Abstract
The pathophysiology of irritable bowel syndrome (IBS) is believed to involve alterations in the brain-gut axis; however, the etiological triggers and mechanisms by which these changes lead to symptoms of IBS remain poorly understood. Although IBS is often considered a condition without an identified “organic” etiology, emerging evidence suggests that alterations in the gastrointestinal microbiota and altered immune function may play a role in the pathogenesis of the disorder. These recent data suggest a plausible model in which changes in the intestinal microbiota and activation of the enteric immune system may impinge upon the brain-gut axis, causing the alterations in gastrointestinal function and the clinical symptoms observed in patients with IBS. This review summarizes the current evidence for altered intestinal microbiota and immune function in IBS. It discusses the potential etiological role of these factors, suggests an updated conceptual model for the pathogenesis of the disorder, and identifies areas for future research.
Keywords: inflammation, gastrointestinal, microbiota
irritable bowel syndrome (IBS) is the most common and best studied condition of a larger group of functional gastrointestinal (GI) disorders. Functional GI disorders refer to the presence of a variable combination of chronic or recurrent GI symptoms not explained by structural or biochemical abnormalities (130, 159). IBS is characterized by chronic or recurring abdominal pain or discomfort that is associated with altered bowel habits (53, 99). The condition is often associated with other GI symptoms (e.g., bloating, distention, and gas), other functional GI disorders (e.g., functional dyspepsia), other non-GI disorders (e.g., fibromyalgia, interstitial cystitis, migraine headache), and psychological disorders (e.g., depression, anxiety, somatization) (96, 146, 167). Traditionally, IBS has been subtyped on the basis of the patient's predominant symptom/stool pattern (99). These subtypes include diarrhea predominant (IBS-D), constipation predominant (IBS-C), and mixed type (IBS-M), in which patients experience alternating periods of diarrhea and constipation. Unlike other conditions that can be associated with abdominal pain and abnormal bowel patterns such as inflammatory bowel diseases (IBD), IBS is not associated with any overt histopathology, structural, or biochemical abnormalities. IBS has been generally considered to be caused by alterations in the brain-gut axis (the bidirectional communication network involving the enteric nervous system, the autonomic nervous system, and the central nervous system) (60, 108, 125), and the pathophysiological mechanisms behind this condition remain unclear. Traditionally, IBS is considered to be a multifactorial condition in which multiple environmental, genetic, physiological, and psychosocial factors contribute to the development of the disorder through direct or indirect effects on the brain-gut axis (65, 125, 146). Bacterial infection, altered GI microbiota, dysregulated immune function, and low-grade inflammation are additional factors that have been gaining more interest as evidence supporting their contribution to the pathophysiology of IBS continues to emerge. What remains unclear is how these factors converge to cause the observed abnormal GI function and functional GI symptoms reported in patients with IBS. This review focuses on the potential roles of the intestinal microbiota and enteric immune function in the pathogenesis of IBS and will discuss the evidence for how these factors may influence the brain-gut axis, thus leading to the altered GI function and symptoms of IBS.
THE INTESTINAL MICROBIOTA
Gastrointestinal bacteria play a critical role in the normal physiological and immunological functions of the GI tract, and alterations in GI microbiota can lead to various GI and non-GI conditions (5, 145, 164). Specifically with regard to IBS, animal studies have shown that alterations in the GI microbiota can lead to changes in GI functions, e.g., altered intestinal motility and visceral hypersensitivity that are often observed in patients with IBS and considered important factors in the pathophysiology of the disorder (12, 20, 25, 43, 80). Additional evidence supporting the role of altered intestinal microbiota in the pathogenesis of IBS has come from epidemiological observations linking bacterial gastroenteritis and small intestinal bacterial overgrowth (SIBO) to IBS, microbiology studies describing quantitative and qualitative alterations in the GI microbiota in patients with IBS compared with healthy controls, and clinical studies demonstrating the efficacy of antimicrobial and probiotic treatments in patients with IBS.
Postinfectious IBS
Postinfectious IBS (PI-IBS) refers to the development of IBS following an episode of acute gastroenteritis. PI-IBS occurs in ∼10% of patients with acute gastroenteritis, and acute gastroenteritis increases the odds of developing IBS six- to sevenfold (76, 157, 172). The extended duration of IBS symptoms in these patients indicates that the condition persists long after the initial infection has cleared. The precise mechanisms by which IBS symptoms persist are not clear, although researchers have speculated that genetic polymorphisms in genes related to host immune response to microbial pathogens, ongoing altered immune function, or chronic low-grade inflammation may be possible factors (26, 145, 170).
Small Intestinal Bacterial Overgrowth
The symptoms of SIBO and IBS overlap since SIBO can lead to similar symptoms and altered intestinal motility that are often observed in patients with IBS (140). Detecting SIBO in patients with IBS remains a challenge. Historically, SIBO has been defined as ≥105 colony-forming units (cfu)/ml intestinal fluid (14). Using this definition, a study of bacterial cultures of aspirates from patients with IBS compared with cultures from healthy volunteers showed no statistically significant differences in the incidence of SIBO (141). However, when a lower cutoff concentration of ≥104 cfu/ml was used, significant differences were observed, suggesting increased numbers of bacteria in the small intestine of patients with IBS.
Because of the limitations in obtaining intestinal cultures, SIBO is often diagnosed indirectly by hydrogen breath testing (HBT). A meta-analysis of studies using HBT reported a pooled prevalence of SIBO in patients with IBS of 54–64% (61). However, the wide range of prevalence of SIBO reported in individual studies [from 10% (186) to 84% (130)] reflects the lack of standardization of HBT and the possibility that lactulose HBT results may reflect changes in small bowel transit time rather than the true presence of SIBO (194). These diagnostic limitations led to some controversies regarding the real prevalence and the importance of SIBO in the pathogenesis of IBS.
Nonetheless, the data from meta-analyses indicating increased prevalence of SIBO in IBS, the symptom overlap between the two conditions, and the improvement of symptoms with antibiotic treatment (47, 49, 59, 103, 131, 135, 136, 193) further support the hypothesis that alterations in the intestinal microbiota may have a role in the pathogenesis of IBS, at least in some patients.
Alterations in Intestinal Microbiota
Several studies have investigated and compared the composition of intestinal microbiota of patients with IBS with that of healthy individuals. Although early studies were relatively small with noticeable methodological limitations, their overall findings suggested differences in the intestinal microbiota of patients with IBS and healthy controls (145, 153). These early findings are further supported by recent studies using advanced molecular biology techniques that are on the basis of the bacterial 16S ribosomal DNA (rDNA) gene. These include genetic fingerprinting (e.g., denaturing gradient gel electrophoresis and terminal restriction fragment length polymorphism), 16S rDNA high-throughput sequencing (e.g., 454-pyrosequencing and Illumina), and phylogenetic microarray [e.g., human intestinal tract chip (HITChip) and PhyloChip]. These techniques enable detailed qualitative and quantitative information regarding the most abundant bacterial communities in the intestinal microbiota (63). Recent studies using these advanced molecular biology techniques have demonstrated quantitative alterations of specific bacterial groups (33, 34, 81, 85, 142, 154) and reduced diversity in gut microbial populations in patients with IBS compared with healthy controls (33, 40). Limited data also suggest differences in mucosa-associated microbiota (32, 33, 86, 129), reduced stability over time (106, 107), rDNA, and differences between IBS subtypes (81, 104, 129, 142). Overall, it appears that the composition of the intestinal microbiota and the relative abundance of specific bacterial species are altered in patients with IBS. Emerging data demonstrate associations between altered intestinal microbiota and abnormal intestinal functions that contribute to the pathophysiology of IBS as detailed below.
Altered Fermentation Processes
Poorly absorbed short-chain carbohydrates (e.g., fructose and dietary starch) provide substrate for generation of short-chain fatty acids (SCFA) by colonic bacterial fermentation. Fecal SCFA have been shown to be increased in patients with IBS (175). In addition, a recent study reported higher counts of acetic- and propionic-acid-producing bacteria (Veillonella and Lactobacillus) in IBS patients and an association between the higher levels of these SCFA and GI symptoms and quality of life (171). From a mechanistic perspective, animal studies demonstrate that SCFA can initiate high-amplitude propagated colonic contractions (82) and increase intestinal transit and motility via intestinal release of 5-hydroxytryptamine (66). The association between certain dietary products and GI symptoms (e.g., abdominal bloating, distention, and pain) in patients with IBS (161) and the emerging data on the beneficial effects of dietary manipulation, particularly elimination of highly fermentable short-chain carbohydrates (127), further support the importance of altered fermentation processes by the intestinal microbiota in the pathogenesis of IBS.
Microbiota and the Intestinal Barrier
The gut microbiota have an important role in promoting the development, maintenance, and function of the intestinal barrier by increasing IgA production and mucin expression, preventing intestinal epithelial cell apoptosis, inhibiting colonization by enteric pathogens, and promoting physiological immune responses (113, 173, 178). Several in vivo studies in children and adults with IBS (56, 128, 162) and in vitro studies of mucosal samples from IBS patients (132) demonstrated increased intestinal permeability in IBS compared with healthy controls. The mechanisms related to the increased permeability in IBS are not clear but alterations in intestinal microbiota and mucosal inflammation have been suggested (28). The increased gut permeability of colonic biopsies from patients with IBS was found to be associated with decreased expression of a tight junction protein, zonula occludens-1 (ZO-1). When fecal supernatants from IBS patients were applied to colonic mucosa from mice (67) or to Caco-2 cells (132), the expression of ZO-1 decreased and permeability increased. Furthermore, in patients with D-IBS, this phenomenon was found to be associated with increased bacterial-related protease activity and their epithelial receptor PAR-2 (proteinase activated receptor 2) (67). Additional support for the role of enteric bacteria in maintaining normal barrier function is provided by data that SCFA (e.g., butyrate and acetate) improve intestinal barrier function (64, 84). One small study found that administration of butyrate enemas in healthy volunteers resulted in decreased in visceral perception (180).
Microbiota and Enteric Sensorimotor Function
Intestinal microbiota may affect gut motility and pain perception. In mice, perturbation of the microbiota by exposure to antibiotics resulted in increased visceromotor responses to colonic distension (182). Interestingly, in this model, treatment of mice with Lactobacillus ameliorated inflammatory indexes in the gut wall and reduced the antibiotic-induced visceromotor response. Direct effects of bacterial products on gut sensorimotor functions have been shown in several in vitro studies. For example, stimulation of smooth muscle cells by supernatants from Escherichia coli Nissle 1917 enhances colonic contractility (9) and Lactobacillus rhamnosus and Bifidobacterium lactis increases intestinal myoelectrical activity in rats (97). In addition, exposure of rats to L. reuteri (83) or L. acidophilus NCFM (151) reduce their pain response to balloon distension, through targeting ion channels in the enteric nervous system (83, 91) or increasing the expression of μ-opioid and cannabinoid receptors in intestinal epithelial cells (151).
Several recent studies have demonstrated bile acid malabsorption in a subset of patients with IBS (1, 29, 93, 192). Furthermore, reduced ileal absorption and excess of bile acid in the colon can lead to acceleration of colonic transit by stimulating motility and secretion (8, 109). Since the intestinal microbiota are directly responsible for the transformation of primary into secondary bile acid it is tempting to hypothesize that the intestinal dysbiosis observed in patients with IBS can affect intestinal motility and lead to IBS symptoms through its effect(s) on bile acid metabolism. Indeed, a recent small study in patients with IBS demonstrated increase of primary bile acid in the feces of patients with IBS-D compared with healthy subjects (54). Interestingly, these differences correlated with stool consistency and frequency and were associated with decreased levels of the Clostridium leptum group, which contains many of the bacteria that can transform primary into secondary bile acid.
Emerging observations of perturbed composition of the intestinal microbiota and altered microbiota-related fermentation, barrier, and sensorimotor functions in patients with IBS support the hypothesis that the intestinal microbiota contribute to the pathogenesis of the disorder. However, two key questions remained unanswered. First, are the observed alterations in the intestinal microbiota in IBS a cause or a consequence of the disorder? Relatedly, since the majority of the reported studies were done at a single time point, it is not clear whether and how the altered intestinal microbiota correlate with the clinical presentation and the IBS symptoms over time. Second, what are the relative contributions of the abnormal composition vs. function of the intestinal microbiota and mechanisms by which these changes contribute to the pathogenesis of IBS?
Current advances in the area of microbiome research including advance in nucleic acid sequencing, metagenomics, and metaproteomics allow investigation of structural and functional aspects of the intestinal microbiome. Incorporating microbial response variables (e.g., taxonomy, microbial diversity, richness, and stability) into future clinical studies in IBS is crucial to advance this field forward. However, controlling for all the variables that influence the intestinal microbiota (e.g., diet and interpatient variability) poses challenges to the design of clinical studies. Animal models may be useful by controlling for host genotype, baseline microbiota, and diet. Furthermore, since the results from animal models cannot be generalized to humans because of differences in the intestinal microbiota, it is possible to improve mouse models of IBS by colonizing germ-free animals with human-derived microbiota. The ability of these systems to mimic human clinical conditions is unclear at present, but they can be useful to test hypotheses particularly regarding causation.
In addition, the fact that genome sequence data for many of the bacterial species in the human gut microbiota are not available limits taxonomic assignments of the data with clinical, physiological, and metabolomic data. The anticipated reference genome sequences generated by the Human Microbiome Reference Genomes initiative, and the rapidly developing analytic tools and bioinformatic technologies, are required to ensure progress.
THE INTESTINAL IMMUNE SYSTEM
Altered Intestinal Immune Function in IBS
Although IBS is not generally considered an inflammatory disease, there is growing appreciation for the hypothesis that IBS may be a condition of low-grade inflammation and/or abnormal immune function, despite a lack of detectable inflammation on routine endoscopy or with conventional histology (48). Evidence supporting this hypothesis includes increased concentrations of inflammatory/immune cells in intestinal tissue from IBS patients, altered levels of pro- and anti-inflammatory cytokines in the GI tract and peripheral blood of patients with IBS (Table 1) (11, 13, 24, 36, 46, 50, 51, 62, 73, 75, 89, 90, 94, 98, 102, 118, 121–124, 168, 174, 185, 187), and IBS-associated polymorphisms in genes involved in immune and inflammatory responses (Table 2) (15, 16, 70, 179, 184). This topic has been thoroughly reviewed recently (27).
Table 1.
Evidence for immune activation in patients with IBS or PI-IBS
| Evidence | Change in IBS or PI-IBS vs. Healthy Controls |
|---|---|
| Altered concentrations or activation of immune cells | |
| GI tract | ↑ Activated (tryptase-positive) mast cells (11, 13, 24, 46, 89, 94, 121, 187) |
| ↑ IELs (36, 73, 89, 174, 185) | |
| T cells | |
| ↑ CD3+ (36, 46, 89, 94, 168)a | |
| ↑ CD4+ (46) | |
| ↑ CD8+ (46, 89, 123) | |
| B cells (62) | |
| ↓ IgA+ | |
| Peripheral blood | T cells (122) |
| ↑ activated CD4+ | |
| ↑ activated CD8+ | |
| B cells (124)a | |
| ↑ IgG+CD80+ | |
| ↑ IgG+CD86+ | |
| Altered levels of cytokines | |
| GI tract* | ↑ IL-1β (75, 187) |
| ↓ IL-10 (102) | |
| Peripheral blood | |
| Baseline | ↑ IL-1β (98) |
| ↑ IL-6 (50, 51, 98) | |
| ↑ IL-8 (50, 51) | |
| ↓ IL-10 (118) | |
| ↑ IL-12 (118) | |
| ↑ TNF-α (98) | |
| In vitro stimulation | ↑ IL-1β (122) |
| ↑ IL-5 (90) | |
| ↑ IL-6 (98) | |
| ↓ IL-12 (90) | |
| ↑ IL-13 (90) |
GI, gastrointestinal; IBS, irritable bowel syndrome; IELs, intraepithelial lymphocytes; IL, interleukin; PI-IBS, postinfectious irritable bowel syndrome; TNF-α, tumor necrosis factor α.
Messenger RNA levels quantified by reverse-transcriptase polymerase chain reaction.
Table 2.
Gene polymorphisms in patients with IBS
| IL-2 |
|---|
| IL-4 |
| IL-6 |
| IL-10 |
| TLR9 |
| TNF-α |
Based on Refs. 15, 16, 70, 179, 184. TLR9, Toll-like receptor 9.
IMMUNE CELLS.
MAST CELLS.
Several studies have demonstrated increased concentrations of activated mast cells in patients with IBS compared with controls (11, 13, 24, 46, 89, 94, 121, 187). Interestingly, the activated mast cells were found to be in close proximity to enteric nerves and correlated with abdominal pain/discomfort (11). Other studies have reported elevated levels of histamine and tryptase release in patients with IBS compared with controls (11, 13, 35, 73). Notably, some studies demonstrated an increase in activated mast cells in biopsies from specific regions of the GI tract (i.e., cecum and terminal ileum) but not from other regions (e.g., ascending colon, descending colon, rectum) (89, 121, 187), whereas other studies have found activated mast cells in the descending colon (11, 13). The abundance of mast cells in patients with IBS may correlate with some symptoms of IBS, including bloating and abdominal pain (2, 46). However, some studies failed to demonstrate increased activated mast cells in IBS patients (35, 36, 57, 58, 168) and there are no consistent differences in the tissue concentrations of activated mast cells in patients with different IBS subtypes (36, 189).
LYMPHOCYTES.
The concentrations of intestinal epithelial lymphocytes (IELs) may be increased in the small intestine (73, 174, 185), colon, and rectum (36, 89) of patients with IBS or PI-IBS. Additionally, increased concentrations of CD3+ T cells in the colon and rectum and in the myenteric plexus of the small intestine were increased in these patients compared with normal controls (36, 46, 57, 58, 89, 94, 133, 168, 174). However, as with mast cells, IELs are not consistently elevated in IBS patients (57, 58, 133). Moreover, CD3+ T cells in the rectum of patients with PI-IBS were not elevated compared with patients who had gastroenteritis but did not develop IBS (57). Similarly, increased levels of CD4+ T helper cells were observed in the colon of patients with IBS in one study (46) but not in another (123), leaving the role for this T cell subtype in IBS unclear. Concentrations of cytotoxic CD8+ T cells, which comprise most of the intraepithelial T cell population (126) may also be elevated in the lamina propria of the colon of patients with IBS (46, 123) or PI-IBS (89) although this finding was disputed by others (36). The reasons for the differences in some of the results are unknown, but, overall, it appears that T cell concentrations are increased in patients with IBS or PI-IBS relative to normal controls.
In addition to elevated concentrations of T cells, patients with IBS may also have an increased numbers of activated T cells in the colon (36) and peripheral blood (122) compared with controls. Peripheral blood T cells from patients with IBS express elevated levels of integrin β7 (122, 123), which is important for homing of cells to the intestines. Levels of MAdCAM-1, the ligand of integrin β7, are also elevated on colonic endothelial cells (123), creating an environment for enhanced recruitment of CD4+ and CD8+ T cells to the intestines of patients with IBS.
Although no differences in total B cell numbers were observed in the colons of IBS patients (46), a single study reported reduced levels of IgA+ B cells in the ascending but not sigmoid colon compared with controls, whereas levels of IgG+, IgM+, and IgE+ B cells in both the ascending and sigmoid colon were normal (62). This altered pattern of B cells suggests that there may be a modified defense mechanism against pathogens in the gut of patients with IBS. In addition, patients with IBS may have increased expression of IgG and the costimulatory molecules CD80 and CD86 on B cells and an increased frequency of peripheral blood IgG+CD80+ and IgG+CD86+ B cells (124). Furthermore, B cells from patients with IBS had an impaired ability to upregulate CD80 in response to in vitro lipopolysaccharide (LPS) stimulation, suggesting that B cells from patients with IBS may have an altered ability to costimulate and regulate T cells.
INFLAMMATORY CYTOKINES.
GI TRACT.
In parallel with altered immune cell concentrations and activation, the levels and relative proportions of certain pro- and anti-inflammatory cytokines have been reported to be altered in the small intestine, colon, and rectum of patients with IBS and PI-IBS compared with controls. Levels of interleukin (IL)-1β mRNA may be increased in the small intestine, colon, and rectum of patients with PI-IBS compared with healthy controls (75, 187), supporting the hypothesis that low-grade inflammation is present in patients with PI-IBS. In contrast, a study by Macsharry and colleagues (102) found reduced levels of IL-1β mRNA in colonic biopsies from patients with IBS. However, in this study a decreased level of mRNA encoding the anti-inflammatory IL-10 gene was observed, as well as an altered IL-10:IL-12 ratio in colonic biopsies from patients with IBS compared with controls, supporting the hypothesis that patients with IBS have a dysfunctional immunological response in the mucosa. Results from examination of cytokine protein levels have been conflicting. In one study, levels of TNF-α were elevated, but levels of IL-6 and interferon-γ were decreased in mucosa of patients with IBS compared with controls (41). Ex vivo examination of cytokine secretion by the colon failed to show elevation of proinflammatory cytokines or chemokines in patients with IBS compared with controls (102).
PERIPHERAL BLOOD.
A recent study demonstrated significantly higher levels of high-sensitivity C-reactive protein in patients with IBS-D but not in patients with IBS-C or IBS-M compared with healthy controls (77). IL-6, which is a major regulator of serum C-reactive protein levels, and IL-8 were also found to be increased in the serum of patients with IBS compared with controls, supporting a proinflammatory profile in these patients (50, 51).
Additionally, IL-1β, IL-6, and TNF-α released from peripheral blood mononuclear cells (PBMCs) were elevated in patients with IBS-D but not in patients with IBS-C or IBS-M (98). Additionally, a decreased ratio of IL-10 to IL-12, indicative of a proinflammatory response, was demonstrated in supernatants of cultured PBMCs obtained from patients with IBS compared with controls (118). In this same study, normalization of the IL-10:IL-12 ratio in patients with IBS following probiotic treatment was paralleled by improvement in the symptoms of IBS, thus supporting an association between changes in the GI microbiota, the IL-10:IL-12 ratio, and symptoms in patients with IBS. However, other studies did not observe differences in IL-10 levels between IBS patients and controls (50, 51, 90), leaving the relevance of IL-10 levels in patients with IBS unclear.
Cytokine secretion following in vitro stimulation of peripheral blood lymphocytes/monocytes may also be altered in patients with IBS (90, 98, 122). Increased secretion of TNF-α, IL-1β, and IL-6 by LPS-stimulated PBMCs was observed only in cells from patients with IBS-D but not in patients with IBS-C or IBS-M (98). In contrast, only IL-1β was secreted following LPS stimulation of PBMCs obtained from IBS-C patients (98). Furthermore, increases in the secretion of TNF-α following LPS stimulation of PBMCs and of IL-1β following T-cell anti-CD3/CD28 antibodies stimulation were significantly associated with anxiety (98) and bowel habit dissatisfaction and overall IBS symptoms (122), respectively. Overall, it seems that alterations in cytokine secretion by stimulated peripheral blood cells appear to favor an activated immune state (98, 122), and an altered immune response shifted toward a T-helper 2 phenotype(90) in IBS patients.
Immune-Related Genetic Alterations in IBS
Gene polymorphisms (188) in TNF-α (15, 179), IL-2 (16), IL-4 (16), IL-6 (15), and IL-10 (16, 70) have been associated with IBS or PI-IBS (Table 2). For example, IBS patients are more likely to carry a combination of polymorphisms that lead to increased expression of TNF-α and decreased expression of IL-10 compared with healthy controls (179). However, the prevalence of specific polymorphisms in the IL-10 gene in patients with IBS are not consistent across studies and may reflect the variety of ethnic groups in which these studies were conducted (16). Genetic variation in promoter regions of the IL-6 and Toll-like receptor 9 (TLR9) genes were also identified as independent risk factors for the development of PI-IBS (26, 184). Altered levels of IL-6, IL-10, and TNF-α in colon tissue from IBS and PI-IBS patients suggest that the genetic polymorphisms in immune genes may have functional consequences in patients with IBS. The presence of polymorphisms in the promoter of TLR9 suggests that patients with PI-IBS may have an inappropriate regulation of the immune response to microbial products.
Immune-Mediated Food Hypersensitivity in IBS
The majority of IBS patients believe that their IBS symptoms are initiated or worsened by certain foods and many of them report intolerance (i.e., hypersensitivity) to a variety of foods (68). The mechanisms by which dietary factors lead to IBS symptoms are currently unknown. However, several studies have documented IgG-mediated food hypersensitivity and demonstrated that food-elimination diets based on IgG antibodies to specific food items improved IBS symptoms (4, 195, 196), perhaps through amelioration of the immune response. In addition, few studies demonstrated increased peripheral blood basophil activation following stimulation with food antigens (30) and elevated levels of fecal tryptase and eosinophil cationic protein (31) in patients with IBS and food hypersensitivity, thus further suggesting possible inflammatory and altered immune etiology.
Although the mechanisms for food hypersensitivity in patients with IBS are not clear, it can be postulated that dysregulated immune responses and possibly altered intestinal mucosal barrier function may be involved.
Inflammation and Sensorimotor Function
The effects of inflammation on sensorimotor enteric function have been demonstrated in IBDs, where patients suffer from abnormal intestinal motor function and enhanced sensory perception, even when inflammation is restricted to the mucosa (44, 143). In addition, human and animal studies have shown anatomical changes in the enteric nervous system (ENS) around inflammatory infiltrates in the gut wall (2, 11, 13). Functionally, intestinal inflammation is associated with increased expression of neuropeptides and mediators that are also involved with gut motility and sensation (e.g., VIP and substance P) [for review, see Vasina et al. (181)]. Similar observations have been made in IBS patients. One study that supports this association demonstrated a strong correlation between number of infiltrated colonic mast cells around nerves and the severity and frequency of abdominal pain in patients with IBS (11).
MICROBIOTA, IMMUNE SYSTEM, AND THE BRAIN-GUT AXIS
The current understanding of IBS as a disorder of malfunction in the bidirectional brain-gut communication suggests that both peripheral (GI) and central nervous system (CNS) factors are involved in the pathophysiology of the disorder. Indeed, at the gut level, the intestinal mucosal neuroimmune system identifies and responds to intestinal luminal food products, nutrients, bacteria, metabolites, and toxins. At the CNS level, the brain-gut axis connects environmental, cognitive, and emotional states with GI functions (191). For example, it has been shown that physical and psychological stress (112), cognition (e.g., attention vs. distraction to aversive stimulus) (55), and early life experience (e.g., sexual and physical abuse) (39, 149) can affect intestinal sensory perception. Furthermore, treatments targeting the CNS, such as antidepressants and psychological interventions, can reduce intestinal sensation/sensitivity and improve GI symptoms in patients with IBS (21, 111, 150, 165).
The Intestinal Microbiota and Brain-Gut Axis
Recent data from animal and human studies have demonstrated that alterations in the intestinal microbiota have substantial effects on the brain-gut axis at both the peripheral and central levels (Fig. 1). In the periphery, intestinal microbiota can affect the ENS directly via release of bacterial substances or metabolites, or indirectly by inducing the release of host-derived immune mediators that in turn impact the ENS (10, 155). For example, production of the SCFA butyrate by bacteria affects gene expression and the phenotype of enteric neurons in a similar manner to that observed in the colons of patients treated with prokinetic agents (166, 169). Gastrointestinal microbiota may also contribute to the affective symptoms associated with GI disorders by impacting the CNS via immune, hormonal, and neural mechanisms (17, 69, 155). Preliminary studies have demonstrated that alterations in the GI microbiota can lead to long-term increased anxiety (69, 115, 116) and altered exploratory behavior in mice (18, 19). Similarly, psychosocial stressors can change the composition of the intestinal microbiota and affect the brain-gut axis function (6, 7, 119). For example, it has been shown that maternal separation, a well-established rodent model of early life stress, can lead to significant alterations in the intestinal microbiota as well as in brain-gut axis function, including increased plasma corticosterone levels, systemic immune responses, and visceral sensation compared with a control group (119).
Fig. 1.
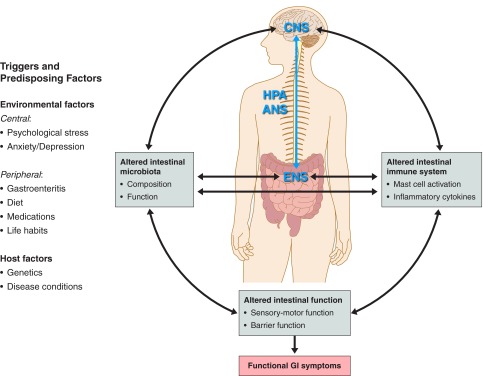
Interactions between the intestinal microbiota, immune system and brain-gut axis, and their effects on intestinal function and functional gastrointestinal (GI) symptoms. A number of factors and triggers, from both the environment and the host, combine to drive the complex interactions between the brain-gut axis, intestinal microbiota, and immune system toward altered intestinal functions and functional GI symptoms. Altered intestinal microbiota may affect the brain-gut axis directly via effect on the mucosal barrier and intestinal neuroimmune system or indirectly via generation of bacterial-related metabolites. Immune activation (e.g., increased levels of proinflammatory cytokines) may affect the mucosal barrier, enteric nervous system (ENS) and peripheral nervous system [i.e., autonomic nervous system (ANS), hypothalamic-pituitary-adrenal (HPA) axis, central nervous system (CNS)]. The outcome of these complex interactions may alter the intestinal sensorimotor function and lead to functional GI symptoms.
The Enteric Immune System and Brain-Gut Axis
The connection between altered intestinal immune function and abnormal brain-gut axis in patients with IBS remains unclear. However, some of the suggested mechanisms involve stress and psychological disturbances and disruption of mucosal barrier function (120). For example, rats exposed to chronic stress exhibited activation of the hypothalamic-pituitary-adrenal (HPA) axis, intestinal mucosal inflammation, increased epithelial permeability, and colonic hyperalgesia (183). At the peripheral level, the close interaction between the immune system and the ENS in IBS is demonstrated by the increased infiltration of mast cells in the colonic mucosa in close proximity to enteric nerve fibers and by the increased number of sensory nerve fibers surrounding the mast cells (2, 11, 13). In addition, several studies support the hypothesis that elevated levels of proinflammatory cytokines may affect the brain-gut axis. For example, in rats elevated IL-1β and TNF-α secretion in response to the bacterial endotoxin LPS leads to visceral hyperalgesia via activation of sensory nerve pathways (42). Others showed that the proinflammatory cytokines IL-6, IL-1, and TNF-α affect the HPA axis through elevated release of arginine vasopressin (AVP), corticotropin-releasing hormone (CRH), and adrenocorticotropic hormone (ACTH) (38, 176). AVP and CRH, by stimulating ACTH secretion, may affect the autonomic nervous system (38) and colonic sensorimotor functions (125, 152). Additionally, CRH may act directly on peripheral receptors of the ENS, independent of alterations in the HPA axis, to impact sensorimotor functions in patients with IBS (152). Thus proinflammatory cytokines (e.g., IL-1β, IL-6, and TNF-α) may interact with the brain-gut axis at different levels, modulate its function, and lead to altered visceral and motility function in patients with IBS (Fig. 1).
Interactions of the Intestinal Microbiota and Immune Function in IBS
The effects of the intestinal microbiota on the development, function, and homeostasis of the enteric immune system are well documented (5, 158). Enteric microbiota are crucial to the host in numerous aspects including maturation of the systemic and enteric immune response, maintenance of the mucosal barrier function, digestion of food, metabolism, and more (for review, see Ref. 78). Although evidence is rapidly accumulating that dysregulated immune responses to normal and “dysbiotic” enteric microbiota contribute to the pathogenesis of IBD (105), there are limited data on the interactions between the intestinal microbiota and the enteric immune system in IBS. However, some recent data suggest that IBS patients also exhibit dysregulated immune responses to commensal and/or pathogenic intestinal bacteria. For example, recognition of bacterial components through Toll-like receptors (TLRs) is increased in IBS patients. Expression of TLR4 (recognizes bacterial LPS) and TLR5 (recognizes flagellin, a common bacterial antigen present in most motile bacteria in the gut) are increased in colons of IBS patients compared with controls. TLRs have a central role in the mucosal innate immune response and their increased expression is also associated with IBDs (23). In this regard, anti-flagellin antibodies were found in almost 30% of IBS patients (mostly in PI-IBS) as opposed to only 7% of healthy controls (156). The importance of TLRs in IBS is further supported by a recent study in a mouse model, indicating that TLR4 stimulation by enteric microbiota can also affect the enteric nervous system and intestinal motility (3). Moreover, the antimicrobial peptide β-defensin-2, which is an innate immune molecule expressed by certain enteric epithelial cells, is elevated in feces collected from IBS patients (similarly to ulcerative colitis) compared with healthy controls (92).
The importance of the interaction between the intestinal microbiota and immune system in IBS patients is demonstrated by a few studies in patients with PI-IBS, indicating activation of the GI immune system following the acute GI infection (57, 58, 75, 89, 94, 124, 168, 170, 187), and the recent animal studies showing that stress-induced intestinal microbial changes are associated with altered immune response (6) and increased susceptibility to an enteric pathogen (183).
Clinical Implications
Manipulation of intestinal microbiota.
The possible role of the altered intestinal microbiota, immune function, and the abnormal interaction between the two systems in the pathogenesis of IBS has led to the increased interest in targeting the intestinal microbiota and immune system in the treatment of this disorder. Early studies demonstrated symptom improvement in IBS patients whose SIBO was successfully treated with antibiotics, including neomycin, metronidazole, and the nonabsorbable antibiotic rifaximin (47, 49, 59, 103, 131, 135, 136, 193). Other studies have demonstrated beneficial effect of antibiotics in patients with IBS regardless of either the diagnosis of SIBO or the effect of antibiotics on HBT (95, 100, 114, 134, 137–139, 160).
Several systematic reviews and meta-analyses suggest that modulation of intestinal microbiota with probiotics may improve some of the symptoms of IBS (22, 79, 110, 147, 148). The largest reported study was a randomized, double-blinded, placebo-controlled, multicenter study of 362 patients that demonstrated beneficial effects of Bifidobacterium infantis 35624 over placebo on IBS composite score and individual symptoms of IBS (e.g., pain and bloating) (190). Although the effects were modest, they were significant. Other smaller studies reported some beneficial effects with other probiotic strains including Bifidobacterium animalis DN-173 010 (74), Bifidobacterium bifidum MIMBb75 (72), Lactobacillus plantarum 299V (117), Saccharomyces boulardii (37), and combination of Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 (144) and multispecies probiotic mixtures (71, 87, 88). Other approaches targeting the intestinal microbiota that have shown benefit in the treatment of IBS include prebiotics (163) and dietary manipulation (127).
Currently, despite the demonstrated efficacy in subgroups of patients with IBS, antibiotics are not FDA-approved medications for treatment of IBS, and there are no clear guidelines for the use of probiotics, prebiotics, and diets in this disorder. Nevertheless, although the mechanisms of action are unclear, the available data suggest potential therapeutic benefit in targeting the intestinal microbiota in the treatment of IBS. The data underscore the need for further preclinical studies, including the use various animal model systems and well-designed human trials in this area. Future research should help direct patients and healthcare providers in making evidence-based decisions regarding the selection of patients, preferred products and treatment regimens.
Anti-inflammatory treatment in IBS.
An alternative, and perhaps complementary, therapeutic approach is to target the dysregulated immune response in IBS patients. Few studies have investigated the use of anti-inflammatory medications in IBS. A small, double-blind, randomized study of 20 IBS patients showed that mesalamine 2.4 g/day for 8 wk failed to show improvement in IBS symptoms despite a reduction in the number of total immune cells in colonic biopsies and improvement in general well-being (45, 177). Similarly, mesalamine 3.2 g/day for 12 wk (168) and prednisolone 30 mg/day for 3 wk (52) were not effective in improving the GI symptoms in patients with PI-IBS. A single small study suggests a possible benefit to targeting mast cells with sodium cromoglycate, a mast cell stabilizer, in patients with IBS and food intolerance (101).
Taken together, the current available data do not support the clinical use of anti-inflammatory agents in the treatment of IBS. However, the documented low-grade inflammation in IBS, the suggestive preliminary data from animal studies (52), and the noticeable paucity of clinical data in this area emphasize the need for further investigation to enable more solid conclusions regarding this treatment option in IBS. Understanding the role of the immune system in IBS pathophysiology may further clarify the potential therapeutic role of targeting the immune system in the treatment of IBS.
CONCLUSIONS
IBS has historically been considered a condition without an identifiable organic etiology. However, accumulating observations in animal models and in patients with IBS suggest a conceptual model in which environmental factors at both the peripheral (e.g., diet, GI infections, antibiotics) and central (e.g., psychological stress, anxiety, depression) levels can affect the intestinal microbiota and enteric immune system, which interact with each other and the brain-gut axis (Fig. 1). Alterations in these systems or in their interactions can affect GI function and lead to functional GI symptoms. Future research in this area may provide further insight into the relevance and relative importance of these interactions in the pathogenesis of IBS and improve our understanding of how manipulating relevant environmental factors (e.g., diet and intestinal microbiota), and the immune and inflammatory processes may affect the brain-gut axis and improve patient outcomes.
GRANTS
Y. Ringel is supported by National Institute of Diabetes and Digestive and Kidney Diseases grants DK084294 and DK075621. N. Maharshak is supported by the Crohn's and Colitis Foundation of America Research Fellowship Award and the American Physicians Fellowship for Medicine in Israel.
DISCLOSURES
Y. Ringel discloses Research Grant: Salix Pharmaceuticals, Danisco USA, Proctor & Gamble. Consultant: Salix Pharmaceuticals; Pfizer Inc.; GlaxoSmithKline; Prometheus Laboratories; Schwabe North America. Travel grants: Nestlé; Institute of Health Sciences; Danone. Consultant: Proctor & Gamble, Ironwood Pharmaceuticals, Inc., Forest Laboratories, Inc. General Mills. Research grant: General Mills.
AUTHOR CONTRIBUTIONS
Y.R. conception and design of research; Y.R. and N.M. prepared figures; Y.R. and N.M. drafted manuscript; Y.R. and N.M. edited and revised manuscript; Y.R. and N.M. approved final version of manuscript.
REFERENCES
- 1.Abrahamsson H, Ostlund-Lindqvist AM, Nilsson R, Simren M, Gillberg PG. Altered bile acid metabolism in patients with constipation-predominant irritable bowel syndrome and functional constipation. Scand J Gastroenterol 43: 1483–1488, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 57: 923–929, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology 143: 1006–1016.e4, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut 53: 1459–1464, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bäckhed F, Fraser-Liggett C, Ringel Y, Sanders ME, Sartor RB, Sherman P, Versalovic J, Young V, Finlay BB. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe 12: 611–622, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 25: 397–407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immun 78: 1509–1519, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol 282: G443–G449, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Bar F, Von Koschitzky H, Roblick U, Bruch HP, Schulze L, Sonnenborn U, Bottner M, Wedel T. Cell-free supernatants of Escherichia coli Nissle 1917 modulate human colonic motility: evidence from an in vitro organ bath study. Neurogastroenterol Motil 21: 559–566.e16–e17, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Barbara G, Stanghellini V, Brandi G, Cremon C, Di Nardo G, De Giorgio R, Corinaldesi R. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol 100: 2560–2568, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126: 693–702, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Barbara G, Vallance BA, Collins SM. Persistent intestinal neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology 113: 1224–1232, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132: 26–37, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Bardhan PK, Gyr K, Beglinger C, Vogtlin J, Frey R, Vischer W. Diagnosis of bacterial overgrowth after culturing proximal small-bowel aspirate obtained during routine upper gastrointestinal endoscopy. Scand J Gastroenterol 27: 253–256, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Barkhordari E, Rezaei N, Ansaripour B, Larki P, Alighardashi M, Ahmadi-Ashtiani HR, Mahmoudi M, Keramati MR, Habibollahi P, Bashashati M, Ebrahimi-Daryani N, Amirzargar AA. Proinflammatory cytokine gene polymorphisms in irritable bowel syndrome. J Clin Immunol 30: 74–79, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Barkhordari E, Rezaei N, Mahmoudi M, Larki P, Ahmadi-Ashtiani HR, Ansaripour B, Alighardashi M, Bashashati M, Amirzargar AA, Ebrahimi-Daryani N. T-helper 1, T-helper 2, and T-regulatory cytokines gene polymorphisms in irritable bowel syndrome. Inflammation 33: 281–286, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Bércik P. The microbiota-gut-brain axis: learning from intestinal bacteria? Gut 60: 288–289, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141: 599–609, 609.e1–e3, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Bergonzelli GE, Collins SM. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139: 2102–2112, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Bercík P, Wang L, Verdú EF, Mao YK, Blennerhassett P, Khan WI, Kean I, Tougas G, Collins SM. Visceral hyperalgesia and intestinal dysmotility in a mouse model of postinfective gut dysfunction. Gastroenterology 127: 179–187, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Brandt LJ, Chey WD, Foxx-Orenstein AE, Quigley E, Schiller L, Schoenfeld P, Speigel B, Talley NJ, Moayyedi P. An evidence-based systematic review on the management of irritable bowel syndrome. Am J Gastroenterol 104, Suppl 1: s8–s35, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol 104: 1033–1049, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Brint EK, MacSharry J, Fanning A, Shanahan F, Quigley EM. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am J Gastroenterol 106: 329–336, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H, Michel K, Schemann M. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 137: 1425–1434, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Caenepeel P, Janssens J, Vantrappen G, Eyssen H, Coremans G. Interdigestive myoelectric complex in germ-free rats. Dig Dis Sci 34: 1180–1184, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Camilleri M, Carlson P, McKinzie S, Zucchelli M, D'Amato M, Busciglio I, Burton D, Zinsmeister AR. Genetic susceptibility to inflammation and colonic transit in lower functional gastrointestinal disorders: preliminary analysis. Neurogastroenterol Motil 23: 935–943.e398, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camilleri M, Katzka DA. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Genetic epidemiology and pharmacogenetics in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 302: G1075–G1084, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 24: 503–512, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camilleri M, Nadeau A, Tremaine WJ, Lamsam J, Burton D, Odunsi S, Sweetser S, Singh R. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil 21: 734–739.e43, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroccio A, Brusca I, Mansueto P, Pirrone G, Barrale M, Di Prima L, Ambrosiano G, Iacono G, Lospalluti ML, La Chiusa SM, Di Fede G. A cytologic assay for diagnosis of food hypersensitivity in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 8: 254–260, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Carroccio A, Brusca I, Mansueto P, Soresi M, D'Alcamo A, Ambrosiano G, Pepe I, Iacono G, Lospalluti ML, La Chiusa SM, Di Fede G. Fecal assays detect hypersensitivity to cow's milk protein and gluten in adults with irritable bowel syndrome. Clin Gastroenterol Hepatol 9: 965–971.e3, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Carroll IM, Chang YH, Park J, Sartor RB, Ringel Y. Luminal and mucosal-associated intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Gut Pathog 2: 19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carroll IM, Ringel-Kulka T, Keku TO, Chang YH, Packey CD, Sartor RB, Ringel Y. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 301: G799–G807, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 24: 521–530.e248, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest 117: 636–647, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 122: 1778–1783, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Choi CH, Jo SY, Park HJ, Chang SK, Byeon JS, Myung SJ. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol 45: 679–683, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 332: 1351–1362, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Chung EK, Zhang X, Li Z, Zhang H, Xu H, Bian Z. Neonatal maternal separation enhances central sensitivity to noxious colorectal distention in rat. Brain Res 1153: 68–77, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Codling C, O'Mahony L, Shanahan F, Quigley EM, Marchesi JR. A molecular analysis of fecal and mucosal bacterial communities in irritable bowel syndrome. Dig Dis Sci 55: 392–397, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Coeffier M, Gloro R, Boukhettala N, Aziz M, Lecleire S, Vandaele N, Antonietti M, Savoye G, Bole-Feysot C, Dechelotte P, Reimund JM, Ducrotte P. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am J Gastroenterol 105: 1181–1188, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Coelho AM, Fioramonti J, Buéno L. Systemic lipopolysaccharide influences rectal sensitivity in rats: role of mast cells, cytokines, and vagus nerve. Am J Physiol Gastrointest Liver Physiol 279: G781–G790, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Collins S, Verdu E, Denou E, Bercik P. The role of pathogenic microbes and commensal bacteria in irritable bowel syndrome. Dig Dis 27, Suppl 1: 85–89, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Collins SM. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology 111: 1683–1699, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Corinaldesi R, Stanghellini V, Cremon C, Gargano L, Cogliandro RF, De Giorgio R, Bartesaghi G, Canovi B, Barbara G. Effect of mesalazine on mucosal immune biomarkers in irritable bowel syndrome: a randomized controlled proof-of-concept study. Aliment Pharmacol Ther 30: 245–252, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol 104: 392–400, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Cuoco L, Salvagnini M. Small intestine bacterial overgrowth in irritable bowel syndrome: a retrospective study with rifaximin. Minerva Gastroenterol Dietol 52: 89–95, 2006 [PubMed] [Google Scholar]
- 48.De Giorgio R, Barbara G. Is irritable bowel syndrome an inflammatory disorder? Curr Gastroenterol Rep 10: 385–390, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Dear KLE, Elia M, Hunter JO. Do interventions which reduce colonic bacterial fermentation improve symptoms of irritable bowel syndrome? Dig Dis Sci 50: 758–766, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Dinan TG, Clarke G, Quigley EMM, Scott LV, Shanahan F, Cryan J, Cooney J, Keeling PW. Enhanced cholinergic-mediated increase in the proinflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol 103: 2570–2576, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Dinan TG, Quigley EMM, Ahmed SMM, Scully P, O'Brien S, O'Mahony L, O'Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology 130: 304–311, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Russo G, Caliendo G, Santagada V, Cirino G, Wallace JL, Fiorucci S. 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H-[1,2]dithiol-3yl)-phenyl ester (ATB-429), a hydrogen sulfide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of postinflammatory hypersensitivity. J Pharmacol Exp Ther 319: 447–458, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology 123: 2108–2131, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Duboc H, Rainteau D, Rajca S, Humbert L, Farabos D, Maubert M, Grondin V, Jouet P, Bouhassira D, Seksik P, Sokol H, Coffin B, Sabate JM. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 24: 513–520, e246–e247, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Dunckley P, Aziz Q, Wise RG, Brooks J, Tracey I, Chang L. Attentional modulation of visceral and somatic pain. Neurogastroenterol Motil 19: 569–577, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 101: 1288–1294, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology 125: 1651–1659, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Dunlop SP, Jenkins D, Spiller RC. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am J Gastroenterol 98: 1578–1583, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Esposito I, de Leone A, Di Gregorio G, Giaquinto S, de Magistris L, Ferrieri A, Riegler G. Breath test for differential diagnosis between small intestinal bacterial overgrowth and irritable bowel disease: an observation on non-absorbable antibiotics. World J Gastroenterol 13: 6016–6021, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng B, La JH, Schwartz ES, Gebhart GF. Irritable Bowel Syndrome: Methods, Mechanisms, and Pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 302: (10) G1085–G1098, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ford AC, Spiegel BMR, Talley NJ, Moayyedi P. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol 7: 1279–1286, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Forshammar J, Isaksson S, Strid H, Stotzer PO, Sjovall H, Simren M, Ohman L. A pilot study of colonic B cell pattern in irritable bowel syndrome. Scand J Gastroenterol 43: 1461–1466, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Fraher MH, O'Toole PW, Quigley EM. Techniques used to characterize the gut microbiota: a guide for the clinician. Nat Rev Gastroenterol Hepatol 9: 312–322, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469: 543–547, 2011 [DOI] [PubMed] [Google Scholar]
- 65.Fukudo S, Kanazawa M. Gene, environment, and brain-gut interactions in irritable bowel syndrome. J Gastroenterol Hepatol 26, Suppl 3: 110–115, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol 284: R1269–R1276, 2003 [DOI] [PubMed] [Google Scholar]
- 67.Gecse K, Roka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztoczy A, Izbeki F, Fioramonti J, Wittmann T, Bueno L. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut 57: 591–599, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Gibson PR. Food intolerance in functional bowel disorders. J Gastroenterol Hepatol 26, Suppl 3: 128–131, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Goehler LE, Lyte M, Gaykema RPA. Infection-induced viscerosensory signals from the gut enhance anxiety: implications for psychoneuroimmunology. Brain Behav Immun 21: 721–726, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gonsalkorale WM, Perrey C, Pravica V, Whorwell PJ, Hutchinson IV. Interleukin 10 genotypes in irritable bowel syndrome: evidence for an inflammatory component? Gut 52: 91–93, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guandalini S, Magazzù G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, Sibal A, Romano C, Canani RB, Lionetti P, Setty M. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr 51: 24–30, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther 33: 1123–1132, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Guilarte M, Santos J, de Torres I, Alonso C, Vicario M, Ramos L, Martinez C, Casellas F, Saperas E, Malagelada JR. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut 56: 203–209, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, Matuchansky C. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther 26: 475–486, 2007 [DOI] [PubMed] [Google Scholar]
- 75.Gwee KA, Collins SM, Read NW, Rajnakova A, Deng Y, Graham JC, McKendrick MW, Moochhala SM. Increased rectal mucosal expression of interleukin 1β in recently acquired post-infectious irritable bowel syndrome. Gut 52: 523–526, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome—a meta-analysis. Am J Gastroenterol 101: 1894–1899, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Hod K, Dickman R, Sperber A, Melamed S, Dekel R, Ron Y, Halpern Z, Berliner S, Maharshak N. Assessment of high-sensitivity CRP as a marker of micro-inflammation in irritable bowel syndrome. Neurogastroenterol Motil 23: 1105–1110, 2011 [DOI] [PubMed] [Google Scholar]
- 78.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science 336: 1268–1273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol 9: 15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Husebye E, Hellström PM, Sundler F, Chen J, Midtvedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol 280: G368–G380, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Jeffery IB, O'Toole PW, Ohman L, Claesson MJ, Deane J, Quigley EM, Simren M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 61: 997–1006, 2012 [DOI] [PubMed] [Google Scholar]
- 82.Kamath PS, Hoepfner MT, Phillips SF. Short-chain fatty acids stimulate motility of the canine ileum. Am J Physiol Gastrointest Liver Physiol 253: G427–G433, 1987 [DOI] [PubMed] [Google Scholar]
- 83.Kamiya T, Wang L, Forsythe P, Goettsche G, Mao Y, Wang Y, Tougas G, Bienenstock J. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut 55: 191–196, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kannampalli P, Shaker R, Sengupta JN. Colonic butyrate—algesic or analgesic? Neurogastroenterol Motil 23: 975–979, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kassinen A, Krogius-Kurikka L, Makivuokko H, Rinttila T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 133: 24–33, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Kerckhoffs APM, Samsom M, van der Rest ME, de Vogel J, Knol J, Ben-Amor K, Akkermans LM. Lower Bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World J Gastroenterol 15: 2887–2892, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ki Cha B, Mun Jung S, Hwan Choi C, Song ID, Woong Lee H, Joon Kim H, Hyuk J, Kyung Chang S, Kim K, Chung WS, Seo JG. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol 46: 220–227, 2012 [DOI] [PubMed] [Google Scholar]
- 88.Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, Thomforde G, Zinsmeister AR. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil 17: 687–696, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Kim HS, Lim JH, Park H, Lee SI. Increased immunoendocrine cells in intestinal mucosa of postinfectious irritable bowel syndrome patients 3 years after acute Shigella infection-an observation in a small case control study. Yonsei Med J 51: 45–51, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kindt S, Van Oudenhove L, Broekaert D, Kasran A, Ceuppens JL, Bossuyt X, Fischler B, Tack J. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil 21: 389–398, 2009 [DOI] [PubMed] [Google Scholar]
- 91.Kunze WA, Mao YK, Wang B, Huizinga JD, Ma X, Forsythe P, Bienenstock J. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med 13: 2261–2270, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Langhorst J, Junge A, Rueffer A, Wehkamp J, Foell D, Michalsen A, Musial F, Dobos GJ. Elevated human beta-defensin-2 levels indicate an activation of the innate immune system in patients with irritable bowel syndrome. Am J Gastroenterol 104: 404–410, 2009 [DOI] [PubMed] [Google Scholar]
- 93.Le Gall G, Noor SO, Ridgway K, Scovell L, Jamieson C, Johnson IT, Colquhoun IJ, Kemsley EK, Narbad A. Metabolomics of fecal extracts detects altered metabolic activity of gut microbiota in ulcerative colitis and irritable bowel syndrome. J Proteome Res 10: 4208–4218, 2011 [DOI] [PubMed] [Google Scholar]
- 94.Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol 23: 1689–1694, 2008 [DOI] [PubMed] [Google Scholar]
- 95.Lembo A, Zakko SF, Ferreira NL, Ringel Y, Bortey E, Courtney K, Corse E, Forbes W, Pimentel M. Rifaximin for the treatment of diarrhea-associated irritable bowel syndrome: short term treatment leading to long term sustained response [AGA abstract T1390]. Gastroenterology 134, Suppl 1: A-545, 2008 [Google Scholar]
- 96.Lembo T, Naliboff B, Munakata J, Fullerton S, Saba L, Tung S, Schmulson M, Mayer EA. Symptoms and visceral perception in patients with pain-predominant irritable bowel syndrome. Am J Gastroenterol 94: 1320–1326, 1999 [DOI] [PubMed] [Google Scholar]
- 97.Lesniewska V, Rowland I, Laerke HN, Grant G, Naughton PJ. Relationship between dietary-induced changes in intestinal commensal microflora and duodenojejunal myoelectric activity monitored by radiotelemetry in the rat in vivo. Exp Physiol 91: 229–237, 2006 [DOI] [PubMed] [Google Scholar]
- 98.Liebregts T, Adam B, Bredack C, Roth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ, Holtmann G. Immune activation in patients with irritable bowel syndrome. Gastroenterology 132: 913–920, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 130: 1480–1491, 2006 [DOI] [PubMed] [Google Scholar]
- 100.Low K, Hwang L, Hua J, Zhu A, Morales W, Pimentel M. A combination of rifaximin and neomycin is most effective in treating irritable bowel syndrome patients with methane on lactulose breath test. J Clin Gastroenterol 44: 547–550, 2010 [DOI] [PubMed] [Google Scholar]
- 101.Lunardi C, Bambara LM, Biasi D, Cortina P, Peroli P, Nicolis F, Favari F, Pacor ML. Double-blind cross-over trial of oral sodium cromoglycate in patients with irritable bowel syndrome due to food intolerance. Clin Exp Allergy 21: 569–572, 1991 [DOI] [PubMed] [Google Scholar]
- 102.Macsharry J, O'Mahony L, Fanning A, Bairead E, Sherlock G, Tiesman J, Fulmer A, Kiely B, Dinan TG, Shanahan F, Quigley EM. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol 43: 1467–1476, 2008 [DOI] [PubMed] [Google Scholar]
- 103.Majewski M, McCallum RW. Results of small intestinal bacterial overgrowth testing in irritable bowel syndrome patients: clinical profiles and effects of antibiotic trial. Adv Med Sci 52: 139–142, 2007 [PubMed] [Google Scholar]
- 104.Malinen E, Rinttilä T, Kajander K, Matto J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol 100: 373–382, 2005 [DOI] [PubMed] [Google Scholar]
- 105.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol 9: 599–608, 2012 [DOI] [PubMed] [Google Scholar]
- 106.Mättö J, Maunuksela L, Kajander K, Palva A, Korpela R, Kassinen A, Saarela M. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome—a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol 43: 213–222, 2005 [DOI] [PubMed] [Google Scholar]
- 107.Maukonen J, Satokari R, Mättö J, Söderlund H, Mattila-Sandholm T, Saarela M. Prevalence and temporal stability of selected clostridial groups in irritable bowel syndrome in relation to predominant faecal bacteria. J Med Microbiol 55: 625–633, 2006 [DOI] [PubMed] [Google Scholar]
- 108.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med 62: 381–396, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mekjian HS, Phillips SF, Hofmann AF. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest 50: 1569–1577, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein A, Brandt L, Quigley E. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut 59: 325–332, 2010 [DOI] [PubMed] [Google Scholar]
- 111.Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut 54: 601–607, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murray CDR, Flynn J, Ratcliffe L, Jacyna MR, Kamm MA, Emmanuel AV. Effect of acute physical and psychological stress on gut autonomic innervation in irritable bowel syndrome. Gastroenterology 127: 1695–1703, 2004 [DOI] [PubMed] [Google Scholar]
- 113.Natividad JM, Petit V, Huang X, de Palma G, Jury J, Sanz Y, Philpott D, Garcia Rodenas CL, McCoy KD, Verdu EF. Commensal and probiotic bacteria influence intestinal barrier function and susceptibility to colitis in Nod1−/−; Nod2−/− mice. Inflamm Bowel Dis 18: 1434–1446, 2012 [DOI] [PubMed] [Google Scholar]
- 114.Nayak AK, Karnad DR, Abraham P, Mistry FP. Metronidazole relieves symptoms in irritable bowel syndrome: the confusion with so-called ‘chronic amebiasis.’ Indian J Gastroenterol 16: 137–139, 1997 [PubMed] [Google Scholar]
- 115.Neufeld KA, Kang N, Bienenstock J, Foster JA. Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol 4: 492–494, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 23: 255–264, e119, 2011 [DOI] [PubMed] [Google Scholar]
- 117.Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol 13: 1143–1147, 2001 [DOI] [PubMed] [Google Scholar]
- 118.O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O'Sullivan GC, Kiely B, Collins JK, Shanahan F, Quigley EM. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 128: 541–551, 2005 [DOI] [PubMed] [Google Scholar]
- 119.O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 65: 263–267, 2009 [DOI] [PubMed] [Google Scholar]
- 120.O'Malley D, Quigley EM, Dinan TG, Cryan JF. Do interactions between stress and immune responses lead to symptom exacerbations in irritable bowel syndrome? Brain Behav Immun 25: 1333–1341, 2011 [DOI] [PubMed] [Google Scholar]
- 121.O'Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, O'Morain CA. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil 12: 449–457, 2000 [DOI] [PubMed] [Google Scholar]
- 122.Ohman L, Isaksson S, Lindmark AC, Posserud I, Stotzer PO, Strid H, Sjovall H, Simren M. T-cell activation in patients with irritable bowel syndrome. Am J Gastroenterol 104: 1205–1212, 2009 [DOI] [PubMed] [Google Scholar]
- 123.Ohman L, Isaksson S, Lundgren A, Simrén M, Sjovall H. A controlled study of colonic immune activity and β7+ blood T lymphocytes in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 3: 980–986, 2005 [DOI] [PubMed] [Google Scholar]
- 124.Ohman L, Lindmark AC, Isaksson S, Posserud I, Strid H, Sjovall H, Simren M. B-cell activation in patients with irritable bowel syndrome (IBS). Neurogastroenterol Motil 21: 644–650, 2009 [DOI] [PubMed] [Google Scholar]
- 125.Ohman L, Simrén M. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig Liver Dis 39: 201–215, 2007 [DOI] [PubMed] [Google Scholar]
- 126.Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol 7: 163–173, 2010 [DOI] [PubMed] [Google Scholar]
- 127.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR, Muir JG. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol 25: 1366–1373, 2010 [DOI] [PubMed] [Google Scholar]
- 128.Park DH, Kim MH, Chari ST. Recent advances in autoimmune pancreatitis. Gut 58: 1680–1689, 2009 [DOI] [PubMed] [Google Scholar]
- 129.Parkes GC, Rayment NB, Hudspith BN, Petrovska L, Lomer MC, Brostoff J, Whelan K, Sanderson JD. Distinct microbial populations exist in the mucosa-associated microbiota of sub-groups of irritable bowel syndrome. Neurogastroenterol Motil 24: 31–39, 2012 [DOI] [PubMed] [Google Scholar]
- 130.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, Ringel Y, Kim HP, Dibonaventura MD, Carroll CF, Allen JK, Cook SF, Sandler RS, Kappelman MD, Shaheen NJ. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143: 1179–1187e1–e3, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peralta S, Cottone C, Doveri T, Almasio PL, Craxi A. Small intestine bacterial overgrowth and irritable bowel syndrome-related symptoms: experience with rifaximin. World J Gastroenterol 15: 2628–2631, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, Neunlist M. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 58: 196–201, 2009 [DOI] [PubMed] [Google Scholar]
- 133.Piche T, Saint-Paul MC, Dainese R, Marine-Barjoan E, Iannelli A, Montoya ML, Peyron JF, Czerucka D, Cherikh F, Filippi J, Tran A, Hebuterne X. Mast cells and cellularity of the colonic mucosa correlated with fatigue and depression in irritable bowel syndrome. Gut 57: 468–473, 2008 [DOI] [PubMed] [Google Scholar]
- 134.Pimentel M, Chatterjee S, Chow EJ, Park S, Kong Y. Neomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: subanalysis of a double-blind randomized controlled study. Dig Dis Sci 51: 1297–1301, 2006 [DOI] [PubMed] [Google Scholar]
- 135.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol 95: 3503–3506, 2000 [DOI] [PubMed] [Google Scholar]
- 136.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol 98: 412–419, 2003 [DOI] [PubMed] [Google Scholar]
- 137.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 364: 22–32, 2011 [DOI] [PubMed] [Google Scholar]
- 138.Pimentel M, Morales W, Chua K, Barlow G, Weitsman S, Kim G, Amichai MM, Pokkunuri V, Rook E, Mathur R, Marsh Z. Effects of rifaximin treatment and retreatment in nonconstipated IBS subjects. Dig Dis Sci 56: 2067–2072, 2011 [DOI] [PubMed] [Google Scholar]
- 139.Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med 145: 557–563, 2006 [DOI] [PubMed] [Google Scholar]
- 140.Pimentel M, Soffer EE, Chow EJ, Kong Y, Lin HC. Lower frequency of MMC is found in IBS subjects with abnormal lactulose breath test, suggesting bacterial overgrowth. Dig Dis Sci 47: 2639–2643, 2002 [DOI] [PubMed] [Google Scholar]
- 141.Posserud I, Stotzer PO, Björnsson ES, Abrahamsson H, Simrén M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 56: 802–808, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rajilić-Stojanović M, Biagi E, Heilig HGHJ, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 141: 1792–1801, 2011 [DOI] [PubMed] [Google Scholar]
- 143.Rao SS, Read NW, Brown C, Bruce C, Holdsworth CD. Studies on the mechanism of bowel disturbance in ulcerative colitis. Gastroenterology 93: 934–940, 1987 [DOI] [PubMed] [Google Scholar]
- 144.Ringel-Kulka T, Palsson OS, Maier D, Carroll I, Galanko JA, Leyer G, Ringel Y. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol 45: 518–525, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ringel Y, Carroll IM. Alterations in the intestinal microbiota and functional bowel symptoms. Gastrointest Endosc Clin N Am 19: 141–150, 2009 [DOI] [PubMed] [Google Scholar]
- 146.Ringel Y, Drossman DA. Irritable bowel syndrome. In: Netter's Textbook of Internal Medicine (2nd ed.), edited by Runge MS, Greganti MA. Philadelphia, PA: Saunders Elsevier, 2009, p. 419–425 [Google Scholar]
- 147.Ringel Y, Quigley E, Lin HC. Probiotics and gastrointestinal disorders. Am J Gastroenterol Suppl 1: 34–40, 2012 [Google Scholar]
- 148.Ringel Y, Ringel-Kulka T. The rationale and clinical effectiveness of probiotics in irritable bowel syndrome. J Clin Gastroenterol 45 Suppl: S145–S148, 2011 [DOI] [PubMed] [Google Scholar]
- 149.Ringel Y, Whitehead WE, Toner BB, Diamant NE, Hu Y, Jia H, Bangdiwala SI, Drossman DA. Sexual and physical abuse are not associated with rectal hypersensitivity in patients with irritable bowel syndrome. Gut 53: 838–842, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Roberts L, Wilson S, Singh S, Roalfe A, Greenfield S. Gut-directed hypnotherapy for irritable bowel syndrome: piloting a primary care-based randomised controlled trial. Br J Gen Pract 56: 115–121, 2006 [PMC free article] [PubMed] [Google Scholar]
- 151.Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, Ouwehand A, Leyer G, Carcano D, Colombel JF, Ardid D, Desreumaux P. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med 13: 35–37, 2007 [DOI] [PubMed] [Google Scholar]
- 152.Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut 53: 958–964, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Salonen A, de Vos WM, Palva A. Gastrointestinal microbiota in irritable bowel syndrome: present state and perspectives. Microbiology 156: 3205–3215, 2010 [DOI] [PubMed] [Google Scholar]
- 154.Saulnier DM, Riehle K, Mistretta TA, Diaz MA, Mandal D, Raza S, Weidler EM, Qin X, Coarfa C, Milosavljevic A, Petrosino JF, Highlander S, Gibbs R, Lynch SV, Shulman RJ, Versalovic J. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 141: 1782–1791, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Saulnier DM, Ringel Y, Heyman MB, Foster JA, Bercik P, Shulman R, Versalovic J, Verdu E, Dinan TG, Hecht G, Guarner F. The intestinal microbiome, probiotics, and prebiotics in neurogastroenterology. Gut Microbes 4: 17–27, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schoepfer AM, Schaffer T, Seibold-Schmid B, Muller S, Seibold F. Antibodies to flagellin indicate reactivity to bacterial antigens in IBS patients. Neurogastroenterol Motil 20: 1110–1118, 2008 [DOI] [PubMed] [Google Scholar]
- 157.Schwille-Kiuntke J, Enck P, Zendler C, Krieg M, Polster AV, Klosterhalfen S, Autenrieth IB, Zipfel S, Frick JS. Postinfectious irritable bowel syndrome: follow-up of a patient cohort of confirmed cases of bacterial infection with Salmonella or Campylobacter. Neurogastroenterol Motil 23: e479–e488, 2011 [DOI] [PubMed] [Google Scholar]
- 158.Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev 90: 859–904, 2010 [DOI] [PubMed] [Google Scholar]
- 159.Shaheen NJ, Hansen RA, Morgan DR, Gangarosa LM, Ringel Y, Thiny MT, Russo MW, Sandler RS. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol 101: 2128–2138, 2006 [DOI] [PubMed] [Google Scholar]
- 160.Sharara AI, Aoun E, Abdul-Baki H, Mounzer R, Sidani S, ElHajj I. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol 101: 326–333, 2006 [DOI] [PubMed] [Google Scholar]
- 161.Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol 6: 765–771, 2008 [DOI] [PubMed] [Google Scholar]
- 162.Shulman RJ, Eakin MN, Czyzewski DI, Jarrett M, Ou CN. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr 153: 646–650, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther 29: 508–518, 2009 [DOI] [PubMed] [Google Scholar]
- 164.Simren M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 62: 159–176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Simrén M, Ringström G, Björnsson ES, Abrahamsson H. Treatment with hypnotherapy reduces the sensory and motor component of the gastrocolonic response in irritable bowel syndrome. Psychosom Med 66: 233–238, 2004 [DOI] [PubMed] [Google Scholar]
- 166.Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 138: 1772–1782, 2010 [DOI] [PubMed] [Google Scholar]
- 167.Sperber AD, Dekel R. Irritable bowel syndrome and co-morbid gastrointestinal and extra-gastrointestinal functional syndromes. J Neurogastroenterol Motil 16: 113–119, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 47: 804–811, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Suply E, de Vries P, Soret R, Cossais F, Neunlist M. Butyrate enemas enhance both cholinergic and nitrergic phenotype of myenteric neurons and neuromuscular transmission in newborn rat colon. Am J Physiol Gastrointest Liver Physiol 302: G1373–G1380, 2012 [DOI] [PubMed] [Google Scholar]
- 170.Swan C, Duroudier NP, Campbell E, Zaitoun A, Hastings M, Dukes GE, Cox J, Kelly FM, Wilde J, Lennon MG, Neal KR, Whorwell PJ, Hall IP, Spiller RC. Identifying and testing candidate genetic polymorphisms in the irritable bowel syndrome (IBS): association with TNFSF15 and TNFalpha. Gut 62: 985–994, 2013 [DOI] [PubMed] [Google Scholar]
- 171.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil 22: 512–519, e114–e115, 2010 [DOI] [PubMed] [Google Scholar]
- 172.Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther 26: 535–544, 2007 [DOI] [PubMed] [Google Scholar]
- 173.Tlaskalova-Hogenova H, Stepankova R, Kozakova H, Hudcovic T, Vannucci L, Tuckova L, Rossmann P, Hrncir T, Kverka M, Zakostelska Z, Klimesova K, Pribylova J, Bartova J, Sanchez D, Fundova P, Borovska D, Srutkova D, Zidek Z, Schwarzer M, Drastich P, Funda DP. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol 8: 110–120, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Törnblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology 123: 1972–1979, 2002 [DOI] [PubMed] [Google Scholar]
- 175.Treem WR, Ahsan N, Kastoff G, Hyams JS. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr 23: 280–286, 1996 [DOI] [PubMed] [Google Scholar]
- 176.Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev 79: 1–71, 1999 [DOI] [PubMed] [Google Scholar]
- 177.Tuteja AK, Fang JC, Al-Suqi M, Stoddard GJ, Hale DC. Double-blind placebo-controlled study of mesalamine in post-infective irritable bowel syndrome — a pilot study. Scand J Gastroenterol 47: 1159–1164, 2012 [DOI] [PubMed] [Google Scholar]
- 178.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 141: 769–776, 2011 [DOI] [PubMed] [Google Scholar]
- 179.van der Veek PPJ, van den Berg M, de Kroon YE, Verspaget HW, Masclee AAM. Role of tumor necrosis factor-α and interleukin-10 gene polymorphisms in irritable bowel syndrome. Am J Gastroenterol 100: 2510–2516, 2005 [DOI] [PubMed] [Google Scholar]
- 180.Vanhoutvin SA, Troost FJ, Kilkens TO, Lindsey PJ, Hamer HM, Jonkers DM, Venema K, Brummer RJ. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil 21: 952–957.e76, 2009 [DOI] [PubMed] [Google Scholar]
- 181.Vasina V, Barbara G, Talamonti L, Stanghellini V, Corinaldesi R, Tonini M, De Ponti F, De Giorgio R. Enteric neuroplasticity evoked by inflammation. Auton Neurosci 126–127: 264–272, 2006 [DOI] [PubMed] [Google Scholar]
- 182.Verdu EF, Bercik P, Verma-Gandhu M, Huang XX, Blennerhassett P, Jackson W, Mao Y, Wang L, Rochat F, Collins SM. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 55: 182–190, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vicario M, Alonso C, Guilarte M, Serra J, Martínez C, González-Castro AM, Lobo B, Antolín M, Andreu AL, García-Arumí E, Casellas M, Saperas E, Malagelada JR, Azpiroz F, Santos J. Chronic psychosocial stress induces reversible mitochondrial damage and corticotropin-releasing factor receptor type-1 upregulation in the rat intestine and IBS-like gut dysfunction. Psychoneuroendocrinology 37: 65–77, 2012 [DOI] [PubMed] [Google Scholar]
- 184.Villani AC, Lemire M, Thabane M, Belisle A, Geneau G, Garg AX, Clark WF, Moayyedi P, Collins SM, Franchimont D, Marshall JK. Genetic risk factors for post-infectious irritable bowel syndrome following a waterborne outbreak of gastroenteritis. Gastroenterology 138: 1502–1513, 2010 [DOI] [PubMed] [Google Scholar]
- 185.Walker MM, Talley NJ, Prabhakar M, Pennaneac'h CJ, Aro P, Ronkainen J, Storskrubb T, Harmsen WS, Zinsmeister AR, Agreus L. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther 29: 765–773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Walters B, Vanner SJ. Detection of bacterial overgrowth in IBS using the lactulose H2 breath test: comparison with 14C-d-xylose and healthy controls. Am J Gastroenterol 100: 1566–1570, 2005 [DOI] [PubMed] [Google Scholar]
- 187.Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut 53: 1096–1101, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Waterer GW, Wunderink RG. Science review: genetic variability in the systemic inflammatory response. Crit Care 7: 308–314, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Weston AP, Biddle WL, Bhatia PS, Miner PB., Jr Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci 38: 1590–1595, 1993 [DOI] [PubMed] [Google Scholar]
- 190.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O'Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 101: 1581–1590, 2006 [DOI] [PubMed] [Google Scholar]
- 191.Wilhelmsen I. Brain-gut axis as an example of the bio-psycho-social model. Gut 47, Suppl IV: iv5–iv7, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wong BS, Camilleri M, Busciglio I, Carlson P, Szarka LA, Burton D, Zinsmeister AR. Pharmacogenetic trial of a cannabinoid agonist shows reduced fasting colonic motility in patients with nonconstipated irritable bowel syndrome. Gastroenterology 141: 1638–1647e31–e37, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang J, Lee HR, Low K, Chatterjee S, Pimentel M. Rifaximin versus other antibiotics in the primary treatment and retreatment of bacterial overgrowth in IBS. Dig Dis Sci 53: 169–174, 2008 [DOI] [PubMed] [Google Scholar]
- 194.Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut 60: 334–340, 2011 [DOI] [PubMed] [Google Scholar]
- 195.Zar S, Mincher L, Benson MJ, Kumar D. Food-specific IgG4 antibody-guided exclusion diet improves symptoms and rectal compliance in irritable bowel syndrome. Scand J Gastroenterol 40: 800–807, 2005 [DOI] [PubMed] [Google Scholar]
- 196.Zuo XL, Li YQ, Li WJ, Guo YT, Lu XF, Li JM, Desmond PV. Alterations of food antigen-specific serum immunoglobulins G and E antibodies in patients with irritable bowel syndrome and functional dyspepsia. Clin Exp Allergy 37: 823–830, 2007 [DOI] [PubMed] [Google Scholar]


