Abstract
Objective. Vitamin D deficiency is common in SLE. Cardioprotective effects of vitamin D have been postulated due to modulation of inflammatory cytokines. However, the effects of vitamin D supplementation on inflammatory cytokines in trials have been inconsistent. We determined whether levels of vitamin D at baseline were associated with subclinical measures of atherosclerosis, or with changes in subclinical measures over 2 years.
Methods. Of the 200 patients enrolled in the Lupus Atherosclerosis Prevention Study, complete baseline and follow-up data [including coronary artery calcium (CAC), carotid intima–media thickness (IMT), 25-hydroxy vitamin D [25(OH)D] and high-sensitivity CRP (hsCRP) levels] were available for 154 patients. Assessments were repeated 2 years later.
Results. 25(OH)D values ranged from 4 to 79 ng/ml. Among African American patients, 25(OH)D values ranged from 4 to 55 ng/ml. With low 25(OH)D (vitamin D <21 ng/ml), a higher proportion had a CAC score >100 (11%) compared with those with vitamin D insufficiency (21–32 ng/ml) (10%) and normal (≥32 ng/ml) 25(OH)D (3%), which was not statistically significant. 25(OH)D was neither associated with nor did it predict progression of CAC or carotid IMT over 2 years. The mean hsCRP decreased over 2 years.
Conclusion. 25(OH)D was not associated with any measure of subclinical atherosclerosis. 25(OH)D deficiency was associated with higher hsCRP at baseline, but did not predict a change in hsCRP over 2 years.
Keywords: systemic lupus erythematosus, atherosclerosis, vitamin D
Introduction
Vitamin D deficiency has increased throughout the world [1]. It has been linked to coronary artery disease, heart failure and renal disease [2–4]. In the prospective Framingham Offspring Study, a hazard ratio of 1.62 for cardiovascular disease was found among individuals with low 25-hydroxy vitamin D [25(OH)D] vs without [5]. In the nested case–control Health Professionals Follow-up Study, a relative risk of 2.09 for myocardial infarction among men with low 25(OH)D levels was found [6]. A prospective study of 3258 participants reported a hazard ratio of 2.22 vs 1.82 for cardiovascular mortality for lower vs higher vitamin D levels [7].
Calcitriol, the active form of vitamin D, plays an important role in regulating cardiac muscle protein myotrophin. Calcitriol exerts its effects through the cytosolic vitamin D receptor (VDR), which is widely found in murine as well as human tissues and cells, including cardiac myocytes [8]. Murine studies in VDR knockout mice found significant underexpression of matrix metalloproteinase inhibitors. Matrix metalloproteinases destabilize atherosclerotic plaques and also play a role in myocardial dysfunction, including heart failure [9]. In other murine models, vitamin D supplementation had a beneficial effect on collagen-induced arthritis, inflammatory bowel disease and transplant rejection [10–12].
Vitamin D deficiency is common in SLE. Earlier studies suggested a prevalence of 8%, whereas recent studies have shown a prevalence as high as 67% [13, 14]. Vitamin D deficiency is associated with other autoimmune diseases, including type 1 diabetes mellitus, rheumatoid arthritis and multiple sclerosis [15–17].
The association of vitamin D with subclinical measures of atherosclerosis in SLE have been contradictory, with some studies suggesting a positive association [18–20], while another study was negative [21]. We investigated whether low 25(OH)D was associated with subclinical measures of atherosclerosis or a major inflammatory marker, high-sensitivity CRP (hsCRP). In addition, we investigated whether low vitamin D can predict a change in subclinical measures of atherosclerosis or hsCRP over 2 years.
Methods
Study participants
Participants in the Lupus Atherosclerosis Prevention Study (LAPS) were members of the Hopkins Lupus Cohort who chose to participate in a randomized, double-blind, placebo-controlled trial of atorvastatin 40 mg vs matching placebo [22]. Patients with a history of an atherosclerotic event (such as angina or myocardial infarction) were excluded. Of the 200 patients enrolled in this trial, complete baseline and follow-up data [including coronary artery calcium (CAC), carotid intima–media thickness (IMT), 25(OH)D and hsCRP levels] were available for 154 patients. All patients gave informed consent for the LAPS, which was approved by the Johns Hopkins University School of Medicine Institutional Review Board. Data from the LAPS were analysed for this study.
At baseline and after 2 years, CAC was assessed by multidetector CT and carotid IMT assessed by carotid duplex ultrasound. We combined both treatment groups for the analysis of progression (those on statin and those on placebo), as statin use did not affect progression.
Measurement of vitamin D
Vitamin D levels at baseline were measured on stored sera by chemiluminescence immunoassay on a Liaison instrument (DiaSorin, Stillwater, MN, USA). The Liaison 25(OH)D assay is a direct competitive chemiluminescent immunoassay for the quantitative determination of total 25(OH)D in serum. During the first incubation, 25(OH)D was dissociated from its binding protein and bound to the specific antibody on the solid phase. After 10 min the tracer (vitamin D linked to an isoluminol derivative) was added. After the second 10 min incubation, the unbound material was removed with a wash cycle. Subsequently the starter reagents were added to initiate a flash chemiluminescent reaction. The light signal was measured by a photomultiplier as relative light units and was inversely proportional to the concentration of 25(OH)D present in calibrators, controls or samples. We did not have follow-up data on vitamin D levels during the study period.
Vitamin D assays
The inter-assay (between run) precision was determined by running 10 samples using the manufacturer’s low and high quality control (QC) material over a period of 10 days. We established the coefficient of variation (CV) based on a 2 s.d. calculation from the QC range for the low and high controls manufactured by DiaSorin. The established CV for the low control was 15% and 12% for the high control. The CVs that resulted from the testing were 10.6% (low) and 9.7% (high), both of which were within the measured range. The intra-assay (within run) precision was determined by running the manufacturer’s low and high QC material 20 times each within 1 day. The CVs were 5.3% (low) and 4.4% (high), which were well within the measured range (15% for the low control and 12% for the high control). Both inter- and intra-assay results demonstrated a precision within the expected calculated CV range from the manufacturers’ QC material of 15% and 12%, respectively.
Measurement of coronary artery calcification
Coronary artery calcification scores were calculated using Agatston scoring. Coronary artery calcification was assessed by multidetector CT with a Siemens Volume Zoom Scanner (Siemens Medical Solutions, Malvern, PA, USA) using a 2.5-mm collimation and a slice width of 3 mm, with both scans done on the same machine. Data were reloaded into a Siemens Leonardo workstation using Siemens calcium scoring software. Coronary artery calcification was quantified using a standard scoring system, available as part of the scanner software package [23]. There is excellent reproducibility of coronary artery calcification using CT [24].
Measurement of carotid IMT
Carotid IMT was measured using high-resolution B-mode ultrasound at baseline and 24 months to image the right and left common carotid arteries using a single ultrasound machine (Sonos 5500, Philips Medical Systems) with a linear array 8-MHz scan head with standardized image settings, including resolution mode, depth of field, gain and transmit focus. Digital imaging and communications in medicine images from a diastolic frame of the cine-loop recording were electronically stored and transferred via optical disk to an off-line workstation for analysis. The carotid IMT was measured between the lumen intima and media adventitia interfaces of the far wall of the common carotid artery (the 1 cm segment proximal to the bifurcation) by a single reader using an automated edge detection system. The mean IMT of this 1 cm segment was measured on two separate images of the left and right common carotid artery at the peak of the R wave on a simultaneous electrocardiogram tracing. The mean of these four measurements was used as the IMT. We chose this location because of its demonstrated reproducibility compared with measurements of carotid IMT at other sites [25, 26]. Both patients and providers were blinded to the IMT results until the 2-year follow-up examination was completed and each examination was considered as an independent study. The provider did not know the previous IMT results.
Statistical analysis
Subgroups of patients defined by vitamin D levels were compared with respect to baseline measures of subclinical atherosclerosis and hsCRP. The groups were also compared with respect to changes in subclinical atherosclerosis and hsCRP. Pearson chi-square tests and analysis of variance (ANOVA) methods were used to assess the statistical significance of observed associations.
Results
The SLE patients were 92% female, 62% Caucasian, 32% African American and had a mean age of 46 years. Cumulative revised ACR classification criteria included malar rash 62%, discoid rash 23%, photosensitivity 64%, oral ulcers 60%, arthritis 82%, serositis 53%, renal disorder 43%, neurological disorder 10%, haematological disorder 73%, immunological disorder 90% and ANA positivity 97%.
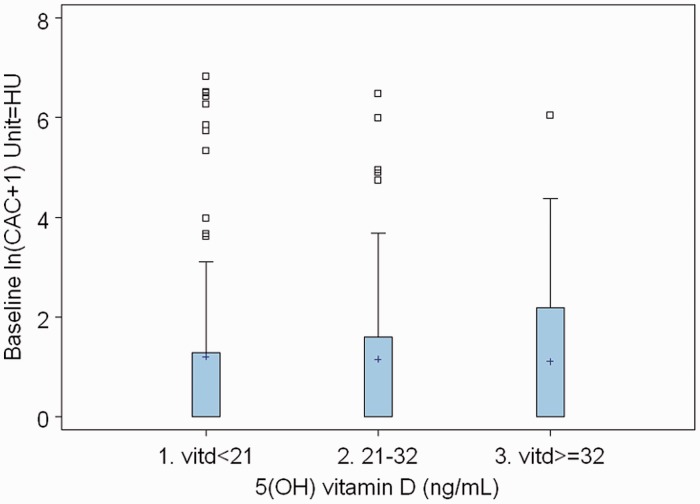
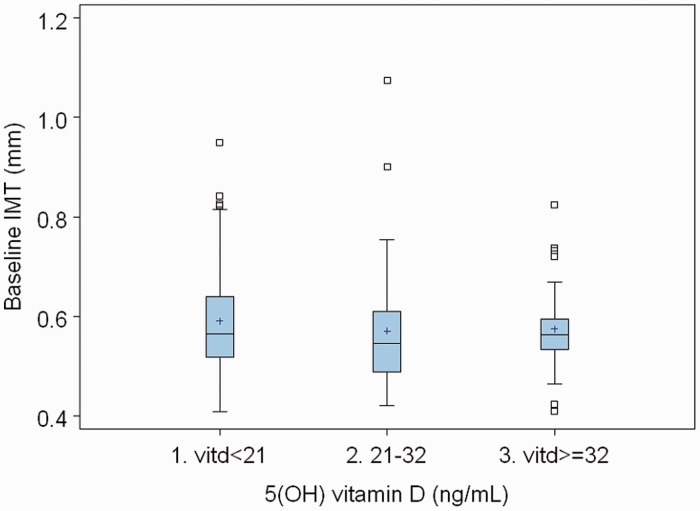
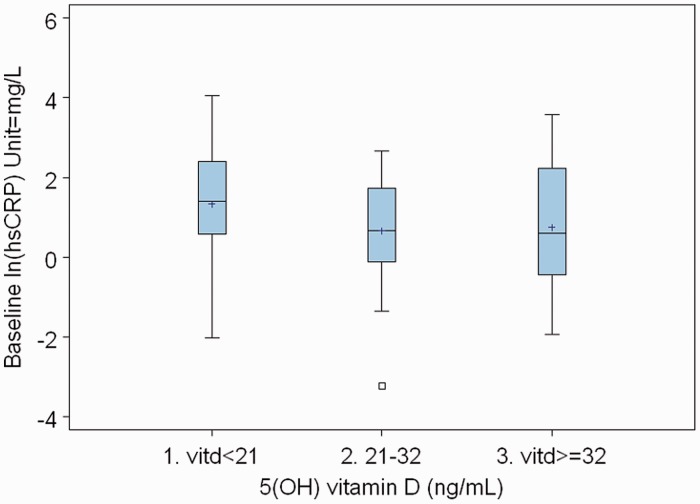
Table 1 shows the baseline characteristics by 25(OH)D status. Caucasian patients had more vitamin D levels ≥32 ng/ml than African American patients (78% vs 16%) (P = 0.0001). Table 2 shows the CAC by vitamin D status. Of those with 25(OH)D deficiency, a higher proportion had a coronary calcium score >100 (11%) compared with those with 25(OH)D insufficiency (10%) and normal vitamin D (3%). We then looked at changes in CAC, carotid IMT and hsCRP between the 25(OH)D insufficiency, deficiency and normal groups. There was no statistically significant difference in the change over 2 years between these groups (Table 3). We did another analysis using vitamin D as a continuous variable. There was no change in our conclusions. We have shown some of these data, described above, in the form of boxplots in Figs 1–3. There were no atherosclerotic events during the follow-up period of the study.
Table 1.
Baseline characteristics by 25(OH)D status
| Characteristic by vitamin D levels, n (%) |
||||
|---|---|---|---|---|
| Variable | <21 ng/ml (n = 71) | 21–32 ng/ml (n = 51) | >32 ng/ml (n = 32) | P-value |
| Age, years | ||||
| 18–30 | 5 (7) | 6 (12) | 3 (9) | 0.84 |
| 31–49 | 42 (59) | 28 (55) | 16 (50) | |
| 50+ | 24 (34) | 17 (33) | 13 (41) | |
| Ethnicity | ||||
| Caucasian | 31 (44) | 39 (76) | 25 (78) | 0.0001 |
| African American | 37 (52) | 8 (16) | 5 (16) | |
| Other | 3 (4) | 4 (8) | 2 (6) | |
| Gender | ||||
| Female | 67 (94) | 46 (90) | 29 (91) | 0.66 |
| Male | 4 (6) | 5 (10) | 3 (9) | |
Table 2.
Levels of CAC by 25(OH)D status
| Vitamin D status | CAC score |
||
|---|---|---|---|
| All patients | 0 | 0.1–100 | >100 |
| <21 ng/ml | 41 (58) | 22 (31) | 8 (11) |
| 21–32 ng/ml | 28 (55) | 18 (35) | 5 (10) |
| 32+ ng/ml | 20 (63) | 11 (34) | 1 (3) |
All values are n (%).
Table 3.
Baseline and changes in CAC, carotid IMT and hsCRP by baseline 25(OH)D level
| Measure | Mean at baseline | P-value for differences in baselinea | Mean after 2 years | Mean change | P-value for change | P-value for differences in changea |
|---|---|---|---|---|---|---|
| Loge (CAC score +1) | ||||||
| Vitamin D <21 | 1.20 | 0.96 | 1.38 | 0.18 | 0.13 | 0.10 |
| Vitamin D 21–32 | 1.15 | 1.05 | −0.09 | 0.62 | ||
| Vitamin D ≥32 | 1.11 | 1.30 | 0.19 | 0.25 | ||
| Carotid IMT | ||||||
| Vitamin D <21 | 0.59 | 0.99 | 0.69 | 0.10 | <0.0001 | 0.19 |
| Vitamin D 21–32 | 0.57 | 0.64 | 0.07 | <0.0001 | ||
| Vitamin D ≥32 | 0.58 | 0.65 | 0.07 | <0.0001 | ||
| Loge (hsCRP) | ||||||
| Vitamin D <21 | 1.33 | 0.017 | 1.04 | −0.29 | 0.011 | 0.89 |
| Vitamin D 21–32 | 0.66 | 0.33 | −0.33 | 0.049 | ||
| Vitamin D ≥32 | 0.75 | 0.32 | −0.43 | 0.036 | ||
aAdjusted for ethnicity, drug treatments including HCQ and prednisone, and serum creatinine. 25(OH)D is shown in ng/ml.
Fig. 1.
Box plot of baseline CAC score vs vitamin D.
Fig. 2.
Box plot of baseline carotid IMT vs vitamin D.
Fig. 3.
Box plot of baseline hsCRP vs vitamin D.
Discussion
In the general population, several studies have highlighted the association of lower vitamin D with subclinical measures of atherosclerosis, including CAC and carotid IMT [27–29]. In the general population, a positive association was found between aortic plaque and low vitamin D levels among African Americans [30]. Bruce et al. [20] found that low 25(OH)D was associated with aortic stiffness, but not with carotid IMT. Wu et al. [18] found no association between low vitamin D levels and carotid IMT. Our study is the first in SLE patients, but also the first to look at 25(OH)D and CAC, and the first to look at progression over 2 years. We did find a higher proportion of patients with CAC scores >100 and low vitamin D levels, but this was not statistically significant.
Inflammation plays an important role in the pathogenesis of atherosclerosis in SLE. In the general population, multiple studies have shown hsCRP to be an independent predictor of future stroke and myocardial infarction [31–33]. With each increasing quartile of baseline hsCRP, the relative risk for first myocardial infarction and stroke increased. In a cohort of 27 939 women, hsCRP was found to be a strong indicator of cardiovascular disease risk [33]. Using National Health and Nutrition Examination Survey (NHANES) data, an inverse relationship was found between low 25(OH)D (≤21 ng/ml) and hsCRP. Higher levels (≥21 ng/ml) were associated with an increase in hsCRP [34].
We have previously reported that hsCRP was associated with higher SLE disease activity, measured by either the physician’s global assessment or the Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA)–SLEDAI [35]. Other studies in SLE have found that hsCRP levels were increased with serositis and arthritis [36, 37]. In a study of newly diagnosed SLE patients, hsCRP levels were reliable indicators of disease activity [38].
Several cross-sectional studies in SLE have shown inconsistent results between hsCRP and subclinical atherosclerosis as measured by carotid IMT, CAC or carotid plaque [39–43]. In a multi-ethnic prospective SLE study of 546 patients, hsCRP levels were associated with vascular events [44]. However, we did not find any association of hsCRP with CAC [22]. One of the limitations of the study is we have missing data on patients, leading to potential bias.
In this study, low 25(OH)D was associated with high hsCRP but did not predict the progression of hsCRP over 2 years. We found lower hsCRP levels with 25(OH)D levels ≥21 ng/ml. This is in contrast to another study, in the general population, that showed higher hsCRP levels with vitamin D levels ≥21 ng/ml [34]. Neither vitamin D deficiency nor high hsCRP predicts progression of subclinical atherosclerosis in SLE.
Rheumatology key messages.
Neither vitamin D deficiency nor high hsCRP predicts progression of subclinical atherosclerosis in SLE.
There was no association of hsCRP with CAC in SLE.
Acknowledgements
LAPS was supported by the Alliance for Lupus Research. The Hopkins Lupus Cohort is supported by a grant from the National Institutes of Health (NIH AR 43727). This research was also supported by grant number UL1 RR 025005 from the National Center for Research Resources (NCRR). There are no relevant financial disclosures.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Lee JH, O’Keefe JH, Bell D, et al. Vitamin D deficiency: an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–56. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E, Liu Y, Hollis BW, et al. 25-Hydroxyvitamin D and risk of myocardial infarction in men. Arch Intern Med. 2008;168:1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–13. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 5.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannucci E, Liu Y, Hollis BW, et al. A prospective study of 25-hydroxyvitamin D in relation to risk of myocardial infarction in men. Arch Intern Med. 2008;168:1174–80. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 8.Nibbelink KA, Tishkoff DX, Hershey SD, et al. 1,25(OH)2-vitamin D3 actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol. 2007;103:533–7. doi: 10.1016/j.jsbmb.2006.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman A, Hershey S, Ahmed S, et al. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103:416–9. doi: 10.1016/j.jsbmb.2006.12.081. [DOI] [PubMed] [Google Scholar]
- 10.Larsson P, Mattsson L, Klareskog L, et al. A vitamin D analogue (MC1288) has immunomodulatory properties and suppresses collagen-induced arthritis (CIA) without causing hypercalcemia. Clin Exp Immunol. 1998;114:277–83. doi: 10.1046/j.1365-2249.1998.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantorna MT, Munsick C, Bemiss C, et al. 1,25 Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–52. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 12.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med. 2002;8:174–9. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 13.Bultink IE, Lems WF, Kostense PJ. Prevalence of and risk factors for low bone mineral density and vertebral fractures in patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52:2044–50. doi: 10.1002/art.21110. [DOI] [PubMed] [Google Scholar]
- 14.Kamen DL, Cooper GS, Bouali H. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev. 2006;5:114–7. doi: 10.1016/j.autrev.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Littorin B, Blom P, Scholin A. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS) Diabetologia. 2006;49:2847–52. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 16.Merlino LA, Curtis J, Mikuls TR. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50:72–7. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 17.Nieves J, Cosman F, Herbert J. High prevalence of vitamin D deficiency and reduced bone mass in multiple sclerosis. Neurology. 1994;44:1687–92. doi: 10.1212/wnl.44.9.1687. [DOI] [PubMed] [Google Scholar]
- 18.Wu PW, Rhew EY, Dyer AR. 25-hydroxyvitamin D and cardiovascular risk factors in women with systemic lupus erythematosus. Arthritis Care Res. 2009;61:1387–95. doi: 10.1002/art.24785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravenell RL, Kamen DL, Spence JD, et al. Premature atherosclerosis is associated with hypovitaminosis D and angiotensin-converting enzyme inhibitor non-use in lupus patients. Am J Med Sci. 2012;344:268–73. doi: 10.1097/MAJ.0b013e31823fa7d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds JA, Haque S, Berry JL, et al. 25-Hydroxyvitamin D deficiency is associated with increased aortic stiffness in patients with systemic lupus erythematosus. Rheumatology. 2012;51:544–51. doi: 10.1093/rheumatology/ker352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mok CC, Birmingham DJ, Leung HW. Vitamin D levels in Chinese patients with systemic lupus erythematosus: relationship with disease activity, vascular risk factors and atherosclerosis. Rheumatology. 2012;51:644–52. doi: 10.1093/rheumatology/ker212. [DOI] [PubMed] [Google Scholar]
- 22.Kiani AN, Magder LS, Petri M. Coronary calcium in systemic lupus erythematosus is associated with traditional cardiovascular risk factors but not with disease activity. J Rheumatol. 2008;35:1300–6. [PubMed] [Google Scholar]
- 23.Budoff MJ, Georgiou D, Brody A, et al. Ultrafast computed tomography as a diagnostic modality in the detection of coronary artery disease: a multicenter disease: a multicenter study. Circulation. 1996;93:898–904. doi: 10.1161/01.cir.93.5.898. [DOI] [PubMed] [Google Scholar]
- 24.Kopp AF, Ohnesorge B, Becker C, et al. Reproducibility and accuracy of coronary calcium measurements with multi-detector row versus electron-beam CT. Radiology. 2002;225:113–9. doi: 10.1148/radiol.2251010173. [DOI] [PubMed] [Google Scholar]
- 25.Bots ML, Mulder PG, Hofman A. Reproducibility of carotid vessel wall thickness measurements: the Rotterdam study. J Clin Epidemiol. 1994;47:921–30. doi: 10.1016/0895-4356(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 26.Smilde TJ, Wollersheim H, Van Langen H. Reproducibility of ultrasonographic measurements of different carotid and femoral artery segments in healthy subjects and in patients with increased intima-media thickness. Clin Sci (Lond) 1997;93:317–24. doi: 10.1042/cs0930317. [DOI] [PubMed] [Google Scholar]
- 27.Doherty TM, Tang W, Dascalos S, et al. Ethnic origin and serum levels of 1α,25-dihydroxyvitamin D3 are independent predictors of coronary calcium mass measured by electron-beam computed tomography. Circulation. 1997;96:1477–81. doi: 10.1161/01.cir.96.5.1477. [DOI] [PubMed] [Google Scholar]
- 28.Reis JP, von Muhlen D, Michos ED, et al. Serum vitamin D, parathyroid hormone levels, and carotid atherosclerosis. Atherosclerosis. 2009;207:589–90. doi: 10.1016/j.atherosclerosis.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson KE, Abrolat ML, Malone LL, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–60. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 30.Freedman BI, Wageknecht LE, Hairston KG, et al. Vitamin D, adiposity and calcified atherosclerotic plaque in African-Americans. J Clin Endocrinol Metab. 2010;95:1076–83. doi: 10.1210/jc.2009-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 32.Bassuk SS, Rifai N, Ridker PM. High sensitivity C reactive protein: clinical importance. Curr Probl Cardiol. 2004;29:439–93. [PubMed] [Google Scholar]
- 33.Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 34.Amer M, Qayyum R. Relation between serum 25-hydroxyvitamin D and C-reactive protein in asymptomatic adults (from the continuous national health and nutrition examination survey 2001-2006) Am J Cardiol. 2012;109:226–30. doi: 10.1016/j.amjcard.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 35.Lee SS, Singh S, Magder LS, et al. Predictors of high sensitivity C-reactive protein levels in patients with systemic lupus erythematosus. Lupus. 2008;17:114–23. doi: 10.1177/0961203307085878. [DOI] [PubMed] [Google Scholar]
- 36.Mochizuki T, Aotsuka S, Satoh T. Clinical and laboratory features of lupus patients with complicating pulmonary disease. Respir Med. 1999;93:95–101. doi: 10.1016/s0954-6111(99)90297-4. [DOI] [PubMed] [Google Scholar]
- 37.Moutsopoulos HM, Mavridis AK, Acritidis NC, et al. High C-reactive protein response in lupus polyarthritis. Clin Exp Rheumatol. 1983;1:53–5. [PubMed] [Google Scholar]
- 38.Liou LB. Serum and in vitro production of IL-1 receptor antagonist correlate with C-reactive protein levels in newly diagnosed, untreated lupus patients. Clin Exp Rheumatol. 2001;19:515–23. [PubMed] [Google Scholar]
- 39.Selzer F, Sutton-Tyrrell K, Fitzgerald SG, et al. Comparison of risk factors for vascular disease in the carotid artery and aorta in women with systemic lupus erythematosus. Arthritis Rheum. 2004;50:151–9. doi: 10.1002/art.11418. [DOI] [PubMed] [Google Scholar]
- 40.Roman MJ, Shanker BA, Davis A, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 41.Asanuma Y, Oeser A, Shintani AK, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–15. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 42.Svenungsson E, Jensen-Urstad K, Heimburger M, et al. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation. 2001;104:1887–93. doi: 10.1161/hc4101.097518. [DOI] [PubMed] [Google Scholar]
- 43.Manzi S, Selzer F, Sutton-Tyrrell K, et al. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:51–60. doi: 10.1002/1529-0131(199901)42:1<51::AID-ANR7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 44.Toloza SM, Uribe AG, McGwin G, Jr, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA). XXIII. Baseline predictors of vascular events. Arthritis Rheum. 2004;50:3947–57. doi: 10.1002/art.20622. [DOI] [PubMed] [Google Scholar]