Abstract
Epithelial cells are continuously exposed to mechanical forces including shear stress and stretch, although the effect these forces have on tight junction (TJ) organization and function are poorly understood. Umbrella cells form the outermost layer of the stratified uroepithelium and undergo large cell shape and surface area changes during the bladder cycle. Here we investigated the effects of bladder filling and voiding on the umbrella cell TJ. We found that bladder filling promoted a significant increase in the length of the TJ ring, which was quickly reversed within 5 min of voiding. Interestingly, when isolated uroepithelial tissue was mounted in Ussing chambers and exposed to physiological stretch, we observed a 10-fold drop in both transepithelial electrical resistance (TER) and the umbrella cell junctional resistance. The effects of stretch on TER were reversible and dependent on the applied force. Furthermore, the integrity of the umbrella cell TJ was maintained in the stretched uroepithelium, as suggested by the limited permeability of biotin, fluorescein, and ruthenium red. Finally, we found that depletion of extracellular Ca2+ by EGTA completely disrupted the TER of unstretched, but not of stretched uroepithelium. Taken together, our studies indicate that the umbrella cell TJ undergoes major structural and functional reorganization during the bladder cycle. The impact of these changes on bladder function is discussed.
Keywords: bladder, claudins, stretch, tight junction, uroepithelium
tight junctions encircle adjacent epithelial cells preventing the unregulated flux of ions, organic solutes, and water across the paracellular space. Tight junctions (TJs) are formed by the close apposition of anastomosing filamentous strands between the plasma membranes of neighboring cells. Claudins, major components of the strands, promote cell-cell adhesion and control paracellular permeability by forming anion-selective, cation-selective, or occluding pores (2, 7, 8, 19, 20, 32, 33, 45, 46, 52). A critical feature of TJs is that they must maintain their function in the face of mechanical stretch or shear stress, forces that are commonplace as air fills the lungs, as solids move along the gastrointestinal tract, as fluid passes through the nephrons of the kidney, or as fluid accumulates in sac-like organs such as the bladder. At present, surprisingly little is understood about how mechanical forces affect TJ activity (i.e., permeability), remodeling, and stability.
The umbrella cells form the urine-contacting layer of the stratified uroepithelium that lines the mucosal surface of the urinary bladder, ureters, and renal pelvis. Critically, these cells must maintain a high-resistance barrier in the face of cyclical changes in stretch as the bladder fills and empties. This is believed to be mediated in part by profound cell shape changes as they transition from a roughly inverted umbrella parasol shape when voided bladders are examined in cross section, to one that is flat and squamous in filled bladders (2a, 10). Furthermore, during filling, the apical surface area of the umbrella cell is dramatically augmented by the Rab8a-, Rab11a-, Myo5B-, and possibly Rab27b- and MAL-dependent exocytosis of discoidal/fusiform-shaped vesicles (6, 15, 16, 53, 55), which is reversed by compensatory endocytosis soon after voiding (17). Even so, almost nothing is known about how the TJ adapts to accommodate the structural changes that occur during filling and voiding or how the function of the umbrella cell paracellular barrier is altered during bladder filling and voiding.
Here, we examined the effect of stretch on TJ organization and paracellular barrier function in the uroepithelium. Our in vivo studies indicated that in addition to the well-described cell shape changes in the umbrella cells and subjacent cell layers of the uroepithelium, bladder filling caused an expansion of the umbrella cell TJ ring, which was reduced soon after voiding. Surprisingly, when isolated uroepithelium was mounted in an Ussing chamber and hydrostatic pressure in the mucosal hemichamber was increased (which mimics bladder filling), there was a significant reduction in the junctional resistance (RJ). These effects were dependent on the applied force, did not affect the integrity of the TJ, and were reversible. In summary, our studies show that stretch promotes a reorganization of the umbrella cell TJ, coupled with an increase in paracellular ion permeability. Possible reasons for increasing TJ conductance during bladder filling are discussed.
EXPERIMENTAL PROCEDURES
Reagents.
Unless described otherwise, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Rabbit polyclonal antibodies to claudin-4, claudin-8, and ZO-1 were purchased from Life Technologies (Grand Island, NY), whereas rabbit polyclonal antibodies to actin were purchased from Sigma-Aldrich. Polyclonal rabbit antibodies to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from the Proteintech Group (Chicago, IL). Alexa-488-coupled or horseradish peroxidase-coupled goat-anti-rabbit secondary antibodies were purchased from Jackson Immunoresearch (West Grove, PA). ToPro-3 and Alexa-488-streptavidin were purchased from Life Technologies.
Animals.
Urinary bladders were obtained from female Sprague-Dawley rats (250–300 g; Harlan Laboratories, Indianapolis, IN) or from female New Zealand white rabbits (3.5–4.0 kg; Covance Laboratories, Madison, WI). Following perfusion fixation (see below), rat euthanization was confirmed by a thoracotomy. Rabbits were euthanized by an intravenous injection of sodium pentobarbital into the ear vein. All animal studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Preparation of filled, voided, and quiescent bladders.
Rats were sedated by inhalation of 3% (vol/vol) isoflurane and then 1.2 g/kg urethane, dissolved in H2O, was injected subcutaneously. Isoflurane treatment was ceased, and 2 h later a toe/tail pinch was used to confirm that the animals had reached an appropriate anesthetic depth. Anesthetized rats were separated into three groups (n = 4 for each group). In the full group, we catheterized the bladder by inserting PE50 tubing into the urethra. The catheter outlet was clamped closed and the bladders were allowed to fill for 2.5 h, resulting in the accumulation of approximately 0.5–0.6 ml of urine. The animals were then perfusion-fixed as described below. In the second group (voided), the animals were catheterized as described above, the bladders were allowed to fill for 2.5 h, and then the catheter was opened to allow voiding. The animal was perfusion-fixed 5 min later. In the third group (quiescent), bladders were catheterized but the catheter outlet remained open, which prevented them from filling during the 2.5-h incubation time.
At the end of the experiment, the still-anesthetized animals in each group were perfusion-fixed as follows. A midline incision was made exposing the peritoneum, followed by exposure of the thoracic cavity. A catheter connected to a flask of warm PBS, held 20 cm above the animal, was inserted into the left ventricle and the caudal vena cava was snipped with sharp scissors. Following a 5-min perfusion with PBS, the perfusate was switched to 100 mM sodium cacodylate pH 7.4 containing 4% (wt/vol) paraformaldehyde. The bladder was then excised, placed in fixative for an additional 30 min, and then either processed for whole mount microscopy (see below) or cut into pieces, cryoprotected in 35% (wt/vol) sucrose, and then frozen on dry ice in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA). Tissue blocks were stored at −70°C prior to sectioning.
Immunofluorescent labeling, image capture, and determination of TJ length.
Whole-mount labeling of the bladder tissue was performed as described previously (1, 17). Cryopreserved tissues were sectioned using a Leica CM1950 cryostat. Sections (4 μm in thickness) were then stained with phalloidin to label the cortical actin cytoskeleton; claudin-4, claudin-8, or ZO-1 antibodies to label the umbrella cell TJ; and ToPro-3 to label the nuclei. Images were captured using a Leica HCX PL APO 63× 1.3 NA glycerol objective or a Leica STED 100× 1.4 NA oil objective and the appropriate laser lines of a Leica TCS SP5 CW-STED confocal microscope (in normal confocal mode). The photomultipliers were set at 600–1,200V, and 8-bit images were collected using 4–8 line averages combined with 4–8 frame averages. Serial 0.25-μm Z-sections were acquired. For whole-mounted tissues, images were imported into Volocity 4-D software (Perkin Elmers, Waltham, MA), and following image reconstruction in three-dimension (3-D), were exported as TIFF files. For quantitation of TJ length, confocal Z-sections were collected from a total of 40 randomly chosen regions (10 regions from each of 4 animals), the tissue was reconstructed in 3-D (to aid in identifying the apico-lateral junctions of the cell), and then the length of five randomly chosen umbrella cell TJs from each region was measured using the calibrated line tool available in the Volocity Quantitation Module. Because the TJ describes the outer margins of each umbrella cell, we were in essence making measurements of the cell circumference. For publication, images were contrast-corrected using Photoshop CS6 (Adobe Systems, San Jose, CA), and the composite images were prepared in Adobe Illustrator CS6.
Western blotting.
This analysis was performed using our previously described methods (43, 54).
Scanning electron microscopy analysis.
For scanning electron microscopy (SEM), bladders from perfusion-fixed rats were plunged into EM fixative (2.0% vol/vol glutaraldehyde, 2% wt/vol paraformaldehyde, 2 mM CaCl2, 1 mM MgCl2 in 100 mM sodium cacodylate pH 7.4) and incubated for 15 min. Afterward, the bladder was carefully cut open and then further fixed for 45 min. The tissue was washed with 100 mM sodium cacodylate pH 7.4, cut into small blocks (2–5 mm in size), and then postfixed in 1.0% wt/vol OsO4 and 1.0% wt/vol K4Fe(CN)6 in 100 mM sodium cacodylate pH 7.4. The tissues were then dehydrated in a graded series of ethanol, critical-point dried, and sputter-coated with a gold-palladium mixture. Samples were viewed in a JEOL T-300 scanning electron microscope and photographed using an attached Nikon D40 digital camera. Digital images were imported into Adobe Lightroom 4.0, and the images processed by using the brightness, contrast, and clarity controls. Images were sharpened using a radius of 0.5 and luminance noise was reduced. The composite images were assembled using Adobe Illustrator CS6.
Preparation of rabbit uroepithelium and electrophysiological measurements.
Urinary bladders were harvested from euthanized animals through an abdominal incision and then mounted in a dissection chamber (49). The tissue was maintained in Krebs solution containing (in mM) NaCl 110, NaHCO3 25, KCl 5.8, MgSO4 1.2, KH2PO4 1.2, glucose 11, CaCl2 2 buffered at pH 7.4 by gassing with a mixture of 95% O2/5% CO2. The mucosa was carefully dissected of the muscle layers as described previously (49), and the uroepithelium was mounted onto modified Ussing chamber P2315 tissue sliders (Physiologic Instruments, San Diego, CA) with a 1.27-cm diameter opening surrounded by eight 3.5-mm sharp pins placed at ∼3 mm from the opening. Silicone grease (Dow Corning, Midland, MI) was carefully applied to the pinned edges of the tissue to prevent edge damage. The tissue sliders were assembled into the chambers of an EM-CSYS Ussing system (Physiologic Instruments) equipped with a heat block for temperature control. We have shown that the mucosal surface of the bladder unfolds during bladder filling and then refolds upon voiding (53). The unfolding of the mucosa during filling stimulates the exocytosis of discoidal/fusiform-shaped vesicles and can be mimicked in vitro by bowing Ussing chamber-mounted uroepithelium outward (away from the bladder lumen). The refolding of the mucosal uroepithelium during voiding occurs in response to the contractions of the detrusor, along with the loss of fluid, and can be simulated in vitro by bowing the tissue inward. This last procedure stimulates endocytosis, decreases short circuit current, and decreases transepithelial conductance. In the current study, the mucosal and serosal hemichambers were filled with 3 and 5 ml of Krebs solution, respectively. Under these conditions the difference in hydrostatic pressure between the serosal and mucosal sides is ∼0.6 cmH2O, causing the tissue to bow slightly inward. We found that this treatment promotes high transepithelial electrical resistance (TER). The temperature inside the chambers was kept at 37°C. The hemichambers were continuously bubbled with 95% O2/5% CO2. The tissue was allowed to equilibrate for at least 1 h before the experimental manipulations. Only preparations that exhibited a resistance >5,000 Ω/cm2 were used in this study. Electrodes for voltage sensing and current passing were constructed of silver wire embedded in agar containing 5 M NaCl. TER was measured under current-clamp conditions with a VCC MC6 Multichannel Voltage/Current Clamp (Physiologic Instruments). Data were acquired at 100 Hz using a Powerlab 8/30 analog-to-digital converter (AD Instruments, Colorado Springs, CO). To calculate the TER of the uroepithelium, a square pulse of 5–10 μA current with duration of 250 ms was applied every 60 s. In some experiments the volume in the mucosal hemichamber was increased by sequentially adding fifteen 0.2-ml aliquots of prewarmed and gassed Krebs solution, which augmented the hydrostatic pressure by ∼1 cmH2O.
Measurement of RJ.
To determine RJ we used a method previously described by Lewis and Wills (25). Briefly, rabbit bladder mucosa was mounted in Ussing chambers and equilibrated in Krebs solution as described above. The mucosal solution was then isovolumetrically replaced with a K-Krebs solution containing (in mM) KCl 115.8, NaHCO3 25, MgSO4 1.2, KH2PO4 1.2, glucose 11, and CaCl2 2 buffered at pH 7.4 and gassed with 95% O2/5% CO2. After equilibration, the uroepithelium was left in a quiescent, unstretched state (control) or subjected to stretch by raising the pressure head as described above. At the indicated time, gramicidin D (GrD; MP Biomedicals, Solon, OH), dissolved in ethanol and used at a final concentration of 50 μM, was added to the mucosal hemichamber, a treatment that selectively permeabilizes the apical membrane without altering the electromotive force (25). To calculate RJ we used the following equations previously derived by Lewis (22): where VT is the transepithelial voltage, Ec is the cellular electromotive source, and Rc is the resistance of the transcellular pathway. The epithelial transcellular and paracellular pathways are arranged in parallel, such that:
Reorganizing equation 1 and equation 2 gives the following:
The addition of GrD to the mucosal compartment perturbs the cell resistance, causing a gradual decrease in TER and an increase in VT. To estimate RJ, we plotted paired values of VT and TER following GrD addition and fitted the data using linear regression. The X- and Y-intercepts of the fit line equal RJ and Ec, respectively.
Ruthenium red permeability.
The uroepithelium was mounted in Ussing chambers and was either left unstretched or was stretched by increasing the pressure head as described above. The tissue was fixed in situ by adding 0.2% ruthenium red and 2% glutaraldehyde dissolved in Krebs buffer to the mucosal hemichamber for 15 min. Fixed samples were processed for transmission electron microscopy (TEM) as previously described (43). Briefly, the tissue was postfixed for 15 min at room temperature with 0.2% ruthenium red, 2% OsO4 in 100 mM sodium cacodylate buffer pH 7.4. Following a wash with water, the samples were en bloc stained overnight in 0.5% wt/vol uranyl acetate. The tissue were then dehydrated in a graded series of ethanol, and following incubation in propylene oxide, the tissue blocks were embedded in the epoxy resin LX-112 (Ladd, Burlington, VT) and cured for 2 days at 60°C. Embedded tissue was sectioned with a diamond knife (Diatome, Fort Washington, PA), and sections, silver to pale gold in color, were mounted on butvar-coated nickel grids, contrasted with uranyl acetate and lead citrate, and viewed at 80 kV in a JEOL (Japan) 100 CX electron microscope. Images were captured using an L9C Peltier-cooled TEM camera system (Scientific Instruments and Applications, Duluth, GA). Images were processed and figures generated as described above for the SEM analysis.
Biotin permeability assay.
Rabbit uroepithelium mounted in the Ussing chambers and equilibrated in Krebs solution as indicated above, was either left unstretched or was stretched. EZ-link sulfo-NHS-biotin (Thermo Scientific) dissolved in DMSO was then added to the mucosal hemichamber to give a final concentration of 1 mg/ml. Following an incubation for 15 min, the tissue was fixed in situ with 4% paraformaldehyde for 30 min, embedded in OCT compound, cryosectioned, labeled with Alexa-488-streptavidin, TRITC-phalloidin and ToPro-3, and images were captured using confocal microscopy as described above.
Flux measurements.
The rabbit uroepithelium was mounted in Ussing chambers as described above and was either left unstretched or was stretched by increasing the pressure head. Fluorescein (sodium salt) was added to the mucosal hemichamber to give a final concentration of 100 μM. Aliquots were collected at time zero and after 1 h. Fluorescence signal was measured in a 96-well GloMax microplate reader (Promega, Madison, WI). A standard curve was prepared using defined dilutions of fluorescein in Krebs solution, and linear regression was used to determine the amount of fluorescein in the samples. Fluorescein flux was expressed in nmoles (corrected by dilution) per unit of time and tissue area (nmol/h/cm2).
Data and statistical analysis.
Data are expressed as mean ± SE (n), where n equals the number of independent experiments analyzed. Parametric or nonparametric tests were employed as appropriate. P < 0.05 was considered statistically significant. Fitting and statistical comparisons were performed with Sigmaplot 11.0 (Systat Software, Chicago, Illinois) or GraphPad 5.03 (GraphPad Software, San Diego, CA).
RESULTS
Stretch promotes an expansion of the umbrella cell TJ ring.
The uroepithelium is known to undergo characteristic morphological changes during bladder filling, which are reversed soon after voiding (10, 14). These include an apparent thinning of the overall tissue height and transition of the umbrella cells from a roughly cuboidal shape to one that is flat and squamous. However, few studies have examined the tissue en face, and we are not aware of reports that describe the effects that bladder filling and voiding have on the TJ ring. We examined three populations of bladders: 1) full bladders were obtained from urethane-anesthetized rats whose bladders were allowed to fill for 2.5 h prior to perfusion fixation; 2) voided bladders from rats whose bladders filled for 2.5 h but were then perfusion-fixed 5 min after voiding; and 3) quiescent bladders rats that were unable to fill their bladders because of placement of an open transurethral catheter and were perfusion-fixed after 2.5 h. This latter set of bladders gave us a baseline to determine whether the voided samples had sufficient time to recover from the previous cycle of filling.
We first examined the uroepithelial tissues in cross section. Consistent with previous reports we observed that the uroepithelium was relatively thin in filled bladders compared with tissue from voided or quiescent bladders (Fig. 1, A and B). In these experiments, the borders of the cells were defined using rhodamine-phalloidin, which labels the cortical actin cytoskeleton of the cells. Furthermore, we observed that the umbrella cells of filled bladders were exceptionally flat and squamous, and fewer umbrella cells could fit in the field of view compared with those in voided and quiescent bladders. We also examined the distribution of claudin-8, a membrane-associated TJ protein, and ZO-1, a protein associated with the cytoplasmic surface of the TJ. Consistent with our previous report, both claudin-8 and ZO-1 where found to accumulate at the apical-lateral junction of the umbrella cell layer in what appeared as a punctate spot (Fig. 1, A and B) (1). We did not note any obvious deviation in the TJ association of claudin-8 or ZO-1 in the three populations of bladders; however, we did observe an increased amount of cytoplasmic claudin-8 in the umbrella cells of voided and quiescent bladders (Fig. 1B). No obvious differences in the amount of claudin-8 (or claudin-4) protein were detected when lysates of uroepithelial tissue from the three populations of bladders were subjected to Western blotting (Fig. 1C).
Fig. 1.
Cell shape changes and claudin and ZO-1 expression in full, voided, and quiescent rat bladders. A: cross-sections of bladder tissue were stained with antibodies to ZO-1 (green), TRITC-phalloidin (red), and ToPro-3 (blue). B: tissues were stained with an antibody to claudin-8 (green) and TRITC-phalloidin (red). The position of the TJ is indicated by arrows. Umbrella cells are outlined with dashed lines. Bottom: higher magnification views of the boxed region. C: Western blots of claudin-8 and -4 in full (F), voided (V), and quiescent (Q) bladders. GAPDH and actin expression were used as loading controls.
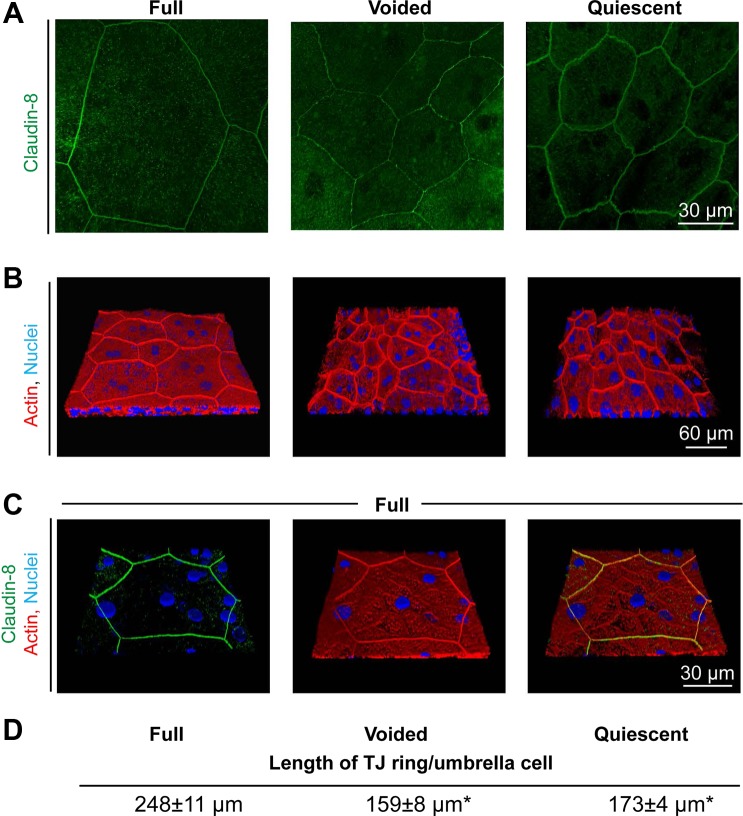
The impact of filling and voiding on the umbrella cell was also studied in whole mounted uroepithelium viewed en face. When examining the distribution of claudin-8 we observed that the outer boundaries of the umbrella cells in full tissues were exceptionally large with diameters often approaching 100 μm or more along the long axis (Fig. 2A). In contrast, those of voided and quiescent tissues were ∼45 μm (Fig. 2A). Not surprisingly then, the number of umbrella cells that fit in the image frame of full tissues was far fewer than that observed in the voided and quiescent tissues. Similar results were observed when we stained the whole-mounted tissue with phalloidin (Fig. 2B), which is enriched at the apico-lateral junction of the umbrella cell and shows strong concordance with TJ markers (1). This was confirmed in this study by showing that in full tissues, claudin-8 colocalized with the apical portion of the cortical actin cytoskeleton (Fig. 2C). Strikingly, we observed a large increase in the apparent length of the TJ encircling the umbrella cells of the full tissues (Fig. 2, A and B). By measuring the apical-most portion of the actin ring we observed that the length of the TJ ring per umbrella cell was 248 ± 11 μm in the full bladder, 159 ± 8 μm in the voided bladder, and 173 ± 4 μm in the quiescent bladder (Fig. 2D). There was a significant difference between the length of the TJ ring per umbrella cell in full and either voided or quiescent tissues, but the difference between voided and quiescent was not significant. The latter finding indicated that the increase in TJ ring length observed upon filling was rapidly reversed, and that at 5 min after voiding the tissue had reached baseline values for this parameter.
Fig. 2.
Filling promotes expansion of the TJ ring, which is reversed upon voiding. A: en face view of the umbrella cell layer in full, voided, and quiescent bladders stained with an antibody to claudin-8. Image is a projection of a confocal Z-series. B: 3-D reconstruction of bladder epithelium stained with TRITC-phalloidin (red) and ToPro-3 (blue). C: 3-D reconstruction of umbrella cell layer from stretched tissues stained with antibodies to claudin-8 (green), TRITC-phalloidin (red), and ToPro-3 (blue). D: average length of TJ per umbrella cell (mean ± SE, n = 4). Values for voided and quiescent tissues are significantly different from those of full bladders (P < 0.05 using ANOVA and a Dunnett's post hoc test). The average length of TJ is not significantly different between voided and quiescent samples.
Previous analysis using high-resolution atomic force microscopy and TEM showed that at sites of cell-cell contact the apical membrane of adjacent umbrella cells interdigitates (zippers) directly above the TJ (within a few nanometers of the first kissing point) (18). This region of zippered membrane is also visible via SEM where it appears as a very fine, raised ridge between adjacent umbrella cells (Fig. 3). We used the latter to establish whether our treatments had any morphological effects on TJ continuity or structure. Indeed, no gross morphological changes were observed in any condition, suggesting that stretch does not obviously compromise the integrity of the TJ ring (Fig. 3, A–C). Furthermore, we observed that the zippered apical membrane was not obviously folded under any condition, possibly indicating that the TJ is not folded during voiding and then unfurled during filling.
Fig. 3.
SEM analysis of the zippered apical membrane between adjacent umbrella cells. Tissues from full (A), voided (B), and quiescent (C) bladders were fixed and processed for SEM analysis. The boxed regions (left) are magnified on the right. The location and path of the zippered apical membrane is indicated by a dashed line.
Taken together, our results demonstrate that the TJ ring of the umbrella cell is significantly expanded during bladder filling, and that this increase is rapidly reversed upon voiding.
Stretch reduces paracellular resistance.
To determine whether the observed changes in TJ length affected paracellular permeability, we mounted dissected rabbit uroepithelium in Ussing chambers and measured the effects of stretch on TER (Fig. 4). We used rabbit tissues in these experiments because the electrical features of this tissue have been well studied. This tissue preparation achieves a very high TER and is amenable to ex vivo studies in Ussing chambers (22, 25, 49). When mounted in Ussing chambers, increasing the level of fluid in the mucosal hemichamber causes the tissue to bow outward, simulating filling (53). After equilibration, the TER of the uroepithelium was 14.6 ± 1.7 kΩ/cm2. However, bowing the tissue outward by the addition of 0.2-ml aliquots of buffer to the mucosal hemichamber caused the TER to drop in a stepwise fashion to 1.6 ± 0.2 kΩ/cm2, a decrease that was reversed when the added solution was slowly removed (Fig. 4, A and B). A similar recovery after experimental filling was observed when the fluid in the mucosal compartment was rapidly removed (Fig. 4C), which we previously showed simulates bladder voiding (53). In summary, filling-induced changes in TER were reversible and dependent on the applied force.
Fig. 4.
Stretch augments uroepithelial ion transport. The rabbit bladder mucosa was mounted in Ussing chambers and equilibrated with a small, negative mucosal hydrostatic pressure, causing the tissue to bow inward. A: changes in the transepithelial voltage (VT) in response to the addition (gray) or removal (black) of 0.2-ml aliquots of buffer from the mucosal compartment were recorded. A square current pulse of 10 μA was applied every 60 s to allow for determination of the TER. A representative tracing is shown. B: uroepithelial TER was plotted as a function of the volume in the mucosal compartment. Values are mean ± SE (n = 13 independent experiments). C: to mimic bladder filling and voiding, the uroepithelium was stretched by adding 15 aliquots of 0.2-ml of Krebs solution to the mucosal compartment over a period of 45 min and then the added fluid was removed at a rate of 1.5 ml/min. TER was measured 5 min after fluid removal. Values are mean ± SE (n = 7).
TER measurements reflect the overall resistance of the epithelium to ion flow. We and others previously reported increased transcellular ion transport in response to stretch (21, 23, 24, 48, 53). To assess the impact that stretch has on the paracellular pathway, we estimated the RJ in quiescent and stretched uroepithelium using a method previously described by Lewis and Wills (25). In this technique, GrD is used to permeabilize the apical membrane, causing a fall in TER and an increase in VT, allowing one to estimate RJ and Ec (see experimental procedures). To prevent changes in the ionic composition of the intracellular compartment, the permeabilization of the apical membrane was performed in the presence of a K+-rich Krebs solution. Figure 5A shows representative tracings from control (unstretched) or stretched uroepithelium (i.e., bowed outward) exposed to GrD. Whereas the estimated RJ for control uroepithelium was 21.1 ± 8.5 kΩ/cm2, that of the stretched uroepithelium was 1.8 ± 0.4 kΩ/cm2. Taken together, our studies indicate that the stretch associated with bladder filling promotes an increase in the paracellular permeability of the uroepithelium.
Fig. 5.
Stretch increases the junctional permeability of the uroepithelium. Isolated rabbit uroepithelium was mounted in Ussing chambers and the junctional resistance (RJ) was estimated as described in experimental procedures. A: representative recording showing the effect of gramicidin D (GrD) addition on the transepithelial voltage (VT) of unstretched (control) and stretched uroepithelium. A square current pulse of 10 μA was applied every 60 s to allow for determination of TER. Gray arrows indicate the addition of 0.2-ml aliquots to the mucosal compartment; black arrows indicate the addition of GrD. B: estimation of the uroepithelial junctional resistance. VT was plotted as function of TER following GrD addition for unstretched (open circles) and stretched uroepithelium (closed circles). The X- and Y-intercepts of the fit line equal the RJ and cellular electromotive force, respectively. C: RJ of unstretched (control) and stretched uroepithelium. Values are mean ± SE (n = 6–7 independent experiments; P < 0.001, using a Mann-Whitney test).
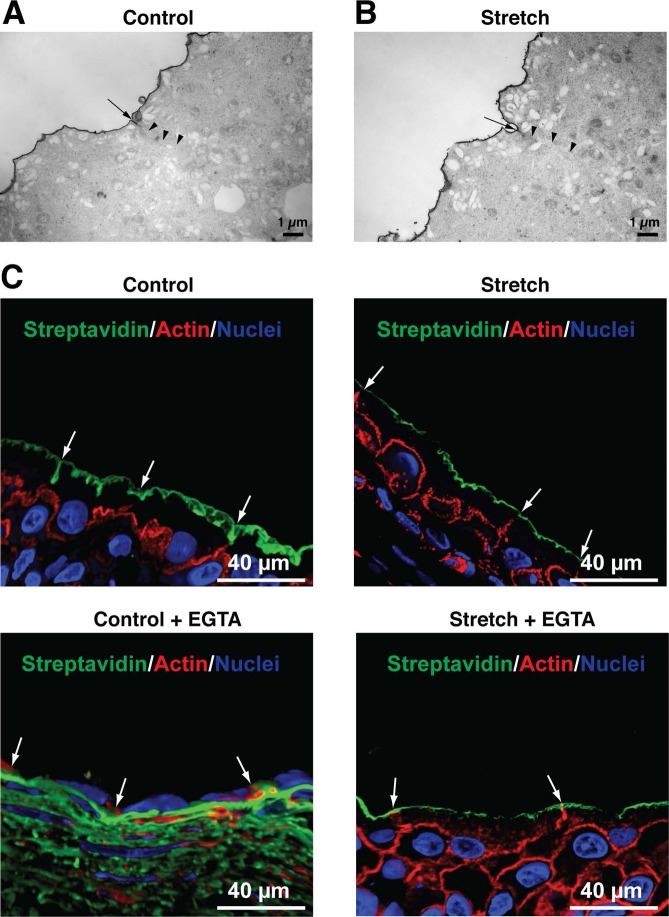
The paracellular pathway includes both an ill-defined leak pathway, which is low capacity and allows for transport of larger ions and molecules regardless of charge, and a claudin-based pore pathway that is high capacity, somewhat charge selective, and which facilitates the passage of small ions (40). To determine whether the stretch of the uroepithelium affects the leak pathway, we examined the diffusion of the membrane impermeant inorganic dye ruthenium red (molecular weight ∼860 Da) in control and stretched uroepithelium. This dye, which forms a complex with surface glycan moieties during aldehyde fixation and electron microscopy processing, was added to the mucosal surface of the tissue mounted in Ussing chambers. Following 15 min of incubation, the uroepithelium was fixed in situ and the samples were processed for TEM (Fig. 6A). We observed no ruthenium red along the lateral surfaces of the cells in either condition, indicating that the paracellular pathway remained intact in the stretched uroepithelium. To further confirm this finding, we assessed the permeability of the control and stretched uroepithelium to sulfo-NHS-biotin. This reagent is a noncleavable water-soluble biotin-derivative that reacts with primary amines and has a molecular weight of 443.4. The tissue was mounted in Ussing chambers and was left unstretched (control) or was bowed outward (stretch). Sulfo-NHS-biotin was added to the apical compartment and incubated for 15 min. The uroepithelium was fixed in situ and the biotin modification detected using Alexa-488-conjugated streptavidin. Similar to the ruthenium red experiment, the streptavidin remained at the apical plasma membrane and none was observed along the lateral membranes of the umbrella cell or in the underlying cell layers (Fig. 6B). Because the ruthenium red and biotin assays were qualitative in nature, we also measured the permeability of fluorescein (molecular weight of 332.3) added to the mucosal hemichamber of control (unstretched) or stretched tissue. The flux of fluorescein across the unstretched and stretched uroepithelium was 0.003 ± 0.002 and 0.051 ± 0.030 nmol−1·h−1·cm2, respectively. These differences were not statistically significant (P > 0.05) (Fig. 7C).
Fig. 6.
TJ integrity is maintained in stretched uroepithelium. Bladder mucosa mounted in Ussing chambers was maintained in a quiescent, unstretched condition (A) or subjected to stretch (B). Ruthenium red permeability was used to assess the integrity of the TJs by TEM. The location of the TJ is indicated by arrows, and the upper portion of the lateral membrane is indicated by arrowheads. C: bladder uroepithelium mounted in Ussing chambers remained in a quiescent state (control) or stretched in the absence or presence of EGTA. Following 30 min, sulfo-NHS-biotin was added to the mucosal hemichamber for 15 min, and the tissue was then fixed in situ. Cryosections of frozen tissue were labeled with Alexa-488-streptavidin (green), TRITC-phalloidin (red), and ToPro-3 (blue). Note the diffusion of biotin into the underlying tissues in control samples treated with EGTA. Images are representative of three independent experiments.
Fig. 7.
Remodeling of the paracellular pathway during experimental filling. A: representative recording showing the effect of EGTA on stretched uroepithelium. A square current pulse of 10 μA was applied every 60 s to allow for determination of TER. Gray arrows indicate the addition of 0.2-ml aliquots to the mucosal compartment; black arrow indicates the addition of EGTA. B: bladder uroepithelium mounted in Ussing chambers remained in an unstretched (control) state or was stretched (stretch). TER was measured at the beginning of the experiment (open bars; 1), after remaining unstretched or stretched (gray bars; 2), and 30 min after EGTA addition (closed bars; 3) (also see arrows in A). EGTA was added to the mucosal and serosal hemichambers to give a final concentration of 3 mM. Values are mean ± SE (n = 13–14 independent experiments; Mann-Whitney test, * P < 0.01 and **P < 0.001). C: tissue was mounted in Ussing chambers and remained in an unstretched (control) state or was stretched (stretch). Fluorescein was added to the mucosal hemichamber immediately after EGTA addition. Where indicated, EGTA was added to the mucosal and serosal hemichambers to give a final concentration of 3 mM. Fluorescein fluxes across the bladder uroepithelium were measured as described in experimental procedures. Values are mean ± SE (n = 4–6 independent experiments; Kruskal-Wallis followed by a Dunn's post hoc test, *P < 0.01). Differences between unstretched and stretched uroepithelium were not statistically significant (P > 0.05).
In summary, the stretch associated with outward bowing caused a marked and reversible decrease in RJ, apparently without affecting permeation across the leak pathway.
Stretch alters the sensitivity of the TJ to EGTA.
Extracellular Ca2+ is necessary for the assembly and maintenance of the TJ complex (9, 26, 37, 38), and when chelated the junctional complex opens, causing a rapid decrease in the TER of epithelial monolayers (38). The mechanism of this effect is not completely understood, and has been ascribed to changes in intracellular Ca2+ as well as effects on cadherin-mediated cell-cell contact (9, 12, 26, 51). We sought to determine whether mechanical force influences the process of EGTA-dependent TJ opening. We observed that in unstretched tissues Ca2+ chelation caused a large, significant drop in the TER from 19.0 ± 1.5 to 0.2 ± 0.1 kΩ/cm2 (Fig. 7B). Consistent with these findings, we observed that EGTA treatment had a negative effect on the overall structure of the umbrella cell layer, and sulfo-NHS-biotin readily permeated into the basolateral layers of control, unstretched tissues (Fig. 6B). Unexpectedly, when EGTA was added to tissue after stretch, the TER fell from 1.7 ± 0.1 to 0.8 ± 0.1 kΩ/cm2 (Fig. 7, A and B), a value that was four times higher than that observed in control, unstretched tissues. Furthermore, there was no leakage of sulfo-NHS-biotin observed into the underlying tissues under these conditions (Fig. 6B). Finally, we observed that in unstretched, control uroepithelium, treatment with EGTA caused a large and significant increase in fluorescein flux (Fig. 7C). In contrast, EGTA had no significant effect on fluorescein flux in stretched tissues. Together, these results suggest that Ca2+ removal nearly completely obviates the paracellular pathway in unstretched, but not stretched uroepithelium.
DISCUSSION
Despite the constant exposure of epithelial cells to mechanical forces, there are few insights into how these stimuli affect TJ organization and function. Work to date shows that strand reorganization occurs in response to experimental cell stretch or upon lactation (when secretory granules fill the cell cytoplasm), and ligation of the pancreatic or bile duct is accompanied by decreased numbers of junctional strands, discontinuities in the strands, and increased paracellular leakage (5, 29, 30, 34, 35). Still yet, hydrodynamic gene therapy depends on increasing the fluid pressure in thin, stretchable capillaries, which likely promotes DNA/viral delivery by increasing the permeability of endothelial-associated TJs (50). Concordantly, excess stretch leads to junction failure (4). In our studies of the uroepithelium, we observed that filling stimulates an expansion of the umbrella cell TJ ring, a process that is rapidly reversed upon voiding. Furthermore, we observed that experimental filling induced an increase in ion permeability across the umbrella cell TJ and that this increase may be independent of the low capacity, nonselective leak pathway. However, the latter conclusion requires a more thorough examination of the permeability properties of the TJ under unstretched and stretched conditions. The implications of these somewhat unexpected findings for umbrella cell biology and function are discussed.
Reminiscent of the changes in umbrella cell apical surface area (1, 15, 16, 43, 53), we observed a significant increase in umbrella cell TJ length in rats whose bladders were allowed to fill to near capacity vs. those in voided or quiescent samples. Strikingly, TJ length decreased by ∼60% within 5 min of voiding. SEM studies of the zippered apical membrane that sits atop the junction did not show any obvious pleating. However, this technique does not provide information about changes that are occurring below the apical surface, and therefore we cannot rule out that micropleating is occurring below the zippered membrane or that the strand architecture of TJ is being dynamically rearranged to accommodate stretch. An additional mechanism that may contribute to the filling-induced TJ ring expansion is the insertion of newly synthesized or recycled membrane proteins at the TJ. Consistent with this possibility are experiments in other cell systems that show the membrane components of the TJ are highly dynamic, even at steady state (41), and that TJ assembly is dependent on exocytosis (27, 31, 41). Whereas exocytosis controls TJ expansion during filling, we predict an important role for endocytosis during voiding. Several studies reported TJ protein internalization by pathways requiring clathrin-coated pits, caveolae, or macropinosomes (3, 11, 28, 39, 42, 44). We previously showed that voiding stimulates a rapid, clathrin-independent endocytosis of umbrella cell apical membrane (17). Intriguingly, this internalized membrane is delivered to ZO-1-positive apical endosomes that are enriched around the junctional complex. However, whether endocytosed TJ membrane proteins are delivered to these same endosomes is unknown.
In addition to effects on TJ dynamics, we also observed that filling/voiding had a major impact on the TER of the uroepithelial tissue mounted in an Ussing chamber. Outward bowing, which simulates filling, decreased TER, whereas inward bowing, which mimics the refolding of the epithelium during voiding, had the opposite effect (53). We previously ascribed the stretch-induced drop in TER to increased transcellular ion transport (53); however, our current studies demonstrated that stretch also caused a marked increase in paracellular ion transport (with its corresponding decrease in RJ). Intriguingly, the corresponding decrease in RJ was not obviously the result of increased transport across the TJ-associated leak pathway because small molecules such as biotin, fluorescein, and ruthenium red did not penetrate the TJ barrier during experimental filling. Instead, it appears the decreased RJ was a result of ion movements across the other pathway for ion conduction in the TJ: the claudin permeation pathway. We previously reported expression of claudins 2, 4, 8, 12, and 13 in the native mouse uroepithelium (1), and other claudin species are reported in cultured human cells (36, 47). The claudins can form occluding pores, cation-selective pores, or anion-selective pores (19). Of the claudins known to be expressed in rat umbrella cells, claudin-4 and claudin-8 are generally believed to form occluding pores, whereas claudin-2, which is apparently expressed by umbrella cells but not obviously localized to their TJs (1), forms a cation-conducting pore (52). Importantly, claudins are arranged in strands, and stretch apparently affects their organization (5, 29, 30, 34, 35). Thus filling may affect the organization of the umbrella cell TJ strands, changing the conductive properties of the claudin-based pores.
The TJ is but one component of the junctional complex, which also includes the adherens junction (AJ) and the subjacent desmosomes. Although we have not examined these latter two junctions directly, it is likely that the AJ ring also expands to accommodate bladder filling and then shrinks upon voiding. Importantly, the AJ and TJ cell-cell adhesion complexes are functionally interconnected and their assembly and stability depend on extracellular Ca2+ (9, 26, 37, 38, 51). This is consistent with our observation that Ca2+ chelation completely disrupted the paracellular barrier of quiescent bladder tissue, resulting in a dramatic drop in TER and leakage of biotin and fluorescein from the apical to basolateral surfaces of the uroepithelium. In contrast, once the tissue was stretched, Ca2+ depletion caused a relatively modest decrease in TER, and no significant increase in leakage of the tested macromolecules was observed. Although the mechanisms involved remain to be elucidated, the interaction between cadherins on adjacent cell membranes may be stabilized as a result of tension-induced changes in their structure or by interactions with AJ-associated proteins in response to stretch. Finally, although none of the membrane protein constituents of the TJs are Ca2+-binding proteins, increasing intracellular Ca2+ can perturb TJ function (9, 26). Because intracellular Ca2+ apparently increases in the umbrella cell during stretch (49, 53), it is possible that this serves to modulate TJ function and paracellular permeability during bladder filling.
Although the barrier function of the uroepithelium has been appreciated for decades, only recently has it become apparent that the uroepithelium also plays an important role in transmitting information about the bladder mucosa and its milieu to the nervous and muscular tissues of the bladder (14). We hypothesize that it performs this function in part by acting as a tissue-type mechanosensor. Indeed, the uroepithelium responds to stretch by releasing various mediators including ATP, adenosine, and NO (14). In turn, these molecules likely act by altering the function of one or more of the underling cell types, including sensory afferent and efferent neurons that innervate the uroepithelium, the subepithelial interstitial cells, or the smooth muscle cells that comprise the detrusor. Our current studies show that in addition to stimulating release of mediators, stretch also increases ion permeability across the TJ of the umbrella cells. We predict that this increased permeability may alter the function of sensory neurons or muscle cells by altering their ionic milieu. Although our studies focused on the uroepithelium, it should be clear that the epithelial cells in other organs of the body are subjected to similar physical forces. Thus stretch-induced paracellular ion permeability may be a common mechanism for epithelial cells to communicate to the underlying tissues in other sac-like organs.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R37-DK-54425, R01-DK-077777, and P30-DK-079307 to G.A.; R01-DK-084060 to M.D.C.; R01-DK-099196 to G.A and M.D.C.; and by Cellular Physiology and Kidney Imaging Cores of the Pittsburgh Center for Kidney Research through Grant P30-DK-079307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.D.C., H.S.P., and G.A. conception and design of research; M.D.C., H.S.P., W.G.R., D.R.C., M.M., and L.I.G. performed experiments; M.D.C., H.S.P., W.G.R., D.R.C., and G.A. analyzed data; M.D.C., H.S.P., W.G.R., D.R.C., and G.A. interpreted results of experiments; M.D.C., H.S.P., W.G.R., D.R.C., and G.A. prepared figures; M.D.C. and G.A. drafted manuscript; M.D.C., H.S.P., and G.A. edited and revised manuscript; M.D.C., H.S.P., W.G.R., D.R.C., M.M., L.I.G., and G.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Shriya Venkatesh for expert technical assistance.
REFERENCES
- 1.Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, Apodaca G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol 287: F305–F318, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1: a002584, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Apodaca G. Stretch-regulated exocytosis of discoidal vesicles in urinary bladder epithelium. Urology 57: 103–104, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J 19: 923–933, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Cavanaugh KJ, Jr, Margulies SS. Measurement of stretch-induced loss of alveolar epithelial barrier integrity with a novel in vitro method. Am J Physiol Cell Physiol 283: C1801–C1808, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Cavanaugh KJ, Jr, Oswari J, Margulies SS. Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol 25: 584–591, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Guo X, Deng FM, Liang FX, Sun W, Ren M, Izumi T, Sabatini DD, Sun TT, Kreibich G. Rab27b is associated with fusiform vesicles and may be involved in targeting uroplakins to urothelial apical membranes. Proc Natl Acad Sci USA 100: 14012–14017, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuse K, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol 143: 391–401, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol 147: 891–903, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Mariscal L, Contreras RG, Bolívar JJ, Ponce A, Chávez De Ramirez B, Cereijido M. Role of calcium in tight junction formation between epithelial cells. Am J Physiol Cell Physiol 259: C978–C986, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Hicks M. The mammalian urinary bladder: an accommodating organ. Biol Rev 50: 215–246, 1975 [DOI] [PubMed] [Google Scholar]
- 11.Ikari A, Takiguchi A, Atomi K, Sugatani J. Epidermal growth factor increases clathrin-dependent endocytosis and degradation of claudin-2 protein in MDCK II cells. J Cell Physiol 226: 2448–2456, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Jovov B, Lewis SA, Crowe WE, Berg JR, Wills NK. Role of intracellular Ca2+ in modulation of tight junction resistance in A6 cells. Am J Physiol Renal Fluid Electrolyte Physiol 266: F775–F784, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 297: F1477–F1501, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khandelwal P, Prakasam HS, Clayton DR, Ruiz WG, Gallo LI, van Roekel D, Lukianov S, Peranen J, Goldenring JR, Apodaca G. A Rab11a-Rab8a-Myo5B network promotes stretch-regulated exocytosis in bladder umbrella cells. Mol Biol Cell 24: 1007–1019, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khandelwal P, Ruiz G, Balestreire-Hawryluk E, Weisz OA, Goldenring JA, Apodaca G. Rab11a-dependent exocytosis of discoidal/fusiform vesicles in bladder umbrella cells. Proc Natl Acad Sci USA 105: 15773–15778, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khandelwal P, Ruiz WG, Apodaca G. Compensatory endocytosis in bladder umbrella cells occurs through an integrin-regulated and RhoA- and dynamin-dependent pathway. EMBO J 29: 1961–1975, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreplak L, Wang H, Aebi U, Kong XP. Atomic force microscopy of mammalian urothelial surface. J Mol Biol 374: 365–373, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krug SM, Gunzel D, Conrad MP, Lee IF, Amasheh S, Fromm M, Yu AS. Charge-selective claudin channels. Ann NY Acad Sci 1257: 20–28, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Krug SM, Gunzel D, Conrad MP, Rosenthal R, Fromm A, Amasheh S, Schulzke JD, Fromm M. Claudin-17 forms tight junction channels with distinct anion selectivity. Cell Mol Life Sci 69: 2765–2778, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis S, de Moura J. Incorporation of cytoplasmic vesicles into apical membrane of mammalian urinary bladder epithelium. Nature 297: 685–688, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Lewis SA. Epithelial electrophysiology. In: Epithelial Transport—A Guide to Methods and Experimental Analysis, edited by Wills NK, Reuss L, Lewis SA. London: Chapman & Hall, 1996, p. 93–117 [Google Scholar]
- 23.Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol 278: F867–F874, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Lewis SA, Diamond JM. Na+ transport by rabbit urinary bladder, a tight epithelium. J Membr Biol 28: 1–40, 1976 [DOI] [PubMed] [Google Scholar]
- 25.Lewis SA, Wills NK. Electrical properties of the rabbit urinary bladder assessed using gramicidin D. J Membr Biol 67: 45–53, 1982 [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Palomo A, Meza I, Beaty G, Cereijido M. Experimental modulation of occluding junctions in a cultured transporting epithelium. J Cell Biol 87: 736–745, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marzesco AM, Dunia I, Pandjaitan R, Recouvreur M, Dauzonne D, Benedetti EL, Louvard D, Zahraoui A. The small GTPase Rab13 regulates assembly of functional tight junctions in epithelial cells. Mol Biol Cell 13: 1819–1831, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuda M, Kubo A, Furuse M, Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci 117: 1247–1257, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Metz J, Aoki A, Merlo M, Forssmann WG. Morphological alterations and functional changes of interhepatocellular junctions induced by bile duct ligation. Cell Tissue Res 182: 299–310, 1977 [DOI] [PubMed] [Google Scholar]
- 30.Metz J, Merlo M, Billich H, Forssmann WG. Exocrine pancreas under experimental conditions. IV. Alterations of intercellular junctions between acinar cells following pancreatic duct ligation. Cell Tissue Res 186: 227–240, 1978 [DOI] [PubMed] [Google Scholar]
- 31.Morimoto S, Nishimura N, Terai T, Manabe S, Yamamoto Y, Shinahara W, Miyake H, Tashiro S, Shimada M, Sasaki T. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J Biol Chem 280: 2220–2228, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Morita K, Sasaki H, Fujimoto K, Furuse M, Tsukita S. Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J Cell Biol 145: 579–588, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161: 653–660, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitelka DR, Hamamoto ST, Duafala JG, Nemanic MK. Cell contacts in the mouse mammary gland. I. Normal gland in postnatal development and the secretory cycle. J Cell Biol 56: 797–818, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitelka DR, Taggart BN. Mechanical tension induces lateral movement of intramembrane components of the tight junction: studies on mouse mammary cells in culture. J Cell Biol 96: 606–612, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rickard A, Dorokhov N, Ryerse J, Klumpp DJ, McHowat J. Characterization of tight junction proteins in cultured human urothelial cells. In Vitro Cell Dev Biol Anim 44: 261–267, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothen-Rutishauser B, Riesen FK, Braun A, Gunthert M, Wunderli-Allenspach H. Dynamics of tight and adherens junctions under EGTA treatment. J Membr Biol 188: 151–162, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Sedar AW, Forte JG. Effects of calcium depletion on the junctional complex between oxyntic cells of gastric glands. J Cell Biol 22: 173–188, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell 16: 3919–3936, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Ann Rev Physiol 73: 283–309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol 181: 683–695, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamatovic SM, Keep RF, Wang MM, Jankovic I, Andjelkovic AV. Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells. J Biol Chem 284: 19053–19066, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Truschel ST, Wang E, Ruiz WG, Leung SM, Rojas R, Lavelle J, Zeidel M, Stoffer D, Apodaca G. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell 13: 830–846, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell 16: 5040–5052, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Itallie C, Anderson JM. The molecular physiology of tight junction pores. Physiology 19: 331–338, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol 291: F1288–F1299, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Varley CL, Garthwaite AE, Cross WR, Hinley J, Trejdosiewicz LK, Southgate J. PPARg-regulated tight junction development during human urothelial cytodifferentiation. J Cell Physiol 208: 407–417, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang E, Lee JM, Johnson JP, Kleyman T, Bridges R, Apodaca G. Hydrostatic pressure-regulated ion transport in bladder uroepithelium. Am J Physiol Renal Physiol 285: F651–F663, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Wang E, Truschel ST, Apodaca G. Analysis of hydrostatic pressure-induced changes in umbrella cell surface area. Methods 30: 207–217, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Wang G, Zabner J, Deering C, Launspach J, Shao J, Bodner M, Jolly DJ, Davidson BL, McCray PB., Jr Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo. Am J Respir Cell Mol Biol 22: 129–138, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Yoshida C, Takeichi M. Teratocarcinoma cell adhesion: identification of a cell-surface protein involved in calcium-dependent cell aggregation. Cell 28: 217–224, 1982 [DOI] [PubMed] [Google Scholar]
- 52.Yu AS, Cheng MH, Angelow S, Gunzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol 133: 111–127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu W, Khandelwal P, Apodaca G. Distinct apical and basolateral membrane requirements for stretch-induced membrane traffic at the apical surface of bladder umbrella cells. Mol Biol Cell 20: 282–295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu W, Zacharia LC, Jackson EK, Apodaca G. Adenosine receptor expression and function in bladder uroepithelium. Am J Physiol Cell Physiol 291: C254–C265, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Zhou G, Liang FX, Romih R, Wang Z, Liao Y, Ghiso J, Luque-Garcia JL, Neubert TA, Kreibich G, Alonso MA, Schaeren-Wiemers N, Sun TT. MAL facilitates the incorporation of exocytic uroplakin-delivering vesicles into the apical membrane of urothelial umbrella cells. Mol Biol Cell 23: 1354–1366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]