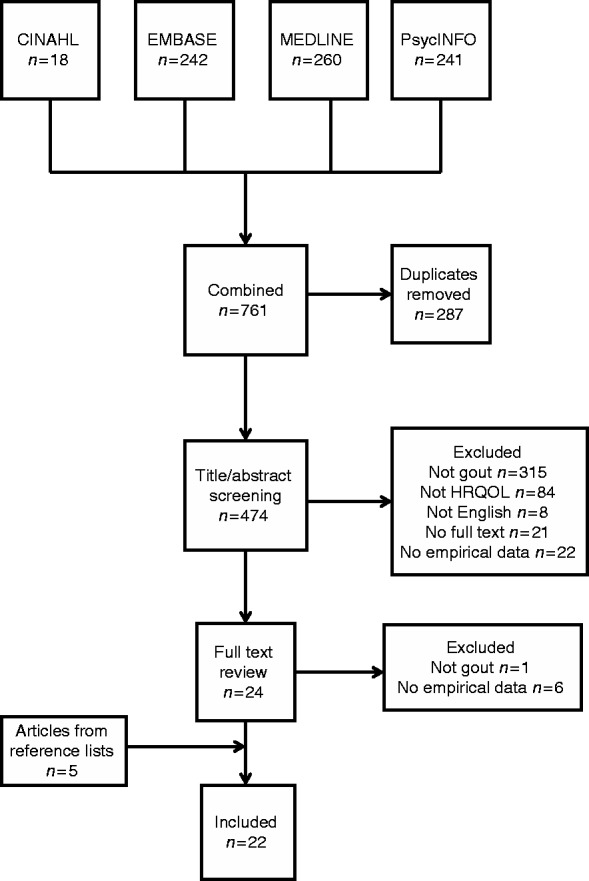
Systematic search and study selection.
Table 1.
Characteristics of studies providing data on HRQOL in gout
| Reference | Study period | Publication year | Location | Source of data/recruitment | Study type | Sample size | Questionnaire to measure HRQOL |
|---|---|---|---|---|---|---|---|
| Singh and Strand [20] | 1996–98 | 2008 | USA | Veterans Affairs database | Cross-sectional | 70 334 | SF-36 (veterans) |
| Colwell et al. [6] | NR | 2006 | USA | Phase 2 clinical trial of febuxostat | Nested prospective cohort | 126 | GAQ 1.0 |
| Taylor et al. [4] | NR | 2008 | New Zealand | Study of hand function in gout and rheumatology clinics | Cross-sectional | 73 | HAQ-DI |
| Hirsch et al. [24] | NR | 2010 | USA | Multispecialty clinics (physician, poster and newspaper advertisement recruitment) | Cross-sectional | 371 | GIS |
| Hirsch et al. [7] | NR | 2008 | USA | Multispecialty clinics (physician, poster and newspaper advertisement recruitment) | Cross-sectional | 371 | GIS |
| Roddy et al. [21] | NR | 2007 | UK | Two GP practices | Cross-sectional | 13 684 | WHOQOL-BREF |
| Dalbeth et al. [25] | NR | 2011 | New Zealand | Advertisements in the community and secondary care clinics | Prospective cohort | 142 | BIPQ, HAQ II |
| Alvarez-Hernandez et al. [16] | NR | 2009 | Spain | Not described | Prospective cohort | 49 | AIMS, MOS 20 |
| Lee et al. [22] | NR | 2009 | USA | Advertisements in primary and secondary care clinics | Cross-sectional | 371 | SF-36 |
| Sarkin et al. [26] | NR | 2010 | USA | Advertisements in community clinics and newspapers | Cross-sectional | 260 | GIS |
| Becker et al. [33] | NR | 2009 | USA | Academic and private rheumatology clinics | Prospective cohort | 110 | SF-36 and HAQ-DI |
| ten Klooster et al. [18] | 2005–08 | 2011 | Netherlands | Outpatient rheumatology clinics | Cross-sectional | 102 | HAQ-DI, HAQ II and SF-36 PF10 |
| Khanna et al. [23] | NR | 2008 | USA | Private clinic and University of Cincinnati Veterans Affairs Medical Center | Cross-sectional | 80 | SF-36, EQ5D and HAQ-DI |
| Alvarez-Hernandez et al. [15] | NR | 2008 | Mexico | Eight rheumatology departments | Prospective cohort | 206 | HAQ-DI |
| Khanna et al. [17] | NR | 2011 | Spain | Gout clinic | Prospective cohort | 99 | SF-36 |
| Lindsay et al. [31] | NR | 2011 | New Zealand | Primary and secondary care clinics | Qualitative interviews | 11 | None |
| Khanna et al. [19] | NR | 2011 | USA | RCT of rilonacept vs placebo | Nested prospective cohort | 73 | GIS |
| van Groen et al. [27] | 2005–08 | 2010 | Netherlands | Outpatient rheumatology clinic | Cross-sectional | 102 | HAQ-DI |
| Alvarez-Nemegyei et al. [28] | 1999 | 2005 | Mexico | Primary care | Nested case-control in a cohort | 90 | HAQ |
| Harrold et al. [32] | 2005–10 | 2010 | USA | Multispecialty practice (Fallon clinic) | Qualitative | 26 | None |
| Singh et al. [29] | NR | 2011 | USA | Multispecialty clinics (physician, poster and newspaper advert recruitment) | Cross-sectional | 298 | Healthcare utilization frequency |
| Khanna et al. [30] | 2010 | 2012 | USA, UK, Germany, France | National Health and Wellness Survey, Lightspeed Research panel | Cross-sectional | 1936 | SF-12v2, SF-6D |
NR: not reported; RCT: randomized controlled trial.
Study characteristics and methodological quality
Of the 22 included studies, 8 evaluated clinimetric properties of instruments used to measure HRQOL [4, 6, 7, 15–19] and the remainder focused on self-reported HRQOL or health care utilization [20, 21–32]. One study reported both the measurement properties as well as the scores of HRQOL as measured by the Health Assessment Questionnaire Disability Index (HAQ-DI) and Short Form 36 (SF-36) [33]. All studies were published in or after 2006. A total of 13 cross-sectional [4, 7, 18, 20–24, 26–30], 7 cohort [6, 15–17, 19, 25, 33] and 2 qualitative studies [31, 32] were identified. The median sample size of the 20 quantitative studies was 134 (range 49–70 334). Only four studies [17, 18, 22, 33] used the diagnostic gold standard of MSU crystal identification from joint or tophus aspirate [34]. Other methods of gout diagnosis in studies included hyperuricaemia (n = 3) [6, 18, 19], ACR classification criteria [35] (n = 11) [4, 7, 15, 16, 23, 25, 26, 28, 29, 31, 35], self-reported gout (n = 4) [19, 21, 22, 30], physician diagnosis (n = 2) [22, 24] and ICD-9 codes (n = 1) [20]. The follow-up period in cohort studies ranged from 8 weeks [16, 19] to 2 years [17]. Five cross-sectional studies reported response rates of >60% [7, 18, 22, 24, 29]. Quality assessment of cohort and cross-sectional studies is summarized in Table 2 (for qualitative studies, see supplementary Table S1 available as supplementary data at Rheumatology Online).
Table 2.
Modified NOS quality assessment for cross-sectional and cohort studies
| Selection |
Comparability |
Assessment of HRQOL in addition to self-reported data |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Study details | Cohort representative of average gout patient in community | Controls from same source as cases | HRQOL associations (CS) or predictors/ change (Cht) | Diagnosis of gout: MSU crystals, ARA criteria (35) or record linkage | Controls matched for age/ gender | Measure of association between gout and HRQOL (OR or RR) | Independent physician assessment, secure records or record linkage | Adequate follow-up period (Cht only) | Response rate >60% (CS) or attrition <30% (Cht) |
| CS | |||||||||
| Singh and Strand [20] | + | + | + | + | − | NR | + | Not relevant | − |
| Hirsch et al. [24] | + | NR | + | + | NR | NR | + | Not relevant | + |
| Hirsch et al. [7] | + | NR | + | + | NR | NR | + | Not relevant | + |
| Roddy et al. [21] | − | + | + | − | − | NR | + | Not relevant | − |
| Lee et al. [22] | + | NR | + | + | NR | NR | + | Not relevant | + |
| Sarkin et al. [26] | + | NR | + | + | NR | NR | + | Not relevant | NR |
| ten Klooster et al. [18] | − | NR | + | + | NR | NR | − | Not relevant | + |
| Khanna et al. [23] | + | NR | + | + | NR | NR | + | Not relevant | NR |
| Taylor et al. [4] | − | NR | + | + | NR | NR | − | Not relevant | NR |
| van Groen et al. [27] | − | + | NR | + | − | NR | − | Not relevant | NR |
| Alvarez-Nemegyei et al. [28] | + | + | + | + | − | NR | + | Not relevant | NR |
| Singh et al. [29] | + | NR | + | + | NR | NR | + | Not relevant | + |
| Khanna et al. [30] | + | NR | + | − | NR | NR | − | Not relevant | − |
| Cht | |||||||||
| Colwell et al. [6] | − | NR | + | − | NR | NR | + | + | + |
| Dalbeth et al. [25] | + | NR | + | + | NR | NR | + | + | + |
| Alvarez-Hernández et al. [16] | − | NR | + | + | NR | NR | + | − | + |
| Becker et al. [33] | − | NR | + | + | NR | NR | + | + | − |
| Alvarez-Hernández et al. [15] | + | NR | + | + | NR | NR | + | + | + |
| Khanna et al. [17] | − | NR | + | + | NR | NR | + | + | − |
| Khanna et al. [19] | − | NR | + | − | NR | NR | − | − | + |
+: positive rating; −: negative rating; Cht: cohort study; CS: cross-sectional study; MSU: monosodium urate; NR: not reported; RR: relative risk.
Instruments used to measure HRQOL in gout
Twelve different instruments to measure HRQOL were identified (five studies employed more than one instrument) [16, 18, 23, 25, 33]. Most commonly used were the HAQ-DI (n = 6) [4, 15, 18, 23, 27, 33], SF-36 (n = 5) [17, 20, 22, 23, 33], GIS (n = 4) [7, 19, 24, 26] and Health Assessment Questionnaire II (HAQ II, n = 2) [18, 25]. The Gout Assessment Questionnaire 1.0 (GAQ 1.0) [6], Arthritis Impact Measurement Scale (AIMS) [16], Medical Outcomes Survey 20 (MOS 20) [16], Brief Illness Perception Questionnaire (BIPQ) [25], SF-36 Physical Function 10 (PF10) [18], Short Form 12 (SF-12v2) [30], HAQ [28], EuroQOL 5D (EQ5D) [23], Short Form 6D (SF-6D) [30] and World Health Organisation Quality of Life (WHOQOL)-BREF [21] were each used once.
Clinimetric properties of instruments used to measure HRQOL in gout
Values of the measurement properties of identified instruments are available in Table 3. Supplementary Tables S2 and S3 (available at Rheumatology Online) present quality ratings assigned to the measurement properties assessed against the modified guidelines by Terwee et al. [12]. Content validity was only established for the gout-specific GIS and GAQ 1.0, which received patient and health care provider input during the development of the questionnaires [6, 7]. The generic SF-36 (except PF10 [18]) and the HAQ-DI [4, 15–17, 33] performed well in the known-group analysis based on self-reported general health, comorbidities and correlation with disease characteristics. The HAQ-DI, HAQ II and SF-36 had significant floor (HAQ-DI 20.5%) and ceiling (HAQ-DI 34%, HAQ II 25.8%, SF-36 18.4%) effects, indicating a weakness in the ability to differentiate between participants at the extreme ends of the scale (no disability and severe disability), leading to limited content validity and responsiveness to change [4, 17]. The GIS showed poor construct validity, with low correlations between the subscales of GIS (except unmet treatment need) and physician-rated severity (r = 0.02–0.34), although moderate correlations were seen with patient-rated severity (r = 0.31–0.45) [7, 19]. Correlations of the SF-36 Mental Component Summary (MCS) (r = −0.17 to −0.43) with the GIS were generally higher than those seen with the Physical Component Summary (PCS) (r = −0.10 to −0.20) (NB correlation coefficients are negative, as higher scores indicate better health status on the SF-36 but worse health status on the GIS [5]). The GAQ 1.0 had better correlation with the MOS health distress questionnaire (r = 0.03–0.46) than the SF-36 (PCS, r = 0.02–0.34; MCS, r = −0.01 to 0.23) [6]. The HAQ-DI and HAQ II correlated with each other (r = 0.87) as well as the SF-36 (HAQ-DI, r = −0.41 to −0.67; HAQ II, r = −0.35 to 0.72) [15, 18]. Most instruments had good or excellent internal consistency (Cronbach’s α = 0.4–1.0), except the GIS (weak correlation between items of the gout medication side effects and unmet treatment needs) [7]. Test–retest reliability was low for the AIMS [intraclass correlation coefficient (ICC) = 0.11–0.70] and the MOS 20 (ICC = 0.27–0.65) [16] but acceptable for the HAQ-DI (ICC = 0.68–0.84) [15]. Responsiveness to clinical change was elicited by the Minimal Clinically Important Difference (MCID) of 5–8 points for the subscales of the GIS [19], SF-36 [17] and GAQ 1.0 (in all subscales except well-being anchored to pain frequency) [6] and a 20% change in scores of the AIMS and MOS 20 [16]. Effect sizes (ESs) of the PCS (SF-36) improved from small (0.3) in the treatment with colchicine only to large (0.99) in the urate lowering treatment (ULT) and colchicine group [17]. The magnitude of the ES was lower for the GIS (0.218–0.376 in the minimally improved and 0.129–0.682 in the markedly improved groups) [19] and moderate (0.62) for the HAQ-DI [15].
Table 3.
Measurement values of instruments used to measure HRQOL
| Reliability |
Validity |
Scale development |
Responsiveness |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Measurement instrument | Internal consistency (Cronbach’s α) | Test–retest (ICC) | Content | Construct (Pearson or Spearman’s r) | Concurrent (Pearson or Spearman’s r) | Hypothesis a priori | Confirmatory factor analysis | Rasch analysis | MCID, SDC, ES, Guyatt’s RR or >20% change in scores |
| GIS [7, 19] | 0.54–0.94 | 0.77–0.89 | Patients and rheumatologists | Patient severity (r = 0.31–0.45), attack freq. (r = 0.06–0.51), attack pain (r = 0.13–0.47), physician severity (r = 0.02–0.34), PCS (r = −0.10 to−0.20), MCS (r = −0.17 to −0.43) | NR | Yes | Yes | Yes | ES 0.218–0.376 in the minimally improved and 0.129–0.682 in the markedly improved groups |
| GAQ 1.0 [6] | 0.78–0.97 | NR | Patients and Rheumatologists | PCS (r = 0.02–0.34), MCS (r = −0.01 to 0.23), MOS (r = 0.03–0.46) | NR | Yes | Yes | No | GRR 0.030 (1 month) to 1.142 (6 months). MCID 1.88–12.33 (not significant in well-being for pain freq.) |
| HAQ-DI [4, 15, 18, 33] | 0.81–0.97 | 0.68–0.84 | Floor 20.5%, Ceiling 34% | Freq. of flares (r = 0.41), physician global (r = 0.42–0.77), swollen joints (r = 0.40–0.62), painful joints (r = 0.46–0.650), joints with limited mobility (r = 0.36), VAS pain (r = 0.56), tophi (r = 0.42), excellent/very good health = 0.16, good = 0.33, fair/poor = 1.25 | SF-36 (r = −0.41 to −0.67), PCS (r = −0.71), MCS (r = −0.56), DASH (r = 0.81), Sollerman (r = −0.79), ACR functional class (r = 0.79), HAQ II (r = 0.87), PF 10 (r = −0.75) | Yes (55.5% true) | Yes | Yes | Mean ES = 0.62 (moderate), SDC = 0.59 and GRR = 1.91 |
| SF-36 [17, 33] | 0.75–0.97 | 0.40–0.90 | Ceiling RP = 18.4%, SF = 32.7%, RE = 58.6% | PCS: tophi (r = 0.277), swollen joints (r = −0.334), painful joints (r = −0.544), flares last year (r = −0.369); MCS: painful joints (r = −0.436), freq. of flares (r = −0.321) | NR | NR | NR | NR | Colchicine: ES for PCS = 0.3 (small), ES for MCS = 0.16 (negligible) |
| Colchicine + ULT: ES for PCS = 0.99 (large) | |||||||||
| ES for MCS = 0.08 (negligible), MCID (all subjects) 70% for PCS and 38% in MCS | |||||||||
| MOS 20 [16] | 0.68–1.0 | 0.27–0.65. | NR | JFL: 23.75–66 | HAQ-DI (r = −0.1 to−0.5) | NR | NR | NR | >20% change: PF, SF, health perception, pain |
| Without JFL: 37.59–81.43 | |||||||||
| AIMS [16] | 0.66–0.96 | 0.11–0.70 | NR | JFL: 3.05–6.62 | HAQ-DI (r = 0.1–0.6) | NR | NR | NR | >20% change: dexterity, daily activity, social development, pain, depression |
| Without JFL: 1.99–5.46 | |||||||||
| HAQ II [18] | 0.94 | NR | Ceiling 25.8% | Excellent/very good health = 0.28, good = 0.44, fair/poor = 1.39 | PF 10 (r = −0.79), SF-36 [−0.35 (MH) to 0.72 (RP)] | Yes | NR | NR | NR |
| PF 10 [18] | 0.94 | NR | NR | Excellent/very good health = 71.91, good = 74.27, fair/poor = 39.33 | HAQ-DI (r = −0.75), SF-36 [0.30 (MH)–0.68 (RP)] | Yes | NR | NR | NR |
SDC: smallest detectable change; GRR: Guyatt's responsiveness ratio; NR: not reported; JFL: joints with functional limitations; SF-36 subscales: RP, role physical; MH, mental health; SF, social function; RE, role emotional; freq.: frequency.
The distribution of HRQOL in gout
No studies were identified that defined or used a cut-off value for poor HRQOL in gout. Higher scores indicate worse HRQOL in the GIS, GAQ 1.0, HAQ-DI, AIMS and BIPQ and better HRQOL in the WHOQOL-BREF, SF-36 including PF10, MOS 20 and SF-12v2. Four studies identified instruments with scores lower than controls (SF-36 physical functioning, role physical, bodily pain, general health, role emotional, PCS P < 0.001 [20]; WHOQOL-BREF P = 0.003 [21]) and USA normative distribution (SF-36 PCS P = 0.007 [22], P < 0.001 [30]), representative of poor HRQOL in gout. One cohort study of treatment-failure gout showed lower scores in all SF-36 domains (except mental health and MCS) compared with age- and sex-matched US normative distributions (PCS and MCS normative distributions have a mean of 50 and a standard deviation of 10 for the US population) [33]. One cohort [17] and two cross-sectional studies [21, 22] highlighted the greater impact of gout on physical HRQOL as measured by the SF-36 [17] and WHOQOL-BREF (P < 0.001) [21], with a lesser reduction seen in the MCS compared with US norms (P < 0.001) [22]. However, the impact on physical function was mild, as shown in two studies using the HAQ-DI, with a baseline HAQ-DI of 1 for those with treatment-failure gout [33] and 0.43 in chronic tophaceous gout [16]. (Consensus-based cut-off for mild disability as measured by the HAQ-DI is a score <1, moderate disability 1–2 and severe disability ≥2 [36].) Similarly the average HAQ score (surrogate for musculoskeletal disability) in another study was 0.17 [28]. Two cross-sectional studies [27, 30] comparing the impact of gout with that of other rheumatic diseases showed substantially lower levels of disability (mean HAQ-DI 0.54) in patients with gout compared with those with RA (0.97) and OA (1.00) [27]. Those with severe gout (three or more flares in the previous year and confirmed tophi) had similar health utility (SF-6D) scores as patients with average RA or systemic lupus [36]. In two studies that utilized the GIS, participants’ gout concern remained high despite their reporting that they found treatment helpful [19, 24], and in another cohort study using the generic BIPQ, the impact of gout was most severe on perceptions of chronicity [25]. Gout severity was also associated with an increased utilization of primary care clinics in a cross-sectional study of health care resources utilization (P = 0.005) [29].
Factors associated with poor HRQOL in gout
Two studies of physical functioning (measured by the SF-36 and HAQ-DI) as a surrogate marker of HRQOL and another study of health care utilization found that associated comorbidities contribute to poorer HRQOL (PCS, r = −0.18 to −0.43, P < 0.01 [22]; HAQ-DI, P < 0.03 [33]) and a greater number of primary care visits (P = 0.006) [29]. In one study of US veterans, comorbidities were solely responsible for poor HRQOL, with no difference in HRQOL between those with and without gout after comorbidities had been adjusted for [20]. However, in one cross-sectional study the association between gout and poor physical HRQOL of the WHOQOL-BREF remained significant after adjustment for medical (diabetes, hypertension and chronic kidney disease) and musculoskeletal comorbidities (WHOQOL-BREF, P = 0.001 [21]). Cross-sectional association of gout characteristics [presence of tophi (PCS, P < 0.01; MCS, P < 0.05), uncertainty about the presence of tophi (PCS, P < 0.001; MCS, P < 0.01) and four or more flares in the last 12 months (PCS, P < 0.05; MCS, P < 0.05)] with poor HRQOL and high activity impairment also remained significant even after adjustment for comorbidities [30]. In one cohort [25] and four cross-sectional studies [7, 22, 28, 30], gout-specific features, including increasing frequency of flares (P = 0.002 [22], P = 0.044 [25], r = 0.51 [7], P < 0.05 [30]), time with pain between attacks (P < 0.001 [22]), pain during a typical attack (P = 0.023 [22]), number of joints involved in a typical attack (P = 0.004 [22]) and presence of tophi {relative risk (RR) = 4.3, 95% confidence interval (CI) 1.2, 15.1 [28], P < 0.05 [30]} were reported to be associated with worse HRQOL (measured by the GIS, SF-36, SF-12v2, HAQ and BIPQ) even after adjustment for age, gender, gout features and comorbidities. Increased frequency of flares in the previous year (three or more) (PCS, P < 0.05) and confirmed tophi (severe gout) (PCS, P < 0.01; MCS, P < 0.01) led to worse HRQOL compared with asymptomatic patients [30]. The presence of tophi had a significant impact on activity impairment (P < 0.05 [30]) and led to an increased likelihood of consultation with a rheumatologist {odds ratio (OR) = 7.92, 95% CI 2.81, 22.34, P < 0.0001 [29]} in two cross-sectional studies [29, 30]. Other cross-sectional variables such as physician-rated severity (primary care OR = 1.46, 95% CI 1.02, 2.08, P = 0.037; rheumatologist OR = 1.52, 95% CI 1.08, 2.14, P = 0.018), time since last gout attack (primary care OR = 0.65, 95% CI 0.55, 0.76, P < 0.0001; rheumatologist OR = 0.78, 95% CI 0.67, 0.91, P = 0.001) and an attack within the last 3 months (primary care OR = 3.48, 95% CI 1.84, 6.58, P < 0.0001; rheumatologist OR = 2.11, 95% CI 1.22, 3.65, P = 0.008) were also associated with health care resources utilization [29]. While some studies support the association of tophi (GIS, P = 0.029 [24]; PCS, P < 0.01; MCS, P < 0.05 [30]) and serum uric acid (SUA) (P = 0.002) [25] with poor HRQOL, others do not (tophi: patient-severity rating, r = 0.174 [26]; SUA: WHOQOL-BREF, P = 0.750 [21]; GIS, r < 0.29 [24]; patient-severity rating, r = 0.06 [26]). There was a paucity of cross-sectional evidence for positive effects of allopurinol on HRQOL from a patient’s perspective (WHOQOL-BREF, P = 0.618 [21]; HAQ, P = 0.79 [28]), whereas steroid and non-steroidal anti-inflammatory drugs were associated with greater musculoskeletal disability [28]. Although tophi, comorbidities, polyarticular disease and radiographic damage were associated with worse HRQOL at baseline, after multivariate analysis, a reduction in flares (P = 0.001–0.06) and baseline SUA (P = 0.001–0.04) were predictors of improvement in HRQOL in one cohort study [17].
Discussion
Although none of the identified instruments to measure HRQOL in gout in this review were satisfactory in all domains of the assessed clinimetric properties, generic instruments (HAQ-DI, SF-36) received the highest ratings. Correlations with clinical characteristics, other instruments and change in scores coupled with clinical change strengthened their construct and concurrent validity as well as responsiveness. The SF-36 and HAQ-DI have been endorsed by the OMERACT group as validated tools to measure HRQOL and functional disability in gout [37, 38]. While the generic instruments allow comparison between the impact of different diseases, their treatments and cost-effectiveness analyses, they may lack the sensitivity to capture the true impact of gout, especially in those with less severe disease [39]. The disease-specific GIS may focus on HRQOL domains more relevant in gout (hence a better correlation with patient-reported factors) and be more responsive to small changes in health status [40]. However, it may miss any unexpected adverse outcomes and does not allow comparison between disease states. Furthermore, the OMERACT group has not yet unreservedly endorsed the Gout Assessment Questionnaire 2.0 and its GIS subscale as fully validated HRQOL measures in chronic gout [38].
A consistent finding of all the instruments reviewed is that people with gout had lower physical HRQOL compared with the normative distribution [20, 22] as well as study controls [21], even after adjusting for comorbidities [7, 22, 24]. This may be due to the strong emphasis on physical functioning as a surrogate measure for HRQOL in the generic instruments. The impact of SUA and tophi were variable, with some studies reporting an adverse effect on HRQOL [17, 24] but others showing no effect [21, 26]. SUA may have an indirect relationship with HRQOL in gout, as it is positively correlated with the frequency of flares in the last 12 months as well development of tophi [28, 30, 41]. Although allopurinol is not perceived by patients to improve HRQOL [21, 28], its use in primary care is often suboptimal [42] and it has been shown to reduce the number of flares as well as tophi [43, 44]. Patients may be unaware of the rationale behind ULT, with many discontinuing treatment at the onset of flares when ULT is initiated [32].
The robustness of the findings of this review is supported by data extraction and quality assessment using validated tools by two independent reviewers. Our search strategy included a filter that is 90–97% sensitive in retrieving clinimetric articles [12], therefore it is unlikely that we would have missed any such articles. Nevertheless, the findings of the review need to be interpreted in the context of the limitations of the study design as well as those of the literature identified. A limitation is that the articles included in the review were restricted to English (due to a lack of translation facilities) as well as not searching for grey literature (presumed low yield). The generalizability of the results may be limited by the highly selective populations studied (treatment failure or chronic tophaceous gout in the intercritical stage, mainly Caucasian males), study settings (private or specialist clinics) and variable response rates.
Existing studies of HRQOL in gout are limited by their paucity of longitudinal data, recruitment from highly selective secondary care populations and use of mostly generic instruments to measure HRQOL. Hence there is a need for a primary care–based prospective cohort study using both gout-specific and generic questionnaires to determine how HRQOL changes over time in the clinical setting, where most patients with gout are treated, identify which factors (such as disease characteristics, treatment, comorbidities including anxiety and depression) predict changes in HRQOL and also identify those at risk of deterioration to better target their treatment.
Rheumatology key messages.
Existing studies of gout most commonly use generic measures of HRQOL.
Gout is associated with poorer physical HRQOL.
Poor HRQOL in gout is associated with both disease-specific characteristics and comorbidity.
Supplementary Material
Acknowledgements
We would like to thank Professor Danielle van der Windt for methodological guidance. We would like to acknowledge fellowships awarded to PC and LC (National School for Primary Care Research) and CM (Arthritis Research UK Clinician Scientist).
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Annemans L, Spaepen E, Gaskin M, et al. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000–2005. Ann Rheum Dis. 2008;67:960–6. doi: 10.1136/ard.2007.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223. doi: 10.1186/ar3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hickey A, Barker M, McGee H, et al. Measuring health-related quality of life in older patient populations: a review of current approaches. Pharmacoeconomics. 2005;23:971–93. doi: 10.2165/00019053-200523100-00002. [DOI] [PubMed] [Google Scholar]
- 4.Taylor WJ, Colvine K, Gregory K, et al. The Health Assessment Questionnaire Disability Index is a valid measure of physical function in gout. Clin Exp Rheumatol. 2008;26:620–6. [PubMed] [Google Scholar]
- 5.Khanna PP, Khanna D. Health-related quality of life and outcome measures in gout. In: Terkeltaub R, editor. Gout and other crystal arthropathies. 1st edn. Philadelphia: Elsevier; 2011. pp. 217–25. [Google Scholar]
- 6.Colwell HH, Hunt BJ, Pasta DJ, et al. Gout Assessment Questionnaire: initial results of reliability, validity and responsiveness. Int J Clin Pract. 2006;60:1210–7. doi: 10.1111/j.1742-1241.2006.01104.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch JD, Lee SJ, Terkeltaub R, et al. Evaluation of an instrument assessing influence of gout on health-related quality of life. J Rheumatol. 2008;35:2406–14. doi: 10.3899/jrheum.080506. [DOI] [PubMed] [Google Scholar]
- 8.Feinstein AR. An additional basic science for clinical medicine: IV. The development of clinimetrics. Ann Intern Med. 1983;99:843–8. doi: 10.7326/0003-4819-99-6-843. [DOI] [PubMed] [Google Scholar]
- 9.Feinstein AR. Clinimetrics. New Haven, CT: Yale University Press; 1987. [Google Scholar]
- 10.Terwee CB, Jansma EP, Riphagen II, et al. Development of a methodological PubMed search filter for finding studies on measurement properties of measurement instruments. Qual Life Res. 2009;18:1115–23. doi: 10.1007/s11136-009-9528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63:737–45. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Terwee CB, Bot SDM, De Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Critical Appraisal Skills Programme (CASP) Qualitative Research: Appraisal Tool. 10 Questions to Help You Make Sense of Qualitative Research. 2006. www.phru.nhs.uk/Doc_Links/Qualitative_Appraisal_Tool.pdf (1 December 2012, date last accessed)
- 14.Wells GA, Shea B, O'Connell D, et al. 2000. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (1 December 2012, date last accessed) [Google Scholar]
- 15.Alvarez-Hernández E, Peláez-Ballestas I, Vázquez-Mellado J, et al. Validation of the Health Assessment Questionnaire disability index in patients with gout. Arthritis Rheum. 2008;59:665–9. doi: 10.1002/art.23575. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez-Hernandez E, Zamudio-Lerma JA, Burgos-Martinez G, et al. Medicion de la calidad de vida asociada a la salud y a la capacidad funcional en pacientes con gota cronica tofacea [Measurement of health-related quality of life and functional capacity in patients with chronic tophaceous gout] Reumatol Clin. 2009;5:103–8. doi: 10.1016/j.reuma.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Khanna PP, Perez-Ruiz F, Maranian P, et al. Long-term therapy for chronic gout results in clinically important improvements in the health-related quality of life: short form-36 is responsive to change in chronic gout. Rheumatology. 2011;50:740–5. doi: 10.1093/rheumatology/keq346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ten Klooster PM, Oude MAH, Taal E, et al. Comparison of measures of functional disability in patients with gout. Rheumatology. 2011;50:709–13. doi: 10.1093/rheumatology/keq387. [DOI] [PubMed] [Google Scholar]
- 19.Khanna D, Sarkin AJ, Khanna PP, et al. Minimally important differences of the gout impact scale in a randomized controlled trial. Rheumatology. 2011;50:1331–6. doi: 10.1093/rheumatology/ker023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh JA, Strand V. Gout is associated with more comorbidities, poorer health-related quality of life and higher healthcare utilisation in US veterans. Ann Rheum Dis. 2008;67:1310–6. doi: 10.1136/ard.2007.081604. [DOI] [PubMed] [Google Scholar]
- 21.Roddy E, Zhang W, Doherty M. Is gout associated with reduced quality of life? A case-control study. Rheumatology. 2007;46:1441–4. doi: 10.1093/rheumatology/kem150. [DOI] [PubMed] [Google Scholar]
- 22.Lee SJ, Hirsch JD, Terkeltaub R, et al. Perceptions of disease and health-related quality of life among patients with gout. Rheumatology. 2009;48:582–6. doi: 10.1093/rheumatology/kep047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khanna D, Ahmed M, Yontz D, et al. The disutility of chronic gout. Qual Life Res. 2008;17:815–22. doi: 10.1007/s11136-008-9355-0. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch J, Terkeltaub R, Khanna D, et al. Gout disease-specific quality of life and the association with gout characteristics. Patient Relat Outcome Meas. 2010;1:1–8. doi: 10.2147/PROM.S8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalbeth N, Petrie KJ, House M, et al. Illness perceptions in patients with gout and the relationship with progression of musculoskeletal disability. Arthritis Care Res. 2011;63:1605–12. doi: 10.1002/acr.20570. [DOI] [PubMed] [Google Scholar]
- 26.Sarkin AJ, Levack AE, Shieh MM, et al. Predictors of doctor-rated and patient-rated gout severity: gout impact scales improve assessment. J Eval Clin Pract. 2010;16:1244–7. doi: 10.1111/j.1365-2753.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 27.van Groen M, ten Klooster P, Taal E, et al. Application of the health assessment questionnaire disability index to various rheumatic diseases. Qual Life Res. 2010;19:1255–63. doi: 10.1007/s11136-010-9690-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarez-Nemegyei J, Cen-Piste JC, Medina-Escobedo M, et al. Factors associated with musculoskeletal disability and chronic renal failure in clinically diagnosed primary gout. J Rheumatol. 2005;32:1923–7. [PubMed] [Google Scholar]
- 29.Singh JA, Sarkin A, Shieh M, et al. Health care utilization in patients with gout. Semin Arthritis Rheum. 2011;40:501–11. doi: 10.1016/j.semarthrit.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanna P, Nuki G, Bardin T, et al. Tophi and frequent gout flares are associated with impairments to quality of life, productivity, and increased healthcare resource use: results from a cross-sectional survey. Health Qual Life Outcomes. 2012;10:117. doi: 10.1186/1477-7525-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindsay K, Gow P, Vanderpyl J, et al. The experience and impact of living with gout: a study of men with chronic gout using a qualitative grounded theory approach. J Clin Rheumatol. 2011;17:1–6. doi: 10.1097/RHU.0b013e318204a8f9. [DOI] [PubMed] [Google Scholar]
- 32.Harrold LR, Mazor KM, Velten S, et al. Patients and providers view gout differently: a qualitative study. Chronic Illn. 2010;6:263–71. doi: 10.1177/1742395310378761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker MA, Schumacher HR, Benjamin KL, et al. Quality of life and disability in patients with treatment-failure gout. J Rheumatol. 2009;36:1041–8. doi: 10.3899/jrheum.071229. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Doherty M, Pascual E, et al. EULAR evidence based recommendations for gout. Part I: diagnosis. Report of a task force of the standing committee for international clinical studies including therapeutics (ESCISIT) Ann Rheum Dis. 2006;65:1301–11. doi: 10.1136/ard.2006.055251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 36.Krishnan E, Tugwell P, Fries J. Percentile benchmarks in patients with rheumatoid arthritis: Health Assessment Questionnaire as a quality indicator (QI) Arthritis Res Ther. 2004;6:R505–13. doi: 10.1186/ar1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schumacher HR, Taylor W, Edwards L, et al. Outcome domains for studies of acute and chronic gout. J Rheumatol. 2009;36:2342–5. doi: 10.3899/jrheum.090370. [DOI] [PubMed] [Google Scholar]
- 38.Singh J, Taylor WJ, Simon LS, et al. Patient-reported outcomes in chronic gout: a report from OMERACT 10. J Rheumatol. 2011;38:1452–7. doi: 10.3899/jrheum.110271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazur W, Kupiainen H, Pitkaniemi J, et al. Comparison between the disease-specific Airways Questionnaire 20 and the generic 15D instruments in COPD. Health Qual Life Outcomes. 2011;9:4. doi: 10.1186/1477-7525-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brazier JE, Harper R, Munro J, et al. Generic and condition-specific outcome measures for people with osteoarthritis of the knee. Rheumatology. 1999;38:870–7. doi: 10.1093/rheumatology/38.9.870. [DOI] [PubMed] [Google Scholar]
- 41.Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Care Res. 2004;51:321–5. doi: 10.1002/art.20405. [DOI] [PubMed] [Google Scholar]
- 42.Roddy E, Zhang W, Doherty M. Concordance of the management of chronic gout in a UK primary-care population with the EULAR gout recommendations. Ann Rheum Dis. 2007;66:1311–5. doi: 10.1136/ard.2007.070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez-Ruiz F, Lioté F. Lowering serum uric acid levels: what is the optimal target for improving clinical outcomes in gout? Arthritis Care Res. 2007;57:1324–8. doi: 10.1002/art.23007. [DOI] [PubMed] [Google Scholar]
- 44.Briesacher B, Andrade S, Fouayzi H, et al. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28:437–43. doi: 10.1592/phco.28.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


