Abstract
The sensitivity of baroreflex control of heart rate is depressed in subjects with obesity hypertension, which increases the risk for cardiac arrhythmias. The mechanisms are not fully known, and there are no therapies to improve this dysfunction. To determine the cardiovascular dynamic effects of progressive increases in body weight leading to obesity and hypertension in dogs fed a high-fat diet, 24-h continuous recordings of spontaneous fluctuations in blood pressure and heart rate were analyzed in the time and frequency domains. Furthermore, we investigated whether autonomic mechanisms stimulated by chronic baroreflex activation and renal denervation—current therapies in patients with resistant hypertension, who are commonly obese—restore cardiovascular dynamic control. Increases in body weight to ∼150% of control led to a gradual increase in mean arterial pressure to 17 ± 3 mmHg above control (100 ± 2 mmHg) after 4 wk on the high-fat diet. In contrast to the gradual increase in arterial pressure, tachycardia, attenuated chronotropic baroreflex responses, and reduced heart rate variability were manifest within 1–4 days on high-fat intake, reaching 130 ± 4 beats per minute (bpm) (control = 86 ± 3 bpm) and ∼45% and <20%, respectively, of control levels. Subsequently, both baroreflex activation and renal denervation abolished the hypertension. However, only baroreflex activation effectively attenuated the tachycardia and restored cardiac baroreflex sensitivity and heart rate variability. These findings suggest that baroreflex activation therapy may reduce the risk factors for cardiac arrhythmias as well as lower arterial pressure.
Keywords: obesity, hypertension, heart rate variability, baroreflex, sympathetic nervous system
the causal link between obesity and hypertension is well recognized. Clinical and experimental evidence also indicate that obesity has a profound impact on cardiac automaticity (44), which may increase the risk for atrial and ventricular arrhythmias, leading causes of morbidity and mortality in the industrialized world (4, 7, 17, 42, 59, 60). The mechanisms that mediate the proarrhythmogenic effects of obesity have received little attention. Low heart rate variability has been identified not only as a predictor of but also as a risk factor for developing life-threatening cardiac arrhythmias (7, 8, 26, 46, 50, 56, 57) and, together with impaired baroreflex control of heart rate, is a common finding in obese individuals (6, 18, 20, 51, 58). Alterations in cardiac autonomic neural regulation are the substrate for reductions in both heart rate variability and baroreflex sensitivity and, consequently, may increase risk for cardiac arrhythmias (7, 8). While sustained activation of the sympathetic nervous system has been shown to play a critical role in the pathogenesis of obesity-related hypertension (15, 23), the time course and functional significance of alterations in autonomic activity to the heart and the attendant changes in cardiovascular dynamics during the progression of obesity-related hypertension have not been determined, as their investigation has typically relied on single-time point observations after obesity is established. Serial daily determinations of heart rate variability and baroreflex function throughout the progression of diet-induced obesity may lead to a better understanding of the dietary and metabolic factors that contribute to autonomic imbalance during weight gain. An additional consideration is that measurements of cardiovascular dynamics during activities of daily life may better reflect the true role of the autonomic nervous system in the pathogenesis of cardiac dysfunction during weight gain than spot determinations performed acutely under basal, resting conditions.
Despite the clinical relevance of dynamic variables such as impaired baroreflex sensitivity and decreased heart rate variability, there are no established interventions to improve cardiac autonomic imbalance during weight gain. This has precluded a mechanistic understanding of the linkage between the underlying cardiac autonomic dysfunction and these proarrhythmogenic risk factors. Recent technological advances have provided two nonpharmacological approaches that have the potential to restore diminished cardiac baroreflex sensitivity: electrical stimulation of the carotid sinus (10, 31) and endovascular radio frequency ablation of the renal nerves (49, 52). In recent clinical trials, these devices have substantially lowered arterial pressure in patients with resistant hypertension, who are commonly obese (10, 52). Chronic electrical stimulation of the carotid sinus activates the carotid baroreflex and lowers arterial pressure by suppressing central sympathetic outflow (25, 32) and decreases heart rate by mechanisms that may include activation of the parasympathetic nervous system (25, 32, 61). In addition, electrical activation of the carotid sinus has been shown to increase the sensitivity of the spontaneous baroreflex control of heart rate, while lowering blood pressure in normotensive animals (32). In comparison, by selective denervation of the kidneys, catheter-based endovascular radio frequency ablation of the renal nerves lowers arterial pressure by directly diminishing renal efferent sympathetic nerve activity (49). This procedure may also chronically decrease central sympathetic outflow by a reflex mechanism triggered by a reduction in renal afferent nerve traffic (24, 49). Thus we hypothesized that these device-based approaches for lowering arterial pressure may restore cardiac baroreflex dysfunction and therefore improve heart rate variability in obesity by decreasing cardiac sympathetic nerve activity and/or increasing vagal activity.
In the present study, 24-h spontaneous fluctuations in heart rate and blood pressure were analyzed in the time and frequency domains to determine the daily time course of cardiovascular dynamics throughout the progression of weight gain in dogs fed a high-fat diet, an experimental model of hypertension closely reproducing many of the abnormalities of human obesity (22, 23, 33). In addition to time course determinations, an important objective of this study was to evaluate and compare the ability of chronic carotid baroreflex activation and bilateral renal denervation to correct cardiac baroreflex and autonomic dysfunction in obesity hypertension, while ultimately improving heart rate variability. This comparison in the established phase of obesity hypertension may be important clinically because many patients treated with these interventions for resistant hypertension are obese (10, 52) and at risk for adverse cardiac arrhythmias. In light of the established autonomic effects of carotid baroreflex activation and the putative effects of ablation of the renal nerves on global sympathetic activity, we surmise that both interventions may improve cardiac baroreflex sensitivity and cardiovascular autonomic dysfunction in this clinically relevant model of human hypertension.
METHODS
Animal preparation.
All procedures were performed in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center. Six male dogs weighing 23–26 kg were used in this study. Arterial and venous catheters were implanted for continuous measurement of arterial pressure and for continuous intravenous infusion of isotonic saline as previously described (33). Stimulating electrodes were implanted around each carotid sinus, and the lead bodies were connected to a pulse generator provided by CVRx (Maple Grove, MN) (33).
General methods.
The dogs were maintained in metabolic cages, given free access to water, and fed a fixed daily diet (33). Total daily sodium intake from the diet and a continuous intravenous saline infusion was ∼60 mmol throughout the study; potassium intake was ∼55 mmol/day. Steady-state control measurements were made after a 3-wk postoperative period of acclimation and establishment of electrolyte and fluid balance. Subsequently, obesity hypertension was produced with the same protocol that we and others have previously reported (22, 33). During the initial 4 wk of the high-fat feeding (Developing Obesity), the diet was supplemented with 0.6–0.7 kg/day cooked beef fat until body weight increased to ∼150% of control. Once this weight gain was achieved, dietary fat was reduced (on day 32) to 0.1–0.15 kg/day to maintain a constant body weight for the remainder of the study (Established Obesity). Some data obtained in this study have been published previously in a report focused on neurohormonal activity and renal function (33).
Experimental protocol.
After control measurements, obesity hypertension was produced by feeding a high-fat diet for 28 days before reducing fat intake to maintain body weight for the remainder of the study. The experimental protocol consisted of 1) control, days 1–3; 2) days 4–31, high fat (development of obesity and hypertension); 3) days 32–63, reduced fat (established obesity hypertension); 4) days 35–41, baroreflex activation (1 wk); 5) days 42–48, recovery (1 wk); 6) day 49, surgical bilateral renal denervation by procedures previously used in our laboratory (33); and 7) day 63, end of study (2 wk after renal denervation).
Data acquisition and analysis.
Arterial pressure was sampled continuously at 100 samples/s, 24 h/day, with a PowerLab data-acquisition system (ADInstruments). The daily values for mean arterial pressure (MAP) and heart rate were averaged between 11:30 AM and 7:30 AM. Although some MAP and heart rate data have been presented elsewhere (33), the present study investigated the detailed dynamic time-dependent changes of these variables, as well as heart rate variability, during the developmental and established phases of obesity hypertension, including the periods of baroreflex activation and renal denervation. For subsequent time- and frequency-domain analyses, daily time series of beat-to-beat systolic blood pressure (SBP) and pulse intervals (PI) were generated from artifact-free 18-h-long blood pressure waveforms with LabChart 7.0 (ADInstruments) and peak detection algorithms.
Time-domain analyses.
The sequence technique was used to calculate spontaneous baroreflex sensitivity from the slope of the linear regression functions between SBP and the subsequent PI within the next heartbeat, as we have previously described (32). Up and down sequences of at least three intervals with changes in SBP of >1 mmHg and in PI of >0.5 ms were analyzed only if the correlation coefficients were >0.85. The means of the standard deviations calculated over each 5-min segment in this 18-h period for beat-to-beat SBP and PI were used to determine short-term variability.
Frequency-domain analyses.
A sequential spectral analysis of the daily beat-to-beat data was performed by Fourier transformation with automated MATLAB routines (R14; MathWorks, Natick, MA), based on algorithms we have previously described (28). Briefly, the daily 18-h SBP and PI time series were divided into discrete blocks of 256 s, low-pass antialiasing filtered, resampled at 1 Hz, and Hanning windowed. The power of the variations of SBP and PI (periodograms) and the baroreflex sensitivity (estimated as the magnitude of the transfer function between SBP and PI) were constructed for individual segments and plotted against time of day. To capture the time-dependent, intraday variability of the power and baroreflex sensitivity, the resulting plots were integrated, rather than averaged, and the daily volume was computed (power or gain in the area delimited by the spectral frequency up to 0.5 Hz and the daily time range) with the trapezoidal method. The frequency domain < 0.5 Hz was chosen because it contains most of the autonomic nervous system-mediated blood pressure and heart rate oscillations in dog and human (5, 41). In addition, to account for possible contamination of transfer gain estimates with non-baroreflex-mediated influences that may occur during respiration (of mechanical or central oscillator-driven origin) (45), and as routinely performed in clinical studies (25), calculations of gain were also performed in the frequency band < 0.1 Hz. All gain function estimates were used in the calculations irrespective of the point coherence values, with the purpose of obtaining an integrated appreciation of the baroreflex sensitivity and also avoiding reduced measurability inherent with the limitations of the classic coherence criterion (43). To discern the main oscillatory components of heart rate, contour plots of sequential power spectra of PI oscillations for frequencies up to 0.5 Hz throughout the day were constructed for one representative day of each experimental period. Since the overall power of PI oscillations (power volume) was different between experimental periods, every point power spectral estimate used to construct the contour plots was normalized to the overall daily power volume of each animal and then averaged in order to allow for uniform color range coding and therefore discernibility of the main oscillatory components.
Statistical analysis.
Results are expressed as means ± SE. One-way repeated-measures ANOVA, followed by the Holm-Sidak test for multiple comparisons (Prism 6.01, GraphPad Software), was used to compare the following experimental periods: 1) Developing Obesity and initial 3 days of Established Obesity (days 4–34) vs. Control (mean of days 2 and 3); 2) Baroreflex Activation and Recovery (days 35–48) vs. baseline of Established Obesity (mean of days 33 and 34); 3) Renal Denervation (days 49–63) vs. Recovery after Baroreflex Activation (mean of days 47 and 48). Statistical significance was considered to be P < 0.05.
RESULTS
Developmental phase of obesity.
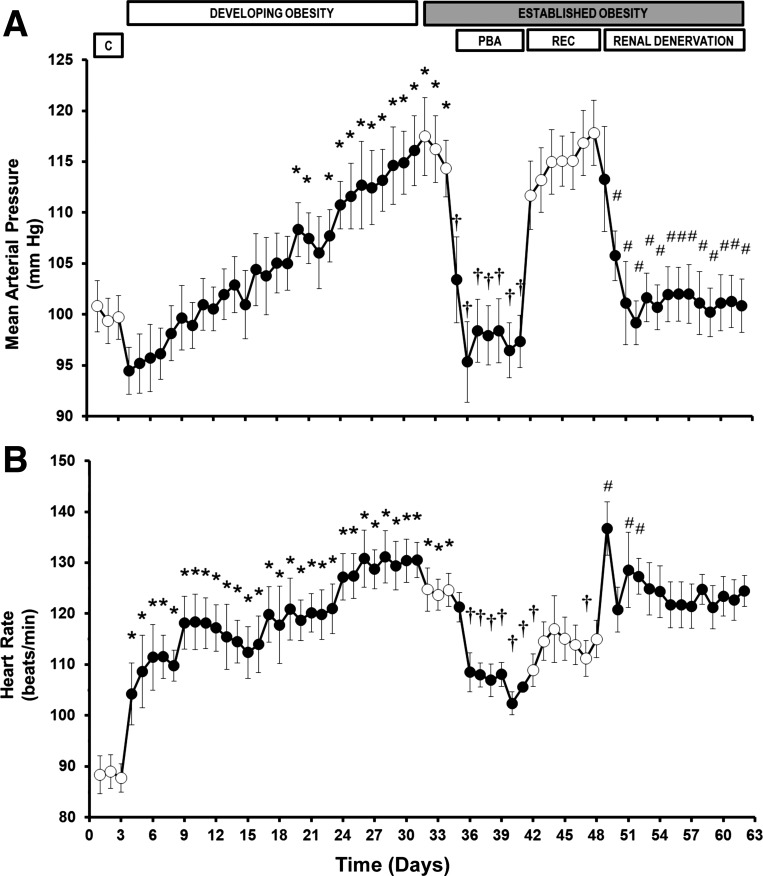
During the 4 wk of high-fat diet, MAP increased progressively, reaching a level of 17 ± 3 mmHg above control on day 31 (Fig. 1), paralleling the weight gain of ∼50%. Heart rate abruptly increased 1 day after initiation of the high-fat diet and progressively thereafter, to 42 ± 5 beats per minute (bpm) higher than control (Fig. 1). Both time- and frequency-domain analyses indicate that baroreflex control of heart rate was significantly suppressed 4 days after initiation of the high-fat diet and continued to decrease to ∼45% of control during the last week of high dietary fat supplementation (Fig. 2). These changes in gain estimates by transfer function analysis were similar in the frequency bands up to either 0.5 or 0.1 Hz (Fig. 2, B and C), indicating that non-baroreflex-mediated respiratory influences at higher frequencies, if any, do not contribute to the observed changes in baroreflex sensitivity spectral estimates. As indicated by Fig. 3, the suppression of the baroreflex function was paralleled by abrupt and sustained reductions of heart rate variability progressively falling to <20% of control by week 4. During the control period, the normalized contour plots of the PI showed two distinct oscillatory components [between 0.1 and 0.2 Hz (high frequency) and between 0.007 and 0.025 Hz (low frequency)]. The reduction in the power of heart rate oscillations during the high-fat diet was evident across the spectrum and involved both the low- and high-frequency components (Fig. 4). The variability of SBP was also diminished by the high-fat diet in both the time and frequency domains (Fig. 5).
Fig. 1.
Changes in mean arterial pressure (A) and heart rate (B) during Developing Obesity and responses to prolonged baroreflex activation (PBA) and renal denervation during Established Obesity. Values are means ± SE (n = 6). *P < 0.05 vs. mean of days 2–3 of Control (C); †P < 0.05 vs. mean of days 33–34 of Established Obesity; #P < 0.05 vs. mean of days 47–48 of Recovery (REC) after PBA.
Fig. 2.
Changes of baroreflex sensitivity indexes in time domain [sequence analysis (A)] and frequency domain [volume of transfer gain throughout the day across frequencies <0.5 Hz (B) and <0.1 Hz (C)] during Developing Obesity and responses to PBA and renal denervation during Established Obesity. Values are means ± SE (n = 6). *P < 0.05 vs. mean of days 2–3 of Control; †P < 0.05 vs. mean of days 33–34 of Established Obesity; #P < 0.05 vs. mean of days 47–48 of Recovery after PBA.
Fig. 3.
Changes in pulse interval (PI) variability indexes in time domain (short-term variability, A) and frequency domain (volume of power across frequencies <0.5 Hz throughout the day, B) during Developing Obesity and responses to PBA and renal denervation during Established Obesity. Values are means ± SE (n = 6). *P < 0.05 vs. mean of days 2–3 of Control; †P < 0.05 vs. mean of days 33–34 of Established Obesity; #P < 0.05 vs. mean of days 47–48 of Recovery after PBA.
Fig. 4.
Contour plots of sequential power spectra of PI oscillations for frequencies up to 0.5 Hz throughout the day (normalized units). The color range is logarithmic, with orange representing the highest and violet the lowest power.
Fig. 5.
Changes in systolic blood pressure (SBP) variability indexes in time domain (short-term variability, A) and frequency domain (volume of power across frequencies <0.5 Hz throughout the day, B) during Developing Obesity and responses to PBA and renal denervation during Established Obesity. Values are means ± SE (n = 6). *P < 0.05 vs. mean of days 2–3 of Control; †P < 0.05 vs. mean of days 33–34 of Established Obesity; #P < 0.05 vs. mean of days 47–48 of Recovery after PBA.
Established phase of obesity.
Reduction of dietary fat intake did not significantly affect the severity of hypertension or tachycardia.
Hemodynamic responses to baroreflex activation.
Baroreflex activation abolished obesity hypertension and significantly diminished the associated tachycardia (Fig. 1). The sensitivity of the baroreflex control of heart rate was completely restored (Fig. 2), while heart rate variability concomitantly increased toward control levels (Fig. 3). SBP variability was not affected by baroreflex activation (Fig. 5). Upon discontinuation of baroreflex activation, MAP quickly returned to prestimulation levels, baroreflex sensitivity decreased to levels observed before carotid sinus stimulation, but recovery of average heart rate and its variability was incomplete.
Hemodynamic responses to renal denervation.
Similar to baroreflex activation, renal denervation also abolished obesity-induced hypertension (Fig. 1). In sharp contrast to electrical activation of the baroreflex, renal denervation did not affect obesity-induced tachycardia (Fig. 1) or the depressed levels of SBP variability (Fig. 5), nor did it improve baroreflex sensitivity (Fig. 2) or heart rate variability (Fig. 3). Reductions in norepinephrine levels in denervated kidneys to <5% of levels present in innervated kidneys attest to the completeness of renal denervation (33).
DISCUSSION
We and others have previously shown that dogs fed a high-fat diet for several weeks exhibit many of the steady-state hemodynamic, neurohormonal, renal, and metabolic changes associated with obesity in human subjects, including weight gain, sodium retention, hypertension, tachycardia, hyperfiltration, hyperinsulinemia, insulin resistance, and activation of the sympathetic and renin-angiotensin systems (22, 23, 33). A major goal of the present study was to assess the time-dependent effects of weight gain on the autonomic control mechanisms of cardiovascular dynamics and to test two potential interventions aimed at reversing any dysfunction. Using complementary time- and frequency-domain analyses of spontaneous beat-to-beat oscillations in arterial pressure and heart rate throughout the day, we showed that in addition to increasing average heart rate, consumption of a high-fat diet had early and sustained effects to markedly reduce dynamic cardiac baroreflex sensitivity in parallel with heart rate variability. A point of emphasis is that these alterations became manifest soon after initiation of the high-fat diet and long before appreciable weight gain or the development of hypertension. Moreover, a particularly important new finding in this study is that electrical stimulation of the carotid sinus had sustained effects to improve autonomic balance and to restore and/or markedly improve the depression in baroreflex sensitivity and heart rate variability associated with weight gain. In contrast, no such improvement in these variables occurred after renal denervation. Finally, blood pressure variability did not increase but actually decreased substantially during consumption of the high-fat diet, and neither baroreflex activation nor renal denervation had additional effects on this measure.
Cardiac baroreflex sensitivity in obesity.
Several previous studies have indicated that cardiac baroreflex sensitivity is depressed in animals and in human subjects after several weeks of weight gain or in subjects with long-standing obesity (6, 18, 20, 51, 58). Conversely, several weeks of weight loss has been shown to improve baroreflex function in obese individuals (1, 19). Despite these important observations, the initiating and sustaining mechanisms that account for baroreflex dysfunction, the site of the dysfunction in the baroreflex arc, and the overall cardiovascular consequences of depressed baroreflex gain in the pathology of obesity hypertension are largely unknown. There are several reasons that contribute to this uncertainty. 1) Virtually all determinations of baroreflex function have been made after established changes in body weight, precluding an understanding of early events that may be important in initiating and sustaining the pathophysiology. 2) Most if not all determinations of baroreflex sensitivity in experimental studies and in human subjects with weight gain/obesity have been conducted under controlled resting conditions over a time course of <1 h and in response to administration of vasoactive drugs. Measurements under these conditions do not reflect the dynamic physiological modulation of heart rate by the baroreflex in response to spontaneous fluctuations in arterial pressure that occur throughout the day, and such determinations may be confounded by pharmacologically induced changes in baroreceptor activity (37). 3) The presence of established hypertension in many studies precludes identification of specific mechanisms, as cardiac baroreflex sensitivity is also depressed in nonobese hypertensive individuals (20). 4) Detailed information on dietary intake is often missing, confounding the relative importance of diet vs. body weight on baroreflex function. 5) Therapy to arrest or reverse baroreflex dysfunction in obesity has not been established, and therefore the benefit of restoring cardiac baroreflex sensitivity is unknown.
One of the novel aspects of the present study was the determination of daily cardiovascular dynamics throughout the evolution of obesity-induced hypertension under conditions of controlled fat intake. To the best of our knowledge, these daily determinations from continuous 24-h recordings of spontaneous fluctuations in arterial pressure and heart rate have not been previously made during progressive increases in body weight that ultimately result in obesity and hypertension. Therefore, these measurements in the present study provide unique insight into the relative influence of diet vs. established obesity and hypertension on baroreflex function and heart rate control. The present study demonstrated marked increases in average heart rate and pronounced reductions in 24-h cardiac baroreflex sensitivity and heart rate variability within days of consumption of the high-fat diet, long before appreciable weight gain and hypertension, although these responses did intensify further with progressive weight gain and attendant hypertension. These data indicate that the high-fat diet itself may cause baroreflex dysfunction and, consequently, lower heart rate variability. However, once established, obesity and hypertension also contribute to the maintenance of these abnormalities, since reduction in fat intake caused only marginal improvement at this stage.
Although this study was not designed to explore the specific mechanisms that account for depressed cardiac baroreflex sensitivity with weight gain, several mechanisms may be proposed. While increased arterial stiffness may contribute to decreased baroreflex sensitivity in the context of long-standing obesity (13, 19, 20), the present study clearly shows an important and more rapid effect of the fat diet itself. This dietary influence may affect a number of factors that impair the processing of signals from baroreceptor afferents by the central nervous system (CNS) (27 34, 62). An early study tested the hypothesis that lipid abnormalities accompanying insulin resistance may depress baroreflex sensitivity (18). In support of this hypothesis, acute infusion of Intralipid-heparin in human subjects was reported to produce rapid decreases in cardiac baroreflex sensitivity within an hour. However, this finding was not confirmed by others using an identical protocol (36). In the present chronic study, significant reductions in cardiac baroreflex sensitivity occurred only after several days of high-fat intake and presumably sustained hyperlipidemia, suggesting that increased circulating lipids from dietary sources may not have immediate direct actions to depress baroreflex function. Given the increased plasma levels of insulin and plasma renin activity measured after 7 days of fat feeding when postabsorptive blood samples were first taken in the present study (33), the early impairment of cardiac baroreflex function during the high-fat diet may be a secondary response to central insulin resistance (12, 62) and/or the central actions of angiotensin II (48). Furthermore, with progressive weight gain, impairment of the cardiac baroreceptor reflex may be attributed to adipose tissue-derived inflammatory cytokines/hormones such as leptin and interleukin-6, which act in the nucleus tractus solitarii to inhibit the baroreflex (2, 53). Clearly, further studies are needed to address the above possibilities.
Baroreflex activation and cardiac autonomic function.
Despite an incomplete understanding of the mechanisms that account for impaired cardiac baroreflex function with weight gain, a most impressive response in the present study was that chronic electrical stimulation of the carotid baroreflex had dramatic effects to diminish the tachycardia and improve the depressed heart rate variability associated with weight gain, while completely restoring cardiac baroreflex sensitivity to control levels. Similarly, in one of our previous studies in normotensive dogs, analysis of 24-h hemodynamic recordings showed that despite delivery of a continuous series of electrical impulses rather than a more physiological pulse-synchronous baroreceptor discharge, electrical stimulation of the carotid sinus did not adversely affect physiological baroreflex regulation. Rather, it actually increased spontaneous baroreflex control of heart rate and heart rate variability over the long term (32). These responses in dogs are consistent with single-time point determinations in patients with resistant hypertension treated with baroreflex activation therapy (25, 61). Therefore, the present findings suggest that continuous electrical stimulation of the carotid sinus has pronounced effects in the CNS to counteract the central mechanisms that lead to baroreflex dysfunction and impaired heart rate variability in obesity.
The prevailing heart rate and its oscillations depend on the intricate interaction of cardiac sympathetic and parasympathetic efferent activities. Despite the recognized role of increased activation of the sympathetic nervous system in mediating obesity hypertension, previous studies using adrenergic and muscarinic blockers in resting dogs and human subjects indicate that weight gain/obesity-induced tachycardia is primarily attributable to suppression of parasympathetic nervous system activity (3, 55, 58). These findings during pharmacological blockade, indicating decreased basal parasympathetic tone, are consistent with observations demonstrating that there is no distinct increase in cardiac norepinephrine spillover in obesity hypertension, despite impaired baroreflex control and increases in sympathetic activity to the kidneys and skeletal muscular vasculature (15, 48). To assess the integrated cardiac baroreflex sensitivity and heart rate variability during daily activity in this model of obesity hypertension, spontaneous fluctuations in heart rate and blood pressure were analyzed in the time and frequency domains. The results from these complementary techniques were concordant, providing confidence in our findings. Furthermore, baroreflex sensitivity estimated by these dynamic analyses correlates well with classical vasoactive drug methods (16). Both analyses (sequence technique and transfer function analysis) indicated that cardiac baroreflex sensitivity was markedly depressed during obesity and completely restored to control levels by baroreflex activation. Similarly, in parallel with changes in baroreflex sensitivity, both analyses (5-min sequential standard deviations and total power volume of PI spectral density) demonstrated pronounced reductions in heart rate variability with weight gain that were largely restored to control levels by baroreflex activation. The clinical significance for the observed changes in heart rate variability of the magnitude found in this study (Fig. 3) can be inferred from human studies showing that the risk of sudden death increases 5.3 times with a decrease in pulse interval variability from >100 ms to <50 ms in patients recovering from myocardial infarction (29). Baroreflex modulation of heart rate depends to a large extent on vagal activity, and the high-frequency component of the PI power spectrum contains most of the oscillatory power, being largely considered as a marker of parasympathetic activity (8, 36, 38, 40, 50). While a precise relative contribution of sympathetic and parasympathetic influences on the heart cannot be accurately quantified by frequency-specific spectral analysis of heart rate oscillations, such as by the low frequency (LF)/high frequency (HF) approach (9, 14), the marked changes in the daily volume of heart rate spectral power during weight gain and subsequent baroreflex activation, manifested across the frequency spectrum, indicate that changes in parasympathetic activity played a major role in mediating the parallel changes in heart rate and heart rate variability that occurred during daily activity in the present study. However, it should be noted that heart rate variability can be affected by events independent of cardiac autonomic nerve activity (8, 9). In fact, for mathematical reasons, reductions in heart rate per se will increase heart rate variability. Therefore, at least some of the increase in heart rate variability with baroreflex activation may be independent of cardiac autonomic regulation.
Renal denervation and cardiac autonomic function.
In contrast to the marked improvements in baroreflex sensitivity, heart rate, and heart rate variability during stimulation of the carotid sinus, these abnormalities remained after renal denervation. These findings are consistent with the previously reported measurements of plasma norepinephrine concentration in these animals (33). As reported previously, while both baroreflex activation and renal denervation completely abolished the hypertension associated with obesity (Fig. 1), only the former decreased plasma norepinephrine concentration, indicating a sustained global sympathoinhibitory effect of baroreflex activation not shared by renal denervation. These observations do highlight the importance of increased efferent renal sympathetic nerve activity in mediating obesity-induced hypertension and the capacity of baroreflex activation, as well as renal denervation, to abrogate renal sympathoexcitation (33). However, they do not support the hypothesis that sensory afferent signals from the kidneys to the CNS contribute to chronic sympathetic overactivity in resistant hypertension (49), despite obesity being a common feature in these individuals (10, 52). In this regard, recent reports in patients with resistant hypertension indicate both unchanged (11) and reduced (24) sympathetic activity to skeletal muscle vasculature several months after renal denervation. Thus these discordant observations in obese patients with resistant hypertension add to this controversial issue. Regardless of these reports, the absence of changes in plasma norepinephrine concentration, baroreflex sensitivity, heart rate, and heart rate variability after renal denervation in the obese dogs of the present study suggests that neither reduced cardiac sympathetic activity nor the concomitant increases in parasympathetic activity that may be associated with central sympathoinhibition (36, 54) contribute to improvement in cardiac autonomic dysfunction as a result of diminishing renal afferent nerve activity.
Blood pressure variability in obesity.
Blood pressure variability usually increases in conditions characterized by sympathetic activation (38, 40), and this increase is considered to be a risk factor for target-organ damage (39). Because our previous findings in normotensive dogs indicated that baroreflex activation decreased blood pressure variability along with suppression of sympathetic activity (32), we determined whether baroreflex activation might diminish increased fluctuations in blood pressure, if present, in the current model of sympathetically induced hypertension. However, both time- and frequency-domain measures actually revealed marked reductions in blood pressure variability with weight gain that were not further altered by baroreflex activation and renal denervation. Decreased blood pressure variability with weight gain may be attributed to factors contributing to the reduced peripheral resistance in obesity (22), presumably mediated, at least in part, by the vasodilator effects of insulin and local influences related to increased metabolic rate.
Study limitations.
Because of the nature of the study, a potential limitation was that randomization of interventions was not possible. That is, baroreflex activation had to precede renal denervation, and time-dependent control studies in obese dogs were not performed. However, dietary intake and body weight were constant throughout the established phase of obesity hypertension, and recovery values 1 wk after baroreflex activation were similar to those measured before carotid sinus stimulation. Furthermore, as previously noted, at least blood pressure and heart rate are stable for at least 5 wk after establishment of obesity hypertension (33). To capture the transient changes following renal denervation, measurements of cardiovascular dynamics were recorded immediately after surgery. While the surgical procedure may have impacted responses during the first 3–4 days after renal denervation, the stable values of variables recorded subsequently indicate that any nonspecific postoperative effects were transient. Therefore, we believe that time-dependent changes were insignificant and unlikely to have influenced the findings in this study. A final point is that one should be cautious in extrapolating the present differential effects of baroreflex activation and renal denervation in this experimental model of obesity hypertension to patients with the more complex pathophysiology of resistant hypertension, particularly when clinical findings are most commonly reported months and years after device-based therapy.
Perspectives
Experimental and clinical studies have demonstrated that impaired cardiac baroreflex sensitivity and decreased heart rate variability are two major negative prognostic indicators of cardiovascular mortality, including sudden cardiac death, in patients with congestive heart failure and chronic kidney disease and in post-myocardial infarction patients (7, 8, 26, 50, 56, 57). These studies have led to the concept that the arrhythmogenic effects of sympathetic hyperactivity can be antagonized by the parasympathetic nervous system and that impairment in vagal activation plays a major role in the occurrence of arrhythmic events. As the ability to augment vagal activity can be quantified by baroreflex sensitivity, the inference from the present study is that impaired baroreflex sensitivity and decreased heart rate variability may help identify individuals with obesity hypertension who are at high risk for atrial fibrillation and other arrhythmic events. If so, an unappreciated benefit of baroreflex activation therapy in patients with resistant hypertension, who are commonly obese, may be diminishing the risk of cardiac arrhythmias not only by lowering arterial pressure but also by improving cardiac baroreflex sensitivity and shifting autonomic balance toward reduced sympathetic drive and increased vagal activity. The potential benefit of global suppression of sympathetic activity and restoration of baroreflex sensitivity in preventing cardiac arrhythmias during baroreflex activation therapy may be especially relevant to patients with resistant hypertension, because increased body weight and arterial pressure increase the risk for developing heart failure (30) and obesity and hypertension exacerbate the levels of sympathoexcitation and baroreflex dysfunction associated with heart failure (21).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-51971.
DISCLOSURES
T. E. Lohmeier and E. D. Irwin are consultants to CVRx, Inc.
AUTHOR CONTRIBUTIONS
Author contributions: R.I. and T.E.L. conception and design of research; R.I., E.D.I., and T.E.L. performed experiments; R.I., I.T., and T.E.L. analyzed data; R.I., I.T., and T.E.L. interpreted results of experiments; R.I. and I.T. prepared figures; R.I., E.D.I., and T.E.L. drafted manuscript; R.I., E.D.I., and T.E.L. edited and revised manuscript; R.I., I.T., E.D.I., and T.E.L. approved final version of manuscript.
REFERENCES
- 1.Alvarez GE, Davy BM, Ballard TP, Beske SD, Davy KP. Weight loss increases cardiovagal baroreflex function in obese and older men. Am J Physiol Endocrinol Metab 289: E665–E669, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Arnold AC, Shaltout HA, Gallagher PE, Diz DI. Leptin impairs cardiovagal baroreflex function at the level of the solitary tract nucleus. Hypertension 54: 1001–1008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronne LJ, Mackintosh R, Rosenbaum M, Leibel RL, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol Regul Integr Comp Physiol 269: R222–R225, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 98: 946–952, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Bernston GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 34: 623–648, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Beske S, Alvarez GE, Ballard TP, Davy KP. Reduced cardiovagal baroreflex gain in visceral obesity: implications for the metabolic syndrome. Am J Physiol Heart Circ Physiol 282: H630–H635, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Billman GE. Cardiac autonomic neural remodeling and susceptibility to sudden cardiac death: effect of endurance exercise training. Am J Physiol Heart Circ Physiol 297: H1171–H1193, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Billman GE. Heart rate variability—a historical perspective. Front Physiol 2: 1–13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol 4: 1–5, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, de Leeuw PW, Sica DA. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled Rheos Pivotal Trial. J Am Coll Cardiol 58: 765–773, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Brinkmann J, Heusser K, Schmidt BM, Menne J, Klein G, Bauersachs J, Haller H, Sweep FC, Diedrich A, Jordan J, Tank J. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients. Prospective case studies. Hypertension 60: 1485–1490, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D'Alessio D, Tso P, Seeley RJ, Woods SC. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav 103: 10–16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dengo AL, Dennis EA, Orr JS, Marinik EL, Ehrlich E, Davy B, Davy KP. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension 55: 855–861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation 96: 3224–3232, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 48: 787–796, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Frankel RA, Metting PJ, Britton SL. Evaluation of spontaneous baroreflex sensitivity in conscious dogs. J Physiol 462: 31–45, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med 118: 489–495, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Gadegbeku CA, Dhandayuthapani A, Sadler ZA, Egan BM. Raising lipids acutely reduces baroreflex sensitivity. Am J Hypertens 15: 479–485, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 97: 2037–2042, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Grassi G, Seravalle G, Dell'Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension 36: 538–542, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Grassi G, Seravalle G, Quarti-Trevano F, Dell'Oro R, Bolla G, Mancia G. Effects of hypertension and obesity on the sympathetic activation of heart failure patients. Hypertension 42: 873–877, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Hall JE, Brands MW, Dixon WN, Smith MJ., Jr Obesity-induced hypertension. Renal function and systemic hemodynamics. Hypertension 22: 292–299, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Hall JE, da Silva AA, Brandon E, Stec DE, Ying Z, Jones DW. Pathophysiology of obesity-induced hypertension and target organ damage. In: Comprehensive Hypertension, edited by Lip GY, Hall JE. New York: Elsevier, 2007, p. 447–468 [Google Scholar]
- 24.Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 61: 457–464, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, Peters T, Sweep FC, Haller H, Pichlmaier A, Luft F, Jordan J. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension 55: 619–626, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Hildreth CM. Prognostic indicators of cardiovascular risk in renal disease. Front Physiol 2: 1–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber DA, Schreihofer AM. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J Physiol 588.9: 1515–1525, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iliescu R, Cazan R, McLemore GR, Jr, Venegas-Pont M, Ryan MJ. Renal blood flow and dynamic autoregulation in conscious mice. Am J Physiol Renal Physiol 295: F734–F740, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59: 256–262, 1987 [DOI] [PubMed] [Google Scholar]
- 30.Lee DS, Massaro JM, Wang TJ, Kannel WB, Benjamin EJ, Kenchaiah S, Levy D, D'Agostino RB, Vasan RS. Antecedent blood pressure, body mass index, and risk of incident heart failure in later life. Hypertension 50: 869–876, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Lohmeier TE, Iliescu R. Chronic lowering of blood pressure by carotid baroreflex activation. Mechanisms and potential for hypertension therapy. Hypertension 57: 880–886, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lohmeier TE, Iliescu R, Dwyer TM, Irwin ED, Cates AW, Rossing MA. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Circ Physiol 299: H402–H409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension 59: 331–338, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCully BH, Brooks VL, Andresen MC. Diet-induced obesity severely impairs myelinated aortic baroreceptor reflex responses. Am J Physiol Heart Circ Physiol 302: H2083–H2091, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monahan KD, Dyckman DJ, Ray CA. Effect of acute hyperlipidemia on autonomic and cardiovascular control in humans. J Appl Physiol 103: 162–169, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'Orto S, Piccaluga E, Turiel M, Baselli G, Cerruti S, Malliani A. Power spectral analysis of heart rate and sympatho-vagal interaction in man and conscious dog. Circ Res 59: 178–193, 1986 [DOI] [PubMed] [Google Scholar]
- 37.Parati G, Di Rienzo M, Castiglioni P, Bouhaddi M, Cerutti C, Cividjian A, Elghozi JL, Fortrat JO, Girard A, Janssen BJ, Julien C, Karemaker JM, Iellamo F, Laude D, Lukoshkova E, Pagani M, Persson PB, Quintin L, Regnard J, Ruediger JH, Saul PJ, Vettorello M, Wesseling KH, Mancia G, European Society of Hypertension Working Group on Blood Pressure and Heart Rate Variability Assessing the sensitivity of spontaneous baroreflex control of the heart: deeper insight into complex physiology. Hypertension 43: e32–e34, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Parati G, Mancia G, Di Rienzo M. Point:Counterpoint: cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol 101: 676–682, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Parati G, Pomidossi G, Albini F, Malaspina D, Manvia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens 5: 93–98, 1987 [DOI] [PubMed] [Google Scholar]
- 40.Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension 25: 1276–1286, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Persson PB, Baumann JE, Ehmke H, Nafz B, Wittmann U, Kirchheim HR. Phasic and 24-h blood pressure control by endothelium-derived relaxing factor in conscious dogs. Am J Physiol Heart Circ Physiol 262: H1395–H1400, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Pietrasik G, Goldenberg I, McNitt S, Moss AJ, Zareba W. Obesity as a risk factor for sustained ventricular tachyarrhythmias in MADIT II patients. J Cardiovasc Electrophysiol 18: 87–95, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Pinna GD, Maestri R. New criteria for estimating baroreflex sensitivity using transfer function method. Med Biol Eng Comput 40: 79–84, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113: 898–918, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Porta A, Baselli G, Rimoldi O, Malliani A, Pagani M. Assessing baroreflex gain from spontaneous variability in conscious dogs: role of causality and respiration. Am J Physiol Heart Circ Physiol 279: H2558–H2567, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Reed MJ, Robertson CE, Addison PS. Heart rate variability measurements and the prediction of ventricular arrhythmias. QJM 98: 87–95, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Reid I. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in the regulation of blood pressure. Am J Physiol Endocrinol Metab 262: E262–E778, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, Wiesner GH, Brunner-La Rocca HP, Esler MD. Neural mechanisms in human obesity-related hypertension. J Hypertens 17: 1125–1133, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension. Novel implications for an old concept. Hypertension 54: 1195–1201, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Schwartz PJ, De Ferrari GM. Sympathetic-parasympathetic interaction in health and disease: abnormalities and relevance in heart failure. Heart Fail Rev 16: 101–107, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Skrapari I, Tentolouris T, Perrea D, Bakoyiannis C, Papazafiropoulou A, Katsilambros N. Baroreflex sensitivity in obesity: relationship with cardiac autonomic nervous system activity. Obesity (Silver Spring) 15: 1685–1693, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Symplicity HTN-2 Investigators Renal sympathetic denervation in patients with treatment resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 376: 1903–1909, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Takagishi M, Waki H, Bhuiyan ME, Gouraud SS, Kohsaka A, Cui H, Yamazaki T, Paton JF, Maeda M. IL-6 microinjected in the nucleus tractus solitarii attenuates cardiac baroreceptor reflex function in rats. Am J Physiol Regul Integr Comp Physiol 298: R183–R190, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Tank J, Diedrich A, Szezech E, Luft F, Jordan J. Alpha-2 adrenergic transmission and human baroreflex regulation. Hypertension 43: 1035–1041, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Truett AA, Borne AT, Poincot MA, West DB. Autonomic control of blood pressure and heart rate in obese hypertensive dogs. Am J Physiol Regul Integr Comp Physiol 270: R541–R549, 1996 [DOI] [PubMed] [Google Scholar]
- 56.Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 94: 2850–2855, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation 90: 878–883, 1994 [DOI] [PubMed] [Google Scholar]
- 58.Van Vliet BN, Hall JE, Mizelle HL, Montani JP, Smith MJ., Jr Reduced parasympathetic control of heart rate in obese dogs. Am J Physiol Heart Circ Physiol 269: H629–H637, 1995 [DOI] [PubMed] [Google Scholar]
- 59.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity—results of a meta-analysis. Am Heart J 155: 310–315, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA 292: 2471–2477, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Wustmann K, Kucera JP, Scheffers I, Mohaupt M, Kroon AA, de Leeuw PW, Schmidli J, Allemann Y, Delacretaz E. Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension 54: 530–536, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Zhao D, McCully BH, Brooks VL. Rosiglitazone improves insulin sensitivity and baroreflex gain in rats with diet-induced obesity. J Pharmacol Exp Ther 343: 206–213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]