Abstract
1, 25-Dihydroxycholechalciferol (calcitriol) and 19-nor-1, 25-dihydroxyvitamin D2 (paricalcitol) are vitamin D receptor (VDR) agonists. Previous data suggest VDR agonists may actually increase renin-angiotensin activity, and this has always been assumed to be mediated by hypercalcemia. We hypothesized that calcitriol and paricalcitol would increase plasma renin activity (PRA) independently of plasma Ca2+ via hypercalciuria-mediated polyuria, hypovolemia, and subsequent increased β-adrenergic sympathetic activity. We found that both calcitriol and paricalcitol increased PRA threefold (P < 0.01). Calcitriol caused hypercalcemia, but paricalcitol did not. Both calcitriol and paricalcitol caused hypercalciuria (9- and 7-fold vs. control, P < 0.01) and polyuria (increasing 2.6- and 2.2-fold vs. control, P < 0.01). Paricalcitol increased renal calcium-sensing receptor (CaSR) expression, suggesting a potential cause of paricalcitol-mediated hypercalciuria and polyuria. Volume replacement completely normalized calcitriol-stimulated PRA and lowered plasma epinephrine by 43% (P < 0.05). β-Adrenergic blockade also normalized calcitriol-stimulated PRA. Cyclooxygenase-2 inhibition had no effect on calcitriol-stimulated PRA. Our data demonstrate that vitamin D increases PRA independently of plasma Ca2+ via hypercalciuria, polyuria, hypovolemia, and increased β-adrenergic activity.
Keywords: renin, PRA, calcium vitamin D, calcitriol, paricalcitol, calcium-sensing receptor (CaSR), PTH, polyuria, hypercalciuria, sympathetic activity, COX-2
1, 25-dihydroxycholechalciferol (calcitriol) is a steroid hormone and is the active metabolite of the vitamin D system. Calcitriol exerts its effects via the vitamin D receptor (VDR), a steroid nuclear receptor (55). Calcitriol is known to play an important role in the regulation of calcium (Ca2+) homeostasis. Calcitriol increases both renal and intestinal Ca2+ reabsorption (27) and also permits parathyroid hormone (PTH)-mediated bone resorption (13), culminating in elevated plasma Ca2+. Calcitriol decreases plasma PTH levels indirectly via its effects on plasma Ca2+ (8), as well as via direct effects on PTH transcription (39).
Vitamin D has been previously shown to stimulate plasma renin activity (PRA) (32, 42). The mechanism by which this occurs remains unknown but has always been assumed to be due to the effects of hypercalcemia. Elevations in plasma Ca2+ are known to inhibit thick ascending limb Na+-K+-2Cl− (NKCC) transport (32) and induce polyuria and hypovolemia (32), both of which could serve as powerful stimuli for increasing PRA. The polyuric effects of hypercalcemia are mediated by the calcium-sensing receptor (CaSR) (9), which impairs tubular transport in different nephron segments (37, 50). Whether vitamin D could stimulate PRA independently of plasma Ca2+ is unknown. 19-nor-1,25-Dihydroxyvitamin D2 (paricalcitol) is a VDR agonist that suppresses plasma PTH, but unlike calcitriol, has minimal effects on plasma Ca2+ (16, 43). To our knowledge, the effects of paricalcitol on renal Ca2+ handling, polyuria, and concentrating defects have not been studied in depth. Paricalcitol could cause polyuria and elevate PRA independently of plasma Ca2+ by upregulating the expression of the renal CaSR and causing hypercalciuria. Activating mutations of the CaSR are known to cause polyuria, hypercalciuria, and hyperreninemia, in part by impairing collecting duct water transport. (37, 46, 47, 52) mediated by aquaporin-2 (AQP2). In support of this notion, paricalcitol is known to increase parathyroid gland CaSR mRNA (54).
Vitamin D-induced hypercalcemia causes its polyuric effects in part through the increased production of prostaglandins (38). Elevated Ca2+ can increase the expression and activity of cyclooxygenase-2 (COX-2) in the renal medulla (49), but whether COX-2 activity actually affects hypercalcemia-mediated polyuria or PRA is unknown. It is well known that loop diuretics and dietary NaCl restriction increase PRA in part by increasing macula densa COX-2 activity and represent a major pathway for renin regulation (18, 19). Whether hypercalciuria increases PRA via natriuresis-mediated elevations in macula densa COX-2 activity is unknown.
Additionally, hypercalcemia-induced polyuria could increase PRA due to reduced circulating volume, which is also a powerful stimulus for elevating PRA. Hypovolemia increases PRA in part due to an effect mediated by β-adrenergic activation (11), and β-adrenergic receptors are integral for normal plasma renin levels (20). In support of this, adrenal medullectomy and renal denervation partially impair the hypovolemia-mediated rise in PRA (6). It is known that plasma catecholamine levels can be elevated in hypercalcemia (41, 48). However, whether calcitriol-induced polyuria increases PRA via β-adrenergic stimulation is unknown.
The aim of this study was to determine whether vitamin D increases plasma renin independently of plasma calcium and to determine the mechanisms by which it does so. We hypothesized that treatment with calcitriol and paricalcitol will both increase PRA. We anticipated that paricalcitol would increase PRA independently of changes in plasma Ca2+ and will coincide elevated renal CaSR expression and hypercalciuria. Last, we anticipated that calcitriol would increase PRA via polyuria-induced hypovolemia mediated by elevated COX-2 activity and/or β-adrenoreceptor-mediated effects.
METHODS
Experimental Methods
Male Sprague-Dawley rats, weighing 200–250 g, singly housed in static caging, fed ad libitum, were used for all studies. Daily food consumption was measured by measuring the remaining food in the cage each day. Water consumption was determined gravimetrically. Both were done to the nearest gram and milliliter, respectively. The day on which treatment protocols began was considered day 1. Rats were placed in metabolic caging on day 3 to equilibrate them before a 24-h urine collection performed on day 4. Rats were returned to their static caging on day 5, and in protocol 1 (see below) systolic blood pressure was measured with an automated tail-cuff system on which the rats had been trained previously three times (see below). In experiments in which PRA was quantified, rats were euthanized on day 6 by decapitation to collect PRA samples unaffected by anesthesia. The first 3 s of free-flowing trunk blood were collected for PRA analyses in chilled tubes containing 50 μl of 3.8% EDTA in 0.9% NaCl. The collection of only the first 3 s of blood is essential to ensure that PRA analyses are not contaminated by baroreceptor-elevated PRA levels from the decapitation. Additional blood samples were collected from free-flowing trunk blood using either 50 μl of 3.8% EDTA (Sigma-Aldrich, St. Louis, MO) or sodium heparin (Sagent Pharmaceuticals, Schaumberg, IL) as anticoagulants. Blood samples were spun at 1,164 g at 4°C for 15 min, and the plasma was aspirated and stored at −20°C until further analysis. The left kidney of each rat was rapidly excised and weighed.
In experiments in which renal CaSR mRNA was quantified, rats were injected with 50 mg/kg Nembutal (pentobarbital sodium ip, Ovation Pharmaceuticals, Deerfield, IL) on day 6. The peritoneal cavity was opened with a midline incision. Under sterile conditions, the left kidney was quickly exposed, clamped, excised, and the capsule was removed. The kidney was bisected and immediately placed in Tri-Reagent (Molecular Research Center, Cincinnati, OH). After homogenization of the kidneys, they were centrifuged at 16,000 g for 10 min at 4°C. The supernatant was aspirated and stored at −80°C until RNA was isolated. Rats were euthanized via aortic transection and bilateral pneumothorax.
Animal Welfare Assurance
All procedures were approved by the Henry Ford Health System Institutional Animal Care and Use Committee and adhered to the guiding principles in the care and use of experimental animals in accordance with the National Institute of Health (NIH) guidelines. Henry Ford Hospital operates an AALAC-certified animal care facility.
Analyses
PRA.
PRA was analyzed by generation of ANG I (ng ANG I·ml−1·h−1·min−1) using a Gamma Coat RIA kit (DiaSorin, Stillwater, MN) as previously described and according to the manufacturer's instructions (3–5).
Plasma ionized Ca2+, plasma Na+, urinary Ca2+, and Na+ quantification.
Plasma Ca2+ and Na+ were measured using a NOVA-8 electrolyte analyzer (NOVA Biomedical, Waltham, MA). Urinary Ca2+ and PO43− were measured with colorimetric (Biovision, Mountain View, CA) assay kits according to the manufacturer's instructions using a colorimetric plate reader (Titertek, Huntsville, AL). Absorbance was measured at 570 and 620 nm, respectively, and values were analyzed with Multiskan Ascent. Urinary Na+ was measured with a NOVA-1 electrolyte analyzer (NOVA Biomedical). The concentrations of Ca2+, PO43− and Na+ in the urine were multiplied by the 24-h urinary volume to achieve the amount of Ca2+ or Na+ excreted in 24 h.
Plasma PTH quantification.
In protocols 1, 2, and 3, plasma PTH 1–84 was quantified using an enzyme-linked immunoassay (Alpco Diagnostics, Salem, NH) according to the manufacturer's instructions as described previously (3–5).
Plasma and urinary creatinine quantification and creatinine clearance calculation.
Plasma and urinary creatinine were determined using a colorimetric assay (BioAssay Systems, Hayward, CA). Creatinine clearance was calculated by multiplying the concentration of urinary creatinine by the 24-h urinary volume, dividing by the plasma creatinine concentration, and then correcting the units of time for clearance to milliliters per minute. Last, clearance values were normalized per gram of kidney weight. The units for creatinine clearance are milliliters per minute per gram kidney weight.
Urine and plasma osmolality.
Urine and plasma osmolality were measured using a model 3300 Advanced Micro Osmometer (Advanced Instruments, Norwood, MA).
Tail-cuff plesmography.
In protocol 1, systolic blood pressure was measured noninvasively using a computerized tail-cuff system (model 1231, IITC, Woodland Hills, CA). Rats were trained over 3 days before systolic blood pressure measurement. Three systolic blood pressure measurements were taken from each rat, and a mean value was calculated for statistical analyses.
Plasma epinephrine.
Plasma epinephrine was determined using a commercially available ELISA kit (Rocky Mountain Diagnostics, Colorado Springs, CO) according to the manufacturer's instructions.
Real-time quantitative RT-PCR.
Quantification of CaSR mRNA was performed by quantitative real-time RT-PCR using a SYBR green method. Custom rat-specific primers from TIB Molbiol (Adelphia, NJ) were used for all of the PCRs. The primer sequences for CaSR, NKCC2, and AQP2 are as follows, respectively: forward 5′-ctgaagagaaggcaacgcta-3′, reverse 5′-tcttgatctttggctgctactc-3′; forward 5′-ggcctcatatgcgctt-3′, reverse 5′-agtgtttggcttcattctcc-3′; and forward 5′-gccacctccttgggatct-3′, reverse 5′-ccagtgatcatcaaacttgcc-3′. Real-time RT-PCR was performed as follows: 1 μg of DNase-treated total RNA sample was reverse transcribed using random primers and Omniscript reverse transcriptase (Qiagen, Valencia, CA) in a total volume of 20 μl for 1 h at 37°C followed by an inactivation step of 95°C for 5 min. Two microliters of the reverse transcription reaction was then amplified in a Roche version 2.0 LightCycler PCR instrument (Roche, Indianapolis, IN) using SYBR green dye (SA Biosciences, Frederick, MD) and specific primers. Reactions were set up in a final volume of 20 μl, which contained 2 μl of sample, 1 μmol/l each of both the primers, and 10 μl of 2× SYBR green PCR mix. After an initial “hot start” at 95°C for 10 min, amplification occurred by denaturation at 95°C for 15 s, annealing at 58°C for 35 s, and extension at 72°C for 35 s, for a total of 30–40 cycles. At the end of PCR cycling, melting curve analyses were performed. A relative quantitation method [ΔΔCt] (53) was used to evaluate expression of CaSR. RT-PCR of GAPDH was used for normalization of all data.
Experimental Protocols
Protocol 1: effect of calcitriol and paricalcitol on PRA and CaSR, NKCC2, and AQP2.
We administered 100 ng of calcitriol once per day (ip) delivered in 100 μl of DMSO. Vehicle control-treated rats were treated with matching volumes of DMSO. This dose of calcitriol was chosen due to the ability of a similar dose to decrease renal renin mRNA in mice (16, 34). To test whether calcitriol was increasing PRA due to its stimulatory effects on plasma Ca2+, we administered a “noncalcemic” analog of vitamin D, paricalcitol, which binds to and activates the VDR (43). Paricalcitol capsules (Zemplar, Abbott Pharmaceuticals) were purchased at the Henry Ford Hospital Pharmacy and were not gifts. Paricalcitol was drained from capsules using a 26-gauge needle and suspended in DMSO. Paricalcitol was injected 100 ng/day (ip) once daily. This dose of paricalcitol was chosen due to its inability to increase plasma Ca2+ (15, 16, 43, 56) and also its reported ability to decrease PRA in spontaneously hypertensive rats (22) and renal renin mRNA in mice (26). The numbers were n = 8 in the control and calcitriol groups and n = 7 for the paricalcitol group.
Additionally, to test whether the polyuria induced by paricalcitol was due to changes in CaSR, NKCC2, or AQP2 expression, rats were treated with 100 ng/day calcitriol (n = 5), paricalcitol (n = 5), or DMSO (control, n = 5). On day 6, rats were euthanized as described in Experimental Methods. Quantitative RT-PCR was performed on RNA from whole-kidney homogenates to test for changes in CaSR, NKCC2, or AQP2 expression.
Protocol 2: effects of volume replacement on calcitriol-stimulated PRA.
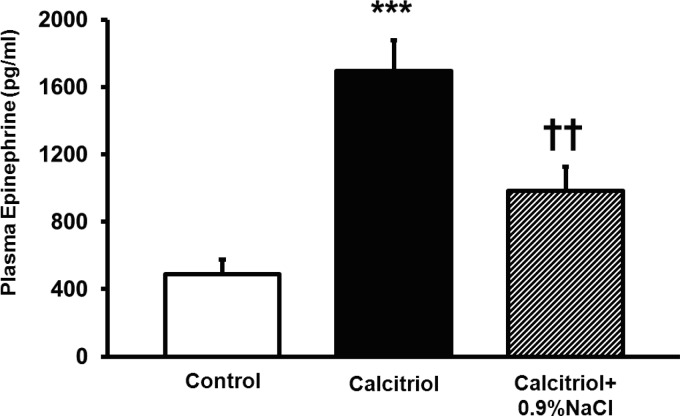
To test whether calcitriol-induced hypercalcemia increased PRA due to its effects on polyuria and hypovolemia, we performed volume replacement in calcitriol-treated rats. Rats were treated with 100 ng/day of calcitriol (ip). One group of calcitriol-treated rats received 25 ml of sterile 0.9% NaCl daily, in divided doses, subcutaneously, except on the final day, on which the rats received all 25 ml 5 h before euthanasia. This dose of saline was chosen to make the rats euvolemic because the calcitriol-treated rats in protocol 1 weighed ∼25 g less than the vehicle control-treated rats at the end of the study. Numbers were n = 8 for the calcitriol group and n = 7 for the calcitriol+0.9% NaCl group. Additionally, to demonstrate that calcitriol clearly increased plasma epinephrine levels compared with control, we sampled plasma from n = 5 control rats to include in Fig. 4.
Fig. 4.
Effects of volume replacement with 0.9% NaCl on calcitriol-elevated plasma epinephrine. Volume replacement with 0.9% NaCl completely normalized calcitriol-stimulated epinephrine and did not differ from control values. Values are means ± SE. ***P < 0.001 vs. control. ††P < 0.01 vs. calcitriol.
Protocol 3: effect of β-adrenoreceptor inhibition on calcitriol-stimulated PRA.
To test whether calcitriol increased PRA due to elevated β-adrenergic activity, we administered the propranolol (Sigma-Aldrich) to calcitriol-treated rats. Rats received 100 ng of calcitriol/day. Starting on day 1, one group of calcitriol-treated rats received 4 mg/kg propranolol daily in divided doses (ip) delivered in 100 μl of sterile 0.9% NaCl (pH = 2). This dose was based on a similar dose shown to inhibit PRA in hypovolemic states (11). A second group received calcitriol and the vehicle for propranolol. A third group received propranolol in the absence of calcitriol, and a final group received both the vehicles for propranolol and calcitriol. Numbers for all groups were n = 10.
Protocol 4: effect of COX-2 inhibition on calcitriol-stimulated PRA.
To test whether calcitriol-induced hypercalcemia increased PRA due to increased COX-2 activity, we administered the COX-2-selective inhibitor NS-398 (Cayman Chemical, Ann Arbor, MI) to calcitriol-treated rats. Rats received 100 ng of calcitriol/day. Starting on day 3, one group (n = 18) received 10 mg/kg NS-398/day, in divided doses, in 50 μl of DMSO (ip). This dose of NS-398 was chosen due to its previously demonstrated ability to inhibit COX-2 (26). The calcitriol control group (n = 18) received calcitriol and the vehicle for NS-398 (DMSO). Additionally, extra groups of rats receiving NS-398 in the absence of calcitriol (n = 8), or only DMSO (n = 8) were included.
Statistical Analyses
Single intergroup comparisons between two groups were performed with a Student's t-test (protocols 1 and 2). When multiple comparisons with one differing factor were performed, one-way ANOVA with a Student-Newman-Keuls post hoc test was performed (protocols 1 and 2). Two-way ANOVA was performed on nested designs where we tested for two-way interactions (protocols 3 and 4). Individual comparisons using a one-way ANOVA design with a Student-Newman-Keuls test was used to examine individual pairwise comparisons post hoc. Pairwise comparisons tested for differences between each treatment within the two factors separately. The treatments within one factor were compared among themselves without regard to the second factor. For simplicity of presentation, all data are presented as means ± SE. In all cases, P < 0.05 was considered statistically significant. The absolute values for PRA, epinephrine, and CaSR/GAPDH, NKCC-2/GAPDH, and AQP-2/GAPDH mRNA ratios are found in the text. All other absolute values are found in tables.
RESULTS
Protocol 1: effect of calcitriol and paricalcitol on PRA and the CaSR, NKCC2, and AQP2.
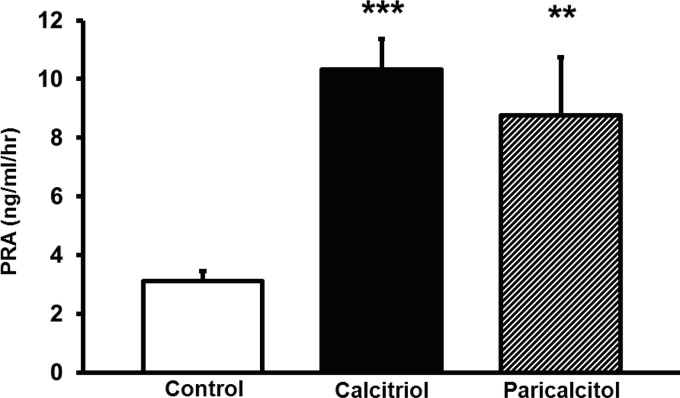
To test whether calcitriol could increase PRA, we administered 100 ng/day of calcitriol. Additionally, to test whether the increases in PRA were independent of changes in plasma Ca2+, we administered 100 ng/day of paricalcitol (a noncalcemic VDR agonist) to an additional group. Calcitriol increased PRA from 3.1 ± 0.3 to 10.3 ± 1.0 ng ANG I·ml−1·h−1 (P < 0.001, Fig. 1). Paricalcitol also similarly increased PRA to 8.8 ± 2.0 ng ANG I·ml−1·h−1 (P < 0.01, Fig. 1).
Fig. 1.
Effects of calcitriol and paricalcitol on plasma renin activity (PRA). Both calcitriol and paricalcitol increased PRA. Values are means ± SE. **P < 0.01 vs. control. ***P < 0.001 vs. control.
Ionized Ca2+ was significantly higher in calcitriol-treated rats by 23% (Table 1), but plasma Ca2+ in the paricalcitol group did not differ from controls (Table 1), demonstrating that vitamin D can increase PRA in the absence of hypercalcemia. Confirming the successful administration of calcitriol and paricalcitol in these rats, plasma PTH was greatly suppressed in both the calcitriol- and paricalcitol-treated groups compared with control (Table 1). The 24-h urinary volume more than doubled in both the calcitriol- and paricalcitol-treated groups (Table 1). Urinary Na+ excretion was higher in the paricalcitol group compared with both control and calcitriol (Table 1). Urinary Ca2+ excretion was significantly higher, and urine osmolality significantly lower in both the calcitriol- and paricalcitol-treated groups compared with controls (Table 1) without significant changes in the creatinine clearance. Urinary PO43− excretion increased in both the calcitriol and paricalcitol groups, but was significantly higher in the calcitriol group compared with paricalcitol (Table 1). The final body weight (at the end of treatment) was lower in the calcitriol-treated group, but not the paricalcitol group (Table 1). Food consumption was slightly, but significantly lower in the calcitriol-treated group, but not the paricalcitol-treated group (Table 1). Thus calcitriol and paricalcitol both increased PRA, but this response was dissociated from changes in plasma Ca2+. However, rats in both groups were characterized by a significant polyuria, hypercalciuria, and an apparent renal concentrating defect.
Table 1.
Effects of calcitriol and paricalcitol on plasma and urinary parameters, body weight, food and water intake, and systolic blood pressure
| Groups |
|||
|---|---|---|---|
| Parameter | Control | Calcitriol | Paricalcitol |
| Plasma Na+, mmol/l | 140 ± 0.2 | 139 ± 0.2 | 139 ± 0.6 |
| Plasma ionized Ca2+, mmol/l | 1.24 ± 0.01 | 1.52 ± 0.03*** | 1.23 ± 0.02††† |
| PTH, pg/ml | 43.5 ± 2.3 | 0.7 ± 0.7*** | 6.4 ± 3.2*** |
| Plasma osmolality, mosmol/kgH2O | 304 ± 4 | 305 ± 4 | 300 ± 3 |
| 24-h Urine volume, ml/24 h | 11.3 ± 1.0 | 29.3 ± 3.4*** | 24.6 ± 3.2** |
| Urinary Na+ excretion, mmol/24 h | 1.48 ± 0.10 | 1.73 ± 0.06 | 2.29 ± 0.23**† |
| Urinary Ca2+ excretion, mg/24 h | 1.31 ± 0.19 | 11.7 ± 2.5** | 9.2 ± 2.6* |
| Urinary PO43− excretion, mmol/24 h | 0.72 ± 0.06 | 1.78 ± 0.16*** | 1.25 ± 0.10**†† |
| Creatinine clearance, ml·min−1·g kidney wt−1 | 0.81 ± 0.07 | 0.89 ± 0.07 | 0.66 ± 0.05 |
| Urine osmolality, mosmol/kgH2O | 1,623 ± 108 | 786 ± 92*** | 1,045 ± 137** |
| Basal body weight, g | 236 ± 5 | 234 ± 5 | 244 ± 4 |
| Final body weight, g | 274 ± 2 | 249 ± 5** | 272 ± 4†† |
| Food consumed, g/day | 17.9 ± 1.0 | 14.4 ± 0.8* | 17.1 ± 0.5† |
| H2O consumed, ml/day | 45 ± 3 | 53 ± 5 | 51 ± 2 |
| Systolic blood pressure, mmHg | 128 ± 9 | 132 ± 8 | 130 ± 4 |
Values are means ± SE.
P < 0.05 vs. control. PTH, parathyroid hormone.
P < 0.01 vs. control.
P < 0.001 vs. control.
P < 0.05 vs. calcitriol.
P < 0.01 vs. calcitriol.
P < 0.001 vs. calcitriol.
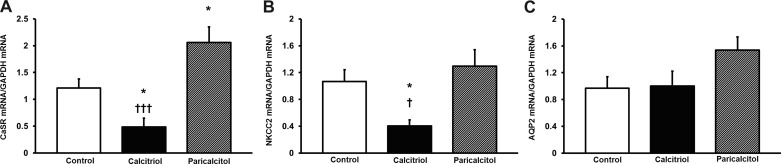
To test a potential mechanism for calcitriol- and paricalcitol-induced hypercalciuria and polyuria, we examined the effects of paricalcitol on whole-kidney homogenate CaSR, NKCC2, and AQP2 expression. Calcitriol decreased renal CaSR/GAPDH mRNA expression from 1.21 ± 0.17 to 0.48 ± 0.17 (Fig. 2A, P < 0.05). Paricalcitol increased renal CaSR/GAPDH mRNA expression to 2.06 ± 0.29 (Fig. 2A), significantly greater than control (P < 0.05) and calcitriol (P < 0.001). Calcitriol also significantly decreased renal NKCC2/GAPDH mRNA from a control level of 1.07 ± 0.18 to 0.40 ± 0.09 (Fig. 2B, P < 0.05). NKCC2/GAPDH mRNA expression was 1.30 ± 0.25 in paricalcitol-treated rats. This was significantly higher than calcitriol (Fig. 2B, P < 0.05), but did not differ from control. Neither calcitriol nor paricalcitol significantly affected AQP2/GAPDH mRNA expression (Fig. 2C).
Fig. 2.
A: effects of calcitriol and paricalcitol on whole-kidney calcium-sensing receptor (CaSR) mRNA. Calcitriol decreased CaSR mRNA. Paricalcitol increased CaSR mRNA compared with control and calcitriol. Values are means ± SE and are corrected by GAPDH expression. *P < 0.05 vs. control. †††P < 0.001 vs. calcitriol. B: effects of calcitriol and paricalcitol on whole-kidney N-K-2 Cl cotransporter (NKCC2) mRNA. Calcitriol decreased NKCC2 mRNA vs. both control and paricalcitol. Values are means ± SE and are corrected by GAPDH expression. *P < 0.05 vs. control. †P < 0.05 vs. calcitriol. C: effects of calcitriol and paricalcitol on whole-kidney aquaporin-2 (AQP2) mRNA. Neither paricalcitol nor calcitriol affected AQP2 mRNA expression. Values are means ± SE and are corrected by GAPDH expression.
Protocol 2: effects of volume replacement on calcitriol-stimulated PRA.
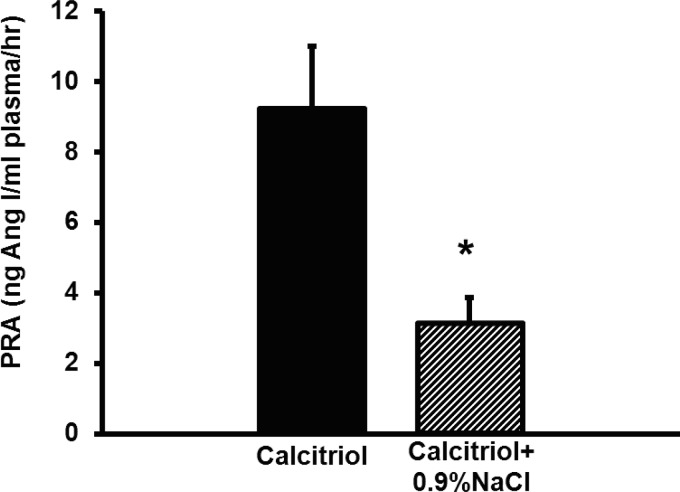
To test whether calcitriol was increasing PRA due to polyuria-mediated hypovolemia, we administered 25 ml of sterile 0.9% NaCl to calcitriol-treated rats daily. To obtain euvolemia, this dose of saline was chosen because the polyuric, calcitriol-treated rats in protocol 1 weighed ∼25 g less than the vehicle control-treated rats at the end of the study. Compared with calcitriol-treated rats (9.2 ± 1.8 ng ANG I·ml−1·h−1), calcitriol+0.9% NaCl-treated rats had completely normalized PRA (3.1 ± 0.8 ng ANG I·ml−1·h−1, P < 0.05, Fig. 3). Twenty-four-hour urine volume and urinary Na+ excretion were significantly higher in 0.9% NaCl-treated rats (Table 2). Plasma epinephrine was significantly elevated in calcitriol-treated rats (control: 487.5 pg/ml; calcitriol: 1,695.0 pg/ml, P < 0.001) and was normalized by 0.9% NaCl administration (985.5 pg/ml, P < 0.01 vs. calcitriol, not significant vs. control, Fig. 4). No difference was detected in any other parameter tested. These data suggest that calcitriol increases PRA via polyuria-induced hypovolemia, which is reversible with volume replacement.
Fig. 3.
Effects of volume replacement with 0.9% NaCl on calcitriol-elevated PRA. Rehydration completely normalized calcitriol-stimulated PRA. Values are means ± SE. *P < 0.05 vs. calcitriol.
Table 2.
Effects of rehydration with 0.9% NaCl on plasma, urinary, and body weight parameters in calcitriol-treated rats
| Groups |
||
|---|---|---|
| Parameter | Calcitriol | Calcitriol +0.9% NaCl |
| Plasma Na+ (mmol/l) | 139 ± 0.4 | 140 ± 0.7 |
| Plasma Ionized Ca2+, mmol/l | 1.39 ± 0.03 | 1.37 ± 0.05 |
| PTHm pg/ml | 35.2 ± 12.6 | 5.6 ± 3.5 |
| Plasma osmolality, mosmol/kgH2O | 304 ± 1 | 302 ± 2 |
| 24-h Urine volume, ml/24 h | 35.6 ± 3.8 | 55.9 ± 4.1** |
| Urinary Na+ excretion, mmol/24 h | 1.70 ± 0.10 | 6.23 ± 0.63*** |
| Urinary Ca2+ excretion, mg/24 h | 17.04 ± 5.71 | 19.31 ± 1.31 |
| Creatinine clearance, ml·min−1·g kidney wt−1 | 0.56 ± 0.04 | 0.56 ± 0.02 |
| Urine osmolality, mosmol/kgH2O | 677 ± 101 | 622 ± 47 |
| Basal body weight, g | 258 ± 4 | 262 ± 4 |
| Final body weight, g | 270 ± 6 | 272 ± 5 |
| Food consumed, g/day | 19.4 ± 0.9 | 19.8 ± 1.0 |
| H2O consumed, ml/day | 69 ± 4 | 61 ± 4 |
| Plasma epinephrine, pg/ml | 1,695.0 ± 182.9 | 985.5 ± 139.5* |
Values are mean ± SE.
P < 0.05 vs. calcitriol.
P < 0.01 vs. calcitriol.
P < 0.001 vs. calcitriol.
Protocol 3: effect of β-adrenoreceptor inhibition on calcitriol-stimulated PRA.
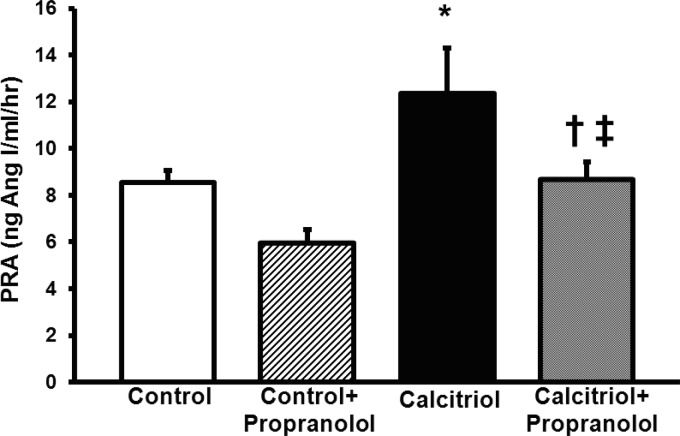
Because calcitriol increased plasma epinephrine in protocol 2, we tested whether calcitriol-mediated polyuria and hypovolemia were increasing PRA via β-adrenergic receptors. We treated rats receiving calcitriol with the β-blocker propranolol. PRA in control rats was 8.5 ± 0.5 ng ANG I·ml−1·h−1. Calcitriol increased this by 46% to 12.4 ± 1.9 ng ANG I·ml−1·h−1 (P < 0.05, Fig. 5). Propranolol given to control rats had no effect (5.9 ± 0.6 ng ANG I·ml−1·h−1) on PRA compared with controls alone. In rats treated with calcitriol+propranolol, PRA did not significantly differ from control (8.7 ± 0.7 ng ANG I·ml−1·h−1, Fig. 5), but was slightly but significantly less than control+propranolol (P < 0.05). PRA in calcitriol+propranolol-treated rats was significantly lower than in rats treated with calcitriol alone (P < 0.05, Fig. 5). No significant interaction term between calcitriol and propranolol was detected. Along with our epinephrine data, this suggests calcitriol-mediated polyuria and hypovolemia increase PRA in part via increased sympathetic activity and stimulation of the β-adrenergic receptors on the juxtaglomerular (JG) cells.
Fig. 5.
Effects of β-blockade with propranolol on calcitriol-stimulated PRA. β-Blockade normalized calcitriol-stimulated PRA. Values are means ± SE. * < 0.05 vs. control. †P < 0.05 vs. calcitriol. ‡P < 0.05 vs. control+propranolol.
Plasma Ca2+, plasma osmolality, and urinary volume were significantly higher in both calcitriol- and calcitriol+propranalol-treated rats compared with controls (Table 3). Urinary Na+ excretion was higher in calcitriol-treated rats vs. both control- and calcitriol+propranolol-treated rats (Table 3). Urinary Ca2+ excretion was significantly higher, and urine osmolality significantly lower, in both calcitriol- and calcitriol+propranolol-treated rats vs. their respective controls (Table 3). Interestingly, body weight was significantly lower in calcitriol+propranolol-treated rats compared with both control and calcitriol alone (Table 3), and this was associated with significantly less food consumption in calcitriol+propranolol-treated rats. Both calcitriol- and calcitriol+propranalol-treated rats consumed more water vs. their respective controls (Table 3).
Table 3.
Effects of β-blockade with propranolol on plasma, urinary, and body weight parameters from control and calcitriol-treated rats
| Control | Control+Propranolol | Calcitriol | Calcitriol+Propranolol | |
|---|---|---|---|---|
| Plasma Na+, mmol/l | 139 ± 0.4 | 139 ± 0.4 | 138 ± 0.4 | 139 ± 0.6 |
| Plasma ionized Ca2+, mmol/l | 1.19 ± 0.02 | 1.19 ± 0.02 | 1.36 ± 0.03*** | 1.39 ± 0.04‡‡‡ |
| Plasma osmolality, mosmol/kgH2O | 296 ± 1 | 296 ± 2 | 308 ± 1*** | 308 ± 2‡‡‡ |
| 24-h Urine volume, ml/24 h | 10.9 ± 1.1 | 10.1 ± 1.2 | 32.6 ± 2.8*** | 34.9 ± 3.0‡‡‡ |
| Urinary Na+ excretion, mmol/24 h | 1.35 ± 0.17 | 1.54 ± 0.14 | 2.28 ± 0.10*** | 1.77 ± 0.18† |
| Urinary Ca2+ excretion, mg/24 h | 1.20 ± 0.40 | 0.91 ± 0.21 | 10.57 ± 2.93** | 8.15 ± 1.83‡ |
| Creatinine clearance, ml·min−1·g kidney wt−1 | 0.48 ± 0.03 | 0.57 ± 0.05 | 0.59 ± 0.05 | 0.62 ± 0.05 |
| Urine osmolality, mosmol/kgH2O | 1,439 ± 157 | 1,580 ± 196 | 809 ± 46** | 691 ± 57‡‡‡ |
| Basal body weight, g | 244 ± 6 | 244 ± 7 | 244 ± 4 | 243 ± 4 |
| Final body weight, g | 272 ± 6 | 270 ± 6 | 259 ± 6 | 243 ± 5‡‡† |
| Food consumed, g/day | 17.0 ± 0.6 | 16.1 ± 0.5 | 16.2 ± 0.4 | 13.4 ± 0.6‡‡††† |
| H2O consumed, ml/day | 39 ± 3 | 39 ± 2 | 62 ± 4*** | 59 ± 2‡‡‡ |
Values are means ± SE.
P < 0.01 vs. control.
P < 0.001 vs. control
P < 0.05 vs. control+propranolol.
P < 0.01 vs. control+propranolol.
P < 0.001 control+propranolol.
P < 0.05 vs. calcitriol.
P < 0.001 vs. calcitriol.
Protocol 4: effect of COX-2 inhibition on calcitriol-stimulated PRA.
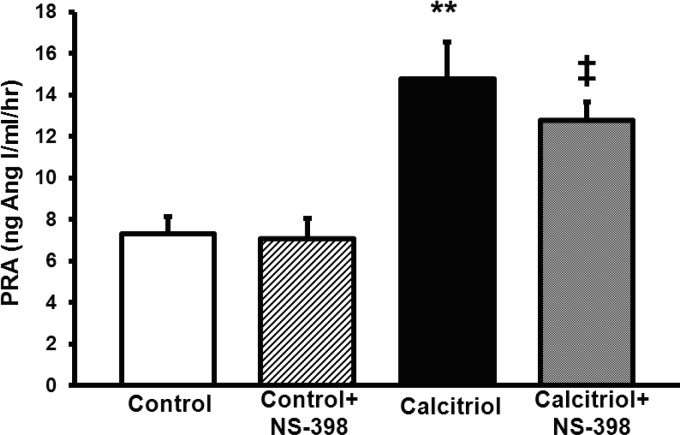
To test whether calcitriol was increasing PRA via elevated COX-2 activity, we tested whether a selective COX-2 inhibitor, NS-398, could ameliorate calcitriol-elevated PRA. Calcitriol elevated PRA compared with untreated controls (14.8 ± 1.8 vs. 7.3 ± 0.8 ng ANG I·ml−1·h−1, P < 0.01, respectively, Fig. 6). PRA was higher in calcitriol+NS-398 rats vs. control+NS-398 rats (12.7 ± 0.9 vs. 7.1 ± 1.0 ng ANG I·ml−1·h−1, respectively, P < 0.05, Fig. 6). PRA did not significantly differ between the calcitriol+NS-398-treated rats and rats treated with calcitriol alone (Fig. 6). No significant interaction term was detected between calcitriol and NS-398, suggesting calcitriol does not increase PRA due to macula densa COX-2-NaCl-transport-mediated mechanisms.
Fig. 6.
Effects of cyclooxygenase (COX)-2 inhibition with NS-398 on calcitriol-stimulated PRA. COX-2 inhibition had no effect on calcitriol-stimulated PRA. Values are means ± SE. **P < 0.01 vs. control. ‡P < 0.05 vs. control+NS-398.
Rats treated with calcitriol and calcitriol+NS-398 had higher plasma Ca2+ and 24-h urinary volumes (Table 4). Additionally, calcitriol- and calcitriol+calcitriol+NS-398-treated rats excreted more Ca2+ and had lower urinary osmolality, consistent with hypercalcemic concentrating defects (Table 4). Calcitriol- and calcitriol+NS-398-treated rats weighed less than their control counterparts and consumed less food, but drank more water (Table 4). The only additional effect of NS-398 treatment on calcitriol-mediated hypercalcemia was to raise plasma osmolality (Table 4). Importantly, NS-398 decreased urinary osmolality in non-calcitriol-treated rats, consistent with previous results (29), demonstrating that the dose of NS-398 we used was sufficient.
Table 4.
Effects of COX-2 inhibition with NS-398 on plasma, urinary, and body weight parameters from control and calcitriol-treated rats
| Control | Control+NS-398 | Calcitriol | Calcitriol+NS-398 | |
|---|---|---|---|---|
| Plasma Na+, mmol/l | 138 ± 1.3 | 138 ± 0.6 | 139 ± 0.5 | 139 ± 0.5 |
| Plasma ionized Ca2+, mmol/l | 1.21 ± 0.03 | 1.21 ± 0.03 | 1.43 ± 0.03*** | 1.43 ± 0.03‡‡‡ |
| Plasma osmolality, mosmol/kgH2O | 303 ± 2 | 302 ± 1 | 303 ± 1 | 308 ± 1‡‡†† |
| 24-h Urine volume, ml/24 h | 9.0 ± 1.7 | 13.0 ± 1.0 | 41.2 ± 2.6*** | 35.2 ± 3.3‡‡‡ |
| Urinary Na+ excretion, mmol/24 h | 1.46 ± 0.09 | 1.79 ± 0.11 | 1.79 ± 0.09 | 1.72 ± 0.08 |
| Urinary Ca2+ excretion, mg/24 h | 1.71 ± 0.71 | 2.44 ± 1.11 | 14.64 ± 2.06*** | 14.23 ± 2.24‡‡‡ |
| Creatinine clearance, ml·min−1·g kidney wt−1 | 0.56 ± 0.06 | 0.64 ± 0.07 | 0.70 ± 0.06 | 0.73 ± 0.06 |
| Urine osmolality, mosmol/kgH2O | 2,103 ± 160 | 1,599 ± 71** | 608 ± 46*** | 780 ± 78‡‡‡ |
| Basal body weight, g | 259 ± 3 | 261 ± 5 | 246 ± 5 | 249 ± 4 |
| Final body weight, g | 286 ± 6 | 287 ± 6 | 259 ± 5** | 264 ± 4‡‡ |
| Food consumed, g/day | 20.4 ± 0.8 | 20.9 ± 0.7 | 16.8 ± 0.7** | 17.8 ± 0.7‡ |
| H2O consumed, ml/day | 43 ± 2 | 44 ± 3 | 65 ± 3*** | 61 ± 3‡‡‡ |
Values are means ± SE. COX, cyclooxygenase.
P < 0.01 vs. control.
P < 0.001 vs. control.
P < 0.05 vs. control+NS-398.
P < 0.01 vs. control+NS-398.
P < 0.001 control+NS-398.
P < 0.01 vs. calcitriol.
DISCUSSION
In this study, we have demonstrated that both calcitriol and paricalcitol increase PRA and were associated with hypercalciuria and polyuria. The paricalcitol-mediated increase in PRA was associated with elevated CaSR mRNA. We demonstrated that the calcitriol-mediated increase in PRA is eliminated by volume replacement with subcutaneous 0.9% NaCl. Additionally, the calcitriol-mediated increase in PRA was ameliorated by β-adrenergic blockade, but not COX-2 inhibition. All of these data support the thesis that vitamin D increases PRA independently of plasma Ca2+ through CaSR-mediated effects on hypercalciuria, polyuria, and volume depletion, which lead to elevated β-adrenergic activity.
It has been previously demonstrated that vitamin D analogs may increase PRA (32, 42). However, the mechanism by which this occurred was not examined, but it was always assumed that vitamin D increases PRA due to its hypercalcemic effects (32). We found that both calcitriol and paricalcitol significantly increase PRA. Importantly, we found that paricalcitol increased PRA in the absence of any changes in ionized plasma Ca2+, demonstrating that the stimulatory effects of vitamin D are not necessarily due to hypercalcemia, a significantly novel finding. It is unlikely the calcitriol- and paricalcitol-mediated increase in PRA was mediated by their inhibitory effects on PTH. PTH actually increases PRA due to indirect effects (2, 5). We found that calcitriol and paricalcitol caused polyuria and hypercalciuria, suggesting that this was the common mechanism by which vitamin D actually increases renin.
To our knowledge, the effects of paricalcitol on urinary Ca2+ handling have not been previously studied. Therefore, we thought it essential to determine how paricalcitol caused hypercalciuria, polyuria, and concentrating defects in the presence of normocalcemia. The effects of plasma Ca2+ on renal Ca2+ reabsorption are mediated by the CaSR (9), which transmits changes in plasma or extracellular Ca2+ into changes in intracellular signaling. The CaSR is ubiquitously expressed along the nephron (35, 36) and is the mediator of Ca2+-induced polyuria (33, 37, 46, 47, 50, 52). Since it had previously been shown that paricalcitol could increase parathyroid CaSR expression (16), we anticipated that paricalcitol was causing the hypercalciuria and polyuria due to elevated renal CaSR expression. As expected, paricalcitol significantly increased CaSR mRNA expression (we did not measure protein or activity), which suggests that elevated CaSR expression is a likely explanation for paricalcitol-mediated hypercalciuria, polyuria, and elevated PRA. In support of this, patients with activating mutations of the CaSR (type-V Bartter syndrome) also present with an identical renal phenotype (hypercalciuria, polyuria, concentrating defect, hyperreninemia) (46, 47, 52). In contrast, calcitriol decreased CaSR expression, but similarly also decreased the expression of NKCC2, consistent with previous studies (32). The discordant effects of calcitriol and paricalcitol were unexpected, as previously it had been shown that calcitriol increases CaSR expression (8). One difference between our data and this study that could account for these differences was the length of time of exposure to calcitriol. Rats in the previous study (8) received two injections over 48 h, while rats in our study were treated over 6 days. Similarly, it is also known that calcitriol significantly increases plasma phosphorus, while paricalcitol has relatively minimal effects. Paricalcitol has minimal effects on calcium and phosphate liberation from bone as well as intestinal uptake (15, 43), and it is thought that these effects contribute to its relative eucalcemic and euphosphatemic effects. Plasma phosphorus is known to decrease CaSR expression (7). While we could not measure plasma phosphorus in our experiments (due to hemolysis that occurred with blood collection), we were able to measure urinary PO43− excretion, which was significantly higher in calcitriol-treated rats compared with paricalcitol and controls. This suggests that the impairment of CaSR expression in calcitriol-treated rats may be due to its hyperphosphatemic effects.
Thus our data crucially demonstrate that stimulation at the VDR with paricalcitol can increase PRA independently of changes in plasma Ca2+ via hypercalciuria, polyuria, and hypovolemia. These chronic changes are in contrast to the acute effects of elevated plasma (in vivo) or media (in vitro) Ca2+ which inhibit renin release from JG cells via impaired synthesis and increased degradation of the stimulatory second messenger, cAMP (3, 4, 30, 31). Thus the effects of Ca2+-mediated processes on renin appear to be a balance of an acute, direct inhibitory effect directly on the JG cell and a chronic, stimulatory effect via volume status.
Factors that regulate renin secretion typically do so via three different classic pathways: 1) by changes in NaCl delivery and reabsorption at the macula densa (14, 17–19); 2) by changes in renal sympathetic nerve activity acting on β-adrenergic receptors (20, 28) on the JG cells; or 3) by changes in perfusion pressure to the kidney (41, 45) via the renal baroreceptor. It has been established that the VDR is not expressed in JG cells, ruling out a direct effect of calcitriol on renin (21, 23, 51). Thus, if calcitriol was increasing PRA, it seemed likely that it would do so through one of these pathways. Rats treated with calcitriol or paricalcitol had higher urinary volumes than their control counterparts, suggesting hypovolemia was the mechanism by which they both increased PRA. Calcitriol and paricalcitol treatment did not alter blood pressure. As such, we were unable to ascertain the role of the baroreceptor in this response. To determine whether volume depletion from the calcitriol-mediated polyuria was causing the elevated PRA, we administered sterile 0.9% NaCl subcutaneously to replace circulating volume lost from the polyuria. Volume replacement in calcitriol-treated rats completely normalized PRA, suggesting that polyuria-mediated hypovolemia is the cause by which calcitriol elevated PRA. Consistent with this result, amelioration of polyuric symptoms also normalized plasma renin levels in a patient with type-V Bartter syndrome (46). The administration of saline to the calcitriol+0.9% NaCl group returned plasma epinephrine levels to control levels without overcorrecting them. This demonstrates that the rats treated with 0.9% NaCl were euvolemic, and not volume expanded.
The elevated plasma epinephrine levels in calcitriol-treated rats suggested that elevated sympathetic activity may account for the effects of polyuria and hypovolemia on calcitriol-elevated PRA. β-Adrenergic receptor activation is a significant element in the elevation of PRA in response to water restriction and hypovolemia (20, 28). Thus it seemed plausible that the β-adrenergic pathway could mediate the response of PRA to calcitriol-mediated polyuria and hypovolemia. Moreover, elevated adrenergic activity has been reported in states of hypercalcemia as well: Patients with hyperparathyroidism have higher levels of circulating epinephrine (48). Consistent with these results, we demonstrated that β-blockade with propranolol reversed calcitriol-stimulated PRA back to control levels. Thus the sum of our data demonstrates that calcitriol elevates PRA due to polyuria-mediated hypovolemia and the resulting elevated β-adrenergic activity.
To further examine how calcitriol-mediated polyuria and hypovolemia elevates PRA, we tested whether it was due to elevated COX-2 activity, and by extension, natriuresis. Macula densa-derived COX-2 elevates PRA in response to loop diuretics or low dietary NaCl (14, 17–19). Additionally, COX-derived metabolites play an integral role in the response of the kidney to hypercalcemia: nonselective COX inhibition ameliorates vitamin D-induced polyuria (24, 38). Emerging research suggested that COX-2 may be responsible, in part, for some of these responses (49). However, our data demonstrated that COX-2 inhibition had no effect on calcitriol-stimulated PRA or polyuria. These data rule out changes in macula densa NaCl reabsorption and COX-2 activity as the cause of the elevation in PRA by calcitriol. Thus the differences in Na+ excretion seen in some of our protocols should not have been the causative factor behind the vitamin D-mediated elevations in PRA. The inability of NS-398 (a COX-2 inhibitor) to decrease PRA is not an issue of inefficient dosing, as this established dose is effective for inhibiting COX-2 (26), and lesser doses of NS-398 can also inhibit renin secretion elevated by NaCl restriction (17, 18). Moreover, our data demonstrate that the administration of NS-398 was successful, as it decreased urine osmolality in non-calcitriol-treated rats, consistent with previous results (29).
Previous data have suggested that calcitriol or other vitamin D metabolites may inhibit renin-angiotensin activity due to a direct suppression of renin transcription in the JG cell (25, 34). This concept is coming under increased scrutiny, as the VDR is not expressed in JG cells (21, 23, 51) or renin-containing As 4.1 cells (25). Thus it is unclear how vitamin D can exert a direct effect on the JG cell in the absence of a nuclear receptor. Calcitriol has been shown to decrease renal renin expression in mice (16, 25, 34). The doses (per gram body weight) used in these studies and ours are similar, and similar increases in plasma Ca2+ were achieved in each. Attention has been paid to mice with genetic deletions of the VDR (25) or 1-α hydroxylase (56) that develop elevated renal renin expression. This phenomenon may solely be due to the lack of cardiac VDR expression, as cardiac-specific VDR knockout mice also develop elevated renal renin expression (10), arguing against a renal-specific effect of VDR agonists on renin suppression. Our findings in a rat model showing that vitamin D can increase renin are consistent with previous data from rats and dogs (32, 42), and since calcitriol and paricalcitol increased PRA in all of our protocols, we are confident in our data.
In conclusion, we have demonstrated that calcitriol and paricalcitol both increase PRA and induce hypercalciuria and polyuria and therefore volume depletion. The increase in PRA is not due to hypercalcemia, but rather mediated by polyuria, hypovolemia, and subsequent elevated β-sympathetic activity. Our data suggest that the therapeutic strategy of treating hyperreninemic states with vitamin D analogs may be ineffective.
Perspectives
A potential criticism of our work is that we have used doses of calcitriol and paricalcitol that solely reflect the effects of vitamin D at levels considered toxic. However, our doses are consistent with previously validated doses in the literature (15, 16, 25, 34, 43). Conversely, we feel that our data may have clinical relevance. It has been shown that clinically relevant doses of paricalcitol induce hypercalciuria, even after only 7 days of administration in human patients (1). Moreover, three separate clinical trials have shown that paricalcitol increases plasma creatinine (1, 12, 44), which would be completely consistent with the hypovolemic effect we see in our study. At clinical doses in predialysis patients, paricalcitol decreases brain-natriuretic peptide (44), further suggesting a hypovolemic effect. Our data suggest that vitamin D analogs may affect cardiovascular parameters via changes in volume status rather than a direct effect on the renin-angiotensin system. Our data do not address whether correcting vitamin D deficiencies alters cardiovascular health, but our data suggest that treating elevated levels of renin with vitamin D may be ineffective.
GRANTS
This research was supported by National Institutes of Health Grants F30DK084654-03 and PPG 5PO1HL090550-03. Paricalcitol was purchased in the Henry Ford Health System Pharmacy and was not a gift. D. K. Atchison is a member of the Wayne State University School of Medicine MD/PhD program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.K.A., P.H., and W.H.B. provided conception and design of research; D.K.A., P.H., and W.H.B. performed experiments; D.K.A., P.H., and W.H.B. analyzed data; D.K.A., P.H., and W.H.B. interpreted results of experiments; D.K.A. and W.H.B. prepared figures; D.K.A. and W.H.B. drafted manuscript; D.K.A., P.H., and W.H.B. edited and revised manuscript; D.K.A., P.H., and W.H.B. approved final version of manuscript.
REFERENCES
- 1.Agarwal R, Hynson JE, Hecht TJ, Light RP, Sinha AD. Short-term vitamin D receptor activation increases serum creatinine due to increased production with no effect on the glomerular filtration rate. Kidney Int 80: 1073–1079, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Atchison DK, Harding P, Cecilia Ortiz-Capisano M, Peterson EL, Beierwaltes WH. Parathyroid hormone stimulates juxtaglomerular cell cAMP accumulation without stimulating renin release. Am J Physiol Renal Physiol 303: F1157–F1165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atchison DK, Harding P, Beierwaltes WH. Hypercalcemia reduces plasma renin via parathyroid hormone, renal interstitial calcium, and the calcium-sensing receptor. Hypertension 58: 604–610, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atchison DK, Ortiz-Capisano MC, Beierwaltes WH. Acute activation of the calcium-sensing receptor inhibits plasma renin activity in vivo. Am J Physiol Regul Integr Comp Physiol 299: R1020–R1026, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atchison DK, Westrick E, Szandzik DL, Gordish KL, Beierwaltes WH. Parathyroid hormone-related protein stimulates plasma renin activity via its anorexic effects on sodium chloride intake. Am J Physiol Endocrinol Metab 303: E457–E463, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair ML, Woolf PD, Felten SY. Sympathetic activation cannot fully account for increased plasma renin levels during water deprivation. Am J Physiol Regul Integr Comp Physiol 272: R1197–R1203, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Brown AJ, Ritter CS, Finch JL, Slatopolsky EA. Decreased calcium-sensing receptor expression in hyperplastic parathyroid glands of uremic rats: role of dietary phosphate. Kidney Int 55: 1284–1292, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Brown AJ, Zhong M, Finch J, Ritter C, McCracken R, Morrissey J, Slatopolsky E. Rat calcium-sensing receptor is regulated by vitamin D but not by calcium. Am J Physiol Renal Fluid Electrolyte Physiol 270: F454–F460, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner DG. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation 124: 1838–1847, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YH, Hisa H, Radke KJ, Izzo JL, Jr, Sladek CD, Blair ML. Adrenergic control of renin in euhydrated and water-deprived conscious dogs. Am J Physiol Endocrinol Metab 255: E793–E800, 1988 [DOI] [PubMed] [Google Scholar]
- 12.DeZeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376: 1543–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Endo H, Kiyoki M, Kawashima K, Naruchi T, Hashimoto Y. Vitamin D3 metabolites and PTH synergistically stimulate bone formation of chick embryonic femur in vitro. Nature 286: 262–264, 1980 [DOI] [PubMed] [Google Scholar]
- 14.Facemire CS, Nguyen M, Jania L, Beierwaltes WH, Kim HS, Koller BH, Coffman TM. A major role for the EP4 receptor in regulation of renin. Am J Physiol Renal Physiol 301: F1035–F1041, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finch JL, Brown AJ, Slatopolsky E. Differential effects of 1,25-dihydroxy-vitamin D3 and 19-nor-1,25-dihydroxy-vitamin D2 on calcium and phosphorus resorption in bone. J Am Soc Nephrol 10: 980–985, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Fryer RM, Rakestraw PA, Nakane M, Dixon D, Banfor PN, Koch KA, Wu-Wong JR, Reinhart GA. Differential inhibition of renin mRNA expression by paricalcitol and calcitriol in C57/BL6 mice. Nephron Physiol 106: 76–81, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Harding P, Carretero OA, Beierwaltes WH. Chronic cyclooxygenase-2 inhibition blunts low sodium-stimulated renin without changing renal haemodynamics. J Hypertens 18: 1107–1113, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Harding P, Sigmon DH, Alfie ME, Sigmon DH, Alfie ME, Huang PL, Fishman MC, Beierwaltes WH, Carretero OA. Cyclooxygenase-2 mediates increased renal renin content induced by low-sodium diet. Hypertension 29: 297–302, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Kammerl MC, Nüsing RM, Richthammer W, Krämer BK, Kurtz A. Inhibition of COX-2 counteracts the effects of diuretics in rats. Kidney Int 60: 1684–1691, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Kim SM, Chen L, Faulhaber-Walter R, Oppermann M, Huang Y, Mizel D, Briggs JP, Schnermann J. Regulation of renin secretion and expression in mice deficient in beta1- and beta2-adrenergic receptors. Hypertension 50: 103–109, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Kong J, Qiao G, Zhang Z, Liu SQ, Li YC. Targeted vitamin D receptor expression in juxtaglomerular cells suppresses renin expression independent of parathyroid hormone and calcium. Kidney Int 74: 1577–1581, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Kong J, Kim GH, Wei M, Sun T, Li G, Liu SQ, Li X, Bhan I, Zhao Q, Thadhani R, Li YC. Therapeutic effects of vitamin D analogs on cardiac hypertrophy in spontaneously hypertensive rats. Am J Pathol 177: 622–631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar R, Schaefer J, Grande JP, Roche PC. Immunolocalization of calcitriol receptor, 24-hydroxylase cytochrome P-450, and calbindin D28k in human kidney. Am J Physiol Renal Fluid Electrolyte Physiol 266: F477–F485, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Levi M, Ellis MA, Berl T. Control of renal hemodynamics and glomerular filtration rate in chronic hypercalcemia. Role of prostaglandins, renin-angiotensin system, and calcium. J Clin Invest 71: 1624–1632, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li YC, Kong J, Wei M, Sun T, Li G, Liu SQ, Li X, Bhan I, Zhao Q, Thadhani R, Li YC. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229–238, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masferrer JL, Zweifel BS, Manning PT, Hauser SD, Leahy KM, Smith WG, Isakson PC, Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci USA 91: 3228–3232, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicolaysen R. Studies upon the mode of action of vitamin D: The absorption of calcium chloride, xylose and sodium sulphate from isolated loops of the small intestine and of calcium chloride from the abdominal cavity in the rat. Biochem J 31: 323–328, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nomura G, Kurosaki M, Takabatake T, Kibe Y, Takeuchi J. Reinnervation and renin release after unilateral renal denervation in the dog. J Appl Physiol 33: 649–655, 1972 [DOI] [PubMed] [Google Scholar]
- 29.Nørregaard R, Madsen K, Hansen PB, Bie P, Thavalingam S, Frøkiær J, Jensen BL. COX-2 disruption leads to increased central vasopressin stores and impaired urine concentrating ability in mice. Am J Physiol Renal Physiol 301: F1303–F1313, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Ortiz-Capisano MC, Liao TD, Ortiz PA, Beierwaltes WH. Calcium-dependent phosphodiesterase 1C inhibits renin release from isolated juxtaglomerular cells. Am J Physiol Regul Integr Comp Physiol 297: R1469–R1476, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortiz-Capisano MC, Ortiz PA, Garvin JL, Harding P, Beierwaltes WH. Expression and function of the calcium-sensing receptor in juxtaglomerular cells. Hypertension 50: 737–743, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Peterson LN. Vitamin D-induced chronic hypercalcemia inhibits thick ascending limb NaCl reabsorption in vivo. Am J Physiol Renal Fluid Electrolyte Physiol 259: F122–F129, 1990 [DOI] [PubMed] [Google Scholar]
- 33.Pollak MR, Brown EM, Chou YH, Hebert SC, Marx SJ, Steinmann B, Levi T, Seidman CE, Seidman JG. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell 75: 1297–1303, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Qiao G, Kong J, Uskokovic M, Li YC. Analogs of 1alpha,25-dihydroxyvitamin D3 as novel inhibitors of renin biosynthesis. J Steroid Biochem Mol Biol 96: 59–66, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol Renal Physiol 274: F611–F622, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Riccardi D, Lee WS, Lee K, Segre GV, Brown EM, Hebert SC. Localization of the extracellular Ca2+-sensing receptor and PTH/PTHrP receptor in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F951–F956, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Sands JM, Naruse M, Baum M, Jo I, Hebert SC, Brown EM, Harris HW. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J Clin Invest 99: 1399–1405, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serros ER, Kirschenbaum MA. Prostaglandin-dependent polyuria in hypercalcemia. Am J Physiol Renal Fluid Electrolyte Physiol 241: F224–F230, 1981 [DOI] [PubMed] [Google Scholar]
- 39.Silver J, Naveh-Many T, Mayer H, Schmelzer HJ, Popovtzer MM. Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. J Clin Invest 78: 1296–1301, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skinner SL, McCubbin JW, Page IH. Renal baroceptor control of renin secretion. Science 141: 814–816, 1963 [DOI] [PubMed] [Google Scholar]
- 41.Sowers JR, Barrett JD. Hormonal changes associated with hypertension in neoplasia-induced hypercalcemia. Am J Physiol Endocrinol Metab 242: E330–E334, 1982 [DOI] [PubMed] [Google Scholar]
- 42.Spangler WL, Gribble DH, Lee TC. Vitamin D intoxication and the pathogenesis of vitamin D nephropathy in the dog. Am J Vet Res 40: 73–83, 1979 [PubMed] [Google Scholar]
- 43.Takahashi F, Finch JL, Denda M, Dusso AS, Brown AJ, Slatopolsky E. A new analog of 1,25-(OH)2D3, 19-NOR-1,25-(OH)2D2, suppresses serum PTH and parathyroid gland growth in uremic rats without elevation of intestinal vitamin D receptor content. Am J Kidney Dis 30: 105–112, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ, Solomon SD. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 307: 674–684, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Vander AJ, Miller R. Control of renin secretion in the anesthetized dog. Am J Physiol 207: 537–546, 1964 [DOI] [PubMed] [Google Scholar]
- 46.Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaître X, Paillard M, Planelles G, Déchaux M, Miller RT, Antignac C. Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol 13: 2259–2266, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Vezzoli G, Arcidiacono T, Paloschi V, Paloschi V, Terranegra A, Biasion R, Weber G, Mora S, Syren ML, Coviello D, Cusi D, Bianchi G, Soldati L. Autosomal dominant hypocalcemia with mild type 5 Bartter syndrome. J Nephrol 19: 525–528, 2006 [PubMed] [Google Scholar]
- 48.Vlachakis ND, Frederics R, Valasquez M, Alexander N, Singer F, Maronde RF. Sympathetic system function and vascular reactivity in hypercalcemic patients. Hypertension 4: 452–458, 1982 [DOI] [PubMed] [Google Scholar]
- 49.Wang D, An SJ, Wang WH, McGiff JC, Ferreri NR. CaR-mediated COX-2 expression in primary cultured mTAL cells. Am J Physiol Renal Physiol 281: F658–F664, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Wang W, Lu M, Balazy M, Hebert SC. Phospholipase A2 is involved in mediating the effect of extracellular Ca2+ on apical K+ channels in rat TAL. Am J Physiol Renal Physiol 273: F421–F429, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Borchert ML, Deluca HF. Identification of the vitamin D receptor in various cells of the mouse kidney. Kidney Int 81: 993–1001, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe S, Fukumoto S, Chang H, Takeuchi Y, Hasegawa Y, Okazaki R, Chikatsu N, Fujita T. Association between activating mutations of calcium-sensing receptor and Bartter's syndrome. Lancet 360: 692–694, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem 270: 41–49, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Wu-Wong JR, Nakane M, Gagne GD, Brooks KA, Noonan WT. Comparison of the pharmacological effects of paricalcitol and doxercalciferol on the factors involved in mineral homeostasis. Int J Endocrinol. [Epub before print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 16: 391–396, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int 74: 170–179, 2008 [DOI] [PubMed] [Google Scholar]