Abstract
Enterohepatic helicobacter species (EHS) infect the intestinal tract and biliary tree, triggering intestinal and hepatic disorders. Helicobacter hepaticus, the prototypic murine EHS, is also associated with inflammation. Necrotizing enterocolitis (NEC) is a devastating disease of premature infants. The cause of NEC is not fully understood, but anomalies of bacterial colonization (dysbiosis) are thought to play an important role in disease onset. To evaluate the effect of H. hepaticus infection on the development of NEC, premature formula-fed rats were kept either in H. hepaticus-free conditions or colonized with H. hepaticus; both groups were exposed to asphyxia and cold stress. The incidence of NEC, expression of Toll-like receptors (TLRs), production of cytokines and mucins, and presence of autophagy regulators were evaluated at the site of injury. H. hepaticus infection increased the incidence of NEC from 39 to 71% and significantly increased levels of TLR4 receptor, expression of proinflammatory cytokines CXCL1, IL-1β, IL-12, and IL-23, and altered activation of autophagy. H. hepaticus induces inflammation and increases the incidence and severity of experimental NEC; this is consistent with observations in neonates of blooms of proinflammatory microbes just before the onset of NEC. Future studies using rodent NEC models should include testing for H. hepaticus infection. Further studies in neonates of early identification and/or diminution of proinflammatory microbes may be beneficial in decreasing the incidence of NEC.
Keywords: autophagy, cytokines, enteral bacteria, mucosal inflammation
the groundbreaking discovery that Helicobacter pylori, which is present in the gastrointestinal tract of roughly half the world's population (34), is the key infectious agent in gastric and duodenal ulceration led to the subsequent identification of Enterohepatic helicobacter species (EHS) as important pathogens in several intestinal and hepatic diseases (22, 59). In contrast to H. pylori, the EHS colonize the lower gastrointestinal tract, including the ileum, cecum, colon, and biliary tree (51). Over 30 Helicobacter organisms have been identified. Among them, H. hepaticus, H. bilis, and H. rodentium have been implicated in murine models of colitis and colorectal cancer (16, 22).
Necrotizing enterocolitis (NEC) is a devastating gastrointestinal disease of prematurely born infants (1, 44). Despite decades of research and extensive clinical experience, its pathogenesis remains unclear. Recent studies suggest that bacterial colonization of newborn intestines plays a critical role in the disease onset. NEC may represent a final pathway for a heterogeneous group of intestinal insults (44), and for this reason it may be impossible to determine the origin and pathogenesis of this disease based purely on clinical observations. During the last two decades, experimental models of NEC have greatly advanced our knowledge of this disease and remain the key scientific tool for the development of novel therapies (17).
As addressed in more detail below, the premature neonate shows alterations in integrity of the epithelial layer, ability to maintain epithelial homeostasis, and formation of the mucus layer. These factors and alterations in regulation of inflammatory responses, including those due to bacteria:Toll-like receptor (TLRs) interactions, are all implicated in the pathogenesis of NEC (24). Evidence from animal models and human NEC tissue studies suggests that induction of TLR4 occurs early in NEC pathogenesis (3, 18, 25, 29–31), inducing expression of proinflammatory cytokines interleukin (IL)-6, IL-12, IL-18, IL-23, CXCL1, and tumor necrosis factor-α (TNF-α) (32, 40) (7). In models of H. hepaticus-induced colitis, TLR4 signaling plays an important role (37). However, the effect of H. hepaticus on the incidence of NEC, TLR4, and cytokine expression in the rat NEC model is not known.
Autophagy is a fundamental process that controls the catabolism of intracellular constituents (9) and has a crucial role in the maintenance of intestinal homeostasis (36). Uncontrolled autophagy leads to increased survival of intracellular bacteria and an uncontrolled proinflammatory response and has been implicated in the pathogenesis of Crohn's disease (48). Our laboratory has discovered the induction of autophagy in the intestinal epithelium of NEC patients and in the rat NEC model (38). Recently, our initial observation was confirmed in the rat and mouse NEC models (43, 60).
The mucus coat is another line of defense protecting the epithelial surface against damage and pathogen invasion. The mucus layer is formed by mucins (Muc) and trefoil factors (Tff). Impaired production of Muc2 and Tff3 has been shown during NEC pathogenesis (5, 26). H. pylori thrives in the mucus layer of the stomach and alters mucin production and mucus clearance to create a more favorable niche for itself (42), but the effect of H. hepaticus on mucus secretion in the small intestine is not known.
We hypothesized that exposure of the developing intestinal mucosa to H. hepaticus infection will exacerbate NEC injury, increase expression of TLR4 and proinflammatory cytokines, induce autophagy, and decrease production of intestinal Muc at the site of intestinal injury, the terminal ileum.
MATERIALS AND METHODS
Bacteria and animal model of NEC.
This protocol was approved by the Animal Care and Use Committee of the University of Arizona (A-324801–95081). H. hepaticus (strain MU 94–1) (10) was obtained from the University of Missouri Research Animal Diagnostics Laboratory (Columbia, MO) by Dr. D. G. Besselsen (Director of University Animal Care, University of Arizona, Tucson, AZ). H. hepaticus isolate was grown in Brucella broth at 37°C with shaking under microaerophilic conditions (90% N2-5% H2-5% CO2) for 1–3 days (10).
Pregnant Sprague-Dawley rats (Harlan Laboratories, Madison, WI) and their offspring were kept either in H. hepaticus-free conditions or colonized with H. hepaticus by being reared in an environment intentionally infected with the H. hepaticus pathogen. The presence of H. hepaticus in the intestinal tract was identified and confirmed by polymerase chain reaction (PCR) analysis (Fig. 1). Forty-one H. hepaticus-free pups (FF; n = 41) and 39 H. hepaticus-infected pups (FF+H.hep; n = 39) were used in this study. Neonatal rats were collected by caesarian section 24 h before their scheduled birth, and the first feeding started 2 h after delivery. Animals were hand fed five times daily with a total volume of 850 μl of rat milk formula per day (13). The rat milk formula used for feeding of neonatal rats was prepared primarily from evaporated milk (13), and composition is described in Table 1.
Fig. 1.
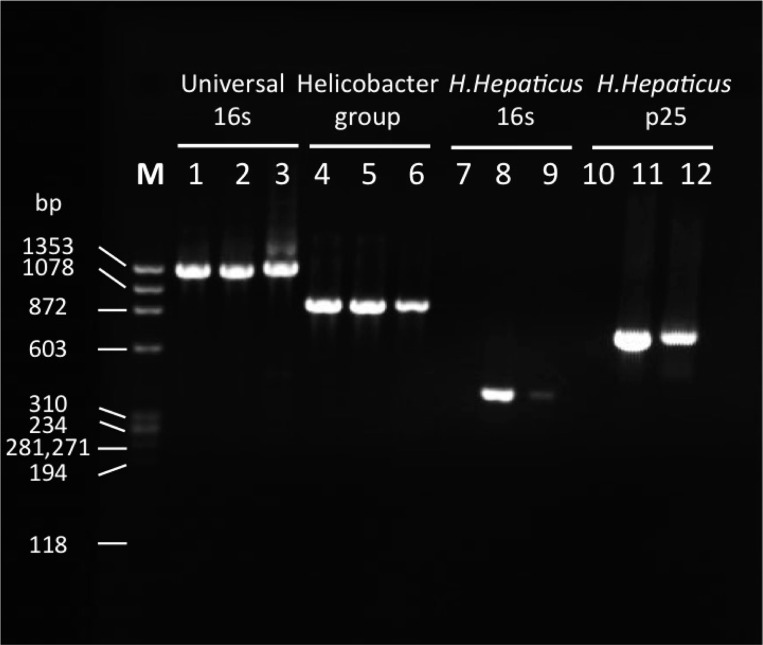
PCR analysis of Helicobacter isolates. The three Helicobacter isolates were H. pylori (lanes 1, 4, 7, and 10) (50), H. hepaticus (lanes 2, 5, 8, and 11) (15), and H. hepaticus (used in the current study, lanes 3, 6, 9, and 12). The PCR primers used were as follows: 16s universal primers (27F and 1391R) (56), lanes 1–3; Helicobacter group specific (274F and 1216R) (50), lanes 4–6; 16s H. hepaticus specific (591F and 979R) (2) lanes 7–9; and p25 H. hepaticus specific (p25F and p25R) (15), lanes 10–12. Phi-X174/HaeIII was used as size standard marker.
Table 1.
Preparation of rat milk formula
| Components | |
|---|---|
| Evaporated milk,1 ml | 695 |
| Bovine serum albumin,2 g | 36 |
| Intralipid 20% i.v. fat emulsion,3 ml | 292 |
| Almond oil, ml | 10 |
| Teklad vitamin mixture CA 40060,4g | 4 |
| Supplemental vitamin mixture,5 g | 0.56 |
| Salt mixture solution,6 ml | 10 |
| Noncalcium mineral mixture,7 g | 6 |
| Calcium lactate solution (40 g/l), ml | 10 |
| CaCO3 solution (40 g/l), ml | 10 |
| CuSO4 solution (30 g/l), ml | 0.57 |
| ZnSO4 solution (380 g/l), ml | 0.07 |
Carnation (Nestle, Solon, OH);
Sigma-Aldrich, St. Louis, MO;
Baxter, Deerfield, IL;
Harlan Laboratories, Madison, WI;
16.7 g/kg riboflavin, 26.0 g/kg niacin, 13.9 g/kg pyridoxal, 929.4 g/kg inositol, and 14.0 g/kg ascorbic acid sodium salt;
257 g/l NaCl and 7 g/l KCl in distilled water;
842 g/kg Na2HPO4, 152 g/kg MgSO4, 4 g/kg FeSO4 · 7H2O, 0.29 g/kg KI, 0.246 g/kg NaF, 0.156 g/kg AlSO4, and 0.042 g/kg MnSO4.
All animals were subjected to asphyxia (breathing 100% nitrogen gas for 60 s) and cold stress (4°C for 10 min) two times daily (12, 26). After 96 h, all surviving animals were terminated via decapitation. All measurements were performed in the ileum from the same set of rat pups that were used for NEC evaluations.
NEC evaluation.
After termination, a 2-cm piece of distal ileum was removed and fixed in 70% ethanol, paraffin embedded, sectioned at 4–6 μm, and stained with hematoxylin and eosin for histological evaluation of NEC. Pathological changes in intestinal architecture were evaluated using our previously published NEC scoring system (12, 26, 46). Histological changes in the ileum were scored by a blinded evaluator and graded as follows: 0 (normal), no damage; 1 (mild), slight submucosal and/or lamina propria separation; 2 (moderate), moderate separation of submucosa and/or lamina propria, and/or edema in submucosal and muscular layers; 3 (severe), severe separation of submucosa and/or lamina propria, and/or severe edema in submucosa and muscular layers, region villous sloughing; and 4 (necrosis), loss of villi and necrosis. Intermediate scores of 0.5, 1.5, 2.5, and 3.5 were also used to more accurately assess levels of ileal damage when necessary (12, 21). To determine the incidence of NEC, only animals with histological scores of 2 or greater were considered to have developed experimental NEC (26, 46).
RNA preparation and real-time PCR.
Total RNA was isolated from ileal tissue (snap-frozen in liquid N2) using the RNeasy Plus Mini Kit (Qiagen, Santa Clarita, CA) as described in the manufacturer's protocol. RNA concentration was quantified by ultraviolet spectrophotometry at 260 nm, and the purity and integrity were determined using a NanoDrop (Thermo Fisher Scientific, Wilmington, DE) (27).
RT real-time PCR assays were performed to quantify steady-state mRNA levels of Muc2, Tff3 and selected cytokines (CXCL1, IL-1β, IL-6, IL-12, IL-17, IL-23, IFN-γ, and TNF-α), and TLRs (TLR2, TLR4, and TLR5). cDNA was synthesized from 0.2 μg of total RNA. Real-time PCR amplification was performed using Primer Express Software (Applied Biosystems). Target probe was labeled with fluorescent reporter dye. The following sequences were used: TNF-α (GenBank X66539): sense primer 5′-gtgatcggtcccaacaagga-3′, anti-sense primer 5′-gggccatggaactgatgaga-3′, and probe 5′-cccatttgggaacttc-3′; Muc2 (GenBank BC036170): sense primer 5′-actgggaatgtgactgctactg-3′, anti-sense primer 5′-accctggtaactgtagtaaagtccat-3′, and probe 5′-acaaagtgtgggtcccc-3′.
PreDeveloped TaqMan primers and probes were used for the detection of CXCL1, IL-1β, IL-6, IL-12, IL-17, IL-23, IFN-γ, Tff3, TLR2, TLR-4, and TLR5. Reporter dye emission was detected by an automated sequence detector combined with ABI Prism 7700 Sequence Detection System software (Applied Biosystems). Real-time PCR quantification was then performed using TaqMan 18S controls.
Immunohistology.
A 2-cm section of distal ileum was collected from each animal and fixed overnight in 70% ethanol, paraffin-embedded, and sectioned at 4–6 μm. Serial sections were stained for TLR4. After deparaffinization and rehydration, sections were blocked in 5% BSA to prevent nonspecific staining and incubated with goat anti-TLR4 polyclonal antibody (sc-30002; Santa Cruz Biotechnology, Santa Cruz, CA) followed by incubation with Alexa Fluor 594-conjugated anti-goat secondary antibody (Molecular Probes, Eugene, OR) for 1 h and mounted with Vectashield Hard Set Mounting Medium containing YOYO-1 as a nuclear counterstain (Vector Laboratories). Negative control sections were treated with the same procedure in the absence of primary antibody; no immunostaining was observed in the negative controls (data not shown). Sections from each experimental group were immunostained for a specific antigen at the same time. Confocal laser scanning microscope (Zeiss LSM 510 NLO/META) equipped with a ×40 oil immersion objective was used to evaluate TLR4 staining.
Western blot analysis.
Individual frozen ileal samples were homogenized with a hand-held homogenizer (Pellet Pestle; Kimble/Kontes, Vineland, NJ) in a 5× volume of ice-cold homogenization buffer (50 mM Tris·HCl, pH 7.4; 150 mM NaCl; 1 mM EDTA; 0.1% SDS; 1% sodium deoxycholic acid; 1% Triton X-100; 50 mM DTT; 50 μg/ml aprotinin; 50 μg/ml leupeptin; and 5 mM phenylmethylsulfonyl fluoride). The homogenates were centrifuged at 10,000 rpm for 5 min at 4°C, and the supernatant was collected.
Total protein concentration was quantified using the Bradford protein assay. For protein analysis, 40 μg of protein were added to an equal volume of 2× Laemmli sample buffer and boiled for 5 min. The samples were run on 10% polyacrylamide gels at 110 V for 1.5 h. Protein was transferred to Immuno-Blot PVDF membranes (Bio-Rad) at 100 mA for 1.5 h. Membranes were blocked with 5% nonfat milk in Tris-buffered saline with 0.1% Tween 20 (Sigma) for 1 h at room temperature and then incubated overnight at 4°C with either a goat polyclonal antibody anti-TLR-4 (1:500, sc-3002; Santa Cruz Biotechnology), anti-TLR-2 (1:500, sc-16237; Santa Cruz Biotechnology), or anti-IL-23 (1:1,000, sc-21083; Santa Cruz Biotechnology). After being washed extensively, the membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated anti-rabbit or anti-goat IgG (Santa Cruz Biotechnology). Proteins were visualized with a chemiluminescent system (Pierce, Rockford, IL) and exposed to X-ray film.
Enumeration of Muc2- and Tff3-positive cells.
Serial sections of the ileum were stained for either Muc2 or Tff3. Briefly, after deparaffinization and rehydration, sections were incubated with either rabbit anti-Muc2 polyclonal antibody (Santa Cruz Biotechnology) or rabbit polyclonal Tff3 antibody (54) for 30 min, washed with PBS three times, and incubated with a biotinylated goat anti-rabbit secondary antibody (Vector Laboratories) for 30 min. Vectastain Elite ABC reagent (Vector Laboratories) was then applied, followed by diaminobenzidine as a substrate. Sections were counterstained with hematoxylin, dehydrated, and mounted on cover slips. Muc2- and Tff3-positive cells were counted from nine animals per experimental group, and the total number of epithelial cells per crypt-villus unit was also enumerated. Fifteen crypt-villus units were counted for each animal.
Statistical evaluation.
All numerical data are expressed as means ± SE. Statistical analyses between FF and FF+H.hep groups were performed by the Student's t-test at the 95% confidence level. Analysis of NEC score between groups was accomplished using the Kruskal-Wallis test for nonparametric values followed by pairwise comparison using the Mann-Whitney test. The Pearson's χ2 test was used to analyze differences in incidence of NEC between groups. All statistical analyses were conducted using the statistical program StatPlus:mac LE for Macintosh computers (AnalystSoft).
RESULTS
Exposure to H. hepaticus infection exacerbates severity and incidence of NEC.
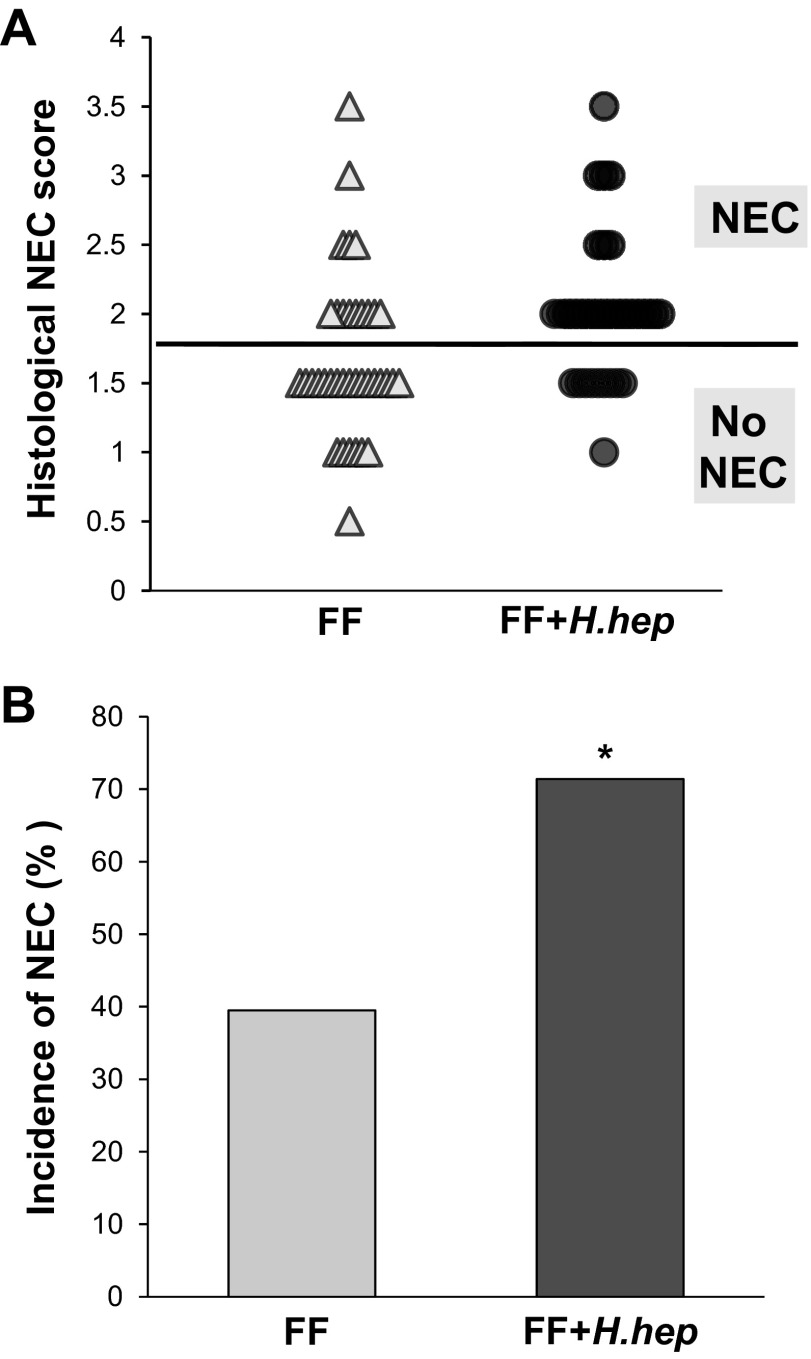
The Helicobacter strain used in this study was confirmed by PCR technique as a strain of H. hepaticus (Fig. 1) (15). There was no statistically significant difference in the survival of caesarian section-delivered premature rats between experimental groups [FF+H.hep 90% (35/39) and FF 93% (38/41)]. Damage to the villi and submucosa in the ileum was significantly higher in the FF+H.hep group with a mean value of NEC injury of 2.01 ± 0.09 compared with 1.68 ± 0.10 in the FF group (P ≤ 0.01; Fig. 2A). The incidence of NEC was higher in the FF+H.hep group [71% (25/35)] than the FF group [39% (15/38); P ≤ 0.01; Fig. 2B].
Fig. 2.
Severity and incidence of necrotizing enterocolitis (NEC) in neonatal rat model. A: histological NEC score in the H. hepaticus-free formula-fed rats (FF) (△; n = 38) and in the H. hepaticus-infected formula-fed rats (FF+H.hep) (●; n = 35). Ileal damage was assessed using the histological scoring scale of 0 (normal) to 4 (necrosis), as previously described (7, 12, 26). B: incidence of NEC in the neonatal rat model of NEC. Animals with scores ≥2 are considered NEC positive, and animals with ileal damage <2 do not have NEC. *P ≤ 0.01 vs. FF, χ2 analysis.
H. hepaticus increases intestinal expression of TLR4 in a rat model of NEC.
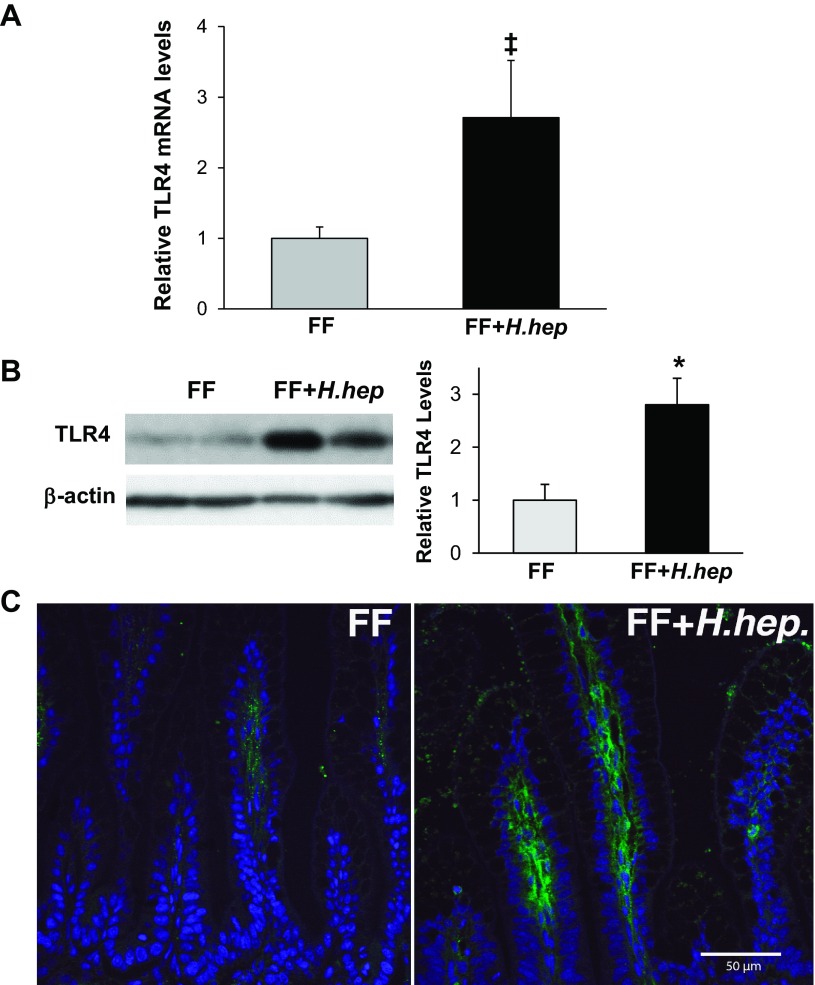
Gene expression of TLR4 in the ileum was increased two- to threefold in the FF+H.hep group compared with the FF group (P ≤ 0.02; Fig. 3A). There were no statistically significant differences in TLR2 and TLR5 mRNA levels between these two groups (results not shown). Western blot analysis showed two- to threefold higher levels of TLR4 protein in the ileum of the FF+H.hep group compared with the FF group (P ≤ 0.01; Fig. 3B). Protein levels of TLR2 were similar in both groups (results not shown). Confocal microscopy revealed that TLR4 signal was localized predominantly in the cells of the lamina propria in the ileum from the FF+H.hep group (Fig. 3C) with significantly fewer TLR4-positive cells observed in the ileum of FF rats (P ≤ 0.01).
Fig. 3.
Effect of H. hepaticus infection on expression and localization of Toll-like receptor-4 (TLR4) in the ileum. A: TLR4 mRNA levels evaluated using real-time PCR. The mean steady-state mRNA level for the FF group was assigned a value of 1.0, and mean mRNA levels for the FF+H.hep group were determined relative to this number. Values are means ± SE; n = 22–23 animals/experimental group. ‡P < 0.02 vs. FF. B: representative TLR4 (95-kDa) bands from Western blot analyses are shown for FF (n = 7) and FF+H.hep (n = 7) groups. All samples were analyzed on the same gel. Densitometric values were normalized to β-actin and to FF value. *P ≤ 0.01 vs. FF. C: representative slides from FF and FF+H.hep groups stained for TLR4 (green) were evaluated using confocal laser scanning microscopy (n = 4 animals/experimental group). In the FF group, a weak TLR4 signal (green) was observed in cells in the lamina propria. In the FF+H.hep group, a strong TLR4 signal was localized mainly in cells in the lamina propria and some enterocytes. Cell nuclei were stained with YOYO-1 (blue). No signal was observed in negative control sections (data not shown). Magnification: ×400.
H. hepaticus increases inflammatory response in the ileum.
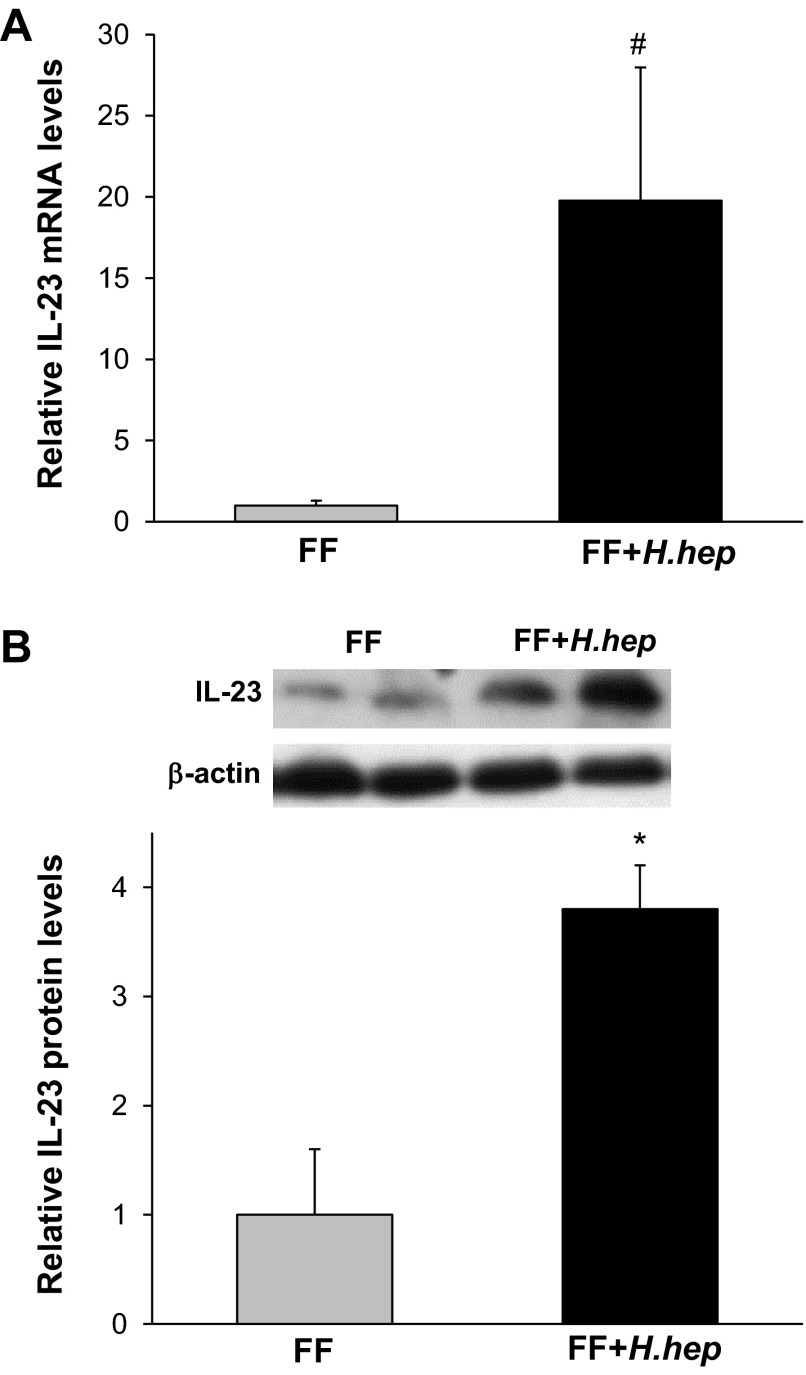
Gene expression of CXCL1, IL-1β, IL-6, IL-12, IL-17, IL-23, IFN-γ, and TNF-α was evaluated in the terminal ileum of studied animals. Exposure to H. hepaticus increased IL-23 mRNA levels in the ileum ∼20 times compared with the FF group (P < 0.05; Fig. 4A). Western blot analysis showed IL-23 protein levels about three to four times higher in the FF+H.hep group compared with the FF group (P < 0.01; Fig. 4B).
Fig. 4.
Effect of H. hepaticus on gene and protein expression of IL-23 in the ileum. A: IL-23 mRNA levels evaluated using real-time PCR. The mean steady-state mRNA level for the FF group was assigned a value of 1.0, and mean mRNA level for the FF+H.hep group was determined relative to this number. Values are means ± SE; n = 12 animals/experimental group. #P ≤ 0.05 vs. FF. B: representative IL-23 (19-kDa) bands from Western blot analyses are shown for FF (n = 6) and FF+H.hep (n = 6) groups. All samples were analyzed on the same gel. Densitometric values were normalized to β-actin and to FF value. *P ≤ 0.01 vs. FF.
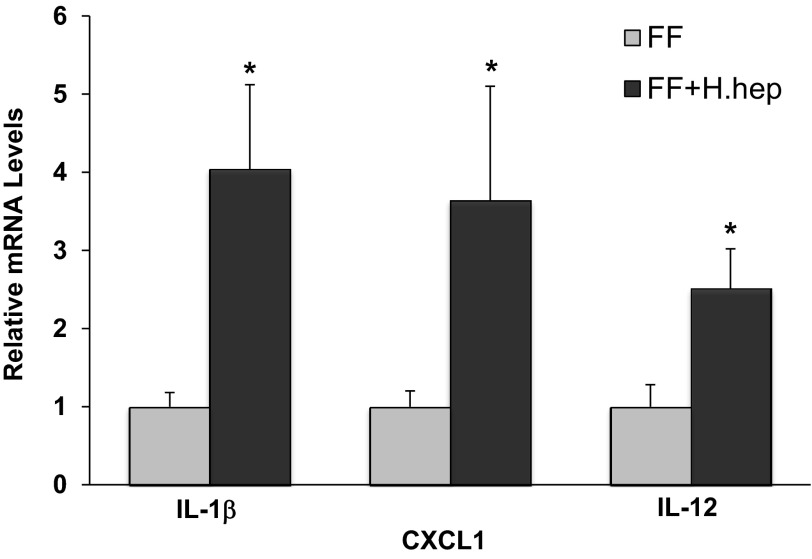
Expression of proinflammatory IL-1β, CXCL1, and IL-12 was significantly increased in rats exposed to H. hepaticus compared with the FF group (Fig. 5). There were no statistically significant differences in IL-6, TNF-α, and IFN-γ mRNA levels between these two groups (results not shown). Gene expression of IL-17 was not detected in the ileum of these animals (results not shown).
Fig. 5.
IL-1β, CXCL1, and IL-12 mRNA levels in neonatal rat ileum. The mean steady-state mRNA levels for the FF group were assigned a value of 1.0, and mean mRNA levels for the FF+H.hep group were determined relative to this number. Values are means ± SE; n = 12–18 animals/experimental group. *P ≤ 0.01 vs. FF.
Evaluation of Muc2 and Tff3 in the ileum.
Ileal Muc2 and Tff3 gene expression and production were evaluated using real-time PCR and immunohistochemistry (Table 2). Gene expression for both Muc2 and Tff3 was similar in all experimental groups. Enumeration of Muc2- and Tff3-positive cells in the terminal ileum showed no differences between these two groups. These results indicate that exposure to H. hepaticus does not affect the formation of the mucus layer in this model.
Table 2.
Effect of H. hepaticus on Muc2 and Tff3 expression in the ileum
| Group | Muc2 mRNA | Muc2-Positive Cells | Tff3 mRNA | Tff3-Positive Cells |
|---|---|---|---|---|
| FF | 1.00 ± 0.49 | 9.5 ± 0.5 | 1.02 ± 0.28 | 5.0 ± 0.6 |
| FF+H.hep | 0.96 ± 0.29 | 9.9 ± 0.3 | 0.82 ± 0.12 | 5.1 ± 0.5 |
Values for Muc2 and Tff3 mRNA are means ± SE; n = 8–10 animals/experimental group. Nos. for Muc2-positive cells and Tff3-positive cells are expressed as mean Muc2- or Tff3-positive cells/100 epithelial cells ± SE. Muc, mucins; Tff, trefoil factor; FF, Helicobacter hepaticus-free condition; FF+H.hep, H. hepaticus infected. The mean steady-state mRNA level for the FF group was assigned a value of 1.00, and mean mRNA level from the FF+H.hep was determined relative to this number.
H. hepaticus induces autophagy in the ileum of a rat model of NEC.
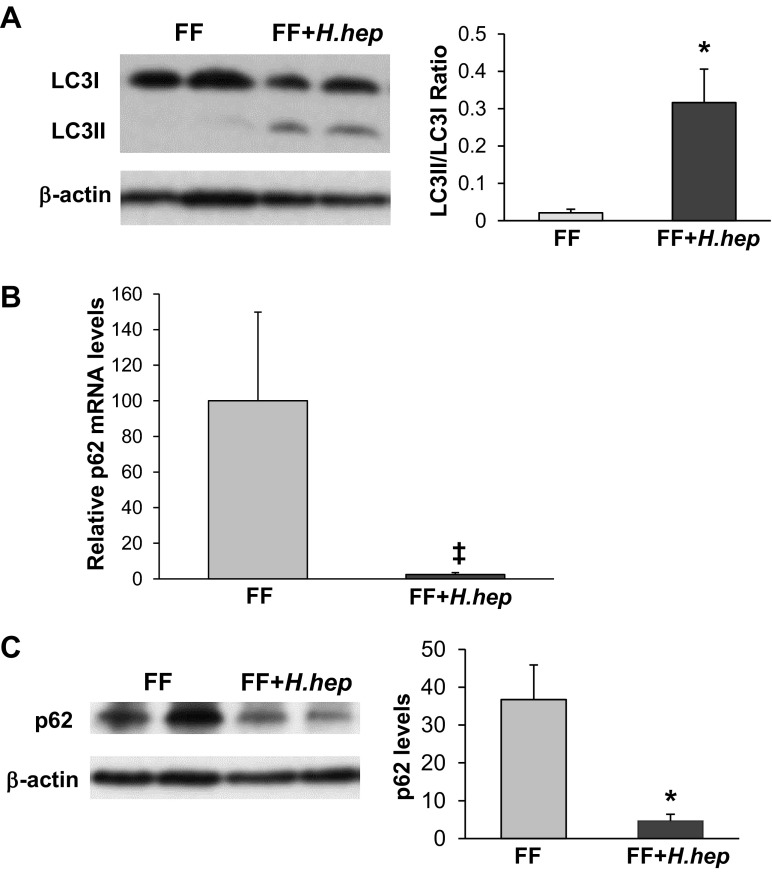
Induction of autophagy is frequently evaluated by following the phospholipid conjugation of LC3I (cytosolic form) to LC3II (membrane-bound form) (47). Detection of LC3II serves as an essential autophagosomal marker, and the ratio between these two LC3 proteins (LC3II/LC3I) correlates with the number of autophagosomes (58). To determine the effect of H. hepaticus on autophagy, LC3 protein levels were evaluated in the ileum of rat pups. In the FF+H.hep group, the expression of the LC3II isoform was markedly higher compared with FF pups (Fig. 6A). The LC3II-to-LC3I ratio in FF+H.hep rats was increased significantly, suggesting the activation of autophagy compared with FF rats (n = 9; P < 0.02; Fig. 6A).
Fig. 6.
Effect of H. hepaticus on expression of LC3II and p62 proteins. A: left, representative LC3 bands from Western blot analyses are shown for the FF and FF+H.hep groups. Conjugation from LC3I (17 kDa) into the LC3II (15 kDa), an autophagosomal membrane-incorporated isoform, is observed in the FF+H.hep group. Right, densitometry quantification of Western blot analysis for LC3 presented as a ratio of LC3II/LC3I (n = 7; *P ≤ 0.01 vs. FF). B: p62 mRNA levels evaluated using real-time PCR. The mean steady-state mRNA level for the FF group was assigned a value of 1.0, and mean mRNA level for the FF+H.hep group was determined relative to this number. Values are means ± SE; n = 14–16 animals/experimental group. ‡P ≤ 0.02 vs. FF. C: left, representative p62 (62-kDa) bands from Western blot analyses are shown for the FF and FF+H.hep groups. Right, densitometric values were normalized to β-actin and to FF value (n = 7; *P ≤ 0.01 vs. FF).
The p62 protein recognizes toxic cellular waste, which is then scavenged from the cell by autophagy. Direct interaction of p62 with LC3 complex leads to the degradation of p62 by the autophagy-lysosome system. Thus, degradation of p62 protein serves as a unique marker of autophagy activation. Protein levels of p62 in the ileum were markedly decreased in the FF+H.hep group compared with rats from the FF group (Fig. 6B), suggesting increased autophagic degradation in the autolysosome (consistent with the changes in the LC3II-to-LC3I ratio).
DISCUSSION
Although most long-term outcomes for prematurely born babies are gradually improving, NEC remains a significant clinical problem. The exact cause of NEC is not known, but critical elements in disease development include immaturity of virtually every aspect of intestinal innate immunity, enteral feeding, and inappropriate gut colonization (dysbiosis) (6, 44). Decreased microbial diversity, alteration in the composition of the intestinal microbiota, and colonization with unfavorable bacteria are frequently observed in feces from NEC patients (57). Indeed, the primary sites of NEC injury (the terminal ileum, ileocecal area, and ascending colon) are niches associated with high numbers and diversity of intestinal microbes. However, a specific pathogen predisposing neonates to develop NEC has not been identified (53).
H. hepaticus is a Gram-negative bacterium of the phylum Proteobacteria, which causes chronic hepatitis, hepatocellular carcinoma, typhlocolitis, and colorectal cancer in murine models (55). Although H. hepaticus has not been found in humans, other EHS (H. bilis, H. fennelliae, and H. pullorum) have been isolated from humans with intestinal diseases such as gallbladder cancer, cholecystitis, pancreatitis, and chronic diarrhea (16, 22, 52). In this study, for the first time, we demonstrate that infection with H. hepaticus increases the incidence of NEC and exacerbates intestinal injury in the rat NEC model.
These observations prompt two compelling questions. First, given that H. hepaticus infection is common and often not clinically evident in laboratory rodents (as high as 59% infection rate) (55), could some of the conflicting or ambiguous results reported from rodent models of NEC be related to undiagnosed H. hepaticus infection? At the very least, these data suggest the importance of regular testing for H. hepaticus in future experimental NEC studies (4). Second, given that Helicobacter species are widespread in the human population (34), could colonization or infection with EHS be contributory to increased susceptibility of premature infants to develop NEC?
In premature babies, NEC typically develops between 2 and 8 wk after delivery, which is similar to the timeline necessary for gut colonization (44) and appears to be associated with dysbiosis. Human studies showed an increase in fecal Proteobacteria in NEC, but predominantly from the family Enterobacteriaceae (33). Recent studies from the feces of premature infants with and without NEC did not identify organisms of the family Helicobacteriaceae (45, 53). While stool specimens may not directly reflect the bacterial population in the distal ileum or proximal colon, it seems unlikely that EHS directly trigger NEC in the neonate. Rather, it is more likely that the proinflammatory effects of H. hepaticus in the rat are analogous to the proinflammatory effects of Enterobacteriaceae in the neonate. Enterobacteriaceae can both trigger a proinflammatory response and outcompete commensal microbes in a proinflammatory environment.
H. hepaticus induces experimental colitis in adult immunocompromized mice with a fully developed gastrointestinal tract (22). However, the effect of H. hepaticus on the developing intestine has not been studied. In our initial experiments, we exposed normal newborn rats nursed by a surrogate dam to H. hepaticus infection during the 1st wk of life. There were no pathological changes observed in the small intestine of these neonatal rats (results not shown). Subsequently, we tested the effect of H. hepaticus on the developing gut in a well-established rat model of NEC, where the immature intestine is challenged with formula feeding and asphyxia followed by cold stress to induce NEC injury. The activation of TLR4 is reported in the intestines of NEC patients and in the experimental NEC models (18, 25, 29–31). Moreover, NEC injury is reduced in TLR4 mutant mice (25, 30) and in the enterocyte-specific TLR4 knockout mice (49). Our data clearly indicate that H. hepaticus infection stimulates TLR4 but does not affect TRL2 expression. These observations are consistent with previous reports of increased TLR4 signaling in models of H. hepaticus-induced colitis (37, 52).
In NEC models, TLR4 signaling induces immune response and secretion of proinflammatory cytokines (8, 29, 31). The small intestine is the largest immune organ in the body, and its activation is critical in the protection against enteric pathogens. In H. hepaticus-induced NEC, the pattern of cytokine induction differed somewhat from reports of standard NEC with marked increases in IL-1β, CXCL1, IL-12, and IL-23 but no significant alteration in IL-6, IFN-γ, or TNF-α, which are commonly increased in the rat NEC model (7, 11, 14, 20, 39). It is challenging to compare the current data with previous reports given the unknown colonization rates of H. hepaticus in previously reported studies.
TLR4 signaling induces IL-23, which is secreted by activated dendritic cells and macrophages and triggers intestinal inflammation and host defense against microbial pathogens (41). IL-23 is an essential driving force for inflammatory response (35), and the depletion of IL-23 prevents the development of H. hepaticus-induced colitis (16, 28, 41). IL-23 amplifies release of proinflammatory cytokines (such as IL-1β, IL-6, and TNF-α), but can also regulate production of Th17 cytokines, especially IL-17 (16). Interestingly, the presence of IL-17 mRNA was not detected in any of studied samples, suggesting that the Th17 response may be delayed in the immature intestine of neonatal rats.
Previously, we reported the activation of autophagy in the intestinal epithelium from babies with NEC and in the rat NEC model (38). In addition, we showed that the inhibition of autophagy reduces experimental NEC (38). Recently, Neal et al. and Yu et al. extended our observation by identifying that increased autophagy is a cause (not a consequence) of NEC and requires TLR4 activation (43, 60). Results from the present study are in agreement with these reports (43, 60) and further support our original hypothesis that inappropriate activation of autophagy exacerbates NEC (38). At a basal level, autophagy acts as a protective mechanism (19), but exaggerated autophagy increases mucosal injury. Because TLR4 signaling not only induces autophagy (43) but also activates the proinflammatory response (23), we speculate that H. hepaticus infection increases NEC via both mechanisms.
We did not observe any alteration in production of Muc2 or Tff3 in response to H. hepaticus infection, suggesting that changes in these contributors to the mucin layer are not part of the mechanism by which H. hepaticus induces NEC and that H. hepaticus infection in the ileum differs from H. pylori infection in the stomach in this regard.
In summary, this study demonstrates that H. hepaticus infection increases the incidence and severity of experimental NEC through several mechanisms involving increased TLR-4 signaling. Future rodent studies of NEC should include testing for infection with H. hepaticus. Whether maternal and/or neonatal exposure to Helicobacter infection may increase the risk of NEC in premature babies is a challenging question given the difficulty of sampling the microbiota of the distal ileum and proximal colon. Helicobacter infections are associated with TH17 and TH1 responses. Analysis of Helicobacter-associated cytokines (e.g., IL-17, IL-23, IFN-γ, and IL-12) in the serum of infants with and without NEC may be valuable in addressing this question. Studies of the microbiota and metagenome of the distal ileum and proximal colon from samples obtained at the time of surgical resection or at autopsy from infants with NEC and other non-NEC intestinal diseases (e.g., spontaneous ileal perforation) would be more definitive.
GRANTS
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant HD-039657 (to B. Dvorak) and a gift from People Acting Now Discover Answers.
DISCLOSURES
B. Dvorak has grants with Mead Johnson, Meiji Dairies Co., and NIH. There are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Author contributions: K.D. and B.D. conception and design of research; K.D., C.F.C.-B., C.L.S., and A.K. performed experiments; K.D., C.F.C.-B., C.L.S., A.K., and B.D. analyzed data; K.D., M.A.U., and B.D. interpreted results of experiments; K.D. and B.D. prepared figures; K.D. and B.D. drafted manuscript; K.D., C.F.C.-B., C.L.S., M.A.U., and B.D. edited and revised manuscript; B.D. approved final version of manuscript.
ACKNOWLEDGMENTS
Current addresses: B. Dvorak, NorthShore University HealthSystem, Evanston, IL 60201; K. Dvorak and C. F. Coursodon-Boyiddle, Ventana Medical Systems, Tucson, AZ 85755; and C. L. Snarrenberg, Vanderbilt University, Nashville, TN 37212.
REFERENCES
- 1.Afrazi A, Sodhi CP, Richardson W, Neal M, Good M, Siggers R, Hackam DJ. New insights into the pathogenesis and treatment of necrotizing enterocolitis: Toll-like receptors and beyond. Pediatr Res 69: 183–188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battles JK, Williamson JC, Pike KM, Gorelick PL, Ward JM, Gonda MA. Diagnostic assay for Helicobacter hepaticus based on nucleotide sequence of its 16S rRNA gene. J Clin Microbiol 33: 1344–1347, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caplan MS, Simon D, Jilling T. The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg 14: 145–151, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Chichlowski M, Hale LP. Effects of Helicobacter infection on research: the case for eradication of Helicobacter from rodent research colonies. Comp Med 59: 10–17, 2009 [PMC free article] [PubMed] [Google Scholar]
- 5.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol 291: G938–G949, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Claud EC, Walker WA. Hypothesis: inappropriate colonization of the premature intestine can cause neonatal necrotizing enterocolitis. FASEB J 15: 1398–1403, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Coursodon-Boyiddle CF, Snarrenberg CL, Adkins-Rieck CK, Bassaganya-Riera J, Hontecillas R, Lawrence P, Brenna JT, Jouni ZE, Dvorak B. Pomegranate seed oil reduces intestinal damage in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 303: G744–G751, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Plaen IG, Liu SX, Tian R, Neequaye I, May MJ, Han XB, Hsueh W, Jilling T, Lu J, Caplan MS. Inhibition of nuclear factor-kappaB ameliorates bowel injury and prolongs survival in a neonatal rat model of necrotizing enterocolitis. Pediatr Res 61: 716–721, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe 5: 527–549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drazenovich NL, Franklin CL, Livingston RS, Besselsen DG. Detection of rodent Helicobacter spp. by use of fluorogenic nuclease polymerase chain reaction assays. Comp Med 52: 347–353, 2002 [PubMed] [Google Scholar]
- 11.Dvorak B, Halpern MD, Holubec H, Dvorakova K, Dominguez JA, Williams CS, Meza YG, Kozakova H, McCuskey RS. Maternal milk reduces severity of necrotizing enterocolitis and increases intestinal IL-10 in a neonatal rat model. Pediatr Res 53: 426–433, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol 282: G156–G164, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Dvorak B, McWilliam DL, Williams CS, Dominguez JA, Machen NW, McCuskey RS, Philipps AF. Artificial formula induces precocious maturation of the small intestine of artificially reared suckling rats. J Pediatr Gastroenterol Nutr 31: 162–169, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics 103: 766–771, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Feng S, Ku K, Hodzic E, Lorenzana E, Freet K, Barthold SW. Differential detection of five mouse-infecting helicobacter species by multiplex PCR. Clin Diagn Lab Immunol 12: 531–536, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol 4: 22–30, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grave GD, Nelson SA, Walker WA, Moss RL, Dvorak B, Hamilton FA, Higgins R, Raju TN. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res 62: 510–514, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Gribar SC, Anand RJ, Sodhi CP, Hackam DJ. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J Leukoc Biol 83: 493–498, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez MG, Saka HA, Chinen I, Zoppino FC, Yoshimori T, Bocco JL, Colombo MI. Protective role of autophagy against Vibrio cholerae cytolysin, a pore-forming toxin from V. cholerae. Proc Natl Acad Sci USA 104: 1829–1834, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halpern MD, Clark JA, Saunders TA, Doelle SM, Hosseini DM, Stagner AM, Dvorak B. Reduction of experimental necrotizing enterocolitis with anti-TNF-α. Am J Physiol Gastrointest Liver Physiol 290: G757–G764, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Halpern MD, Holubec H, Saunders TA, Dvorak K, Clark JA, Doelle SM, Ballatori N, Dvorak B. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology 130: 359–372, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen R, Thomson JM, Fox JG, El-Omar EM, Hold GL. Could Helicobacter organisms cause inflammatory bowel disease? FEMS Immunol Med Microbiol 61: 1–14, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Harris J. Autophagy and cytokines. Cytokine 56: 140–144, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Hoffman SM, Wang H, Pope MR, Fleming SD. Helicobacter infection alters MyD88 and Trif signalling in response to intestinal ischaemia-reperfusion. Exp Physiol 96: 104–113, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, Thomson RB, Soliman A, Arditi M, Caplan MS. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 177: 3273–3282, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, Dvorak B. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 297: G940–G949, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khailova L, Mount Patrick SK, Arganbright KM, Halpern MD, Kinouchi T, Dvorak B. Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 299: G1118–G1127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med 203: 2485–2494, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Mandat Schultz A, Bonnard A, Barreau F, Aigrain Y, Pierre-Louis C, Berrebi D, Peuchmaur M. Expression of TLR-2, TLR-4, NOD2 and pNF-kappaB in a neonatal rat model of necrotizing enterocolitis. PLoS ONE 2: e1102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 179: 4808–4820, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Zhu L, Fatheree NY, Liu X, Pacheco SE, Tatevian N, Rhoads JM. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 297: G442–G450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maheshwari A, Kelly DR, Nicola T, Ambalavanan N, Jain SK, Murphy-Ullrich J, Athar M, Shimamura M, Bhandari V, Aprahamian C, Dimmitt RA, Serra R, Ohls RK. TGF-beta2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 140: 242–253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M, Neu J. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One 6: e20647, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malaty HM. Epidemiology of Helicobacter pylori infection. Best Pract Res Clin Gastroenterol 21: 205–214, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Maloy KJ, Kullberg MC. IL-23 and Th17 cytokines in intestinal homeostasis. Mucosal Immunol 1: 339–349, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474: 298–306, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Matharu KS, Mizoguchi E, Cotoner CA, Nguyen DD, Mingle B, Iweala OI, McBee ME, Stefka AT, Prioult G, Haigis KM, Bhan AK, Snapper SB, Murakami H, Schauer DB, Reinecker HC, Mizoguchi A, Nagler CR. Toll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient mice. Gastroenterology 137: 1380–1390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maynard AA, Dvorak K, Khailova L, Dobrenen H, Arganbright KM, Halpern MD, Kurundkar AR, Maheshwari A, Dvorak B. Epidermal growth factor reduces autophagy in intestinal epithelium and in the rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 299: G614–G622, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McElroy SJ, Weitkamp JH. Innate immunity in the small intestine of the preterm infant. Neoreviews 12: e517–e526, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morecroft JA, Spitz L, Hamilton PA, Holmes SJ. Plasma cytokine levels in necrotizing enterocolitis. Acta Paediatr Suppl 396: 18–20, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Morrison PJ, Ballantyne SJ, Kullberg MC. Interleukin-23 and T helper 17-type responses in intestinal inflammation: from cytokines to T-cell plasticity. Immunology 133: 397–408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navabi N, Johansson ME, Raghavan S, Linden SK. Helicobacter pylori infection impairs the mucin production rate and turnover in the murine gastric mucosa. Infect Immun 81: 829–837, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neal MD, Sodhi CP, Dyer M, Craig BT, Good M, Jia H, Yazji I, Afrazi A, Richardson WM, Beer-Stolz D, Ma C, Prindle T, Grant Z, Branca MF, Ozolek J, Hackam DJ. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J Immunol 190: 3541–3551, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 364: 255–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Normann E, Fahlen A, Engstrand L, Lilja HE. Intestinal microbial profiles in extremely preterm infants with and without necrotizing enterocolitis. Acta Paediatr 102: 129–136, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Ran-Ressler RR, Khailova L, Arganbright KM, Adkins-Rieck CK, Jouni ZE, Koren O, Ley RE, Brenna JT, Dvorak B. Branched chain Fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS One 6: e29032, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakiyama T, Musch MW, Ropeleski MJ, Tsubouchi H, Chang EB. Glutamine increases autophagy under Basal and stressed conditions in intestinal epithelial cells. Gastroenterology 136: 924–932, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scharl M, Rogler G. Inflammatory bowel disease: dysfunction of autophagy? Dig Dis 30, Suppl 3: 12–19, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, Prindle T, Jr, Russo AM, Afrazi A, Good M, Brower-Sinning R, Firek B, Morowitz MJ, Ozolek JA, Gittes GK, Billiar TR, Hackam DJ. Intestinal epithelial toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143: 708–718, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solnick JV, Franceschi F, Roccarina D, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection–other Helicobacter species. Helicobacter 11, Suppl 1: 46–51, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Solnick JV, Schauer DB. Emergence of diverse Helicobacter species in the pathogenesis of gastric and enterohepatic diseases. Clin Microbiol Rev 14: 59–97, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sterzenbach T, Lee SK, Brenneke B, von Goetz F, Schauer DB, Fox JG, Suerbaum S, Josenhans C. Inhibitory effect of enterohepatic Helicobacter hepaticus on innate immune responses of mouse intestinal epithelial cells. Infect Immun 75: 2717–2728, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart CJ, Marrs EC, Magorrian S, Nelson A, Lanyon C, Perry JD, Embleton ND, Cummings SP, Berrington JE. The preterm gut microbiota: changes associated with necrotizing enterocolitis and infection. Acta Paediatr 101: 1121–1127, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Suemori S, Lynch-Devaney K, Podolsky DK. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci USA 88: 11017–11021, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor NS, Xu S, Nambiar P, Dewhirst FE, Fox JG. Enterohepatic Helicobacter species are prevalent in mice from commercial and academic institutions in Asia, Europe, and North America. J Clin Microbiol 45: 2166–2172, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turner S, Pryer KM, Miao VP, Palmer JD. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol 46: 327–338, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, Antonopoulos DA, Chang EB, Claud EC. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J 3: 944–954, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu J, Dang Y, Su W, Liu C, Ma H, Shan Y, Pei Y, Wan B, Guo J, Yu L. Molecular cloning and characterization of rat LC3A and LC3B–two novel markers of autophagosome. Biochem Biophys Res Commun 339: 437–442, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Young VB, Knox KA, Schauer DB. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect Immun 68: 184–191, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu Y, Shiou SR, Guo Y, Lu L, Westerhoff M, Sun J, Petrof EO, Claud EC. Erythropoietin protects epithelial cells from excessive autophagy and apoptosis in experimental neonatal necrotizing enterocolitis. PLoS One 8: e69620, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]