Abstract
NF-κB is a well-known transcription factor that is intimately involved with inflammation and immunity. We have previously shown that NF-κB promotes inflammatory events and mediates adverse cardiac remodeling following ischemia reperfusion (I/R). Conversely, others have pointed to the beneficial influence of NF-κB in I/R injury related to its anti-apoptotic effects. Understanding the seemingly disparate influence of manipulating NF-κB is hindered, in part, by current approaches that only indirectly interfere with the function of its most transcriptionally active unit, p65 NF-κB. Mice were generated with cardiomyocyte-specific deletion of p65 NF-κB. Phenotypically, these mice and their hearts appeared normal. Basal and stimulated p65 expression were significantly reduced in whole hearts and completely ablated in isolated cardiomyocytes. When compared with wild-type mice, transgenic animals were protected from both global I/R by Langendorff as well as regional I/R by coronary ligation and release. The protected, transgenic hearts had less cytokine activity and decreased apoptosis. Furthermore, p65 ablation was associated with enhanced calcium reuptake by the sarcoplasmic reticulum. This influence on calcium handling was related to increased expression of phosphorylated phospholamban in conditional p65 null mice. In conclusion, cardiomyocyte-specific deletion of the most active, canonical NF-κB subunit affords cardioprotection to both global and regional I/R injury. The beneficial effects of NF-κB inhibition are related, in part, to modulation of intracellular calcium homeostasis.
Keywords: ischemia-reperfusion, nuclear factor-κB, calcium, phospholamban
nf-κb consists of a family of DNA binding transcription factors that regulate expression of genes involved in the innate immune response, inflammation, cell survival, and proliferation (3). There are five NF-κB subunits: p65 (also called RelA), RelB, c-Rel, p50, and p52. These NF-κB protein subunits form homo- and heterodimer combinations that bind to gene promoter sequences and support transcription. Classic activation of NF-κB is controlled by inducible phosphorylation and degradation of the inhibitory proteins IκB (IκBα, IκBβ, IκBε) through the IκB kinase (IKK) complex (11). Once phosphorylated, IκB is targeted for polyubiquitination and ultimate degradation by the 26S proteasome (14). The degradation of phospho-IκB frees dimers of p50/p65 NF-κB, unmasking a nuclear localization sequence, and facilitates dimer trafficking to the nucleus and DNA binding to regulate gene expression. Albeit simplistic, within the classic or canonical pathway, NF-κB signaling is considered fulfilled with the binding of the p65 subunit onto a transcriptionally active gene (15).
NF-κB is an important transcription factor in most organ systems, and systemic inhibition might cloud any observations that might exist pertaining to the heart. Functional studies of NF-κB using transgenic approaches are limited by the 100% lethality of RelA-deficient mice. These animals die embryonically at Days 14–16 from massive liver degeneration secondary to uncontrolled apoptosis (5). Alternative genetic methods have been reported and have successfully demonstrated a causal role for NF-κB in the cardiac response to ischemia, endotoxin, and TNF (18). These animals indirectly inhibit NF-κB by cardiac-specific overexpression of IκBα. Nearly all of these studies include nuclear gel shifts of the p65 subunit to validate that NF-κB was upregulated and then downregulated following injury and subsequent therapy. However, NF-κB has several different subunits that are transcriptionally active. Although deletion of p50 decreases myocardial ischemia-reperfusion (I/R) injury (13), the effect of p50 loss-of-function in these animals is not specific to the heart. Despite reliance on p65 subunit assays to describe positive or negative effects of NF-κB activation, no group has specifically examined the role of this subunit with regards to I/R injury.
We and others have used various methods of inhibiting the NF-κB signaling pathways to attenuate an undesirable response to injury (32, 33, 36, 41, 48). Imbedded within these reports is the concept that NF-κB is responsible for creating a bad phenotype (i.e., an injured heart). However, this pattern overlooks that fact that NF-κB is a vital part of our innate immunity and promotes survival signals (47). Indeed, some evidence suggests that NF-κB promotes cardioprotection in models of ischemic preconditioning and coronary ligation (46, 49). Central to reconciling many of these issues is enhanced understanding of the role of its principal subunit. In this report, mice are generated and evaluated with cardiomyocyte-specific deletion of the p65 NF-κB unit. We demonstrate that cardiomyocyte p65 is not required for normal development and that its deletion provides cardioprotection when exposed to I/R. Furthermore, these mice appear resistant to cell death by reversing adverse calcium cycling associated with I/R injury.
MATERIAL AND METHODS
Generation of transgenic mice.
All animal studies were approved by the Institutional Animal Care and Use Committee, University of Utah. We applied the Cre-loxP system to create cardiomyocyte-specific ablation of p65 NF-κB. Mice homozygous for the floxed p65 alleles (p65flox/flox), which are viable and fertile without any obvious abnormalities (generously provided by A. S. Baldwin, University of North Carolina) (42), were crossed with transgenic mice (Jackson Laboratories) in which Cre recombinase is expressed under the control of α-myosin heavy chain promoter (MHCCre/+) (1). Both mice were backcrossed with C57BL/6J mice eight times. Functional Cre activation was examined by crossing p65-MHCCre mice with ROSA26 reporter mice and evaluating X-Gal staining in whole-mount embryos at E9.5.
Histology.
Hearts were harvested and fixed overnight at 10% formaldehyde. After progressive tissue dehydration with ethanol and CitriSolv, the heart samples were embedded in paraffin. Ten micrometer longitudinal or cross sections were subjected to hematoxylin-eosin stain or Periodic Acid-Schiff stain.
Heart and cell isolation Western blots.
Whole hearts or cardiomyocytes were isolated from 8- to 12-wk-old wild-type or p65 knockout mouse. Cardiomyocytes were stimulated with TNF-α (10 ng/ml). Western immunoblotting was performed on tissue and cell preparations with the following primary antibodies: IKKα, IKKβ, NF-κB p65, Phospho-NF-κB p65 (39), IκBα, GAPDH (Cell Signaling Technology), NF-κB p105/p50 (Millipore), sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a; Santa Cruz), phospholamban (Millipore), PLB phospho serine-16, and phospholamban (PLN) phospho Threonine-17 antiserum (Badrilla). Secondary peroxidase-conjugated antibody (Cell Signaling Technology) was applied.
Isolated heart preparation.
Hearts were excised and connected to the aortic cannula using a Langendorff apparatus to perfuse continuously aerated buffer solution at constant pressure. Left ventricular pressure was monitored with a water-filled balloon placed through the left atria appendage. Hearts were perfused for 20 min for stabilization as baseline and then subjected to 45 min of global no-flow ischemia, followed by 60 min reperfusion. Contractile function was continuously recorded and measured every 5 min. Effluent was collected, stored, and measured for LDH (lactate dehydrogenase) and creatine phosphokinase. Infarct size was measured after infusion with TTC (1% solution of 2,3,5-triphenyltetrazolium chloride dissolved in Krebs-Henseleit buffer).
Regional I/R.
I/R of the left anterior descending coronary artery (LAD) was performed as previously described (32). For infarct analysis, TTC staining was quantified 24 h postreperfusion to assess area at risk and area of infarct. Whole heart assessment of NF-κB-related proteins, including apoptosis terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling staining, were assessed in procured mouse hearts 6 h postreperfusion (31).
Calcium studies.
Adult mouse ventricular myocytes were isolated as previously described on laminin-coated chambers (4, 51). After superfusion in a normoxic solution (pO2 = 580 ± 2.0 mmHg), cells were bathed in an ischemic solution (pO2 = 20.5 ± 3.5 mmHg) for 5 min (8). Reperfusion was simulated by superfusion in the normal solution for an additional 5 min.
Cells were field-stimulated at 1-s cycle lengths, and calcium transients (CaTs) were recorded throughout the experiment. Intracellular Ca2+ was monitored with epifluorescence in single cardiomyocytes with the fluorescent indicator, fluo-4. The amplitude and the time course of the decay of CaT were well fit with a single exponential equation, and the time constant was used as measured of the rate of decline of CaT as previously described (12).
Statistics.
Comparisons between experimental groups were made using one-way ANOVA or repeated-measures ANOVA with Bonferroni post hoc testing using the statistical package Prism 4 (GraphPad, San Diego, CA). Data are expressed as means ± SE. Statistical significance was accepted at the 95% confidence interval.
RESULTS
Generation of CMC-specific p65 transgenic mice.
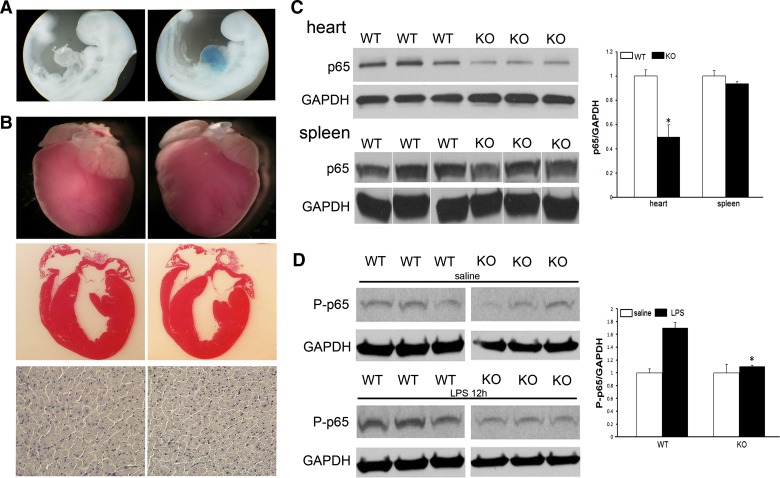
To examine the specific role of p65 NF-κB in cardiac biology, we generated mice with cardiomyocyte-specific deletion of the p65 subunit using the Cre-Lox system. To show the fidelity of our α-MHC-Cre-recombinase, mice were crossed with ROSA26 reporter mice. When stained for X-gal, expression of the transgene was only seen in the hearts of day 9.5 embyros, not in other structures or in the wild-type mice (Fig. 1A).
Fig. 1.
Characterization of mice with cardiomyocyte-specific deletion of p65 NF-κB. A: X-gal staining of wild-type (WT; left) and α-MHC-Cre (right) animals crossed with ROSA26 mice. B: gross, cross-sectional, and microscopic histology of WT and p65 knockout (KO) mice. C: Western blot analysis of whole heart and spleen homogenates for p65 and phospho-p65 NF-κB with densitometry normalized to GADPH expression (spleen blots modified to align with the heart sequence at top). D: mice were treated with intraperitoneal LPS or saline. Hearts were procured 12 h later, and Western blots performed for p65 and phospo-p65. Western blots are representative of 4–6 mice/group. *P < 0.05.
Mice were observed over the course of 12 mo and, when compared with wild-type mice, there were no difference in survival. After 3 mo, heart weight-to-body weight ratios between wild-type and knockout mice were similar (5.32 ± 0.52 vs. 4.96 ± 0.16, P = 0.16). In addition, gross and cross-sectional images appeared similar between groups (Fig. 1B). Structurally, there were also no differences in cardiomyocyte size (228.9 ± 12.1 μm2 vs. 206.3 ± 10.6 μm2, P = 0.16). Echocardiography demonstrated no differences in wall thickness, ventricular dimensions, are function (Table 1).
Table 1.
Baseline echocardiographic parameters comparing 10-wk-old wild-type mice and mice with cardiomyocyte-specific deletion of p65 NF-κB
| Wild-type | Knockout | |
|---|---|---|
| Ejection fraction, % | 48 ± 1.361 | 47 ± 1.265 |
| Left ventricular mass, mm | 93 ± 3.627 | 86 ± 1.471 |
| Fractional shortening, % | 30.193 ± 1.361 | 30.570 ± 1.120 |
| Posterior wall, mm | ||
| During diastole | 1.203 ± 0.041 | 1.198 ± 0.048 |
| During systole | 1.749 ± 0.956 | 0.772 ± 0.032 |
| Left ventricular internal dimension, mm | ||
| During diastole | 2.829 ± 0.109 | 2.830 ± 0.075 |
| During systole | 4.049 ± 0.089 | 4.049 ± 0.064 |
Values are means ± SE; n = 18 for wild-type and knockout groups.
We next looked at p65 protein expression in the respective mice. When compared with wild-type, in knockout animals expression of p65 was decreased. In these unstimulated animals, no changes in phosphorylated p65 were identified. As an internal control, no difference in splenic expression of p65 was observed (Fig. 1C). When stimulated with LPS, a known activator of NF-κB, phosphorylated p65 expression was increased in wild-type mice, but remained at its basal level, and comparatively reduced, in transgenic mice (Fig. 1D). Summarily, the phenotypic appearance of the transgenic animals appear normal despite having baseline decreases in myocardial p65 as well as reduced phosphorylation of p65 following activation.
Characterization of cardiomyocyte NF-κB activity.
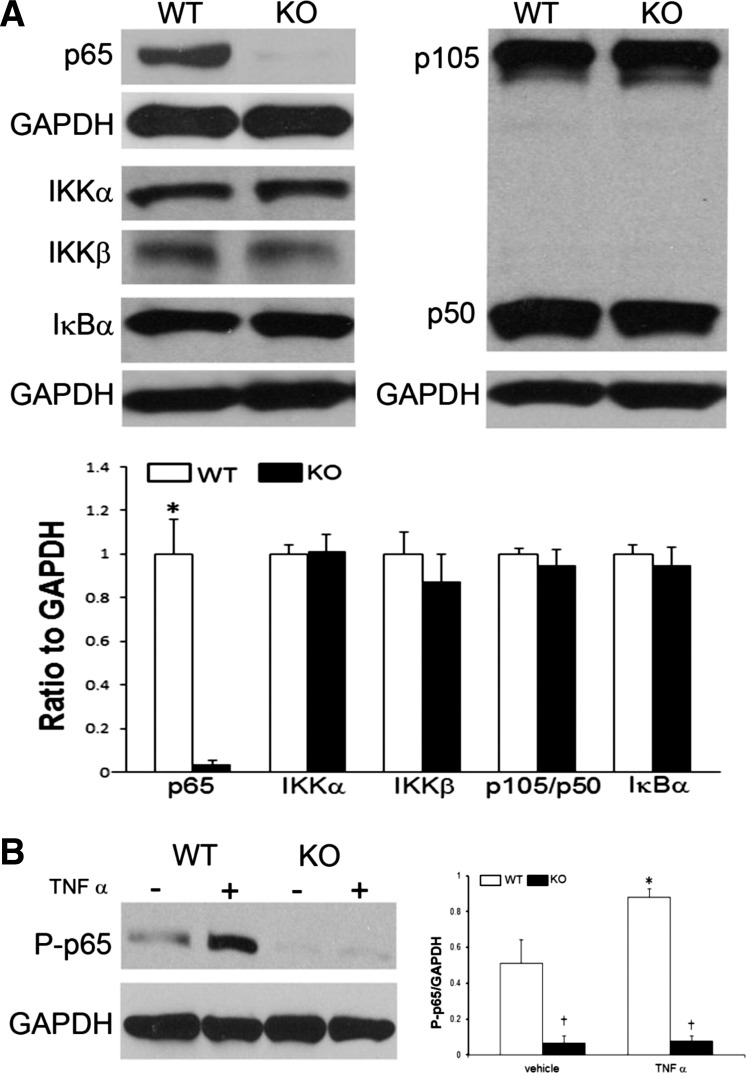
As demonstrated in Fig. 1C, total myocardial p65 was decreased but not completely ablated. This result is to be expected since there are other sources of heart-derived NF-κB, including fibroblasts, macrophages, and endothelial cells. To more purely test our transgenic mouse, we isolated cardiomyocytes and examined the NF-κB signaling pathway (Fig. 2A). No differences in protein expression were observed in its upstream kinase proteins, IKKβ and IKKα, or its heterodimer inhibitor, IκBα. In addition, there were no differences in expression of its companion subunits, p50 and p105. We next looked at the response of the cardiomyocytes to stress (Fig. 2B). After stimulation with TNF-α, wild-type cardiomyocytes expectantly increased phosphorylated p65 expression that was essentially ablated in the transgenic mice. Taken together, functional absence of cardiomyocyte p65 NF-κB did not result in any compensatory upregulation of its upstream regulator or sister protein subunits.
Fig. 2.
Cardiomyocyte NF-κB activity in vitro. A: cardiomyocytes were cultured, and Western blots were performed for NF-κB-related proteins including proximal kinase, IKKα, IKKβ, and IκBα, as well as its sister subunits, p50 and p105. B: cardiomyocytes were stimulated with TNF-α (10 ng/ml) for 30 min, and Western blots performed for phospho-p65. Western blots are representative of 4–6 mice/group (*vs. vehicle; †vs. WT; P < 0.05).
Cardiomyocyte deletion of p65 NF-κB and the response to global I/R.
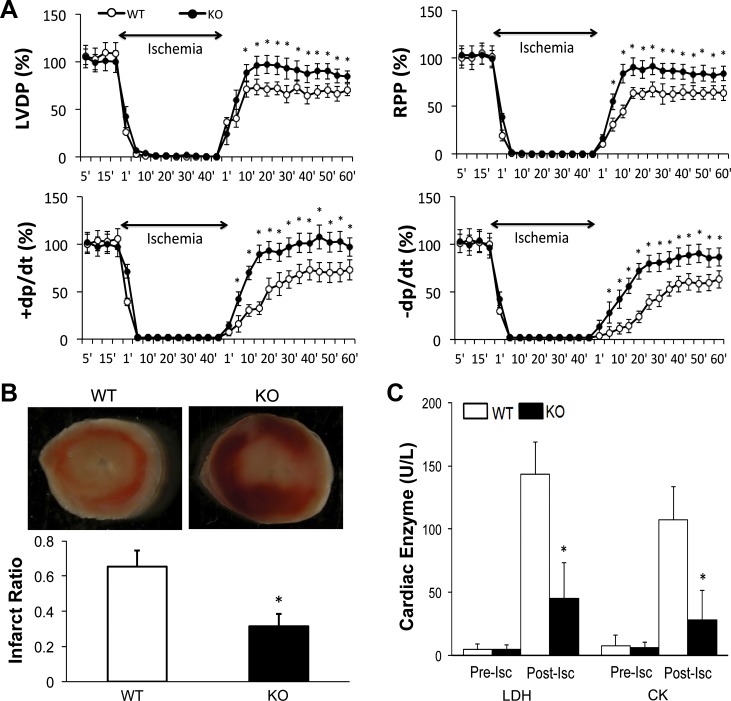
We next sought to physiologically examine the effects of cardiomyocyte p65 ablation on the response of the mouse heart to global I/R by ex vivo perfusion of isolated mouse hearts (Fig. 3). No differences were noted with respect to baseline and ischemic contraction. With reperfusion, the developed pressure started to recover after 1 min and reached stable levels after 10 min. When compared with that of wild-type mice, p65 deletion resulted in a faster and more robust return of myocardial function with reperfusion (wild-type, 64–73% recovery vs. knockout, 84–97%, P < 0.05). In addition, p65 deleted hearts had enhanced rate of contraction [rate of rise of LV pressure per unit time (+dP/dt)] and relaxation [rate of fall of LV pressure (−dP/dt)] following reperfusion.
Fig. 3.
Ex vivo perfusion of WT and CMC p65-deficient mouse hearts undergoing simulated global ischemia-reperfusion. A: pressure measurements during the experimental period as a percentage of contractile function. n = 7–9/group. LVDP, left ventricular (LV) developed pressure; RPP, rate pressure product; +dP/dt, rate of rise of LV pressure per unit time; −dP/dt, rate of fall of LV pressure. B: triphenyltetrazolium chloride (TTC) staining and infarct size depicted as a ratio of infarcted area to whole heart tissue (n = 5/group). C: myocardial enzyme release for lactate dehydrogenase (LDH) and creatine phosphokinase (LDH, CK). n = 7–9. Pre-isc, preischemic; post-isc, postischemic. For each experiment, *P < 0.05, vs. WT.
These changes in contractile function were associated with differences in myocardial viability (Fig. 3B). When compared with the p65-deleted hearts, wild-type mice had a nearly twofold increase in infarct size (65% vs. 32%, P < 0.05). This histologic evidence was supported by the significant reduction in cardiac enzyme release observed in the p65 knockout mice. Low levels of both LDH and CK were detected before ischemia that subsequently increased over baseline following the ischemic insult. Transgenic mouse hearts released lower levels of both LDH and CK (45 ± 28 u/l and 28 ± 23 u/l, respectively) compared with wild-type (143 ± 26 u/l and 107 ± 26 u/l, respectively).
Cardiomyocyte-specific deletion of p65 NF-κB and the response to regional I/R.
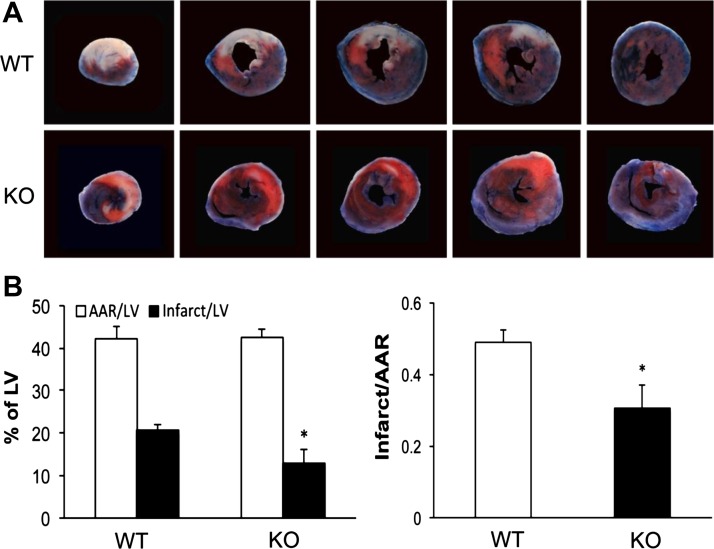
To confirm the Langendorff ex vivo observations that p65 ablation is cardioprotective, we performed in vivo regional I/R with LAD occlusion and release in our respective animals. Mice were subjected to 30 min of ischemia and 24 h of reperfusion before analyzing infarct size by TTC (Fig. 4). Qualitatively, p65 knockout animals appeared to have less necrotic tissue as seen on serial cross-sections from apex to the base of the heart. Quantitatively, both animals had similar area at risk to left ventricular ratios, suggesting consistent site of LAD occlusion. The infarct area in p65 knockout hearts was smaller than that of wild-type hearts (infarct mass/left ventricular mass 13.0 ± 3.0% vs. 20.6 ± 1.5%, P < 0.05). When compared by area at risk, p65 depletion afforded even lower relative size of infarct compared with that of wild-type mice (0.31 ± 0.06 vs. 0.49 ± 0.03, P < 0.05; Fig. 4B). Taken together with the Langendorff studies, hearts with absence of cardiomyocyte p65 NF-κB show less contractile impairment and less myocardial injury after both global and regional I/R.
Fig. 4.
In vivo ischemia-reperfusion with LAD ligation and release in WT and CMC p65-deficient mice. A: twenty-four hours after reperfusion, hearts were analyzed after TTC staining in vivo (n = 7/group). Infarct area stains white, injured area stains red, and viable areas stain blue. B: quantitative analysis of infarct area, area at risk (AAR), and viable area (n = 7/group; *P < 0.05). LV, left ventricular.
Influence of p65 NF-κB absence on apoptosis.
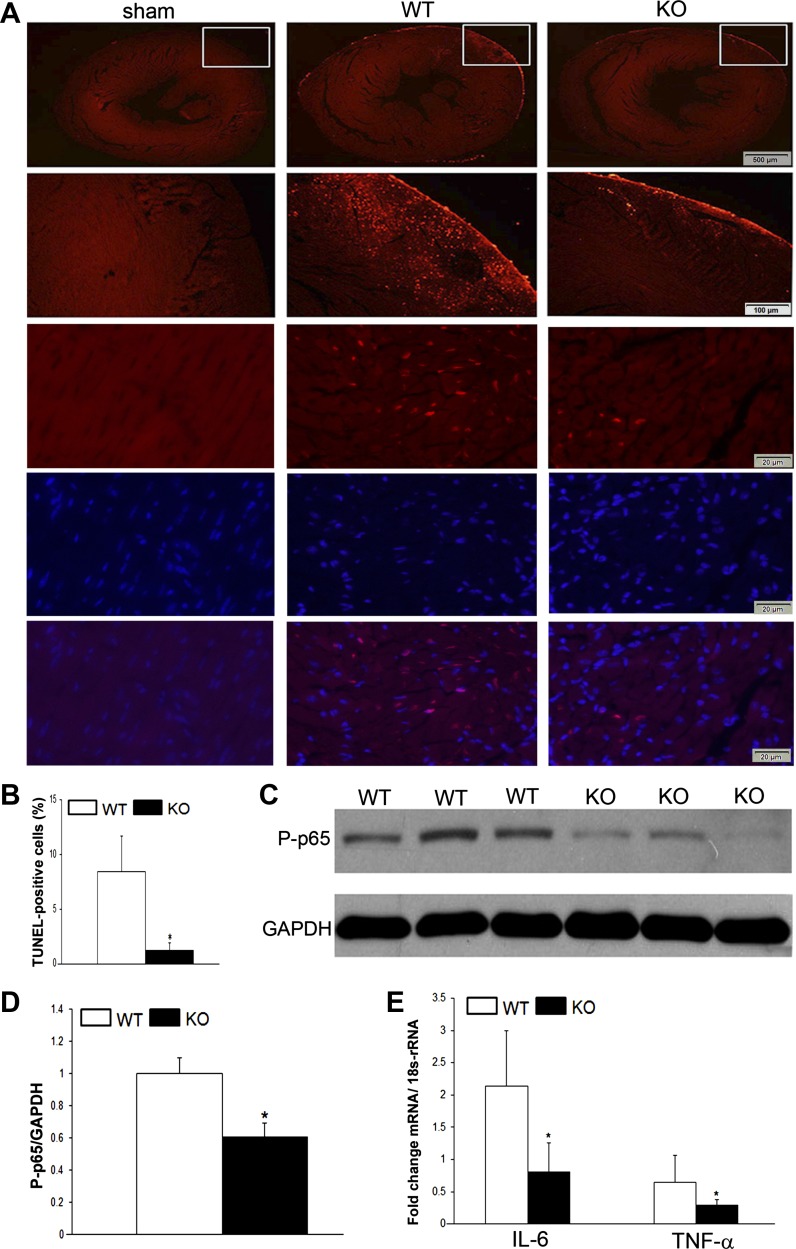
NF-κB regulates cell life and death via its role in either promoting or protecting from apoptosis (16). As such, we next examined the influence of p65-deletion on apoptosis following I/R. Mice underwent LAD occlusion and release and were harvested 6 h after injury. Terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling staining was performed on midpapillary sections and demonstrated increased apoptosis in the peri-infarct region in wild-type mice compared with p65 knockout and sham-operated animals (Fig. 5, A and B). These observations were associated with decreased expression of activated p65 at the same time point (Fig. 5, C and D). We examined two classic downstream transcriptional targets for NF-κB, TNF and IL-6, that are implicated in the injury response. When compared with wild-type animals, both IL-6 and TNF were downregulated in p65-deficient animals following I/R injury. Taken together, the cardioprotective influence of p65-absence is due, in part, to decreased cytokine production and diminished apoptosis.
Fig. 5.
In vivo ischemia-reperfusion 6 h after LAD ligation and release. A: terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining for apoptosis in area at risk of sham-operated, WT, and CMC p65-deficient mice. Included are macroscopic views demonstrating the area at risk as well as high-powered views with nuclear counterstaining. B: quantification of apoptotic cells. C and D: Western blot for active p65 NF-κB expression and its quantification. E: RT-PCR expression of IL-6 and TNF-α. *P < 0.05; n = 3–5.
NF-κB and calcium homeostasis.
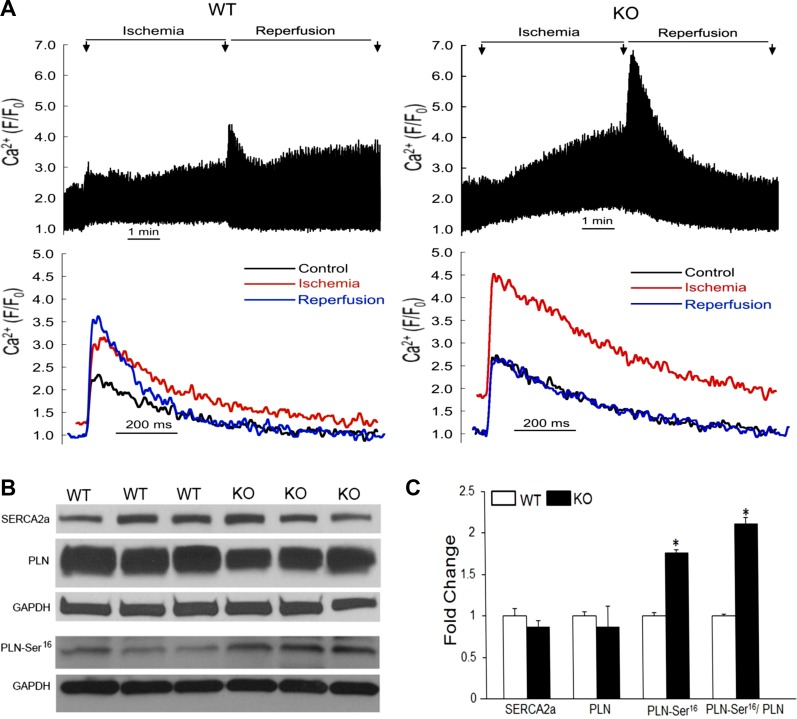
Intracellular calcium overload is associated with cell death (34, 54). To better understand the role of NF-κB as it relates to myocardial infarction and I/R injury, we next sought to determine the influence of NF-κB on calcium homeostasis during conditions of simulated ischemia. Electrically evoked CaT were examined in cardiomyocytes undergoing simulated I/R in vitro (Fig. 6A). During superfusion in the normal bathing solution, there were no significant differences in amplitude of CaTs (F/F0, wild-type = 2.99 ± 0.73; knockout = 2.21 ± 0.19), maximum rate of rise of CaTs (wild-type = 165 ± 60 ms−1; knockout = 103 ± 16 ms−1), or decay of CaTs (time constant, wild-type = 301 ± 14.1 ms; knockout = 346 ± 28 ms) between wild-type and knockout (n = 7/group). After 5 min of simulated reperfusion, wild-type cardiomyocytes displayed sustained elevations in CaT amplitude. In contrast, cardiomyocytes without p65 displayed rapid recovery of CaT amplitude to basal conditions (percent change relative to control, wild-type 42.9 ± 15.5% vs. knockout −4.85 ± 12.6%, P < 0.05).
Fig. 6.
NF-κB and calcium handling. A: calcium transients during simulated ischemia-reperfusion. Myocytes were field stimulated (cycle length = 1 s), and transients were recorded. Representative calcium transients during control (black), after 5 min of ischemia followed by 5 min of reperfusion (blue) (n = 7), are shown. Western blot analysis (B) and densitometry (C) of whole mouse hearts for sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a), phospholamban (PLN), and phosphorylated PLN-Ser16 6 h after in vivo regional ischemia-reperfusion (n = 4 to 5). *P < 0.05 compared with WT.
Although a number of factors contribute to intracellular calcium shuttling (22), sequestration of calcium by the sarcoplasmic reticulum (SR) is responsible for nearly the entire decline in cytoplasmic calcium following the peak of the CaT (43). SR calcium uptake is mediated by SERCA2a and its activity is tonically braked by phospholamban (PLN). When phosphorylated, PLN releases its inhibitory action on SERCA2a, thus stimulating SR calcium pumping (25). To assess the influence of NF-κB on the SR in whole heart tissues following in vivo I/R injury by LAD ligation and release, we assessed expression of SERCA2a and PLN (Fig. 6B). There were no significant differences in SERCA2a expression between the respective animals and a trend toward decreased total PLN in the transgenic mice. Importantly, when compared with wild-type mice, p65-ablated mice had increased expression of the serine-16 phosphorylated form of PLN and an increased ratio of PLN-Ser16 to total PLN. Concurrently, we did not identify any differences in expression in the threonine-17 phosphorylated form of PLN (data not shown). Taken together, these results suggest that p65 NF-κB influences intracellular calcium regulation via PLN, and its absence favorably enhances calcium reuptake by the SR.
DISCUSSION
Because of its well-known role in the inflammatory response, numerous studies have identified NF-κB as a mediator of myocardial injury (15, 48). Many of these reports are based on pharmacologic interventions in which the cardioprotection provided by the drug or agent was proposed to be mediated, in part, by NF-κB. In other studies, various methods have been used (pharmacologic, gene therapy, and transgenic mice) to block upstream elements of NF-κB activation to demonstrate cardioprotection with I/R injury. Within the most common activation pathway, translocation of the p65 NF-κB subunit and its binding to its gene target is considered the ultimate transcriptional event. Yet, to date, this dominant activity has only been indirectly addressed.
Currently, the most distally targeted approach in the p65 signaling pathway involves transgenic manipulation of IκBα, thereby preventing the translocation of the p65/p50 dimer. Although these mice, indeed, provided cardioprotection (10, 17), the relative contributions of the individual p50 and p65 subunits cannot be distinguished. This point is potentially important since conditional deletion of the p50 subunit has demonstrated conflicting results: worsening of postinfarction heart failure (13, 24) versus ischemic cardioprotection (45). Similarly, inhibition of the p65 subunit has also produced, admittedly rare, conflicting results regarding its effect on ischemic injury (46, 49). Taken together, we felt there was a need to directly assess the role of the p65 subunit not only to determine its influence on cardiac function but also provide a more direct method to investigate the mechanism of NF-κB-mediated cardioprotection.
In this report, we demonstrate that cardiomyocyte-specific deletion of p65 NF-κB protects the heart after both global and regional I/R. Our data expands on recently published work in mice with cardiac p65 deletion using a Nkx2.5 promoter. After transverse aortic constriction, their mice had decreased left ventricular hypertrophy and preservation of contractile function (27). Similar to that study, our genetically modified mice did not have any apparent developmental changes in heart phenotype or function, suggesting that p65, on its own, is not necessary for cardiac development. This lack of baseline cardiac alteration was similarly seen in conditionally deleted IKK-β mice and mice that conditionally overexpress IκBα (17, 20). Interestingly, conditional deletion of one member of the IKK complex, NF-κB essential modulator (NEMO) or IKK-γ, resulted in dilated cardiomyopathy with enhanced apoptosis (26). Herein lies the paradox of NF-κB in that it can produce opposing effects based on its stimulus and environment. Toggling of NF-κB through IKK kinase and activation of either canonical or noncanonical pathways possibly contribute to the diverse phenotypes induced through this transcription factor (15, 40). Furthermore, phosphorylation of the p65 subunit can occur at numerous sites and drive differential NF-κB-dependent gene expression (21). Although we observed decreases in downstream expression of NF-κB-dependent cytokines, we did not see any changes in NF-κB-related protein expression, suggesting that there were minimal compensatory changes in NF-κB-related activity except for the quantitative decrease in the p65 subunit.
Ablation of cardiomyocyte p65 NF-κB was associated with decreased infarct size with both global and regional I/R, and less apoptosis than wild-type mice. Although decreased NF-κB activity was shown to be protective in a chronic ligation model (17), other acute I/R models have also demonstrated a cardioprotective role of NF-κB (30). To extend the paradox, and competing with our findings, NF-κB was found to be essential for cell survival following acute hypoxic stress by regulating Bnip3 transcription (37). This paradigm suggests that the nature of the stimulus (acute vs. chronic) can direct the phenotypic pathway of NF-κB. Quite possibly, our cardioprotective and anti-apoptotic observations within an acute model of I/R are related to the method of NF-κB ablation. Manipulation of the IκB protein complex is the most common method described for genetically altering NF-κB signaling. Yet this complex is still an upstream to the dominant transcriptional event with NF-κB signaling, thereby reinforcing the need to directly examine p65 NF-κB signaling in our conditionally knocked out mouse.
Although we demonstrate the expected decrease in IL-6 and TNF, honing down on the mechanism of tissue protection led us to examine the role of NF-κB and calcium homeostasis. Calcium regulation is a central determinant of multiple aspects of cell death, including apoptosis (35, 54). We hypothesized that cardiac dysfunction and cell death are related to Ca2+ overload and that this is regulated, in part, by NF-κB. Indeed, the interplay of NF-κB and calcium signaling has not been fully investigated. Cardiac injury secondary to intracellular Ca2+ overload has been indirectly attributed to increased production of TNF and activation of NF-κB (53). By virtue of its central position in inflammation and immunity, NF-κB is mutually involved with multiple signaling pathways and transcription factors that mediate calcium homeostasis including calcineurin-NFAT, HDACs, and IP3 (19, 23, 44, 50). However, these interactions are indirectly inferred. Only a few reports have demonstrated more direct influence of NF-κB on calcium maintenance in cardiomyocytes (7, 38).
Herein, we demonstrate for the first time that absence of cardiac p65 promotes full recovery of the CaT following simulated ischemia. We fully acknowledge that we only show an associative relationship between cytotoxicity and calcium overload. That said, mice deficient in p65 NF-κB had a reduction in the rate of the decay of the calcium transient, thereby decreasing the duration of cytosolic exposure to higher levels of calcium. Several mechanisms may account for this, including increased calcium extrusion via sodium-calcium exchange (6, 52) and/or increased uptake by the SR (43). We did not examine sodium-calcium exchange activity or expression in our experiments, and there were no significant differences in absolute expression of either SERCA2a or its inhibitory protein PLN. Although SERCA2a can interact with several other proteins in the SR lumen, PLN remains the major regulator of SERCA2a activity (25, 28). Dephosphorylated PLN acts to tonically inhibit SERCA2a. When PLN is phosphorylated either at Ser16 or Thr17, SERCA2a activity is enhanced. Phosphorylation of Ser16 occurs by cAMP-dependent protein kinases, whereas phosphorylation of Thr17 exclusively occurs via Ca2+-CaM-dependent protein kinases (9, 29). When compared with wild-type, p65-deficient cardiomyocytes displayed markedly increased expression of the phosphorylated form of Ser16 but not Thr17. This finding suggests that enhanced phosphorylation of PLN may contribute to the nearly complete recovery of the CaT in knockout mice and suggests that NF-κB modulates cAMP-dependent protein kinase activity (PKA, protein kinase A). Mechanistically, it remains speculative if changes in PKA activity are related to a direct effect of p65 NF-κB absence (protein-protein interaction or direct transcriptional effect). Quite possibly, these findings could reflect the influence of altered downstream products of NF-κB expression (cytokines and immunomodulating proteins) or even changes in β-adrenergic receptor function (2). Further elaboration of cross-talk between NF-κB and intracellular calcium regulation provides exciting avenues for future studies.
GRANTS
This work was funded in part by National Heart, Lung, and Blood Institute Grants R01HL-089592 (to C. H. Selzman) and 5R37HL-042873-24 (to K. W. Spitzer) and by grants from the American College of Surgeons (to C. H. Selzman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.Q.Z., R.T., K.W.S., and C.H.S. completed conception and design of research; X.Q.Z., R.T., L.L., A.s., H.J., N.S., and K.W.S. performed experiments; X.Q.Z., R.T., L.L., A.s., H.J., N.S., K.W.S., and C.H.S. analyzed data; X.Q.Z., R.T., L.L., A.s., H.J., K.W.S., and C.H.S. interpreted results of experiments; X.Q.Z. and C.H.S. drafted manuscript; L.L., H.J., K.W.S., and C.H.S. prepared figures; K.W.S. and C.H.S. edited and revised manuscript; C.H.S. approved final version of manuscript.
REFERENCES
- 1.Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest 100: 169–179, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aksoy MO, Bin W, Yang Y, Yun-You D, Kelsen SG. Nuclear factor-κB augments β2-adrenergic receptor expression in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 281: L1271–L1278, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Baldwin AS., Jr Series introduction: the transcription factor NF-κB and human disease. J Clin Invest 107: 3–6, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry WH, Zhang XQ, Halkos ME, Vinten-Johansen J, Saegusa N, Spitzer KW, Matsuoka N, Sheets M, Rao NV, Kennedy TP. Nonanticoagulant heparin reduces myocyte Na+ and Ca2+ loading during simulated ischemia and decreases reperfusion injury. Am J Physiol Heart Circ Physiol 298: H102–H111, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376: 167–170, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Bridge JH, Smolley JR, Spitzer KW. The relationship between charge movements associated with ICa and INa-Ca in cardiac myocytes. Science 248: 376–378, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Camandola S, Cutler RG, Gary DS, Milhavet O, Mattson MP. Suppression of calcium release from inositol 1,4,5-trisphosphate-sensitive stores mediates the anti-apoptotic function of nuclear factor-kappaB. J Biol Chem 280: 22287–22296, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Cordeiro JM, Ferrier GR, Howlett SE. Effects of adenosine in simulated ischemia and reperfusion in guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol 269: H121–H129, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Davis BA, Edes I, Gupta RC, Young EF, Kim HW, Steenaart NA, Szymanska G, Kranias EG. The role of phospholamban in the regulation of calcium transport by cardiac sarcoplasmic reticulum. Mol Cell Biochem 99: 83–88, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Dawn B, Xuan YT, Marian M, Flaherty MP, Murphree SS, Smith TL, Bolli R, Jones WK. Cardiac-specific abrogation of NF-kappa B activation in mice by transdominant expression of a mutant I kappa B alpha. J Mol Cell Cardiol 33: 161–173, 2001 [DOI] [PubMed] [Google Scholar]
- 11.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388: 548–554, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Duan J, Zhang HY, Adkins SD, Ren BH, Norby FL, Zhang X, Benoit JN, Epstein PN, Ren J. Impaired cardiac function and IGF-I response in myocytes from calmodulin-diabetic mice: role of Akt and RhoA. Am J Physiol Endocrinol Metab 284: E366–E376, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Frantz S, Hu K, Bayer B, Gerondakis S, Strotmann J, Adamek A, Ertl G, Bauersachs J. Absence of NF-kappaB subunit p50 improves heart failure after myocardial infarction. FASEB J 20: 1918–1920, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell 109: S81–S96, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-kappaB in the heart: to be or not to NF-kappaB. Circ Res 108: 1122–1132, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Hall G, Hasday JD, Rogers TB. Regulating the regulator: NF-kappaB signaling in heart. J Mol Cell Cardiol 41: 580–591, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn B, Prabhu SD. Cardiomyocyte NF-kappaB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovasc Res 89: 129–138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haudek SB, Spencer E, Bryant DD, White DJ, Maass D, Horton JW, Chen ZJ, Giroir BP. Overexpression of cardiac IκBα prevents endotoxin-induced myocardial dysfunction. Am J Physiol Heart Circ Physiol 280: H962–H968, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Hikoso S, Yamaguchi O, Nakano Y, Takeda T, Omiya S, Mizote I, Taneike M, Oka T, Tamai T, Oyabu J, Uno Y, Matsumura Y, Nishida K, Suzuki K, Kogo M, Hori M, Otsu K. The IκB kinase β/nuclear factor-κB signaling pathway protects the heart from hemodynamic stress mediated by the regulation of manganese superoxide dismutase expression. Circ Res 105: 70–79, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Hochrainer K, Racchumi G, Anrather J. Site-specific phosphorylation of the p65 protein subunit mediates selective gene expression by differential NF-kappaB and RNA polymerase II promoter recruitment. J Biol Chem 288: 285–293, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoshijima M, Knoll R, Pashmforoush M, Chien KR. Reversal of calcium cycling defects in advanced heart failure toward molecular therapy. J Am Coll Cardiol 48: A15–A23, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, Adcock IM. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. The Journal of experimental medicine 203: 7–13, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawano S, Kubota T, Monden Y, Kawamura N, Tsutsui H, Takeshita A, Sunagawa K. Blockade of NF-kappaB ameliorates myocardial hypertrophy in response to chronic infusion of angiotensin II. Cardiovasc Res 67: 689–698, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phopholamban/SERCA2a regulatome. Circ Res 110: 1646–1660, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kratsios P, Huth M, Temmerman L, Salimova E, Al Banchaabouchi M, Sgoifo A, Manghi M, Suzuki K, Rosenthal N, Mourkioti F. Antioxidant amelioration of dilated cardiomyopathy caused by conditional deletion of NEMO/IKKgamma in cardiomyocytes. Circ Res 106: 133–144, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Chen Y, Auger-Messier M, Molkentin JD. Interaction between NFkappaB and NFAT coordinates cardiac hypertrophy and pathological remodeling. Circ Res 110: 1077–1086, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 4: 566–577, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Mattiazzi A, Mundina-Weilenmann C, Guoxiang C, Vittone L, Kranias E. Role of phospholamban phosphorylation on Thr17 in cardiac physiological and pathological conditions. Cardiovasc Res 68: 366–375, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Misra A, Haudek SB, Knuefermann P, Vallejo JG, Chen ZJ, Michael LH, Sivasubramanian N, Olson EN, Entman ML, Mann DL. Nuclear factor-kappaB protects the adult cardiac myocyte against ischemia-induced apoptosis in a murine model of acute myocardial infarction. Circulation 108: 3075–3078, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki Y, Kaikita K, Endo M, Horio E, Miura M, Tsujita K, Hokimoto S, Yamamuro M, Iwawaki T, Gotoh T, Ogawa H, Oike Y. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler Thromb Vasc Biol 31: 1124–1132, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Moss NC, Stansfield WE, Willis MS, Tang RH, Selzman CH. IKKβ inhibition attenuates myocardial injury and dysfunction following acute ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 293: H2248–H2253, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Moss NC, Tang RH, Willis M, Stansfield WE, Baldwin AS, Selzman CH. Inhibitory kappa B kinase-beta is a target for specific nuclear factor kappa B-mediated delayed cardioprotection. J Thorac Cardiovasc Surg 136: 1274–1279, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Murgia M, Giorgi C, Pinton P, Rizzuto R. Controlling metabolism and cell death: at the heart of mitochondrial calcium signalling. J Mol Cell Cardiol 46: 781–788, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4: 552–565, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Pye J, Ardeshirpour F, McCain A, Bellinger DA, Merricks E, Adams J, Elliott PJ, Pien C, Fischer TH, Baldwin AS, Jr, Nichols TC. Proteasome inhibition ablates activation of NF-κB in myocardial reperfusion and reduces reperfusion injury. Am J Physiol Heart Circ Physiol 284: H919–H926, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Shaw J, Yurkova N, Zhang T, Gang H, Aguilar F, Weidman D, Scramstad C, Weisman H, Kirshenbaum LA. Antagonism of E2F-1 regulated Bnip3 transcription by NF-kappaB is essential for basal cell survival. Proc Natl Acad Sci USA 105: 20734–20739, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh MV, Kapoun A, Higgins L, Kutschke W, Thurman JM, Zhang R, Singh M, Yang J, Guan X, Lowe JS, Weiss RM, Zimmermann K, Yull FE, Blackwell TS, Mohler PJ, Anderson ME. Ca2+/calmodulin-dependent kinase II triggers cell membrane injury by inducing complement factor B gene expression in the mouse heart. J Clin Invest 119: 986–996, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solt LA, Madge LA, May MJ. NEMO-binding domains of both IKKalpha and IKKbeta regulate IkappaB kinase complex assembly and classical NF-kappaB activation. J Biol Chem 284: 27596–27608, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solt LA, May MJ. The IkappaB kinase complex: master regulator of NF-kappaB signaling. Immunol Res 42: 3–18, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stansfield WE, Moss NC, Willis MS, Tang R, Selzman CH. Proteasome inhibition attenuates infarct size and preserves cardiac function in a murine model of myocardial ischemia-reperfusion injury. Ann Thorac Surg 84: 120–125, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol 180: 2588–2599, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Su Z, Li F, Spitzer KW, Yao A, Ritter M, Barry WH. Comparison of sarcoplasmic reticulum Ca2+-ATPase function in human, dog, rabbit, and mouse ventricular myocytes. J Mol Cell Cardiol 35: 761–767, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Tiangco DA, Lattanzio FA, Jr, Osgood CJ, Beebe SJ, Kerry JA, Hargrave BY. 3,4-Methylenedioxymethamphetamine activates nuclear factor-kappaB, increases intracellular calcium, and modulates gene transcription in rat heart cells. Cardiovasc Toxicol 5: 301–310, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Timmers L, van Keulen JK, Hoefer IE, Meijs MF, van Middelaar B, den Ouden K, van Echteld CJ, Pasterkamp G, de Kleijn DP. Targeted deletion of nuclear factor kappaB p50 enhances cardiac remodeling and dysfunction following myocardial infarction. Circ Res 104: 699–706, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Tranter M, Ren X, Forde T, Wilhide ME, Chen J, Sartor MA, Medvedovic M, Jones WK. NF-kappaB driven cardioprotective gene programs; Hsp70.3 and cardioprotection after late ischemic preconditioning. J Mol Cell Cardiol 49: 664–672, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valen G. Innate immunity and remodelling. Heart Fail Rev 16: 71–78, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valen G. Signal transduction through nuclear factor kappa B in ischemia-reperfusion and heart failure. Basic Res Cardiol 99: 1–7, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Wilhide ME, Tranter M, Ren X, Chen J, Sartor MA, Medvedovic M, Jones WK. Identification of a NF-kappaB cardioprotective gene program: NF-kappaB regulation of Hsp70.1 contributes to cardioprotection after permanent coronary occlusion. J Mol Cell Cardiol 51: 82–89, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94: 110–118, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Yamada S, Zhang XQ, Kadono T, Matsuoka N, Rollins D, Badger T, Rodesch CK, Barry WH. Direct toxic effects of aqueous extract of cigarette smoke on cardiac myocytes at clinically relevant concentrations. Toxicol Appl Pharmacol 236: 71–77, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Yao A, Matsui H, Spitzer KW, Bridge JH, Barry WH. Sarcoplasmic reticulum and Na+/Ca2+ exchanger function during early and late relaxation in ventricular myocytes. Am J Physiol Heart Circ Physiol 273: H2765–H2773, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Zhang M, Xu YJ, Saini HK, Turan B, Liu PP, Dhalla NS. TNF-alpha as a potential mediator of cardiac dysfunction due to intracellular Ca2+-overload. Biochem Biophys Res Commun 327: 57–63, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Zhivotovsky B, Orrenius S. Calcium and cell death mechanisms: a perspective from the cell death community. Cell Calcium 50: 211–221, 2011 [DOI] [PubMed] [Google Scholar]