Abstract
The inbred genetic hypercalciuric stone-forming (GHS) rats exhibit many features of human idiopathic hypercalciuria and have elevated levels of vitamin D receptors (VDR) in calcium (Ca)-transporting organs. On a normal-Ca diet, 1,25(OH)2D3 (1,25D) increases urine (U) Ca to a greater extent in GHS than in controls [Sprague-Dawley (SD)]. The additional UCa may result from an increase in intestinal Ca absorption and/or bone resorption. To determine the source, we asked whether 1,25D would increase UCa in GHS fed a low-Ca (0.02%) diet (LCD). With 1,25D, UCa in SD increased from 1.2 ± 0.1 to 9.3 ± 0.9 mg/day and increased more in GHS from 4.7 ± 0.3 to 21.5 ± 0.9 mg/day (P < 0.001). In GHS rats on LCD with or without 1,25D, UCa far exceeded daily Ca intake (2.6 mg/day). While the greater excess in UCa in GHS rats must be derived from bone mineral, there may also be a 1,25D-mediated decrease in renal tubular Ca reabsorption. RNA expression of the components of renal Ca transport indicated that 1,25D administration results in a suppression of klotho, an activator of the renal Ca reabsorption channel TRPV5, in both SD and GHS rats. This fall in klotho would decrease tubular reabsorption of the 1,25D-induced bone Ca release. Thus, the greater increase in UCa with 1,25D in GHS fed LCD strongly suggests that the additional UCa results from an increase in bone resorption, likely due to the increased number of VDR in the GHS rat bone cells, with a possible component of decreased renal tubular calcium reabsorption.
Keywords: vitamin D, calcium, kidney stones, intestinal absorption
the most common metabolic abnormality in patients who form calcium-based kidney stones is hypercalciuria (14, 50). The increased urine (U) calcium (Ca) excretion enhances nucleation and growth of calcium hydrogen phosphate (CaHPO4; brushite) and/or calcium oxalate (CaOx) crystals into stones (14). Idiopathic hypercalciuria (IH) typically manifests as hypercalciuria with normal serum (S) Ca, normal or elevated S1,25(OH)2D3 (1,25D), normal or elevated S parathyroid hormone (PTH), normal or low S phosphate (P), and low bone mass (14, 48, 55) and is polygenic (48, 49, 55).
To study the pathophysiology of hypercalciuria and stone formation, we established a strain of hypercalciuric rats by selectively inbreeding Sprague-Dawley (SD) rats for increased UCa excretion (3–5, 11–13, 15–19, 21–24, 27, 32, 35, 36, 40, 41, 43, 45, 52, 57, 61, 62). After more than 80 generations, each rat consistently excretes ∼8- to 10-fold more UCa than SD controls (3–5, 11–13, 15–19, 21–24, 27, 32, 35, 36, 40, 41, 43, 45, 52, 57, 61, 62) and forms kidney stones (3, 17, 18, 22); these animals are termed genetic hypercalciuric stone-forming (GHS) rats (3–5, 11–13, 15–19, 21, 23, 24, 27, 32, 35, 36, 40, 41, 43, 45, 52, 57, 61, 62).
GHS rats exhibit many features of human IH including normal SCa (15), increased intestinal Ca absorption (45) and bone resorption (43), decreased renal tubule Ca reabsorption (57), and normal S1,25D levels in addition to decreased bone mineral density (24, 32) and have a polygenic mode of inheritance (35). GHS rats have elevated levels of vitamin D receptor (VDR) protein in Ca-transporting organs including the kidney, intestine, and bone (30, 43, 45, 57).
In humans, the changes in intestine, kidney, and bone Ca transport in IH may be reproduced by the administration of 1,25D to normals leading to hypercalciuria (1, 47). This increase in UCa indicates that the effect of 1,25D to increase intestinal Ca absorption (34) and bone Ca resorption (1, 47) overwhelms any 1,25D-mediated increase in renal tubular Ca reabsorption (8). While elevated S1,25D levels may account for the phenotype in some (6, 10, 38, 53), most IH patients have normal S1,25D levels (63). In one study, high-VDR levels have been found in male IH stone formers (28), suggesting elevated VDR levels may play a role in hypercalciuria in human stone formers.
We previously showed that administration of 1,25D to GHS and SD rats fed a normal-Ca diet (NCD) leads to a greater increment in hypercalciuria in GHS rats (29). This result is consistent with a model in which the greater number of VDR in GHS is relatively undersaturated with 1,25D under basal conditions, but with addition of more 1,25D, the mass action of the increased VDR leads to enhanced UCa. Alteration in renal reabsorption can control circulating Ca levels but cannot, by itself, lead to sustained hypercalciuria. An increase in UCa must originate either from an increase in intestinal absorption or from an increase in bone resorption, the only significant sources of Ca. To determine which, in this study a low-Ca diet (LCD) was utilized to remove the contribution of increased intestinal Ca absorption from 1,25D-mediated hypercalciuria. We hypothesized that due to the increased 1,25D receptors in GHS rat bones, administration of 1,25D to rats fed LCD would increase hypercalciuria to a greater extent in GHS than SD.
METHODS
Animals.
The GHS rats were derived from SD rats (Charles River Laboratories, Kingston, NY) by successively inbreeding the most hypercalciuric progeny of each generation (12, 15, 17, 27, 32, 41, 43, 45, 57). Eight-week-old male GHS rats from the 88th generation and 8-wk-old male SD rats (Charles River Laboratories) were used in this study.
Experimental conditions.
At the start of the study [day 0 (d 0)], 16 SD and 16 GHS rats were placed in metabolic cages, fed 13 g/day LCD (0.02% Ca, Harlan-Teklad, Indianapolis, IN), and given deionized, distilled water ad libitum. Also starting on d 0, by random allocation, eight rats in each group were injected daily with 1,25D (25 ng/100 g body wt, American Regent, Shirley, NY) in saline and eight rats in each group with only saline. This dose of 1,25D elicits a maximal physiologic response (61). Starting on d 8, U was collected for four 24-h periods. On d 9 and d 11, U was acidified with HCl and on d 10 and d 12, U was collected in thymol. Collections in thymol were used for pH and Cl and collections in HCl for all other measurements. On days 14, 15, and 16, rats were anesthetized and blood was collected by cardiac puncture. Rats were killed and kidneys were quickly removed. Any animal that ate <10 g/day food or drank <15 ml/day water would have been excluded from further analysis; however, all rats met these prespecified criteria during the entire study. All procedures were approved by the University of Rochester Committee for Animal Resources.
Urine and serum chemistries.
Urine Ca, Mg, P, ammonia, and creatinine were measured spectrophotometrically using a Beckman CX5 Pro autoanalyzer (Beckman Instruments, Brea, CA). Change in UCa (ΔUCa) was estimated by pairing each SD+1,25D rat with a randomly assigned SD+saline rat, and each GHS+1,25D with a GHS+saline rat, and calculating the differences. Urine K, Cl, and Na were measured by ion-specific electrodes on the Beckman CX5. Urine pH was measured using a glass electrode and citrate, oxalate, and sulfate were measured by ion chromatography using a Dionex ICS 2000 system (Dionex, Sunnyvale, CA). Serum Ca and P were determined colorimetrically (BioVision, Milpitas, CA). Serum PTH was determined by EIA for intact-PTH (ALPCO, Salem, NH). We have used these methods previously (3, 4, 24, 29).
Urine supersaturation.
The CaOx and CaHPO4 (CaP) ion activity product were calculated using the computer program EQUIL 2 (60) as we have done previously (11–13, 16, 19, 21–23). Ratios of 1 denote a sample at equilibrium, >1 denotes supersaturation, and <1 denotes undersaturation. We found excellent correspondence between calculated and experimentally measured saturation in U and blood and in bone culture medium (3, 4, 24).
RNA harvest and purification.
Kidneys were bisected and placed in 2 ml RNAlater (Ambion, Grand Island, NY) at 4°C overnight and then transferred to −70°C until purification. Each kidney was homogenized in 6 ml TRIzol (Invitrogen, Grand Island, NY) using a glass homogenizer, and RNA purification was conducted according to the manufacturer's instructions. Aqueous and phenol phases were separated by centrifugation after addition of 1-bromo-3-chloropropane. RNA was precipitated from the aqueous layer with isopropyl alcohol and washed with 75% ethanol. DNA contaminants were removed by on-column digestion with DNase 1 followed by purification using Qiagen RNeasy mini columns (Qiagen, Valencia, CA).
Quantitative real-time PCR.
Kidney RNA was transcribed to cDNA using an iScript kit (BioRad, Hercules, CA). Gene-specific targets were amplified and analyzed by real-time PCR with a MyIQ cycler (BioRad) and Sybr Green (IQ Supermix, BioRad). To normalize gene expression, the geometric mean of expression of RNA for β-actin, ribosomal protein L13a, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide and succinate dehydrogenase complex, subunit a, flavoprotein was calculated for each sample (58). Primer sets used were described previously (29). All expression values were calculated relative to the mean expression in SD+saline.
Statistics.
Values were compared by ANOVA using the Bonferroni correction for multiple comparisons, with a conventional computer program (Statistica, StatSoft, Tulsa, OK). Values are expressed as means ± SE, with P ≤ 0.05 considered significant.
RESULTS
Serum and urine chemistry.
When fed LCD, there was no difference in SCa between the GHS and control SD rats without exogenous 1,25D, as previously observed (41) (Table 1). 1,25D induced an increase in SCa in GHS (compared with SD) but not SD rats. There were no differences in SP in any group. Serum PTH was numerically higher, but not significantly different, in the GHS compared with SD. 1,25D led to suppression of PTH in both groups.
Table 1.
Serum and urine values
| SD | GHS | SD +1,25D | GHS +1,25D | |
|---|---|---|---|---|
| SCa, mg/dl | 9.3 ± 1.1 | 10.4 ± 0.6 | 11.0 ± 0.5 | 12.6 ± 0.5* |
| SP, mg/dl | 10.2 ± 0.4 | 10.2 ± 0.6 | 11.4 ± 0.3 | 10.6 ± 0.4 |
| SPTH, pg/ml | 753 ± 112 | 1,212 ± 312 | 40 ± 23*† | 245 ± 76† |
| UV, ml | 28.8 ± 2.6 | 36.2 ± 3.7 | 52.1 ± 10.2 | 66.4 ± 10.6*† |
| UpH | 6.13 ± 0.04 | 5.84 ± 0.02* | 5.58 ± 0.06*† | 5.46 ± 0.03*† |
| Ucitrate, mg/24 h | 3.6 ± 0.6 | 28.8 ± 1.8* | 43.5 ± 4.5*† | 92.5 ± 3.1*†‡ |
| Uphosphate, mg/24 h | 72.1 ± 2.4 | 69.9 ± 2.0 | 75.1 ± 3.4 | 80.4 ± 3.2 |
| Uoxalate, mg/24 h | 0.68 ± 0.05 | 0.78 ± 0.04 | 0.86 ± 0.03* | 0.92 ± 0.05* |
| Uchloride, mmol/24 h | 1.87 ± 0.09 | 1.75 ± 0.05 | 1.67 ± 0.05 | 1.75 ± 0.08 |
| UNH4+, mmol/24 h | 1.02 ± 0.04 | 0.88 ± 0.03 | 0.90 ± 0.06 | 1.14 ± 0.05†‡ |
| Upotassium, mmol/24 h | 1.43 ± 0.04 | 1.3 ± 0.04 | 1.33 ± 0.06 | 1.26 ± 0.05 |
| Usulfate, meq/24 h | 0.70 ± 0.03 | 0.72 ± 0.03 | 0.68 ± 0.04 | 0.85 ± 0.04*†‡ |
| UCr, mg/24 h | 11.4 ± 0.4 | 9.8 ± 0.3 | 9.9 ± 0.6 | 9.9 ± 0.5 |
Results are means ± SE. Values for selected serum (S) and urine (U) components in Sprague-Dawley (SD) and genetic hypercalciuric stone-forming (GHS) rats fed low-Ca diet (LCD), without or with exogenous 1,25(OH)2D3 (1,25D).
P < 0.05 compared with SD.
P < 0.05 compared with GHS.
P < 0.05 compared with SD +1,25D. PTH, parathyroid hormone.
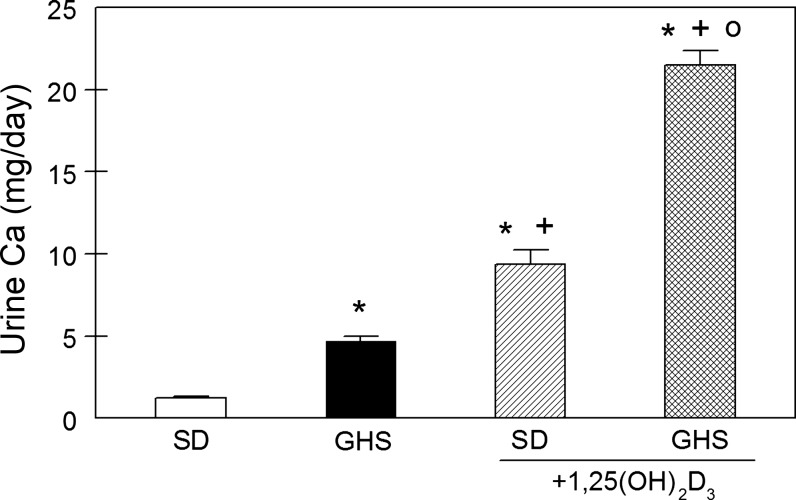
Without exogenous 1,25D, UCa in GHS was higher (4.7 ± 0.3 mg/day) than that of SD (1.2 ± 0.1 mg/day; Fig. 1), as we reported previously (19, 22, 32, 41, 57). 1,25D increased UCa to 9.3 ± 0.9 mg/day in SD and to 21.5 ± 0.9 mg/day in GHS. While 1,25D increased UCa in both groups, there was a significantly greater increase in GHS (ΔUCa = 16.8 ± 0.8 mg/day) than in SD (ΔUCa = 8.1 ± 1.0 mg/day, P < 0.001) consistent with a greater biological response to 1,25D in GHS than in SD. With SD+1,25D and with GHS with or without 1,25D, UCa was far greater than total dietary Ca intake (2.6 mg/day), indicating that the rats were in negative total body Ca balance. The source of the additional UCa must be bone mineral, the only appreciable reservoir of Ca in the body.
Fig. 1.
Urine Ca (UCa) in Sprague-Dawley (SD) and genetic hypercalciuric stone-forming (GHS) rats fed low-Ca diet (LCD), without or with exogenous 1,25(OH)2D3 (1,25D). UCa was measured in 24-h urine collections and expressed as mg/day (means ± SE). *P < 0.05 compared with SD. +P < 0.05 compared with GHS. oP < 0.05 compared with SD+1,25D.
Urine volume (V) was numerically greater in GHS than in SD but the difference did not reach significance (Table 1). 1,25D increased UV significantly in GHS. UP did not change with 1,25D in either SD or GHS (Table 1).
Urine supersaturation.
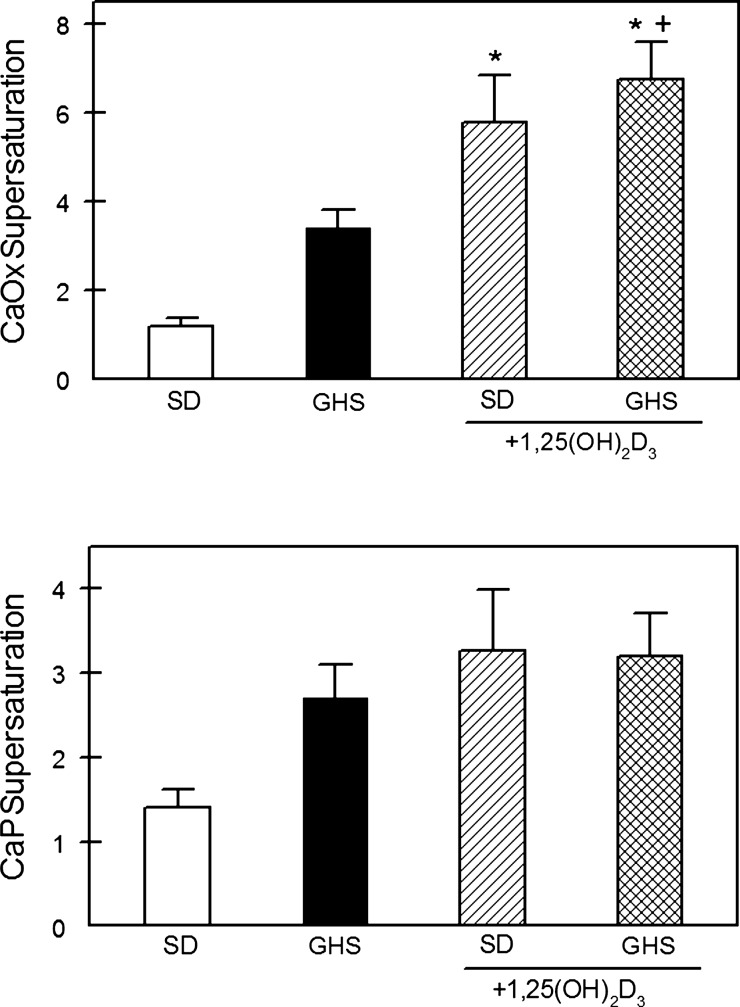
When fed LCD without exogenous 1,25D, CaOx supersaturation (SS) was numerically, but not significantly, greater in the GHS than in SD (Fig. 2). 1,25D led to an increase in CaOx SS in both SD and GHS which were not different from each other. Without exogenous 1,25D, CaP SS was also numerically greater, but not significantly different, in GHS compared with SD. 1,25D did not increase CaP SS in either SD or GHS (Fig. 2).
Fig. 2.
Supersaturation of calcium oxalate (CaOx) and Ca phosphate (CaP) in U from SD and GHS rats fed LCD, without or with exogenous 1,25D. Supersaturation for CaOx (top) and CaP (bottom) was calculated from U measurements using EQUIL2 as indicated in methods. Results are expressed as a unitless ratio (means ± SE). *P < 0.05 compared with SD. +P < 0.05 compared with GHS.
Expression of markers of transcellular Ca transport.
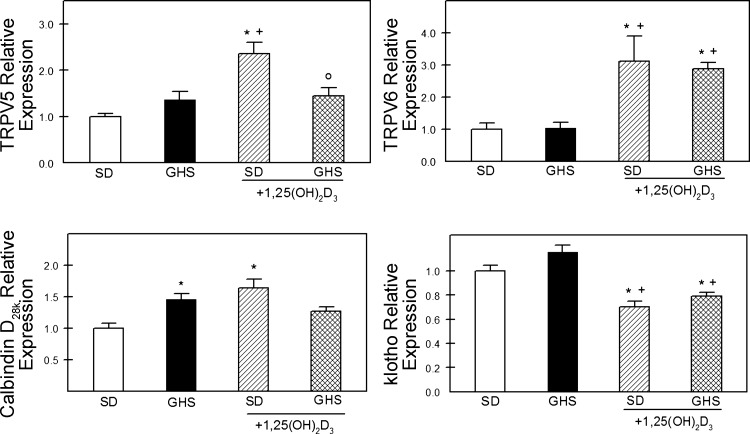
To determine whether renal tubular Ca reabsorptive pathways were regulated differently in GHS compared with SD rats fed LCD, renal expression of genes related to Ca transport was examined. Without additional 1,25D, there was no difference in the RNA expression of transient receptor potential vanilloid (TRPV) 5 or TRPV6 between GHS and SD rats (Fig. 3). Basal expression of calbindin D28k was elevated in GHS rats but there was no difference in basal expression of klotho.
Fig. 3.
Expression of transient receptor potential vanilloid (TRPV)5, TRPV6, calbindin D28k, and klotho in kidney from SD and GHS fed LCD, without or with 1,25D. Each sample's RNA content was normalized to the geometric mean of 4 markers (β-actin, rpl13a, YHAWAH, and sdh) and then expressed as relative to the level of that particular gene product in SD as a unitless ratio (means ± SE). *P < 0.05 compared with SD. +P < 0.05 compared with GHS. oP < 0.05 compared with SD+1,25D.
1,25D increased expression of TRPV5, TRPV6, and calbindin D28K in SD rats (Fig. 3). In GHS rats, 1,25D increased expression only of TRPV6. Klotho expression was decreased by 1,25D in both SD and GHS. Expression of the basolateral Na/Ca exchanger, NCX1, and the plasma membrane Ca-ATPase, PMCA, was not different between GHS and SD (Table 2).
Table 2.
Relative RNA expression of components of renal calcium transport
| SD | GHS | SD +1,25D | GHS +1,25D | |
|---|---|---|---|---|
| Claudin 14 | 1.00 ± 0.07 | 1.51 ± 0.13* | 1.08 ± 0.10† | 0.97 ± 0.06† |
| Claudin 16 | 1.0 ± 0.07 | 1.27 ± 0.06* | 1.16 ± 0.10 | 1.46 ± 0.06*‡ |
| Claudin 19 | 1.00 ± 0.12 | 0.80 ± 0.13 | 1.76 ± 0.21*† | 1.08 ± 0.05‡ |
| CaR | 1.0 ± 0.09 | 1.4 ± 0.1 | 1.12 ± 0.13 | 1.5 ± 0.1* |
| NCX1 | 1.0 ± 0.07 | 1.14 ± 0.07 | 1.05 ± 0.07 | 1.15 ± 0.12 |
| PMCA | 1.0 ± 0.06 | 1.02 ± 0.11 | 1.04 ± 0.05 | 1.07 ± 0.06 |
| ROMK | 1.0 ± 0.1 | 1.16 ± 0.11 | 1.1 ± 0.07 | 1.3 ± 0.09 |
| NKCC2 | 1.0 ± 0.08 | 1.18 ± 0.09 | 0.9 ± 0.09 | 1.13 ± 0.1 |
Results are means ± SE. Relative RNA expression of selected components of renal Ca transport in SD and GHS rats fed LCD, without or with exogenous 1,25D.
P < 0.05 compared with SD.
P < 0.05 compared with GHS.
P < 0.05 compared with SD +1,25D.
Expression of markers of paracellular Ca transport.
Renal Ca reabsorption occurs in the thick ascending limb of Henle (TALH), via paracellular transport through tight junctions that contain claudin 16 and claudin 19 (37), and their cation permeability is regulated by claudin 14 (31). Basal expression of claudins 16 and 14 was elevated in GHS, while expression of claudin 19 was not different between GHS and SD (Table 2).
1,25D increased expression of claudin 19 in SD but not GHS (Table 2). In GHS, 1,25D decreased expression of claudin 14 to a level not different from SD. With 1,25D, claudin 16 in GHS remained elevated relative to SD. Expression of the calcium-sensing receptor (CaR) was increased in GHS+1,25D compared only with SD alone. Expression of the outward modulating K channel (ROMK) and the Na-K-2Cl transporter (NKCC2) did not differ between SD and GHS with or without 1,25D (Table 2).
DISCUSSION
GHS rats exhibit many features of human IH including increased intestinal Ca absorption (45), increased bone resorption (43), and decreased renal tubule Ca reabsorption (57) and have elevated levels of VDR protein in these Ca-transporting organs (43, 45, 57). We previously showed that administration of 1,25D to GHS and SD fed NCD (1.2% Ca) led to a larger increment in hypercalciuria in GHS (29), suggesting that the increased VDR induced a greater biological response. The sustained increase in UCa must originate from increased intestinal absorption and/or bone resorption. In this study, LCD (0.02% Ca) was utilized to remove the contribution of any increase in intestinal Ca absorption to the additional 1,25D-mediated hypercalciuria in GHS. We found that 1,25D administered to rats fed LCD led to a far greater increase in UCa in GHS than in SD, indicating that the increased VDR in GHS rat bone was biologically active and that bone was the source of the additional UCa.
While being fed LCD, GHS excreted more UCa than SD rats, as we reported previously (19, 22, 32, 41, 57). 1,25D led to a further increase in UCa in both groups; however, GHS rats had a significantly greater increase than SD. The 1,25D-mediated increase in UCa could be the result of a primary increase in bone resorption and/or a reduction in renal tubular Ca reabsorption which then leads to enhanced bone resorption mediated through a fall in SCa and a rise in PTH. However, with 1,25D we did not observe a fall in SCa nor a rise in PTH but, in GHS, an increase in SCa and a decrease in PTH, indicating that the increase in UCa was due primarily to increased 1,25D-mediated bone resorption. This is consistent with our previous observation that alendronate decreases hypercalciuria in GHS by inhibiting bone resorption (22).
The LCD contains 0.02% calcium and would provide 2.6 mg Ca/day if all of this Ca were absorbed. With LCD, GHS rats excreted more Ca (4.7 ± 0.03 mg/day) than they consumed, indicating that they were in negative total body Ca balance. 1,25D led to a further increase in UCa (21.5 ± 0.09) in GHS rats, indicating an even more negative Ca balance. In vitro, neonatal GHS rat calvariae have increased bone resorption in response to graded amounts of 1,25D compared with SD (43). In vivo, we previously found that GHS rats are in negative total body Ca balance (41) and have decreased bone mineral density (24, 32), supporting our hypothesis that GHS rats exhibit enhanced bone resorption.
Since GHS rats have normal levels of S1,25D, the elevated tissue levels of VDR in the Ca-transporting organs (43, 45, 57) would be relatively undersaturated with 1,25D compared with SD (29). LCD alone increases 1,25D (54) and 1,25D is known to increase expression of VDR (26, 33). In this study, the greater increase in UCa in GHS compared with SD with LCD+1,25D indicates that the GHS rat VDR could not have been saturated with endogenous 1,25D even while fed LCD. The increased VDR in GHS are clearly biologically active and any increase in VDR induced by 1,25D must be greater in GHS than SD.
With LCD, SCa increased only in GHS+1,25D compared with SD, indicating that increased bone resorption must have exceeded the ability of the GHS rat kidney to excrete this additional Ca. We previously found with NCD (29) that SCa increased in both SD+1,25D and GHS+1,25D, perhaps due to the 1,25D stimulation of intestinal Ca absorption which was limited here with LCD. There was no difference in SPTH between GHS and SD rats with LCD, in contrast to the finding with rats fed NCD where SPTH is lower in GHS rats than in SD (19). The lower PTH in GHS fed NCD (19) suggested that increased intestinal Ca absorption and/or increased bone resorption, rather than a failure to adequately reabsorb filtered Ca, are the more prominent metabolic abnormalities in GHS rats. However, once dietary Ca is limited, as in this study, sufficient Ca cannot be absorbed nor resorbed to overcome the Ca lost due to the defect in renal tubular Ca reabsorption in GHS rats, which would normally have led to the reduction in PTH.
Although there was no significant difference in UOx between GHS and SD, numerically UOx was higher in GHS; patients with IH may exhibit mild hyperoxaluria (7). While there was no difference in CaOx SS between GHS and SD without exogenous 1,25D, administration of 1,25D led to a marked increase in CaOx SS in both groups. The paucity of dietary Ca and increased absorption of any available Ca with 1,25D would lead to greater free Ox in the intestine, greater absorption and increased UOx (44). Despite a marked increase in UCa in GHS rats, especially those given 1,25D, we did not observe a commensurate increase in UP as might be expected with increased bone resorption. This result may be due to greater loss of Ca carbonate, rather than apatite, from the bone. Indeed, we previously showed that H+ preferentially causes resorption of bone CaCO3 rather than apatite (20). There was no significant difference in CaP SS in any group, due to the lack of increase in UP and perhaps to the parallel increase in UV and the fall in urine pH. Hypercalcemia and hypercalciuria in rats and humans are associated with polyuria (39, 42, 59).
Urine citrate (Cit) excretion is higher in GHS than SD and increases to a greater extent in GHS+1,25D than in SD+1,25D. The progressive increase in UCit parallels that of the increase in UCa (Fig. 1). This increase in UCit may represent the release of anionic proton buffers from bone and supports the hypothesis that the increase in UCa is derived from resorption of bone mineral. In GHS rats, administration of 1,25D also led to an increase in H+ excretion in the form of increased UNH4+ and Usulfate, resulting in a fall in UpH. Since we did not measure titratable acidity, we cannot calculate net acid excretion and reconcile the apparent discrepancy between the increase in citrate and the fall in UpH.
While the greater excess in UCa in GHS rats fed LCD must be derived from bone mineral stores, at baseline GHS also have decreased renal tubular Ca reabsorption (57) similar to many humans with IH (14) and nonhypercalciuric humans given 1,25D (1, 47). As the GHS rats have more VDR than control SD rats, we then determined whether additional 1,25D would further decrease renal tubular reabsorption of this bone-derived Ca by examining RNA expression for components of Ca reabsorption. We administered sufficient 1,25D to significantly suppress PTH, minimizing any effect of this hormone on Ca reabsorption (8).
In humans, mutations in the paracellular proteins claudin 16 or 19 cause familial hypercalciuria and nephrocalcinosis (9). A genome-wide association study in kidney stone patients identified sequence variants in the gene CLDN14 (encoding claudin 14) associated with hypercalciuria (56). The observed increase in claudin 14 in GHS is consistent with their previously described defect in renal tubular Ca reabsorption (57). We previously reported increased levels of CaR mRNA and protein in GHS kidneys at baseline and after acute stimulation with 1,25D (61). In this study, CaR was numerically, although not statistically, increased in GHS. 1,25D increased SCa and UCa in GHS rats and led to upregulation of CaR which should reduce Ca reabsorption despite no demonstrable increase in claudin 14. Renal Ca transport in GHS is more sensitive to chlorothiazide and less sensitive to furosemide than in SD, suggesting decreased Ca reabsorption in the TALH (57), consistent with our current observations.
Further Ca reabsorption occurs in the distal nephron through active transepithelial reabsorption via TRPV5. A second apical Ca transporter, TRPV6, is found in the TALH (51). The FGF23 coreceptor klotho activates either TRPV5 or TRPV6 (25, 46). There was no difference in the basal expression of TRPV5, its regulator klotho, or TRPV6 between GHS and SD rats, suggesting that hypercalciuria in GHS was not due to differences in active transepithelial Ca transport. 1,25D led to a similar increase in TRPV6 and decrease in klotho between GHS and SD rats. In a prior study of rats fed NCD, we also found that 1,25D decreased klotho in both GHS and SD rats (29). The phenotype of klotho−/− mice includes hypercalciuria (2). Even a modest decrease in klotho could lead to marked inhibition of TRPV5 resulting in decreased Ca reabsorption, allowing Ca released from bone to be more readily excreted. However, the 1,25D-induced increase in TRPV6 would tend to increase renal tubular Ca reabsorption and decrease hypercalciuria. Protein abundance and transporter activity have not been studied in these rats and the extent of reabsorption may differ from estimates based on RNA abundance.
Previously, we showed that administration of 1,25D to GHS and SD rats fed NCD leads to a greater increment in hypercalciuria in GHS, which must originate from an increase in intestinal Ca absorption and/or an increase in bone resorption (29). In this study, we utilized LCD to remove the contribution of intestinal Ca absorption. We found that administration of 1,25D to rats fed LCD increased hypercalciuria to a greater extent in GHS than in SD controls. By severely limiting intestinal Ca absorption, and demonstrating negative Ca balance in GHS rats even without additional 1,25D, we now provide clear support for enhanced bone resorption as the source of the additional UCa, which is further enhanced by exogenous 1,25D. The current findings are consistent with the greater number of VDR in GHS rat bone being biologically active under both basal and stimulated conditions.
GRANTS
This work was supported by National Institutes of Health Grant RO1 DK075462.
Some of these findings were presented as an oral communication at the annual meeting of the American Society for Nephrology, 2011.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.K.F., N.S.K., and D.A.B. conception and design of research; K.K.F., N.S.K., C.D.C., and D.M.A. performed experiments; K.K.F., J.R.A., N.S.K., C.D.C., and D.A.B. analyzed data; K.K.F., J.R.A., N.S.K., and D.A.B. interpreted results of experiments; K.K.F. and D.A.B. prepared figures; K.K.F. and D.A.B. drafted manuscript; K.K.F., J.R.A., N.S.K., and D.A.B. edited and revised manuscript; K.K.F., J.R.A., N.S.K., C.D.C., and D.A.B. approved final version of manuscript.
REFERENCES
- 1.Adams ND, Gray RW, Lemann JJ. Effects of calcitriol administration on calcium metabolism in healthy men. Kidney Int 21: 90–97, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Alexander RT, Woudenberg-Vrenken TE, Buurman J, Dijkman H, van der Eerden BCJ, van Leeuwen JPTM, Bindels RJ, Hoenderop JG. Klotho prevents renal calcium loss. J Am Soc Nephrol 20: 2371–2379, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asplin JR, Bushinsky DA, Singharetnam W, Riordon D, Parks JH, Coe FL. Relationship between supersaturation and crystal inhibition in hypercalciuric rats. Kidney Int 51: 640–645, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Asplin JR, Donahue SE, Lindeman C, Michalenka A, Strutz KL, Bushinsky DA. Thiosulfate reduces calcium phosphate nephrolithiasis. J Am Soc Nephrol 20: 1246–1253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai S, Wang H, Shen J, Zhou R, Bushinsky DA, Favus MJ. Elevated vitamin D receptor levels in genetic hypercalciuric stone-forming rats are associated with downregulation of Snail. J Bone Miner Res 25: 830–840, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bataille P, Bouillion R, Fournier A, Renaud H, Gueris J, Idrissi A. Increased plasma concentrations of total and free 1,25(OH)2D3 in calcium stone formers with idiopathic hypercalciuria. Contr Nephrol 58: 137–142, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Bergsland KJ, Zisman AL, Asplin JR, Worcester EM, Coe FL. Evidence for net renal tubule oxalate secretion in patients with calcium kidney stones. Am J Physiol Renal Physiol 300: F311–F318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bindels RJM, Hartog A, Timmermans J, Van Os CH. Active Ca2+ transport in primary cultures of rabbit kidney CCD: stimulation by 1,25-dihydroxyvitamin D3 and PTH. Am J Physiol Renal Fluid Electrolyte Physiol 261: F799–F807, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Blanchard A, Jeunemaitre X, Coudol P, Dechaux M, Froissart M, May A, Demontis R, Fournier A, Paillard M, Houillier P. Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int 59: 2206–2215, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Broadus AE, Horst RL, Lang R, Littledike ET, Rasmussen H. The importance of circulating 1,25(OH)2D in the pathogenesis of hypercalciuria and renal stone formation in primary hyperparathyroidism. N Engl J Med 302: 421–426, 1980 [DOI] [PubMed] [Google Scholar]
- 11.Bushinsky DA, Asplin JR. Thiazides reduce brushite, but not calcium oxalate, supersaturation and stone formation in genetic hypercalciuric stone-forming rats. J Am Soc Nephrol 16: 417–424, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Bushinsky DA, Asplin JR, Grynpas MD, Evan AP, Parker WR, Alexander KM, Coe FL. Calcium oxalate stone formation in genetic hypercalciuric stone-forming rats. Kidney Int 61: 975–987, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Bushinsky DA, Bashir MA, Riordon DR, Nakagawa Y, Coe FL, Grynpas MD. Increased dietary oxalate does not increase urinary calcium oxalate saturation in hypercalciuric rats. Kidney Int 55: 602–612, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Bushinsky DA, Coe FL, Moe OW. Nephrolithiasis. In: The Kidney, edited by Brenner BM. Philadelphia: W. B. Saunders, 2012, p. 1455–1507 [Google Scholar]
- 15.Bushinsky DA, Favus MJ. Mechanism of hypercalciuria in genetic hypercalciuric rats: inherited defect in intestinal calcium transport. J Clin Invest 82: 1585–1591, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bushinsky DA, Grynpas MD, Asplin JR. Effect of acidosis on urine supersaturation and stone formation in genetic hypercalciuric stone forming rats. Kidney Int 59: 1415–1423, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Bushinsky DA, Grynpas MD, Nilsson EL, Nakagawa Y, Coe FL. Stone formation in genetic hypercalciuric rats. Kidney Int 48: 1705–1713, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Bushinsky DA, Kim M, Sessler NE, Nakagawa Y, Coe FL. Increased urinary saturation and kidney calcium content in genetic hypercalciuric rats. Kidney Int 45: 58–65, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Bushinsky DA, LaPlante K, Asplin JR. Effect of cinacalcet on urine calcium excretion and supersaturation in genetic hypercalciuric stone-forming rats. Kidney Int 69: 1586–1592, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Bushinsky DA, Lechleider RJ. Mechanism of proton-induced bone calcium release: calcium carbonate-dissolution. Am J Physiol Renal Fluid Electrolyte Physiol 253: F998–F1005, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Bushinsky DA, Michalenka AC, Strutz KL, Donahue S, Asplin JR. Effect of bolus and divided feeding on urine ions and supersaturation in genetic hypercalciuric stone-forming rats. Kidney Int 73: 423–429, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Bushinsky DA, Neumann KJ, Asplin J, Krieger NS. Alendronate decreases urine calcium and supersaturation in genetic hypercalciuric rats. Kidney Int 55: 234–243, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Bushinsky DA, Parker WR, Asplin JR. Calcium phosphate supersaturation regulates stone formation in genetic hypercalciuric stone-forming rats. Kidney Int 57: 550–560, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Bushinsky DA, Willett T, Asplin JR, Culbertson C, Che SPY, Grynpas M. Chlorthalidone improves vertebral bone quality in genetic hypercalciuric stone-forming rats. J Bone Miner Res 26: 1904–1912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro-o M, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA 105: 9805–9810, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornet A, Baudet C, Neveu I, Baron-Van Evercooren A, Brachet P, Naveilhan P. 1,25-Dihydroxyvitamin D3 regulates the expression of VDR and NGF gene in Schwann cells in vitro. J Neurosci Res 53: 742–746, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Evan AP, Bledsoe SB, Smith SB, Bushinsky DA. Calcium oxalate crystal localization and osteopontin immunostaining in genetic hypercalciuric stone-forming rats. Kidney Int 65: 154–161, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Favus MJ, Karnauskas AJ, Parks JH, Coe FL. Peripheral blood monocyte vitamin D receptor levels are elevated in patients with idiopathic hypercalciuria. J Clin Endocrinol Metab 89: 4937–4943, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Frick K, Asplin J, Favus M, Culbertson C, Krieger N, Bushinsky D. Increased biological response to 1,25(OH)2D3 in genetic hypercalciuric stone-forming rats. Am J Physiol Renal Physiol 304: F718–F726, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frick KK, Bushinsky DA. Molecular mechanisms of primary hypercalciuria. J Am Soc Nephrol 14: 1082–1095, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J. Claudin-14 regulates renal Ca2+ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J 31: 1999–2012, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grynpas M, Waldman S, Holmyard D, Bushinsky DA. Genetic hypercalciuric stone-forming rats have a primary decrease in bone mineral density and strength. J Bone Miner Res 24: 1420–1426, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Healy KD, Zella JB, Prahl JM, DeLuca HF. Regulation of the murine renal vitamin D receptor by 1,25-dihydroxyvitamin D3 and calcium. Proc Natl Acad Sci USA 100: 9733–9737, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoenderop JGJ, Nilius B, Bindels RJM. Calcium absorption across epithelia. Physiol Rev 85: 373–422, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Hoopes RR, Jr, Middleton FA, Sen S, Hueber PA, Reid R, Bushinsky DA, Scheinman SJ. Isolation and confirmation of a calcium excretion quantitative trait locus on chromosome 1 in genetic hypercalciuric stone-forming congenic rats. J Am Soc Nephrol 17: 1292–1304, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Hoopes RR, Reid R, Sen S, Szpirer C, Dixon P, Pannet A, Thakker RV, Bushinsky DA, Scheinman SJ. Quantitative trait loci for hypercalciuria in a rat model of kidney stone disease. J Am Soc Nephrol 14: 1844–1850, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci USA 106: 15350–15355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Insogna KL, Broadus AE, Dryer BE, Ellison AF, Gertner JM. Elevated production rate of 1,25-dihydroxyvitamin D in patients with absorptive hypercalciuria. J Clin Endocrinol Metab 61: 490–495, 1985 [DOI] [PubMed] [Google Scholar]
- 39.Joshi R. Hypercalcemia due to hypervitaminosis D: report of seven patients. J Trop Pediatr 55: 396–398, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Karnauskas AJ, van Leeuwen JP, van den Bemd GJ, Kathpalia PP, DeLuca HF, Bushinsky DA, Favus MJ. Mechanism and function of high vitamin D receptor levels in genetic hypercalciuric stone-forming rats. J Bone Miner Res 20: 447–454, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Kim M, Sessler NE, Tembe V, Favus MJ, Bushinsky DA. Response of genetic hypercalciuric rats to a low calcium diet. Kidney Int 43: 189–196, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Koul PA, Ahmad SH, Ahmad F, Jan RA, Shah SU, Khan UH. Vitamin d toxicity in adults: a case series from an area with endemic hypovitaminosis d. Omam Med J 26: 201–204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krieger NS, Stathopoulos VM, Bushinsky DA. Increased sensitivity to 1,25(OH)2D3 in bone from genetic hypercalciuric rats. Am J Physiol Cell Physiol 271: C130–C135, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Lemann J, Jr, Pleuss JA, Worcester EA, Hornick L, Schrab D, Hoffman RG. Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney Int 49: 200–208, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Li XQ, Tembe V, Horwitz GM, Bushinsky DA, Favus MJ. Increased intestinal vitamin D receptor in genetic hypercalciuric rats: a cause of intestinal calcium hyperabsorption. J Clin Invest 91: 661–667, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu P, Boros S, Chang Q, Bindels RJ, Hoenderop JG. The β-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6. Nephrol Dial Transplant 23: 3397–3402, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Maierhofer WJ, Gray RW, Cheung HS, Lemann J., Jr Bone resorption stimulated by elevated serum 1,25-(OH)2-vitamin D3 concentrations in healthy men. Kidney Int 24: 555–560, 1983 [DOI] [PubMed] [Google Scholar]
- 48.Moe OW, Bonny O. Genetic hypercalciuria. J Am Soc Nephrol 16: 729–745, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Monico CG, Milliner DS. Genetic determinants of urolithiasis. Nat Rev Nephrol 8: 151–162, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monk RD, Bushinsky DA. Kidney stones. In: Williams Textbook of Endocrinology, edited by Kronenberg HM, Melmed S, Polonsky KS, Larsen PR. Philadelphia: W. B. Saunders, 2011, p. 1350–1367 [Google Scholar]
- 51.Nijenhuis T, Hoenderop JGJ, van der Kemp AWCM, Bindels RJM. Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J Am Soc Nephrol 14: 2731–2740, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Perry GML, Nehrke KW, Bushinsky DA, Reid R, Lewandowski KL, Hueber P, Scheinman SJ. Sex modifies genetic effects on residual variance in urinary calcium excretion in rat (Rattus norvegicus). Genetics 191: 1003–1013, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen FH, Baylink DJ, Nielsen RL. Increased serum 1,25-dihydroxy cholecalciferol (1,25 diOHD3) in patients with idiopathic hypercalciuria (IH). Clin Res 23: 423A, 1975 [Google Scholar]
- 54.Shinki T, Shimada H, Wakino S, Anazawa H, Hayashi M, Saruta T, DeLuca HF, Suda T. Cloning and expression of rat 25-hydroxyvitamin D3-1α-hydroxylase cDNA. Proc Natl Acad Sci USA 94: 12920–12925, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stechman MJ, Loh NY, Thakker RV. Genetics of hypercalciuric nephrolithiasis: renal stone disease. Acad Sci 1116: 461–484, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, Sulem P, Halldorsson BV, de VF, d'Ancona FC, den HM, Franzson L, Christiansen C, Alexandersen P, Rafnar T, Kristjansson K, Sigurdsson G, Kiemeney LA, Bodvarsson M, Indridason OS, Palsson R, Kong A, Thorsteinsdottir U, Stefansson K. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 41: 926–930, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Tsuruoka S, Bushinsky DA, Schwartz GJ. Defective renal calcium reabsorption in genetic hypercalciuric rats. Kidney Int 51: 1540–1547, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: research0034.1–research0034.11, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang W, Kwon TH, Li C, Frøkiær J, Knepper MA, Nielsen S. Reduced expression of Na-K-2Cl cotransporter in medullary TAL in vitamin D-induced hypercalcemia in rats. Am J Physiol Renal Physiol 282: F34–F44, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Werness PG, Brown CM, Smith LH, Finlayson B. Equil2: a BASIC computer program for the calculation of urinary saturation. J Urol 134: 1242–1244, 1985 [DOI] [PubMed] [Google Scholar]
- 61.Yao J, Karnauskas AJ, Bushinsky DA, Favus MJ. Regulation of renal calcium-sensing receptor gene expression in response to 1,25(OH)2D3 in genetic hypercalciuric stone forming rats. J Am Soc Nephrol 16: 1300–1308, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Yao J, Kathpalia P, Bushinsky DA, Favus MJ. Hyperresponsiveness of vitamin D receptor gene expression to 1,25-dihydroxyvitamin D3: a new characteristic of genetic hypercalciuric stone-forming rats. J Clin Invest 101: 2223–2232, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zerwekh JE, Reed BY, Heller HJ, Gonzalez GB, Haussler MR, Pak CY. Normal vitamin D receptor concentration and responsiveness to 1,25-dihydroxyvitamin D3 in skin fibroblasts from patients with absorptive hypercalciuria. Miner Electrolyte Metab 24: 307–313, 1998 [DOI] [PubMed] [Google Scholar]