Abstract
The Na+ taurocholate (TC) cotransporting polypeptide Ntcp/NTCP mediates TC uptake across the sinusoidal membrane of hepatocytes. Previously, we demonstrated that nitric oxide (NO) inhibits TC uptake through S-nitrosylation of a cysteine residue. Our current aim was to determine which of the eight cysteine residues of Ntcp is responsible for NO-mediated S-nitrosylation and inhibition of TC uptake. Thus, we tested the effect of NO on TC uptake in HuH-7 cells transiently transfected with cysteine-to-alanine mutant Ntcp constructs. Of the eight mutants tested, only C44A Ntcp displayed decreased total and plasma membrane (PM) levels that were also reflected in decreased TC uptake. C266A Ntcp showed a decrease in TC uptake that was not explained by a decrease in total expression or PM localization, indicating that C266 is required for optimal uptake. We speculated that NO would target C266 since a previous report had shown the thiol reactive compound [2-(trimethylammonium) ethyl] methanethiosulfonate bromide (MTSET) inhibits TC uptake by wild-type NTCP but not by C266A NTCP. We confirmed that MTSET targets C266 of Ntcp, but, surprisingly, we found that C266 was not responsible for NO-mediated inhibition of TC uptake. Instead, we found that C96 was targeted by NO since C96A Ntcp was insensitive to NO-mediated inhibition of TC uptake. We also found that wild-type but not C96A Ntcp is S-nitrosylated by NO, suggesting that C96 is important in regulating Ntcp function in response to elevated levels of NO.
Keywords: nitric oxide, S-nitrosylation, sodium-dependent taurocholate cotransporting polypeptide
the transcellular hepatic transport of bile acids is driven by various transporters present at sinusoidal and canalicular membranes of hepatocytes. The Na+-dependent taurocholate (TC) cotransporting polypeptide (NTCP/Ntcp) is the major sinusoidal bile salt transporter of hepatocytes. Ntcp mediates the transport of conjugated bile salts such as TC and taurochenodeoxycholate in a Na+-dependent fashion using the Na+ gradient produced by the Na+-K+-ATPase (1, 16, 24, 39). Orthologs of the rat Ntcp have been identified for human, mouse, and rabbit (42).
Cholestasis, the pathological condition marked by a decrease in bile formation, can be caused by a number of factors, one of them being bacterial infections (6, 12, 24). Sepsis-associated cholestasis is most common with systemic gram-negative bacterial infections. Sepsis-induced cholestasis occurs as a result of the effect of lipopolysaccharide (LPS) on the liver (12). During LPS-induced cholestasis, bile flow is decreased, resulting from both plasma membrane (PM) retrieval of bile transporters (short-term regulation) and ultimately a decrease in the expression of bile transporters (long-term regulation) (6, 12, 24). At the same time, sepsis-associated cholestasis is associated with a burst of nitric oxide (NO) production by inducible nitric oxide synthase in all major cell types of the liver (12).
A recent report demonstrated that NO can inhibit TC uptake in isolated rat hepatocytes (37). One of the ways that NO exerts its cellular effects is through a posttranslational modification termed S-nitrosylation, the binding of NO to reactive thiol groups on cysteine residues. We have previously shown that NO noncompetitively inhibits TC uptake by NTCP. This inhibition by NO was reversible by dithiothreitol (DTT), which supports the notion that NO blocks uptake by modifying cysteine residues. Furthermore, we found that NO treatment results in S-nitrosylation of NTCP under these conditions (33). These results led to the suggestion that NO inhibits TC uptake by S-nitrosylating NTCP. However, the cysteine residue (s) of Ntcp S-nitrosylated by NO has not been determined. Identification of the cysteine residues targeted by NO would lead to an improved understanding of the posttranscriptional regulation of Ntcp/NTCP.
Ntcp has eight cysteines, and one of these, Cys266, is highly conserved not only across several species but also with the NTCP/Ntcp homolog, apical Na+-dependent bile acid transporter (Asbt) (32, 42). A previous study reported that the thiol reactive compound [2-(trimethylammonium) ethyl] methanethiosulfonate bromide (MTSET) inhibits TC uptake by wild-type (WT) human NTCP but not by the C266A NTCP mutant, indicating that the C266 residue is responsible for the sensitivity to the MTSET compound (17). Thus, we hypothesized that cysteine 266 of Ntcp/NTCP could be S-nitrosylated by NO. The aim of this study was to determine which of the eight cysteine residues of Ntcp is responsible for NO-mediated inhibition of TC uptake. Surprisingly, we found that C266 is not responsible for NO-mediated inhibition of TC uptake. Rather, we found that mutation of C96 is targeted by NO and is important for NO-mediated inhibition of TC uptake.
MATERIALS AND METHODS
Materials.
TC (Na+ salt), cysteine, (+)-sodium l-ascorbate, DTT, S-methyl methanethiosulfonate (MMTS), and streptavidin agarose were purchased from Sigma-Aldrich (St. Louis, MO). MTSET was purchased from Affymetrix (Maumee, OH). Sodium nitrite was purchased from JT Baker Chemical (Phillipsburg, NJ). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA). Biotin-HPDP and sulfo-NHS-LC-biotin were purchased from Pierce (Rockford, IL). E-cadherin antibody was purchased from BD Transduction Laboratories (San Jose, CA). Actin antibody was purchased from Calbiochem (San Diego, CA). Antibody for Ntcp was a gift from Dr. Alan Wolkoff (New York, NY). [3H]TC was purchased from Perkin Elmer (Shelton, CT). S-nitrosocysteine (CysNO) was prepared fresh by combining equal volumes of 600 mM NaNO2 in H2O with 600 mM l-cysteine in 0.5 M HCl to yield a 300 mM stock solution of CysNO. The QuickChange II Site-Directed Mutagenesis Kit was purchased from Agilent Technologies (Lexington, MA). Mutagenesis primers were purchased from Integrated DNA Technologies (Coralville, IA). A pcDNA3-based Ntcp expression construct was a gift from Dr. Ralf Kubitz (Dusseldorf, Germany).
Site-directed mutagenesis.
Cysteine-to-alanine mutations for each Ntcp cysteine were generated using standard conditions with the QuickChange II Site-Directed Mutagenesis Kit from Agilent. Primers for each mutant were previously reported (42). Each mutation was confirmed by sequencing.
HuH-7 cell culture and transfection.
HuH-7 cells were from a gift from Dr. Ananthanaraynan (New York, NY). Cells were cultured in Dulbeco's minimum essential medium supplemented with 10% fetal bovine serum, 100,000 U/l penicillin, and 100 mg/l streptomycin at 37°C under 5% CO2. HuH-7 cells were transfected with an empty vector (pcDNA3), WT, or cysteine mutant Ntcp by using Lipofectamine 2000 according to the manufacturer's instructions. Briefly, the culture medium was changed to OptiMem containing Lipofectamine and empty vector, WT, or mutant Ntcp and incubated at 37°C for 24 h. Cells were briefly washed and then incubated with complete media for another 24 h. On experiment days, cells were incubated for 3 h in serum-free media and then incubated in a HEPES assay buffer, pH 7.4 (20 mM HEPES, 140 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1 mM CaCl2, 0.8 mM KH2PO4, and 5 mM glucose), before determining TC uptake, biotinylation of PM-bound proteins, or NTCP S-nitrosylation.
S-nitrosylation assay.
The biotin switch assay was performed as described previously with minor modifications (10, 33). Following transient transfection of HuH-7 cells with WT or mutant Ntcp, cells were treated with or without CysNO. Lysates were prepared, and then samples were incubated with a final concentration of 2.5% SDS and 0.2% MMTS for 20 min at 50°C with frequent vortexing to block free thiols. MMTS was removed by acetone precipitation, and then the samples were resuspended in HENS buffer (100 mM HEPES, 1 mM EDTA, 0.1 mM neocuproine, 1% SDS, pH 8.0) with biotin-HPDP and ascorbic acid (0.25 mg/ml and 20 mM final concentrations, respectively). Samples were rotated in the dark for 1 h at room temperature. After another acetone precipitation, samples were resuspended in 0.25 ml HENS/10 (HENS buffer diluted 1:10 in H2O) buffer followed by the addition of 0.75 ml of neutralization buffer (25 mM HEPES, 100 mM NaCl, 1 mM EDTA, and 0.5% Triton X-100, pH 7.5). Streptavidin agarose beads (50 μl) were added, and then samples were rocked overnight at 4°C. Samples were washed four times with wash buffer (neutralization buffer + 600 mM NaCl) and then eluted with 30 μl elution buffer (HEN/10 buffer containing 1% β-mercaptoethanol). After addition of an equal volume of 2× SDS sample buffer plus β-mercaptoethanol the samples were processed for immunoblot.
Immunoblot.
Following SDS-PAGE, proteins were transferred to nitrocellulose. Membranes were blocked with 5% milk in 0.1% Tween-TBS (TTBS) for 1 h at room temperature while rocking. Antibodies for either Ntcp, actin, or E-cadherin were then added to 5% milk-TTBS and incubated with the membrane for 2 h at room temperature while rocking. After three washes with TTBS, membranes were incubated with a horseradish peroxidase-conjugated secondary antibody in 5% milk-TTBS for 1 h at room temperature. Following five washes in TTBS, ECL was performed, and proteins were detected by autoradiography. Blots were scanned into Adobe Photoshop and subjected to computerized densitometric scanning using SigmaGel.
TC uptake.
TC uptake was determined as previously described (33). Following transfection with pcDNA3, WT or mutant Ntcp cells were treated with or without CysNO or MTSET for the indicated doses and then incubated with 20 μM TC containing [3H]TC for 2 min. The cells were then washed thee times with ice-cold HEPES assay buffer and lysed in lysis buffer (20 mM Tris, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, plus protease inhibitors, pH 7.5). Aliquots of cell lysate were counted for radioactivity and used for protein determination. Each individual experiment was performed in triplicate. TC uptake was calculated after correcting for background uptake determined from cells transfected with pcDNA3, and relative TC uptake was expressed by setting WT Ntcp control samples as one.
Translocation of NTCP.
A previously described cell surface biotinylation method was used to assess Ntcp translocation in cells transfected with WT or mutant Ntcp (33). After treatment with or without CysNO, cell surface proteins were biotinylated by exposing cells to sulfo-NHS-LC-biotin (0.5 mg/ml) in PBS, pH 8.0 for 45 min on ice. Cells were then washed with PBS + 100 mM glycine, pH 8.0, and cell lysates were prepared. Biotinylated proteins were isolated with streptavidin-agarose beads followed by immunoblot analysis. Immunoblotting with E-cadherin, a PM protein, was used for a loading control. The absence of biotinylated GAPDH was routinely monitored to ensure that intracellular proteins were not biotinylated. In some experiments, total Ntcp was determined and normalized against actin expression.
Data analysis.
Values are presented as means ± SE, unless otherwise indicated. Differences between groups were analyzed using Student's t -test with P < 0.05 considered significant.
RESULTS
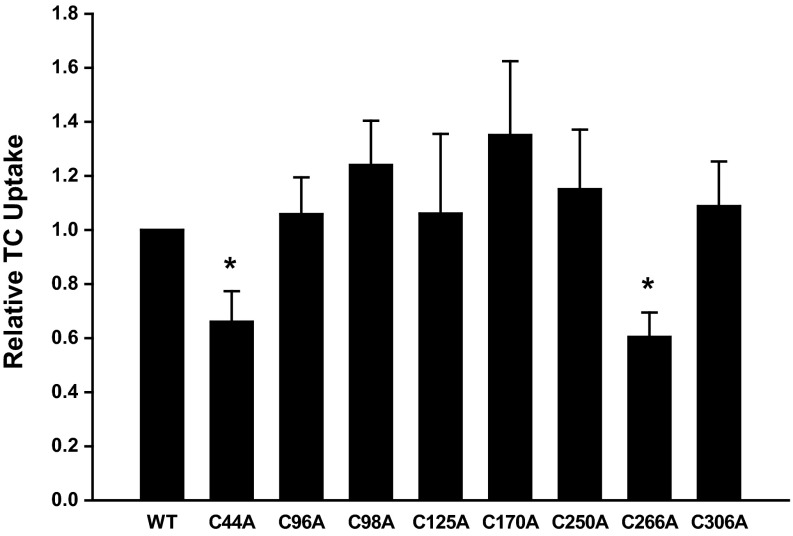
To investigate which cysteine residue(s) might serve as a target for NO, we created cysteine-to-alanine mutations for each of the eight Ntcp cysteines. We then determined the effect of these mutations on basal TC uptake. We found that C44A and C266A mutations led to a decrease in TC uptake compared with WT Ntcp (Fig. 1).
Fig. 1.
C44A and C266A Na+-dependent taurocholate (TC) cotransporting polypeptides (Ntcp) show a decrease in TC uptake. HuH-7 cells were transfected with wild-type (WT) and cysteine mutant Ntcp constructs. Later (48 h), TC uptake was determined. Data are expressed as means ± SE (n = 7 experiments). *Significantly different from WT Ntcp control values (P < 0.05).
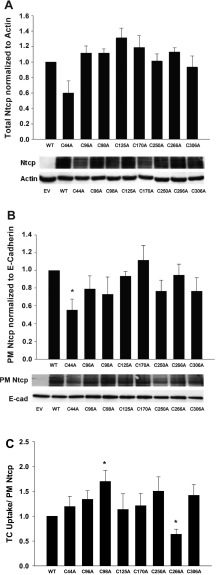
It is possible that a decrease in TC uptake may be due to a decrease in total expression of Ntcp or a decrease in the amount of Ntcp localized to the PM. To control for these possibilities, we performed immunoblots to measure the total expression levels of Ntcp in our transient transfections. Ntcp is highly glycosylated, and immunoblots with this antibody recognize multiple glycosylated forms of Ntcp (19). We and others have shown that Ntcp/NTCP immunoblots appear as three to four bands in close proximity depending on the amount of lysate loaded on gels and the length of film exposure time (19, 23, 33, 35). Mutation of each cysteine did not significantly change the expression level of total Ntcp, although mutation of cysteine 44 did show a nonsignificant decrease in expression (P = 0.0629) (Fig. 2A). We also measured the amount of WT and cysteine mutant Ntcp localized at the PM by using a cell-surface protein biotinylation technique. As with total levels of expression, there was a significant decrease in the PM levels of C44A Ntcp (P = 0.024) (Fig. 2B). This decrease in uptake (33.9 ± 11.3%) was comparable to the decrease in PM Ntcp levels (44.7 ± 12.6%) (Figs. 1 and 2B). Thus, the decrease in TC uptake by C44A is more likely to be due to decreased PM localization (a reflection of decreased total expression) rather than an effect of the mutation. Total and PM levels of all other cysteine mutants were similar to WT Ntcp (Fig. 2, A and B).
Fig. 2.
Effects of cysteine-to-alanine mutation on total and plasma membrane (PM) Ntcp expression. A: empty vector (EV), WT, and cysteine mutant Ntcp were transiently transfected into HuH-7 cells. Later (48 h), cells were collected, and lysates were prepared to measure total Ntcp expression levels by immunoblot. Relative expression levels were normalized against actin levels. Data are expressed as means ± SE (n = 5). B: EV, WT, and cysteine mutant Ntcp were transiently transfected into HuH-7 cells. Later (48 h), PM-bound proteins were selectively biotinylated, cells were collected, and lysates were prepared. PM-bound proteins were purified by streptavidin pulldown, and PM Ntcp levels were determined by immunoblot. Relative PM levels were normalized against E-cadherin levels. Data are expressed as means ± SE (n = 5). *Significantly different from WT Ntcp control values (P < 0.05). C: TC uptake values in Fig. 1 were normalized for the amount of PM Ntcp present in B. Data are expressed as means ± SE (n = 7). *Significantly different from WT Ntcp control values (P < 0.05).
The C266A mutation exhibited a defect in TC uptake (Fig. 1). Unlike C44A Ntcp, however, this defect cannot be explained by a decrease in total or PM expression of C266A Ntcp (Fig. 2, A and B). Indeed, when the TC uptake data shown in Fig. 1 are normalized to the amount of PM Ntcp, the C266A Ntcp mutant still showed a decrease in TC uptake (Fig. 2C). Thus, it would appear that C266 is necessary for optimal function of the transporter. We also found that mutation of C98 to alanine resulted in an increase in TC uptake (Fig. 2C). This result is in agreement with an earlier study that looked at the effect of cysteine mutations of mouse Ntcp on TC uptake in COS-7 cells (32). In contrast, when C98 rat Ntcp was expressed in Xenopus laevis oocytes, uptake levels were similar to WT Ntcp (42). Perhaps the various expression systems used to measure Ntcp function could explain these differing results.
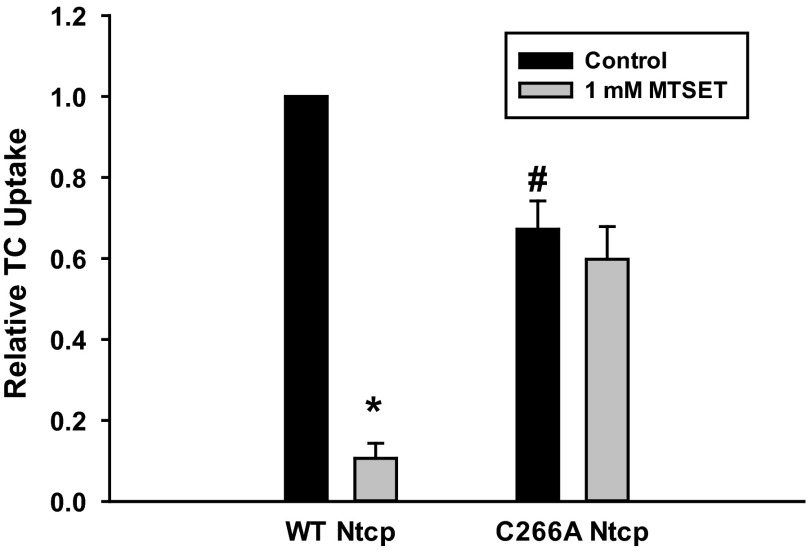
A previous study suggested that inhibition of TC uptake by the thiol-modifying agent MTSET involves C266 of human NTCP (11). This result raised the possibility that NO-mediated inhibition of TC uptake may involve S-nitrosylation of C266. This study was conducted in nonhepatic cell lines (COS-7 and HEK293 cells) transfected with C266A NTCP. The present study was conducted in a hepatic cell line (HuH-7) transfected with rat Ntcp. Thus, to confirm that the effect of MTSET is preserved in our system, we determined the effect of MTSET on TC uptake in HuH-7 cells transfected with WT and C266A Ntcp. Results confirmed the previous finding that WT Ntcp was sensitive to MTSET-mediated inhibition of TC uptake while C266A Ntcp was insensitive to MTSET-mediated inhibition (Fig. 3).
Fig. 3.
C266 is required for [2-(trimethylammonium) ethyl] methanethiosulfonate bromide (MTSET)-mediated inhibition of TC uptake. Cells were transfected with either WT or C266A Ntcp. Later (48 h), cells were treated with (gray bars) or without (black bars) 1 mM MTSET for 30 min, and then relative TC uptake was determined. Data are expressed as means ± SE (n = 4). *Significantly different from untreated sample (P < 0.05). # Significantly different from WT Ntcp control values (P < 0.05).
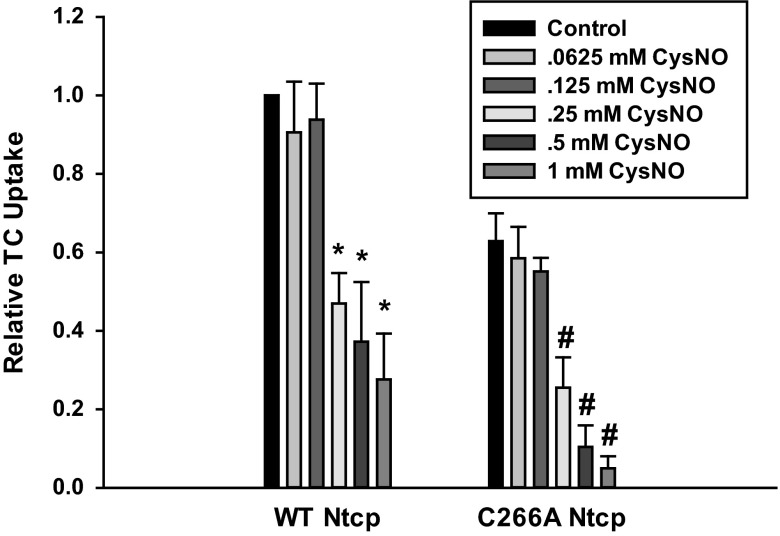
Because C266 was targeted by MTSET, we hypothesized that NO-mediated inhibition of TC uptake may involve C266. We determined dose-dependent effects of NO on TC uptake in HuH-7 cells transfected with WT and C266A Ntcp using the NO donor CysNO. Our expectation was that TC uptake by C266A Ntcp would be insensitive to NO. Surprisingly, we found that both WT and C266A Ntcp were susceptible to NO-mediated inhibition of TC uptake, with significant inhibition observed at doses of 0.25 mM CysNO and higher (Fig. 4). Doses within this range are routinely used to mimic pathological situations where nitrosative stress occurs (2, 7, 21, 22, 41). These results would suggest that NO-mediated inhibition of TC uptake by Ntcp involves cysteine residues other than C266.
Fig. 4.
Nitric oxide (NO)-mediated inhibition of TC uptake does not occur through C266. Cells were transfected with either WT or C266A Ntcp. Later (48 h), cells were treated with or without various doses of S-nitrosocysteine (CysNO, 0.0625–1 mM) for 30 min, and then relative TC uptake was determined. Data are expressed as means ± SE (n = 3). *Significantly different from WT Ntcp control values (P < 0.05). # Significantly different from C266A Ntcp control values (P < 0.05).
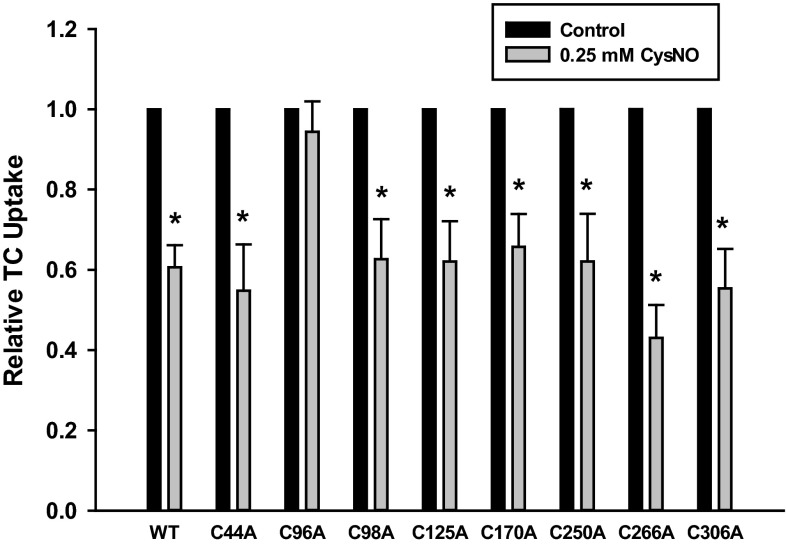
Because C266 is not targeted by NO, we tested if any other cysteine mutant was insensitive to NO-mediated inhibition of uptake. Insensitivity to NO-mediated inhibition would indicate that NO acts by modification of that residue. Uptake assays revealed that NO inhibited uptake by every cysteine mutant except for the C96A mutant (Fig. 5). Thus, as opposed to C266 which is targeted by MTSET (Fig. 4), NO-mediated inhibition of uptake occurs through C96.
Fig. 5.
C96A Ntcp is insensitive to NO-mediated inhibition. Cells were transfected with WT or cysteine mutant Ntcp. Later (48 h), cells were treated with (gray bars) or without (black bars) 0.25 mM CysNO, and relative TC uptake was determined. Data are presented as means ± SE (n = 8). *Statistically different from control samples not treated with CysNO (P < 0.05).
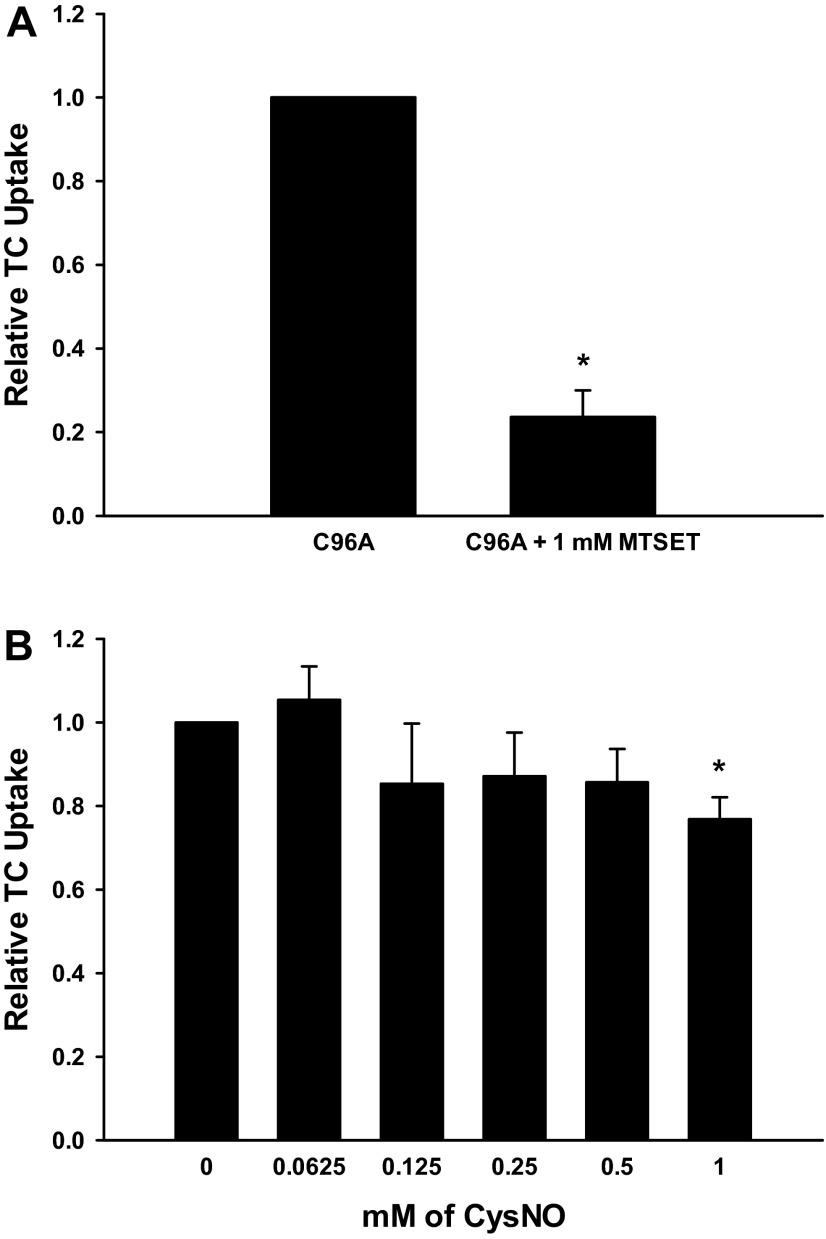
To further characterize the sensitivity of the C96A Ntcp mutant, we tested the effect of MTSET on C96A Ntcp function. As opposed to C266A Ntcp, MTSET had no effect on TC uptake by C96A Ntcp (Fig. 6A). We also performed a more detailed dose response of CysNO on uptake by C96A Ntcp. Strikingly, the C96A Ntcp mutant is resistant to NO-mediated inhibition of TC uptake (Fig. 6B). At a dose of 1 mM CysNO there is a small decrease in TC uptake by the C96A Ntcp mutant (23 ± 5.24%). However, even at this dose, the inhibition by NO does not approach the NO-mediated inhibition seen with WT or C266A Ntcp (72.4 ± 11.66 and 95.0 ± 3.06%, respectively) (Fig. 4). Thus, we conclude that NO-mediated inhibition of TC uptake, but not MTSET-mediated inhibition of TC uptake, occurs through C96.
Fig. 6.
NO-mediated inhibition of TC uptake but not MTSET-mediated inhibition of TC uptake occurs through C96. A: cells were transfected with C96A Ntcp. Later (48 h), cells were treated with or without 1 mM MTSET for 30 min, and then relative TC uptake was determined. Data are expressed as means ± SE (n = 3). *Significantly different from untreated sample (P < 0.05). B: cells were transfected with C96A Ntcp. Later (48 h), cells were treated with or without various doses of CysNO (0.0625–1 mM) for 30 min, and then relative TC uptake was determined. Data are expressed as means ± SE (n = 3). *Significantly different from WT Ntcp control values (P < 0.05).
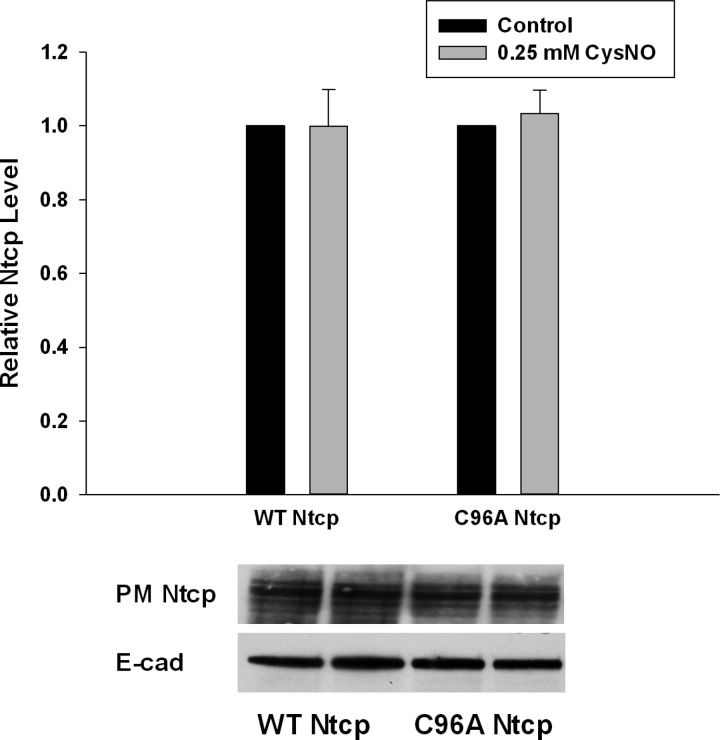
It is possible that CysNO treatment may inhibit TC uptake by decreasing the amount of Ntcp present at the PM. To account for this possibility, we tested the effect of CysNO treatment on the PM levels of both WT and C96A Ntcp. In this study, neither WT nor C96A Ntcp PM levels were affected by CysNO treatment (Fig. 7). Thus, the inhibition of WT rat Ntcp does not appear to occur by decreasing PM levels of the transporter.
Fig. 7.
CysNO does not alter PM levels of WT or C96A Ntcp. WT and C96A Ntcp were transiently transfected into HuH-7 cells. Later (48 h), cells were treated with 0.25 mM CysNO (grey bars) and without CysNO (black bars). PM-bound proteins were selectively biotinylated, cells were collected, and lysates were prepared. PM-bound proteins were purified by streptavidin pulldown, and PM Ntcp levels were determined by immunoblot. Relative PM levels were normalized against E-cadherin levels. Data are expressed as means ± SE (n = 4).
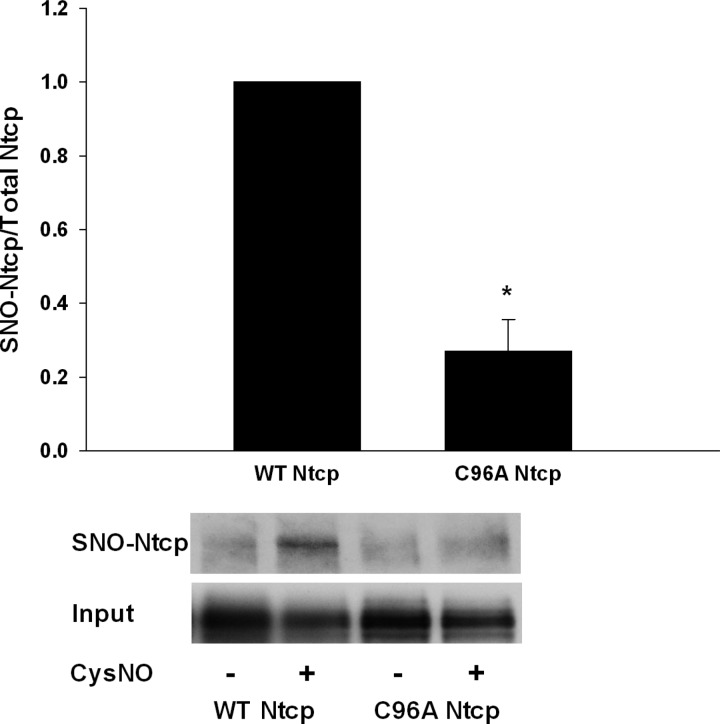
NO mediates many of its cellular effects through binding of reactive cysteine thiol groups in a posttranslational modification termed S-nitrosylation. Indeed, we have previously demonstrated that human NTCP can be S-nitrosylated by NO (33). Because C96A Ntcp was insensitive to NO-mediated inhibition of TC uptake, we tested whether C96A Ntcp is S-nitrosylated by NO. When cells were transfected with either WT or C96A Ntcp and then treated with or without CysNO, we found that WT Ntcp but not C96A Ntcp was S-nitrosylated (Fig. 7). This result would indicate that C96 is S-nitrosylated by NO, and this may explain NO-mediated inhibition of TC uptake.
DISCUSSION
The aim of this study was to determine which of the eight cysteine residues of Ntcp is responsible for NO-mediated S-nitrosylation and inhibition of TC uptake. We found that C44A Ntcp showed a decrease in total expression and PM localization. It is possible that this mutation resulted in decreased stability of the protein. We found that C266 of rat Ntcp is required for optimal TC uptake (Fig. 1). MTSET-mediated inhibition of TC uptake also occurs through modification of C266 (Fig. 3). Surprisingly, however, C266 of rat Ntcp was not targeted during NO-mediated inhibition of TC uptake (Fig. 4). Instead, we found that C96 of rat Ntcp is responsible for NO-mediated inhibition (Figs. 5 and 6). This finding was confirmed by virtue of the fact that C96A Ntcp was not S-nitrosylated in response to NO treatment (Fig. 8).
Fig. 8.
WT Ntcp but not C96A Ntcp is S-nitrosylated by NO. Cells were transfected with WT or cysteine mutant Ntcp. Later (48 h), cells were treated with (−) or without (+) 0.25 mM CysNO, and S-nitrosylation status was determined using the biotin switch assay. Blots showing S-nitrosylated Ntcp (SNO-Ntcp) and a control blot showing levels of input lysate (Input) are shown. Data are expressed as means ± SE (n = 3). *Significantly different from WT Ntcp control values (P < 0.05).
The present study suggests that C266 is necessary for optimal function of Ntcp. However, previous studies that have investigated the importance for C266 in Ntcp/NTCP function are contradictory. Based on kinetic analysis, one study suggested that C266 of human NTCP is not essential for function (17). However, expression of rat C266A Ntcp in X. laevis oocytes demonstrated that C266 was required for optimal uptake (42). Another group expressed cysteine mutations of mouse Ntcp in COS-7 cells. They likewise found that mouse C266A Ntcp had a diminished TC uptake capacity (32). Our results are in agreement with these latter two studies. We found that C266 of rat Ntcp is required for optimal TC uptake (Figs. 1 and 2). It is possible that there is a species difference between human and rat/mouse isoforms of NTCP/Ntcp and that C266 is not essential for human NTCP but is essential for rat and mouse Ntcp function. Alternatively, the various expression systems employed might have led to differences in C266A Ntcp/NTCP function.
We previously found that NO-mediated inhibition of TC uptake is reversible with DTT (33). This indicates that NO most likely mediates this inhibitory effect through modification of a cysteine residue(s). Indeed, earlier work in isolated rat hepatocytes had shown that several thiol-binding reagents [p-chloromercuribenzenesulfonate (PCMBS), bromosuccinimide, N-ethylmaleimide (NEM), and 2,2′-dithio-bis(5-nitropyridine) (DTNP)] reversibly blocked TC uptake (3, 4). A study using the membrane-impermeant sulfhydryl reacting reagents MTSET and 2-sulfonatoethylmethanethiosulfonate (MTSES) also blocked uptake. Moreover, they demonstrated that C266 of human NTCP is the cysteine residue modified by these reagents (17). We confirmed this finding for rat Ntcp by demonstrating that C266A Ntcp is also resistant to MTSET-mediated inhibition of TC uptake (Fig. 3).
Because both MTSET and NO can bind to thiol groups and because C266 is targeted by MTSET in both human NTCP and rat Ntcp, we hypothesized that NO may also inhibit NTCP/Ntcp function via modification of C266. Our study showed that, while C266 is targeted by MTSET, it is not by NO. On the other hand, C96 is targeted by NO but not by MTSET (Fig. 6). The reason for this difference may be due to the predicted location of C266 and C96 in Ntcp. Membrane-impermeant methanethiosulfonates like MTSET have been used to identify extracellular cysteine residues (17, 30). C266 lies in an extracellular loop as predicted by several topology models of NTCP/Ntcp (17, 28, 42). Thus, C266 is accessible to MTSET binding due to the fact that it is exposed to agents acting extracellularly. These same topology models predict that C96 lies in one of several transmembrane domains of NTCP/Ntcp (17, 28, 42). Therefore, it follows that C96 is inaccessible to MTSET. Efforts to identify a consensus sequence motif for S-nitrosylation have been unsuccessful (18, 25). However, hydrophobicity analyses have indicated that the majority of S-nitrosylation sites lie within hydrophobic pockets (15, 25). Hence, it would appear that C96 resides in a region of NTCP/Ntcp that is susceptible to S-nitrosylation, whereas C266 may lie in an area that does not favor S-nitrosylation.
The mechanism by which S-nitrosylation of C96 leads to TC uptake inhibition is not known but can be speculated based on a recent study that reported the crystal structure of a Ntcp homolog, the bacterial ASBTNM (20). Using this crystal structure, another group has produced a three-dimensional homology model for human NTCP (8). This model predicts that C96 lies in a possible permeation pathway for Na+. We and others have demonstrated that, when C96, which is conserved among human, mouse, and rat, is mutated to alanine, the mutant is still functional (Fig. 1) (42). However, when C96 is mutated to tryptophan, the mutant becomes functionally inactive (42). It is suggested that insertion of the bulky tryptophan may block the permeation pathway for Na+. It is possible that S-nitrosylation of C96 may result in steric hindrance that blocks the permeation pathway for Na+ and thereby inhibits Ntcp function. Molecular modeling will be needed to evaluate the validity of this hypothesis.
In our earlier study of human NTCP, we found that high doses of CysNO (1 mM) caused a decrease in the amount of NTCP present in the PM (33). This finding was in agreement with our kinetic studies which showed that NO-mediated inhibition of TC uptake was noncompetitive (33). We did not observe the same effect on PM levels with rat Ntcp and a lower dose of CysNO although comparable inhibition of TC uptake was observed (Fig. 7). This discrepancy may be due to species differences between rat and human Ntcp/NTCP or the difference in CysNO dose employed. Species differences may be a likely explanation, since taurolithocholate-induced inhibition of TC uptake is associated with a decrease in PM rat Ntcp but not human NTCP (34). Also, inhibition of TC uptake by bosentan, a dual antagonist of endothelin receptors A and B, is noncompetitive for rat Ntcp and competitive for human NTCP (26).
The role of NO in the liver is complex in that NO can be protective as well as toxic. Thus, NO can be both pro-apoptotic and anti-apoptotic in the liver (31) and is implicated in alcoholic liver disease and ischemia-reperfusion injury (31, 36). While lower levels of NO have been linked to increased bile flow, higher levels of NO are associated with cholestasis and hepatocellular injury (5, 9, 12, 29, 38, 40). Increased production of NO has been demonstrated in experimental cholestasis as well as in primary biliary cirrhosis (14), and inhibition of NOS has been shown to ameliorate hepatocellular injury in cholestasis induced by bile duct ligation (27). These studies suggest that overproduction of NO may activate cellular mechanisms that are deleterious to hepatocytes. S-nitrosylation has emerged as an important posttranslational modification that has critical roles in regulating protein function in physiology and pathophysiology (11). S-nitrosylation of NTCP/Ntcp has been identified in NO donor-treated HuH-NTCP cells and also in liver from bile duct-ligated rats (13, 33). It would be attractive to speculate that S-nitrosylation of Ntcp at C96 occurs when hepatocytes are exposed to pathological levels of NO and further that this modification may contribute to the development of cholestasis by inhibiting Ntcp function.
In summary, the present study showed that C266 of Ntcp is required for optimal TC uptake. In addition, MTSET-mediated inhibition of TC uptake also occurs through modification of C266. NO-mediated inhibition of TC uptake, however, is mediated through S-nitrosylation of C96.
GRANTS
This study was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-090010 and DK-33436 (M. S. Anwer) and a New Investigator Grant from the Massachusetts Life Sciences Center (C. M. Schonhoff).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: U.R. and C.M.S. performed experiments; M.S.A. and C.M.S. interpreted results of experiments; M.S.A. and C.M.S. edited and revised manuscript; M.S.A. and C.M.S. approved final version of manuscript; C.M.S. conception and design of research; C.M.S. analyzed data; C.M.S. prepared figures; C.M.S. drafted manuscript.
ACKNOWLEDGMENTS
We thank the Liver Function Laboratory at Tufts Cummings School of Veterinary Medicine for helpful discussions.
REFERENCES
- 1.Anwer MS. Cellular regulation of hepatic bile acid transport in health and cholestasis. Hepatology 39: 581–590, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science 320: 1050–1054, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumrich M, Petzinger E. Membrane transport of conjugated and unconjugated bile acids into hepatocytes is susceptible to SH-blocking reagents. Biochim Biophys Acta 1029: 1–12, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Blumrich M, Petzinger E. Two distinct types of SH-groups are necessary for bumetanide and bile acid uptake into isolated rat hepatocytes. Biochim Biophys Acta 1149: 278–284, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Burgstahler AD, Nathanson MH. NO modulates the apicolateral cytoskeleton of isolated hepatocytes by a PKC-dependent, cGMP-independent mechanism. Am J Physiol Gastrointest Liver Physiol 269: G789–G799, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Chand N, Sanyal AJ. Sepsis-induced cholestasis. Hepatology 45: 230–241, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science 304: 1328–1331, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Doring B, Lutteke T, Geyer J, Petzinger E. The SLC10 carrier family: transport functions and molecular structure. Curr Topics Membr 70: 105–168, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Dufour JF, Turner TJ, Arias IM. Nitric oxide blocks bile canalicular contraction by inhibiting inositol trisphosphate-dependent calcium mobilization. Gastroenterology 108: 841–849, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Forrester MT, Foster MW, Benhar M, Stamler JS. Detection of protein S-nitrosylation with the biotin-switch technique. Free Radic Biol Med 46: 119–126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol Med 9: 160–168, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Geier A, Fickert P, Trauner M. Mechanisms of disease: mechanisms and clinical implications of cholestasis in sepsis. Nat Clin Pract Gastroenterol Hepatol 3: 574–585, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez R, Cruz A, Ferrin G, Lopez-Cillero P, Fernandez-Rodriguez R, Briceno J, Gomez MA, Rufian S, Mata Mde L, Martinez-Ruiz A, Marin JJ, Muntane J. Nitric oxide mimics transcriptional and post-translational regulation during alpha-tocopherol cytoprotection against glycochenodeoxycholate-induced cell death in hepatocytes. J Hepatol 55: 133–144, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Grattagliano I, Portincasa P, Palmieri VO, Palasciano G. Mutual changes of thioredoxin and nitrosothiols during biliary cirrhosis: results from humans and cholestatic rats. Hepatology 45: 331–339, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Greco TM, Hodara R, Parastatidis I, Heijnen HF, Dennehy MK, Liebler DC, Ischiropoulos H. Identification of S-nitrosylation motifs by site-specific mapping of the S-nitrosocysteine proteome in human vascular smooth muscle cells. Proc Natl Acad Sci USA 103: 7420–7425, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagenbuch B, Dawson P. The sodium bile salt cotransport family SLC10. Pflugers Arch 447: 566–570, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Hallen S, Fryklund J, Sachs G. Inhibition of the human sodium/bile acid cotransporters by side-specific methanethiosulfonate sulfhydryl reagents: substrate-controlled accessibility of site of inactivation. Biochemistry (Mosc) 39: 6743–6750, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Hao G, Derakhshan B, Shi L, Campagne F, Gross SS. SNOSID, a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proc Natl Acad Sci USA 103: 1012–1017, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hata S, Wang P, Eftychiou N, Ananthanarayanan M, Batta A, Salen G, Pang KS, Wolkoff AW. Substrate specificities of rat oatp1 and ntcp: implications for hepatic organic anion uptake. Am J Physiol Gastrointest Liver Physiol 285: G829–G839, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Hu NJ, Iwata S, Cameron AD, Drew D. Crystal structure of a bacterial homologue of the bile acid sodium symporter ASBT. Nature 478: 408–411, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE. NOS2 regulation of NF-kappaB by S-nitrosylation of p65. J Biol Chem 282: 30667–30672, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 310: 1966–1970, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kuhlkamp T, Keitel V, Helmer A, Haussinger D, Kubitz R. Degradation of the sodium taurocholate cotransporting polypeptide (NTCP) by the ubiquitin-proteasome system. Biol Chem 386: 1065–1074, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology 126: 322–342, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Lam YW, Yuan Y, Isaac J, Babu CV, Meller J, Ho SM. Comprehensive identification and modified-site mapping of S-nitrosylated targets in prostate epithelial cells. PloS One 5: e9075, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leslie EM, Watkins PB, Kim RB, Brouwer KL. Differential inhibition of rat and human Na+-dependent taurocholate cotransporting polypeptide (NTCP/SLC10A1)by bosentan: a mechanism for species differences in hepatotoxicity. J Pharmacol Exp Ther 321: 1170–1178, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Sanchez LM, Corrales FJ, Barcos M, Espejo I, Munoz-Castaneda JR, Rodriguez-Ariza A. Inhibition of nitric oxide synthesis during induced cholestasis ameliorates hepatocellular injury by facilitating S-nitrosothiol homeostasis. Lab Invest 90: 116–127, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Mareninova O, Shin JM, Vagin O, Turdikulova S, Hallen S, Sachs G. Topography of the membrane domain of the liver Na+-dependent bile acid transporter. Biochemistry (Mosc) 44: 13702–13712, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Myers NC, Grune S, Jameson HL, Sawkat-Anwer M. cGMP stimulates bile acid-independent bile formation and biliary bicarbonate excretion. Am J Physiol Gastrointest Liver Physiol 270: G418–G424, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Omoto JJ, Maestas MJ, Rahnama-Vaghef A, Choi YE, Salto G, Jr, Sanchez RV, Anderson CM, Eskandari S. Functional consequences of sulfhydryl modification of the gamma-aminobutyric acid transporter 1 at a single solvent-exposed cysteine residue. J Membr Biol 245: 841–857, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rockey DC, Shah V. Nitric oxide biology and the liver: report of an AASLD research workshop. Hepatology 39: 250–257, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Saeki T, Kuroda T, Matsumoto M, Kanamoto R, Iwami K. Effects of Cys mutation on taurocholic acid transport by mouse ileal and hepatic sodium-dependent bile acid transporters. Biosci Biotechnol Biochem 66: 467–470, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Schonhoff CM, Ramasamy U, Anwer MS. Nitric oxide-mediated inhibition of taurocholate uptake involves S-nitrosylation of NTCP. Am J Physiol Gastrointest Liver Physiol 300: G364–G370, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schonhoff CM, Yamazaki A, Hohenester S, Webster CR, Bouscarel B, Anwer MS. PKCε-dependent and -independent effects of taurolithocholate on PI3K/PKB pathway and taurocholate uptake in HuH-NTCP cell line. Am J Physiol Gastrointest Liver Physiol 297: G1259–G1267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shneider BL, Fox VL, Schwarz KB, Watson CL, Ananthanarayanan M, Thevananther S, Christie DM, Hardikar W, Setchell KD, Mieli-Vergani G, Suchy FJ, Mowat AP. Hepatic basolateral sodium-dependent-bile acid transporter expression in two unusual cases of hypercholanemia and in extrahepatic biliary atresia. Hepatology 25: 1176–1183, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Siriussawakul A, Zaky A, Lang JD. Role of nitric oxide in hepatic ischemia-reperfusion injury. World J Gastroenterol 16: 6079–6086, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song IS, Lee IK, Chung SJ, Kim SG, Lee MG, Shim CK. Effect of nitric oxide on the sinusoidal uptake of organic cations and anions by isolated hepatocytes. Arch Pharm Res 25: 984–988, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Spirli C, Fabris L, Duner E, Fiorotto R, Ballardini G, Roskams T, Larusso NF, Sonzogni A, Okolicsanyi L, Strazzabosco M. Cytokine-stimulated nitric oxide production inhibits adenylyl cyclase and cAMP-dependent secretion in cholangiocytes. Gastroenterology 124: 737–753, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Takikawa H. Hepatobiliary transport of bile acids and organic anions. J Hepatobiliary Pancreat Surg 9: 443–447, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Trauner M, Nathanson MH, Mennone A, Rydberg SA, Boyer JL. Nitric oxide donors stimulate bile flow and glutathione disulfide excretion independent of guanosine 3′,5′-cyclic [corrected] monophosphate in the isolated perfused rat liver. Hepatology 25: 263–269, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441: 513–517, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Zahner D, Eckhardt U, Petzinger E. Transport of taurocholate by mutants of negatively charged amino acids, cysteines, and threonines of the rat liver sodium-dependent taurocholate cotransporting polypeptide Ntcp. Eur J Biochem 270: 1117–1127, 2003 [DOI] [PubMed] [Google Scholar]