Abstract
Nephrogenic diabetes insipidus (NDI) is the most common renal side effect in patients undergoing lithium therapy for bipolar affective disorders. Approximately 2 million US patients take lithium of whom ∼50% will have altered renal function and develop NDI (2, 37). Lithium-induced NDI is a defect in the urinary concentrating mechanism. Lithium therapy also leads to proliferation and abundant renal cysts (microcysts), commonly in the collecting ducts of the cortico-medullary region. The mTOR pathway integrates nutrient and mitogen signals to control cell proliferation and cell growth (size) via the mTOR Complex 1 (mTORC1). To address our hypothesis that mTOR activation may be responsible for lithium-induced proliferation of collecting ducts, we fed mice lithium chronically and assessed mTORC1 signaling in the renal medulla. We demonstrate that mTOR signaling is activated in the renal collecting ducts of lithium-treated mice; lithium increased the phosphorylation of rS6 (Ser240/Ser244), p-TSC2 (Thr1462), and p-mTOR (Ser2448). Consistent with our hypothesis, treatment with rapamycin, an allosteric inhibitor of mTOR, reversed lithium-induced proliferation of medullary collecting duct cells and reduced levels of p-rS6 and p-mTOR. Medullary levels of p-GSK3β were increased in the renal medullas of lithium-treated mice and remained elevated following rapamycin treatment. However, mTOR inhibition did not improve lithium-induced NDI and did not restore the expression of collecting duct proteins aquaporin-2 or UT-A1.
Keywords: aquaporin-2, urea transporter, GSK3β, collecting ducts
nephrogenic diabetes insipidus (NDI) is the most common renal side effect in patients undergoing lithium therapy for bipolar affective disorders. Approximately 2 million US patients take lithium of whom ∼50% will have altered renal function and develop NDI (2, 37). Lithium-induced NDI is a defect in the urinary concentrating mechanism. In addition to NDI, patients can develop chronic renal disease, including focal interstitial nephritis. When combined with the progressive and often nonreversible defect in urinary concentrating ability, chronic renal insufficiency can occur (3, 39). The appearance of lithium nephropathy has been evaluated by magnetic resonance imaging (10); in patients diagnosed for chronic renal insufficiency secondary to lithium therapy, abundant renal cysts (microcysts) are seen (10) and commonly occur in the cortico-medullary region (14).
Lithium-induced proliferation occurs in the kidneys of rodents (7) and is highly localized to renal collecting ducts predominantly in the initial inner medulla. Indeed, chronic lithium treatment in rats substantially modifies the cellular composition of the medullary collecting duct. Furthermore, gene array analyses have identified lithium-induced changes in the expression of cell cycle modulators associated with an increase in proliferation (28). Proteomic analyses of inner medullary collecting ducts in lithium-treated rats identified proteins with an altered abundance, including an increase in phosphorylated PKB/Akt (25); GSK-3β, as previously reported (27); and confirmed our previous observations that lithium decreases P27/Kip1 in the renal medulla (28), suggesting an attenuation of its cell cycle inhibitory effect.
However, although a gap remains in our understanding of the proliferative mechanisms of lithium on collecting duct cells, studies in the polycystic kidney have identified mTOR activation as a leading contributor of proliferation in collecting duct cells and in cyst formation (32, 38). The mTOR pathway integrates nutrient and mitogen signals to control cell proliferation and cell growth (size) via the mTOR Complex 1 (mTORC1) (41), which is sensitive to rapamycin and mTORC2, which is mostly rapamycin insensitive (41). Activation of mTORC1 leads to phosphorylation and activation of the kinase S6K (S6K1) (13, 15, 16, 22). Active S6K1 stimulates the initiation of protein synthesis through activation of S6 ribosomal protein (rS6) and other components of the translational machinery (9, 26). mTORC1 activation can also phosphorylate eukaryotic translation initiation factor 4E (eIF4E) binding proteins (4EBPs).
Here, we hypothesize that mTOR activation may be responsible for lithium-induced proliferation of collecting ducts. To address this hypothesis, we fed mice lithium chronically and assessed mTORC1 signaling in the renal medulla. Our data show that mTOR signaling is activated in the renal collecting ducts of lithium-treated mice and that treatment with an allosteric inhibitor of mTOR, rapamycin, reversed lithium-induced proliferation of medullary collecting duct cells.
MATERIALS AND METHODS
Animals.
Eight-week-old male ICR mice were purchased from Harlan Laboratories (Indianapolis, IN). Animals were housed under standard light cycles and humidity in standard cages. Animals were allowed ad libitum access to food and water. Body weight was measured weekly before dosing and then daily once dosing regimen started. All experiments were approved by the University of Arizona Institutional Animal Care and Use Committee. Experimental mice were switched from the standard University Animal Care diet (Harlan Teklad 7013) to a 0.2% LiCl (40 mM·kg−1·food−1) in 7013 diet (Teklad TD09326). Controls remained on the standard diet. Animals were fed their respective diets for the entire duration of the study. At the end of the study, animals were euthanized and kidneys were harvested.
Rapamycin treatment.
Rapamycin (LC laboratories R-5000) was solubilized in DMSO and then diluted daily to a concentration of 0.55 mg/ml in PBS. Two studies utilizing rapamycin were conducted. For the first study, mice were fed 0.2% LiCl for a week before daily injections of rapamycin (ip) were initiated at a concentration of 5 mg/kg. In the second study, mice were fed 0.2% LiCl diet for 2 wk before daily injections of rapamycin were initiated which continued for 14 days. In both studies, control groups were injected with 5% DMSO in PBS vehicle and fed either the 0.2% LiCl or the regular diet. An additional rapamycin group was included which was maintained on the regular diet.
Urine analysis.
Twenty-four-hour urine was collected weekly for urine osmolality measurement. Osmolality was measured by a vapor pressure osmometer (Wescor model 5600).
RNA isolation and real-time quantitative PCR.
Kidney medullas were dissected and RNA was isolated using the Qiagen RNeasy Mini Kit (74104) according to the manufacturer's instructions and per our previous studies (6, 24). A DNase (Qiagen, 79254) incubation was performed to remove potential DNA contamination. RNA was quantified using a Nanodrop ND1000 spectrophotometer (Wilmington, DE).
Real-time PCR was performed as previously described (24). Briefly, 2.5 μg of RNA were reverse transcribed and resulting cDNA was diluted to 8 ng/μl for real-time PCR which was performed on a RotorGene RG3000 (Qiagen, Valencia, CA). Primer sequences for c-Ret, c-Ret9, c-Ret51, cyclin D1, egr-1, and dynactin are previously published (6, 20). Primer sequences for cyclooxygenase (COX)-2 were forward: ttaggctgttggaatttacgc, reverse: cttacagggccttcaaaatgtc. Ct values were used to calculate the expression levels of genes of interest relative to the expression of dynactin mRNA, ran in parallel (24).
Protein isolation and Western blot.
Renal medulla and cortex were dissected and protein isolation and semiquantitative immunoblotting were performed as previously published (4, 5).
Antibodies used were as follows: aquaporin-2 (AQP2; 1:2,000 dilution, Novus Biologicals, NB110–74682), phospho-mTOR (Ser2448, 1:1,000 dilution, Cell Signaling 5536), mTOR (1:1,000 dilution, Cell Signaling 2972), phospho-S6 ribosomal protein (Ser240/244, 1:1,000 dilution, Cell Signaling 2215), S6 ribosomal protein (1:3,000 dilution, Cell Signaling 2217), phospho-4EBP1 (Thr37/46, 1:500 dilution, Cell Signaling 9459), 4EBP1 (1:1,000 dilution, Cell Signaling 9452), phospho-Tuberin/TSC2 (Thr1462, 1:500 dilution, Cell Signaling 3617), Tuberin/TSC2 (1:1,000 dilution, Cell Signaling 3990), phospho-Akt (Ser473, 1:1,000 dilution, Cell Signaling 4060), Akt (1:1,000 dilution, Cell Signaling 9272), phospho-GSK-3α/β (Ser21/9, 1:1,000 dilution, Cell Signaling 9331), GSK3β (1:1,000, Cell Signaling 9315), and UTA1–3 (1:1,000 dilution, gifts of Dr. M. A. Knepper, National Institutes of Health). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:2,000 dilution, Cell Signaling 7074) was used for 1 h at room temperature for all the above antibodies. Mouse anti-proliferating cell nuclear antigen (PCNA; 1:1,000 dilution, Santa Cruz Biotechnology sc-25280) was used with goat anti-mouse IgG (1:2,000 dilution, Cell Signaling Technology 7076). Horseradish peroxidase was visualized using Supersignal West Femto Maximum Sensitivity Substrate (Fisher, 34095). Images were obtained and quantified by Quantity One (Bio-Rad, Hercules, CA). To facilitate comparisons, intensity values were normalized, such that the mean value of the control group is defined as 100%.
Immunohistochemistry.
Kidneys were fixed in 4% paraformaldehyde overnight, embedded in paraffin, and sectioned (4 μm) by the University of Arizona Pathology Laboratory. Sections were processed as previously published (6). Nonspecific binding was blocked with 2% BSA in PBS. Sections were incubated at 4°C overnight in anti-PCNA (1:400 dilution; Santa Cruz SC-25280) or phospho-mTOR (Ser2448, 1:100 dilution, Cell Signaling 2976), followed by biotin-rabbit anti-mouse IgG (1:400 dilution) for 30 min at 37°C and horseradish peroxidase-streptavidin conjugate (1:400 dilution) for 30 min at 37°C or by SignalStain Boost IHC detection reagent HRP rabbit (Cell Signaling 8114) for 1 h at room temperature. Labeling was visualized with chromogen diaminobenzidine (Invitrogen 00–2020), and sections were counterstained with hematoxylin (Zymed 00–8011) and dehydrated in graded ethanol. Coverslips were mounted with Histomount mounting solution (Invitrogen 00–8030).
Statistics.
Data were analyzed using Student's t-test or one-way ANOVA followed by a Student-Newman Keuls post hoc test to identify differences between groups. Significance was determined as P ≤ 0.05. Results are represented as means ± SE.
RESULTS
Lithium-induced NDI.
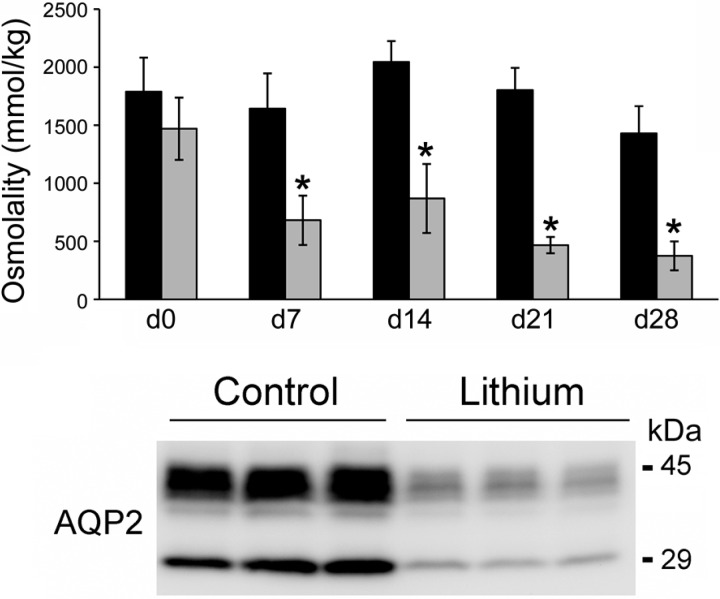
To ensure that lithium treatment was consistent with previous studies in rats, ICR mice were treated with lithium (diet) for 28 days to evaluate the time course of induction of NDI. Urine osmolality decreased significantly after a week of lithium treatment, and continued to decline throughout the 28-day study (Fig. 1). AQP2 expression was significantly decreased in the renal medulla following 2 wk of lithium (Fig. 1).
Fig. 1.
Decreased urine osmolality and aquaporin-2 (AQP2) expression in mice on a lithium diet. Top: mice were fed a 0.2% LiCl diet for 28 days (d28). Urine was collected and osmolality was measured using a vapor pressure osmometer. Data are presented as means ± SE; n = 4. *Significant difference between control mice and lithium-treated mice (t-test, P < 0.05). Bottom: immunoblots examining AQP2 protein abundance in inner medulla homogenates of control and lithium-treated mice (2 wk). Each lane represents a homogenate from an individual medulla.
Lithium activates the mTOR signaling pathway in renal medullary collecting duct cells.
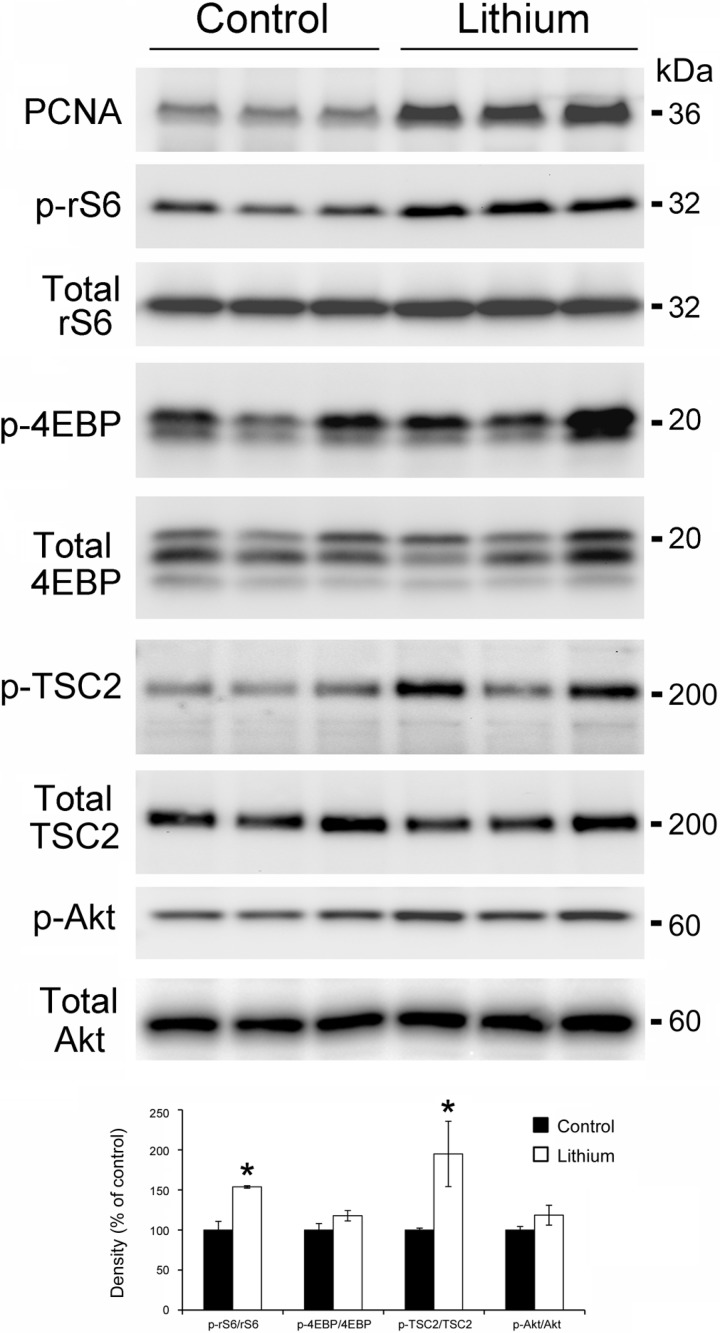
Lithium treatment is known to induce a proliferative response in the principal cells of renal medullary collecting ducts, and this was consistent in our model; protein expression of PCNA was significantly increased after 2 wk of lithium (Fig. 2). Therefore, we asked whether lithium-induced renal cell proliferation was associated with an increase in mTOR signaling.
Fig. 2.
mTOR signaling is activated in the renal medulla of lithium-treated mice. Mice were fed a 0.2% LiCl diet for 2 wk. Top: Western blot analysis of protein homogenates from control and lithium-treated mice was probed for PCNA, p-rS6, p-4EBP, p-TSC2, and p-Akt. Total rS6, 4EBP, TSC2, and Akt were used as loading control for densitometry. Each lane represents a homogenate from an individual medulla. Bottom: densitometry results for top. Each phospho protein was normalized to total protein, n = 3 vs. 3. *Significant difference between control mice and lithium-treated mice (t-test, P < 0.05).
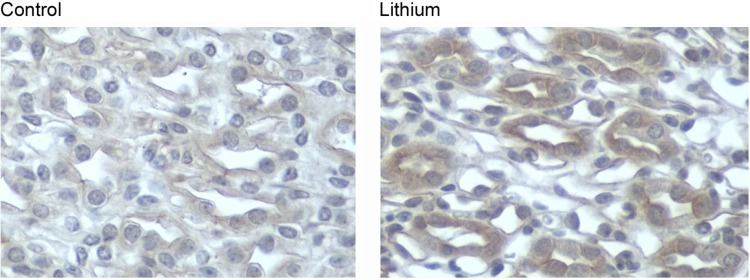
Using phosphospecific antibodies, we examined the phosphorylation of rS6 and 4EBP, downstream targets of mTOR, in the renal medulla of lithium-treated mice. Lithium treatment for 2 wk was associated with an increase in p-rS6; however, it had no effect on p-4EBP in the renal medulla (Fig. 2). In a quiescent state, TSC2 (tuberin) inhibits mTOR; however, phosphorylation of TSC2 at Thr 1462 (23) by Akt can release this inhibition, activating the mTOR pathway. Consistent with our hypothesis, p-TSC2 (T1462) levels in the renal medulla of lithium-fed mice were significantly increased (Fig. 2). p-Akt (S473) abundance increased following 2 wk of lithium treatment, although when normalized to total Akt levels the increase was not significant. Immunohistochemistry data presented in Fig. 3 demonstrate that mTOR activation in the renal medulla of lithium-treated mice was localized to collecting ducts. Faint p-mTOR staining seen in renal collecting ducts from control mice increased in the renal medullary collecting ducts following lithium treatment.
Fig. 3.
Localization of lithium-induced phospho-mTOR expression in mouse renal inner medulla. Phospho-mTOR immunoperoxidase labeling was performed in paraffin-embedded mouse kidney sections from control mice and lithium-treated mice. Representative photographs depict phospho-mTOR staining, demonstrating an increase in the renal medulla following lithium treatment (magnification ×400).
Rapamycin reverses lithium-induced renal medullary cell proliferation.
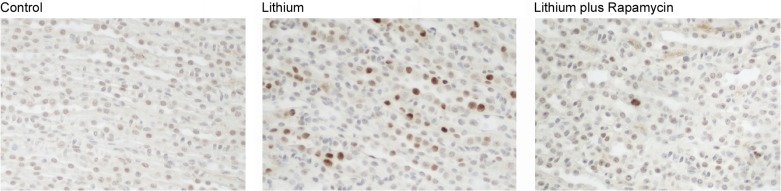
Rapamycin is an inhibitor of mTOR and has been demonstrated to reduce the rate of cell proliferation in polycystic kidney disease (PKD). To test whether inhibition of mTOR could reverse lithium-induced medullary cell proliferation, we treated lithium-fed mice with rapamycin. Mice were fed lithium for 14 days, with rapamycin treatment (daily ip) starting on day 7. Control mice received a placebo injection. One week of rapamycin treatment reduced lithium-induced medullary cell proliferation (Fig. 4).
Fig. 4.
Rapamycin reverses lithium-induced cell proliferation in medullary collecting ducts. Proliferating cell nuclear antigen (PCNA) immunoperoxidase labeling was performed in paraffin-embedded mouse kidney sections from control mice, lithium-treated mice, and lithium/rapamycin-treated mice. Representative photographs depict PCNA-positive cells in the renal medulla, demonstrating that rapamycin reverses lithium-induced proliferation in the initial mouse renal medulla (magnification ×200).
Rapamycin inhibited mTOR signaling in the renal medulla.
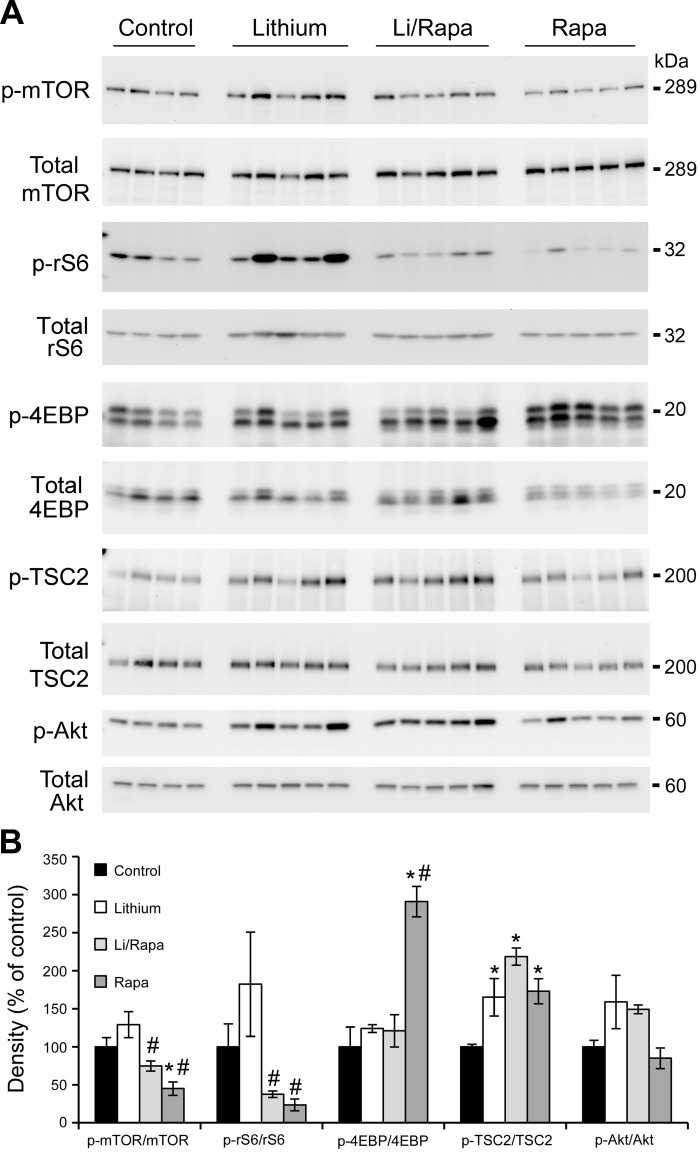
Rapamycin is an allosteric inhibitor of mTOR and acts to decrease the activity of rS6 by reducing the level of phosphorylated rS6. We examined whether mTOR inhibition by rapamycin was sufficient to reduce p-rS6 in the renal medullas of lithium-fed mice. Lithium was fed to mice for 28 days, and rapamycin (ip) was initiated at day 14 of lithium (which correlated with an increase of proliferation and activation of mTOR). Following 28 days of lithium treatment, the phosphorylation of rS6 remained elevated in the renal medulla of lithium-treated mice compared with control mice (Fig. 5) and rapamycin treatment reversed this increase in p-rS6. Phosphorylated mTOR (p-mTOR) levels in the renal medulla were reduced by rapamycin both in the presence and absence of lithium (Fig. 5). Chronic lithium treatment had no effect on medullary p-4EBP levels, and remained unchanged when combined with rapamycin. In animals treated with rapamycin alone, p-4EBP levels were significantly elevated and p-rS6 levels were significantly reduced.
Fig. 5.
Rapamycin inhibition reduces mTOR activity in renal medulla of lithium-treated mice. Mice were fed lithium (0.2%) or control diet for 4 wk and injected daily with rapamycin or placebo for 2 wk. Western blot analysis of protein homogenates from control, lithium-, lithium/rapamycin-, and rapamycin-treated mice was probed for p-mTOR, p-rS6, p-4EBP, p-TSC2, and p-Akt. Total mTOR, rS6, 4EBP, TSC2, and Akt were used as loading controls for densitometry. A: representative blots. Each lane represents a homogenate from an individual mouse. B: densitometry results for A. Each phospho protein was normalized to total protein; results are expressed as means ± SE, n = 4 in control vs. 5 experimental groups. *Significant difference vs. control mice. #Significant difference vs. lithium-treated mice as determined by Student-Newman-Keuls post hoc test following 1-way ANOVA.
Rapamycin, a direct inhibitor of mTOR, had no effect on the levels of phosphorylated TSC2; levels of p-TSC2 (T1462) were significantly increased in lithium-fed mice, and remained elevated in rapamycin-treated lithium-fed mice. p-Akt (S473) levels were elevated in the renal medulla after 4 wk of lithium and remained elevated with rapamycin treatment (Fig. 5).
Lithium-induced increases in cell cycle modulators.
We examined the mRNA expression of cell cycle modulators, Egr-1 and cyclin D1, in the renal medulla of lithium-treated mice, along with the tyrosine kinase receptor proto-oncogene c-RET. Our previous studies identified Egr-1 and cyclin D1 as genes associated with medullary collecting duct proliferation (6, 28) and studies in a medullary collecting duct cell line (mIMCD3) identified c-Ret as inducible by lithium (20). The mRNA expression of Egr-1 and cyclin D1 was significantly increased by lithium. In contrast, c-Ret9 expression, the most highly expressed isoform of c-Ret, was significantly decreased (Table 1). Egr-1 expression was reduced by rapamycin treatment; however, cyclin D1 levels remain elevated (Table 1).
Table 1.
Effect of lithium on gene expression in mouse renal medulla
| Gene | C vs. Li | C vs. Li-Rapa | C vs. Rapa | Li vs. Li-Rapa |
|---|---|---|---|---|
| AQP2 | 0.66 | 0.49* | 1.20 | 0.82 |
| COX-2 | 1.09 | 0.47* | 0.67 | 0.43* |
| Egr-1 | 5.46* | 1.27 | 0.93 | 0.23 |
| Cyclin D1 | 1.73* | 2.09 | 1.64* | 1.20 |
| c-Ret | 0.75 | 0.59 | 0.50 | 0.79 |
| c-Ret9 | 0.36* | 0.35* | 0.34* | 0.96 |
| c-Ret51 | 0.63 | 0.55 | 0.45 | 0.88 |
SYBR green real-time PCR assay was used to derive Ct values for calculation of mRNA abundance. Data shown are mean relative gene expression normalized to control group. AQP2, aquaporin-2; COX-2, cyclooxygenase-2; C, control; Li, lithium; Rapa, rapamycin.
Significant difference between groups (t-test, P < 0.05).
Rapamycin did not prevent lithium-induced loss of AQP2 mRNA or protein expression and subsequent NDI.
Rapamycin treatment clearly prevented lithium's ability to cause a proliferative response in the kidney, as shown in Fig. 4. An additional side effect of lithium treatment is NDI, involving a significant decrease in AQP2 expression in the renal medullary collecting ducts (Fig. 1). We examined whether rapamycin treatment would improve lithium-induced NDI. AQP2 protein expression was significantly reduced after 4 wk of lithium as shown in Fig. 6, and treatment with rapamycin did not reverse the loss of AQP2 expression in the renal medulla. Indeed, when mRNA expression levels were examined, the combination of rapamycin and lithium caused a further reduction in AQP2 mRNA in the renal medulla: AQP2 mRNA was significantly reduced in the LiRa group vs. lithium alone (Table 1).
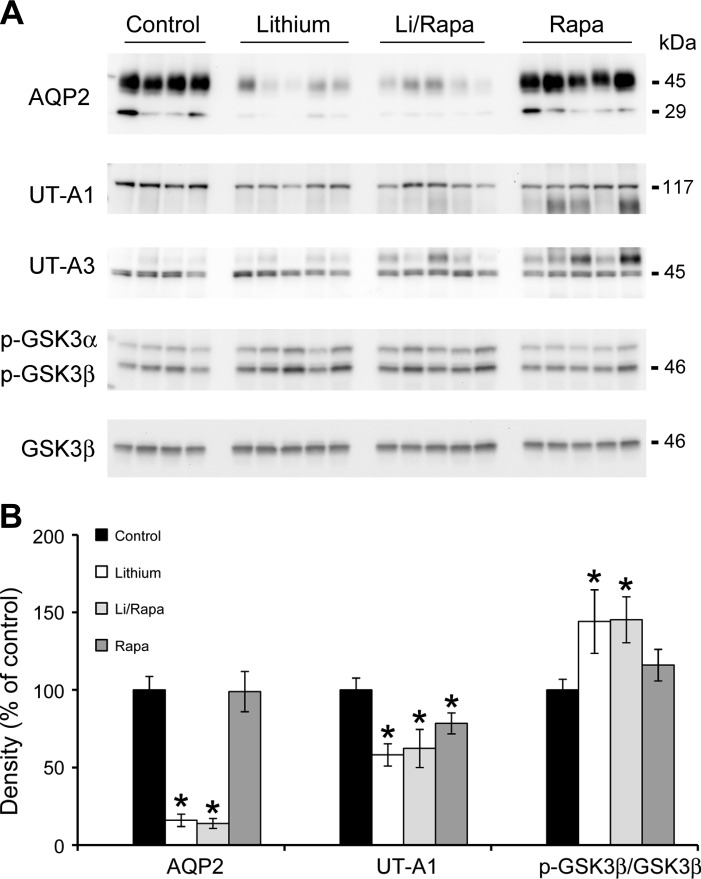
Fig. 6.
Rapamycin inhibition did not restore AQP2 or UT-A1 expression. Mice were fed lithium (0.2%) or control diet for 4 wk and injected daily with rapamycin or placebo for 2 wk. Western blot analysis of protein homogenates from control, lithium-, lithium/rapamycin-, and rapamycin-treated mice was probed for AQP2, UT-A1, UT-A3, and p-GSK3α and β. Total GSK3β was used as loading controls for densitometry. A: representative blots. Each lane represents a homogenate from an individual mouse. B: densitometric analysis of results for AQP2, UT-A1, and p-GSK3β is expressed as means ± SE, n = 4 in control vs. 5 experimental groups. *Significant difference vs. control mice (t-test, P < 0.05).
A reduction in apical urea transporter expression was also seen with lithium treatment; UT-A1 protein expression was significantly reduced by lithium treatment, and remained at reduced levels when lithium-treated mice were given rapamycin. Interestingly, rapamycin alone significantly decreased UT-A1 expression yet had no effect on AQP2 expression. UT-A3 expression did not alter across all groups (Fig. 6).
Lithium is known to inactivate GSK3β via phosphorylation of serine-9. Medullary levels of p-GSK3β were increased in the renal medullas of lithium-treated mice and remained elevated following rapamycin treatment (Fig. 6). However, COX-2 mRNA abundance was not increased in the renal medullas of lithium-fed mice compared with control mice. Furthermore, COX-2 mRNA abundance was significantly reduced in the renal medullas of lithium-fed rapamycin-treated mice compared with control mice (Table 1).
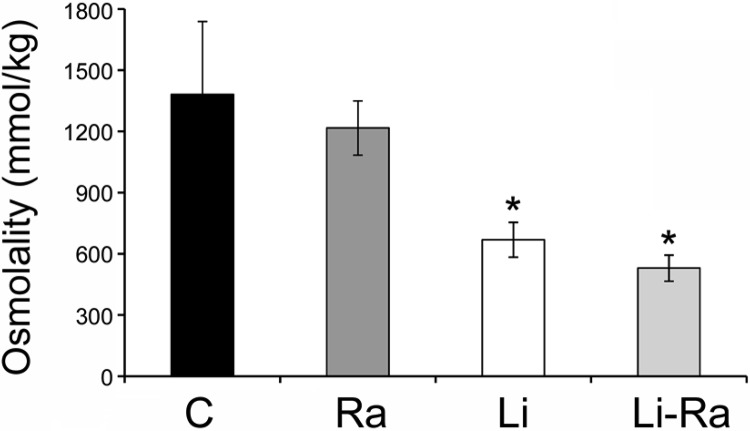
Finally, rapamycin had no significant effect to improve lithium-induced NDI as measured via urine osmolality; urinary osmolality was significantly reduced after 4 wk of lithium treatment, and remained significantly reduced in the rapamycin-treated lithium group (Fig. 7).
Fig. 7.
Rapamycin inhibition did not increase urine osmolality in lithium-treated mice. Mice were fed lithium (0.2%) or control diet for 4 wk and injected daily with rapamycin or placebo for 2 wk. Urine was collected and osmolality was measured using a vapor pressure osmometer. Data are presented as means ± SE; n = 5. *Significant difference vs. control mice as determined by Student-Newman-Keuls post hoc test following 1-way ANOVA.
DISCUSSION
In addition to lithium's ability to cause NDI, via the downregulation of collecting duct AQP2 expression, lithium is also known to induce proliferation of collecting duct cells in the renal medulla. Lithium-induced proliferative signaling mechanisms are little understood, reviewed in Ref. 17, and were the focus of this study. The present study shows that lithium, when fed to mice, leads to cellular proliferation of medullary collecting ducts via the activation of mTORC1 pathway. Rapamycin, by inhibition of mTORC1, reversed lithium-induced proliferation. In contrast, rapamycin did not reverse the loss of AQP2 expression in the renal medulla and had no impact on lithium-induced NDI.
Lithium-induced cell proliferation: role for mTOR activation.
Renal cell proliferation has long been known to occur in response to the administration of lithium, leading to the appearance of renal microcysts in humans (10) and rodents (7). Gene array and proteomic analyses of collecting ducts have identified lithium-induced changes in the expression of cell cycle modulators, associated with an increase in proliferation (28), and the activation of PKB/Akt (25) and phosphorylation of GSK3β (27). Here, we add mTOR activation to the list of signaling pathways involved in lithium-induced renal cell proliferation. Using Western blot analysis, immunohistochemistry, and gene expression analysis, we demonstrate that lithium rapidly activates mTORC1, increasing the phosphorylation of TSC2 and rS6 in the renal medulla.
It is well-known that mTOR is a serine threonine kinase that exists in two structurally distinct signaling complexes, mTORC1 and mTORC2. The Tuberous sclerosis complex (TSC) is itself a complex of TSC1/TSC2 proteins (also known as hamartin and tuberin) which, when quiescent, binds to an additional protein Rheb and inhibits mTOR activity. Akt phosphorylation of TSC2 (T1462) releases Rheb, leading to the activation of mTORC1. Once activated, mTORC1-mediated phosphorylation of 4EBP and the ribosomal protein S6 kinases (S6K1/2) (13, 15, 16, 22) increases mRNA translation leading to an increase in cell proliferation and growth (12, 16).
The data in this study are compatible with this pathway as rapamycin treatment reduced the activity of rS6 and reversed renal cell proliferation in lithium-fed mice. Rapamycin is a potent inhibitor of S6K phosphorylation, whereas recent studies showed its effect on 4EBP is less robust and more transient perhaps explaining why rapamycin did not reduce 4EBP levels in our study, indeed rapamycin alone significantly increased 4EBP in the renal medulla (11, 36).
Paradoxically, while lithium increased the expression levels of cyclin D1 and Egr-1, confirming our previous studies associating increased expression of cell cycle modulators with an increase in medullary cell proliferation (6), rapamycin did not reduce cyclin D1 expression. Cyclin D1 serves as a regulator of continued cell cycle progression and increased cyclin D1 levels are an early indicator of carcinogenesis (1). Phosphatidylinositol 3-kinase (PI3K)/PKB (Akt) activation can trigger a network that alters the regulation of cell cycle progression leading to an increase in cyclin D1, via GSK3β inhibition (21). mTOR activation via TSC2 phosphorylation participates in this network and rapamycin is under intense investigation as an anti-proliferative cancer therapy. However, recent studies have suggested that rapamycin inhibition of mTOR does not always result in a reduction of cyclin D1 expression, as sustained inhibition of S6K can lead to feedback activation of the Akt pathway and elevated cyclin D1 levels (40). Phosphorylated levels of Akt did remain high in the renal medulla of lithium-fed mice following prolonged treatment with rapamycin (2 wk) supporting the ability of rapamycin to enhance Akt activity.
Lithium is itself a known inhibitor of GSK3β, a serine threonine kinase. GSK3 has two isoenzymes, beta and alpha, and is constitutively active in cells under resting conditions. GSK3β phosphorylates cyclin D1 on threonine-286 leading to its rapid proteasomal degradation (8). However, phosphorylation of GSK3 (serine-9) inhibits GSK3β activity and thus its ability to degrade cyclin D1, which in turn can increase proliferation. Lithium has been shown to phosphorylate ser-9 in the renal medulla, both in collecting duct cells and in renal interstitial cells (18, 25, 27). Furthermore, administration of lithium in utero induced an increase in p-GSK3β expression, an increase in cell proliferation, and led to structural injury of the kidney along with attenuation of the urinary concentrating capacity in adult life (18).
An additional known effect of GSK3β inhibition (either by Wnt activation or by direct inhibition) is to stabilize β-catenin which allows its translocation to the nucleus where it can then activate the transcription of a large number of genes. A study in a medullary collecting duct cell line (mIMCD3) demonstrated that lithium activated c-Ret, a tyrosine kinase receptor and proto-oncogene (20). Cells treated with lithium for 5–10 days increased cRet9 mRNA abundance. This effect was mimicked using another inhibitor of GSK3β, 6-bromomindirubin-3′-oxime. However, lithium's ability to activate c-Ret was suggested to be independent of β-catenin/TCF signaling pathway, despite lithium's ability to increase cellular β-catenin levels. Indeed, the authors suggested that mTOR activation could contribute to c-Ret activation (20). These studies again demonstrate the possible overlap of mechanisms behind lithium's ability to activate mTOR, inhibit GSK3β, and possibly activate PI3K/PKB (Akt). However, in contrast to the in vitro study where lithium increased c-Ret expression and activation, we did not see an increase in c-Ret expression in the renal medulla of lithium-treated mice. Indeed, c-Ret9 mRNA expression decreased significantly with lithium treatment in our study, highlighting key differences between in vivo physiological models of disease and in vitro studies.
The degradation of cytoplasmic proteins or organelles is mediated via autophagy. mTOR activity negatively regulates autophagy, and inhibition of mTOR via rapamycin upregulates autophagy (reviewed in Ref. 29). It is known that lithium has opposing effects on autophagy (31) via its targets inositol monophosphatase (IMPase) and GSK3β. Initial studies in a Drosophila model of Huntingdon's disease demonstrated that lithium induced autophagy in an mTOR-independent manner via the inhibition of IMPase (30). Subsequent studies demonstrated that lithium's ability to inhibit GSK3β and activate mTOR suppressed autophagy (31). Combined use of lithium with rapamycin, where rapamycin inhibited mTOR downstream of GSK3β, resulted in overall induction of autophagy. We did not examine autophagy in our current study; however, we can hypothesize that autophagic pathways would be induced following the combined treatment of lithium and rapamycin, and future studies should clarify whether autophagy plays a critical role in this model of renal cell proliferation.
It is clear that constitutively activated mTORC1 leads to uncontrolled cell proliferation in many cell types. In renal collecting duct cells, the role of mTOR has been investigated as a signaling mechanism involved in the proliferation of principal cells in PKD. Although a genetic disease in origin, PKD is characterized by an increase in renal cell proliferation, leading to cyst formation and progressive renal dysfunction. Experimental studies in rodent models of PKD have used rapamycin (Sirolimus) to inhibit mTOR signaling, with beneficial results. Specifically in a mouse model with a deletion of PKD1, rapamycin inhibited epithelial cell proliferation, cyst formation, and inhibited fibrosis (33). Somewhat disappointingly, recent clinical trials on the use of mTOR inhibitors in PKD were negative as they failed to reduce the progressive increase in single cyst and total kidney volume (34).
Lithium and NDI.
Several pathways have been identified to play a role in lithium's ability to cause NDI. Lithium's ability to increase urinary PGE2 excretion (35) and COX2 expression has been attributed to the inhibition of medullary GSK3β activity. Indeed, indomethacin, a nonspecific COX-inhibiting NSAID, reduced PGE2 excretion and improved polyuria in lithium-treated mice (35). An increase in COX2 and local PGE2 excretion would antagonize the collecting duct cells response to vasopressin and reduce AQP2 expression.
Our data confirmed the downregulation of AQP2 protein expression in the renal medulla of lithium-treated mice; however, lithium had no significant effect on AQP2 mRNA expression in the renal medulla. Rapamycin treatment of lithium-fed mice did not reverse this decrease in protein expression; however, rapamycin in combination with lithium significantly reduced AQP2 mRNA expression, along with a further small reduction in urinary osmolality. As discussed above, inhibition of mTOR with rapamycin activates autophagy. Whether autophagy is playing a role in the lithium-induced loss of expression of AQP2 in the principal cell of the collecting duct has not been investigated.
In addition to a significant decrease in AQP2 protein expression, lithium is known to decrease the expression of the collecting duct urea transporter, UT-A1 (19). Our data confirmed these observations. Indeed, rapamycin alone is sufficient to significantly decrease UT-A1 expression in the renal medulla of mice, identifying a possible role for mTOR in the regulation of UT-A1 expression.
In summary, our study identifies mTOR as a signaling pathway that is activated by lithium in the medullary collecting ducts of the kidney. We suggest that mTOR activation is responsible for lithium-induced renal cell proliferation, which was reversed by inhibition of the mTOR pathway with rapamycin. However, mTOR inhibition did not improve lithium-induced NDI and did not restore the expression of collecting duct proteins AQP2 or UT-A1. In fact, rapamycin alone may have unwanted effects to reduce the expression of key components of the renal concentrating mechanism.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK073611 (to H. L. Brooks) and NS065926 (to T. J. Price).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.G., M.J.R.-A., Q.C., T.J.P., and H.L.B. conception and design of research; Y.G., M.J.R.-A., and Q.C. performed experiments; Y.G., M.J.R.-A., Q.C., and H.L.B. analyzed data; Y.G., M.J.R.-A., Q.C., T.J.P., and H.L.B. interpreted results of experiments; Y.G., M.J.R.-A., Q.C., and H.L.B. prepared figures; Y.G., M.J.R.-A., Q.C., and H.L.B. drafted manuscript; Y.G., M.J.R.-A., Q.C., T.J.P., and H.L.B. edited and revised manuscript; Y.G., M.J.R.-A., Q.C., T.J.P., and H.L.B. approved final version of manuscript.
REFERENCES
- 1.Arber N, Hibshoosh H, Moss SF, Sutter T, Zhang Y, Begg M, Wang S, Weinstein IB, Holt PR. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology 110: 669–674, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Bedford JJ, Weggery S, Ellis G, McDonald FJ, Joyce PR, Leader JP, Walker RJ. Lithium-induced nephrogenic diabetes insipidus: renal effects of amiloride. Clin J Am Soc Nephrol 3: 1324–1331, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis 10: 329–345, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Brooks HL, Sorensen AM, Terris J, Schultheis PJ, Lorenz JN, Shull GE, Knepper MA. Profiling of renal tubule Na+ transporter abundances in NHE3 and NCC/TSC null mice using targeted proteomics. J Physiol 530: 359–366, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks HL, Ageloff S, Kwon TH, Brandt W, Terris JM, Seth A, Michea L, Nielsen S, Fenton R, Knepper MA. cDNA array identification of genes regulated in rat renal medulla in response to vasopressin infusion. Am J Physiol Renal Physiol 284: F218–F228, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Cai Q, McReynolds MR, Keck M, Greer KA, Hoying JB, Brooks HL. Vasopressin receptor subtype 2 activation increases cell proliferation in the renal medulla of AQP1 null mice. Am J Physiol Renal Physiol 293: F1858–F1864, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Christensen BM, Kim YH, Kwon TH, Nielsen S. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol 291: F39–F48, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Diehl JA, Zindy F, Sherr CJ. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev 11: 957–972, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, Kozma SC, Thomas G, Sonenberg N. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 328: 1172–1176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farres MT, Ronco P, Saadoun D, Remy P, Vincent F, Khalil A, Le Blanche AF. Chronic lithium nephropathy: MR imaging for diagnosis. Radiology 229: 570–574, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol 7: e38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol 24: 200–216, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev 12: 502–513, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golshayan D, Nseir G, Venetz JP, Pascual M, Barbey F. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int 81: 601, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 12: 9–22, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 18: 1926–1945, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Kishore BK, Ecelbarger CM. “Lithium” a versatile tool to understand renal physiology. Am J Physiol Renal Physiol 304: F1139–F1149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjaersgaard G, Madsen K, Marcussen N, Christensen S, Walter S, Jensen BL. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3 beta-positive epithelium. Am J Physiol Renal Physiol 302: F455–F465, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Klein JD, Gunn RB, Roberts BR, Sands JM. Downregulation of urea transporters in the renal inner medulla of lithium-fed rats. Kidney Int 61: 995–1002, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Kojima N, Saito H, Nishikawa M, Yuri S, Jo OD, Pham PC, Yanagawa N, Yanagawa N. Lithium induces c-Ret expression in mouse inner medullary collecting duct cells. Cell Signal 23: 371–379, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2: 339–345, 2003 [PubMed] [Google Scholar]
- 22.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J 441: 1–21, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell 10: 151–162, 2002 [DOI] [PubMed] [Google Scholar]
- 24.McReynolds MR, Taylor-Garcia KM, Greer KA, Hoying JB, Brooks HL. Renal medullary gene expression in aquaporin-1 null mice. Am J Physiol Renal Physiol 288: F315–F321, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci USA 105: 3634–3639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC, Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature 371: 762–767, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Rao R, Zhang MZ, Zhao M, Cai H, Harris RC, Breyer MD, Hao CM. Lithium treatment inhibits renal GSK-3 activity and promotes cyclooxygenase 2-dependent polyuria. Am J Physiol Renal Physiol 288: F642–F649, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Rojek A, Nielsen J, Brooks HL, Gong H, Kim YH, Kwon TH, Frokiaer J, Nielsen S. Altered expression of selected genes in kidney of rats with lithium-induced NDI. Am J Physiol Renal Physiol 288: F1276–F1289, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov 6: 304–312, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 170: 1101–1111, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarkar S, Krishna G, Imarisio S, Saiki S, O'Kane CJ, Rubinsztein DC. A rational mechanism for combination treatment of Huntington's disease using lithium and rapamycin. Hum Mol Genet 17: 170–178, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 103: 5466–5471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shillingford JM, Piontek KB, Germino GG, Weimbs T. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol 21: 489–497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stallone G, Infante B, Grandaliano G, Bristogiannis C, Macarini L, Mezzopane D, Bruno F, Montemurno E, Schirinzi A, Sabbatini M, Pisani A, Tataranni T, Schena FP, Gesualdo L. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD-study): a randomized, controlled study. Nephrol Dial Transplant 27: 3560–3567, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Sugawara M, Hashimoto K, Ota Z. Involvement of prostaglandin E2, cAMP, and vasopressin in lithium-induced polyuria. Am J Physiol Regul Integr Comp Physiol 254: R863–R869, 1988 [DOI] [PubMed] [Google Scholar]
- 36.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284: 8023–8032, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Timmer RT, Sands JM. Lithium intoxication. J Am Soc Nephrol 10: 666–674, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Wahl PR, Serra AL, Le HM, Molle KD, Hall MN, Wuthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transplant 21: 598–604, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Walker RG. Lithium nephrotoxicity. Kidney Int Suppl 42: S93–S98, 1993 [PubMed] [Google Scholar]
- 40.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26: 1932–1940, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 124: 471–484, 2006 [DOI] [PubMed] [Google Scholar]