Abstract
Active transcellular oxalate transport in the mammalian intestine contributes to the homeostasis of this important lithogenic anion. Several members of the Slc26a gene family of anion exchangers have a measurable oxalate affinity and are expressed along the gut, apically and basolaterally. Mouse Slc26a6 (PAT1) targets to the apical membrane of enterocytes in the small intestine, and its deletion results in net oxalate absorption and hyperoxaluria. Apical exchangers of the Slc26a family that mediate oxalate absorption have not been established, yet the Slc26a3 [downregulated in adenoma (DRA)] protein is a candidate mediator of oxalate uptake. We evaluated the role of DRA in intestinal oxalate and Cl− transport by comparing unidirectional and net ion fluxes across short-circuited segments of small (ileum) and large (cecum and distal colon) intestine from wild-type (WT) and DRA knockout (KO) mice. In WT mice, all segments demonstrated net oxalate and Cl− absorption to varying degrees. In KO mice, however, all segments exhibited net anion secretion, which was consistently, and solely, due to a significant reduction in the absorptive unidirectional fluxes. In KO mice, daily urinary oxalate excretion was reduced 66% compared with that in WT mice, while urinary creatinine excretion was unchanged. We conclude that DRA mediates a predominance of the apical uptake of oxalate and Cl− absorbed in the small and large intestine of mice under short-circuit conditions. The large reductions in urinary oxalate excretion underscore the importance of transcellular intestinal oxalate absorption, in general, and, more specifically, the importance of the DRA exchanger in oxalate homeostasis.
Keywords: large intestine, small intestine, kidney stones, anion exchange, hyperoxaluria, chloride-losing diarrhea, nephrolithiasis
the mammalian intestine plays an important role in systemic oxalate homeostasis as a source for dietary oxalate absorption and as an extrarenal avenue for oxalate excretion (17–19). Like most solutes, transepithelial oxalate transport occurs through transcellular and paracellular pathways to produce net oxalate absorption or secretion, often in a segment-specific manner (18, 19). On the basis of stilbene sensitivity of transepithelial oxalate transport and ion-substitution experiments, our earlier studies using rat and rabbit intestine (18, 19) suggest that absorptive and secretory pathways involve ion-exchange mechanisms at the apical and basolateral poles of the enterocyte. These views were consistent with numerous studies utilizing apical and basolateral membrane vesicles of intestinal and renal epithelia (28), further indicating a multiplicity of anion-exchange mechanisms mediating oxalate transport. However, because of coexpression of oxalate exchangers at apical and/or basolateral membranes of enterocytes in intact epithelia, it is difficult to discern their specific functional significances and relative contributions to net oxalate transport.
With the identification of several gene families that mediate anion exchange (e.g., SLC26A and SLC4A) in the mammalian genome, the prospect of resolving the importance of various transporters became feasible in the last 10 years. In terms of oxalate transport, the SLC26A family has received the most attention on the basis of physiological properties revealed in studies employing heterologous expression systems (6, 7, 38, 41). With the generation of transgenic mice lacking gene products expressed by members of the SLC26A family, it has become possible to explore the specific contribution of a given exchanger to transepithelial oxalate transport in vitro. For example, targeted disruption of slc26a6 [alias PAT1 (putative anion transporter 1)] in mice enhanced the ileal absorption of oxalate, and, importantly, this single deletion of an apically targeted ileal transporter was associated with a marked increase in urinary oxalate excretion, leading to our proposal that PAT1 normally mediates apical secretion of oxalate in this intestinal segment (9). Similar findings based on a different PAT1 knockout (KO) mouse strain corroborated the importance of Slc26a6 in oxalate secretion in the duodenum, and this KO strain exhibited nephrolithiasis (26).
While it is clear that PAT1 mediates ileal oxalate secretion and that its deletion results in net oxalate absorption by this segment, the exchanger protein(s) mediating the transcellular absorption of oxalate has not been identified. Another member of the SLC26A family, SLC26A3 [alias DRA (downregulated in adenoma), the gene responsible for Cl−-losing diarrhea], is also expressed apically in the mammalian intestine (24, 39, 41). This ion exchanger mediates Cl−-HCO3− countertransport and has been shown to have an affinity for oxalate in rat and rabbit duodenal brush border vesicles (37) and some exogenous expression systems (3, 6). Consequently, we hypothesized that the DRA protein mediates transcellular oxalate absorption along the mouse intestine. Here we report results of our studies comparing oxalate and Cl− transport by isolated, short-circuited segments of ileum, cecum, and distal colon from wild-type (WT) and DRA KO mice. We found that Slc26a3 is, indeed, a significant contributor to net transcellular oxalate absorption and that its deletion results in a significant reduction of urinary oxalate excretion.
MATERIALS AND METHODS
Animals and tissue preparation.
Mice heterozygous for targeted gene disruption of Slc26a3 (DRA) on a C57BL/6j background were kindly provided by Dr. M. Soleimani (University of Cincinnati, Cincinnati, OH). This founder population was subsequently outbred with C57BL/6 WT mice (Charles River Laboratories), and the colony was expanded in the Animal Care Facility at the University of Florida to generate the WT (Slc26a3+/+) and DRA KO (Slc26a3−/−) mice used in this study. Protocols were approved by the University of Florida Institutional Animal Care and Use Committee. Mice were given free access to food (diet no. 2018S, Harlan Teklad, Indianapolis, IN) and drinking water; KO mice received 50% pediatric electrolyte solution (Pedialyte, Walgreens, Deerfield, IL) to mitigate diarrhea-related volume loss. We have determined (by assay) that Pedialyte does not contain oxalate; thus it is unlikely that Pedialyte, per se, will result in changes in urinary oxalate excretion. Pedialyte is also unlikely to result in the generation of metabolic oxalate, in that it consists simply of glucose and the salts NaCl, KCl, ZnSO4, and Na3citrate, but no oxalate precursors. Animals of either sex were used in the transport experiments; mean body weights were 25.4 ± 0.9 and 20.7 ± 0.9 g for WT and KO mice, respectively. Animals were euthanized by exposure to 100% CO2 followed by exsanguination; then the intestinal segments were removed and placed in chilled, buffered saline. The distal ileum (3–4 cm proximal to the ileocecal valve), the cecum, and the distal colon (terminal 30% of the large intestine from the peritoneal border) were studied. Each segment was flushed with saline, opened along the mesenteric border, and further washed with chilled saline before it was mounted in a modified Ussing chamber with a 0.3-cm2 aperture. Mucosal and serosal surfaces of the tissues were bathed in 4.0 ml of standard buffer (pH 7.4) containing (mM) 139.4 Na+, 5.4 K+, 1.2 Ca2+, 1.2 Mg2+, 123.2 Cl−, 21.0 HCO3−, 0.6 H2PO4−, 2.4 HPO42−, 10 glucose, and a total of 1.5 × 10−3 oxalate (labeled and unlabeled). Endogenous prostanoid production was nominally reduced by inclusion of 5 μM indomethacin in the standard buffer. These solutions were maintained at 37°C and circulated by vigorous gassing with a hydrated mixture of 95% O2-5% CO2.
Transepithelial transport measurements.
Transepithelial potentials generated by the tissues were continuously clamped to 0 mV by means of an automatic voltage clamp (model VCCMC6, Physiologic Instruments, San Diego, CA), except for brief interruption for recording of open-circuit potential (VT, mV). Tissue conductance (GT, mS/cm2) was calculated as the ratio of the measured short-circuit current (Isc, μA/cm2) to VT from Ohm's law. Isc values are presented as μeq·cm−2·h−1, with a negative sign representing net cation absorption and/or net anion secretion.
Unidirectional oxalate and Cl− fluxes [mucosal-to-serosal (MS) = Jms and serosal-to-mucosal (SM) = Jsm] were measured on separate tissues as follows. Radioisotopes ([14C]oxalate or 36Cl; Amersham, Piscataway, NJ) were added to the mucosal or serosal reservoir 20 min after the tissues were mounted, and sampling of the opposing reservoir commenced 20 min thereafter. At 15-min intervals, the electrical properties were recorded and 1-ml samples were taken from the appropriate reservoir and replaced with warmed/gassed standard buffer. Specific activities were determined from the average isotope activity of 50-μl samples taken during the initial and final sampling periods. Samples were dissolved in 5 ml of Ecoscint A (National Diagnostics, Atlanta, GA), and isotope activity was determined with quench correction by liquid scintillation spectrometry (model LS 9000, Beckman, Fullerton, CA). Fluxes were calculated from the change in counts per minute between successive 15-min samples, with correction for the changes in activity due to dilution of the sampling reservoir with replacement buffer. Results from three to four successive samples were averaged to establish a given unidirectional flux. Net ion fluxes (Jnet) were calculated as the difference between Jms and Jsm with use of conductance-matched tissue pairs (GT less than ±15%). The chemical integrity of the [14C]oxalate was routinely tested by comparison of 14C activity in a standard sample before and after hydrolysis with the substrate-specific enzyme oxalate decarboxylase following volatilization of 14CO2 under acidic conditions.
Urine collections.
Mice were housed in pairs in metabolic cages and had free access to food and water; 24-h urine collections were made under mineral oil in vessels containing 10 μl of 2% sodium azide as a preservative. Urinary oxalate was determined in each collected specimen, which was acidified with HCl to ensure complete solubilization of crystallized oxalate following measurement of urine pH.
Fecal oxalate degradation assay.
Fecal samples were collected directly from the mouse intestinal lumen at the time the tissues were being prepared for the flux studies. The presence of anaerobic oxalate-degrading bacteria in the luminal contents was determined by inoculation of anaerobically sealed vials containing 20 mM oxalate with ∼20 mg of fecal material (20). Any change in oxalate concentration by microbial action in these vials incubated at 37°C was determined 1 wk later by our routine enzymatic oxalate assay. None of the mice included in the study were found to be colonized with oxalate-degrading bacteria.
Analytic methods and reagents.
Urinary oxalate in the acidified 24-h specimens was determined using a kit assay (catalog no. 591, Trinity Biotech, St. Louis, MO). Urinary Ca2+ concentration was determined with a Ca2+ assay kit (Point Scientific, Canton, MI), and urinary creatinine was determined using a modification of the Jaffé reaction, as previously described (12). Blood, collected by cardiac puncture, was handled immediately, with the appropriate precautions to prevent oxalogenesis, and serum pools from two to five mice were prepared for oxalate determination using a coupled enzymatic (oxalate decarboxylase and formate dehydrogenase) assay procedure used routinely in our laboratory (14). Reagent-grade salts used in these experiments were purchased from Sigma-Aldrich (St. Louis, MO).
Statistical analyses.
Comparisons between WT and KO mice were made using Student's t-test (2-tailed, unpaired). Values are means ± SE. Differences between means were judged significant if P ≤ 0.050.
RESULTS
We performed the oxalate and Cl− flux studies on separate mice. Because there were no differences in the electrical characteristics between these two populations, we have combined the Isc and GT measures from each series.
Ileal oxalate and Cl− fluxes.
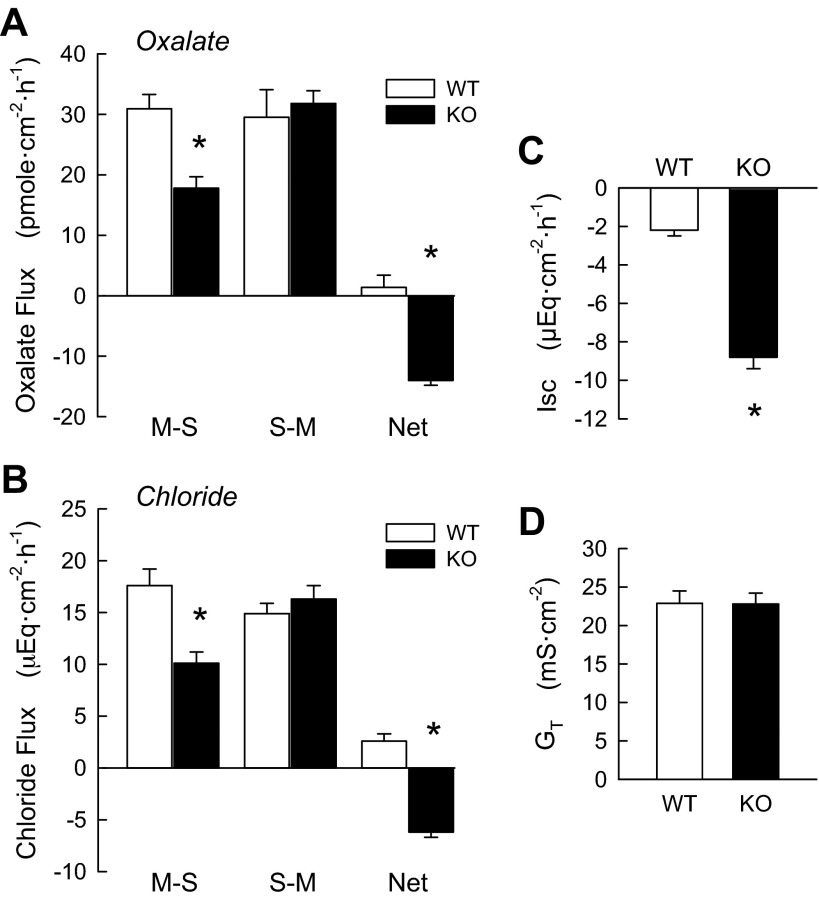
In WT mice, the short-circuited distal ileum exhibited little difference between oxalate Jms (JmsOx) and oxalate Jsm (JsmOx), resulting in no significant net transport of oxalate (JnetOx; Fig. 1A). In striking contrast, DRA KO mice demonstrated a very significant net secretion of oxalate (−14.0 ± 0.5 μeq·cm−2·h−1), which was solely due to a significant 42% decrease in JmsOx compared with WT mice. This large depression of the absorptive flux indicates that a substantial fraction of the transepithelial flux of oxalate is mediated by transcellular avenues that are dependent on expression of the DRA protein in the apical membrane. The WT mouse ileum exhibited a small net absorption of Cl− (2.6 μeq·cm−2·h−1; Fig. 1B), while in the absence of DRA there was a strong net secretion of −6.2 ± 0.5 μeq·cm−2·h−1 (Fig. 1B). This was the result of a significant reduction in Cl− Jms (JmsCl) (43%) and a small (10%) increase in Cl− Jsm (JsmCl).
Fig. 1.
DRA deletion [knockout (KO)] results in net oxalate and Cl− secretion in mouse ileum compared with wild-type (WT) littermates. A and B: unidirectional [mucosal-to-serosal (MS) and serosal-to-mucosal (SM)] and net fluxes of oxalate [n = 18 (WT) and 13 (KO)] and Cl− (n = 7 WT and KO) in separate populations of mice. C and D: combined short-circuit currents (Isc) and conductances (GT) for all pairs used in the flux experiments. *Significantly different from WT (P ≤ 0.05 by unpaired t-test).
While conductances in WT and KO mice were the same (Fig. 1D), there was a large increase (2.7-fold more negative) in Isc in KO mice (Fig. 1C). Given the recording conventions used here, this DRA-dependent change in current represents an increase in net electrogenic anion secretion, net electrogenic cation absorption, or some combination thereof.
Oxalate and Cl− fluxes across the cecum.
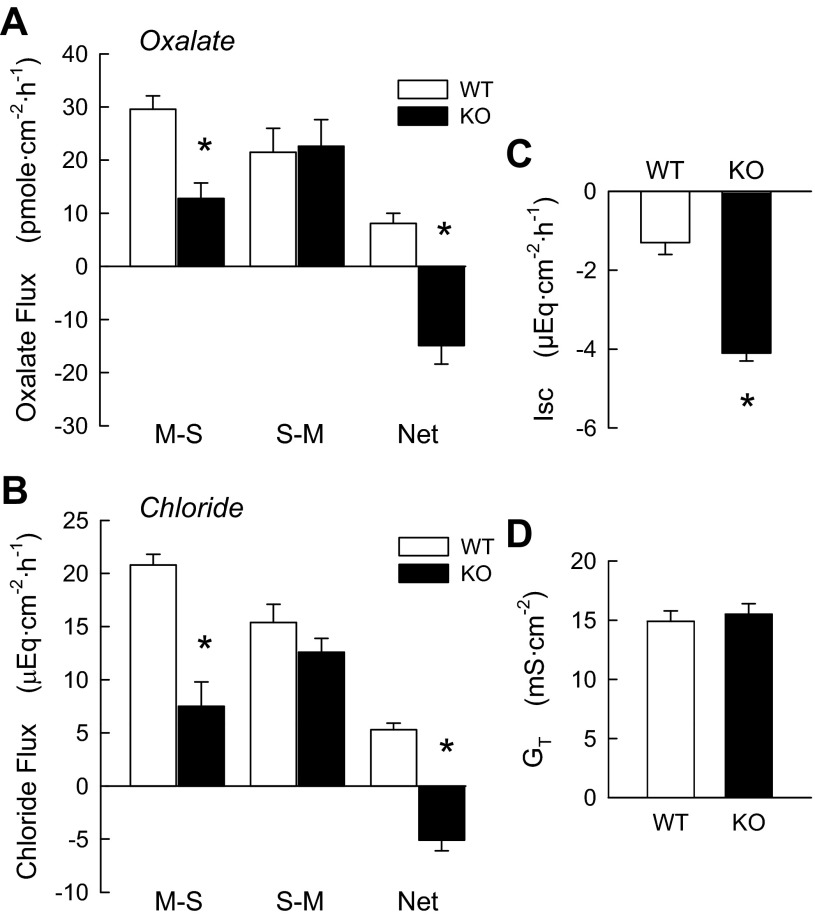
In cecal epithelia, a significant net absorption of oxalate was observed (8.1 ± 1.9 pmol·cm−2·h−1) in WT mice (Fig. 2A), which represented 27% of the unidirectional absorptive flux of oxalate (JmsOx). As shown in Fig. 2A, in the DRA KO mouse cecum, net transport of oxalate was significantly reversed to a net secretion of −14.9 ± 3.5 pmol·cm−2·h−1. This reversal of net transport was a sole consequence of a significant 57% reduction in JmsOx, indicating that DRA mediates an important transcellular component of oxalate absorption in native mouse cecum. WT cecum exhibited a significant net absorption of Cl− (4.3 ± 0.6 μeq·cm−2·h−1), which was changed to a significant net secretion in DRA KO mice (Fig. 2A). This was principally the result of a large (67%) and significant decrease in JmsCl accompanied by a smaller, insignificant reduction (23%) in JsmCl. Again, DRA appears to be the principal mediator of Cl− absorption in this intestinal segment. There were no significant differences in GT between WT and DRA KO cecal epithelia, but the magnitude of Isc in DRA KO mice was almost twice that of the WT tissues.
Fig. 2.
DRA deletion (KO) inhibits net oxalate and Cl− absorption, resulting in net secretion of these anions in mouse cecum compared with WT littermates. A and B: unidirectional and net fluxes of oxalate [n = 19 (WT) and 10 (KO)] and Cl− [n = 6 (WT) and 8 (KO)] in separate populations of mice. C and D: combined Isc and GT values for all pairs used in the flux experiments. *Significantly different from WT (P ≤ 0.05 by unpaired t-test).
Oxalate and Cl− fluxes across the distal colon.
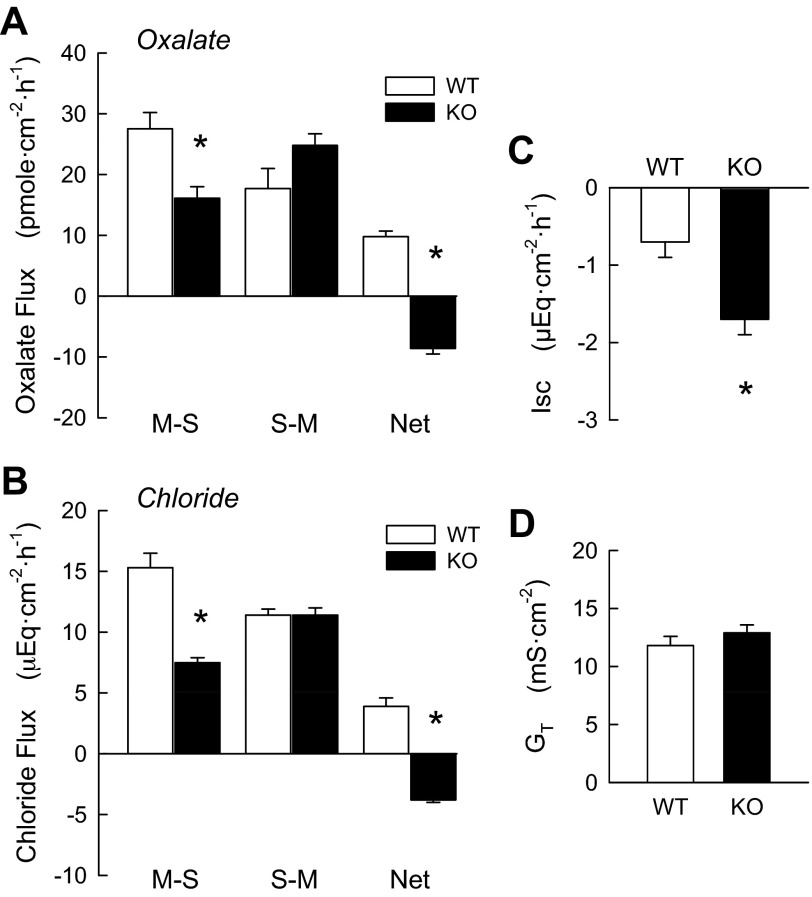
The WT mouse distal colon exhibited a net absorption of oxalate (9.8 ± 0.9 pmol·cm−2·h−1; Fig. 3A) comparable in magnitude to that in the WT cecum (Fig. 2A). The net flux of oxalate (JnetOx) represented 35% of JmsOx in the colon, indicative of a substantial transcellular component to oxalate absorption. This absorptive, transcellular component of JmsOx was significantly reduced by 41% in DRA KO mouse colon, while JsmOx exhibited a slight increase that did not reach significance (P = 0.10). These changes in unidirectional oxalate fluxes in DRA KO colon resulted in a significant net secretion of oxalate (−8.6 ± 0.9 pmol·cm−2·h−1). As shown in Fig. 3B, net Cl− transport (JnetCl) in the WT distal colon was absorptive (3.9 ± 0.7 μeq·cm−2·h−1), whereas JnetCl was reversed to net secretion in KO mice (−3.8 ± 0.2 μeq·cm−2·h−1). The reversal in net transport was the result of a 50% reduction in JnetCl, as the opposing unidirectional flux was unaltered in KO mouse colon (Fig. 3B). DRA KO colon also exhibited a twofold change in Isc (Fig. 3C) with no associated changes in GT.
Fig. 3.
DRA deletion (KO) inhibits net oxalate and Cl− absorption in mouse distal colon, resulting in net secretion of these anions compared with tissues from WT littermates. A and B: unidirectional and net fluxes of oxalate [n = 16 (WT) and 12 (KO)] and Cl− (n = 7 WT and KO) in separate populations of mice. C and D: combined Isc and GT values for all pairs used in the flux experiments. *Significantly different from WT (P ≤ 0.05 by unpaired t-test).
DRA-dependent changes in urinary oxalate excretion.
The major finding revealed by the preceding results is that the absorptive flux of oxalate is abolished in DRA KO mouse ileum, cecum, and distal colon. Such a striking reduction in net intestinal absorption and the emergence of net intestinal secretion of oxalate in the DRA KO mouse should impact oxalate homeostasis, as reflected in the daily urinary excretion of the oxalate anion. To evaluate the contribution of DRA to oxalate homeostasis, we compared the excretory patterns of oxalate by WT mice with those of DRA KO mice. As shown in Table 1, urine volume was slightly (15%), but not statistically, smaller in KO than WT mice, which likely reflects the intestinal water losses associated with this phenotype (36, 40). Urine pH was also significantly reduced in the KO mice, whereas urinary excretion patterns of creatinine and Ca2+ were similar between these genotypes. Urinary oxalate excretion in KO mice was 30% of that measured in WT mice, highlighting the contribution of transcellular intestinal absorption to oxalate homeostasis. Since there is little evidence for a significant presence of the DRA protein in renal tissues (43), the reduction of oxalate excretion in DRA KO mice is most likely associated with the decrease in intestinal absorption. Finally, DRA KO mice exhibited a 60% reduction of serum oxalate, which was significantly lower than in the controls.
Table 1.
Selected urinary parameters and serum oxalate in DRA KO mice and their WT littermates
| WT | KO | |
|---|---|---|
| Urine volume, ml/24 h | 0.80 ± 0.12 | 0.68 ± 0.10 |
| Urine pH | 6.11 ± 0.01 | 5.29 ± 0.09* |
| Urinary oxalate excretion, μmol/24 h | 1.64 ± 0.16 | 0.50 ± 0.06* |
| Urinary Ca2+ excretion, μmol/24 h | 1.42 ± 0.19 | 1.71 ± 0.26 |
| Urinary creatinine excretion, μmol/24 h | 5.75 ± 0.44 | 6.06 ± 0.51 |
| Serum oxalate, μmol/l | 11.61 ± 2.30 | 4.65 ± 1.76* |
Values are means ± SE of duplicate measurements of 12–20 urine pools in each group with 2 mice per pool. Serum oxalate was measured in 11 wild-type (WT) and 8 DRA (downregulated in adenoma) knockout (KO) pools with 2–5 mice per pool.
Significantly different from WT (P ≤ 0.05).
DISCUSSION
The major objective of the present study was to establish the role of Slc26a3 (DRA) in the intestinal transport of the divalent anion oxalate. Oxalic acid is the simplest dicarboxylic acid and exists as the fully ionized divalent anion at physiological pH values. In mammals, the oxalate anion is a metabolic end-product that is chiefly excreted in the urine without consequence. However, elevated urinary oxalate concentrations (hyperoxaluria) thermodynamically favor the formation of insoluble oxalate salts with divalent metal cations such as Ca2+, possibly leading to the formation of insoluble calcium oxalate kidney stones in the pathological state. Systemic oxalate homeostasis represents the balance between oxalate input (via hepatic oxalogenesis and intestinal absorption of dietary oxalate) and oxalate output (via renal excretory pathways and intestinal secretory avenues). Because intestinal absorption and secretion impact oxalate homeostasis, an understanding of the mechanisms underlying these net transport processes is an important component in the etiology and management of nephrolithiasis.
As a member of the Slc26a gene family, DRA belongs to a group of transport proteins that mediate transmembrane exchange of a variety of inorganic and small organic anions, typically in a countertransport mechanism (2, 35). While these exchangers are usually, and reasonably, viewed as principally involved in acid-base regulation (i.e., in Cl−/HCO3− exchange mode), they assume additional functional significance by virtue of their relatively broad substrate/solute selectivity. For example, various members of this gene family mediate, to varying degrees, the transport of Cl−, HCO3−, SO42−, NO3−, formate (HCO2−), and oxalate (C2O42−). In general, Slc26a3 (DRA), Slc26a6 (PAT1), Slc26a2 [diastrophic dysplasia sulfate transporter (DTDST)], and Slc26a1 [sulfate anion transporter 1 (SAT1)] are expressed along the mouse intestine to varying degrees, with all but SAT1 targeted to the apical membrane.
DRA mediates oxalate and Cl− absorption across mouse ileum.
WT mouse ileum under short-circuit conditions exhibited a small net absorption of oxalate that was not significantly different from zero, whereas the DRA KO mice supported a significant net secretion of oxalate. These changes in net transport in the absence of DRA were the result of a 42% reduction in JmsOx, which indicates that the net secretion of oxalate in DRA KO mice was due to an inhibition of an ongoing absorptive process in WT mice, rather than an enhancement of a secretory process in KO mice. At least 40% of the unidirectional absorptive flux is mediated by, or dependent on, a transcellular pathway through the DRA exchanger located at the apical membrane. These findings further suggest that the mouse ileum supports active transcellular oxalate absorption and oxalate secretion simultaneously, since JsmOx was unaffected by DRA removal. On the basis of our previous study of oxalate transport in mouse ileum (9), the secretory oxalate flux remaining in DRA KO ileum is likely mediated by PAT1 (Slc26a6).
WT ileum exhibited a net absorptive flux of Cl− of 2.6 ± 0.7 μeq·cm−2·h−1, but in the absence of DRA we observed a strong net Cl− secretion (−6.3 ± 0.5 μeq·cm−2·h−1). The Cl− fluxes and electrical characteristics in our WT strain are comparable to those reported for Balb/C mouse ileum by Charney et al. (5) in standard HCO3− buffer. As with oxalate, the reversal of the direction of net Cl− transport in DRA KO ileum was primarily a consequence of a 43% reduction of absorptive unidirectional flux. Given that the location of the Slc26a3 protein is targeted to the luminal membrane, these results indicate that DRA mediates Cl− uptake at the apical membrane of ileal enterocytes engaged in Cl− absorption. While this is the first report of ileal Cl− transport in DRA KO mice, 36Cl− flux measurements in mouse jejunum of WT and DRA KO mice (40) also indicate that DRA mediates apical Cl− uptake and, hence, net Cl− absorption by exchange of luminal Cl− with intracellular HCO3− (Cl−out/HCO3−in exchange). In the jejunum, however, no significant net Cl− secretion was observed in the KO mice, unlike our finding of secretion in the ileum.
Initial expression studies of DRA along the mouse intestine suggested a relatively minor role for this anion exchanger in the small intestine compared with segments of the large intestine (37, 42). In other species, however, DRA expression in the ileum is quite significant. For example, Jacob et al. (24) reported DRA mRNA expression as follows: ileum > colon = duodenum in rabbit, and colon > ileum > jejunum in rat. Moreover, DRA mRNA expression has recently been reported to be 10-fold that of PAT1 in rabbit ileal villous cells, and immunofluorescence studies localized the DRA protein to the apical border of these enterocytes (32). In our laboratory, we have found comparable expression levels of DRA mRNA in WT mouse distal ileum and distal colon (unpublished observations). The current finding that DRA mediates a substantial fraction of Cl− transport in the distal ileum shows that, functionally, the DRA exchanger is an important component of anion exchange in the mouse distal, as well as proximal, small intestine (40, 41).
DRA mediates oxalate and Cl− absorption across mouse cecum.
Cecal epithelia from WT mice exhibited a prominent net absorption of oxalate (8 pmol·cm−2·h−1) under short-circuit conditions, as we reported earlier (20). The unidirectional oxalate fluxes in this segment were similar in magnitude to those measured in the ileum, even though the conductance of cecal tissues was 30% lower. This indicates a higher transcellular throughput of oxalate in cecal epithelia than ileal enterocytes (see above) and correlates with a higher transcellular throughput of Cl− in this tissue (see below). The net absorptive flux of oxalate in the WT cecum represents 27% of the unidirectional MS flux of this ion under short-circuit conditions. In DRA KO mouse cecum, there was a 60% reduction in JmsOx with no significant reduction in JsmOx, resulting in a large net secretion of oxalate in the absence of a change in GT. This distinctive reversal of net oxalate transport solely via JmsOx clearly indicates that transcellular oxalate absorption is dependent on expression of the DRA protein. The reversal of net transport direction further suggests that active transcellular secretion and absorption of oxalate occur simultaneously in WT mouse cecum, as in the ileum.
The native mouse cecum has been previously shown to support a very large net absorption of Cl− (11–20 μeq·cm−2·h−1) under short-circuit conditions (1, 21, 44) that is approximately fourfold greater than that observed here. In one of these earlier studies (1), JnetCl was completely abolished when examined in a DRA KO mouse. In our hands, DRA removal reduced JmsCl by 64% with no significant change in JsmCl or GT, which resulted in a significant net secretion of Cl− of −5 μeq·cm−2·h−1. As there is abundant DRA expressed in the cecum but relatively little Na+/H+ exchanger 3 (NHE3) (39), the DRA protein contributes significantly to alkalinization of the luminal contents of this fermentative organ while absorbing considerable amounts of Cl− and, as shown here, oxalate.
DRA mediates oxalate and Cl− absorption across mouse distal colon.
In the present study, the distal colon of WT mice exhibited a net absorption of oxalate, whereas we previously observed a small net oxalate secretion in another strain/colony with the C57BL/6 background (20). The basis of this difference is not clear but resides in the higher secretory component observed in the latter study, as the magnitudes of JmsOx were similar in both strains. The DRA KO mouse colon exhibited net oxalate secretion chiefly through a significant decrease (42%) in JmsOx and an increase (40%) in JsmOx that did not reach statistical significance.
The WT mice employed here exhibited a significant net absorption of Cl− that was similar in magnitude (∼3 μeq·cm−2·h−1) to that reported in earlier studies of short-circuited distal colon from different mouse strains (5, 11). In contrast to the net Cl− absorption in WT colon, the DRA KO mouse colon exhibited a significant net secretion of Cl− solely as a consequence of a reduction in JmsCl. As with the cecum, DRA is abundant in the mouse distal colon, but there is little NHE3 expression (39), so the overall transport activity of this segment results in luminal alkalinization and Cl− absorption.
Contribution of DRA to oxalate homeostasis in mouse.
The principal effect of DRA removal in all the segments examined here was a reduction of JmsOx with no significant impact on JsmOx. The overall result of depressed JmsOx is the appearance of a net secretory flux of oxalate in all segments. In a previous study comparing oxalate transport across WT and PAT1 KO mouse ileum, we concluded that the PAT1 gene product mediates oxalate secretion, since PAT1 KO mice exhibited enhanced ileal oxalate absorption and elevated urinary oxalate excretion (9).
Table 1 presents the first information on urinary parameters in DRA KO mice and shows that urinary oxalate excretion was significantly reduced (70%), whereas creatinine and Ca2+ excretion were unaffected, by DRA deletion. DRA KO mice also exhibited a significantly lower serum oxalate concentration, consistent with reduced intestinal oxalate absorption. While daily urine volume was lower in KO mice, as might be expected due to the persistent Cl− diarrhea exhibited by these animals, this was not statistically significant, implying no major fluid imbalance. Previously, DRA KO mice displayed characteristics of severe volume depletion, including activation of the renin-angiotensin-aldosterone system and a high blood urea nitrogen, indicative of reduced glomerular filtration (36). The difference here may be accounted for by the oral rehydration supplementation given to our mice as part of their husbandry. This would be consistent with matching hematocrit readings for DRA KO and WT mice following the addition of Pedialyte to their drinking water (40). Even though the volume deficit may be offset, the chronic Cl−-rich diarrhea of KO mice due to the absence of DRA produces an elevated blood HCO3− concentration and Pco2, suggestive of a metabolic alkalosis with respiratory compensation (40). This presents something of a paradox in relation to the urine pH determined for KO mice, which was significantly more acidic by 0.82 pH unit than the urine of their WT counterparts (Table 1). This might be explained in part by the increased Pco2 (40), which can directly stimulate renal H+ secretion and HCO3− reabsorption (31, 45). Moreover, characteristic of metabolic alkalosis caused by persistent gastrointestinal Cl− losses, the normal response of the kidney to such an acid-base disturbance (increased urinary HCO3− excretion) becomes impaired by the secondary stimulation of net acid secretion along the nephron (4, 10). This unique situation will not only enhance HCO3− reabsorption, sustaining the alkalotic state, but may have contributed to the acidic urine produced by DRA KO mice seen here.
Absorptive and secretory processes for oxalate along the nephron could participate in determining urinary oxalate excretion, but the contribution of DRA to this is likely to be minimal, as it is frequently not detected in renal tissues. DRA expression has only been reported for the human kidney (43), whereas studies with mouse (37; unpublished observations), rat (3), and rabbit (24) renal cortex failed to detect the Slc26a3 gene product. The impact of intestinal DRA on oxalate homeostasis in humans does not appear to be as dramatic as seen here in mice, since Cl−-losing diarrhea patients have been reported to have a median daily excretion ratio of oxalate to creatinine of 0.012 (43), which is similar to that reported (30) for a population of healthy adults (0.016).
Perspectives on transcellular oxalate absorption.
The most prominent overall feature observed in DRA KO mice in the present study was the significant reduction of JmsOx and JmsCl in all segments in the absence of significant changes in the secretory unidirectional fluxes for oxalate or Cl−. Walker et al. (40) reported similar findings concerning Cl− transport in the proximal small intestine from WT and DRA KO mice. In the study of Walker et al., a strong net absorption of Cl− in WT jejunum (12 μeq·cm−2·h−1) was abolished solely by a decrease (39%) in JmsCl compared with WT without a change in JsmCl or GT. These findings afford compelling evidence that most, if not all, transcellular Cl− (JmsCl) along the mouse intestine is DRA-mediated and, from the present study, that transcellular oxalate absorption is similarly mediated by DRA. While we have long argued that oxalate and Cl− share similar transport characteristics in rat and rabbit intestinal segments (13, 18, 19), for the first time, we can specifically identify a single transporter that mediates the transcellular absorptive flux of Cl− and oxalate simultaneously.
DRA-mediated apical uptake of Cl− is primarily in exchange with, and driven by, efflux of intracellular HCO3− to the luminal compartment (Cl−out/HCO3−in exchange) (2, 3, 6, 24, 29, 33, 40). We propose a similar exchange process for the oxalate anion, namely oxalateout/HCO3−in exchange as the first step in transcellular, DRA-mediated oxalate absorption. In our experiments, the concentration ratio of oxalate to Cl− is 1:82,000, so the oxalate affinity of mouse DRA must be significant, given the dramatic difference in the relative abundance of the translocated species. There are few previous studies of oxalate affinity for DRA, and the conclusions have been varied (3, 6, 37), which may reflect technical or species variability. Some insight into the relative affinity of oxalate and Cl− for DRA may be gleaned from our current results by comparing the relative apparent permeabilities derived from DRA-dependent oxalate and Cl− fluxes, as described below.
Normalization of the observed fluxes for Cl− and oxalate to their respective molar concentrations provides a practical, albeit rough, estimate of the relative affinities of these two substrates under the steady-state conditions of our experiments. Normalization of the flux of a solute (mol·cm−2·h−1) by dividing by the solute concentration (mol/l) yields an apparent permeability coefficient for that solute (cm/s). Of the three segments we examined in this study, the cecum exhibited the largest steady-state, DRA-dependent (JmsOx in WT mice − JmsOx in KO mice) absorptive unidirectional fluxes for oxalate and Cl− (16.8 pmol·cm−2·h−1 and 13.3 μmol·cm−2·h−1, respectively; Fig. 2, A and B). Using these DRA-dependent unidirectional MS fluxes in the cecum, the calculated apparent transcellular permeability coefficients are 0.31 × 10−5 and 3.0 × 10−5 cm/s for oxalate and Cl−, respectively. Certainly, DRA-mediated oxalate permeability is low relative to Cl− permeability in the mouse cecum, as reported elsewhere (6). Nonetheless, even this low relative affinity is entirely sufficient to contribute 57% of the total transepithelial (transcellular + paracellular) JmsOx; i.e., [(JmsOx in WT − JmsOx in DRA KO)]/JmsOx in WT = 57%. Calculated relative oxalate and Cl− permeability coefficients for the DRA-mediated absorptive unidirectional fluxes in the other segments were similar: 0.24 × 10−5 and 1.7 × 10−5 cm/s, respectively, in the ileum and 0.21 × 10−5 and 1.8 × 10−5 cm/s, respectively, in the distal colon. From the latter values, we estimate that the contribution of DRA-mediated oxalate flux to JmsOx was 42% in the ileum and 41% in the distal colon. So, while the affinities of oxalate for DRA are low relative to those for Cl−, they are sufficient to account for a major component of mediated transcellular oxalate transport in the intestine under short-circuit conditions.
Role of paracellular oxalate transport.
Our conclusion regarding the role of DRA in transcellular oxalate transport in the mouse intestine is strikingly different from the recent proposal of Knauf et al. (27) that transepithelial oxalate absorption (JmsOx) is predominantly passive and paracellular in all major intestinal segments. The key observation in their report was that, in isolated, short-circuited segments of WT mouse duodenum, oxalate and mannitol permeabilities (POx and PMan, respectively) were numerically similar when measured in the MS direction, but POx > PMan when measured in the SM direction. This was interpreted to mean that the SM permeability coefficient differences in WT duodenum reflected transcellular oxalate secretion, while the similarity of MS permeability coefficients demonstrated an absence of significant transcellular oxalate transport. In PAT1 KO mice, which exhibit large reductions of JsmOx in the ileum (9) and duodenum (26), POx = PMan in the SM direction, which they argued supported their proposal.
The interpretation presented by Knauf et al. (27) is confounding for the following theoretical and technical reasons. 1) An apparent numerical equivalency of POx and PMan in mouse intestine does not imply that oxalate and Cl− share a common transport pathway. 2) Important electrophysiological metrics are not presented for any experimental series or intestinal segment, so it is not possible to evaluate the integrity of their tissue preparations. 3) The concentration(s) employed or the duration of exposure to DIDS is not given, making it impossible to evaluate the sensitivity of their preparations to this inhibitor, which is fairly important, given the reported differential sensitivities of Slc26a exchangers to DIDS (2, 3, 6, 25, 33, 34, 44). 4) Only oxalate transport in the MS direction was examined in the other segments (jejunum, ileum, and proximal and distal colon). Thus it must be assumed that their conclusions on duodenal handling can be extrapolated to the entire intestine. Furthermore, this ignores previous studies clearly demonstrating segmental heterogeneity of intestinal oxalate transport in mouse (20), rat (8, 15), and rabbit (13, 16). 5) Their observation of no net oxalate absorption in the duodenum of PAT1 KO mice is in striking contrast to the initial report (26) from the same laboratory characterizing their PAT1 KO mouse model. In their initial report (Table 2), WT duodenum exhibited a net secretion of oxalate (−73 pmol·cm−2·h−1), but a net oxalate absorption (+28 pmol·cm−2·h−1) was revealed in the PAT1 KO mouse duodenum. As indicated below, this net absorptive flux reported in their PAT1 KO model was obtained using a sampling protocol that commenced 2 h earlier than that employed in the later report of Knauf et al. (27). In WT mouse ileum, we previously observed (9) a small net secretion of oxalate under short-circuit conditions (−10 pmol·cm−2·h−1), but in PAT1 KO mouse ileum there was a very large net absorption (+75 pmol·cm−2·h−1), yet conductances were the same (28 mS/cm2) between WT and KO mice. 6) Results presented by Knauf et al. were derived from experiments that began 2.5–3.5 h after tissues were mounted, a length of time that may produce marked changes in histology and electrophysiology of the mouse small intestine maintained in Ussing chambers for such prolonged periods (22). On the basis of these points, we suggest that the difference between the present findings of a net transcellular component to oxalate absorption and the solely paracellular model of Knauf et al. is principally a consequence of technical differences, since shunt permeability in the latter study was likely exaggerated.
It is important to reemphasize that unidirectional oxalate transport will take place through parallel pathways (transcellular and paracellular) and that these avenues may contribute differentially in various intestinal segments, with a greater paracellular component provided by leakier than by tight epithelia.
Final comments and conclusions.
Two possibilities need to be considered in the interpretation of changes to oxalate and Cl− transport resulting from the deletion of DRA. 1) Particularly in the case of oxalate, it is possible to interpret a reduction of JmsOx in DRA KO mice in an indirect manner. For example, apical oxalate uptake (and, hence, transcellular oxalate absorptive flux) could be mediated by a separate anion exchanger that is coupled to, and driven by, an electrochemical gradient generated by the transport activity of DRA. In this case, depression of oxalate uptake via this “tertiary” component would be indirect, and oxalate transport would be more accurately described as DRA-dependent than DRA-mediated. In the absence of additional information, the simplest explanation of our current findings is that DRA directly mediates oxalate and Cl− absorption at the apical membrane, and we have interpreted our experiments in this manner. 2) It is possible that compensatory adaptations in the abundance of other transporters could confound the comparison of flux measurements between WT and KO mice. For example, because DRA and PAT1 are Cl−/HCO3− exchangers residing in the apical pole of enterocytes, one might imagine a reciprocal compensatory abundance of one or the other. However, no consistent body of published evidence supports this proposal in the intestine. In a previous study on mouse ileum (9), we found no significant differences in the mRNA expression levels of Slc26a1, Slc26a2, Slc26a3, or CFTR in WT and PAT1 KO animals, whereas DRA has been reported to increase fivefold in the pancreatic duct of PAT1 KO mice (23). No change in PAT1 expression was detected in the colon of DRA KO compared with WT mice, while in the same study the authors noted (but did not show) that PAT1 increased threefold in the duodenum and jejunum (36). In the mouse duodenum, mRNA expression levels for PAT1, CFTR, and NHE3 in DRA KO animals were not different from those in WT animals (41), and mRNA expression levels of these genes were unaltered in jejuna of DRA or PAT1 KO mice (40). Consequently, there is no compelling reason to attempt to explain our current findings (the reduction of JmsOx and JmsCl in DRA KO mice) in terms of changes in PAT1, CFTR, or NHE3 expression levels. Finally, since the mouse ileum exhibits PAT1-mediated oxalate secretion (9), as well as DRA-mediated oxalate absorption (present study), any reciprocal upregulation of PAT1 in our DRA KO mice might have been expected to elevate JsmOx, but this was not realized experimentally (Fig. 1A).
In conclusion, the DRA anion exchanger contributes to transcellular oxalate and Cl− absorption in small and large intestine in the mouse. Deletion of the DRA protein in KO mice results in a significant reduction of urinary oxalate excretion and serum oxalate concentration, attesting to its importance as an avenue for intestinal oxalate uptake. Together with earlier conclusions regarding the secretory function of PAT1 (Slc26a6) in the mouse ileum (9) and duodenum (26), these observations highlight the importance of these two members of the Slc26a gene family in oxalate homeostasis and, hence, nephrolithiasis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-056245 to M. Hatch.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.W.F. and M.H. are responsible for conception and design of the research; R.W.F., J.M.W., and M.H. analyzed the data; R.W.F., J.M.W., and M.H. interpreted the results of the experiments; R.W.F. and M.H. prepared the figures; R.W.F. and M.H. drafted the manuscript; R.W.F., J.M.W., and M.H. edited and revised the manuscript; R.W.F., J.M.W., and M.H. approved the final version of the manuscript; M.H. performed the experiments.
ACKNOWLEDGMENTS
We thank Sonia Gabrilovich and Shreya Mishra for excellent technical assistance and animal husbandry.
REFERENCES
- 1.Alper SL, Stewart AK, Vandorpe DH, Clark JS, Horack RZ, Simpson JE, Walker NM, Clarke LL. Native and recombinant Slc26a3 (downregulated in adenoma, Dra) do not exhibit properties of 2Cl−/1HCO3− exchange. Am J Physiol Cell Physiol 300: C276–C286, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alper SL, Sharma AK. The SLC26 gene family of anion transporters and channels. Mol Aspects Med 34: 494–515, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barmeyer C, Ye JH, Sidani S, Geibel J, Binder HJ, Rajendran VM. Characteristics of rat downregulated in adenoma (rDRA) expressed in HEK 293 cells. Pflügers Arch 454: 441–450, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Charney AN, Goldfarb DS, Dagher PC. Metabolic disorders associated with gastrointestinal disease. In: Fluid, Electrolyte and Acid-Base Disorders (2nd ed.), edited by Arieff AI, DeFronzo RA. New York: Churchill-Livingstone, 1995, p. 813–837 [Google Scholar]
- 5.Charney AN, Egnor RW, Steinbrecher KA, Cohen MB. Effect of secretagogues and pH on intestinal transport in guanylin-deficient mice. Biochim Biophys Acta 1671: 79–86, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Chernova MN, Jiang L, Shmukler BE, Schweinfest CW, Blanco P, Freedman SD, Stewart AK, Alper SL. Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes. J Physiol 549: 3–19, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernova MN, Jiang L, Friedman DJ, Darman RB, Lohi H, Kere J, Vandorpe DH, Alper SL. Functional comparison of mouse slc26a6 anion exchanger with human SLC26A6 polypeptide variants: differences in anion selectivity, regulation, and electrogenicity. J Biol Chem 280: 8564–8580, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Freel RW, Hatch M, Earnest DL, Goldner AM. Oxalate transport across the isolated rat colon. A re-examination. Biochim Biophys Acta 600: 838–843, 1980 [DOI] [PubMed] [Google Scholar]
- 9.Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290: G719–G728, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Gennari FJ. Pathophysiology of metabolic alkalosis: a new classification based on the centrality of stimulated collecting duct ion transport. Am J Kidney Dis 58: 626–636, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Goldfarb DS, Sly WS, Waheed A, Charney AN. Acid-base effects on electrolyte transport in CA II-deficient mouse colon. Am J Physiol Gastrointest Liver Physiol 278: G409–G415, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Green ML, Hatch M, Freel RW. Ethylene glycol induces hyperoxaluria without metabolic acidosis in rats. Am J Physiol Renal Physiol 289: F536–F543, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Hatch M, Freel RW, Goldner AM, Earnest DL. Oxalate and chloride absorption by the rabbit colon: sensitivity to metabolic and anion transport inhibitors. Gut 25: 232–237, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatch M. Spectrophotometric determination of oxalate in whole blood. Clin Chim Acta 193: 199–202, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Hatch M, Freel RW, Vaziri ND. Intestinal excretion of oxalate in chronic renal failure. J Am Soc Nephrol 5: 1339–1343, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Hatch M, Freel RW, Vaziri ND. Mechanisms of oxalate absorption and secretion across the rabbit distal colon. Pflügers Arch 426: 101–109, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Hatch M, Freel RW. Renal and intestinal handling of oxalate following oxalate loading in rats. Am J Nephrol 23: 18–26, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Hatch M, Freel RW. Intestinal transport of an obdurate anion: oxalate. Urol Res 33: 1–16, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Hatch M, Freel RW. The roles and mechanisms of intestinal oxalate transport in oxalate homeostasis. Semin Nephrol 28: 143–151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatch M, Gjymishka A, Salido EC, Allison MJ, Freel RW. Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am J Physiol Gastrointest Liver Physiol 300: G461–G469, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homaidan FR, Tripodi J, Cheng P, Donovan V, Burakoff R. Ion transport across the cecum in normal and colitic mice. Dig Dis Sci 44: 1539–1546, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Inagaki E, Natori Y, Ohgishi Y, Hayashi H, Suzuki Y. Segmental difference of mucosal damage along the length of a mouse small intestine in an Ussing chamber. J Nutr Sci Vitaminol (Tokyo) 51: 406–412, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Ishiguro H, Namkung W, Yamamoto A, Wang Z, Worrell RT, Xu J, Lee MG, Soleimani M. Effect of Slc26a6 deletion on apical Cl−/HCO3− exchanger activity and cAMP-stimulated bicarbonate secretion in pancreatic duct. Am J Physiol Gastrointest Liver Physiol 292: G447–G455, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl−/HCO3− exchange in rabbit, rat, and human duodenum. Gastroenterology 122: 709–724, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem 277: 33963–33967, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Knauf F, Ko N, Jiang Z, Robertson WG, Van Itallie CM, Anderson JM, Aronson PS. Net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion. J Am Soc Nephrol 22: 2247–2255, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo SM, Aronson PS. Pathways for oxalate transport in rabbit renal microvillus membrane vesicles. J Biol Chem 271: 15491–15497, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Lamprecht G, Schaefer J, Dietz K, Gregor M. Chloride and bicarbonate have similar affinities to the intestinal anion exchanger DRA (down regulated in adenoma). Pflügers Arch 452: 307–315, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Lemann J, Jr, Pleuss JA, Worcester EM, Hornick L, Schrab D, Hoffmann RG. Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney Int 49: 200–208, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Madias NE, Adrogue HJ, Cohen JJ. Maladaptive renal response to secondary hypercapnia in chronic metabolic alkalosis. Am J Physiol Renal Fluid Electrolyte Physiol 238: F283–F289, 1980 [DOI] [PubMed] [Google Scholar]
- 32.Manoharan P, Coon S, Baseler W, Sundaram S, Kekuda R, Sundaram U. Prostaglandins, not the leukotrienes, regulate Cl−/HCO3− exchange (DRA, SLC26A3) in villus cells in the chronically inflamed rabbit ileum. Biochim Biophys Acta 1828: 179–186, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse down-regulated in adenoma (DRA) is an intestinal Cl−/HCO3− exchanger and is up-regulated in colon of mice lacking the NHE3 Na+/H+ exchanger. J Biol Chem 274: 22855–22861, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Moseley RH, Hoglund P, Wu GD, Silberg DG, Haila S, de la Chapelle A, Holmberg C, Kere J. Downregulated in adenoma gene encodes a chloride transporter defective in congenital chloride diarrhea. Am J Physiol Gastrointest Liver Physiol 276: G185–G192, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflügers Arch 447: 710–721, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281: 37962–37971, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Silberg DG, Wang W, Moseley RH, Traber PG. The down regulated in adenoma (dra) gene encodes an intestine-specific membrane sulfate transport protein. J Biol Chem 270: 11897–11902, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Soleimani M. Expression, regulation and the role of SLC26 Cl−/HCO3− exchangers in kidney and gastrointestinal tract. Novartis Found Symp 273: 91–106, 2006 [PubMed] [Google Scholar]
- 39.Talbot C, Lytle C. Segregation of Na/H exchanger-3 and Cl/HCO3 exchanger SLC26A3 (DRA) in rodent cecum and colon. Am J Physiol Gastrointest Liver Physiol 299: G358–G367, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Walker NM, Simpson JE, Yen PF, Gill RK, Rigsby EV, Brazill JM, Dudeja PK, Schweinfest CW, Clarke LL. Down-regulated in adenoma Cl/HCO3 exchanger couples with Na/H exchanger 3 for NaCl absorption in murine small intestine. Gastroenterology 135: 1645–1653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL. Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterology 136: 893–901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Petrovic S, Mann E, Soleimani M. Identification of an apical Cl−/HCO3− exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol 282: G573–G579, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Wedenoja S, Ormala T, Berg UB, Halling SF, Jalanko H, Karikoski R, Kere J, Holmberg C, Hoglund P. The impact of sodium chloride and volume depletion in the chronic kidney disease of congenital chloride diarrhea. Kidney Int 74: 1085–1093, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Whittamore JM, Freel RW, Hatch M. Sulfate secretion and chloride absorption are mediated by the anion exchanger DRA (Slc26a3) in the mouse cecum. Am J Physiol Gastrointest Liver Physiol 305: G172–G184, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y, Zhao J, Bouyer P, Boron WF. Evidence from renal proximal tubules that HCO3− and solute reabsorption are acutely regulated not by pH but by basolateral HCO3− and CO2. Proc Natl Acad Sci USA 102: 3875–3880, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]