Abstract
The inner medullary collecting duct (IMCD) is the nephron segment with the highest production of endothelin-1 (ET-1) and the greatest expression of ET-1 receptors that function to adjust Na+ and water balance. We have reported that male rats have reduced natriuresis in response to direct intramedullary infusion of ET-1 compared with female rats. Our aim was to determine whether alterations of ET-1 receptor expression and downstream intracellular Ca2+ signaling within the IMCD could account for these sex differences. IMCDs from male and female rats were isolated for radioligand binding or microdissected for intracellular Ca2+ ([Ca2+]i) measurement by fluorescence imaging of fura-2 AM. IMCD from male and female rats had similar ETB expression (655 ± 201 vs. 567 ± 39 fmol/mg protein, respectively), whereas male rats had significantly higher ETA expression (436 ± 162 vs. 47 ± 29 fmol/mg protein, respectively; P < 0.05). The [Ca2+]i response to ET-1 was significantly greater in IMCDs from male compared with female rats (288 ± 52 vs. 118 ± 32 AUC, nM × 3 min, respectively; P < 0.05). In IMCDs from male rats, the [Ca2+]i response to ET-1 was significantly blunted by the ETA antagonist BQ-123 but not by the ETB antagonist BQ-788 (control: 137 ± 27; BQ-123: 53 ± 11; BQ-788: 84 ± 25 AUC, nM × 3 min; P < 0.05), consistent with greater ETA receptor function in male rats. These data demonstrate a sex difference in ETA receptor expression that results in differences in ET-1 Ca2+ signaling in IMCD. Since activation of ETA receptors is thought to oppose ETB receptor activation, enhanced ETA function in male rats could limit the natriuretic effects of ETB receptor activation.
Keywords: inner medullary collecting duct, endothelin
it has been well established that men are more susceptible to hypertension and cardiovascular disease than premenopausal women and that females are protected against high blood pressure in a number of hypertensive animal models (3, 22). For instance, male rats are more sensitive to angiotensin II (AngII)-induced hypertension than female rats, and male spontaneously hypertensive rats have higher blood pressure than their female counterparts. As the prevalence of uncontrolled hypertension becomes greater, understanding the mechanisms behind these observations becomes increasingly important to define future treatments.
Endothelin-1 (ET-1) is an important regulator of blood pressure through activation of its two receptor subtypes, ETA and ETB (18). Our laboratory has recently determined that sex differences in renal ET-1 signaling may mediate sex differences in blood pressure regulation (9, 11). For instance, AngII, whether stimulated by a low-salt diet or given exogenously, inhibits ETB receptor function in male rats but to a much lesser degree than in female rats (9, 10). Furthermore, acute infusion of an ETB agonist directly into the renal medulla enhances sodium and water excretion in both male and female rats. However, infusion of ET-1, the endogenous ligand, only produces natriuresis in female rats. Although the mechanisms for these sex differences are still speculative, one possibility is an altered functional distribution of ET receptors within the kidney.
The collecting duct is an important site for both endogenous ET-1 production and long-term blood pressure control. In fact, a series of publications from the Kohan laboratory has demonstrated that the absence of ET-1 and ETB receptor expression in the collecting duct leads to an attenuated ability to handle a high-salt diet, resulting in salt-dependent hypertension (1, 4). Furthermore, the inner medullary collecting duct (IMCD) produces considerably more ET-1 and has a much higher expression of ETB receptors compared with all other segments within the kidney (12, 13). The importance of ET-1 from the IMCD in blood pressure regulation is due in large part to ETB-mediated increases in nitric oxide production, resulting in reductions in Na+ and H2O reabsorption in the IMCD and other nephron segments (8, 15, 17, 19). In general, activation of ETA receptors opposes the actions of ETB receptors. Both ETA and ETB receptor subtypes are located on IMCD, and, given their key role in Na+ and water homeostasis, differences in ET-1 signaling within IMCD could potentially mediate sex differences in arterial pressure; however, little is known regarding sex differences in ET-1 signaling in IMCD.
We hypothesize that the distribution and downstream signaling of ET-1 receptors in IMCD is different in male and female rats. To determine whether the expression of ET-1 receptors could account for the functional difference between sexes, we isolated IMCD from male and female Sprague-Dawley rats and compared ETA and ETB receptor expression using radioligand binding assays. To assess whether any sex differences in receptor expression in IMCD were functional and result in altered cellular responses to ET-1, we determined intracellular Ca2+ ([Ca2+]i) responses to ET-1 activation in live, freshly isolated IMCD from male and female Sprague-Dawley rats.
METHODS
Experimental Animals
All protocols were approved by the Institutional Animal Care and Use Committee at Georgia Regents University. Male (n = 43) and female (n = 47) Sprague-Dawley (SD; Harlan, Indianapolis, IN) rats were obtained at 10 wk of age and maintained on a standard rat chow containing 0.4% NaCl with free access to water. Rats were housed in temperature- and humidity-controlled, 12:12-h light-cycled quarters.
Bulk IMCD Isolation
Renal inner medullae were dissected under sodium brevital anesthesia and chopped into 1-mm sections following the methods of Hyndman et al. (7). The minced sections from two animals were pooled and digested in sucrose buffer (250 mM sucrose and 10 mM triethanolamine, pH 7.4) plus 2 mg/ml collagenase type I, 2 mg/ml hyaluronidase type IV. The suspension was incubated in a 37°C shaking water bath for 60–90 min. After 30 min, 0.5 mg of DNAse I was added to the suspension. After digestion, the solution was filtered over a 100-μm filter (Fisher Scientific). The supernatant was centrifuged at 300 g for 2.5 min. The supernatant was removed, and the pellet was washed twice with sucrose buffer, centrifuging at 300 g for 1 min between washes to repellet the IMCD. After the final wash, the supernatant was removed, and the pellet was resuspended in Hank's balanced saline solution (HBSS; Cellgro). It was then centrifuged for 5 min at 300 g, and the HBSS was removed. The pellet was stored at −80°C until binding assay was performed.
ET-1 Receptor Binding in IMCD
IMCD pellets were suspended in 200 μl of homogenization buffer (50 mM Tris·HCl, 5 mM EDTA, 250 mM sucrose, 15 μM PMSF, pH 7.4). The IMCDs were disrupted by brief sonication and spun at 1,000 g for 30 min at 4°C to pellet any large debris. To the supernatant, 1.3 mM phenylmethanesulfonyl fluoride was added, and the supernatant was then centrifuged at 30,000 g and 4°C for 45 min to pellet membranes. The supernatant was discarded, and the pellet (cell membrane enriched) was suspended in 100 μl of homogenization buffer and disrupted. Total protein was measured using the Bradford method.
The binding assay was performed in duplicate on a 96-well Packard optiplate. Samples were diluted in binding buffer (20 mM Tris·HCl, 100 mM NaCl, 10 mM MgCl2, 3 mM EDTA, 0.1 mM PMSF, 5 μg/ml pepstatin A, 0.025% bacitracin, 0.2% BSA) to a final protein concentration of 0.3 μg/50 μl. Sample (50 μl) and 25 μl of 40 mg/ml wheatgerm agglutinin-coated PVT SPA beads (Perkin Elmer) were added to each well. The plate was incubated on a shaker for 3 h at room temperature. Total binding was determined by adding 50 μl of 125I-ET-1 or 125I-ET-3 (Perkin Elmer, Waltham, MA) to each well at final concentrations of 0.6, 0.3, 0.15, 0.075, 0.03, and 0.01 nM. Nonspecific binding was determined by adding 10 μM of nonlabeled ET-1 or ET-3 (American Peptide, Sunnyvale, CA) to the total binding mix. The plates were incubated on a shaker for 18 h at room tempertature, and scintillation was measured by using a Packard Top Count apparatus.
Preparation of IMCD for Ca2+ Signaling Detection
Isolation of IMCDs was performed as described previously (16). Rats were anesthetized with isoflurane, the abdominal aorta was cannulated, and the kidney was flushed with 10 ml of chilled HBSS with 20 mM HEPES (Sigma, St. Louis, MO) (pH 7.40) at 3 ml/min. The left kidney was then excised and sagittally sectioned, and a portion of the inner medulla was removed. IMCD tissue was placed on a cooled stage in HBSS and visualized using a Nikon SMZ 745T stereomicroscope. IMCD tissue was micro dissected using fine needles to isolate a single layer of collecting ducts. The thin layer of tissue containing IMCD was placed on a glass coverslip coated with the tissue adhesive Cell-tak (BD Biosciences, San Jose, CA) and loaded with fura 2-AM fluorescent dye (5 μM, Molecular Probes, Grand Island, NY) in HBSS for 1 h before imaging. Pluronic F127 (Molecular Probes) was used to dissolve the fura-2 AM dye to prevent dye compartmentalization upon loading.
Fluorescence Detection
Fluorescence measurements were made using an Olympus IX81 inverted microscope with a ×40 objective lens. The signal was detected using a high-resolution digital camera (EVOLVE, Photometrics Technology). Excitation was provided by a Sutter DG-4 175-W xenon arc lamp (Sutter Instrument, Novato, CA) that allowed high-speed excitation wavelength switching. IMCDs isolated on coverslips were washed to remove excess dye before being placed in the imaging chamber. Fura 2 fluorescent signal was stimulated by dual-wavelength excitation at 340 and 380 nm. A 510-nm band-pass emission filter was used to collect fura 2 signals at 3-s intervals. Ratios between the fluorescence intensity stimulated by 340/380-nm excitation were calculated, and the excitation intensity was adjusted on the DG-4 to minimize fura 2 fluorescence bleaching and to balance 340/380 excitation intensities. At the end of the experiment, maximum and minimum fura 2 signals were collected for the calibration of [Ca2+]i concentration. The tissue bath solution was exchanged to 5 μM of the calcium ionophore 4-bromo-A23187 (Molecular Probes) with known solution of Ca2+ (Calcium buffer kit; Molecular Probes) to establish maximum and minimum fura 2 signals. [Ca2+]i was calculated as equation 1, [Ca2+]i = Kd * (R − Rmin)/(Rmax − R) * F, where Kd is the dissociation constant of fura 2, R is the actual ratio of intensities at excitation wavelengths 340 and 380 nm, Rmax and Rmax are the maximal and minimal fura 2 ratios in the presence and absence of Ca2+, and F is the ratio of fura 2 intensities at 380 nm in the presence and absence of Ca2+. Kd of fura 2 at 37°C was 224 nM as reported (6). The maximum cytosolic Ca2+ response (Δ[Ca2+i]) from baseline as well as the integrated Ca2+ response [3 min of area under the curve (AUC) immediately following stimulation] were used for the comparison of the responses.
Experimental Protocol
Ca2+ imaging.
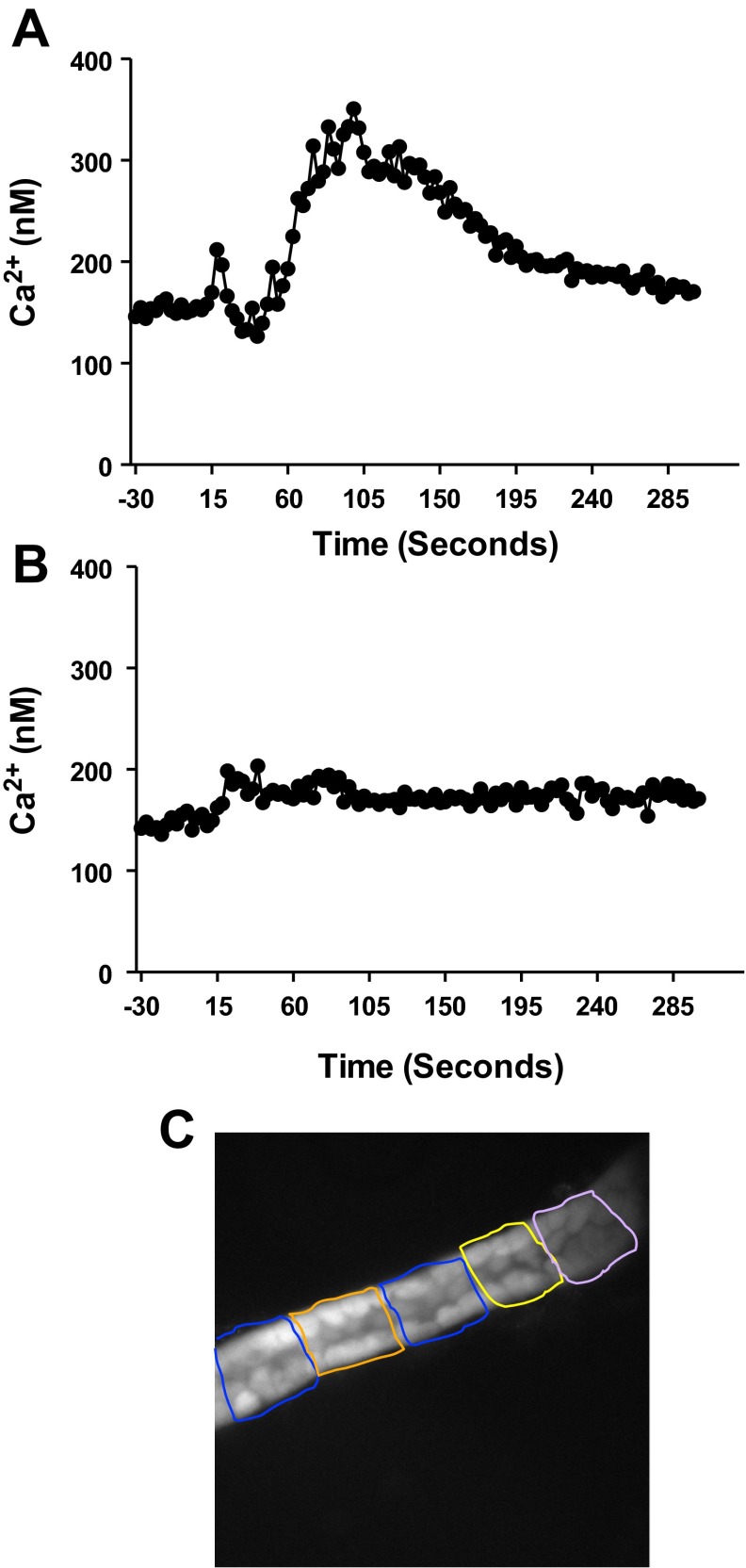
Coverslips containing IMCD were placed in an imaging chamber (maintained at 37°C) mounted on the stage of the inverted microscope that allowed the superfusion of the experimental buffer and buffer containing drugs. Five to eight regions, as shown in Fig. 2C, containing only collecting duct cells, were selected within each IMCD to quantify changes in fluorescent intensity of fura-2 AM dyes using Metafluor imaging software (Universal Imaging, Bedford Hills, NY). Fluorescent signals were recorded for 200 s (baseline) before bath exchange. Bath media containing either IMCDs from male or female rats was then rapidly exchanged with media containing 1) buffer only or 2) ET-1 (1 nM; American Peptide) and fluorescent signal recorded for a further 300 s. Because maximal [Ca2+]i responses occurred within the first 3 min, data are presented as AUC by 180 s. All exchanged solutions were prewarmed to 37°C using an in-line solution heater (Warner Instruments, Hamden, CT).
Fig. 2.
Typical intracellular Ca2+ concentration ([Ca2+]i) tracing before and after treatment with endothelin-1 (ET-1; 1 nM) in freshly isolated inner medullary collecting duct (IMCD) from male (A) and female (B) rats. Baseline [Ca2+]i is measured for 2 min, followed by treatment with ET-1. Shaded area represents the area under the curve used to quantify [Ca2+]i response to ET-1. C: an image of an IMCD loaded with fura 2. Boxes represent fields from which [Ca2+]i was measured.
In experiments in which antagonists were used, IMCD were incubated with antagonists for 15 min before stimulation with ET-1. The response to ET-1 (1 nM) was determined in IMCD from both male and female rats for 300 s following incubation of IMCD with either the ETA receptor antagonist BQ-123 (1 μM; Calbiochem/EMD chemical, Darmstadt, Germany) or the ETB receptor antagonist BQ-788 (1 μM; Calbiochem/EMD chemical). In a separate group of experiments, IMCD from both male and female rats were stimulated using the ETB receptor-specific agonist sarafotoxin 6c (S6c;1 nM; American Peptide) in place of ET-1. To confirm [Ca2+]i responses were mediated by receptor activation, in some experiments, IMCD were incubated with the phospholipase C (PLC) inhibitor U73122 (3 μM; Sigma) for 15 min before stimulation with ET-1 (1 nM).
Statistical Analysis
All data are expressed as means ± SE. Comparisons between antagonist treatments were made by one-way ANOVA, followed by Dunnett's multiple comparison test. All other comparisons were determined using unpaired t-test. P < 0.05 was considered statistically significant.
RESULTS
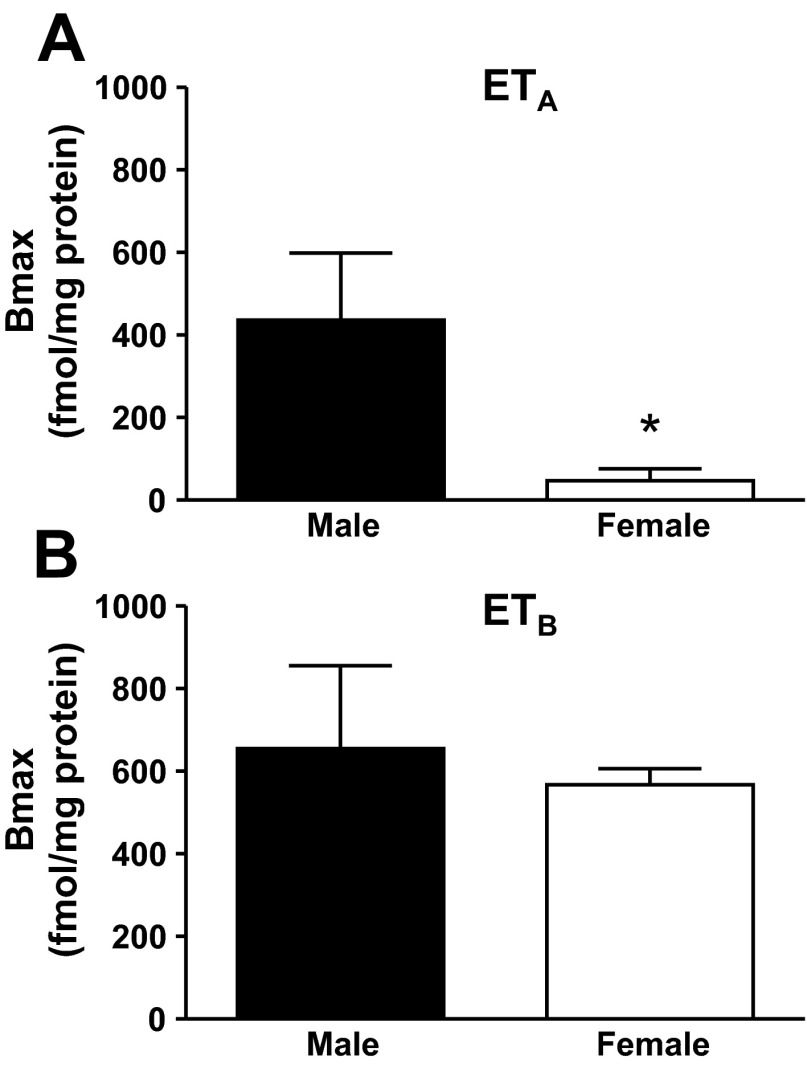
To help explain sex differences in medullary ET-1 receptor function observed in vivo (14), receptor binding was determined on freshly isolated IMCD. ETB receptor expression in IMCD as determined by ET-3 binding was similar between male (n = 4) and female (n = 5) rats (655 ± 201 vs. 567 ± 39 Bmax, respectively). In contrast, female rats had significantly reduced ETA receptor binding as measured by the difference in ET-1 and ET-3 binding (434 ± 162 vs. 47 ± 29 Bmax, male vs. female rats, P < 0.05; Fig. 1). Thus the ratio of ETB to ETA receptors in the IMCD is 60/40 for male rats but 90/10 for female rats.
Fig. 1.
Maximum binding (Bmax) for ETA receptors (A) and ETB receptors (B) measured by [I125]-ET-1 and [I125]-ET-3 in the IMCD of male and female rats (n = 4–5 rats). *Significant difference (P < 0.05).
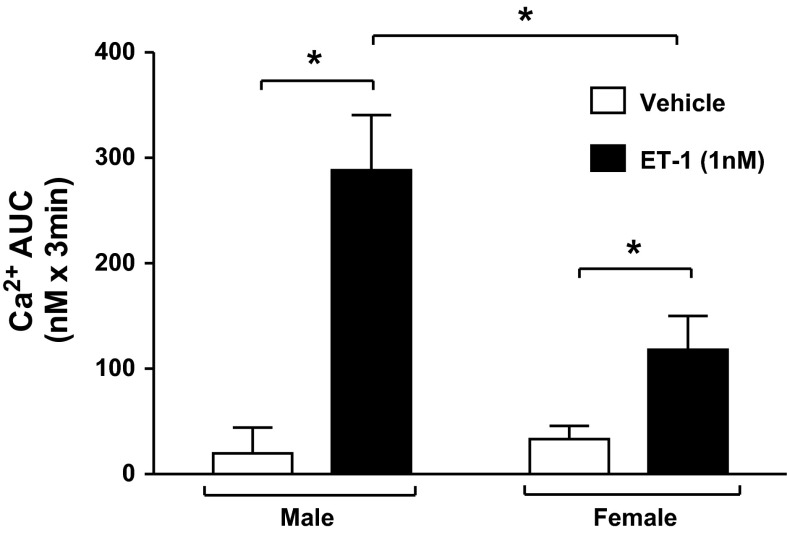
To determine whether differences in ET-1 receptor expression in IMCD between male and female rats could account for functional differences, intracellular calcium responses to ET-1 were measured in freshly isolated IMCD. Baseline [Ca2+]i before stimulation with ET-1 was 156 ± 30 and 124 ± 26 nM in male and female rats, respectively. In IMCDs from both male and female rats, addition of ET-1 (1 nM) resulted in a rapid increase in [Ca2+]i over the following 60 s followed by a prolonged period (>200 s) in which [Ca2+]i fluctuated but remained elevated above baseline levels (Fig. 2). The total calcium response calculated as AUC was significantly greater than that of vehicle treatment in both male and female rats (treatment P < 0.0001; Fig. 3). However, [Ca2+]i responses to ET-1 were significantly greater in IMCD from male rats compared with female rats, averaging 288 ± 52 and 118 ± 32 AUC (sex P < 0.05), respectively, over 180 s following treatment with ET-1 (1 nM).
Fig. 3.
Represents [Ca2+]i response as area under the curve (AUC) to either vehicle (open bars) or ET-1 (filled bars) in freshly isolated IMCD from male and female rats (n = 6–9 rats per group). *Significant difference by two-way ANOVA (P < 0.05).
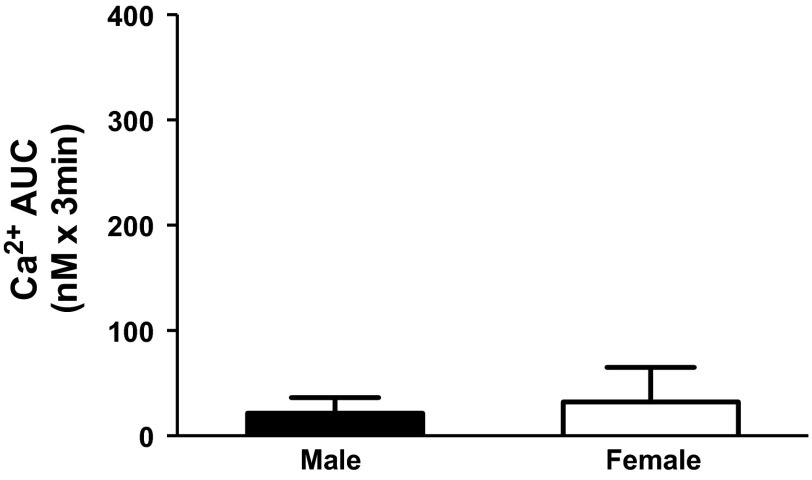
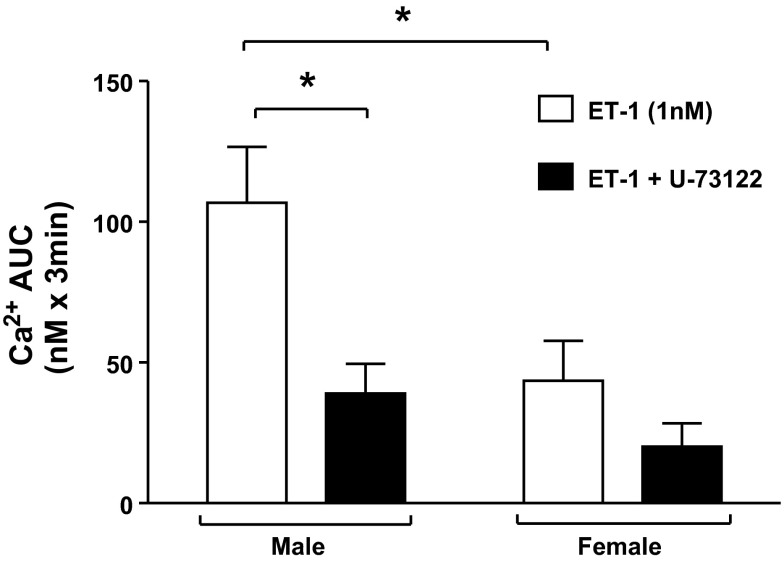
When stimulated with the potent and selective ETB receptor agonist S6c (1 nM) rather than ET-1, no significant change in [Ca2+]i was observed in either male or female rats, indicating that [Ca2+]i responses were not stimulated by activation of the ETB receptor in either sex (Fig. 4). Furthermore, prior (15 min) incubation with the phospholipase C inhibitor U-73122 (3 μM) significantly blunted the [Ca2+]i response in male (P = 0.04) but not female rats, indicating that ET-1 increases [Ca2+]i in IMCD via a G-protein-coupled receptor pathway (Fig. 6). These data together are consistent with activation of the ETA receptor.
Fig. 4.
Represents [Ca2+]i response as AUC in freshly isolated IMCD from male and female rats in response to the ETB agonist sarafotoxin 6c.
Fig. 6.
[Ca2+]i response in freshly isolated IMCD of male or female rats to ET-1 (1 nM) in the presence or absence of a PLC inhibitor (U-73122; 3 μM).
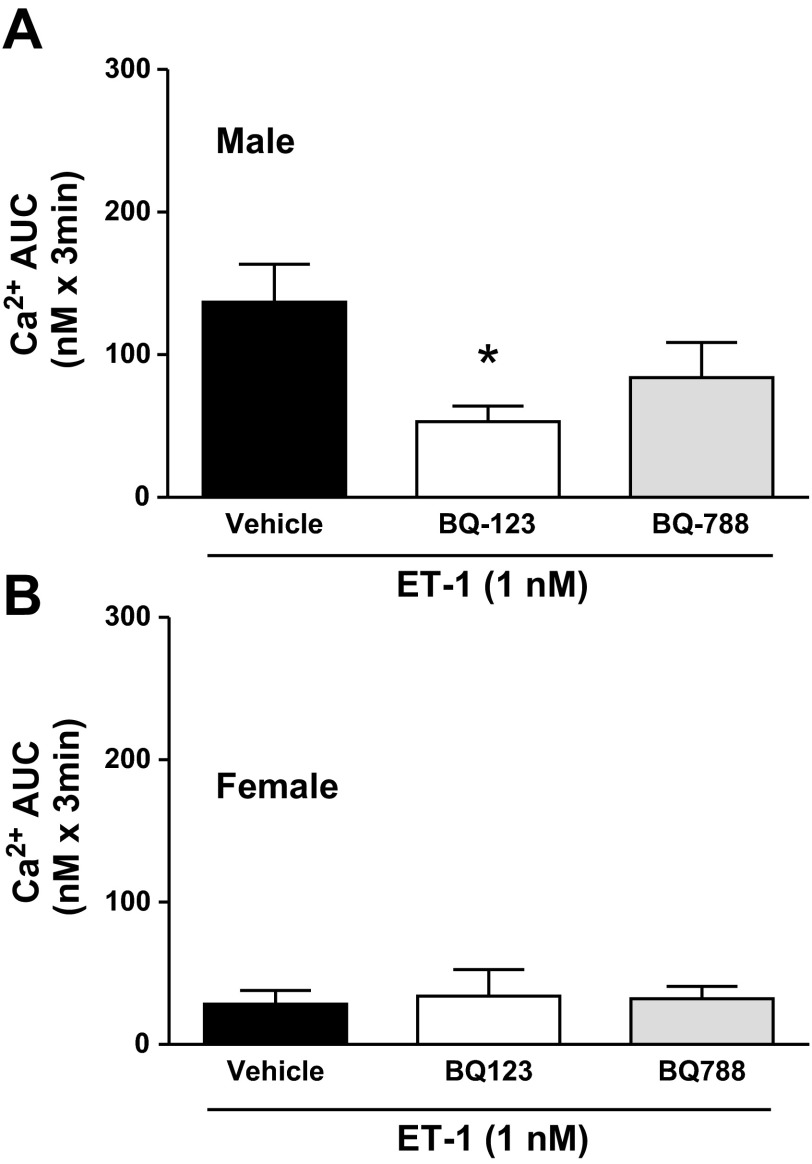
To further elucidate which receptor on the IMCD is responsible for the increase in [Ca2+]i in response to ET-1, IMCD taken from the same rat were treated with ET-1 in the presence of the specific antagonists BQ-123 (ETA antagonist; 1 μM) or BQ-788 (ETB antagonist; 1 μM) given 15 min before ET-1 stimulation. Baseline [Ca2+]i in IMCD was higher in male rats compared with female rats, and treatment with BQ-123 lowered baseline [Ca2+]i in IMCD from male rats but had no effect in female rats. Treatment with BQ-788 did not affect baseline [Ca2+]i in either male or female rats (Table 1). Interestingly, in the presence of BQ-123, the increase in [Ca2+]i following addition of ET-1 was significantly blunted (P < 0.05; Fig. 5), such that, in the presence of BQ-123, the response of IMCD from male rats to ET-1 did not differ from that of IMCD from female rats. Incubation with BQ-788 did not significantly reduce the [Ca2+]i response to ET-1 in male rats, consistent with this response being independent of the ETB receptor.
Table 1.
Baseline intracellular Ca2+ concentrations (in nM) in isolated inner medullary collecting ducts pretreated with ETA (BQ-123) or ETB (BQ-788) antagonists
| Untreated | BQ-123 | BQ-788 | |
|---|---|---|---|
| Male (n = 8) | 114 ± 16* | 75 ± 14 | 98 ± 18 |
| Female (n = 6) | 53 ± 8 | 53 ± 10 | 48 ± 6 |
Values are means ± SE.
Significant difference vs. females (P < 0.05).
Fig. 5.
[Ca2+]i response to ET-1 (1 nM) in the presence or absence of an ETA antagonist (BQ-123; 1 μM) or an ETB antagonist (BQ-788; 1 μM) in IMCD of male (A; n = 8) or female (B; n = 6) rats. *Significant difference by one-way ANOVA (P < 0.05).
DISCUSSION
The major finding of this study is that IMCD ETA receptor expression and intracellular Ca2+ signaling are greater in male compared with female rats. Alterations in IMCD ET-1 signaling could explain sex differences in renal medullary ETB receptor function that several laboratories, including our own, have previously observed (9, 10, 14, 21, 23). We recently reported that direct infusion of an ETB agonist into the renal medulla produces diuresis and natriuresis in both male and female rats, whereas infusion of ET-1, the endogenous ligand, produces natriuresis only in female rats. These differences could be explained at least in part by counteracting ETA (anti-natriuretic) and ETB (natriuretic) receptor function in IMCD, which contribute to Na+ and water excretion by the kidney. Our data, for the first time, demonstrate sex differences in ET receptor expression and Ca2+ signaling in this key nephron segment and implicate increased ETA signaling in male rats as a probable source of functional differences in Na+ excretion in response to ET-1 between male and female rats.
Our finding that both ETA and ETB receptors are expressed in IMCD of male rats is consistent with previously published reports in both freshly isolated and cultured IMCD (20). Although it has been well established that the ETB receptor within the IMCD is a major contributor to the maintenance of Na+ and water balance, the role of the ETA receptor in this nephron segment is largely unknown. Our data, together with our previous finding that male rats have a natriuretic response to an ETB agonist but not to ET-1, a combined ETA/ETB agonist, suggest that IMCD ETA receptors oppose ETB receptors and are anti-natriuretic. Although studies of collecting duct-specific knockout mice suggest that ETA receptors may contribute to the natriuretic response to ET-1, this action of the ETA receptor has only been observed in the absence of the ETB receptor (5). Therefore, it is likely that removal of ETB receptor function, as seen in collecting duct-specific ETB knockout mice, from the ETA receptor on the IMCD may compensate to promote natriuresis, although this hypothesis has yet to be tested. Furthermore, it has yet to be directly determined whether sex differences in the renal medullary response to ET-1 exist for mice.
Previously, we reported female rats have an ETA-dependent natriuresis in response to exogenous ET-1 infusion into the renal medulla. Here, we present that females have fewer ETA receptors on the IMCD. Taken together, we postulate that ETA-dependent natriuresis in female rats is related to ETA receptors located on other cell types, such as the vasa recta, the thick ascending limb, or perhaps renomedullary interstitial cells, but the mechanism for natriuresis remains unclear (14).
To confirm that ETA receptors were responsible for enhanced Ca2+ signaling in male compared with female IMCD, we quantified [Ca2+]i responses to pharmacological agonists/antagonists of the ET system in IMCD. Interestingly, we found that baseline [Ca2+]i in untreated IMCD from female rats was significantly lower than in IMCD from male rats. Treatment with the potent and selective ETA receptor antagonist BQ-123 reduced baseline [Ca2+]i in IMCD from male rats compared with levels observed in IMCD from female rats. These data are consistent with our finding that the male rats have more ETA on the IMCD than the female rats and indicate that endogenous ET-1 signaling within IMCD leads to sex differences in resting [Ca2+]i levels within this nephron segment.
In response to exogenous ET-1 (1 nM), an initial Ca2+ spike followed by a prolonged increase in [Ca2+]i was observed. As observed following ET-1 administration, initial Ca2+ spikes were quite variable in magnitude and were sometimes absent all together; therefore, we determined [Ca2+]i as AUC, integrating the [Ca2+]i signal over the duration of the response to better quantify Ca2+ responses in IMCD. In IMCD from male rats, ETA receptor antagonism blunted Ca2+ responses to ET-1. Confirming that [Ca2+]i responses were mediated by receptor signaling, the phospholipase C inhibitor U-73122 also greatly blunted the response to ET-1 (Fig. 6). Our data indicating that ETA receptor activation drives increased intracellular Ca2+ in IMCD of rats is in contrast to data from porcine IMCD indicating Ca2+ responses are mediated by ETB receptors. Our data do not completely exclude the possibility that ETB receptors participate in Ca2+ signaling in rat IMCD, because neither ETA nor ETB receptor antagonism completely abolished responses to ET-1 in freshly isolated rat IMCD. Furthermore, in some individual preparations, we did observe reduced [Ca2+]i responses following ETB receptor inhibition with BQ-778. This, however, did not reach statistical significance (Fig. 5A). Although we cannot rule out Ca2+ signaling through the ETB receptor, our data is in agreement with reports that there is increased ETA-mediated calcium signaling in the thin limb of Henle's loop of hypertensive rats (2), and we are confident that sex differences in [Ca2+]i are mediated by ETA receptor signaling, because differences in the response of male and female rats were completely abolished in the presence of an ETA antagonist BQ-123 (AUC [Ca2+]i = 53 ± 11 vs. 34 ± 19 N·S in male and female rats, respectively). Furthermore, S6c (1 nM), a potent ETB selective agonist, did not elicit measureable [Ca2+]i responses in either male or female rats, indicating that Ca2+ signaling by ETB receptors is minimal.
Our data clearly demonstrate a sex difference in ET-1 receptor expression and signaling in rats; however, it is unknown whether ET-1 peptide levels are different within the IMCD of male and female rats. Although tissue concentrations have proven difficult to accurately measure, previous data from our laboratory demonstrates that urinary ET-1 levels, an indicator of renal production, are not different between male and female ETB-deficient rats or wild-type rats (23). Thus it is unlikely that IMCD ET-1 concentrations differ among the sexes.
Our data demonstrate a clear sex difference in this important cell type for ETA expression and signaling that affects both renal function and ET signaling. Since ETB receptor expression was not different in IMCD of male and female rats, nor was the [Ca2+]i response to ET-1 affected by ETB blockade, these data prompt us to examine the role and the functional consequences of ETA receptor activation at both the cellular and whole animal level. Although several mechanisms are implicated in fluid and electrolyte balance malfunction, differences in ETA signaling could potentially explain sex differences in associated pathologies such as hypertension and may provide a clinical target for sex-specific treatment of these diseases. Moreover, there is a need to determine whether sex differences observed in this study play a role in experimental models of hypertension. In perspective, these results provide further mechanistic information that could potentially explain the reduced incidence of salt-sensitive hypertension in women vs. men.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-69999 and HL-95499, an American Heart Association Scientist Development grant (P. O'Connor), and an American Heart Association postdoctoral fellowship (J. Speed).
DISCLOSURES
D. M. Pollock has received research support from Takeda Pharmaceuticals and Abbott Laboratories.
AUTHOR CONTRIBUTIONS
Author contributions: C.J., J.S.S., K.A.H., P.M.O., and D.M.P. conception and design of research; C.J., J.S.S., K.A.H., and P.M.O. performed experiments; C.J., J.S.S., K.A.H., P.M.O., and D.M.P. analyzed data; C.J., J.S.S., K.A.H., P.M.O., and D.M.P. interpreted results of experiments; C.J., J.S.S., K.A.H., P.M.O., and D.M.P. prepared figures; C.J., J.S.S., K.A.H., P.M.O., and D.M.P. drafted manuscript; C.J., J.S.S., K.A.H., P.M.O., and D.M.P. edited and revised manuscript; C.J., J.S.S., K.A.H., P.M.O., and D.M.P. approved final version of manuscript.
REFERENCES
- 1.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest 114: 504–511, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey MA, Haton C, Orea V, Sassard J, Bailly C, Unwin RJ, Imbert-Teboul M. ETA receptor-mediated Ca2+ signaling in thin descending limbs of Henle's loop: impairment in genetic hypertension. Kidney Int 63: 1276–1284, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bhatia K, Elmarakby AA, El-Remessy AB, Sullivan JC. Oxidative stress contributes to sex differences in angiotensin II-mediated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 302: R274–R282, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol 291: F1274–F1280, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Ge Y, Bagnall A, Stricklett PK, Webb D, Kotelevtsev Y, Kohan DE. Combined knockout of collecting duct endothelin A and B receptors causes hypertension and sodium retention. Am J Physiol Renal Physiol 295: F1635–F1640, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 7.Hyndman KA, Musall JB, Xue J, Pollock JS. Dynamin activates NO production in rat renal inner medullary collecting ducts via protein-protein interaction with NOS1. Am J Physiol Renal Physiol 301: F118–F124, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyndman KA, Pollock JS. Nitric oxide and the A and B of endothelin of sodium homeostasis. Curr Opin Nephrol Hypertension 22: 26–31, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kittikulsuth W, Looney SW, Pollock DM. Endothelin ETB receptors contribute to sex differences in blood pressure elevation in angiotensin II hypertensive rats on a high-salt diet. Clin Exp Pharmacol Physiol 40: 362–370, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kittikulsuth W, Pollock JS, Pollock DM. Sex differences in renal medullary endothelin receptor function in angiotensin II hypertensive rats. Hypertension 58: 212–218, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kittikulsuth W, Sullivan JC, Pollock DM. ET-1 actions in the kidney: evidence for sex differences. Br J Pharmacol 168: 318–326, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohan DE. Endothelin and collecting duct sodium and water transport. Contributions Nephrol 172: 94–106, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91: 1–77, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakano D, Pollock DM. Contribution of endothelin A receptors in endothelin 1-dependent natriuresis in female rats. Hypertension 53: 324–330, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakano D, Pollock JS, Pollock DM. Renal medullary ETB receptors produce diuresis and natriuresis via NOS1. Am J Physiol Renal Physiol 294: F1205–F1211, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Connor PM, Cowley AW., Jr Vasopressin-induced nitric oxide production in rat inner medullary collecting duct is dependent on V2 receptor activation of the phosphoinositide pathway. Am J Physiol Renal Physiol 293: F526–F532, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Plato CF, Pollock DM, Garvin JL. Endothelin inhibits thick ascending limb chloride flux via ET(B) receptor-mediated NO release. Am J Physiol Renal Physiol 279: F326–F333, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281: F144–F150, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Pollock JS, Pollock DM. Endothelin and NOS1/nitric oxide signaling and regulation of sodium homeostasis. Curr Opin Nephrol Hypertension 17: 70–75, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Sullivan JC, Goodchild TT, Cai Z, Pollock DM, Pollock JS. Endothelin(A) [ET(A)] and ET(B) receptor-mediated regulation of nitric oxide synthase 1 (NOS1) and NOS3 isoforms in the renal inner medulla. Acta Physiol (Oxf) 191: 329–336, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Sullivan JC, Pollock JS, Pollock DM. Superoxide-dependent hypertension in male and female endothelin B receptor-deficient rats. Exp Biol Med (Maywood) 231: 818–823, 2006 [PubMed] [Google Scholar]
- 22.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 293: R1573–R1579, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Gender differences in ET and NOS systems in ETB receptor-deficient rats: effect of a high salt diet. Hypertension 41: 657–662, 2003 [DOI] [PubMed] [Google Scholar]