Abstract
The individual effects of estrogen and progesterone on baroreflex function remain poorly understood. We sought to determine how estradiol (E2) and progesterone (P4) independently alter the carotid-cardiac and carotid-vasomotor baroreflexes in young women by using a hormone suppression and exogenous add-back design. Thirty-two young women were divided into two groups and studied under three conditions: 1) after 4 days of endogenous hormone suppression with a gonadotropin releasing hormone antagonist (control condition), 2) after continued suppression and 3 to 4 days of supplementation with either 200 mg/day oral progesterone (N = 16) or 0.1 to 0.2 mg/day transdermal 17β-estradiol (N = 16), and 3) after continued suppression and 3 to 4 days of supplementation with both hormones. Changes in heart rate (HR), mean arterial pressure (MAP), and femoral vascular conductance (FVC) were measured in response to 5 s of +50 mmHg external neck pressure to unload the carotid baroreceptors. Significant hormone effects on the change in HR, MAP, and FVC from baseline at the onset of neck pressure were determined using mixed model covariate analyses accounting for P4 and E2 plasma concentrations. Neither P4 (P = 0.95) nor E2 (P = 0.95) affected the HR response to neck pressure. Higher P4 concentrations were associated with an attenuated fall in FVC (P = 0.01), whereas higher E2 concentrations were associated with an augmented fall in FVC (P = 0.02). Higher E2 was also associated with an augmented rise in MAP (P = 0.01). We conclude that progesterone blunts whereas estradiol enhances carotid-vasomotor baroreflex sensitivity, perhaps explaining why no differences in sympathetic baroreflex sensitivity are commonly reported between low and high combined hormone phases of the menstrual cycle.
Keywords: menstrual cycle, hormones, estrogen, arterial baroreceptors, blood pressure
both the cardiovagal and sympathetic branches of the baroreflex are important for the short-term control of blood pressure. Clear differences in cardiovagal and sympathetic baroreflex sensitivity have been documented between men and women (1, 2, 26, 42, 43), and women experience higher rates of orthostatic hypotension compared with men (7, 32, 39). Furthermore, the incidence of hypertension and cardiovascular disease is greatly increased in woman after menopause (23). These data provide evidence that the female sex hormones, estrogen and progesterone, influence the cardiovascular system; however, these influences are still poorly understood.
In young women, the cardiovascular effects of the female sex hormones have predominantly been studied across the natural menstrual cycle. The majority of studies have found no differences in both cardiovagal (8, 17, 29) and sympathetic (6, 14, 22) baroreflex sensitivity between the early follicular (low hormone) and mid-luteal (high estrogen and progesterone) phases, but this is not true of all studies (20, 42, 44). It is possible that estrogen and progesterone have opposing influences on the baroreflex, necessitating study of the individual actions of the hormones. For example, sympathetic baroreflex sensitivity is enhanced by estrogen replacement in postmenopausal women (21) and is positively correlated with blood estradiol concentrations in young naturally menstruating women (29), whereas progesterone administration decreases sympathetic baroreflex sensitivity in animals (3, 19). Few studies to date have studied the independent effects of estrogen on baroreflex function in young women, and none have studied the effects of progesterone. The study of high progesterone in conjunction with low estrogen is important, not only to discern the separate roles of endogenous estrogen and progesterone but also because large numbers of women are now using progestin-only treatments (e.g., depot medroxyprogesterone acetate injections and subcutaneous etonogestrel implants, such as Implanon and Nexplanon) for contraception and treatment of gynecological conditions.
The effects of the female sex hormones on baroreflex sensitivity have most commonly been assessed using the Modified Oxford method, in which bolus doses of vasoactive drugs are administered to simulate hypotension and hypertension. However, this technique does not allow for the measurement of resultant changes in blood pressure or vascular conductance following baroreceptor stimulation due to the influence of the vasoactive drugs on these responses. Sympatho-excitatory maneuvers, such as externally applied neck pressure, can be used to study cardiovagal and sympathetic baroreflex sensitivity, as well as vascular transduction. Application of external pressure, through the use of a neck collar, unloads the carotid baroreceptors, thus simulating a hypotensive state. Unloading of the carotid baroreceptors with neck pressure results in a robust increase in heart rate (HR) and muscle sympathetic nerve activity (MSNA) rapidly following the onset of external pressure (37).
One study recently used external neck pressure to examine differences in baroreflex sensitivity across the early follicular, ovulatory, and mid-luteal phases of the menstrual cycle (24). The study found no differences in the response of HR across phases, but researchers did observe an augmented increase in mean arterial pressure (MAP) to neck pressure during the mid-luteal phase when both estrogen and progesterone are high compared with the early-follicular and ovulatory phases. These results suggest either an effect of progesterone or an interactive effect of combined progesterone and estrogen on the MAP response. Testing the effects of high progesterone in conjunction with low estrogen is required to determine which interpretation is accurate. Furthermore, testing the response of vascular conductance to neck pressure is required to attribute alterations in carotid-MAP baroreflex sensitivity to changes in either the cardiac or vasomotor arms of the carotid baroreflex.
Thus the present study sought to determine the independent and interactive effects of estradiol and progesterone on baroreflex sensitivity in response to unloading of the carotid baroreceptors. We measured the changes in HR, MAP, and femoral vascular conductance (FVC) in response to a stimulus of +50 mmHg external neck pressure. To study the independent effects of the hormones, we used a hormone add-back model in which we suppressed endogenous sex hormones and then supplemented either estradiol or progesterone, followed by supplementation with both hormones to observe the combined effects. We hypothesized the magnitude of the change in HR in response to carotid baroreceptor unloading would not be altered by the administration of either estradiol or progesterone, but that administration of estradiol would increase the magnitudes of the change in MAP and FVC (increased sensitivity) and that administration of progesterone would decrease the magnitudes of the change in MAP and FVC (decreased sensitivity). Furthermore, we hypothesized that the magnitudes of the MAP and FVC responses during combined hormone administration would not significantly differ from those during hormone suppression.
MATERIALS AND METHODS
All protocols were approved by the Institutional Review Board of the University of Oregon. All subjects gave oral and written informed consent before participation in the study as set forth by the Declaration of Helsinki.
Thirty-two healthy young (age, 21.5 ± 0.4 years; body mass index, 22.3 ± 0.5 kg/m2) female subjects participated in the study. All subjects were nonsmokers and were not taking prescription medications, with the exception of combined contraceptives, which were discontinued at least 48 h before participation in the study. All subjects were screened by a physician specializing in gynecology and reproductive endocrinology with extensive clinical experience with GnRH analog use. Subjects were excluded if they had cardiovascular disease, hypertension, hypercholesterolemia, metabolic disease, menstrual disorders, or a personal or family history of blood clots. All subjects were required to provide a negative pregnancy test before beginning supplementation with study drugs and on each experimental day. On the same study days, flow-mediated dilation was assessed in 26 of our subjects, the results of which are published elsewhere (28).
Hormone manipulation.
To study the individual effects of estrogen and progesterone, we used a hormone add-back model in which all endogenous sex hormones were suppressed, and then estradiol and progesterone were administered exogenously (40). Subjects were studied under three conditions: first after 3 to 4 days of hormone suppression (Study Day 1), second after 3 to 4 days of supplementation with either estradiol or progesterone (Study Day 2), and third after another 3 to 4 days of supplementation with both hormones (Study Day 3). Sex hormones were suppressed using a daily 250 μg/0.5 ml subcutaneous injection of gonadotropin-releasing hormone antagonist (GnRHa, Ganirelix acetate; Organon International, Roseland, NJ). Ganirelix competitively inhibits GnRH receptors in the anterior pituitary, reducing the release of follicle stimulating hormone (FSH) and luteinizing hormone (LH), and thus downregulating production of estrogen and progesterone. Endogenous estrogens and progesterone are suppressed within 36 to 48 h of treatment with GnRHa (34). Suppression was initiated within 1 to 4 days of the start of menses, such that endogenous hormones were already low, and was continued throughout the time course of the study (10–12 days total). Subjects were trained by the research staff on how to self-administer the injection. After Study Day 1, subjects were divided into two groups. Subjects in group 1 (N = 16) were supplemented with 200 mg/day oral progesterone (P4, Prometrium; Solvay Pharmaceuticals, Marietta, GA). Progesterone was taken at the same time each evening. Subjects in Group 2 (N = 16) were supplemented with 0.1 to 0.2 mg/day 17β-estradiol (E2, Estradiol; Mylan Pharmaceuticals, Morgantown, WV) via transdermal patch. Subjects were studied a third time after 3 to 4 days of supplementation with both hormones to examine the combined effects of the hormones (Study Day 3). Subjects underwent identical protocols on all three study days. Subjects were initially supplemented with 0.1 mg/day estradiol (single-dose: group 1, N = 9; Group 2, N = 8). This dose resulted in a substantial increase in E2 by Study Day 2; however, by Study Day 3, E2 was significantly lower than in Study Day 2, although still significantly elevated above suppression levels. This may have been caused by the characteristics of the transdermal patch to deliver E2. Although patches were marketed to deliver equal doses of E2 each day for a week, it is possible the dose was reduced toward the end of the week. Thus we doubled the dose to 0.2 mg/day in subsequent subjects and required subjects to change the patches after Study Day 2 (double-dose: Group 1, N = 7; Group 2, N = 8).
Measurements.
On experimental days, HR and blood pressure were monitored throughout the protocol. HR was measured using 5-lead electrocardiogram (CardioCap; Datex-Ohmeda, Louisville, CO). Beat-by-beat blood pressure was measured on a finger using photoplethysmography (Nexfin; BMEYE, Amsterdam, The Netherlands) and was verified via brachial oscillation (CardioCap).
The femoral artery was imaged above the bifurcation using high-resolution Doppler ultrasound (Terason t3000cv; Teratech, Burlington, MA). The entire width of the artery was insonated at 60 degrees using a 10.0-MHz linear array ultrasound transducer probe. The image and velocity tracing were captured at 20 frames/s for later analysis (described below; Camtasia Studio; TechSmith, Okemos, MI).
Experimental protocol.
On all three experimental days, subjects arrived at the laboratory after overnight fast and having abstained from alcohol and caffeine for 12 h and all over-the-counter medications and supplements for 24 h. Subjects rested supine for at least 40 min before the beginning of the protocol. Also before the protocol, venous blood samples were taken from an antecubital vein and transferred to vacutainer (BD Vacutainer, Franklin, NJ). They were centrifuged at 1,300 g relative centrifugal force for 15 min at 4°C. Plasma was separated from the formed content and stored in a subzero freezer until the samples were shipped for testing (Oregon Clinical and Translational Research Institute, Portland, OR). Plasma samples were analyzed for concentrations of estradiol via ultrasensitive radioimmunoassay (Beckman Coulter, Brea, CA; lower sensitivity, 2.2 pg/ml) and progesterone via chemiluminescent-based immunoassay (Immulite 1000 analyzer; Siemens Medical Solutions, Malvern, PA; lower sensitivity, 0.2 ng/ml).
A neck collar was fitted around the anterior two-thirds of the subjects' necks. The collar was fit to produce a seal around the mandible, clavicles, and sternum. The neck collar was set to deliver 50 mmHg of external positive pressure for 5 s using a programmable pressure controller (PPC-1000; Engineering Development Laboratory, Newport News, VA), thus unloading the baroreceptors and simulating a decrease in arterial pressure. Before placement of the neck collar, the common carotid artery was imaged using Doppler ultrasound (Terason) to ensure the bifurcation was within the area that would receive the neck pressure.
Five to eight trials of neck pressure were performed. For each trial, subjects held their breath following end-tidal exhalation. Subjects held their breath for 3 s before neck pressure, for the 5 s of neck pressure, and for 5 s following the release of neck pressure. The onset of neck pressure was timed to the R wave of the ECG. Between trials, breathing was paced to 12 breaths/min to minimize the effects of ventilation on HR and sympathetic activity. Two trials of just a breath hold were interspersed among the neck pressure trials. For the breath-hold trials, subjects held their breath for the same amount of time as in the neck pressure trials (13 s), but external neck pressure was not delivered. Breath hold trials were performed to ensure the changes in HR, MAP, and FVC seen in the neck pressure trials were the result of carotid baroreceptor unloading, and not the result of the breath hold.
Data analysis.
HR and blood pressure data were digitized and stored on a computer for analysis offline using signal-processing software (Windaq; Dataq Instruments, Akron, OH). HR was calculated from the R-R intervals from the ECG. Mean arterial blood pressure was calculated as the average blood pressure per cardiac cycle and was corrected back to the blood pressure attained from brachial oscillation.
Femoral ultrasound data were transferred to a computer with a custom-designed edge-detection and wall-tracking analysis software (DICOM; Perth, Australia). The software detects vessel wall diameter and peak blood velocity in real time. Together, these measurements provide a calculation of blood flow (the product of cross-sectional area and blood velocity) (45). FVC was calculated as (peak blood velocity/2) × vessel cross-sectional area (from diameter)/MAP.
Beat-by-beat values for HR, MAP, and FVC were zero-hold interpolated to 5 Hz. These data were then presented as a change from time zero (the onset of neck pressure). Neck pressure trials were excluded if they did not exhibit a blood pressure response consistent with proper stimulation of the baroreceptors. Appropriate baroreflex-mediated responses were determined as an increase in MAP ≥ 2 mmHg, which reached a peak at a time consistent with other trials within each subject (range 3–5 s following the onset of neck pressure). Data were averaged across all appropriate trials and then down-sampled to 1 Hz before determination of the average baroreflex response for each subject for each study day.
The HR, MAP, and FVC responses to breath hold were analyzed and characterized in the same manner, including being presented as a change from time zero, which was determined as 3 s following the onset of breath hold to match with time zero in the neck pressure trials. In the majority of subjects, we saw no change in MAP across the duration of the breath hold. However, some subjects exhibited deviations in MAP of up to 4 mmHg. In these subjects, there was no consistency or pattern across trials. Thus the average HR, MAP, and FVC breath hold responses were subtracted from the average neck pressure responses to separate the effects of breath hold from those of baroreceptor unloading.
In addition to presenting the data across time, responses to neck pressure were characterized by calculating the following parameters: peak change in HR during neck pressure (ΔHRpeak), peak change in MAP (ΔMAPpeak), and nadir change in FVC from baseline (ΔFVCnadir). Changes in HR and MAP in response to neck pressure were plotted against carotid sinus pressure to determine HR and MAP carotid-baroreflex gain. Carotid sinus pressure was calculated as resting MAP minus 50 mmHg.
Statistical analysis.
HR, MAP, and FVC across time, time to peak HR and MAP, and baseline cardiovascular and hemodynamic data were compared across study days using repeated-measures ANOVA. Pairwise interactions were analyzed using the Student-Newman-Keuls post hoc test. Student's unpaired two-tailed t-tests were used to compare demographic data across groups.
Outcome parameters (ΔHRpeak, HR barorelex gain, ΔMAPpeak, MAP baroreflex gain, and ΔFVCnadir) were compared across hormone conditions in three ways: 1) repeated-measures ANOVA was used to determine significant differences within groups across study days. Significant interactions were determined using the Student-Newman-Keuls post hoc test; 2) E2 and P4 were modeled as time-varying covariates to examine the predictive effects of these time-specific measurements on time-specific outcomes, after accounting for any study day and group effects as well as resting HR and resting MAP (time-varying covariates mixed model). E2 and P4 were considered as covariates since other measures of autonomic function have previously been shown to depend on the relative concentrations of E2 and P4 (4); 3) the outcome parameters were expressed as a change from Study Day 1 (GnRHa) and modeled as a function of the change in E2 and P4 using approaches that completely partition between and within subject effects (change in predictors and change in outcomes mixed model) (18, 33), and after accounting for any study day and group effects. Random-intercept linear mixed models with unconstructed covariance and maximum likelihood estimation were specified for the latter two sets of models, first for all subjects combined and then separately by group.
All data are presented as means ± SE. Statistical significance was set to α = 0.05.
RESULTS
Age, BMI, and resting MAP were not different for subjects across the treatment groups. There were no differences across study days in resting mean blood velocity, diameter, femoral blood flow, or FVC in either group. There were also no differences in resting HR across study days except for a slight increase in HR that was observed in the E2-first group on Study Day 3 (combined hormones). These results are summarized in Table 1.
Table 1.
Baseline hemodynamic data and hormone concentrations across study days
|
Study Day 1 |
Study Day 2 |
Study Day 3 |
Study Day 1 |
Study Day 2 |
Study Day 3 |
|
|---|---|---|---|---|---|---|
| GnRHa | GnRHa + P4 | GnRHa + E2 + P4 | GnRHa | GnRHa + E2 | GnRHa + E2 + P4 | |
| HR, beats/min | 61.7 ± 1.9 | 61.1 ± 1.7 | 63.7 ± 2.2 | 66.3 ± 2.2‡ | 64.2 ± 2.7 | 69.5 ± 2.5†‡ |
| MAP, mmHg | 83.7 ± 1.9 | 83.9 ± 1.6 | 82.7 ± 1.6 | 84.1 ± 1.4 | 82.4 ± 1.0 | 81.6 ± 1.2 |
| Mean blood velocity, cm/s | 12.4 ± 1.2 | 10.4 ± 0.9 | 11.9 ± 1.5 | 14.2 ± 1.9 | 12.1 ± 1.0 | 14.1 ± 1.5 |
| Diameter, mm | 8.4 ± 0.2 | 7.9 ± 0.3 | 7.8 ± 0.3 | 7.9 ± 0.2 | 7.9 ± 0.3 | 7.8 ± 0.3 |
| Femoral blood flow, ml/min | 208.5 ± 27.5 | 154.0 ± 15.5 | 174.5 ± 22.2 | 220.9 ± 35.0 | 176.6 ± 21.2 | 204.9 ± 23.5 |
| FVC, ml·min−1· mmHg−1 | 2.5 ± 0.3 | 1.9 ± 0.2 | 2.1 ± 0.3 | 2.6 ± 0.4 | 2.1 ± 0.2 | 2.5 ± 0.3 |
| Estradiol, pg/ml | ||||||
| 0.1 mg/day E2 | 16.0 ± 2.4 | 20.3 ± 2.9 | 87.9 ± 14.7*† | 16.0 ± 1.7 | 113.9 ± 25.0* | 65.3 ± 9.5*† |
| 0.2 mg/day E2 | 16.8 ± 2.1 | 20.7 ± 3.2 | 223.3 ± 32.4*† | 15.6 ± 2.2 | 192.5 ± 37.0* | 207.3 ± 43.9* |
| All subjects combined | 16.3 ± 1.6 | 20.5 ± 2.1 | 147.1 ± 23.4*† | 15.8 ± 1.3 | 153.2 ± 23.8* | 141.0 ± 29.8* |
| Progesterone, ng/ml | 1.7 ± 0.2 | 5.5 ± 0.5* | 6.3 ± 0.9* | 1.6 ± 0.2 | 1.6 ± 0.2 | 5.6 ± 0.4*† |
Values are means ± SE. Subjects were supplemented with one of two doses of estradiol (E2): 0.1 mg/day (Group 1, N = 9; Group 2, N = 7) or 0.2 mg/day (Group 1, N = 8; Group 2, N = 8). Data for all other parameters includes all subjects combined (Group 1, N = 16; Group 2, N = 16). HR, heart rate; MAP, mean arterial pressure; FVC, femoral vascular conductance.
P < 0.05 compared with Study Day 1;
P < 0.05 compared with Study Day 2;
P < 0.05 compared with the same study day for Group 1.
In both groups, P4 and E2 concentrations were low in all subjects on the first study day, consistent with values observed during the early follicular phase of the menstrual cycle, and indicating adequate suppression of the sex hormones using the GnRH antagonist. In the group that was administered P4 first, P4 increased significantly between Study Day 1 (GnRHa only) and Study Day 2 and remained high for Study Day 3. E2 concentration remained low for Study Days 1 and 2 and was elevated for Study Day 3. In the group that was administered E2 first, E2 concentration was significantly elevated for Study Days 2 and 3; P4 remained suppressed for Study Days 1 and 2 and was then elevated for Study Day 3. E2 concentration was significantly higher in the subjects who received the double dose of E2. However, there were no subgroup differences in resting hemodynamic variables nor in the HR, MAP, and FVC responses to neck pressure. Furthermore, our statistical analyses account for E2 concentration. Therefore, all data from subjects who received both doses of E2 have been combined. E2 and P4 concentrations were significantly related to several of the outcomes. The results are summarized in Table 2 (time-varying covariate analysis) and Table 3 (change in predictors and change in outcomes mixed model analysis), and described below. In-text descriptions indicate whether the results were derived from the analyses highlighted in Tables 2 or 3.
Table 2.
Results of mixed models predicting time-specific outcome levels from time-specific hormone levels
| All Subjects | Group 1 (P4 First) | Group 2 (E2 First) | |
|---|---|---|---|
| N, female | 32 | 16 | 16 |
| ΔHRpeak | |||
| E2 | 0.59 (0.45) | 1.07 (0.31) | 0.35 (0.56) |
| P4 | 0.03 (0.86) | 0.56 (0.46) | 0.02 (0.90) |
| HR BR gain | |||
| E2 | 0.65 (0.42) | 0.83 (0.37) | 0.35 (0.56) |
| P4 | 0.02 (0.89) | 0.51 (0.48) | 0.02 (0.90) |
| ΔMAPpeak | |||
| E2 | 7.43 (0.008)* | 3.33 (0.08) | 3.23 (0.08) |
| P4 | 0.24 (0.63) | 1.40 (0.25) | 0.25 (0.62) |
| MAP BR gain | |||
| E2 | 6.98 (0.01)* | 2.63 (0.11) | 3.09 (0.09) |
| P4 | 0.24 (0.62) | 0.004 (0.95) | 0.13 (0.72) |
| ΔFVCnadir | |||
| E2 | 3.68 (0.06) | 1.64 (0.21) | 1.63 (0.21) |
| P4 | 5.08 (0.03)* | 17.57 (<0.001)* | 0.02 (0.88) |
Values are F(p). Concentrations of estradiol (E2) and progesterone (P4) were modeled as time-varying covariates on the time-specific outcomes, after accounting for effects of study day, group, resting HR, and resting MAP. Outcomes include the change in HR in response to 5 s of +50 mmHg neck pressure (ΔHRpeak), the carotid baroreflex gain between HR and estimated carotid sinus pressure (HR BR gain), the change in MAP in response to neck pressure (ΔMAPpeak), the carotid baroreflex gain between MAP and estimated carotid sinus pressure (MAP BR gain), and the fall in FVC in response to neck pressure (ΔFVCnadir).
P < 0.05.
Table 3.
Results of mixed models predicting change in outcomes from change in hormone
| All Subjects | Group 1 (P4 First) | Group 2 (E2 First) | |
|---|---|---|---|
| N, female | 32 | 16 | 16 |
| Difference in ΔHRpeak from Study Day 1 | |||
| ΔE2 | 0.003 (0.95) | 0.31 (0.59) | 0.003 (096) |
| ΔP4 | 0.005 (0.95) | 0.06 (0.81) | 0.07 (0.79) |
| Difference in HR BR gain from Study Day 1 | |||
| ΔE2 | 0.03 (0.86) | 0.09 (0.77) | 0.07 (0.79) |
| ΔP4 | 0.53 (0.47) | 0.06 (0.82) | 0.07 (0.80) |
| Difference in ΔMAPpeak from Study Day 1 | |||
| ΔE2 | 1.10 (0.30) | 3.62 (0.07) | 0.04 (0.85) |
| ΔP4 | 0.61 (0.44) | 0.58 (0.45) | 0.58 (0.46) |
| Difference in MAP BR gain from Study Day 1 | |||
| ΔE2 | 0.92 (0.34) | 0.96 (0.34) | 0.17 (0.68) |
| ΔP4 | 0.59 (0.45) | 0.10 (0.75) | 0.26 (0.61) |
| Difference in ΔFVCnadir from Study Day 1 | |||
| ΔE2 | 6.58 (0.01)* | 2.73 (0.11) | 5.46 (0.03)* |
| ΔP4 | 6.44 (0.02)* | 16.56 (<0.001)* | 0.02 (0.89) |
Values are F(p). The change in outcome variables from Study Day 1 modeled as a function of the change in estradiol (ΔE2) and progesterone (ΔP4) concentrations from each subject's personal mean. Effects of study day, group, resting HR, and resting MAP were accounted for. Outcome variables include the change in ΔHRpeak, HR BR gain, ΔMAPpeak, MAP BR gain, and ΔFVCnadir.
P < 0.05.
Heart rate.
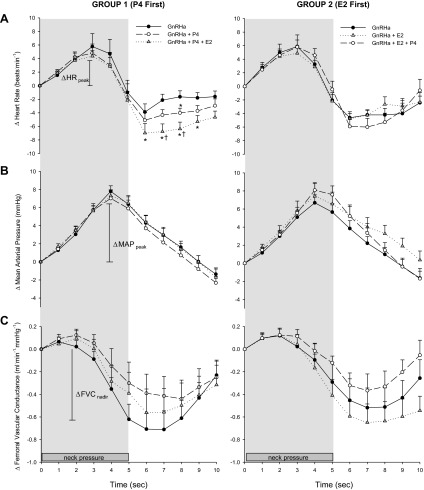
Simulated hypotension from the application of external pressure to the neck resulted in an increase in HR, which reached a peak at 2.9 ± 0.1 s after the onset of neck pressure. Release of neck pressure resulted in a drop in HR below baseline, which returned back to baseline shortly thereafter (Fig. 1A). Time-to-peak HR did not significantly differ across study days or between groups. No significant effects of the hormones on the HR response to neck pressure were found using any of the three statistical analysis methods; however, ΔHRpeak was significantly related to resting MAP in all subjects combined (P = 0.03; time varying covariates mixed model, data not shown in tables), such that subjects with a higher resting MAP had a greater HR response to neck pressure. Interestingly, HR fell significantly lower after the release of neck pressure in subjects in Group 1 (P4 first) under combined hormone supplementation compared with suppression and P4 only (Fig. 1A). This effect may represent the response to rapid baroreceptor loading.
Fig. 1.
The change in heart rate (A), mean arterial pressure (B), and femoral vascular conductance (C) in both groups of subjects in response to 5 s of neck pressure across the 3 study days: 1) sex hormones suppression with a gonadotropin-releasing hormone antagonist (GnRHa), 2) administration of progesterone (P4; Group 1) or estradiol (E2; Group 2), and 3) administration of both P4 and E2. Data are presented as means ± SE. *P < 0.05 from Study Day 1; †P < 0.05 from Study Day 2.
MAP.
Neck pressure resulted in an increase in MAP, which reached a peak at 4.2 ± 0.08 s after the onset of neck pressure and then returned back to baseline following the release of neck pressure (Fig. 1B). Time-to-peak MAP did not significantly differ across study days or between groups. No significant effects of the hormones on the MAP response to neck pressure were found across study days. When accounting for hormone concentrations, E2 was positively related to ΔMAPpeak, such that E2 augmented the rise in MAP. This relationship was observed across both groups, but only approached significance within each group (P = 0.08 for both groups; Table 2). E2 was also related to a steeper MAP carotid baroreflex gain, meaning that administration of E2 resulted in an augmented MAP response for the same drop in carotid sinus pressure. There were no significant effects of P4 on the MAP response. Resting MAP was related to the magnitude of the rise in MAP in response to neck pressure in Group 2 only (P < 0.05; Table 3) and was related to MAP baroreflex gain in all subjects (P < 0.05; Table 2) and in Group 2 (P = 0.04, Table 2; P = 0.01, Table 3).
FVC.
Neck pressure resulted in a decrease in FVC, which reached a nadir 7.3 ± 0.2 s after the onset of neck pressure (Fig. 1C). No significant effects of the hormones on the response in FVC to neck pressure were observed across study days. When hormone concentrations in the mixed models were accounted for (Table 2), P4 was related to ΔFVCnadir, such that P4 attenuated the FVC response to neck pressure. However, this observation was only seen in Group 1 (P4 first) and with all subjects combined. When subjects received E2 first, P4 had no effect on the FVC response. When outcomes were presented as a change from Study Day 1 (Table 3), E2 also had a significant effect on ΔFVCnadir, but the opposite effect of P4. Administration of E2 augmented the FVC response to neck pressure. However, again, this observation was only significant in Group 2 (E2 first), indicating that when the subject received P4 first, administration of E2 had no effect on the FVC response.
DISCUSSION
Simulated hypotension via the application of external neck pressure produces a robust increase in HR, MAP, and MSNA and a decrease in vascular conductance (36, 37). The present study tested the effects of exogenous administration of estradiol and progesterone on isolated carotid baroreflex sensitivity by measuring the responses of HR, MAP, and FVC to 5 s of externally applied neck pressure. As hypothesized, we found that the HR response to neck pressure was not affected by either estradiol or progesterone, the response of MAP was augmented by estradiol but was not affected by progesterone, and the response of FVC was augmented by estradiol and attenuated by progesterone.
Carotid-baroreflex control of HR.
Prior work regarding the effects of the female sex hormones on baroreflex control of HR has been mixed. Most studies have found no effect of estrogen or progesterone on cardiac baroreflex sensitivity in women, including across the phases of the menstrual cycle (8, 17, 21, 29), in young women using combined oral contraceptives (5), and in postmenopausal women receiving estrogen-replacement therapy (21). However, some studies have observed differences across hormone conditions. For example, Wenner et al. (44) found an increase in sensitivity with estradiol administration, but only in women with low orthostatic tolerance. Tanaka et al. (42) found a decrease in sensitivity in response to hypotensive stimuli near ovulation (high estrogen but low progesterone) compared with the early follicular and mid-luteal phases, but an increase in sensitivity in response to hypertensive stimuli. Minson et al. (30) found a decrease in sensitivity in women taking combined oral contraceptives. It is possible differences in techniques for measuring cardiac baroreflex sensitivity account for the disparity in findings. Studies using the neck pressure technique have shown no differences in sensitivity across the menstrual cycle (8, 24), consistent with our results. We are the first to assess progesterone independently.
Carotid-baroreflex control of vascular conductance.
Baroreflex control of the vasculature is conducted through the sympathetic nervous system. The sex hormones may alter either sympathetic activity or the transduction of that sympathetic activity to the blood vessels. In young women, there have been mixed results as to whether the fluctuations in ovarian hormones alter baroreflex control of sympathetic activity. Minson et al. (29) reported an increase in MSNA sensitivity in the mid-luteal phase compared with the early follicular phase using the Modified Oxford method, whereas others have reported no changes across the same menstrual phases using a variety of techniques (6, 14, 22, 27). Similarly, combined oral contraceptives have been shown to either decrease (30) or have no effect on (5, 27) sympathetic baroreflex sensitivity. Studies in postmenopausal women and animals have shown estradiol and progesterone to have opposing effects on sympathetic baroreflex sensitivity, perhaps explaining why many studies have shown no differences in MSNA baroreflex sensitivity when comparing only low and high combined hormone states. Estrogen has been found to increase MSNA baroreflex sensitivity in postmenopausal women (11, 21). This effect is likely mediated in the brainstem, as demonstrated in ovariectomized rats (31, 38). No studies have documented the independent effects of progesterone on MSNA baroreflex sensitivity in humans; but in animal models, a metabolite of progesterone, 3α-hydroxy-dihydroprogesterone (3α-OH-DHP), has been shown to act in the brainstem to suppress sympathetic outflow, thus decreasing sympathetic baroreflex sensitivity (3, 19). It is also possible the changes in baroreflex sensitivity are dependent upon the relative plasma concentrations of estrogen and progesterone, which was recently shown to be true of resting MSNA in a study by Carter et al. (4).
Sympathetic activity is transduced to the blood vessels via adrenergic receptors. The vasoconstrictor response to neck pressure is mediated by α-adrenergic vasoconstriction, but limited by β-adrenergic vasodilation, since β-adrenergic blockade with propranolol enhances the response (36). Altering the sensitivity of adrenergic receptors is a possible means by which estrogen and progesterone modify the changes in vascular conductance observed in response to baroreceptor unloading, and there is evidence to suggest the hormones reduce α-adrenergic sensitivity and increase β-adrenergic sensitivity. However, because these studies either studied women under low hormone conditions (15) or did not control for menstrual cycle phase (13, 25), these sex differences cannot be attributed to estrogen and/or progesterone. In ovariectomized rats, estrogen supplementation has been documented to increase β-adrenergic sensitivity (12). However, this effect would produce the opposite results from what we observed (a blunting of the fall in FVC in response to neck pressure instead of an augmentation of the response), meaning that changes in β-adrenergic sensitivity are not driving the changes in FVC observed in the present study, but might be opposing them, i.e., the augmentation of vasomotor baroreflex sensitivity with E2 occurs despite possible opposing effects of changes in β-adrenergic sensitivity. To our knowledge, the actions of progesterone on β-adrenergic receptors have not been studied.
Changes in blood volume can also impact the carotid-baroreflex. Decreases in central blood volume, as simulated by lower body negative pressure and head-up tilt, increase carotid-cardiac and carotid-vasomotor baroreflex sensitivity (9, 10, 35). Studies by Stachenfeld and Taylor (40, 41) have shown estradiol and progesterone to both impact fluid regulation and to independently increase plasma volume using the same suppression and hormone add-back design used in the present study. In those studies, plasma volume increased within 4 days of administration of E2 and P4 (40, 41), indicating that our subjects likely also experienced increases in plasma volume with hormone administration. Assuming the relationship between central blood volume and carotid-baroreflex sensitivity is also true in light of increases in central volume, increases in plasma volume with the hormones would have contributed to a decrease in carotid-vasomotor baroreflex sensitivity, which is consistent with what we recorded following P4 administration. Thus, changes in plasma volume may, at least partially, explain our results regarding P4 administration. We observed an increase in carotid-vasomotor baroreflex sensitivity after E2 administration, despite the fact that predicted changes in plasma volume would have the opposite effect. Presumably, the mechanisms driving the increase in baroreflex sensitivity must have had a great enough effect on carotid-baroreflex control of vascular conductance to overcome the effects of increased plasma volume.
Because we did not measure MSNA or plasma volume in the present study, the mechanisms behind the observed changes in carotid-vasomotor baroreflex sensitivity are purely speculative. It is most likely that estrogen and progesterone affect the cardiovascular system via a combination of mechanisms. Based on the current literature, we believe estradiol increased carotid-vasomotor sensitivity by enhancing sympathetic outflow, despite possible opposing effects of increased β-adrenergic sensitivity and increased plasma volume. We propose progesterone blunted carotid-vasomotor sensitivity via a combination of blunted sympathetic outflow and increased plasma volume. Further studies are required to determine the mechanisms behind our observed results.
Carotid-baroreflex control of MAP.
We found E2 concentration to be significantly related to the response of MAP to neck pressure, such that E2 augmented the MAP response. This was observed both in the peak response of MAP to neck pressure and in the MAP carotid baroreflex gain, as determined by comparing changes in MAP to changes in estimated carotid sinus pressure. Furthermore, this effect of E2 on MAP was observed across both groups, including Group 2, which received E2 first (thus E2 was high both alone in Study Day 2 and in combination with P4 in Study Day 3), and Group 1, which received P4 first (thus E2 was only high in combination with P4). In normally menstruating young women, Kim et al. (24) found the MAP response to neck pressure to be augmented only when both hormones were high, and not during the ovulatory phase when only E2 was high, which indicates either the effect is due to progesterone or an interactive effect between the two hormones. We found no effect of progesterone on the MAP response to neck pressure when administered alone (Group 1, Study Day 2) or in combination with E2 (Groups 1 and 2, Study Day 3), suggesting that the observations of Kim et al. (24) reflect the actions of estrogen, and not of progesterone. The inconsistencies between our data and that of Kim et al. (24) may also be attributable to differing E2 concentrations or differences in endogenous versus exogenous hormones.
Both HR and FVC affect baroreflex control of MAP. Because there were no effects of the hormones on carotid-cardiac baroreflex sensitivity, it appears the augmentation of the MAP response by estrogen is the result of an increased vasoconstrictor response to neck pressure (as determined by FVC). Progesterone attenuated carotid-vasomotor baroreflex sensitivity, but had no effect on control of MAP, indicating that another mechanism must also be affected by progesterone. It may be that progesterone augments baroreflex control of stroke volume.
Limitations.
Previous studies have used a range of both neck pressure and neck suction to assess carotid baroreflex sensitivity and construct full baroreflex curves (24, 35, 37). We only assessed the responses to +50 mmHg of neck pressure. We adopted this approach because the study of baroreflex responses to hypotensive stimuli is more clinically relevant in young women than the study of baroreflex responses to hypertensive stimuli, since women experience much higher rates of orthostatic intolerance than men (32, 39), suggesting a role of the female sex hormones in that response. However, because we assessed only the responses to +50 mmHg of neck pressure, our results cannot be applied to hypertensive stimuli or to a wider spectrum of positive neck pressures.
Significant effects of the hormones were found only when accounting for hormone concentration in our mixed models. Differences across study days were nonsignificant, despite some trends. High levels of interindividual variability in the data reduced our ability to detect statistical significance. The high degree of variability in our data is consistent with two notions regarding baroreflex control that have been brought forward recently. First, there is a large amount of interindividual variability in baroreflex sensitivity, which is highly dependent on both sex and age (16, 26) and affects the balance between both neural and hemodynamic influences on baroreflex control. In young women, some of this interindividual variability may be explained by their susceptibility for orthostatic intolerance. In an intriguing study, Wenner et al. (44) recently found that young women with low orthostatic tolerance respond differently to hypotensive stimuli than women with high orthostatic tolerance. Specifically, administration of estradiol blunted the vasoconstrictor response to baroreflex unloading (with lower body negative pressure) in women with low orthostatic tolerance but had no effect in women with high orthostatic tolerance. Because we did not determine orthostatic tolerance in our subjects before enrolling them in the study, we must assume we had subjects with both low and high tolerance. Second, the magnitude of changes in baroreflex sensitivity induced by estrogen and progesterone is likely dependent on the relative concentrations of each. This has been shown to be true regarding resting MSNA (4). Despite administration of identical doses of E2 and P4 across subjects, we recorded a large degree of variability in serum hormone concentration (Study Day 2 hormone concentration ranges: Group 1, progesterone 3.7–9.3 ng/ml; Group 2, 0.1 mg/day estradiol 49.4–238.4 pg/ml; 0.2 mg/day estradiol 64.2–379.8 pg/ml), which may represent individual differences in hormone metabolism. This variability presumably accounts for the large amount of variation in the HR, MAP, and FVC responses to neck pressure.
Finally, we observed significant effects of the hormones on FVC only when the hormone was administered first (i.e., P4 has a significant effect in Group 1-P4 first, and E2 had a significant effect in Group 2-E2 first). This suggests either a possible order effect or an effect of the duration of exposure.
Perspectives.
The neurovascular influences of the female sex hormones in young women have commonly been studied across the menstrual cycle and using techniques that alter blood pressure, such as the Modified Oxford method, and thus rule out the option to study vascular transduction. Very little research has examined the individual effects of estrogen and progesterone, and the present study is the first to study the effects of exogenous hormone administration on the baroreflex-mediated response to neck pressure. Although high progesterone in concurrence with low estrogen does not reflect a normal physiological state, the study of the separate effects of the hormones is important to discern the effects of progesterone from those of estrogen under conditions of high combined hormones. For example, in the current study, we found E2 and P4 to have opposing effects on the change in vascular conductance to baroreceptor unloading, shedding light on the reasons why many studies have found the ovarian hormones to have no effect on sympathetic baroreflex sensitivity when studied in combination in the mid-luteal phase of the menstrual cycle and in combined oral contraceptives. Furthermore, these findings not only advance our understanding of the naturally occurring ovarian hormones but also provide insight into the effects of hormonal treatments on baroreflex function, particularly progestin-only treatments. It is important to understand the influences of estrogen and progesterone on blood pressure regulation, not only to understand how the hormones impact naturally menstruating women but also to minimize cardiovascular risk in the large number of women currently taking combined and progestin-only contraceptives and in postmenopausal women, who are at significantly elevated risk of cardiovascular disease and hypertension.
GRANTS
Blood sample analyses were provided by Oregon Clinical and Translational Research Institute (OCTRI; Portland, OR), supported by a NCRR/NCATS-funded CTSA grant UL1TR000128. This study was supported by National Heart, Lung, and Blood Institute Grant HL-081671.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.E.B. and J.A.M. performed experiments; V.E.B. and L.A.S. analyzed data; V.E.B., L.A.S., and C.T.M. interpreted results of experiments; V.E.B. prepared figures; V.E.B. drafted manuscript; V.E.B., J.A.M., P.F.K., J.R.H., L.A.S., and C.T.M. edited and revised manuscript; V.E.B., J.A.M., P.F.K., J.R.H., L.A.S., and C.T.M. approved final version of manuscript; J.A.M., P.F.K., J.R.H., and C.T.M. conception and design of research.
ACKNOWLEDGMENTS
We thank Emily Martini for help with subject recruitment and data collection, Cory Miner for help with data collection, and the subjects for their participation.
REFERENCES
- 1.Abdel-Rahman AR, Merrill RH, Wooles WR. Gender-related differences in the baroreceptor reflex control of heart rate in normotensive humans. J Appl Physiol 77: 606–613, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Beske SD, Alvarez GE, Ballard TP, Davy KP. Gender difference in cardiovagal baroreflex gain in humans. J Appl Physiol 91: 2088–2092, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Brooks VL, Dampney RAL, Heesch CM. Pregnancy and the endocrine regulation of the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 299: R439–R451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter JR, Fu Q, Minson CT, Joyner MJ. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension 61: 395–399, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter JR, Klein JC, Schwartz CE. Effects of oral contraceptives on sympathetic nerve activity during orthostatic stress in young, healthy women. Am J Physiol Regul Integr Comp Physiol 298: R9–R14, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab 297: E85–E91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 275: R1909–R1920, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Cooke WH, Ludwig DA, Hogg PS, Eckberg DL, Convertino VA. Does the menstrual cycle influence the sensitivity of vagally mediated baroreflexes? Clin Sci 102: 639–644, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Cooper VL, Hainsworth R. Carotid baroreceptor reflexes in humans during orthostatic stress. Exp Physiol 86: 677–681, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Cooper VL, Hainsworth R. Effects of head-up tilting on baroreceptor control in subjects with different tolerances to orthostatic stress. Clin Sci 103: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 11.De Meersman RE, Zion AS, Giardina EG, Weir JP, Lieberman JS, Downey JA. Estrogen replacement, vascular distensibility, and blood pressures in postmenopausal women. Am J Physiol Heart Circ Physiol 274: H1539–H1544, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Ferrer M, Meyer M, Osol G. Estrogen replacement increases beta-adrenoceptor-mediated relaxation of rat mesenteric arteries. J Vasc Res 33: 124–131, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Freedman RR, Sabharwal SC, Desai N. Sex differences in peripheral vascular adrenergic receptors. Circ Res 61: 581–585, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol 587: 2019–2031, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol 589: 5285–5297, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart ECJ, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol 590: 2069–2079, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi K, Miyachi M, Seno N, Takahashi K, Yamazaki K, Sugawara J, Yokoi T, Onodera S, Mesaki N. Fluctuations in carotid arterial distensibility during the menstrual cycle do not influence cardiovagal baroreflex sensitivity. Acta Physiol (Oxf) 186: 103–110, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hedeker D, Gibbons R. Mixed-effects regression models for continuous outcomes. In: Longitudinal Data Analyses. Hoboken, New Jersey: Wiley & Sons, 2006, p. 69–76 [Google Scholar]
- 19.Heesch CM, Foley CM. CNS effects of ovarian hormones and metabolites on neural control of circulation. Ann N Y Acad Sci 940: 348–360, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Hirshoren N, Tzoran I, Makrienko I, Edoute Y, Plawner MM, Itskovitz-Eldor J, Jacob G. Menstrual cycle effects on the neurohumoral and autonomic nervous systems regulating the cardiovascular system. J Clin Endocrinol Metab 87: 1569–1575, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Hunt BE, Taylor JA, Hamner JW, Gagnon M, Lipsitz LA. Estrogen replacement therapy improves baroreflex regulation of vascular sympathetic outflow in postmenopausal women. Circulation 103: 2909–2914, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Jarvis SS, VanGundy TB, Galbreath MM, Shibata S, Okazaki K, Reelick MF, Levine BD, Fu Q. Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. Am J Physiol Regul Integr Comp Physiol 301: R193–R200, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med 85: 447–452, 1976 [DOI] [PubMed] [Google Scholar]
- 24.Kim A, Deo SH, Fisher JP, Fadel PJ. The effect of sex and ovarian hormones on carotid baroreflex resetting and function during dynamic exercise in humans. J Appl Physiol 112: 1361–1371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Laitinen T, Hartikainen J, Vanninen E, Niskanen L, Geelen G, Länsimies E. Age and gender dependency of baroreflex sensitivity in healthy subjects. J Appl Physiol 84: 576–583, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Middlekauff HR, Park J, Gornbein JA. Lack of effect of ovarian cycle and oral contraceptives on baroreceptor and non-baroreceptor control of sympathetic nerve activity in healthy women. Am J Physiol Heart Circ Physiol 302: H2560–H2566, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miner JA, Martini ER, Smith MM, Brunt VE, Kaplan PF, Halliwill JR, Minson CT. Short-term oral progesterone administration antagonizes the effect of transdermal estradiol on endothelium-dependent vasodilation in young healthy women. Am J Physiol Heart Circ Physiol 301: H1716–H1722, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Minson CT, Halliwill JR, Young TM, Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation 102: 1473–1476, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Mohamed MK, El-Mas MM, Abdel-Rahman AA. Estrogen enhancement of baroreflex sensitivity is centrally mediated. Am J Physiol Regul Integr Comp Physiol 276: R1030–R1037, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Montgomery LD, Kirk PJ, Payne PA, Gerber RL, Newton SD, Williams BA. Cardiovascular responses of men and women to lower body negative pressure. Aviat Space Environ Med 48: 138–145, 1977 [PubMed] [Google Scholar]
- 33.Neuhaus JM, Kalbfleisch JD. Between- and within-cluster covariate effects in the analysis of clustered data. Biometrics 54: 638–645, 1998 [PubMed] [Google Scholar]
- 34.Oberyé JJ, Mannaerts BM, Huisman JA, Timmer CJ. Pharmacokinetic and pharmacodynamic characteristics of ganirelix (Antagon/Orgalutran). Part II. Dose-proportionality and gonadotropin suppression after multiple doses of ganirelix in healthy female volunteers. Fertil Steril 72: 1006–1012, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Pawelczyk JA, Raven PB. Reductions in central venous pressure improve carotid baroreflex responses in conscious men. Am J Physiol Heart Circ Physiol 257: H1389–H1395, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Pellinger TK, Halliwill JR. Effect of propranolol on sympathetically mediated leg vasoconstriction in humans. J Physiol 583: 797–809, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rea RF, Eckberg DL. Carotid baroreceptor-muscle sympathetic relation in humans. Am J Physiol Regul Integr Comp Physiol 253: R929–R934, 1987 [DOI] [PubMed] [Google Scholar]
- 38.Saleh MC, Connell BJ, Saleh TM. Autonomic and cardiovascular reflex responses to central estrogen injection in ovariectomized female rats. Brain Res 879: 105–114, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Savage DD, Corwin L, McGee DL, Kannel WB, Wolf PA. Epidemiologic features of isolated syncope: the Framingham Study. Stroke 16: 626–629, 1985 [DOI] [PubMed] [Google Scholar]
- 40.Stachenfeld NS, Taylor HS. Effects of estrogen and progesterone administration on extracellular fluid. J Appl Physiol 96: 1011–1018, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Stachenfeld NS, Taylor HS. Progesterone increases plasma volume independent of estradiol. J Appl Physiol 98: 1991–1997, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Tanaka M, Sato M, Umehara S, Nishikawa T. Influence of menstrual cycle on baroreflex control of heart rate: comparison with male volunteers. Am J Physiol Regul Integr Comp Physiol 285: R1091–R1097, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Tank J. Baroreflex regulation of heart rate and sympathetic vasomotor tone in women and men. Hypertension 45: 1159–1164, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Wenner MM, Haddadin AS, Taylor HS, Stachenfeld NS. Mechanisms contributing to low orthostatic tolerance in women: the influence of oestradiol. J Physiol 591: 2345–2355, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001 [DOI] [PubMed] [Google Scholar]