Abstract
Chronic ANG II infusion in rodents is widely used as an experimental model of hypertension, yet very limited data are available describing the resulting blood pressure-renal blood flow (BP-RBF) relationships in conscious rats. Accordingly, male Sprague-Dawley rats (n = 19) were instrumented for chronic measurements of BP (radiotelemetry) and RBF (Transonic Systems, Ithaca, NY). One week later, two or three separate 2-h recordings of BP and RBF were obtained in conscious rats at 24-h intervals, in addition to separate 24-h BP recordings. Rats were then administered either ANG II (n = 11, 125 ng·kg−1·min−1) or phenylephrine (PE; n = 8, 50 mg·kg−1·day−1) as a control, ANG II-independent, pressor agent. Three days later the BP-RBF and 24-h BP recordings were repeated over several days. Despite similar increases in BP, PE led to significantly greater BP lability at the heart beat and very low frequency bandwidths. Conversely, ANG II, but not PE, caused significant renal vasoconstriction (a 62% increase in renal vascular resistance and a 21% decrease in RBF) and increased variability in BP-RBF relationships. Transfer function analysis of BP (input) and RBF (output) were consistent with a significant potentiation of the renal myogenic mechanism during ANG II administration, likely contributing, in part, to the exaggerated reductions in RBF during periods of BP elevations. We conclude that relatively equipressor doses of ANG II and PE lead to greatly different ambient BP profiles and effects on the renal vasculature when assessed in conscious rats. These data may have important implications regarding the pathogenesis of hypertension-induced injury in these models of hypertension.
Keywords: hypertension, hemodynamics, blood pressure variability
increased activity of the renin-angiotensin-aldosterone system (RAAS) (40, 56, 73) is postulated to be a major contributor to chronic kidney disease progression through both blood pressure (BP)-dependent and -independent mechanisms (7, 28, 39, 73). Accordingly, chronic ANG II infusion is extensively used to investigate mechanisms that mediate renal damage in hypertensive states characterized by enhanced RAAS activation (3, 20, 42, 65, 71). Renal parenchymal injury with significant tubulointerstitial fibrosis and a propensity to develop salt-sensitive hypertension has been observed after ANG II infusions (44, 57). Both barotrauma and renal vasoconstriction-mediated tissue ischemia have been postulated to initiate the pathogenic cascades that lead to renal injury after chronic ANG II infusions. However, despite its wide use, there is a paucity of experimental data describing the BP-renal blood flow (RBF) relationships in conscious ANG II-infused animals, and the relative contribution of these two initiating mechanisms has remained uncertain and controversial. Most of the renal vascular responses to ANG II have been investigated in terms of steady-state relationships and usually in anesthetized animals. Although such data have undoubtedly provided important insights into the directional changes, they nevertheless have significant limitations given the effects of anesthesia on RBF (23, 82) and the associated abrogation of the considerable time-dependent variability in BP-RBF relationships that is normally observed in conscious rats (23, 58, 69, 81). Thus, the effects of ANG II on such time-dependent variability in BP-RBF relationships remain unknown. The present studies were performed to examine the effects of continuous infusion of ANG II on ambient BP and RBF profiles, as well as the variability of BP-RBF relationships in the presence of modest increases in BP. As phenylephrine (PE) administration has also been reported to have similar effects on BP and renal injury in rats (18, 43), experiments were also performed in conscious rats continuously administered PE to evaluate the effects of an ANG II-independent pressor agent on such BP and renal hemodynamic profiles.
MATERIALS AND METHODS
Animals.
All experiments were performed on male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 250–350 g and fed a standard 1% NaCl Purina chow (Purina no. 5008) and provided water ad libitum. All animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Hines VA Institutional Animal Care and Use Committee.
Surgical procedures.
Rats were anesthetized with pentobarbital sodium (50 mg/kg ip) anesthesia, and all surgeries were performed on a temperature-controlled warming board using aseptic techniques. During the initial surgery, a BP radiotransmitter (model TA11PA-C40; Data Sciences, St. Paul, MN) was inserted, via the femoral artery, into the abdominal aorta just below the level of the renal arteries and a RBF transducer (model 1RB; Transonic Systems) was placed around the left renal artery and packed in Dacron mesh to ensure proper alignment of the transducer and vessel (8, 29). The transducer cable was secured to the back muscles, routed subcutaneously, exteriorized at the back of the neck, and connected to a flowmeter (model T106; Transonic Systems) during RBF recordings. ANG II and PE were purchased from Sigma and administered via the implantation of osmotic minipumps (Durect, Cupertino, CA) positioned subcutaneously between the scapulae.
Experimental design.
Rats were allowed to recover for 1 wk following the implantation of the BP transmitter and RBF probe. In conscious rats, BP and RBF were then obtained for 2 h at a sampling rate of 200 Hz on 1–3 separate occasions at 24-h intervals. In addition to these simultaneous BP and RBF recordings, BP was separately sampled at 200 Hz 1 or 2 times for 24 h for analysis of ambient BP profiles and BP power spectra. Following baseline BP-RBF measurements, rats were anesthetized and implanted with osmotic minipumps to chronically deliver either ANG II (125 ng·kg−1·min−1; n = 11) or PE (50 mg·kg−1·day−1; n = 8) for 1 wk, doses that elicit modest increases in BP in normal-salt diet-fed rats (32, 43). Starting ∼48 to 72 h later, 1–3 simultaneous BP and RBF recordings were again obtained at 200 Hz for a 2-h period. Similar to baseline measurements, BP was separately sampled at 200 Hz on 1–2 separate occasions for 24 h to examine the effects of ANG II and PE on ambient BP profiles and on BP power spectra. Twenty-four-hour BP recordings were made in 7 of the 11 rats administered ANG II, and in all of the rats administered PE.
Ambient heart rate and BP load profiles.
The effect of ANG II and PE on heart rate and BP load was estimated using power spectral analysis. The total BP load or power (energy/unit time) can be separated into two major components consisting of its mean value [direct current (DC) BP power] and that because of its fluctuations from the mean because of heart beat and other slower neurohumoral mechanisms [alternating current (AC) BP power] (6, 8, 9). Accordingly, individual 24-h BP recordings (200 Hz) were resampled to 20 Hz after being low pass filtered to remove signal components with frequencies greater than 10 Hz. The recording was then divided into ∼100 segments of 32,768 samples with 50% overlap of segments. The BP power spectra were determined using Welch's averaged periodogram method with a fast Fourier transform applied to each segment after linear detrending and multiplication by a Hanning window. The results from the separate recordings in each rat obtained at baseline and after ANG II or PE were averaged before calculating the mean group data. The integrated BP spectral power is presented over specified frequency bands consisting of a very low frequency range (VLF; 0.0006–0.1 Hz), a low-frequency range (LF; 0.1–1 Hz), a high-frequency range (HF; 1–3 Hz), and at the heart beat frequency (∼6 Hz). The heartbeat frequency was determined on the basis of the location of the peak of the BP power spectral density observed within the 4- to 8-Hz frequency range. The BP power at the heartbeat frequency was then calculated within a frequency range centered at the peak between 4 and 8 Hz with its edges defined by the points where the spectral density drops to 1% of the maximum power (27). These frequency bins were selected on the basis of our previous study (27) and their inclusion of various cardiovascular regulatory components (e.g., respiratory, Meyer waves, and myogenic activity) that have been suggested to contribute to BP oscillations (53, 72).
Ambient renal hemodynamics.
Results from the two or three separate 2-h recordings of BP and RBF in each rat before and after ANG II and PE were averaged for analysis of the effects on mean arterial pressure (MAP), renal vascular resistance (RVR), and RBF.
Ambient pulse pressure and renal pulse flow analysis.
Subsegments of ∼30-min duration from each 2-h recording that were free of noise or other artifacts were selected for analysis. Within each contiguous segment, every pressure and flow pulse amplitude was calculated by the identification of the peak systolic pressure and flow point subtracted by the subsequent nadir diastolic pressure and flow point. The average pulse pressure and pulse flow over the multiple recordings for an individual rat at baseline and during ANG II or PE infusion were then averaged. Finally, the average pulse pressure and pulse flow were averaged across rats.
Time-varying BP-RBF relationships.
Each BP and RBF recording was downsampled (20 Hz) and divided into segments of varying lengths (1, 10, 30, 60, and 100 s), with 50% overlap between successive segments. For each segment, we averaged the samples of BP and RBF over the whole segment. The resulting average RBF values were associated with a 10-mmHg range (bin), in which the associated average BP value falls. We also performed similar analysis for 5- and 20-mmHg BP bins. We then averaged the RBF-BP bin data over multiple baseline recordings for an individual rat, and we did the same for recordings during ANG II or PE infusion. Finally, the RBF-BP bin data at baseline and during ANG II or PE infusion were averaged across rats. RBF values are expressed as a percentage of the respective RBF observed when BP was between 90 and 100 mmHg during baseline recordings.
Transfer function analysis.
As previously described (2, 8, 26, 29), transfer function analysis of the dynamic relationship between BP (input) and RBF (output) was performed at baseline and after ANG II and PE. Subsegments of 30-min duration from each 2-h recording that were free of noise or other artifacts were selected for analysis. The 30-min recordings were resampled at 20 Hz using a 10-Hz low-pass antialiasing filter. Each time sequence of 36,000 data points was then subjected to linear trend removal. The BP and RBF power spectra were determined using Welch's averaged periodogram method. Input and output autopower spectra and cross-power spectra were calculated for each segment and then averaged and from these, the admittance function, coherence, and fractional gain in admittance (FGA) were computed (2, 8, 29). The natural frequencies of the myogenic and tubuloglomerular feedback (TGF) mechanisms were determined from their characteristic signature resonance peaks in FGA between 0.1 and 0.3 Hz and between 0.025 and 0.05 Hz, respectively, by inspection of individual records and averaged across each record. We used several other components of the transfer function analyses that have been considered potential indices of the strength of the renal myogenic mechanism (26, 80, 84). Among these is the slope of admittance magnitude reduction immediately below the myogenic peak (49, 69, 84). On a logarithmic scale (log gain vs. log frequency), the gain is approximately linear between the frequency where the fractional gain is unity (or 0 dB, ∼0.1 Hz) and the frequency of the myogenic peak (∼0.25 Hz). The slope of this linear region, when in units of decibels per decade, may be interpreted as being indicative of the order of the dynamic relationship between BP and RBF, with 20 dB/decade corresponding to first order and 40 dB/decade to second order (49, 69, 80, 84). Note that the slope value in decibels per decade is independent of whether the gain is measured in absolute units (as for admittance gain) or normalized units (as for the dimensionless fractional gain).
Statistical analysis.
Results are expressed as means ± SE. Statistical comparisons between the groups were performed using a two-way repeated-measures ANOVA. A one-way repeated-measures ANOVA was used to evaluate the time-varying BP-RBF relationships within baseline, ANG II, and PE groups. If necessary, post hoc comparisons were made using a Student-Newman-Keuls test. P < 0.05 was considered significant.
RESULTS
Ambient heart rate and BP profiles in ANG II- and PE-infused rats.
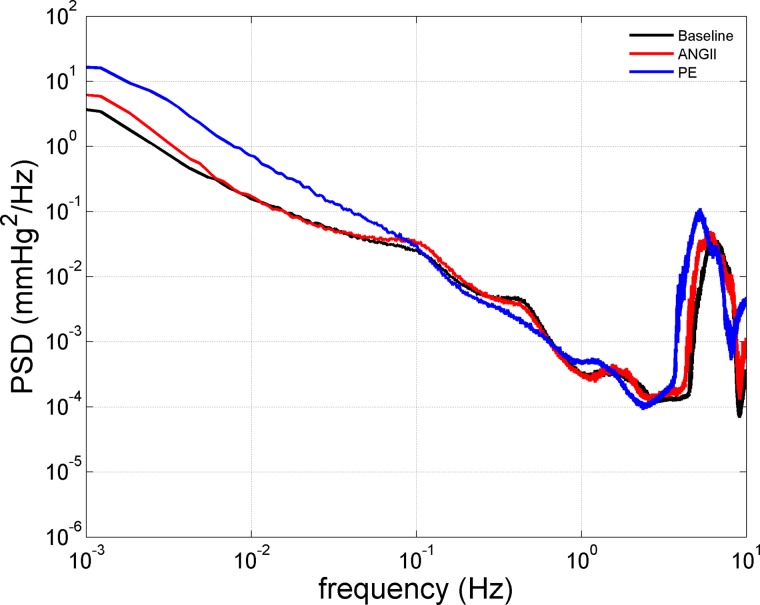
As shown in Table 1, ANG II and PE both led to reductions in heart rate (HR), but the decrease was significantly greater in PE-infused rats. ANG II and PE increased the average 24-h MAP compared with baseline but were not significantly different from each other. Total AC BP power and its individual components at the heart beat frequency (4–8 Hz), VLF, LF, and HF bands are summarized in Table 1 and are illustrated in Fig. 1. ANG II and PE increased total AC BP power, which was evident at several of the individual AC BP power components, including VLF, HF, and the heart beat frequency. Yet, PE led to more robust increases in total AC BP power manifested mainly within the VLF and heart beat frequency ranges. Interestingly, whereas ANG II increased LF BP power, PE administration led to reduced BP power in this range. Fluctuations in the Meyer waves (∼0.1 Hz) and that due to the myogenic mechanism of RBF autoregulation (∼0.25 Hz) are thought to operate within this frequency band (53, 72). Thus, despite the relatively modest effects of PE on MAP (DC BP power), it had a more potent effect on AC BP power compared with ANG II.
Table 1.
Twenty-four-hour HR, MAP, and BP power distribution in ANG II- and PE-infused rats
| BP Power, mmHg2 |
|||||||
|---|---|---|---|---|---|---|---|
| HR, bpm | MAP, mmHg | VLF 0.0006-0.1 Hz | LF 0.1–1 Hz | HF 1–3 Hz | at Heart Beat | Total AC | |
| Baseline | 370 ± 11 | 103 ± 3 | 18 ± 1 | 4.6 ± 0.3 | 0.7 ± 0.1 | 99 ± 8 | 129 ± 9 |
| ANG II | 355 ± 9* | 120 ± 6 | 31 ± 5 | 5.7 ± 0.4 | 0.8 ± 0.1 | 129 ± 9 | 173 ± 12 |
| Baseline | 364 ± 11 | 98 ± 2 | 23 ± 3 | 5.1 ± 0.5 | 0.8 ± 0.2 | 85 ± 11 | 119 ± 11 |
| PE | 306 ± 8*† | 111 ± 4 | 97 ± 16*† | 4.2 ± 0.4 | 0.8 ± 0.1 | 204 ± 22*† | 309 ± 31*† |
| ANOVA effects | |||||||
| Baseline vs. drug | P < 0.001 | P < 0.005 | P < 0.005 | NS | NS | P < 0.01 | P < 0.001 |
| ANG II vs. PE | P < 0.05 | NS | P < 0.005 | NS | NS | P = 0.09 | P < 0.05 |
| Interaction | P < 0.005 | NS | P < 0.05 | P < 0.05 | NS | P < 0.01 | P < 0.001 |
Values are expressed as means ± SE. ANG II (n = 7; 125 ng·kg−1·min−1), phenylephrine (PE; n = 8; 50 mg·kg−1·day−1). HR, heart rate; MAP, mean arterial pressure; BP, blood pressure; VLF, very low frequency; LF, low frequency; HF, high frequency; NS, not significant.
P < 0.05 vs. baseline.
P < 0.05 vs. ANG II.
Fig. 1.
Computed 24-h ambient blood pressure (BP) power spectral density [PSD, (mmHg)2/Hz] at baseline (n = 15) and during ANG II (ANG II; n = 7) and phenylephrine (PE; n = 8) administration. Because no significant differences were observed with respect to the BP PSD obtained during baseline recordings, these data have been averaged between groups for presentation clarity. Significant differences in the effects of ANG II and PE were observed at several different frequency ranges of the 24 h. BP spectra (see Table 1 for details).
Ambient renal hemodynamics in ANG II- and PE-infused rats.
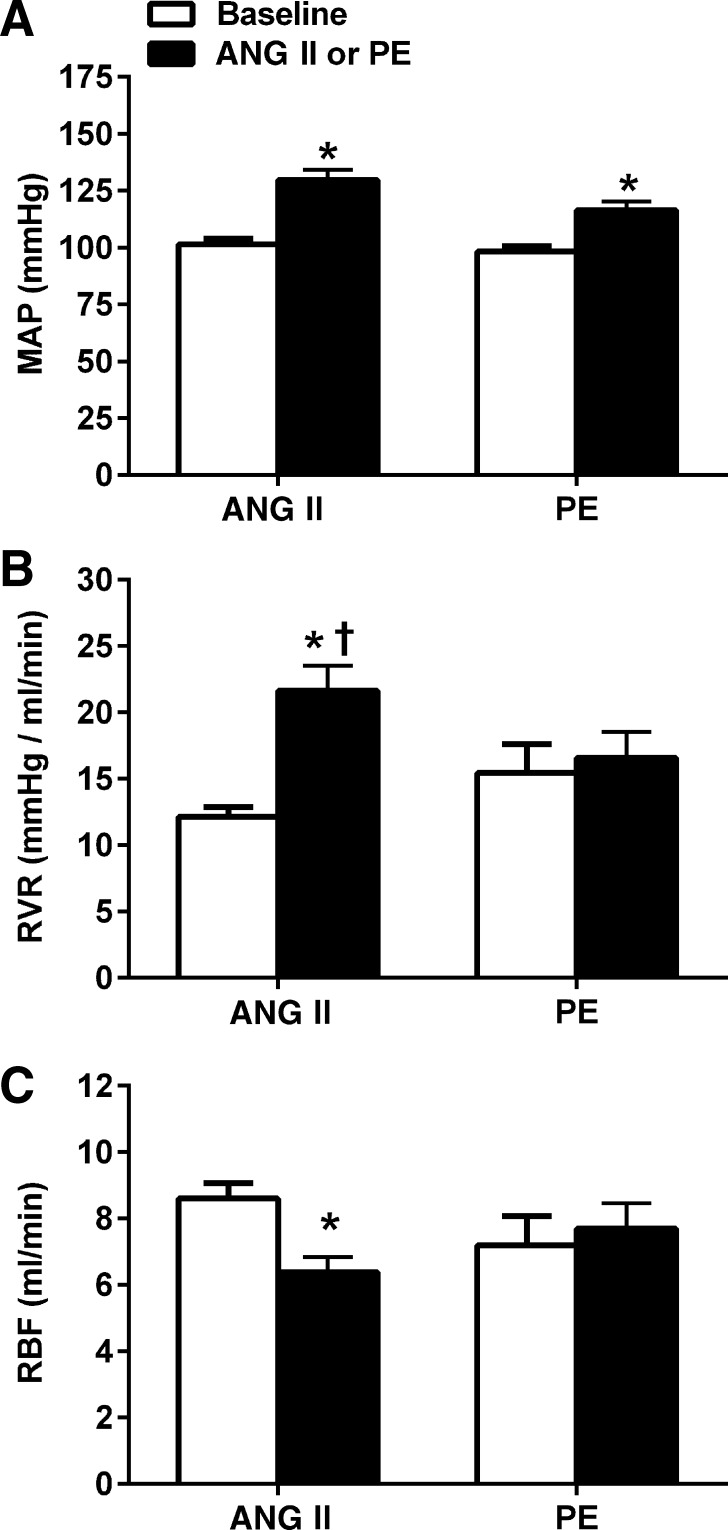
The average MAP, RVR, and RBF obtained from the simultaneous BP-RBF recordings (∼2 h) are presented in Fig. 2. ANG II and PE led to a statistically similar 28% and 19% increase in MAP, respectively; however, significant differences were noted in the RVR and RBF responses. Whereas RVR and RBF were not significantly altered during PE, ANG II led to a 62% increase in RVR (P < 0.05) and 21% decrease in RBF (P < 0.05) compared with baseline values. These data indicate that ANG II is a more potent renal vasoconstrictor in conscious rats compared with PE at similar pressor doses.
Fig. 2.
Renal hemodynamic responses at baseline and during chronic administration of ANG II (n = 11; 125 ng·kg−1·min−1) and PE (n = 8; 50 mg·kg−1·day−1) in conscious chronically instrumented rats. Despite similar increases in mean arterial pressure, only ANG II led to significant increases in renal vascular resistance (P < 0.001) and decreases in renal blood flow (RBF) (P < 0.05). Values are expressed as means ± SE. A: mean arterial pressure (MAP). B: renal vascular resistance (RVR). C: renal blood flow (RBF). *P < 0.05 vs. baseline. †P < 0.05 vs. PE-infused rats.
Ambient pulse pressure and renal pulse flow in ANG II- and PE-infused rats.
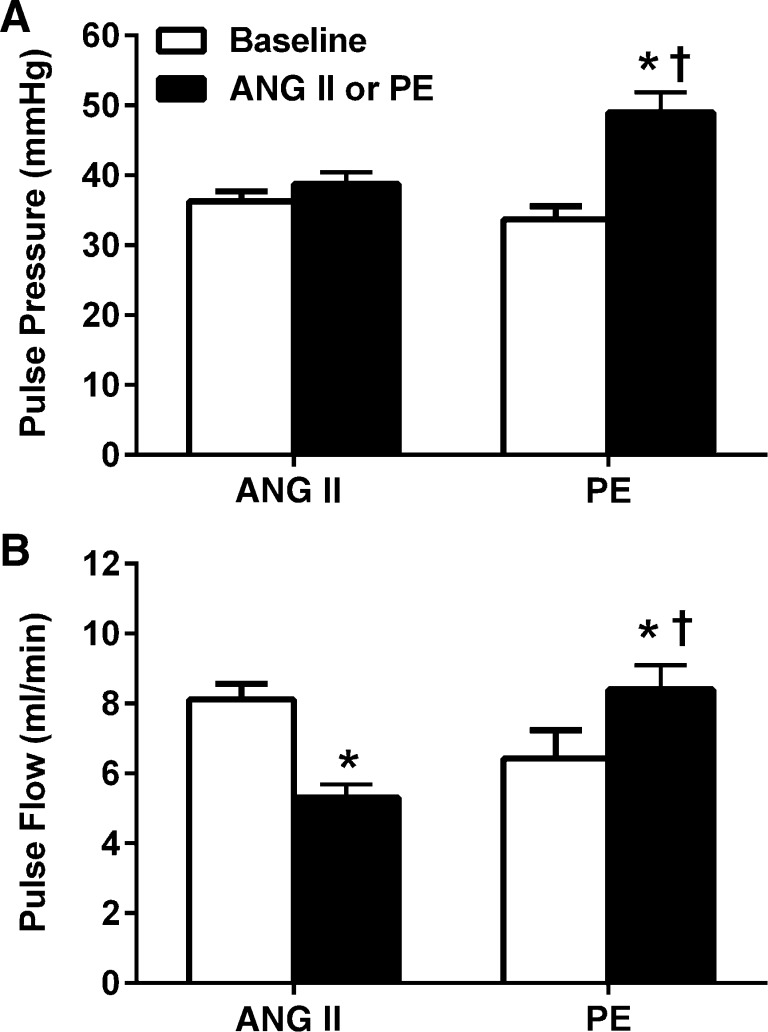
To gain additional insights into the effects of ANG II and PE on the renal vasculature, the changes in pulsatility of BP and RBF were evaluated (Fig. 3). As shown in Fig. 3, A and B, striking differences were noted between ANG II and PE with respect to both pulse pressure and pulse flow. PE led to a robust 46% increase in pulse pressure (P < 0.05) and 41% increase in pulse flow (P < 0.05). Conversely, ANG II led to a very modest 7% increase in pulse pressure (NS) but a significant 35% decrease in pulse flow (P < 0.05). Such data provide further evidence indicating significant differences with respect to the effects of ANG II and PE on the renal vasculature.
Fig. 3.
Pulse pressure and renal pulse flow at baseline and during chronic administration of ANG II (n = 11; 125 ng·kg−1·min−1) and PE (n = 8; 50 mg·kg−1·day−1) in conscious chronically instrumented rats. PE led to significant increases in both pulse pressure and renal pulse flow compared with baseline (P < 0.001) and ANG II-infused rats (P < 0.001). ANG II did not have any significant effects on pulse pressure but led to a significant reduction in pulse flow (P < 0.001 vs. baseline). Values are expressed as means ± SE. A: pulse pressure. B: renal pulse flow. *P < 0.05 vs. baseline. †P < 0.05 vs. ANG II-infused rats.
Time-varying BP-RBF relationships in conscious ANG II- and PE-infused rats.
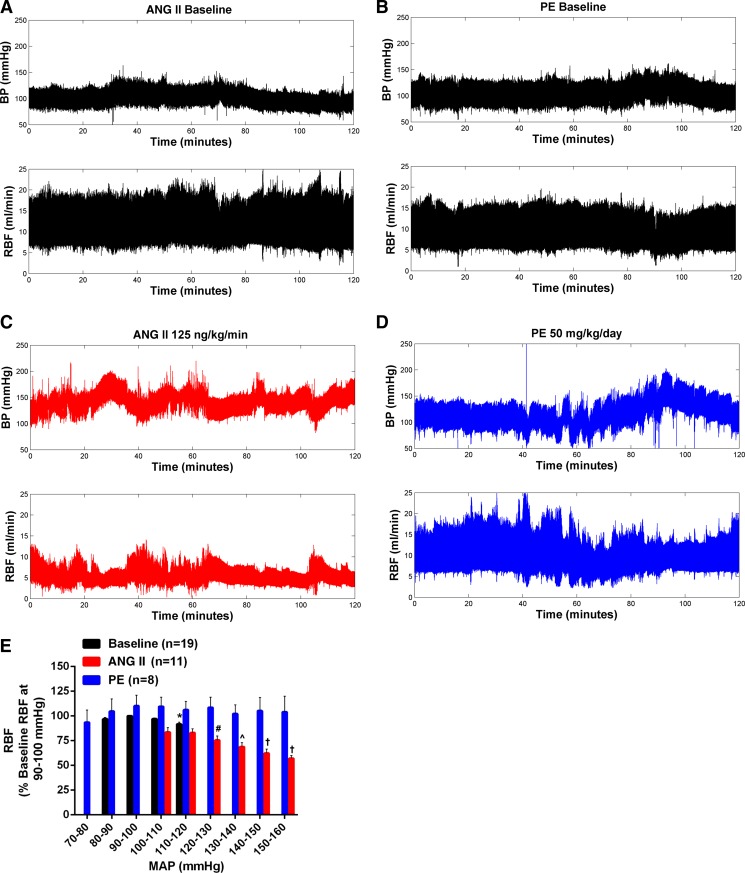
Fig. 4 shows the results of the BP-RBF segment analysis at baseline and during ANG II and PE infusion in conscious rats. Because no significant differences in the BP-RBF relationships were observed at baseline between ANG II and PE groups, these data have been averaged between groups for presentation clarity in Fig. 4E, which shows the BP-RBF analysis for 10-s segments and 10-mmHg BP bins. Similar patterns of BP-RBF relationships were observed regardless of the length of the time window (1–100 s) or width of the BP bin (5–20 mmHg) (data not shown). During baseline recordings, RBF was well preserved over the smaller range of BP fluctuations (representative images shown in Fig. 4, A and B), although a modest, but significant, 9% decrease in RBF was observed during periods of BP elevations (110–120 mmHg) compared with RBF when BP was within baseline levels (90–100 mmHg). During ANG II and PE administration, there was considerable time-dependent variability in the BP-RBF relationships observed between animals, on different days in the same animal, and even more strikingly within a single data record of an individual animal. Although the time-dependent variability in BP was considerably exaggerated by both PE and ANG II, the pattern of associated RBF changes was very different. In the case of PE, RBF was largely similar across a wide range of spontaneous changes in BP (Fig. 4E with representative image shown in Fig. 4D). Conversely, RBF exhibited significant reductions during periods of BP increases in ANG II-infused rats (Fig. 4E with representative image shown in Fig. 4C). In summary, the BP-RBF bin analysis demonstrates that chronic ANG II infusion is associated with exaggerated renal vasoconstriction during episodes of spontaneous BP increases.
Fig. 4.
Effects of ANG II and PE on BP-RBF relationships in conscious chronically instrumented rats. Representative BP-RBF recordings at baseline (A and B) and during ANG II (C) or PE (D). When expressed as % change from RBF when BP was within 90–100 mmHg (E), only ANG II led to marked reductions in RBF during episodes of BP elevations. No differences were seen in BP-RBF relationships during baseline recordings in both groups so these data have been averaged. Values are expressed as means ± SE. *P < 0.05 vs. respective RBF at baseline BP (90–100 mmHg). #P < 0.05 vs. respective RBF at 100–110- and 110–120-mmHg BP bins. ^P < 0.05 vs. respective RBF at 100–110-, 110–120-, and 120–130-mmHg BP bins. †P < 0.05 vs. respective RBF at 100–110-, 110–120-, 120–130-, and 130–140-mmHg BP bins.
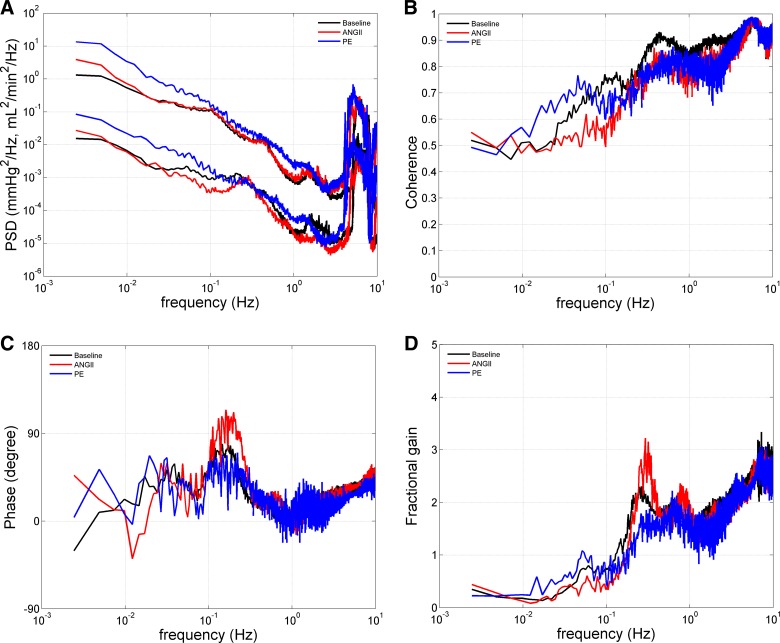
Transfer functions in conscious ANG II- and PE-infused rats.
Transfer function analyses between fluctuations in BP (input) and RBF (output) from the 2-h BP-RBF recordings are summarized in Table 2. No significant differences in any component of the transfer functions were detected at baseline between ANG II and PE groups; therefore, these data have been averaged between groups for presentation clarity (Fig. 5). We have previously suggested that the use of such transfer function analysis has limitations with respect to the assessment of autoregulatory capacity; however, it may allow one to investigate the effects of various hormonal and/or neural inputs on the operational characteristics of the two major components of autoregulation, the TGF, and the myogenic response (2, 8, 29). Significant effects were noted with respect to the myogenic mechanism. The only directionally similar effect elicited by ANG II and PE was an increase in the operating frequency of the myogenic mechanism, as evidenced by a rightward shift in the signature resonance peak of the myogenic mechanism in both groups (Fig. 5D). ANG II administration resulted in several changes that are consistent with a strengthening of the myogenic mechanism and thus a reduction of transmission of BP fluctuations to the kidney. For example, ANG II led to significant increases in the fractional gain of the signature resonance peak of the myogenic mechanism (Fig. 5D), phase peak (Fig. 5C), and a tendency (P = 0.08) to increase the slope-of-gain reduction associated with the signature resonance peak of the myogenic mechanism (Fig. 5D). ANG II also led to a significant decrease in the coherence at 0.05–0.1 Hz (Fig. 5B), again indicating enhanced buffering of renal microvasculature pressure transmission. Conversely, PE led to a significant reduction in the fractional gain of the signature resonance peak of the myogenic mechanism (Fig. 5D). Furthermore, directionally opposite changes compared with ANG II were observed in the phase peak (Fig. 5C) and slope-of-gain reduction (Fig. 5D) with minimal changes observed in the coherence at 0.05–0.1 Hz (Fig. 5B). These changes are consistent with a reduced buffering of systemic BP fluctuations to the renal vasculature during PE administration compared with baseline (26). The only significant change observed with respect to the TGF response was the directionally opposite effects of ANG II to attenuate and PE to potentiate the fractional gain of the TGF signature resonance peak. In summary, the transfer function analysis suggests that ANG II enhances the myogenic response of the renal microvasculature to oscillations of BP, whereas such changes were not observed during PE at the doses administered.
Table 2.
Effects of ANG II and PE on transfer functions related to myogenic and TGF autoregulatory mechanisms
| Myogenic |
TGF |
||||||
|---|---|---|---|---|---|---|---|
| Frequency, Hz | Fractional Gain | Phase Peak, degrees | Coherence at 0.05-0.1 Hz | Slope of Gain Reduction, db/decade | Frequency, Hz | Fractional Gain | |
| Baseline | 0.23 ± 0.01 | 2.4 ± 0.2 | 78 ± 7 | 0.7 ± 0.03 | 42 ± 10 | 0.05 ± 0.001 | 0.7 ± 0.1 |
| ANG II | 0.27 ± 0.01 | 3.0 ± 0.2* | 106 ± 6* | 0.5 ± 0.03* | 62 ± 6 | 0.04 ± 0.003 | 0.6 ± 0.1 |
| Baseline | 0.24 ± 0.01 | 2.3 ± 0.2 | 75 ± 7 | 0.7 ± 0.05 | 40 ± 3 | 0.04 ± 0.002 | 0.6 ± 0.1 |
| PE | 0.25 ± 0.01 | 1.6 ± 0.1*† | 59 ± 3† | 0.7 ± 0.06† | 24 ± 12† | 0.04 ± 0.003 | 0.8 ± 0.1† |
| ANOVA effects | |||||||
| Baseline vs. Drug | P < 0.005 | NS | NS | P < 0.05 | NS | NS | NS |
| ANG II vs. PE | NS | P < 0.005 | P < 0.005 | NS | P < 0.05 | NS | NS |
| Interaction | P = 0.07 | P < 0.001 | P < 0.005 | P < 0.05 | P < 0.05 | NS | P < 0.05 |
Values are expressed as means ± SE. ANG II (n = 11; 125 ng·kg−1·min−1), PE (n = 8; 50 mg·kg−1·day−1). TGF, tubuloglomerular feedback; NS, not significant.
P < 0.05 vs. baseline.
P < 0.05 vs. ANG II.
Fig. 5.
Effects of ANG II (n = 11) and PE (n = 8) on transfer functions in conscious chronically instrumented rats. Because no significant differences were observed with respect to the transfer functions obtained during baseline recordings, these data have been averaged between groups for presentation clarity. A: BP and RBF power spectral density (PSD) at baseline and during ANG II (125 ng·kg−1·min−1) and PE (50 mg·kg−1·day−1) in units of (mmHg)2/Hz for BP and (ml/min)2/Hz for RBF. Directionally opposite effects consistent with an augmentation and attenuation of the renal myogenic mechanism during ANG II and PE administration, respectively, were observed in the coherence at 0.05–0.1 Hz (B), phase peak (C), and signature resonance peak of the myogenic mechanism (D). Conversely, PE administration was associated with an increase in the signature resonance peak of the TGF mechanism, while a decrease was observed during ANG II (D). See text and Table 2 for statistical analysis.
DISCUSSION
The precise pathogenesis of renal damage in hypertensive states, including those characterized by an increased activity of RAAS, continues to be the subject of ongoing investigations. The present studies in conscious chronically instrumented rats provide new insights and demonstrate marked differences in the ambient BP, renal hemodynamic, and BP-RBF relationship response to modestly pressor doses of ANG II and PE. Such data may have considerable pathophysiological implications for the investigation of mechanisms of hypertension-induced renal injury.
Ambient BP profiles.
Although the pathogenic significance of BP variability patterns remains controversial (25, 35, 60, 74), striking differences were observed in 24-h ambient BP profiles in ANG II vs. PE-infused animals. Whereas ANG II led to more robust increases in the average 24-h BP (DC BP Power), PE resulted in significantly greater BP lability (AC BP power), which was primarily manifested within two frequency bandwidths: 1) BP fluctuations due to the heart beat (∼6 Hz) and 2) the slower BP fluctuations occurring within the VLF band (≤0.1 Hz). Increased amplitude of BP fluctuations at the heart beat frequency (i.e., pulse pressure) is largely dependent on stroke volume and arterial compliance (4, 50). Because PE is a selective α1-adrenergic agonist with minimal direct cardiac effects, the PE-induced amplification of pulse pressure was most likely mediated by a reduced arterial compliance (17, 55). This is consistent with the association between increased arterial stiffness (i.e., reduced compliance) and pulse pressure amplification that is observed with aging (63).
The most dramatic increase in AC BP power in PE-infused rats was observed within the VLF bandwidth. Compared with the more frequently investigated and better understood origin of the LF and HF ranges of BP variability, the potential sources of BP variability within the VLF range are only beginning to emerge. A recent study by Radaelli et al. (72) reported that α2 adrenergic receptors contribute to VLF BP oscillations via activation of L-type Ca2+- and Ba2+-sensitive K+ channels. A subsequent study by Langager et al. (53) similarly reported a role of sympathetically mediated activation of L-type Ca2+ channels in the generation of VLF BP oscillations. Conversely, the increased VLF BP variability observed in PE-infused rats could have resulted from a reduction in BP buffering. For example, previous studies have reported an increase in VLF BP oscillations following baroreflex denervation (46, 49). Administration of PE may be analogous to baroreflex denervation in that baroreflex-mediated changes in peripheral vascular resistance may be largely attenuated because of the chronic stimulation of α1-adrenergic receptors. Other factors have also been reported to modulate VLF BP oscillations, including the RAAS and nitric oxide (15, 22). To this extent, our data are also in agreement with these previous studies, as evidenced by the ANG II-induced increase in VLF BP power, although to a significantly lesser extent compared with PE-infused rats.
Contrary to the effects of PE on BP oscillations within the VLF range, PE administration led to a decrease in the amplitude of oscillations within the LF range (0.1–1 Hz); whereas ANG II increased the magnitude of such BP oscillations. The reasons remain to be defined (45, 54); however, vascular myogenic responses have been implicated in the generation and buffering of LF BP fluctuations (1, 24, 60, 66). Of particular relevance, parallel differences between ANG II and PE were observed with respect to the fractional gain of the myogenic response in the renal transfer function data (see Table 2).
Ambient renal hemodynamics.
In the present study, continuous ANG II administration led to sustained reductions in RBF measured over several days. Although the acute renal vasoconstrictive effects of ANG II have been extensively documented in rodents, previous studies at single time points after chronic ANG II infusion have failed to show significant RBF reductions in anesthetized or conscious rats, possibly because of the counteracting effects of increased perfusion pressure (67, 68, 83). Brands et al. (13), however, recently reported a reduction of RBF measured over several days in conscious ANG II-infused mice, although the dose of ANG II required to elicit such RBF responses was significantly higher than that administered to rats in the present study, and the effects on time-dependent variability in BP-RBF relationships were not reported. In any event, given the spontaneous and often large fluctuations of arterial BP and RBF that were found in the present study, acute single assessments of hemodynamics may not be sufficient to fully characterize the chronic effects of ANG II on RBF.
In contrast to ANG II-infused rats, RBF was unchanged from baseline levels during PE infusion. Although the renal vasoconstrictive effects of catecholamines are well documented (38, 48), there is evidence that the effects of adrenergic agents on renal vascular function are dependent on the dose, route of administration, and the state of the animal during investigation. For instance, Kleinjans et al. (52) demonstrated drastic reductions in glomerular filtration rate (GFR) and RBF during intrarenal infusion of high doses of norepinephrine (NE) in anesthetized but not conscious rats (52). Furthermore, intrarenal NE administration has been used to produce ischemic acute renal failure in anesthetized animals (14, 16). By contrast, increases in RBF were observed during acute intravenous and intrarenal administration of NE in conscious dogs (5). Although PE, unlike NE, acts mainly on the α-adrenergic receptors, the lack of reduction in RBF during PE administration in the present studies is consistent with such observations. Similarly, adrenergic agents, including PE, in appropriate doses have been used clinically to support BP without compromising RBF and renal function (21). Differential renal vascular responses to ANG II and NE have also been observed in humans (78).
Ambient pulse pressure and renal pulse flow.
The increase in renal pulse flow during PE was likely a secondary consequence of the PE-induced increase in pulse pressure, given there were no significant effects of PE on RBF (an important determinant of renal pulse flow). The decrease in pulse flow in ANG II-infused rats, thus, likely stemmed from the significant reduction in RBF. These data suggest that modification of vascular compliance may be an important target of sympathetically mediated α1-adrenergic stimulation. This observation is in agreement with previous studies that have found sympathetically mediated changes in vascular compliance, independent of diameter, in conduit vessels (11, 61, 76, 86). Alternatively, the greater reduction in HR observed in PE- vs. ANG II-infused rats could have contributed, in part, to parallel differences in stroke volume and, thus, pulse pressure.
Time-dependent variability in BP-RBF relationships.
A striking time-associated variability of RBF was observed at any given BP in individual rats in the present study, which has also been observed previously (58, 70, 81). Nevertheless, despite this variability in RBF, the BP-RBF bin analysis in the present study (Fig. 4E) and in the previous study of Pires et al. (69) revealed an overall constancy of average RBF over the autoregulatory range of pressures not only during single recordings but over several days as well. Such a pattern observed at baseline and even after PE is consistent with the concept of a successful achievement of overall RBF autoregulation. However, when the beat-to-beat BP-RBF relationships are examined, Skarlatos et al. (81) reported that an autoregulatory like pattern was observed, at the most, 35% of the time. Qualitatively similar BP-RBF patterns were observed when segments of 10 or 100 s were used, as also noted in our analyses. Likewise, Pires et al. (70) concluded that an autoregulatory like pattern of spontaneous BP-RBF relationships could not be modeled in normal, sinoaortic baroreceptor-innervated rats using 1-s segment lengths and 2.5-mmHg BP bins. In a similar manner, we have previously noted that assessment of dynamic autoregulation in conscious rats with intact or reduced renal mass yields estimates that are significantly lower than those obtained during steady-state autoregulatory studies (8). We have, therefore, suggested that such spontaneous fluctuations in RBF represent the composite responses to various neurohumoral inputs at multiple frequencies, thus making it difficult to separate, define, and quantify autoregulatory responses in conscious rats (8, 58). Similar concerns were noted by Skarlatos et al. (81). We have additionally suggested that while autoregulatory responses to BP changes provide one mechanism for stabilization of RBF, there are likely other mechanisms that may also mediate an overall stability and set point regulation of RBF independent of autoregulatory responses (58). These inferences were based on the lack of differences in ambient RBF and GFR in 5/6 ablated rats despite large differences in BP and autoregulatory capacity (31, 58). We had also noted that in the absence of such compensatory set point regulation, antihypertensive therapy in individuals with autoregulatory impairment would not be feasible and would result in acute renal hypoperfusion and declines in GFR (58). The identity of such mechanisms remains to be identified, but it seems to be intimately related to body surface area and metabolic needs (30).
Given these considerations, the striking progressive and consistent reductions in RBF with increasing BP in ANG II-infused rats indicates qualitative and/or quantitative differences in the interactions between various factors that influence time-associated RBF variability in these rats vs. PE-infused rats. For example, it is possible that increases in sympathetic activity may be contributing to the episodes of exaggerated RBF responses during BP elevations in ANG II-infused rats (19, 59, 87). A similar mechanism may be responsible for the small, but significant, decreases in RBF observed during BP elevations in control rats. Conversely, the absence of such a pattern in the PE-infused rats may be due to a reduction of baroflex input due to constant α1-adrenergic stimulation. However, it is also possible that the interaction between ANG II and the myogenic response triggered by increasing BP results in an exaggerated and dysregulated vasoconstriction (34, 41, 81). Such observations are consistent with the previously described enhancement of myogenic activity by ANG II (36, 51, 62). In contrast, others have reported reduced autoregulatory responses in the vasoconstricted renal vasculature of anesthetized ANG II-infused rats (33, 37, 83). However, it is possible that additional interactions may occur under anesthesia and not be replicative of the responses in conscious animals. Of particular relevance, the implications of impaired autoregulation for renal microvasculature BP transmission and tissue injury are directionally opposite in a vasoconstricted (i.e., ANG II) vs. a vasodilated (i.e., remnant kidney) vasculature (7, 10). Further support for our interpretations of the interaction with ANG II and the myogenic response comes from the transfer function data obtained during dynamic autoregulation studies.
Transfer functions.
Significant changes in transfer functions were observed with both ANG II and PE. The most robust effects were seen on the myogenic response. Directionally opposite effects of ANG II and PE were observed on the myogenic resonance peak with ANG II potentiating and PE attenuating it. These changes were paralleled by significant changes in phase peak, coherence, and slope-of-gain reduction. Just et al. (47) similarly reported a significant potentiation of the myogenic resonance peak during ANG II infusion in conscious dogs. These effects of ANG II on the myogenic mechanism are consistent with the observed pattern of the BP-RBF relationships that we observed. The disproportionate and exaggerated reductions in RBF during acute BP elevations indicate a possible dysregulated and ANG II-sensitized myogenic mechanism. The underlying cellular mechanisms by which ANG II potentiates renal myogenic activity have been previously described and include the modulation of PKC (51) and G protein-coupled receptor (36, 62) pathways. By contrast, the cellular mechanisms responsible for the apparently reduced myogenic responses after PE remain to be elucidated. In contrast to the myogenic response noted in the conscious rat (present studies) and in the conscious dog (47), Saeed et al. (75) observed a reduced slope of gain reduction and diminished phase peak in anesthetized rats following 2 wk of ANG II at a higher dose (250 ng·kg−1·min−1) than used in the present study. These effects were further exaggerated in ANG II-infused rats fed a high-sodium diet (4% NaCl). The reasons for such differences remain to be defined but may involve the differences in dosage and duration of ANG II, dietary salt intake, and the potential effects of anesthesia.
Both ANG II and PE administration led to a significant increase in the operating frequency of the myogenic mechanism. Just et al. (47) did not observe a significant effect of ANG II on the operating frequency of the myogenic mechanism in conscious, resting dogs. While the reasons for such differences between studies are not readily apparent, some potential explanations include acute vs. chronic ANG II administration, differences in the level of BP achieved during ANG II, as well as species differences. However, an enhanced myogenic response during nitric oxide synthase (NOS) blockade has previously been reported (84). Furthermore, a subsequent study demonstrated that perfusion pressure directly modulates the frequency response of the myogenic mechanism and that the increased operating frequency of the myogenic mechanism observed during NOS inhibition was a consequence of the concurrent elevation in BP (85). This provides a potential mechanism, whereby both ANG II and PE can increase the operating frequency of myogenic mechanism, as shown in the present study, although future studies are required to validate such pressure-induced pathways, as well as the underlying mechanisms.
The observed effects of ANG II and PE on the TGF mechanism in the present study are at some variance to previous results. In contrast to the attenuation of the TGF resonance peak by ANG II, Just et al. (47) noted a trend for ANG II to enhance and angiotensin-converting enzyme inhibition to attenuate the TGF resonance peak, although statistical significance was not achieved. Similarly, an enhancement of TGF responses by ANG II has been noted during micropuncture studies (12, 64, 79). The mechanisms responsible for such differences remain to be defined. Similarly, in contrast to the significant enhancement of the TGF resonance peak by PE in the present study in conscious rats, no effects were seen during intravenous or intrarenal PE administration in anesthetized rats (80). Such effects of PE on the TGF resonance peak are consistent with either an enhancement in gain or a reduction in the damping of the TGF response and will require future studies to elucidate the underlying mechanisms.
In summary, the present studies demonstrate substantial differences in the ambient BP profiles and renal vascular responses at comparable pressor doses of ANG II and PE. The renal vascular effects of ANG II in conscious rats in the present study indicate a potential attenuation of intrarenal BP transmission, thereby counteracting its BP-independent deleterious effects. These results are consistent with previous studies reporting a very modest degree of hypertensive glomerular injury in chronic ANG II-infused rodents (42, 65, 71, 77). Future studies investigating the quantitative relationships between chronic radiotelemetrically measured BP and renal damage will be needed to validate these insights.
GRANTS
This work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases Grants DK-40426 and DK-61653, by a Merit Review Award (K.A.G.) and by a Career Development Award 1IK2BX001285 (A.J.P.) from the Office of Research and Development of the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.J.P., K.A.G., J.L., G.A.W., and A.K.B. analyzed data; A.J.P., K.A.G., G.A.W., and A.K.B. interpreted results of experiments; A.J.P., K.A.G., J.L., G.A.W., and A.K.B. prepared figures; A.J.P., K.A.G., and A.K.B. drafted manuscript; A.J.P., K.A.G., G.A.W., and A.K.B. edited and revised manuscript; A.J.P., K.A.G., J.L., G.A.W., and A.K.B. approved final version of manuscript; K.A.G. and A.K.B. conception and design of research; K.A.G. performed experiments.
ACKNOWLEDGMENTS
We acknowledge Theresa Herbst for technical support and Martha Prado for secretarial support.
REFERENCES
- 1.Abu-Amarah I, Ajikobi DO, Bachelard H, Cupples WA, Salevsky FC. Responses of mesenteric and renal blood flow dynamics to acute denervation in anesthetized rats. Am J Physiol Regul Integr Comp Physiol 275: R1543–R1552, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Abu-Amarah I, Bidani AK, Hacioglu R, Williamson GA, Griffin KA. Differential effects of salt on renal hemodynamics and potential pressure transmission in stroke-prone and stroke-resistant spontaneously hypertensive rats. Am J Physiol Renal Physiol 289: F305–F313, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Aizawa T, Ishizaka N, Kurokawa K, Nagai R, Nakajima H, Taguchi J, Ohno M. Different effects of angiotensin II and catecholamine on renal cell apoptosis and proliferation in rats. Kidney Int 59: 645–653, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Alfie J, Waisman GD, Galarza CR, Camera MI. Contribution of stroke volume to the change in pulse pressure pattern with age. Hypertension 34: 808–812, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Anderson WP, Korner PI, Selig SE. Mechanisms involved in the renal responses to intravenous and renal artery infusions of noradrenaline in conscious dogs. J Physiol 321: 21–30, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bidani AK, Griffin KA. Long-term renal consequences of hypertension for normal and diseased kidneys. Curr Opin Nephrol Hypertens 11: 73–80, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension 44: 595–601, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Bidani AK, Hacioglu R, Abu-Amarah I, Williamson GA, Loutzenhiser R, Griffin KA. “Step” vs“ dynamic” autoregulation: implications for susceptibility to hypertensive injury. Am J Physiol Renal Physiol 285: F113–F120, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Bidani AK, Picken M, Hacioglu R, Williamson G, Griffin KA. Spontaneously reduced blood pressure load in the rat streptozotocin-induced diabetes model: potential pathogenetic relevance. Am J Physiol Renal Physiol 292: F647–F654, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA. Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens 22: 1–9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boutouyrie P, Lacolley P, Girerd X, Beck L, Safar M, Laurent S. Sympathetic activation decreases medium-sized arterial compliance in humans. Am J Physiol Heart Circ Physiol 267: H1368–H1376, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Braam B, Koomans HA. Nitric oxide antagonizes the actions of angiotensin II to enhance tubuloglomerular feedback responsiveness. Kidney Int 48: 1406–1411, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Brands MW, Banes-Berceli AK, Inscho EW, Al-Azawi H, Allen AJ, Labazi H. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension 56: 879–884, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conger JD, Robinette JB, Guggenheim SJ. Effect of acetylcholine on the early phase of reversible norepinephrine-induced acute renal failure. Kidney Int 19: 399–409, 1981 [DOI] [PubMed] [Google Scholar]
- 15.Cordero JJ, Gonzalez J, Feria M. Effects of N omega-monomethyl-l-arginine on short-term RR interval and systolic blood pressure oscillations. J Cardiovasc Pharmacol 24: 323–327, 1994 [PubMed] [Google Scholar]
- 16.Cronin RE, Erickson AM, de Torrente A, McDonald KM, Schrier RW. Norepinephrine-induced acute renal failure: a reversible ischemic model of acute renal failure. Kidney Int 14: 187–190, 1978 [DOI] [PubMed] [Google Scholar]
- 17.Dabire H, Lacolley P, Chaouche-Teyara K, Fournier B, Safar ME. Relationship between arterial distensibility and low-frequency power spectrum of blood pressure in spontaneously hypertensive rats. J Cardiovasc Pharmacol 39: 98–106, 2002 [DOI] [PubMed] [Google Scholar]
- 18.deBlois D, Schwartz SM, van Kleef EM, Su JE, Griffin KA, Bidani AK, Daemen MJ, Lombardi DM. Chronic alpha 1-adrenoreceptor stimulation increases DNA synthesis in rat arterial wall. Modulation of responsiveness after vascular injury. Arterioscler Thromb Vasc Biol 16: 1122–1129, 1996 [DOI] [PubMed] [Google Scholar]
- 19.DiBona GF. Nervous kidney. Interaction between renal sympathetic nerves and the renin-angiotensin system in the control of renal function. Hypertension 36: 1083–1088, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Galis ZS, Thrasher T, Reid DM, Stanley DV, Oh YS. Investing in high blood pressure research: a national institutes of health perspective. Hypertension 61: 757–761, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gooneratne NMS. UpToDate, Waltham, MA, www.uptodate.com, 2012 [Google Scholar]
- 22.Gouedard O, Blanc J, Gaudet E, Ponchon P, Elghozi JL. Contribution of the renin-angiotensin system to short-term blood pressure variability during blockade of nitric oxide synthesis in the rat. Br J Pharmacol 119: 1085–1092, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grady HC, Bullivant EM. Renal blood flow varies during normal activity in conscious unrestrained rats. Am J Physiol Regul Integr Comp Physiol 262: R926–R932, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Grassi G, Bertoli S, Seravalle G. Sympathetic nervous system: role in hypertension and in chronic kidney disease. Curr Opin Nephrol Hypertens 21: 46–51, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Grassi G, Bombelli M, Brambilla G, Trevano FQ, Dell'oro R, Mancia G. Total cardiovascular risk, blood pressure variability and adrenergic overdrive in hypertension: evidence, mechanisms and clinical implications. Curr Hypertens Rep 14: 333–338, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Griffin K, Polichnowski A, Licea-Vargas H, Picken M, Long J, Williamson G, Bidani A. Large BP-dependent and -independent differences in susceptibility to nephropathy after nitric oxide inhibition in Sprague-Dawley rats from two major suppliers. Am J Physiol Renal Physiol 302: F173–F182, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffin KA, Abu-Naser M, Abu-Amarah I, Picken M, Williamson GA, Bidani AK. Dynamic blood pressure load and nephropathy in the ZSF1 (fa/fa cp) model of type 2 diabetes. Am J Physiol Renal Physiol 293: F1605–F1613, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Griffin KA, Bidani AK. Progression of renal disease: renoprotective specificity of renin-angiotensin system blockade. Clin J Am Soc Nephrol 1: 1054–1065, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Griffin KA, Hacioglu R, Abu-Amarah I, Loutzenhiser R, Williamson GA, Bidani AK. Effects of calcium channel blockers on “dynamic” and “steady-state step” renal autoregulation. Am J Physiol Renal Physiol 286: F1136–F1143, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Griffin KA, Kramer H, Bidani AK. Adverse renal consequences of obesity. Am J Physiol Renal Physiol 294: F685–F696, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest 96: 793–800, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffin SA, Brown WC, MacPherson F, McGrath JC, Wilson VG, Korsgaard N, Mulvany MJ, Lever AF. Angiotensin II causes vascular hypertrophy in part by a non-pressor mechanism. Hypertension 17: 626–635, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Guan Z, Fuller BS, Yamamoto T, Cook AK, Pollock JS, Inscho EW. Pentosan polysulfate treatment preserves renal autoregulation in ANG II-infused hypertensive rats via normalization of P2X1 receptor activation. Am J Physiol Renal Physiol 298: F1276–F1284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan Z, Willgoss DA, Matthias A, Manley SW, Crozier S, Gobe G, Endre ZH. Facilitation of renal autoregulation by angiotensin II is mediated through modulation of nitric oxide. Acta Physiol Scand 179: 189–201, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Bjorklund-Bodegard K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Imai Y, Wang J, Ibsen H, O'Brien E, Staessen JA. Prognostic value of reading-to-reading blood pressure variability over 24 h in 8938 subjects from 11 populations. Hypertension 55: 1049–1057, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Hercule HC, Tank J, Plehm R, Wellner M, da Costa Goncalves AC, Gollasch M, Diedrich A, Jordan J, Luft FC, Gross V. Regulator of G protein signalling 2 ameliorates angiotensin II-induced hypertension in mice. Exp Physiol 92: 1014–1022, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Inscho EW, Imig JD, Deichmann PC, Cook AK. Candesartan cilexetil protects against loss of autoregulatory efficiency in angiotensin II-infused rats. J Am Soc Nephrol 10 Suppl 11: S178–S183, 1999 [PubMed] [Google Scholar]
- 38.Insel PA, Snavely MD. Catecholamines and the kidney: receptors and renal function. Annu Rev Physiol 43: 625–636, 1981 [DOI] [PubMed] [Google Scholar]
- 39.Izuhara Y, Nangaku M, Inagi R, Tominaga N, Aizawa T, Kurokawa K, van Ypersele de Strihou C, Miyata T. Renoprotective properties of angiotensin receptor blockers beyond blood pressure lowering. J Am Soc Nephrol 16: 3631–3641, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 139: 244–252, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Johnson PC. Autoregulation of blood flow. Circ Res 59: 483–495, 1986 [DOI] [PubMed] [Google Scholar]
- 42.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM. Renal injury from angiotensin II-mediated hypertension. Hypertension 19: 464–474, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Johnson RJ, Gordon KL, Suga S, Duijvestijn AM, Griffin K, Bidani A. Renal injury and salt-sensitive hypertension after exposure to catecholamines. Hypertension 34: 151–159, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med 346: 913–923, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Joles JA, Koomans HA. Causes and consequences of increased sympathetic activity in renal disease. Hypertension 43: 699–706, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Julien C, Zhang ZQ, Barres C. Role of vasoconstrictor tone in arterial pressure lability after chronic sympathectomy and sinoaortic denervation in rats. J Auton Nerv Syst 42: 1–10, 1993 [DOI] [PubMed] [Google Scholar]
- 47.Just A, Ehmke H, Wittmann U, Kirchheim HR. Role of angiotensin II in dynamic renal blood flow autoregulation of the conscious dog. J Physiol 538: 167–177, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Just A, Olson AJ, Whitten CL, Arendshorst WJ. Superoxide mediates acute renal vasoconstriction produced by angiotensin II and catecholamines by a mechanism independent of nitric oxide. Am J Physiol Heart Circ Physiol 292: H83–H92, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Just A, Wittmann U, Nafz B, Wagner CD, Ehmke H, Kirchheim HR, Persson PB. The blood pressure buffering capacity of nitric oxide by comparison to the baroreceptor reflex. Am J Physiol Heart Circ Physiol 267: H521–H527, 1994 [DOI] [PubMed] [Google Scholar]
- 50.Kelly RP, Tunin R, Kass DA. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ Res 71: 490–502, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Kirton CA, Loutzenhiser R. Alterations in basal protein kinase C activity modulate renal afferent arteriolar myogenic reactivity. Am J Physiol Heart Circ Physiol 275: H467–H475, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Kleinjans JC, Smits JF, Kasbergen CM, van Essen H, Struyker-Boudier H. An evaluation of renal function during intrarenal norepinephrine infusion in conscious rats. Renal Physiol 7: 243–250, 1984 [DOI] [PubMed] [Google Scholar]
- 53.Langager AM, Hammerberg BE, Rotella DL, Stauss HM. Very low-frequency blood pressure variability depends on voltage-gated L-type Ca2+ channels in conscious rats. Am J Physiol Heart Circ Physiol 292: H1321–H1327, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Levy BI, Benessiano J, Poitevin P, Safar ME. Endothelium-dependent mechanical properties of the carotid artery in WKY and SHR. Role of angiotensin converting enzyme inhibition. Circ Res 66: 321–328, 1990 [DOI] [PubMed] [Google Scholar]
- 55.Levy BI, Poitevin P, Safar ME. Effects of alpha 1-blockade on arterial compliance in normotensive and hypertensive rats. Hypertension 17: 534–540, 1991 [DOI] [PubMed] [Google Scholar]
- 56.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 57.Lombardi D, Gordon KL, Polinsky P, Suga S, Schwartz SM, Johnson RJ. Salt-sensitive hypertension develops after short-term exposure to angiotensin II. Hypertension 33: 1013–1019, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malpas SC, Leonard BL. Neural regulation of renal blood flow: a re-examination. Clin Exp Pharmacol Physiol 27: 956–964, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Mancia G. Short- and long-term blood pressure variability: present and future. Hypertension 60: 512–517, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Mangoni AA, Mircoli L, Giannattasio C, Mancia G, Ferrari AU. Effect of sympathectomy on mechanical properties of common carotid and femoral arteries. Hypertension 30: 1085–1088, 1997 [DOI] [PubMed] [Google Scholar]
- 62.Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 27: 3092–3103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 43: 1239–1245, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Mitchell KD, Navar LG. Enhanced tubuloglomerular feedback during peritubular infusions of angiotensins I and II. Am J Physiol Renal Fluid Electrolyte Physiol 255: F383–F390, 1988 [DOI] [PubMed] [Google Scholar]
- 65.Mori T, Cowley AW., Jr Role of pressure in angiotensin II-induced renal injury: chronic servo-control of renal perfusion pressure in rats. Hypertension 43: 752–759, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn PJ. Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int 65: 1568–1576, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Nishiyama A, Fujisawa Y, Fukui T, Rahman M, Kondo N, Ogawa Y, Fanzhu L, Guoxing Z, Kimura S, Abe Y. Role of nitric oxide in regional blood flow in angiotensin II-induced hypertensive rats. Hypertens Res 24: 421–427, 2001 [DOI] [PubMed] [Google Scholar]
- 68.Nishiyama A, Fukui T, Fujisawa Y, Rahman M, Tian RX, Kimura S, Abe Y. Systemic and regional hemodynamic responses to Tempol in angiotensin II-infused hypertensive rats. Hypertension 37: 77–83, 2001 [DOI] [PubMed] [Google Scholar]
- 69.Pires SL, Barres C, Sassard J, Julien C. Renal blood flow dynamics and arterial pressure lability in the conscious rat. Hypertension 38: 147–152, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Pires SL, Julien C, Chapuis B, Sassard J, Barres C. Spontaneous renal blood flow autoregulation curves in conscious sinoaortic baroreceptor-denervated rats. Am J Physiol Renal Physiol 282: F51–F58, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Polichnowski AJ, Cowley AW., Jr Pressure-induced renal injury in angiotensin II vs. norepinephrine-induced hypertensive rats. Hypertension 54: 1269–1277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Radaelli A, Castiglioni P, Centola M, Cesana F, Balestri G, Ferrari AU, Di Rienzo M. Adrenergic origin of very low-frequency blood pressure oscillations in the unanesthetized rat. Am J Physiol Heart Circ Physiol 290: H357–H364, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl S57–S65, 2005 [DOI] [PubMed]
- 74.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 375: 938–948, 2010 [DOI] [PubMed] [Google Scholar]
- 75.Saeed A, Dibona GF, Marcussen N, Guron G. High-NaCl intake impairs dynamic autoregulation of renal blood flow in ANG II-infused rats. Am J Physiol Regul Integr Comp Physiol 299: R1142–R1149, 2010 [DOI] [PubMed] [Google Scholar]
- 76.Salzer DA, Medeiros PJ, Craen R, Shoemaker JK. Neurogenic-nitric oxide interactions affecting brachial artery mechanics in humans: roles of vessel distensibility vs. diameter. Am J Physiol Regul Integr Comp Physiol 295: R1181–R1187, 2008 [DOI] [PubMed] [Google Scholar]
- 77.Sasser JM, Moningka NC, Cunningham MW, Jr, Croker B, Baylis C. Asymmetric dimethylarginine in angiotensin II-induced hypertension. Am J Physiol Regul Integr Comp Physiol 298: R740–R746, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schachinger H, Klarhofer M, Linder L, Drewe J, Scheffler K. Angiotensin II decreases the renal MRI blood oxygenation level-dependent signal. Hypertension 47: 1062–1066, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Schnermann J, Briggs JP. Single nephron comparison of the effect of loop of Henle flow on filtration rate and pressure in control and angiotensin II-infused rats. Miner Electrolyte Metab 15: 103–107, 1989 [PubMed] [Google Scholar]
- 80.Shi Y, Wang X, Chon KH, Cupples WA. Tubuloglomerular feedback-dependent modulation of renal myogenic autoregulation by nitric oxide. Am J Physiol Regul Integr Comp Physiol 290: R982–R991, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Skarlatos S, Metting PJ, Britton SL. Spontaneous pressure-flow patterns in the kidney of conscious rats. Am J Physiol Heart Circ Physiol 265: H2151–H2159, 1993 [DOI] [PubMed] [Google Scholar]
- 82.Walker LA, Buscemi-Bergin M, Gellai M. Renal hemodynamics in conscious rats: effects of anesthesia, surgery, and recovery. Am J Physiol Renal Fluid Electrolyte Physiol 245: F67–F74, 1983 [DOI] [PubMed] [Google Scholar]
- 83.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol 279: F319–F325, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Wang X, Cupples WA. Interaction between nitric oxide and renal myogenic autoregulation in normotensive and hypertensive rats. Can J Physiol Pharmacol 79: 238–245, 2001 [PubMed] [Google Scholar]
- 85.Wang X, Loutzenhiser RD, Cupples WA. Frequency modulation of renal myogenic autoregulation by perfusion pressure. Am J Physiol Regul Integr Comp Physiol 293: R1199–R1204, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Zamir M, Goswami R, Salzer D, Shoemaker JK. Role of vascular bed compliance in vasomotor control in human skeletal muscle. Exp Physiol 92: 841–848, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Zimmerman BG, Sybertz EJ, Wong PC. Interaction between sympathetic and renin-angiotensin system. J Hypertens 2: 581–587, 1984 [DOI] [PubMed] [Google Scholar]