Abstract
Cilia, membrane-enclosed organelles protruding from the apical side of cells, can be divided into two classes: motile and primary cilia. During the past decades, motile cilia have been intensively studied. However, it was not until the 1990s that people began to realize the importance of primary cilia as cellular-specific sensors, particularly in kidney tubular epithelial cells. Furthermore, accumulating evidence indicates that primary cilia may be involved in the regulation of cell proliferation, differentiation, apoptosis, and planar cell polarity. Many signaling pathways, such as Wnt, Notch, Hedgehog, and mammalian target of rapamycin, have been located to the primary cilia. Thus primary cilia have been regarded as a hub that integrates signals from the extracellular environment. More importantly, dysfunction of this organelle may contribute to the pathogenesis of a large spectrum of human genetic diseases, named ciliopathies. The significance of primary cilia in acquired human diseases such as hypertension and diabetes has gradually drawn attention. Interestingly, recent reports disclosed that cilia length varies during kidney injury, and shortening of cilia enhances the sensitivity of epithelial cells to injury cues. This review briefly summarizes the current status of cilia research and explores the potential mechanisms of cilia-length changes during kidney injury as well as provides some thoughts to allure more insightful ideas and promotes the further study of primary cilia in the context of kidney injury.
Keywords: primary cilia, kidney injury, IFT, planar cell polarity, ciliopathy
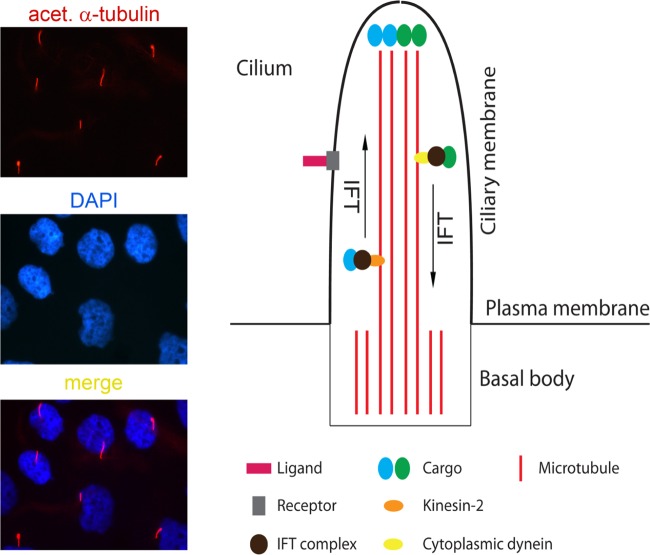
cilia or flagella (here used interchangeably) contain nine sets of microtubule doublets arranged in a circular pattern with (9+2) or without (9+0) a central pair of microtubule singlets. They are largely membrane-enclosed organelles that project from the apical surface of cells (133, 185, 187). The majority of cells in the human body have either one or multiple cilia. A single or monocilium in one cell is called the primary or nonmotile cilium (9+0), since it is immotile (for cells with primary cilia, see http://www.bowserlab.org/primarycilia/cilialist.html), apart from the exceptions such as motile nodal cilia (9+0) and nonmotile olfactory sensory cilia (9+2) (108, 120, 136). However, epithelial cells in some organs, for instance the respiratory tract and reproductive system, harbor multiple cilia (9+2) on the apical side of cells, which can beat upon stimulation, and therefore are named motile cilia although a chemosensory function has been recently suggested (179). Inside the cilium is the microtubule-based axoneme, in connection with bidirectional microtubule motors and associated protein complexes. Outside the axoneme is the enclosed ciliary membrane, which is generally believed to be specific and different from the rest of the plasma membrane. The structure connected to the cilium at the bottom is the basal body, and it is derived from the mother centriole of the centrosome, which provides a docking site for the cilium and transforms into a centriole during mitosis (Fig. 1). The basal body is structurally different from the daughter centriole, owing to its additional distal and subdistal appendages (79).
Fig. 1.
Left: anti-acetylated tubulin-stained primary cilia of human proximal tubular epithelial cells (HK-2). DAPI, 4',6-diamidino-2-phenylindole. Right: basic structure of a primary cilium.
It has been known that cilia play pivotal roles in embryo development, cell and tissue homeostasis, and human diseases. Although specialized cilia in the retina and kinocilia in the inner ear have been recognized for their specific roles in photoreception and cell polarization, the functions of primary cilia in humans have been obscure for more than a century. It was not until the observation of expression of the polycystin-1 (PC1) homolog Lov-1 in sensory neurons of Caenorhabditis elegans and the generation of Tg737°rpk mice that researchers began to realize the importance of primary cilia, since Lov-1 is required for C. elegans mating and Tg737°rpk mice unexpectedly die of polycystic kidney disease (PKD) shortly after birth. Importantly, primary cilia in the kidney of mice with the Tg737 mutation are stunted (13, 219). These discoveries, for the first time, link the primary cilia to PKD. Later, Nauli et al. (132, 208) found that dysfunctional primary cilia are responsible for cystogenesis in human autosomal dominant (AD) and recessive (AR) PKD. These studies disclosed that primary cilia in the kidney epithelial cells are potentially the mechanosensors to fluid flow (132, 158, 208). During recent years, studies of primary cilia have been expanded to a spectrum of human genetic diseases, collectively termed the ciliopathies (61, 83), as well as to a few nongenetic disorders such as kidney injury, obesity, hypertension, and diabetes (125, 172) (Table 1). In addition, cilia have been proposed to function in exocytosis (11) in the ciliary pocket of the flagella and kinetoplastid protozoa (66, 126).
Table 1.
Cilia-associated human diseases and genes
| Human Diseases | Genes |
|---|---|
| Alstrom syndrome | ALMS1 |
| Asphyxiating thoracic dystrophy | IFT80, DYNC2H1, TTC21B, WDR19 |
| Bardet-Biedl syndrome | BBS1-15 |
| Joubert syndrome | TMEM67, 138, 216, 231, 237; CC2D2A, ARL13B, NPHP1, NPHP6/CEP290, INPP5E, AHI1, RPGRIP1L, CXORF5, TTC21B, KIF7, TCTN1, CEP41 |
| Kartegener syndrome | DNAI1, DNAH11, DNAH5 |
| Meckel-Gruber syndrome | TMEM67, 216; CEP290, RPGRIP1L, CC2D2A, NPHP3, TCTN2, B9D1, 2; MKS1 |
| Nephrononphthisis | NPHP1-13, NEK8, GLIS2 |
| Orofaciodigital syndrome 1 | OFD1 |
| Polycystic kidney disease | PKD1, PKD2, PKHD1 |
| Primary ciliary dyskinesis | DNAI1, 2; DNAH5, TXNDC3, DNAH11, KTU, RSPH4A, 9, LRRC50, CCDC39, 40, 103; DNAAF3, |
| Senior-Loken syndrome | NPHP1, 4, 5, 6; SDCCAG8 |
| Sensenbrenner syndrome | IFT122, IFT43, WDR19, 35 |
| Short-rib polydactyly syndrome | NEK1, DYNC2H1, WDR35 |
| Tuberous sclerosis | TSC1, TSC2 |
| von Hippel-Lindau disease | VHL |
| Diabetes | |
| Hypertension | |
| Kidney injury | |
| Obesity |
Cilia or flagella have been studied using different model systems. In addition to zebrafish, C. elegans, Xenopus laevis, and Tetrahymena, the most popular model systems are Chlamydomonas (http://labs.umassmed.edu/chlamyfp/index.php) and mammals (http://v3.ciliaproteome.org/cgi-bin/index.php). Indeed, a large body of knowledge was obtained studying Chlamydomonas. In this review, we will discuss the current status of studies of primary cilia and focus on the potential roles of cilia in kidney injury. A series of excellent reviews are available for more information about cilia and flagella (7, 122, 130, 205).
Ciliogenesis and Intraflagellar Transport
Ciliogenesis generally occurs in differentiated cells and involves a series of steps from cell cycle exit and mother centriole transformation to basal body to axoneme growth and extension. After cells exit the cell cycle, the mother centriole moves to the apical surface of the cell and acquires a series of components necessary for ciliary budding. A microtubule-based axoneme extends from the microtubule of the mature centriole/basal body, and new microtubule units are added to the distal tip by a process called intraflagellar transport (IFT) to lengthen the axoneme (68). When a certain point in its length is reached, an axoneme stops growing and starts maintaining. In most cases, the exit of the cell cycle is correlated with ciliogenesis; however, in hTERT-RPE1 cells, cell spatial confinement seems to be a major regulator of ciliogenesis (157). Convincing experiments showed that the cell cycle per se regulates cilium length (80). During cell proliferation, cilium length fluctuates in parallel with the four phases of the cell cycle (G1, S, G2, and M). In the M phase, cilia are resorbed to facilitate cell division while in the G1, S, and early G2 phases cilia can still be observed (22). The molecular mechanisms underlying ciliogenesis during the cell cycle have begun to emerge. Cdc14b phosphatase, an antagonist of Cdk1, is required for both motile and primary ciliogenesis in zebrafish in a manner independent of fibroblast growth factor (FGF) (38). Aurora A, a mitotic kinase, induces ciliary disassembly in hTERT-RPE cells (161). Cilia-associated proteins, vice versa, have been found to regulate the cell cycle. A typical example is polycystin-2 (PC2), a transmembrane protein responsible for 15% of cases of patients with ADPKD (189). PC2 has been localized to the primary cilia and is known to regulate the cell cycle (154, 198). Polaris, another protein responsible for ciliary assembly, leads to misorientation of the spindle body during mitosis and hyperproliferative kidney cysts if the normal function is disrupted (49).
Exiting the cell cycle is just the initial step for ciliogenesis. Microtubule formation and posttranslational modifications are all essential. In Chlamydomonas, tubulin levels are significantly upregulated after deflagellation (211). Sharma et al. (180) further explored the role of tubulin in mammalian cells and found that soluble cytosolic tubulin regulates cilium length. Ciliary microtubules, consisting of α- and β-tubulins, can undergo a wide range of posttranslational modifications, including acetylation, glutamylation, glycylation, ubiquitination, methylation, and phosphorylation (93). The former three are unique to the tubulin in cilia and flagella (184). It has been known that acetylation is one characteristic of α-tubulin in the cilia but not in the cytoplasm. Therefore, acetylated α-tubulin is regarded as the standard marker of cilia in cell staining. In Chlamydomonas, acetylation of α-tubulin is associated with flagellar growth and resorption (104, 105) but does not correlate with ciliary growth in the sea urchin (191). In Tetrahymena, mutated glutamylation of β-tubulin leads to abnormal axonemes and lethality (218). CEP41, an evolutionarily conserved polyglutamylase enzyme, is localized to the basal body and primary cilia, and CEP41 was very recently found to be causative of Joubert syndrome, suggesting that tubulin glutamylation is important in the pathogenesis of human ciliary diseases (102). It seems that mammalian cilia function is not dependent on the polymeric state of tubulin glycylation although monomeric glycylation is likely essential (51). TTLL3, a tubulin glycine ligase, appears important for cilia assembly, and in vivo, glutamic acid and glycine ligase oppose each other probably by competition of shared modification sites of tubulin, because in both Tetrahymena and zebrafish, deletion of TTLL3 leads to shortened cilia (217). Ubiquitination and methylation and phosphorylation of tubulin do occur in cilia but also in the cytoplasm. In Chlamydomonas, tubulin, IC2, and dynein have been found to be abnormally ubiquitinated and methylated during flagellar resorption, implying that these two posttranslational modifications are likely involved in ciliogenesis (74, 176). In addition to the effect of microtubules on ciliogenesis, microtubule tip-associated proteins such as EB1 and EB3 also regulate ciliogenesis, because depletion of these genes leads to a significant decrement of cilia number in different mammalian cells (177).
In addition to the regulation of ciliogenesis by the cell cycle and microtubules, actin dynamics is associated with ciliogenesis. Cytochalasin D and jasplakinolide are two reagents that disrupt polymerization of actin filaments, both of which facilitate the lengthening of the primary cilia in different types of cultured cells (20, 180). Bershteyn et al. (20) showed that MIM (Missing-in-Metastasis), an actin-regulatory protein, is required for ciliogenesis at the basal body of mesenchymal cells, and they proposed that MIM promotes ciliogenesis by antagonizing phosphorylation of cortactin. It has been known that cortactin is a monomeric protein and plays an important role in promoting polymerization and rearrangement of the actin cytoskeleton (46). ACTR3, an interaction protein of cortactin identified by functional screening, has been shown to increase the length of primary cilia (89). Filamin A, an actin-binding protein, has been reported to be crucial in ciliogenesis and positioning of the basal body (3), and meckelin, an interaction protein of filamin A, regulates ciliogenesis possibly by affecting the distribution of cytoplasmic stress fibers (47).
IFT has been proven to be an indispensable process for ciliogenesis and is well conserved evolutionally from C. elegans to Chlamydomonas, and to mammals. Kozminski et al. (97) first described IFT as the bidirectional movement of particles along the axoneme of flagella. Later, the same group found that the IFT process is dependent on a protein called FLA10 (96). Kinesin-II, the homolog of FLA10 in mammals, was identified to be important for both motile and primary cilia (110, 127). IFT is a two-parallel process of anterograde transport toward the tip of the axoneme and retrograde transport toward the base of the cilia. Anterograde transport is performed by the heterotrimeric kinesin-II motor protein complex (Kif3a, Kif3b, Kap), and retrograde transport is facilitated by the motor protein cytoplasmic dynein. Thus far, it has been known that IFT particles contain at least 20 polypeptides which are divided into complex A (IFT43, 121/122b, 122/122a, 139, 140, 144) and B (IFT20, 22, 25, 27, 46, 52, 54, 57/55, 70, 74/72, 80, 81, 88, 172) (40, 62, 81, 145). Proteins in complex A and B are distinct in functions because mutations of complex A proteins generally do not affect cilia assembly while mutations of complex B proteins do (162). The typical example for complex B proteins is IFT88 mutation mice, which demonstrate shortened cilia (153). Compared with complex B proteins, mutation of the complex A protein IFT140 causes PKD but does not completely prevent cilia assembly (84).
Cilia Maintenance
Once cilia are established, the next step is to maintain them. This process is also performed by IFT because no protein synthesis machinery is found in the cilia. Thus almost all ciliary components are synthesized in the cell body (167). Two models for cilium/flagellum length control have been described (7, 99, 117). The first one is the limiting-precursor model based on the hypothesis that the quantity of precursors or building blocks in one cell is limited (99). Obviously, this model cannot explain why after flagella are severed, the residues of the flagella can still regenerate to about half of their normal length (167). Although there might be reserved building blocks, the question is where they are inside the cell. Marshall et al. (117) proposed the balance point model in which it was postulated that there is continuous tubulin unit assembly and disassembly at the tip of the cilia, and which one (assembly or disassembly) predominates depends upon a set point. This means when cilia are shorter than the set point, assembly will exceed the disassembly, and when cilia are longer than the set point, disassembly will be more predominant. The intriguing question then is what determines the balance point.
Many factors (physical, chemical, and biological) have been found to modulate cilium length. The regulators of cilium length can be divided into two classes: intrinsic and extrinsic. Intrinsic factors refer to those initiated by any molecules inside the cell, and extrinsic factors point to those from the extracellular environment, while extrinsic factors most likely regulate cilium length by affecting the intrinsic ones. The intrinsic factors can be subclassified as structural and signaling molecules. Kif3a and Pitchfork are two typical examples of the former molecules. Kinzel et al. (90) found that Pitchfork regulates primary cilium disassembly, probably through activating Aurora A. Haploinsufficiency of Pitchfork leads to left-right asymmetry, heart failure, and more importantly, node cilia duplication phenotype. Among the cilium-signaling molecules, calcium and cAMP are two key players in determining cilium length. Besschetnova et al. (21) reported that forskolin and gadolinium can increase cilium length almost twofold in 3 h by activating adenylyl cyclase and decreasing intracellular calcium and subsequent PKA activation in mIMCD3, MEK, and bone mesenchymal cells. Three kinase members of the NIMA family (Nek1, 4, 8) have been known to regulate cilium length. Interestingly, loss of Nek1 shortens cilia in mice whereas loss of Nek8 results in excessively long cilia (39, 188, 195).
Researchers have used different approaches to treat cultured cells or animals and then defined the effect on cilium length. Deflection of the primary cilium by fluid shear stress can shorten its length and consequently ameliorate mechanosensitivity, which coincides with the observations seen with mutated ADPKD gene products, PC1 or PC2 (123, 132). Miyoshi et al. (123) showed that lithium elongates primary cilia in the mouse brain and in cultured NIH3T3 and neuronal cells. Simultaneously, Ou et al. (143) independently found that lithium can elongate the primary cilium length in FLS cells, rat PC12 cells, and human astrocytes and suggested that lithium elongates cilium length partially by the inhibition of adenylyl cyclase III (ACIII) and reduction of the cAMP level. Ouabain, the inhibitor of Na-K-ATPase, at a concentration of 10 nM, promotes ciliogenesis in an ERK1/2-dependent manner (101). Based on the observation that primary cilium length is increased in osteoarthritis, Wann and Knight (209) tested the effect of interleukin-1 (IL-1) on cilium length and found that fibroblasts and chondrocytes exhibited a significant increment in cilium length after incubation with IL-1. They further identified that this elongation depended upon protein kinase A.
If axoneme structure maintenance is the physical basis for cellular function, ciliary membrane proteins are essential for many signaling pathways. It has been known that a number of receptors and channels are located on the ciliary membrane, including PC1, PC2, and fibrocystin/polyductin (FPC), somatostatin receptor 3, serotonin receptor 5, platelet-derived growth factor receptor-α (PDGFRα), and components of Wnt and Hedgehog and Notch signaling pathways (1, 70, 77, 124, 140, 210). One key question is how these receptors and channels arrive at the ciliary membrane.
Three working models have been suggested for ciliary membrane protein trafficking (130). The simplest one is that membrane proteins are transported into the nearby area of the cilium and then fused with the ciliary membrane as occurs with the plasma membrane proteins because the ciliary and plasma membranes are topologically continuous and the ciliary axoneme is connected to the cytoskeleton of the cell body. However, data from a number of experiments do not support this model. For instance, expressed glycosylphosphatidylinositol-fluorescent protein (GPI-FP) is transported to the apical side of the plasma membrane but excluded from an area around the base of the primary cilia in Madin-Darby canine kidney (MDCK) cells, suggesting that there is a special structure at the base of the cilium preventing certain proteins from entering the cilium (206). This observation is further supported by ultrastructural analysis of the ciliary base (173). The second model and probably the most recognized one, is that vesicles containing membrane proteins are transported to the base of the cilium and then, together with the ciliary membrane docking proteins, gradually move to the ciliary membrane (168). This model has been supported by a number of experiments (146, 155). The third model is that membrane proteins first fuse with the plasma membrane and then the proteins move to the ciliary membrane laterally. This model originates from an observation in the Snell lab (78). Additional evidence supporting this model is Smo trafficking to the ciliary membrane (121). Indeed, considering the complexity at the ciliogenesis stage, all these three possibilities may be feasible. The route that the transported protein takes to the ciliary membrane depends on the property of the protein and the stage of ciliogenesis. For instance, at the very early stage, some ciliary membrane proteins may be transported to the preciliary membrane by the first route before the cilium starts to protrude, but disappear from the cilia of well-differentiated cells. At the late stage of ciliogenesis, proteins are transported to the ciliary membrane by the second and third models. Thus proteins on the preciliary membrane may not necessarily be the same ones present after full differentiation of the cell.
Cilia-Associated Signaling Pathways
In the past, many studies, particularly on ligand-receptor signaling pathways, have not focused on the cilia. Currently, cilia have been proven to be a hub involved in many signaling pathways relevant to development and human diseases. Thus it is necessary to reexamine these signaling molecules and determine whether and how they function in terms of cilia.
Calcium signaling.
The association between cilia and calcium has been noticed for decades but has been mainly focused on motile cilia. However, people did not know that calcium can enter into the cell through the primary ciliary membrane of renal tubular epithelial cells until PC2 was localized on this organelle (132, 154). Nauli et al. (132) found that PC1 and PC2 codistribute on the primary cilia where they regulate calcium signaling through PC2 upon fluid flow. Furthermore, PC2 can regulate calcium signaling through genetic or biochemical interactions with other molecules such as FPC (208), the inositol 1,4,5-trisphosphate receptor (107), CAML (131), and CAMK-II (170). It has been known that PC2 can form a channel protein complex with TRPC1/TRPV4 on the primary cilia (10). Because depletion of TRPV4 abolishes flow-induced calcium transients, TRPV4 was considered to be an essential component of the renal ciliary mechanosensor (94). Clearly, the presence of cilia is required for calcium signaling in ARPKD collecting duct cells (183).
Wnt signaling.
The Wnt signaling pathway is best known for its significant roles in embryonic development and cancer (15). Nineteen WNT and 10 Frizzled genes have been found in humans, and the encoded Wnt proteins comprise a large group of secreted proteins of ∼40 kDa in size (31). Based upon the effect pattern, the Wnt signaling pathway can be classified into the canonical and noncanonical types. The canonical signaling pathway exerts its roles via Wnt-Frizzled-Dishevelled-β-catenin-TCF/LEF, while the noncanonical signaling pathway functions through either Wnt-Frizzled-Dishevelled-small GTPase-cytoskeleton or Wnt-Frizzled-Dishevelled-inositol 1,4,5-trisphosphate (IP3)-calcium (15). In the canonical pathway, binding of Wnt proteins to Frizzleds induces phosphorylation of Dishevelled, which prevents β-catenin from phosphorylation by either GSK-3 and/or casein kinase 1. Consequently, stabilized β-catenin triggers downstream effects by translocation to the nucleus and complexes with LEF/TCF (Fig. 2). In the noncanonical pathway, instead of β-catenin, Dishevelled transmits its signals to downstream protein targets and then regulates small GTPases such as Rho, Rac, and Cdc42, or IP3/calcium (Fig. 2).
Fig. 2.
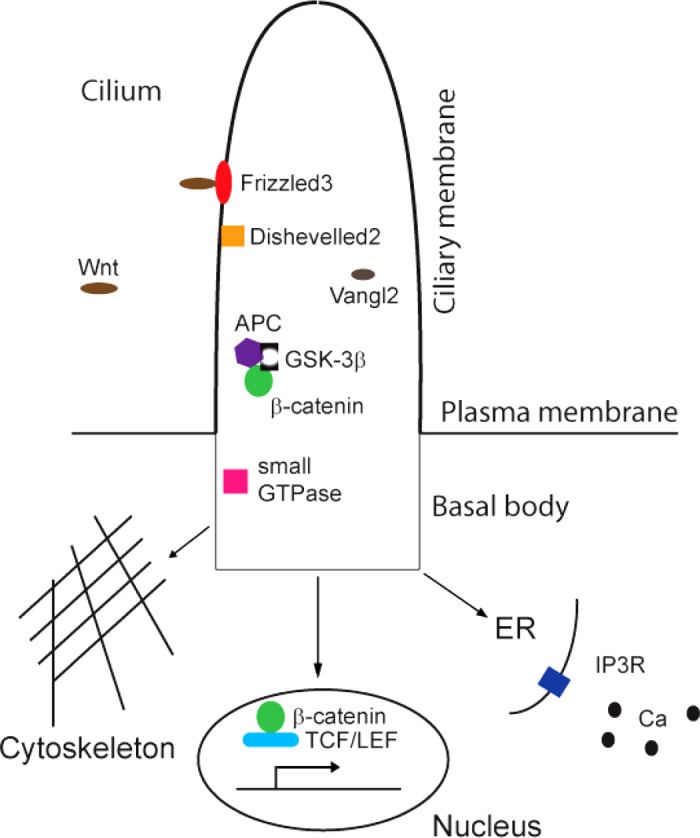
Wnt signaling pathway in the primary cilia. Binding of Wnt to Frizzled3 stabilizes β-catenin, which translocates to the nucleus and activates target genes with TCF/LEF by the canonical pathway. Instead of β-catenin, Dishevelled2 transmits signals to small GTPase or inositol 1,4,5-trisphosphate (IP3) receptor by the noncanonical pathway, which regulates the cytoskeleton and calcium respectively. ER, endoplasmic reticulum.
Several lines of evidence have revealed that the Wnt signaling pathway is coupled to cilia. Probably the earliest report about cilia and Wnt is from the study of Inversin, mutations of which lead to both NPHP and situs inversus (142). It has been identified that several Wnt signaling members [Frizzled3, Dishevelled2, adenomatous polyposis coli (APC), β-catenin, GSK-3β, Vangl2] are located to the cilia, suggesting that both canonical and noncanonical Wnt signaling cascades can occur in the ciliary area (113, 148, 169, 196). Mice with knockout of a few ciliary proteins (Kif3a, Tg737, BBS1, 4, 6) showed abnormal β-catenin level and the dysfunctional canonical Wnt responses (45, 64, 110). The PCP effector proteins Inturned and Fuzzy function importantly in cilia formation and orientation and apical actin assembly (147, 222). All these experiments point to the association of cilia and Wnt signaling. However, it is unknown why the IFT88 mutation in zebrafish displays no cilia but normal Wnt signaling (75).
Hedgehog signaling.
Hedgehog signaling was originally identified by Nusslein-Volhard and Wieschaus (137) and later was proven to be essential in cell proliferation and embryo development. Basically, Hedgehog proteins consist of three kinds of secreted molecules, i.e., Sonic Hedgehog, Indian Hedgehog, and Desert Hedgehog. The best studied is Sonic Hedgehog signaling. Sonic Hedgehog binds to the Hedgehog receptor Patched, which relieves the downstream inhibition of Smo. This then activates the Gli transcription factor. Subsequently, activated Gli trafficking to the nucleus regulates the transcription of Hedgehog target genes.
As early as 2003, the Hedgehog signaling pathway was connected to the cilium. Huangfu et al. (76) performed genetic screening and identified that Wimple, Polaris, and Kif3a are required for Hedgehog signaling in mice, and the Wimple gene was shown to be IFT172 (67). Subsequent experiments done by many groups showed that key Hedgehog components (Patched1, Smo, Gli, and Sufu) are all enriched in the cilia and/or basal body (44, 72, 166) (Fig. 3). In the absence of Hedgehog molecules, Gli proteins are inhibited by the cytoplasmic form of SuFu (Suppressor of Fused). With Hedgehog stimulation, the Gli-SuFu protein complex is quickly recruited to the cilia proximal to Smo and Gli proteins are released to enter into the nucleus to activate certain genes (199). Furthermore, Sufu controls the protein levels of Gli by antagonizing the activity of Spop, a conserved Gli-degrading factor. Interestingly, regulation of Gli by SuFu is cilium independent (36). In mammalian cells, Smo translocation to the primary cilia is necessary for Smo-dependent signaling. With knockdown of Arrestin, it was found that Smo failed to traffic to the primary cilia and Smo-dependent activation of Gli was prevented, suggesting that Arrestin plays a significant role in Hedgehog signaling (95). Very recently, IFT25 was also shown to be essential for movement of Hedgehog components (88). Interestingly, the IFT80 trap mouse exhibits short rib polydactyly syndrome and abnormality of Hedgehog signaling without malformation of cilia (165).
Fig. 3.
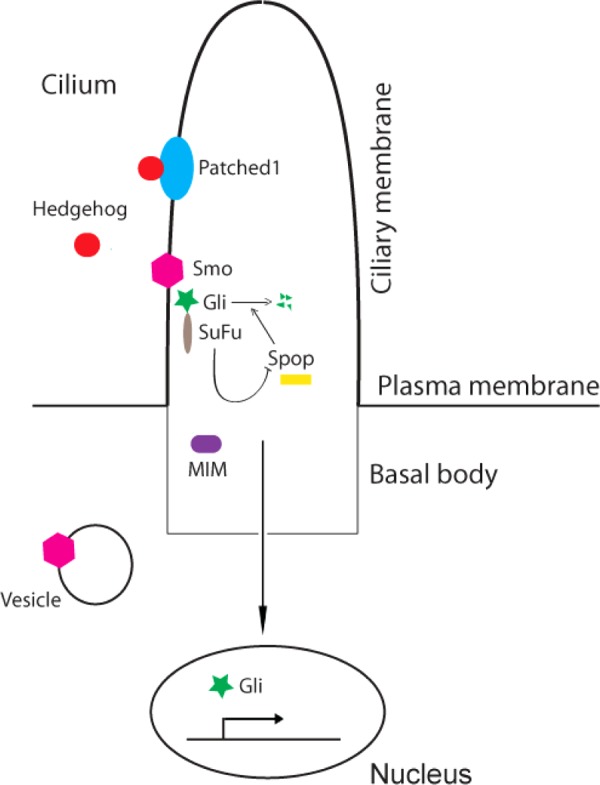
Hedgehog signaling pathway in the primary cilia. In the absence of Hedgehog, Smo is suppressed by an unclear mechanism. After Hedgehog binding to Patched1, Smo is activated and subsequently facilitates the formation of active form of Gli, which enters the nucleus to regulate target genes. SuFu and Spop are all involved in Gli trafficking and stability.
Notch signaling.
Notch signaling is best known for cell-cell communications. In mammals, Notch refers to four different kinds of receptors, from Notch1 to Notch4. The Notch receptor is a single transmembrane protein containing a large extracellular portion associated with calcium, a single membrane pass, and a short intracellular fragment. Notch protein can undergo a series of cleavages upon binding to its ligands. One of the cleaved forms enters into the nucleus to regulate gene expression. It has been reported that the Notch signaling pathway regulates left-right asymmetry by cilium length control and plays a key role in cilium length adjustment (98, 111). Lopes et al. (111) found that in deltaD zebrafish mutants, the cilium length in Kupffer's vesicle is decreased but can be restored by Foxj1a, while cilium length increases if Notch signaling is overactivated. In Tg737 and Kif3a mutant embryos, there are defects of Notch signaling and cell differentiation that can be rescued by activated Notch. Furthermore, Notch receptors are located in cilia. All these findings suggest the roles of primary cilia in the nexus of signaling, proliferation, and differentiation (53). Apart from the primary cilia, Notch signaling regulates multiple cilia formation and controls the balance of ciliated and secretory cell fates in developing airways (116, 197).
Mammalian target of rapamycin signaling.
The mammalian target of rapamycin (mTOR) was identified in 1994 by Sabatini et al. (171). mTOR is a serine/threonine protein kinase that is responsible for a number of cellular functions such as cell growth, proliferation, and survival. By binding to different protein partners, mTOR forms rapamycin-sensitive (mTORC1) and -insensitive (mTORC2) complexes. mTORC1 regulates transcription and protein synthesis through downstream S6 kinase and 4EBP1, and mTORC2 exerts its roles by Akt, Rac1, protein kinase C, and cytoskeleton (114). The detailed subcellular localization of mTOR family members is largely unclear except that Hamartin (TSC1) was localized to the basal body of the primary cilia (71). It was reported that primary cilia regulate mTORC1 activity by Lkb1 (25). Indeed, the most intensive studies about primary cilia and mTOR in the kidney field lie in the PKD-associated proteins (212). It was found that the loss of primary cilia is involved in cystogenesis and kidney hypertrophy signaling (17), and PC1 suppresses mTOR activity through regulation of Tuberin localization (50). Furthermore, mTOR inhibitors are effective for the treatment of PKD in animal models (109). Very recently, CCDC28B, a Bardet-Biedl syndrome-related protein, was reported to interact with SIN1 and modulate mTORC2 function (32).
Others.
The evidence from the zebrafish study raised the possibility that FGF signaling regulates cilium length, since knockdown of Fgfr1 causes short cilia in Kupffer's vesicles (134). Christensen et al. (37) summarized the relationship of primary cilia and receptor tyrosine kinases. PDGFRα has previously been located to the primary cilia, and in growth-arrested fibroblasts primary cilia coordinate PDGFRα-mediated cell migration (174). Furthermore, NHE1 is required for cell migration stimulated with PDGFRα (175).
Role of Cilia in Cell Polarity and Planar Cell Polarity
Recent studies have shown that cilia play important roles in cell and planar cell polarity (PCP) and left-right asymmetry (73). In epithelial cells, there are three major cell polarity protein complexes, i.e., Polarity protein (Par), Crumbs, and Scribble (29).The former two complexes define the apical polarity of the cell, and the latter one functions at the basolateral surface. Pars have been recognized as the fundamental players in animal cell polarity, in coordination with atypical protein kinase C (PKC) and CDC42. Fan et al. (55) reported that Par3, Par6, Crumbs3, atypical PKCξ, and 14-3-3η are all localized in the primary cilia of MDCK and IMCD3 cells by immunostaining and GFP-tagged target protein expression. They also found that Crumbs3, atypical PKCξ, and 14-3-3η are all required for ciliogenesis. Furthermore, they showed that Crumbs3 regulates ciliogenesis by an interaction with Importin β (54). These findings provided convincing evidence of cellular polarity roles in ciliogenesis.
Unlike single-cell polarity, PCP involves a complex coordination of a group of cells. The best studied organism is Drosophila, from which many signaling pathways, such as Wnt, Hedgehog, Notch, and small GTPases, were elucidated and found to regulate PCP (41, 57, 58, 87). Recent work regarding cilia and PCP in cystic kidney diseases has drawn much attention (60, 113, 150). By using Pck rats and HNF1β-deficient mice, Fischer et al. (60) found a distorted mitotic orientation before the onset of cystogenesis in the kidney and suggested that PCP is responsible for PKD. This discovery was further confirmed by two other groups (113, 150). In one study, Patel et al. (150) examined tubular regeneration in Kif3a mutant mice by inducing acute kidney injury and found that the loss of cilia does not promote cell proliferation but causes aberrant PCP in the precystic tubules. They concluded that primary cilia are essential for the maintenance of PCP, and cystic kidney disease is exacerbated by acute kidney injury. Another study by Luyten et al. (113) is in line with the observation of aberrant regulation of PCP in PKD. It is wroth noting that Nishio et al. (135) did not find that precystic tubular cells in Pkd1 and Pkd2 knockout mice lost oriented division but found that distorted orientation of cells in Pkhd1 mice occurred, which did not develop into kidney cysts. We do not know exactly what causes this difference, but different animal models are one factor to be considered.
Inversin, a protein mutated in nephronophthisis type II, has been regarded to play a molecular switch role between canonical and noncanonical Wnt signaling (182). The researchers found that inversin degrades cytoplasmic Dishevelled to inhibit the canonical Wnt pathway and is necessary for convergent extension in X. laevis embryos, which is regulated by noncanonical Wnt signaling. Furthermore, in Xenopus, inversin suppresses Dishevelled-induced axis duplication. Ross et al. (169) recently knocked out BBS protein in mice and found disrupted cochlear stereociliary bundles. They further provided evidence showing the genetic interaction between the PCP gene Vangl2 and the BBS genes. All these findings suggest that cilia are involved in PCP signaling. In a special form of PCP, i.e., left-right asymmetry, nodal cilia have been studied in detail (9, 69). Many proteins have been localized to the nodal cilia, and mutant mice exhibit left-right patterning defects (120). Interestingly, in mice with mutations of a few of the PCP genes, such as Fz3/6, Vangl1, Vangl2, and Dvl1/2, ciliogenesis seems normal (85, 190).
Cilia and Kidney Injury
The earliest report linking kidney injury and cilia is, to our knowledge, the study by Verghese et al. (203). By studying the relationship between kidney injury and cystogenesis in a murine Pkd1 model, Takakura et al. (192) found that inactivation of Pkd1 in the adult kidney increased cellular susceptibility to ischemic injury, which promoted cystogenesis although the cilium length in Pkd1 mutant mice was unknown. The contribution of kidney injury to cystogenesis was also confirmed in a Pkd1 haploinsufficiency model by Bastos et al. (14) and in Pkd2 heterozygous kidneys, in which more neutrophils and macrophages were detected, followed by interstitial inflammation and fibrosis (159). Zhou et al. (224) further explored the mechanism of cystogenesis underlying kidney injury by use of Cys1cpk/cpk mice. They found that induction of heme oxygenase (HO) ameliorated both kidney injury and cystogenesis while inhibition of HO enhanced cystogenesis. Furthermore, component 3 strongly correlated with cystogenesis. All these findings confirmed that kidney injury does play a certain role in cystogenesis, although insightful detailed mechanisms are not fully clear.
Since cilium length regulation and kidney injury bear some common characteristics in cell proliferation, differentiation, and cell death, it would be intriguing to investigate the functional roles of cilia in injured kidneys. Verghese et al. (202, 203) have studied cilium length of kidney tubular epithelial cells in both humans and mice. After ischemia-reperfusion kidney injury in mice, the average length of renal cilia in the proximal tubule decreased at days 1 and 2 (∼3 μm) compared with the control (∼4 μm). During the kidney repair stage at days 4 and 7, the average length of cilia increased in both proximal (∼6 μm day 7) and distal tubule/collecting duct (∼5.5 μm day 7). In a unilateral ureteral obstruction model, at day 8 cilium length in the distal tubule/collecting duct was also lengthened. Thus it was proposed that cilia may play important roles in sensing environmental cues caused by injury and in the repair process for reestablishing a new epithelial layer of differentiated cells. The finding that cilium length was lengthened in the recovery stage was further confirmed in human renal transplants suffering from acute tubular necrosis. By using series biopsies of human renal transplants, it was found that acute tubular necrosis caused more than twofold longer cilia 1 wk after kidney injury, and normalization of cilium length occurred at a late stage. These results indicate that cilium length could be a clinically relevant indicator of kidney injury and repair in patients with kidney transplantation. To further investigate the mechanisms, cultured MDCK cells were treated with bovine serum albumin, cobalt chloride, and tumor necrosis factor-α. Cilium length was only increased in cells treated with cobalt chloride. Because cobalt chloride is a chemical inducer of hypoxia-inducible factor 1α (HIF-1α), HIF-1α may be a regulator of cilium length following renal injury (204). However, data from Lutz and Burk (112) using renal-derived cells did not support this hypothesis. Other indirect evidence is from a study in murine models of PKD and normal ischemic kidneys, in which HIF-1α is upregulated (16, 52). The function of HIF-1α in kidney injury-associated cilium length change is yet to be determined.
The mechanisms for cilium length regulation are probably not the same during early and late phases of kidney injury: cilia retract in the injury phase, while they elongate in the repair phase and then gradually return to normal. It is not surprising that at the early stage of kidney injury, cilia are shortened especially after exposure to different sorts of toxic substances such as ochratoxin A and cisplatin although the mechanism for cilia resorption remains unsolved (163, 207). Many signaling pathways, such as MAPK, p53, reactive oxygen species, NF-κB, AMPK, mTOR, and Lkb1, and many key molecules, such as ATP, interleukins, TNF-α and -β, toll-like receptor 2 and 4, heme oxygenase, and heat shock proteins, are all involved in kidney injury and should be the candidates responsible for cilia resorption. Indeed, some of them have been found to regulate primary cilia (Table 2). For instance, it has been reported that heat shock protein 90 and HDAC6 coordinately regulate cilia resorption in response to extracellular stress (160). In addition, urine flow blockage, augmentation of extracellular pressure in the urogenital tract, cell death, and de-differentiation may also be involved in cilia shortening although which is the earliest event is unknown. Takakura et al. (193) reported the sustained activation of STAT3 in ischemic-injured and uninjured Pkd1 knockout polycystic kidneys and in human ADPKD kidneys, and Olsan et al. (138) found the role of STAT6 in renal cyst growth. These two studies suggest that the STAT signaling pathway may be involved in cilium length regulation upon kidney injury. One interesting report is from the study of kidney injury in Kif3a knockout mice, in which PCP was abnormal before cystogenesis (150). We know that these Kif3a knockout mice harbor very short cilia in the kidney epithelial cells and that Wnt signaling plays a significant role in PCP. However, the question remains as to how short cilia affect PCP through cell polarity and PCP molecules. The elongation of cilia during tubular cell regeneration may not only increase the ability of cilia to sense extracellular cues but may also facilitate the secretion of metabolic wastes from cilia, which are generated during the kidney injury stage. Indeed, cilia have been regarded as a secretary organelle (11). We (207) and others (2) very recently found that Erk1/2 regulates cilia length in renal tubular cells and endothelial cells. The relationship between cilia and many more kidney injury-associated molecules needs to be further elucidated.
Table 2.
Cilia formation and maintenance-associated factors
| Factors (References) | |||
|---|---|---|---|
| Intrinsic | |||
| ACIII (143) | ACTR3 (89) | Arl-3 (106) | Arl-13 (106) |
| AurA (161) | BBS9 (201) | Bromi (91) | Ca+2 (21) |
| cAMP (2, 21) | Cc2d2a (63) | CCDC28B (32) | Cdc14b (38) |
| Cdc42 (226) | Cep41 (102) | Cep70 (214) | Cep89/Cep123 (181) |
| Cep131 (214) | CEP135/BLD10 (33) | CLUAP1 (149) | Cnk2p (27) |
| Collectrin (223) | D2lic (26) | Daf-19 (156) | DCDC2 (119) |
| Dlic1 (92) | DR5 (2) | Dyf-5 (30) | Dynein-2 (5, 164) |
| DYX1C1 (34) | EB1 (177) | EB3 (177) | FGFR1 (134) |
| Filamin A (3) | FOP (103) | Fuzzy (147) | GSK3β (123, 215) |
| HDAC6 (161) | HFH-4 (35) | HSP90 (160) | IFT46 (100) |
| IFT54/DYF-11/TRAF3IP1 (18) | IFT70 (56) | IFT88 (153) | VDAC |
| IFT140 (84) | INPP5E (42) | Inturned (147, 222) | LmxMKK (213) |
| Iqub (100) | KIF3A (118) | KIF3B (136) | Kinesin-13 (24, 48) |
| Klp-6 (128) | LF2p (6, 194) | LF4p (6, 19) | MAK (139) |
| MAP4 (65) | mC21orf2 (100) | Meckelin (47) | MIM (20) |
| Nek1 (195) | Nek4 (39) | Nek8 (186, 188) | Nphp-8 (151) |
| Nrk2p (216) | Nrk17p (216) | Nrk30p (216) | OCRL1 (43) |
| OFD1 (59) | PHLP2 (28) | Pitchfork (90) | PKC (2) |
| Ptpdc1 (100) | Rab GTPase (220) | Rer1p (86) | RFX3 (4) |
| RP1 (139) | S6K1 (221) | Septin (65) | Ssa2 (100) |
| Tctex-1 (144) | Tctn1 (63) | Tctn2 (63) | Tmem67 (63) |
| Torc1 (221) | Traf3ip (18) | TSC1 (71, 221) | TSC2 (71) |
| TTLL3 (217) | VDAC (115) | ||
| Extrinsic | |||
| Cytochalasin D (180) | Forskolin (180) | Gadolinium (21) | Hypoxia (204) |
| Interleukin-1 (209) | Jasplakinolide (180) | KCl (123) | Li2CO3, LiCl (123, 143) |
| LY294002 (129, 178, 200, 225) | Ochratoxin A (163) | ||
| Ouabain (101) | Potassium-bromated (163) | Rapamycin (221) | |
| Taxol (180) | U0126 (2, 178, 207) |
Conclusions and Perspectives
Taken together, we conclude that the primary cilium is an important organelle responsible for integrative signaling from outside cues to normal physiological functions of cells. Mutations of ciliary proteins cause different kinds of human diseases displaying a diversity of clinical features. Probably a larger spectrum of human ciliopathic diseases is related to primary cilia than expected originally.
However, a large number of questions remain to be answered. One basic question is why a majority of, but not all, cells grow cilia. Although different approaches have been used to study the composition of cilia or flagella in different model organisms (8, 12, 23, 141, 152), we have just begun to know the composition of primary cilia (82). With regard to cilia and kidney injury, the key question is the role of primary cilia during kidney injury and recovery stages. Once the detailed mechanisms are determined, we can design different strategies to interfere with the process of kidney injury recovery in animal models by regulating cilia-associated signaling pathways. It is expected that studies on cilia and kidney injury will shed light on identifying novel mechanisms that can be translated into clinical treatment.
GRANTS
This work was supported in part by the National Basic Research Program of China 973, Program No. 2012CB517600 (No. 2012CB517606) and grants from the National Institutes of Health and US Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.W. and Z.D. provided conception and design of research; S.W. prepared figures; S.W. and Z.D. drafted manuscript; S.W. and Z.D. edited and revised manuscript; S.W. and Z.D. approved final version of manuscript.
REFERENCES
- 1.Anonymous. Polycystic kidney disease: the complete structure of the PKD1 gene, and its protein. The International Polycystic Kidney Disease Consortium. Cell 81: 289–298, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Majeed S, Moloney BC, Nauli SM. Mechanisms regulating cilia growth and cilia function in endothelial cells. Cell Mol Life Sci 69: 165–173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams M, Simms RJ, Abdelhamed Z, Dawe HR, Szymanska K, Logan CV, Wheway G, Pitt E, Gull K, Knowles MA, Blair E, Cross SH, Sayer JA, Johnson CA. A meckelin-filamin A interaction mediates ciliogenesis. Hum Mol Genet 21: 1272–1286, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ait-Lounis A, Baas D, Barras E, Benadiba C, Charollais A, Nlend Nlend R, Liegeois D, Meda P, Durand B, Reith W. Novel function of the ciliogenic transcription factor RFX3 in development of the endocrine pancreas. Diabetes 56: 950–959, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Asai DJ, Rajagopalan V, Wilkes DE. Dynein-2 and ciliogenesis in Tetrahymena. Cell Motil Cytoskeleton 66: 673–677, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Asleson CM, Lefebvre PA. Genetic analysis of flagellar length control in Chlamydomonas reinhardtii: a new long-flagella locus and extragenic suppressor mutations. Genetics 148: 693–702, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avasthi P, Marshall WF. Stages of ciliogenesis and regulation of ciliary length. Differentiation 83: S30–S42, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell 117: 527–539, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Aw S, Levin M. Is left-right asymmetry a form of planar cell polarity? Development 136: 355–366, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep 9: 472–479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldari CT, Rosenbaum J. Intraflagellar transport: it's not just for cilia anymore. Curr Opin Cell Biol 22: 75–80, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baron DM, Ralston KS, Kabututu ZP, Hill KL. Functional genomics in Trypanosoma brucei identifies evolutionarily conserved components of motile flagella. J Cell Sci 120: 478–491, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 401: 386–389, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Bastos AP, Piontek K, Silva AM, Martini D, Menezes LF, Fonseca JM, Fonseca II, Germino GG, Onuchic LF. Pkd1 haploinsufficiency increases renal damage and induces microcyst formation following ischemia/reperfusion. J Am Soc Nephrol 20: 2389–2402, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayly R, Axelrod JD. Pointing in the right direction: new developments in the field of planar cell polarity. Nat Rev Genet 12: 385–391, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belibi F, Zafar I, Ravichandran K, Segvic AB, Jani A, Ljubanovic DG, Edelstein CL. Hypoxia-inducible factor-1α (HIF-1α) and autophagy in polycystic kidney disease (PKD). Am J Physiol Renal Physiol 300: F1235–F1243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell PD, Fitzgibbon W, Sas K, Stenbit AE, Amria M, Houston A, Reichert R, Gilley S, Siegal GP, Bissler J, Bilgen M, Chou PC, Guay-Woodford L, Yoder B, Haycraft CJ, Siroky B. Loss of primary cilia upregulates renal hypertrophic signaling and promotes cystogenesis. J Am Soc Nephrol 22: 839–848, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berbari NF, Kin NW, Sharma N, Michaud EJ, Kesterson RA, Yoder BK. Mutations in Traf3ip1 reveal defects in ciliogenesis, embryonic development, and altered cell size regulation. Dev Biol 360: 66–76, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berman SA, Wilson NF, Haas NA, Lefebvre PA. A novel MAP kinase regulates flagellar length in Chlamydomonas. Curr Biol 13: 1145–1149, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Bershteyn M, Atwood SX, Woo WM, Li M, Oro AE. MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev Cell 19: 270–283, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr Biol 20: 182–187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettencourt-Dias M, Carvalho-Santos Z. Double life of centrioles: CP110 in the spotlight. Trends Cell Biol 18: 8–11, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Blacque OE, Perens EA, Boroevich KA, Inglis PN, Li C, Warner A, Khattra J, Holt RA, Ou G, Mah AK, McKay SJ, Huang P, Swoboda P, Jones SJ, Marra MA, Baillie DL, Moerman DG, Shaham S, Leroux MR. Functional genomics of the cilium, a sensory organelle. Curr Biol 15: 935–941, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Blaineau C, Tessier M, Dubessay P, Tasse L, Crobu L, Pages M, Bastien P. A novel microtubule-depolymerizing kinesin involved in length control of a eukaryotic flagellum. Curr Biol 17: 778–782, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Boehlke C, Kotsis F, Patel V, Braeg S, Voelker H, Bredt S, Beyer T, Janusch H, Hamann C, Godel M, Muller K, Herbst M, Hornung M, Doerken M, Kottgen M, Nitschke R, Igarashi P, Walz G, Kuehn EW. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol 12: 1115–1122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonnafe E, Touka M, AitLounis A, Baas D, Barras E, Ucla C, Moreau A, Flamant F, Dubruille R, Couble P, Collignon J, Durand B, Reith W. The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Mol Cell Biol 24: 4417–4427, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley BA, Quarmby LM. A NIMA-related kinase, Cnk2p, regulates both flagellar length and cell size in Chlamydomonas. J Cell Sci 118: 3317–3326, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Bregier C, Krzemien-Ojak L, Wloga D, Jerka-Dziadosz M, Joachimiak E, Batko K, Filipiuk I, Smietanka U, Gaertig J, Fabczak S, Fabczak H. PHLP2 is essential and plays a role in ciliogenesis and microtubule assembly in the ciliate Tetrahymena thermophila. J Cell Physiol 228: 2175–2189, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol 9: 887–901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burghoorn J, Dekkers MP, Rademakers S, de Jong T, Willemsen R, Swoboda P, Jansen G. Dauer pheromone and G-protein signaling modulate the coordination of intraflagellar transport kinesin motor proteins in C. elegans. J Cell Sci 123: 2077–2084, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev 11: 3286–3305, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Cardenas-Rodriguez M, Irigoin F, Osborn DP, Gascue C, Katsanis N, Beales PL, Badano JL. The Bardet-Biedl syndrome-related protein CCDC28B modulates mTORC2 function and interacts with SIN1 to control cilia length independently of the mTOR complex. Hum Mol Genet [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carvalho-Santos Z, Machado P, Alvarez-Martins I, Gouveia SM, Jana SC, Duarte P, Amado T, Branco P, Freitas MC, Silva ST, Antony C, Bandeiras TM, Bettencourt-Dias M. BLD10/CEP135 is a microtubule-associated protein that controls the formation of the flagellum central microtubule pair. Dev Cell 23: 412–424, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Chandrasekar G, Vesterlund L, Hultenby K, Tapia-Paez I, Kere J. The zebrafish orthologue of the dyslexia candidate gene DYX1C1 is essential for cilia growth and function. PLoS One 8: e63123, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Knowles HJ, Hebert JL, Hackett BP. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J Clin Invest 102: 1077–1082, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen MH, Wilson CW, Li YJ, Law KK, Lu CS, Gacayan R, Zhang X, Hui CC, Chuang PT. Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev 23: 1910–1928, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christensen ST, Clement CA, Satir P, Pedersen LB. Primary cilia and coordination of receptor tyrosine kinase (RTK) signalling. J Pathol 226: 172–184, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clement A, Solnica-Krezel L, Gould KL. The Cdc14B phosphatase contributes to ciliogenesis in zebrafish. Development 138: 291–302, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coene KL, Mans DA, Boldt K, Gloeckner CJ, van Reeuwijk J, Bolat E, Roosing S, Letteboer SJ, Peters TA, Cremers FP, Ueffing M, Roepman R. The ciliopathy-associated protein homologs RPGRIP1 and RPGRIP1L are linked to cilium integrity through interaction with Nek4 serine/threonine kinase. Hum Mol Genet 20: 3592–3605, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol 141: 993–1008, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colosimo PF, Tolwinski NS. Wnt, Hedgehog and junctional Armadillo/beta-catenin establish planar polarity in the Drosophila embryo. PLoS One 1: e9, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conduit SE, Dyson JM, Mitchell CA. Inositol polyphosphate 5-phosphatases; new players in the regulation of cilia and ciliopathies. FEBS Lett 586: 2846–2857, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Coon BG, Hernandez V, Madhivanan K, Mukherjee D, Hanna CB, Barinaga-Rementeria Ramirez I., Lowe M, Beales PL, Aguilar RC. The Lowe syndrome protein OCRL1 is involved in primary cilia assembly. Hum Mol Genet 21: 1835–1847, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature 437: 1018–1021, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol 10: 70–76, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Cosen-Binker LI, Kapus A. Cortactin: the gray eminence of the cytoskeleton. Physiology (Bethesda) 21: 352–361, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Dawe HR, Adams M, Wheway G, Szymanska K, Logan CV, Noegel AA, Gull K, Johnson CA. Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. J Cell Sci 122: 2716–2726, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dawson SC, Sagolla MS, Mancuso JJ, Woessner DJ, House SA, Fritz-Laylin L, Cande WZ. Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in Giardia intestinalis. Eukaryot Cell 6: 2354–2364, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delaval B, Bright A, Lawson ND, Doxsey S. The cilia protein IFT88 is required for spindle orientation in mitosis. Nat Cell Biol 13: 461–468, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dere R, Wilson PD, Sandford RN, Walker CL. Carboxy terminal tail of polycystin-1 regulates localization of TSC2 to repress mTOR. PLoS One 5: e9239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dossou SJ, Bre MH, Hallworth R. Mammalian cilia function is independent of the polymeric state of tubulin glycylation. Cell Motil Cytoskeleton 64: 847–855, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eickelberg O, Seebach F, Riordan M, Thulin G, Mann A, Reidy KH, Van Why SK, Kashgarian M, Siegel N. Functional activation of heat shock factor and hypoxia-inducible factor in the kidney. J Am Soc Nephrol 13: 2094–2101, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell 145: 1129–1141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan S, Fogg V, Wang Q, Chen XW, Liu CJ, Margolis B. A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin beta interactions. J Cell Biol 178: 387–398, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan S, Hurd TW, Liu CJ, Straight SW, Weimbs T, Hurd EA, Domino SE, Margolis B. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr Biol 14: 1451–1461, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Fan ZC, Behal RH, Geimer S, Wang Z, Williamson SM, Zhang H, Cole DG, Qin H. Chlamydomonas IFT70/CrDYF-1 is a core component of IFT particle complex B and is required for flagellar assembly. Mol Biol Cell 21: 2696–2706, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fanto M, Mlodzik M. Asymmetric Notch activation specifies photoreceptors R3 and R4 and planar polarity in the Drosophila eye. Nature 397: 523–526, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Fanto M, Weber U, Strutt DI, Mlodzik M. Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr Biol 10: 979–988, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Ferrante MI, Zullo A, Barra A, Bimonte S, Messaddeq N, Studer M, Dolle P, Franco B. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet 38: 112–117, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet 38: 21–23, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol 8: 880–893, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Follit JA, Xu F, Keady BT, Pazour GJ. Characterization of mouse IFT complex B. Cell Motil Cytoskeleton 66: 457–468, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, Garcia-Verdugo JM, Katsanis N, Hildebrandt F, Reiter JF. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet 43: 776–784, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, Badano JL, Katsanis N. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet 39: 1350–1360, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Ghossoub R, Hu Q, Failler M, Rouyez MC, Spitzbarth B, Mostowy S, Wolfrum U, Saunier S, Cossart P, Nelson WJ, Benmerah A. Septins 2, 7, and 9 and MAP4 co-localize along the axoneme in the primary cilium and control ciliary length. J Cell Sci 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghossoub R, Molla-Herman A, Bastin P, Benmerah A. The ciliary pocket: a once-forgotten membrane domain at the base of cilia. Biol Cell 103: 131–144, 2011 [DOI] [PubMed] [Google Scholar]
- 67.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11: 331–344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graser S, Stierhof YD, Lavoie SB, Gassner OS, Lamla S, Le Clech M, Nigg EA. Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol 179: 321–330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamada H, Meno C, Watanabe D, Saijoh Y. Establishment of vertebrate left-right asymmetry. Nat Rev Genet 3: 103–113, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Harris PC, Germino G, Klinger K, Landes G, van Adelsberg J. The PKD1 gene product. Nat Med 1: 493, 1995 [DOI] [PubMed] [Google Scholar]
- 71.Hartman TR, Liu D, Zilfou JT, Robb V, Morrison T, Watnick T, Henske EP. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet 18: 151–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1: e53, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirokawa N, Tanaka Y, Okada Y. Cilia, KIF3 molecular motor and nodal flow. Curr Opin Cell Biol 24: 31–39, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Huang K, Diener DR, Rosenbaum JL. The ubiquitin conjugation system is involved in the disassembly of cilia and flagella. J Cell Biol 186: 601–613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development 136: 3089–3098, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426: 83–87, 2003 [DOI] [PubMed] [Google Scholar]
- 77.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, Gamble V, Harris PC. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet 10: 151–160, 1995 [DOI] [PubMed] [Google Scholar]
- 78.Hunnicutt GR, Kosfiszer MG, Snell WJ. Cell body and flagellar agglutinins in Chlamydomonas reinhardtii: the cell body plasma membrane is a reservoir for agglutinins whose migration to the flagella is regulated by a functional barrier. J Cell Biol 111: 1605–1616, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iftode F, Fleury-Aubusson A. Structural inheritance in paramecium: ultrastructural evidence for basal body and associated rootlets polarity transmission through binary fission. Biol Cell 95: 39–51, 2003 [DOI] [PubMed] [Google Scholar]
- 80.Irigoin F, Badano JL. Keeping the balance between proliferation and differentiation: the primary cilium. Curr Genomics 12: 285–297, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ishikawa H, Marshall WF. Ciliogenesis: building the cell's antenna. Nat Rev Mol Cell Biol 12: 222–234, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Ishikawa H, Thompson J, Yates JR, 3rd, Marshall WF. Proteomic analysis of mammalian primary cilia. Curr Biol 22: 414–419, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jagger DJ, Forge A. Assessing PCP in the cochlea of mammalian ciliopathy models. Methods Mol Biol 839: 239–248, 2012 [DOI] [PubMed] [Google Scholar]
- 84.Jonassen JA, SanAgustin J, Baker SP, Pazour GJ. Disruption of IFT complex A causes cystic kidneys without mitotic spindle misorientation. J Am Soc Nephrol 23: 641–651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones C, Chen P. Planar cell polarity signaling in vertebrates. Bioessays 29: 120–132, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jurisch-Yaksi N, Rose AJ, Lu H, Raemaekers T, Munck S, Baatsen P, Baert V, Vermeire W, Scales SJ, Verleyen D, Vandepoel R, Tylzanowski P, Yaksi E, de Ravel T, Yost HJ, Froyen G, Arrington CB, Annaert W. Rer1p maintains ciliary length and signaling by regulating gamma-secretase activity and Foxj1a levels. J Cell Biol 200: 709–720, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet 41: 793–799, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keady BT, Samtani R, Tobita K, Tsuchya M, San Agustin JT, Follit JA, Jonassen JA, Subramanian R, Lo CW, Pazour GJ. IFT25 links the signal-dependent movement of hedgehog components to intraflagellar transport. Dev Cell 22: 940–951, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464: 1048–1051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kinzel D, Boldt K, Davis EE, Burtscher I, Trumbach D, Diplas B, Attie-Bitach T, Wurst W, Katsanis N, Ueffing M, Lickert H. Pitchfork regulates primary cilia disassembly and left-right asymmetry. Dev Cell 19: 66–77, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ko HW, Norman RX, Tran J, Fuller KP, Fukuda M, Eggenschwiler JT. Broad-minded links cell cycle-related kinase to cilia assembly and hedgehog signal transduction. Dev Cell 18: 237–247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kong S, Du X, Peng C, Wu Y, Li H, Jin X, Hou L, Deng K, Xu T, Tao W. Dlic1 deficiency impairs ciliogenesis of photoreceptors by destabilizing dynein. Cell Res 23: 972, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Konno A, Setou M, Ikegami K. Ciliary and flagellar structure and function-their regulations by posttranslational modifications of axonemal tubulin. Int Rev Cell Mol Biol 294: 133–170, 2012 [DOI] [PubMed] [Google Scholar]
- 94.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science 320: 1777–1781, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol 131: 1517–1527, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA 90: 5519–5523, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krebs LT, Iwai N, Nonaka S, Welsh IC, Lan Y, Jiang R, Saijoh Y, O'Brien TP, Hamada H, Gridley T. Notch signaling regulates left-right asymmetry determination by inducing Nodal expression. Genes Dev 17: 1207–1212, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuchka MR, Jarvik JW. Analysis of flagellar size control using a mutant of Chlamydomonas reinhardtii with a variable number of flagella. J Cell Biol 92: 170–175, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lai CK, Gupta N, Wen X, Rangell L, Chih B, Peterson AS, Bazan JF, Li L, Scales SJ. Functional characterization of putative cilia genes by high-content analysis. Mol Biol Cell 22: 1104–1119, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Larre I, Castillo A, Flores-Maldonado C, Contreras RG, Galvan I, Munoz-Estrada J, Cereijido M. Ouabain modulates ciliogenesis in epithelial cells. Proc Natl Acad Sci USA 108: 20591–20596, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee JE, Silhavy JL, Zaki MS, Schroth J, Bielas SL, Marsh SE, Olvera J, Brancati F, Iannicelli M, Ikegami K, Schlossman AM, Merriman B, Attie-Bitach T, Logan CV, Glass IA, Cluckey A, Louie CM, Lee JH, Raynes HR, Rapin I, Castroviejo IP, Setou M, Barbot C, Boltshauser E, Nelson SF, Hildebrandt F, Johnson CA, Doherty DA, Valente EM, Gleeson JG. CEP41 is mutated in Joubert syndrome and is required for tubulin glutamylation at the cilium. Nat Genet 44: 193–199, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee JY, Stearns T. FOP is a centriolar satellite protein involved in ciliogenesis. PLoS One 8: e58589, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.L'Hernault SW, Rosenbaum JL. Chlamydomonas alpha-tubulin is posttranslationally modified in the flagella during flagellar assembly. J Cell Biol 97: 258–263, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.L'Hernault SW, Rosenbaum JL. Reversal of the posttranslational modification on Chlamydomonas flagellar alpha-tubulin occurs during flagellar resorption. J Cell Biol 100: 457–462, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol 189: 1039–1051, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Y, Wright JM, Qian F, Germino GG, Guggino WB. Polycystin 2 interacts with type I inositol 1, 4, 5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J Biol Chem 280: 41298–41306, 2005 [DOI] [PubMed] [Google Scholar]
- 108.Lidow MS, Menco BP. Observations on axonemes and membranes of olfactory and respiratory cilia in frogs and rats using tannic acid-supplemented fixation and photographic rotation. J Ultrastruct Res 86: 18–30, 1984 [DOI] [PubMed] [Google Scholar]
- 109.Lieberthal W, Levine JS. Mammalian target of rapamycin and the kidney. II. Pathophysiology and therapeutic implications. Am J Physiol Renal Physiol 303: F180–F191, 2012 [DOI] [PubMed] [Google Scholar]
- 110.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA 100: 5286–5291, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lopes SS, Lourenco R, Pacheco L, Moreno N, Kreiling J, Saude L. Notch signalling regulates left-right asymmetry through ciliary length control. Development 137: 3625–3632, 2010 [DOI] [PubMed] [Google Scholar]
- 112.Lutz MS, Burk RD. Primary cilium formation requires von Hippel-Lindau gene function in renal-derived cells. Cancer Res 66: 6903–6907, 2006 [DOI] [PubMed] [Google Scholar]
- 113.Luyten A, Su X, Gondela S, Chen Y, Rompani S, Takakura A, Zhou J. Aberrant regulation of planar cell polarity in polycystic kidney disease. J Am Soc Nephrol 21: 1521–1532, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318, 2009 [DOI] [PubMed] [Google Scholar]
- 115.Majumder S, Fisk HA. VDAC3 and Mps1 negatively regulate ciliogenesis. Cell Cycle 12: 849–858, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marcet B, Chevalier B, Coraux C, Kodjabachian L, Barbry P. MicroRNA-based silencing of Delta/Notch signaling promotes multiple cilia formation. Cell Cycle 10: 2858–2864, 2011 [DOI] [PubMed] [Google Scholar]
- 117.Marshall WF, Rosenbaum JL. Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J Cell Biol 155: 405–414, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci USA 96: 5043–5048, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Massinen S, Hokkanen ME, Matsson H, Tammimies K, Tapia-Paez I, Dahlstrom-Heuser V, Kuja-Panula J, Burghoorn J, Jeppsson KE, Swoboda P, Peyrard-Janvid M, Toftgard R, Castren E, Kere J. Increased expression of the dyslexia candidate gene DCDC2 affects length and signaling of primary cilia in neurons. PLoS One 6: e20580, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114: 61–73, 2003 [DOI] [PubMed] [Google Scholar]
- 121.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol 187: 365–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miyoshi K, Kasahara K, Miyazaki I, Asanuma M. Factors that influence primary cilium length. Acta Med Okayama 65: 279–285, 2011 [DOI] [PubMed] [Google Scholar]
- 123.Miyoshi K, Kasahara K, Miyazaki I, Asanuma M. Lithium treatment elongates primary cilia in the mouse brain and in cultured cells. Biochem Biophys Res Commun 388: 757–762, 2009 [DOI] [PubMed] [Google Scholar]
- 124.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339–1342, 1996 [DOI] [PubMed] [Google Scholar]
- 125.Mok CA, Heon E, Zhen M. Ciliary dysfunction and obesity. Clin Genet 77: 18–27, 2010 [DOI] [PubMed] [Google Scholar]
- 126.Molla-Herman A, Ghossoub R, Blisnick T, Meunier A, Serres C, Silbermann F, Emmerson C, Romeo K, Bourdoncle P, Schmitt A, Saunier S, Spassky N, Bastin P, Benmerah A. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J Cell Sci 123: 1785–1795, 2010 [DOI] [PubMed] [Google Scholar]
- 127.Morris RL, Scholey JM. Heterotrimeric kinesin-II is required for the assembly of motile 9+2 ciliary axonemes on sea urchin embryos. J Cell Biol 138: 1009–1022, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morsci NS, Barr MM. Kinesin-3 KLP-6 regulates intraflagellar transport in male-specific cilia of Caenorhabditis elegans. Curr Biol 21: 1239–1244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129: 1201–1213, 2007 [DOI] [PubMed] [Google Scholar]
- 130.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol 26: 59–87, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nagano J, Kitamura K, Hujer KM, Ward CJ, Bram RJ, Hopfer U, Tomita K, Huang C, Miller RT. Fibrocystin interacts with CAML, a protein involved in Ca2+ signaling. Biochem Biophys Res Commun 338: 880–889, 2005 [DOI] [PubMed] [Google Scholar]
- 132.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 133.Nauli SM, Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays 26: 844–856, 2004 [DOI] [PubMed] [Google Scholar]
- 134.Neugebauer JM, Amack JD, Peterson AG, Bisgrove BW, Yost HJ. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature 458: 651–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nishio S, Tian X, Gallagher AR, Yu Z, Patel V, Igarashi P, Somlo S. Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol 21: 295–302, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95: 829–837, 1998 [DOI] [PubMed] [Google Scholar]
- 137.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 287: 795–801, 1980 [DOI] [PubMed] [Google Scholar]
- 138.Olsan EE, Mukherjee S, Wulkersdorfer B, Shillingford JM, Giovannone AJ, Todorov G, Song X, Pei Y, Weimbs T. Signal transducer and activator of transcription-6 (STAT6) inhibition suppresses renal cyst growth in polycystic kidney disease. Proc Natl Acad Sci USA 108: 18067–18072, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Omori Y, Chaya T, Katoh K, Kajimura N, Sato S, Muraoka K, Ueno S, Koyasu T, Kondo M, Furukawa T. Negative regulation of ciliary length by ciliary male germ cell-associated kinase (Mak) is required for retinal photoreceptor survival. Proc Natl Acad Sci USA 107: 22671–22676, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schoneborn S, Mrug M, Sweeney W, Avner ED, Zerres K, Guay-Woodford LM, Somlo S, Germino GG. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet 70: 1305–1317, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ostrowski LE, Blackburn K, Radde KM, Moyer MB, Schlatzer DM, Moseley A, Boucher RC. A proteomic analysis of human cilia: identification of novel components. Mol Cell Proteomics 1: 451–465, 2002 [DOI] [PubMed] [Google Scholar]
- 142.Otto EA, Schermer B, Obara T, O'Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, Foreman JW, Goodship JA, Strachan T, Kispert A, Wolf MT, Gagnadoux MF, Nivet H, Antignac C, Walz G, Drummond IA, Benzing T, Hildebrandt F. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 34: 413–420, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ou Y, Ruan Y, Cheng M, Moser JJ, Rattner JB, van der Hoorn FA. Adenylate cyclase regulates elongation of mammalian primary cilia. Exp Cell Res 315: 2802–2817, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Palmer KJ, MacCarthy-Morrogh L, Smyllie N, Stephens DJ. A role for Tctex-1 (DYNLT1) in controlling primary cilium length. Eur J Cell Biol 90: 865–871, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pan J, Wang Q, Snell WJ. Cilium-generated signaling and cilia-related disorders. Lab Invest 85: 452–463, 2005 [DOI] [PubMed] [Google Scholar]
- 146.Papermaster DS, Schneider BG, Besharse JC. Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Invest Ophthalmol Vis Sci 26: 1386–1404, 1985 [PubMed] [Google Scholar]
- 147.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet 38: 303–311, 2006 [DOI] [PubMed] [Google Scholar]
- 148.Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet 40: 871–879, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pasek RC, Berbari NF, Lewis WR, Kesterson RA, Yoder BK. Mammalian Clusterin associated protein 1 is an evolutionarily conserved protein required for ciliogenesis. Cilia 1: 20, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Patzke S, Redick S, Warsame A, Murga-Zamalloa CA, Khanna H, Doxsey S, Stokke T. CSPP is a ciliary protein interacting with nephrocystin 8 and required for cilia formation. Mol Biol Cell 21: 2555–2567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol 170: 103–113, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 151: 709–718, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12: R378–380, 2002 [DOI] [PubMed] [Google Scholar]
- 155.Peters KR, Palade GE, Schneider BG, Papermaster DS. Fine structure of a periciliary ridge complex of frog retinal rod cells revealed by ultrahigh resolution scanning electron microscopy. J Cell Biol 96: 265–276, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Phirke P, Efimenko E, Mohan S, Burghoorn J, Crona F, Bakhoum MW, Trieb M, Schuske K, Jorgensen EM, Piasecki BP, Leroux MR, Swoboda P. Transcriptional profiling of C. elegans DAF-19 uncovers a ciliary base-associated protein and a CDK/CCRK/LF2p-related kinase required for intraflagellar transport. Dev Biol 357: 235–247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pitaval A, Tseng Q, Bornens M, Thery M. Cell shape and contractility regulate ciliogenesis in cell cycle-arrested cells. J Cell Biol 191: 303–312, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184: 71–79, 2001 [DOI] [PubMed] [Google Scholar]
- 159.Prasad S, McDaid JP, Tam FW, Haylor JL, Ong AC. Pkd2 dosage influences cellular repair responses following ischemia-reperfusion injury. Am J Pathol 175: 1493–1503, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Prodromou NV, Thompson C, Osborn DP, Cogger KF, Ashworth R, Knight MM, Beales PL, Chapple JP. Heat shock induces rapid resorption of primary cilia. J Cell Sci 125: 4297–4305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent aurora A activation induces disassembly of the primary cilium. Cell 129: 1351–1363, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qin H, Rosenbaum JL, Barr MM. An autosomal recessive polycystic kidney disease gene homolog is involved in intraflagellar transport in C. elegans ciliated sensory neurons. Curr Biol 11: 457–461, 2001 [DOI] [PubMed] [Google Scholar]
- 163.Radford R, Slattery C, Jennings P, Blaque O, Pfaller W, Gmuender H, Van Delft J, Ryan MP, McMorrow T. Carcinogens induce loss of the primary cilium in human renal proximal tubular epithelial cells independently of effects on the cell cycle. Am J Physiol Renal Physiol 302: F905–F916, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rajagopalan V, Subramanian A, Wilkes DE, Pennock DG, Asai DJ. Dynein-2 affects the regulation of ciliary length but is not required for ciliogenesis in Tetrahymena thermophila. Mol Biol Cell 20: 708–720, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rix S, Calmont A, Scambler PJ, Beales PL. An Ift80 mouse model of short rib polydactyly syndromes shows defects in hedgehog signalling without loss or malformation of cilia. Hum Mol Genet 20: 1306–1314, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science 317: 372–376, 2007 [DOI] [PubMed] [Google Scholar]
- 167.Rosenbaum JL, Moulder JE, Ringo DL. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol 41: 600–619, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol 3: 813–825, 2002 [DOI] [PubMed] [Google Scholar]
- 169.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet 37: 1135–1140, 2005 [DOI] [PubMed] [Google Scholar]
- 170.Rothschild SC, Francescatto L, Drummond IA, Tombes RM. CaMK-II is a PKD2 target that promotes pronephric kidney development and stabilizes cilia. Development 138: 3387–3397, 2011 [DOI] [PubMed] [Google Scholar]
- 171.Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78: 35–43, 1994 [DOI] [PubMed] [Google Scholar]
- 172.Saigusa T, Reichert R, Guare J, Siroky BJ, Gooz M, Steele S, Fenton RA, Bell PD, Kolb RJ. Collecting duct cells that lack normal cilia have mislocalized vasopressin-2 receptors. Am J Physiol Renal Physiol 302: F801–F808, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol 69: 377–400, 2007 [DOI] [PubMed] [Google Scholar]
- 174.Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol 15: 1861–1866, 2005 [DOI] [PubMed] [Google Scholar]
- 175.Schneider L, Stock CM, Dieterich P, Jensen BH, Pedersen LB, Satir P, Schwab A, Christensen ST, Pedersen SF. The Na+/H+ exchanger NHE1 is required for directional migration stimulated via PDGFR-alpha in the primary cilium. J Cell Biol 185: 163–176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schneider MJ, Ulland M, Sloboda RD. A protein methylation pathway in Chlamydomonas flagella is active during flagellar resorption. Mol Biol Cell 19: 4319–4327, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schroder JM, Larsen J, Komarova Y, Akhmanova A, Thorsteinsson RI, Grigoriev I, Manguso R, Christensen ST, Pedersen SF, Geimer S, Pedersen LB. EB1 and EB3 promote cilia biogenesis by several centrosome-related mechanisms. J Cell Sci 124: 2539–2551, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Seeley ES, Carriere C, Goetze T, Longnecker DS, Korc M. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res 69: 422–430, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science 325: 1131–1134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sharma N, Kosan ZA, Stallworth JE, Berbari NF, Yoder BK. Soluble levels of cytosolic tubulin regulate ciliary length control. Mol Biol Cell 22: 806–816, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sillibourne JE, Hurbain I, Grand-Perret T, Goud B, Tran P, Bornens M. Primary ciliogenesis requires the distal appendage component Cep123. Biol Open 2: 535–545, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 37: 537–543, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD. Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am J Physiol Renal Physiol 290: F1320–F1328, 2006 [DOI] [PubMed] [Google Scholar]
- 184.Sloboda RD. Posttranslational protein modifications in cilia and flagella. Methods Cell Biol 94: 347–363, 2009 [DOI] [PubMed] [Google Scholar]
- 185.Sloboda RD, Rosenbaum JL. Making sense of cilia and flagella. J Cell Biol 179: 575–582, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Smith LA, Bukanov NO, Husson H, Russo RJ, Barry TC, Taylor AL, Beier DR, Ibraghimov-Beskrovnaya O. Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol 17: 2821–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 187.Snell WJ, Pan J, Wang Q. Cilia and flagella revealed: from flagellar assembly in Chlamydomonas to human obesity disorders. Cell 117: 693–697, 2004 [DOI] [PubMed] [Google Scholar]
- 188.Sohara E, Luo Y, Zhang J, Manning DK, Beier DR, Zhou J. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J Am Soc Nephrol 19: 469–476, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Somlo S, Markowitz GS. The pathogenesis of autosomal dominant polycystic kidney disease: an update. Curr Opin Nephrol Hypertens 9: 385–394, 2000 [DOI] [PubMed] [Google Scholar]
- 190.Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature 466: 378–382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Stephens RE. Tubulin in sea urchin embryonic cilia: post-translational modifications during regeneration. J Cell Sci 101: 837–845, 1992 [DOI] [PubMed] [Google Scholar]
- 192.Takakura A, Contrino L, Zhou X, Bonventre JV, Sun Y, Humphreys BD, Zhou J. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 18: 2523–2531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Takakura A, Nelson EA, Haque N, Humphreys BD, Zandi-Nejad K, Frank DA, Zhou J. Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum Mol Genet 20: 4143–4154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tam LW, Wilson NF, Lefebvre PA. A CDK-related kinase regulates the length and assembly of flagella in Chlamydomonas. J Cell Biol 176: 819–829, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Thiel C, Kessler K, Giessl A, Dimmler A, Shalev SA, von der Haar S, Zenker M, Zahnleiter D, Stoss H, Beinder E, Abou Jamra R, Ekici AB, Schroder-Kress N, Aigner T, Kirchner T, Reis A, Brandstatter JH, Rauch A. NEK1 mutations cause short-rib polydactyly syndrome type majewski. Am J Hum Genet 88: 106–114, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Thoma CR, Frew IJ, Hoerner CR, Montani M, Moch H, Krek W. pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat Cell Biol 9: 588–595, 2007 [DOI] [PubMed] [Google Scholar]
- 197.Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 136: 2297–2307, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tsiokas L, Kim S, Ong EC. Cell biology of polycystin-2. Cell Signal 19: 444–453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tukachinsky H, Lopez LV, Salic A. A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu-Gli protein complexes. J Cell Biol 191: 415–428, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, Spoor MS, You Y, Brody SL, Holtzman MJ. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest 116: 309–321, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Veleri S, Bishop K, Dalle Nogare DE, English MA, Foskett TJ, Chitnis A, Sood R, Liu P, Swaroop A. Knockdown of Bardet-Biedl syndrome gene BBS9/PTHB1 leads to cilia defects. PLoS One 7: e34389, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Verghese E, Ricardo SD, Weidenfeld R, Zhuang J, Hill PA, Langham RG, Deane JA. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol 20: 2147–2153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Verghese E, Weidenfeld R, Bertram JF, Ricardo SD, Deane JA. Renal cilia display length alterations following tubular injury and are present early in epithelial repair. Nephrol Dial Transplant 23: 834–841, 2008 [DOI] [PubMed] [Google Scholar]
- 204.Verghese E, Zhuang J, Saiti D, Ricardo SD, Deane JA. In vitro investigation of renal epithelial injury suggests that primary cilium length is regulated by hypoxia-inducible mechanisms. Cell Biol Int 35: 909–913, 2011 [DOI] [PubMed] [Google Scholar]
- 205.Verhey KJ, Dishinger J, Kee HL. Kinesin motors and primary cilia. Biochem Soc Trans 39: 1120–1125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci USA 103: 18556–18561, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang S, Wei Q, Dong G, Dong Z. ERK-mediated suppression of cilia in cisplatin-induced tubular cell apoptosis and acute kidney injury. Biochim Biophys Acta [Epub before print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol 27: 3241–3252, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wann AK, Knight MM. Primary cilia elongation in response to interleukin-1 mediates the inflammatory response. Cell Mol Life Sci 69: 2967–2977, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30: 259–269, 2002 [DOI] [PubMed] [Google Scholar]
- 211.Weeks DP, Collis PS. Induction of microtubule protein synthesis in Chlamydomonas reinhardi during flagellar regeneration. Cell 9: 15–27, 1976 [DOI] [PubMed] [Google Scholar]
- 212.Weimbs T. Polycystic kidney disease and renal injury repair: common pathways, fluid flow, and the function of polycystin-1. Am J Physiol Renal Physiol 293: F1423–F1432, 2007 [DOI] [PubMed] [Google Scholar]
- 213.Wiese M, Kuhn D, Grunfelder CG. Protein kinase involved in flagellar-length control. Eukaryot Cell 2: 769–777, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wilkinson CJ, Carl M, Harris WA. Cep70 and Cep131 contribute to ciliogenesis in zebrafish embryos. BMC Cell Biol 10: 17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wilson NF, Lefebvre PA. Regulation of flagellar assembly by glycogen synthase kinase 3 in Chlamydomonas reinhardtii. Eukaryot Cell 3: 1307–1319, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wloga D, Camba A, Rogowski K, Manning G, Jerka-Dziadosz M, Gaertig J. Members of the NIMA-related kinase family promote disassembly of cilia by multiple mechanisms. Mol Biol Cell 17: 2799–2810, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wloga D, Webster DM, Rogowski K, Bre MH, Levilliers N, Jerka-Dziadosz M, Janke C, Dougan ST, Gaertig J. TTLL3 Is a tubulin glycine ligase that regulates the assembly of cilia. Dev Cell 16: 867–876, 2009 [DOI] [PubMed] [Google Scholar]
- 218.Xia L, Hai B, Gao Y, Burnette D, Thazhath R, Duan J, Bre MH, Levilliers N, Gorovsky MA, Gaertig J. Polyglycylation of tubulin is essential and affects cell motility and division in Tetrahymena thermophila. J Cell Biol 149: 1097–1106, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Jr, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol 282: F541–F552, 2002 [DOI] [PubMed] [Google Scholar]
- 220.Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol 178: 363–369, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yuan S, Li J, Diener DR, Choma MA, Rosenbaum JL, Sun Z. Target-of-rapamycin complex 1 (Torc1) signaling modulates cilia size and function through protein synthesis regulation. Proc Natl Acad Sci USA 109: 2021–2026, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zeng H, Hoover AN, Liu A. PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev Biol 339: 418–428, 2010 [DOI] [PubMed] [Google Scholar]
- 223.Zhang Y, Wada J, Yasuhara A, Iseda I, Eguchi J, Fukui K, Yang Q, Yamagata K, Hiesberger T, Igarashi P, Zhang H, Wang H, Akagi S, Kanwar YS, Makino H. The role for HNF-1beta-targeted collectrin in maintenance of primary cilia and cell polarity in collecting duct cells. PLoS One 2: e414, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 224.Zhou J, Ouyang X, Schoeb TR, Bolisetty S, Cui X, Mrug S, Yoder BK, Johnson MR, Szalai AJ, Mrug M. Kidney injury accelerates cystogenesis via pathways modulated by heme oxygenase and complement. J Am Soc Nephrol 23: 1161–1171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhu D, Shi S, Wang H, Liao K. Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3–L1 preadipocytes. J Cell Sci 122: 2760–2768, 2009 [DOI] [PubMed] [Google Scholar]
- 226.Zuo X, Fogelgren B, Lipschutz JH. The small GTPase Cdc42 is necessary for primary ciliogenesis in renal tubular epithelial cells. J Biol Chem 286: 22469–22477, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]